Abstract
Background: Iatrogenic nerve injuries (NIs) are an under-recognized complication of urological surgery. Though less common than vascular or organ damage, they may cause lasting sensory and motor deficits, significantly affecting patients’ quality of life. With increasing complexity in pelvic procedures, a consolidated understanding of nerve injuries is essential. Purpose: This review aims to synthesize current knowledge regarding peripheral and autonomic NIs in urological surgery, highlighting mechanisms of injury, associated procedures, preventative strategies, and treatment options. Scope: Focused on common urological interventions such as radical prostatectomy, cystectomy, pelvic lymphadenectomy, and reconstructive techniques, the review explores injuries from positional compression, traction, and intraoperative transection to their surgical management. Key Findings: The review categorizes nerve injuries into crush and transection types and details intraoperative signs and repair techniques. Skeletonization of nerves, avoidance of energy devices near neural structures, and prompt end-to-end anastomosis using 7-0 polypropylene are central to management. Adoption of novel sutureless nerve coaptation devices have also been described with promising outcomes. Early repair offers a better prognosis. New intraoperative technologies like NeuroSAFE during robotic-assisted procedures may enhance nerve preservation. Conclusion: Iatrogenic NIs, although rare, are clinically significant and often preventable. Prompt intraoperative recognition and repair are critical. Further research is warranted to develop standardized preventative protocols and enhance intraoperative nerve monitoring. A multidisciplinary approach, extended across surgical specialties, could improve outcomes and guide timely treatment of nerve injuries.
1. Introduction
Iatrogenic nerve injuries (NIs) represent an underappreciated yet potentially debilitating complication of urological surgery [1]. While less common than vascular or organ damage, nerve lesions can result in long-term sensory or motor deficits, significantly impacting patients’ quality of life [2,3]. The risk of these complications is expected to rise alongside the increasing frequency of aggressive oncological resections and complex pelvic procedures, both open and minimally invasive.
Despite this increasing procedural complexity, the literature offers surprisingly limited comprehensive guidance on the peripheral and autonomic nerves at risk during urological surgery. Most existing data are confined to case reports or mentioned only in passing within broader discussions of surgical complications. This lack of structured insight may hinder clinicians’ ability to identify vulnerable nerves during specific procedures, and to effectively anticipate, prevent, or manage resulting injuries.
Several urological interventions are particularly associated with heightened risk of NIs. Pelvic surgeries such as radical cystectomy (RC), radical prostatectomy (RP), pelvic lymphadenectomy (PL), and reconstructive procedures like colposacropexy or psoas hitch ureteric reimplantation frequently pose threats to pelvic nerve integrity. Likewise, inguinal approaches including orchifunicolectomy, inguinal lymphadenectomy (IL), and varicocelectomy, as well as retroperitoneal lymphadenectomy (RL) and subcostal access operations such as percutaneous nephrolithotomy (PCNL) or open radical nephrectomy (RN), may jeopardize neural structures due to direct trauma, excessive retraction, or postoperative complications.
Beyond direct surgical mechanisms, extrinsic factors such as patient positioning, inadequate padding, obesity, and prolonged operative time are well recognized contributors to positional nerve injuries, which often present postoperatively as sensory paraesthesia or motor deficits [3].
This narrative review aims to synthesize current knowledge on iatrogenic NIs in urological surgery, with a particular focus on the peripheral nerves most commonly affected. We outline relevant anatomical pathways, mechanisms of injury, high-risk procedures, and preventative strategies. By consolidating this information, we seek to enhance awareness among urologists and surgical teams, supporting early recognition and the minimization of these, often avoidable, complications.
2. Materials and Methods
This narrative review was designed to explore surgical nerve injuries within urological practice by analyzing and integrating relevant literature. A comprehensive search was conducted using databases such as PubMed, Scopus, and Web of Science, focusing on publications up to April 2025. The search strategy combined MeSH terms and free-text keywords including “urological surgery,” “nerve injury,” “pelvic nerve damage,” and “nerve-sparing techniques.” Only articles published in English were considered. Priority was given to peer-reviewed studies such as original research, reviews, and clinical guidelines that addressed nerve injury types, mechanisms, surgical approaches, and outcomes related to urological procedures. Articles not directly related to urological surgery, or lacking substantive data, were excluded. Relevant studies were carefully reviewed to extract information on anatomical vulnerabilities, intraoperative techniques to avoid nerve damage, monitoring tools, and recovery outcomes. While no formal quality scoring tool was used, studies with robust methodology and clinical relevance were prioritized. The synthesis was qualitative and thematic, aiming to present a coherent and informative overview of how surgical nerve injuries occur, how they are managed, and what strategies might reduce their incidence in urology. Since the review did not involve new data collection, no ethical approval was required.
2.1. Inclusion Criteria
- Peer-reviewed articles, including original research, systematic reviews, meta-analyses, and clinical guidelines, focusing on nerve injuries in urological surgeries.
- Studies discussing anatomical considerations, surgical techniques, prevention strategies, and management of nerve injuries.
- Publications detailing intraoperative monitoring methods and postoperative outcomes related to nerve preservation.
2.2. Exclusion Criteria
- Studies not directly related to urological surgical procedures.
- Non-peer-reviewed articles, editorials, commentaries, and conference abstracts without sufficient data.
- Articles lacking detailed information on nerve injury mechanisms or management strategies.
2.3. Data Extraction and Synthesis
Data from the selected articles were extracted systematically, focusing on the following:
- Types and classifications of nerve injuries (e.g., neurapraxia, axonotmesis, neurotmesis).
- Anatomical pathways of nerves commonly at risk during specific urological procedures.
- Surgical techniques employed to minimize nerve injury risk, including nerve-sparing approaches.
- Use and efficacy of intraoperative neuromonitoring tools.
- Postoperative outcomes and rehabilitation strategies for nerve injuries.
Given the narrative nature of this review, a qualitative synthesis approach was adopted. Findings were organized thematically to provide a coherent overview of the current understanding and practices related to nerve injuries in urological surgery.
2.4. Quality Assessment
While formal quality assessment tools were not employed due to the narrative design of this review, emphasis was placed on including studies with sound methodology, clear objectives, and relevant findings. Preference was given to recent publications and those with significant contributions to the field.
3. Types and Management of Intraoperative and Positional Nerve Injuries in Urological Surgery (Figure 1)
Nerve injuries (NIs) during urological procedures can be broadly categorized as positional or intraoperative. Positional injuries typically result from compression or stretch and do not necessitate surgical intervention. For example, ESWL is generally safe for urinary stones but can rarely cause nerve injuries due to shock wave impact or improper patient positioning. Cases of neuropathy and nerve palsy have been reported, and animal studies suggest possible subtle spinal cord effects. These findings emphasize the need for careful positioning and monitoring to prevent complications, highlighting that even minimally invasive treatments require caution. Further research is needed to better understand and prevent these injuries [4].
The American Urological Association (AUA) classifies intraoperative nerve injuries into crush or transection injuries, which can be partial or complete. Management depends on the type and extent of the injury, as highlighted in Table 1 and Table 2, with the primary aim being timely anatomical restoration to preserve neural conduction [3]. Below we describe common intraoperative mechanisms and specific nerve involvements relevant to pelvic lymphadenectomy (PL), radical cystectomy (RC), and other urological procedures.

Table 1.
Summary of nerves at risk during common urological procedures.

Table 2.
Evidence-based treatment for nerve injuries in urological surgery.
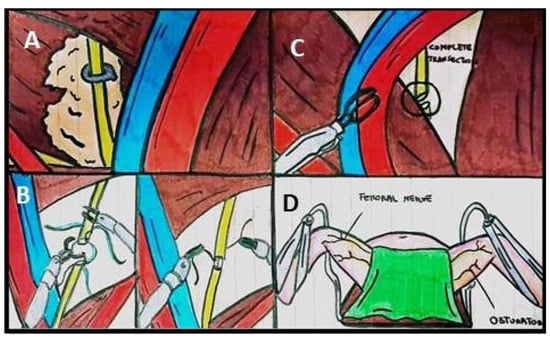
Figure 1.
Illustrations of ways of nerve injuries during urological procedures. (A) Crush injury of obturator nerve with a clip placed transversely during pelvic lymph node packets. (B) Partial Transection with Tension-free approximation (end-to-end obturator nerve). (C) Complete transection. (D) Positional nerve injuries.
4. Crush Injuries with Clips/Suture Ligation
Crush injuries often occur during PL or inguinal lymphadenectomy (IL), particularly when clips (e.g., Hem-o-lok®, Weck Closure Systems, Research Triangle Park, NC, USA) are inadvertently applied near nerves such as the obturator or genitofemoral nerves. These nerves are at risk due to their proximity to lymphatic tissues targeted during dissection.
Intraoperative signs such as abrupt limb movement on the monitor may indicate nerve reflexes and help localize the injury. Once identified, clip removal is the mainstay of treatment. If a dedicated removal tool is unavailable, a needle holder can be used to grip and pry open the clip, though this is technically challenging. Clip destruction using energy devices, despite posing additional risk to nerve function, may also be considered in expert hands.
To prevent such injuries, it is advised that clips or sutures are applied parallel rather than perpendicular to nerve trajectories and when possible after clear visualization of the nerve course. To avoid this type of injury, direct visualization of nerves before placing the clip and proceeding with dissection is recommended [8].
5. Transection Injuries
Transection injuries may result from thermal energy or cold instruments. They often manifest with sudden movement of the innervated region, prompting the surgeon to halt dissection to avoid worsening the lesion. Three scenarios may present in nerve transection injuries:
- Feasible approximation with no tension (partial or complete);
- Challenging approximation, requiring release techniques or grafts;
- Hidden proximal nerve stump, discovered only after further dissection [8].
6. Partial or Complete Transection with Feasible Approximation
In the case of a cold-blade injury where nerve ends are clearly visible, the next step is to attempt a tension-free approximation. Begin by carefully skeletonizing the nerve, ensuring full exposure of the injury site. If the transection is partial, it is crucial not to separate the remaining attached nerve ends, as they will help maintain alignment during repair. Once the nerve injury is adequately exposed, perform a simple end-to-end interrupted epineural anastomosis with 7-0 polypropylene (Prolene®, Ethicon Inc., Cornelia, GA, USA).
In cases of complete transection, the surrounding tissue should be carefully removed to reduce tension at the nerve ends. If feasible, attempt a tension-free approximation of the nerve endings. When this is achieved, perform an end-to-end interrupted epineural anastomosis using 7-0 polypropylene along the periphery of the nerve. This ensures optimal alignment and promotes nerve healing.
7. Complete Transection with Challenging Approximation
If the nerve ends are retracted or the dissection is challenging, positioning maneuvers can help release tension and facilitate approximation. Hip flexion and transitioning from an extended lithotomy Trendelenburg position to a more neutral stance can help reduce spinal tension and align the nerve ends.
In cases where the transection is caused by a stapler, both nerve endings must be properly debrided and prepared before attempting any repair. Once debridement is complete, a simple end-to-end interrupted epineural anastomosis with 7-0 polypropylene (Prolene®, Ethicon Inc.) should be performed for nerve repair.
However, if the nerve injury is extensive and tension-free approximation is not possible, the only viable option is to use a cadaveric nerve graft. This graft, after being cut to the appropriate length, can bridge the gap between the two nerve ends. After aligning the graft, perform two end-to-end epineural anastomoses using 7-0 polypropylene (Prolene®, Ethicon Inc.), one for the proximal nerve stump and one for the distal nerve stump. This procedure provides a suitable pathway for nerve regeneration.
8. Complete Transection with Hidden Proximal Nerve Ending
During urological surgeries, the use of electrocautery can result in thermal damage, which may cause retraction or concealment of nerve endings in narrow or deep spaces. In such cases, the retracted nerve ends can be difficult to identify, requiring careful and meticulous dissection to expose the hidden nerve stump. Once the nerve ends are located, a simple end-to-end interrupted epineural anastomosis should be performed using 7-0 polypropylene (Prolene®, Ethicon Inc.) to restore continuity.
It is essential that the dissection is performed with great caution to avoid further damage, especially when using thermal instruments. The goal is to locate the nerve ends, relieve any tension, and restore proper nerve function through a careful anastomosis [8].
Although sutured neurorrhaphy is widely regarded as the standard approach for repairing peripheral nerves, it presents notable challenges: it demands precision, is time-consuming, and requires advanced microsurgical skills [9].
Nerve Tape is a sutureless device designed to simplify peripheral nerve repair. Made from bioengineered tissue with embedded microhooks, it enables quick and consistent end-to-end nerve approximation. It is easy to apply and available in multiple sizes [9].
In a recent cadaveric study, Eberlin KR et al. compared the nerve repair between senior surgeons and residents obtained through microsuture or application of the nerve tape in terms of time, tensile strength, and grade of alignment and technical repair quality. They demonstrated a substantial reduction in time for repairs with the novel coaptation device combined with significantly higher rates of repair quality and higher average peak tensile force when compared with suture repairs. Interestingly, the suture repair is experience dependent, while the outcomes of the nerve tape repair do not depend on the surgeon experience level, abolishing a learning-curve effect [9]. Transection nerve injuries made by electrocautery are generally classified as blunt transection injuries rather than sharp transection injuries. Blunt transection injuries tend to damage the nerve stumps for some distance along the stump, causing the nerve to degenerate for a distance during the healing process. This can result in failure of the anastomosis. In the extremities, where nerves are easily accessible surgically, one would normally wait 3–4 weeks before repairing blunt transection injuries to allow the devitalized tissue to die back, trim the nerve end back to healthy nerve tissue, and then perform the repair. Unfortunately, this delayed repair strategy becomes more difficult in deep surgical regions such as the abdomen and pelvis, where delayed reoperative exploration of the cut nerve a month or so later would entail significant surgical risks. One compromise that many peripheral nerve surgeons recommend in the setting of a blunt nerve injury that occurs in an area of the body that would be difficult to repair in a delayed fashion (e.g., abdomen and pelvis) is to trim the transected nerve back a few millimeters to healthy tissue and then perform the repair. This removes the potentially thermally damaged nerve tissue before the repair is performed. it is not a perfect solution but it is better than repairing nerve tissue that has been directly cauterized.
9. Specific Nerve Injuries in Urological Surgery (Figure 2)
9.1. Obturator Nerve
The obturator nerve is a mixed nerve originating from the front of the L2–L4 spinal nerves of the lumbar plexus [10,11,12]. It follows the iliopectineal line, descending through the psoas muscle towards the obturator canal, where it divides into two branches. The anterior branch innervates the adductor muscles (Longus, Brevis, and Gracilis), while the posterior branch travels between the Brevis adductor and the Longus and Brevis adductors. The anterior branch also forms anastomoses with the femoral nerve branches, including the inferomedial cutaneous nerve of the thigh and the knee joint [3].
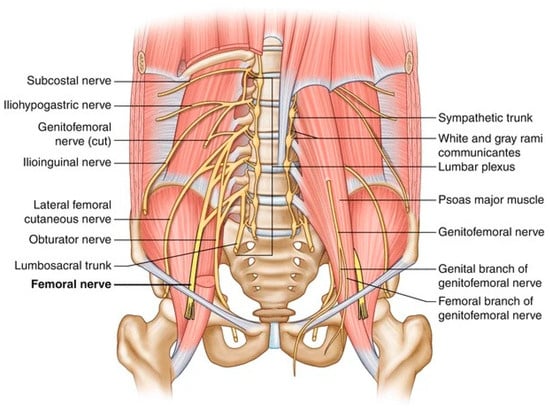
Figure 2.
Illustration of the pathway of the main nerves that could be damaged during urological surgeries.
Injury to the obturator nerve commonly presents as hypoesthesia, paraesthesia, or pain in the medial thigh, groin, or pubic bone. A patient may also experience weakness and a sense of leg instability. A characteristic gait abnormality, including circumduction of the externally rotated hip and reduced adduction and internal rotation of the hip, may be observed.
Although obturator nerve injury is relatively rare (0.2–5.7%), it is a significant complication, particularly during pelvic surgeries such as prostatic adenomectomy (PA) [13,14]. The nerve may also be damaged during dissection of the lateral peduncle in radical cystectomy (RC) [8] or in transobturator tape (TOT) surgery (0.7–0.9%) [15]. In PL, the nerve can be ligated using Hem-o-lock clips or transected with clips and cold Endoshears, as reported by Moinzadeh. When the nerve is transected, the Hem-o-lock clips must be removed using a harmonic scalpel, and the nerve ends should be re-approximated with 7-0 Prolene sutures in interrupted fashion, opposing the perineural sheath. The robotic-assisted suturing technique can facilitate this repair [16,17].
9.2. Genitofemoral Nerve
The genitofemoral nerve, a mixed nerve originating from L1–L2, emerges from the front of the psoas muscle, running downward parallel to the ureter and laterally to the common iliac vessels, ultimately reaching the external iliac artery. At the level of the inguinal ligament, it divides into two branches: the genital branch, which innervates the cremaster muscle and the skin of the external genitalia, and the femoral branch, which is exclusively cutaneous, supplying the anterosuperior thigh [3,18]
Damage to the genitofemoral nerve typically presents as pain and a burning sensation in the groin that radiates into the inner thigh. Symptoms are aggravated by activities such as walking, bending, or hip hyperextension, leading to tenderness and possibly hyperesthesia along the inguinal canal. A prospective cohort study of 616 women undergoing gynecologic surgery reported a single genitofemoral nerve injury, indicating an incidence of approximately 0.16% [18]. Another retrospective review of 1210 major pelvic surgeries identified four genitofemoral nerve injuries, yielding an incidence of about 0.33% [19].
The nerve may be injured during procedures such as distal ureterectomy, psoas hitch of the bladder, or prostatic adenectomy (PA). However, few reports describe nerve sectioning and repair in such cases. Soares-Aquino et al. noted genitofemoral nerve damage as a complication in 3% of varicocelectomies during vessel ligation [20], while Sandorf et al. described a case of nerve injury during living donor nephrectomy involving ureter stapler dissection. In this case, the repair involved removing clips and fibrous tissue surrounding the nerve [15]. Avoiding placing stitches transversally to the axis of the psoas muscle when hitching the bladder may also prevent damage, particularly in cases where the nerve is not visible.
9.3. Femoral Nerve
The femoral nerve is a mixed nerve originating from the L1–4 nerve roots. It runs along the lateral margin of the psoas muscle, crossing the inguinal ligament (dorsally) and passing laterally to the iliopectineal arch. Within the abdomen, it innervates the psoas, iliacus, and pectineus muscles, and provides a branch for the femoral artery. After passing the inguinal ligament, it bifurcates into two branches: one anterior (lateral and medial femoral cutaneous nerves) and one posterior (motor branches for the quadriceps femoris and the saphenous nerve, which innervates the skin of the anterior thigh and medial part of the leg) [3].
Iatrogenic damage to the femoral nerve is a rare but significant complication in urological surgery, particularly during procedures such as inguinal lymphadenectomy (IL) for penile cancer, renal transplantation, and radical cystoprostatectomy (RC) [15]. There have also been reports of femoral neuropathy following nephrectomy, often attributed to excessive nerve elongation in the lateral decubitus position, which may alter the anatomical planes and contribute to injury [21].
Studies have reported incidence rates ranging from 0.7% to 11.6%, influenced by factors such as the use of self-retaining retractors and patient positioning. For instance, the use of self-retaining retractors has been associated with higher incidence rates, with one study reporting a decrease from 7.5% to 0.7% when these retractors were not used. Additionally, improper patient positioning, particularly in the lithotomy position with excessive hip flexion and abduction, can increase the risk of femoral nerve injury [22].
The most common mechanism of femoral nerve injury during surgery is compression by self-locking spreaders, typically used in complex procedures like RC or radical prostatectomy (RP) in the “open” technique. Femoral neuropathy can affect up to 10% of patients undergoing laparotomy [23]. Symptoms include sensory and motor impairment, such as anesthesia of the anterior and medial thigh and weakness in the quadriceps and iliopsoas muscles. While most cases resolve spontaneously, severe damage may result in significant disability, impairing activities like climbing stairs. The prognosis is generally favorable, with most patients experiencing motor and sensory recovery within 6 to 12 months. Neurolysis of nerve grafting may be considered in exceptional cases [24].
9.4. Ilioinguinal and Iliohypogastric Nerves
The ilioinguinal nerve, originating from L1, provides motor innervation to the abdominal wall and sensory innervation to the hypogastric region and medial thigh. It divides at the level of the anterior superior iliac spine into an abdominal branch (innervating the muscles of the abdomen and the skin of the hypogastric region) and a cutaneous genital branch (innervating the medial thigh, external genitalia, and abdominal wall).
The iliohypogastric nerve, originating from T12-L1, provides motor branches to the rectus abdominis and pyramidal muscles, and sensory branches to the hypogastric region, buttocks, and external genitalia. It travels in front of the quadratus lumborum muscle before reaching the transversus abdominis muscle and often anastomoses with the ilioinguinal nerve [3].
Injury to these nerves typically results in acute, burning pain radiating to the genitals and thigh. The trauma can occur during procedures such as orchiectomy, varicocelectomy, nephrectomy and even after abdominal wall closure or trocar placement in the lower abdomen. Pareses of abdominal muscles due to trocar placement are rare due to overlap in innervation and relatively small sizes of trocar incisions [25].
Reported incidence ranges from 0.16% to 4.9%, depending on surgical type and technique higher in laparoscopic procedures with lateral port placement. Injuries typically result from direct trauma or suture entrapment and cause sharp pain and paraesthesia in the groin, thigh, or genital area. Prompt diagnosis is key to preventing chronic pain [26].
Although intraoperative nerve sectioning is rare, entrapment or stretching of these nerves has been reported following abdominal surgery. Hawksworth et al. described bladder pain syndrome after laparoscopic pyeloplasty, attributing the pain to nerve entrapment during abdominal wall closure. The resolution of symptoms required nerve sectioning [27].
9.5. Hypogastric Plexus
The hypogastric plexus, located in front of the sacrum, is responsible for autonomic innervation of pelvic organs. It receives afferents from the hypogastric nerves, which contribute to the sympathetic system. Flynn et al. emphasized the importance of carefully exposing vascular structures in the presacral space before suturing for colposacropexy, as injury to autonomic nerves can lead to urinary and sexual dysfunctions post-surgery [26,27]. These dysfunctions are thought to result from damage to the hypogastric plexus, which provides vital innervation to pelvic organs. Shiozawa et al. recommend a right peritoneal longitudinal incision to protect the hypogastric plexus and improve surgical outcomes in sacrocolpopexy [8]. During RALP, rates of hypogastric plexus injury range from 10% to 30%, often related to nerve-sparing techniques and surgeon experience [28].
During presacral lymphadenectomy, there is a significant risk of injuring the superior hypogastric plexus. Injury to the superior hypogastric plexus during dissection can lead to postoperative complications such as urinary and sexual dysfunctions, including retrograde ejaculation in men. The risk is heightened during extensive lymphadenectomy procedures where the dissection extends close to the aortic bifurcation and sacral promontory, areas where the plexus is most vulnerable [3].
Retroperitoneal lymphadenectomy (RPLND) is a procedure in the management of testicular cancer. During RPLND, lymph nodes are removed from the retroperitoneum to assess and treat potential metastatic spread. However, when the dissection is extensive, especially near the aortic bifurcation and along the interaortocaval region, there is a risk also in this case to damage the superior hypogastric plexus.
Damage during RPLN, particularly in non-nerve-sparing approaches, can result in complications such as retrograde ejaculation or anejaculation, affecting fertility and quality of life in young male patients.
Nerve-sparing techniques have been developed to preserve the sympathetic fibers while still achieving oncologic control. These approaches can significantly reduce the incidence of ejaculatory dysfunction, maintaining normal sexual function in most patients [29].
9.6. Cavernous Nerves
The cavernous nerve originates primarily from the pelvic plexus and contains both sympathetic fibers (from T11-L2) and parasympathetic fibers (from S2–S4). It is a crucial component of the neurovascular bundle that surrounds the prostate and extends to the membranous urethra. The cavernous nerve plays a key role in erectile function and urinary continence by regulating the transverse urethral muscle [28].
Damage to the cavernous nerve can result from procedures such as transurethral resection of the prostate (TURP), radical cystectomy (RC), or robot-assisted laparoscopic prostatectomy (RL). Postoperative erectile dysfunction (ED) has been reported in 4–35% of patients following TURP [29], incidence up to 70% post RALP and ED is a common, albeit often underreported, complication following RC, particularly in women [27]. The injury to the cavernous nerve can be either direct or indirect and may be acute or chronic. It can result from traction, clamping, dissection, freezing, electrocautery, excision, or irradiation. Unfortunately, surgical repair of cavernous nerve damage is not feasible, and the focus is instead on preserving the nerve during dissection. Intrafascial nerve-sparing techniques that present the neural network within the prostatic fascia should be adopted when oncologically safe to enhance nerve-preservation [30,31].
9.7. Intercostal Nerve
Intercostal nerves, part of the somatic nervous system, originate from the anterior branches of the thoracic spinal nerves (T1–T11), innervating the thoracic pleura and abdominal peritoneum. Damage to these nerves can occur during percutaneous supracostal nephrostolithotomy (PCNL) or any other procedure involving intercostal access, such as nephrostomy or posterior approach nephrolithotomy [22,32,33,34,35,36,37,38] or intercostal nephrectomies.
McAllister et al. studied anatomical relationships relevant to percutaneous access and found that the intercostal artery was exposed under the eleventh rib in 30%, 60%, and 70% of cadavers, respectively, with a distance of 6 mm from the rib in 25% of intercostal spaces [37]. To reduce nerve injury, they recommend accessing the paraspinal muscles and lower half of the eleventh intercostal space. Intercostal nerve injury typically presents with acute pain, burning, and decreased skin sensitivity. Abdominal muscle weakness may lead to unilateral abdominal swelling or intercostal hernia. While nerve transection cannot be repaired, most cases of neuropathy are self-limiting and resolve spontaneously over several months, unless the injury is severe.
9.8. Pudendal Nerve
The pudendal nerve is a mixed motor and sensory nerve, innervating the external urethral sphincter (both male and female) and external anal sphincter, as well as providing sensory innervation to the external genitalia, anus, and perineum. Being frequently entrapped, the technique of pudendal nerve release has been described and recently the first robotic-series has been published [31]. Although rare, pudendal nerve injury can occur during procedures such as TOT and TVT. However, there are few documented cases of pudendal nerve injury in the literature [39]. Other authors report pudendal nerve injury during perineal prostatectomy [35] or RALp [36]. A systematic review reported a 1.8% incidence of pudendal nerve injury following hip arthroscopy, with higher rates (up to 4.3%) associated with the use of a perineal post during surgery. In orthopedic procedures, the incidence of pudendal nerve palsy ranges from 1.9% to 27.6%, often due to prolonged traction or improper positioning. Most cases are transient, resolving within weeks to months, but some may lead to persistent neuropathic pain [39].
10. Analysis of the Available Literature on the Mechanisms of Nerve Damage and Innovative Treatment Strategies
NIs in urological surgery commonly result from direct trauma, excessive traction, compression from retractors, or even thermal damage from cautery devices. These injuries are particularly prevalent in complex pelvic procedures, including radical prostatectomy and cystectomy, where structures such as the pelvic plexus or femoral nerve are at risk.
For instance, prolonged use of self-retaining retractors can compress the femoral nerve, resulting in postoperative weakness or numbness. Similarly, patient positioning, especially in lithotomy with excessive hip flexion or abduction may stretch the lateral femoral cutaneous nerve, leading to paresthesia or meralgia paresthetica. Despite advancements in surgical techniques and anatomical understanding, these injuries remain underrecognized and underreported. Intraoperative nerve monitoring and preoperative planning can help mitigate risks, but awareness and early detection post-surgery are crucial. Prompt recognition and multidisciplinary management involving urologists, neurologists, and physiotherapists can significantly improve recovery and reduce the risk of chronic neuropathic pain.
Nerve injuries during urological surgeries represent a significant concern, leading to patient morbidity, increased healthcare costs, and potential legal implications.
The intricate nature of pelvic anatomy and the close proximity of critical neural structures demand meticulous dissection and thorough anatomical knowledge to minimize intraoperative risks. To this end, several innovative strategies have been employed to mitigate nerve injuries, such as intraoperative neurophysiological monitoring (IONM) and advanced imaging modalities. IONM techniques, including electromyography (EMG) and somatosensory evoked potentials (SSEPs), allow for real-time monitoring of nerve function during surgery, enabling immediate detection and correction of potential nerve injuries.
Advanced imaging modalities, including high-resolution magnetic resonance imaging (MRI) or diffusion tensor imaging (DTI) are increasingly employed in preoperative planning to map neural pathways and support nerve-sparing approaches [40].
Robotic-assisted surgery has further contributed to lower rates of nerve injury by enhancing visualization and precision, although careful patient positioning remains essential to prevent positioning-related neuropathies [41]. Nerve-sparing techniques, especially during RP, have shown improved functional outcomes without compromising oncological control. However delayed recognition and referral of nerve injuries remain as significant issues. A study reviewing 100 cases of iatrogenic nerve injuries found that approximately one-third of referrals were made more than a year after the initial surgery. Contributing factors include limited awareness among clinicians, inadequate recognition of early clinical signs, insufficient physical examination, and overreliance on neurophysiological tests. Delayed diagnosis may result in irreversible neurological deficits and chronic neuropathic pain, underscoring the importance of prompt recognition and a multidisciplinary management approach involving urologists, neurologists, and physiotherapists [42].
11. Discussion
The occurrence of iatrogenic nerve injuries (NIs) during urological surgery is challenging to quantify, but approximately 5% of patients may develop clinically significant neuropathic pain postoperatively [1]. This is especially noted in laparoscopic or robotic procedures involving lateral port placements, fascial closures, or “blind” pelvic surgeries such as transvaginal tape (TVT) or transobturator tape (TOT) procedures [43].
Nerve injuries can arise from crushing, stretching, partial or complete transection, electrocoagulation, or ligation. Understanding the mechanisms of these intraoperative adverse events is key to determining the best approach for rapid management and minimizing long-term sequelae [8,41,42,44]. Peripheral nerve damage can be classified as neurapraxia, axonotmesis, or neurotmesis [45]. Neurapraxia is a demyelinating lesion that affects normal nerve impulse conduction without any anatomical interruption. This condition has a good prognosis, with full recovery typically occurring within 6–8 weeks. Axonotmesis involves damage to the axon and myelin sheath, while supporting structures remain intact. Recovery of this injury depends on the length of the damage, ranging from 6 months to 1 year. Neurotmesis, the complete transection of both the axon and supporting structures, has a poor prognosis, but surgical management can improve the chances of regaining partial or complete neuronal function [46] Axonal recovery has been reported up to 6 months postoperatively [47].
To prevent nerve injury during urological procedures requiring tissue dissection, we recommend skeletonizing the nerves to separate them from surrounding structures before applying clips or suture ligation. Titanium clips are more challenging to remove while the harmonic scalpel has been described as a safe option to remove Hem-o-lock clips, reducing the risk of further tissue damage due to crushing [30]. Additionally, bipolar electrocauterization should be used to accurately identify bleeding areas without grasping the tissues blindly, further minimizing nerve damage [48].
Two key considerations should be made when dealing with nerve injuries: considering the possibility of NIs during dissection and performing immediate repair when feasible. This approach promotes neurological healing and decreases the potential severity of permanent sequelae [49,50,51]. Intraoperative nerve repair, when possible, has an excellent prognosis. However, not all nerves can or should be repaired, such as the erecting nerves or intercostal nerves [52]. A significant role in repair is played by a free-tension end-to-end anastomosis. If this is not possible, nerve graft interposition (autograft, allograft, or artificial grafting) is an attractive alternative for restoring function. A systematic review reported that 86% of patients undergoing nerve grafting for obturator nerve injuries achieved full recovery within 6 months [53]. In all cases, urologists should consult with a neurosurgeon to assess the injury and determine the most appropriate course of treatment.
In the field of new technologies applied to the preservation of the neurovascular bundles, NeuroSAFE is an intraoperative technique developed by Martini Klinik to identify and preserve neurovascular bundles during robotic-assisted radical prostatectomy (RARP) [54,55]. Following prostate removal, immediate frozen sections of the surgical margins are performed to study the proximity of the tumor to the neurovascular bundles. Despite some controversy surrounding the technique, current results suggest that it increases the rate of nerve-sparing surgery without negatively affecting oncological outcomes [50]. Increasingly sophisticated techniques, such as robotic surgery, contribute to improved visualization of anatomical structures. However, experience and surgical knowledge remain indispensable in limiting any form of neuronal damage.
Moreover, emerging techniques such as electrical stimulation have demonstrated potential in enhancing axonal growth and functional recovery by modulating neurotrophic signaling pathways. Meanwhile, experimental approaches like nano neuro knitting and photoacoustic silk scaffolds offer futuristic possibilities, promoting axonal extension through scaffold-guided or light-mediated mechanisms. Despite their potential, the application of these techniques varies depending on the nerve type, injury severity, and clinical context, underscoring the need for further comparative studies to optimize outcomes [56,57].
While extensive literature exists for the obturator nerve due to its higher susceptibility to injury, comprehensive data on other nerve injuries remain limited [45] Nerve transection, a severe form of injury, is rare; the majority of reported nerve injuries stem from elongation or entrapment [46]. Typically, these injuries manifest with partial or complete spontaneous resolution within 6 to 12 months, contingent on the severity and nature of the nerve damage [47]. The spectrum of nerves susceptible to injury during urological interventions encompasses the obturator nerve, genitofemoral and femoral nerves, ilioinguinal and iliohypogastric nerves, pudendal nerve, intercostal nerve, cavernous nerves, and the hypogastric plexus [5,7,13,15,58,59,60].
The present review highlights that despite the heterogeneity in nerve injury patterns—mechanical, thermal, traction-based—several key principles remain constant: early identification, skeletonization of nerves, meticulous dissection, and tension-free epineural repair.
A notable strength of this review is the effort made to consolidate fragmented data into a clear, structured summary that is accessible to both general urologists and subspecialists. It provides a practical, anatomy-oriented overview to guide intraoperative prevention and evidence-based treatment strategies. While the limited availability of high-quality data restricts the breadth of the review, this limitation lies within the literature itself, not the review’s design. Importantly, this work may serve as a model for similar cross-disciplinary initiatives to enhance understanding of nerve injuries across other surgical domains.
The lack of standardized protocols for managing iatrogenic nerve injuries has been identified as a significant issue. A study analyzing urinary system iatrogenic injuries highlighted that the absence of unified algorithms and typical procedural mistakes contribute to the occurrence of such incidents. The research concluded that a more profound interdisciplinary interaction in all treatment phases, along with the identification and elimination of procedural mistakes, could partially address this issue [61].
Multidisciplinary collaboration is essential for the effective management of iatrogenic nerve injuries. A study examining collaborative operations involving urology and other surgical specialties over thirteen years found that such collaborations led to better outcomes in managing iatrogenic injuries. The research emphasized the importance of interdisciplinary interaction in interpreting examination results, making comprehensive clinical diagnoses, and choosing effective treatment policies [6].
Therefore, the development of prospective studies and multidisciplinary registries is vital for accurately quantifying the incidence of iatrogenic nerve injuries, refining intraoperative monitoring techniques, and establishing unified management protocols. Collaborative, specialty-agnostic approaches should be pursued to reduce the incidence and consequences of these injuries, ultimately improving patient outcomes in urological practice.
The existing literature on surgical nerve injuries in urology highlights significant concerns regarding the recognition, management, and prevention of iatrogenic nerve damage. A recurring issue is the delay in referral to nerve specialists, with studies indicating that approximately one-third of referrals occur more than a year post-operation, potentially compromising patient outcomes. This delay is often attributed to a lack of awareness or misinterpretation of postoperative nerve palsy as self-resolving, leading to missed opportunities for timely intervention [62].
Moreover, while advancements in surgical techniques, such as nerve-sparing approaches in RALP, have been developed to mitigate nerve damage, their effectiveness is sometimes limited by anatomical complexities and the subjective nature of intraoperative decisions. The literature also underscores the importance of intraoperative neuromonitoring; however, its adoption varies, and its efficacy is still under evaluation [63].
Furthermore, the heterogeneity of nerve injuries and the variability in reporting standards pose challenges in establishing consistent data, making it difficult to draw definitive conclusions or develop standard is ed protocols. This inconsistency hampers the ability to compare outcomes across studies and to formulate evidence-based guidelines [7].
In summary, while strides have been made in understanding and preventing nerve injuries in urological surgeries, the literature reveals gaps in timely diagnosis, standardized reporting, and the implementation of preventive strategies. Addressing these issues requires a concerted effort to enhance clinician education, establish uniform reporting systems, and promote multidisciplinary collaboration to improve patient care and outcomes.
12. Conclusions
Iatrogenic nerve injuries, though rare in urological surgery, can have significant long-term consequences. This review highlights the need for precise anatomical knowledge, early recognition, and timely intervention using techniques such as tension-free epineural anastomosis or nerve grafting. It offers a practical classification of injuries and management strategies to support surgical decision-making. Given the limited data available, further research is essential to establish standardized prevention protocols and enhance intraoperative monitoring. A multidisciplinary approach will be crucial to improving outcomes and reducing the burden of nerve injuries across surgical fields.
Author Contributions
Conceptualization: G.C., S.I. and A.D.A.; methodology: G.C., S.I. and A.D.A.; data Collection: G.C.; writing—original draft: G.C. and S.I.; writing—review, editing: S.I. and A.D.A.; supervision: A.D.A., V.M.A., P.S. and R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to express our sincere gratitude to the Fondazione ‘Enrico ed Enrica Sovena’ (Rome, Italy) for their valuable support and contribution, which, while not directly funding this specific study, has been instrumental to our broader research efforts.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
Iatrogenic nerve injuries (NIs), American Urological Association (AUA), radical cystectomy (RC), radical prostatectomy (RP), pelvic lymphadenectomy (PL), percutaneous nephrolithotomy (PCNL) or open radical nephrectomy (RN), inguinal lymphadenectomy (IL), prostatic adenomectomy (PA), transobturator tape (TOT), Retroperitoneal lymphadenectomy (RPLND), transurethral resection of the prostate (TURP), robot-assisted laparoscopic prostatectomy (RL), Postoperative erectile dysfunction (ED), Transvaginal tape (TVT), robotic-assisted radical prostatectomy (RARP).
References
- Figler, B.D.; Hoffler, C.E.; Reisman, W.; Carney, K.J.; Moore, T.; Feliciano, D.; Master, V. Corrigendum to “Multi-disciplinary update on pelvic fracture associated bladder and urethral injuries” [JINJ 43/8 (2012) 1242–1249]. Injury 2013, 44, 1967. [Google Scholar] [CrossRef]
- Carando, R.; Afferi, L.; Marra, G.; Krajewski, W.; Pagliarulo, V.; Abufaraj, M.; Xylinas, E.; Cathelineau, X.; Sanchez-Salas, R.; Moschini, M. The effectiveness of multiparametric magnetic resonance imaging in bladder cancer (Vesical Imaging-Reporting and Data System): A systematic review. Arab. J. Urol. 2020, 18, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Nerve injuries. American Urological Association. Education and Research, Inc. Available online: https://auau.auanet.org/system/files/6-Raman%20Complications%20WITH%20ANSWERS_0.pdf (accessed on 7 August 2023).
- Konczak, C.R. Ulnar nerve neuropraxia after extracorporeal shock wave lithotripsy: A case report. J. Can. Chiropr. Assoc. 2005, 49, 40–45. [Google Scholar] [PubMed] [PubMed Central]
- Ríos, E.; Martínez-Piñeiro, L. Treatment of posterior urethral distractions defects following pelvic fracture [Review of Treatment of posterior urethral distractions defects following pelvic fracture]. Asian J. Urol. 2017, 5, 164. [Google Scholar] [CrossRef] [PubMed]
- Willand, M.P.; Nguyen, M.-A.; Borschel, G.H.; Gordon, T. Electrical Stimulation to Promote Peripheral Nerve Regeneration [Review of Electrical Stimulation to Promote Peripheral Nerve Regeneration]. Neurorehabilit. Neural Repair. 2015, 30, 490. [Google Scholar] [CrossRef]
- Sahai, A.; Ali, A.; Barratt, R.; Belal, M.; Biers, S.; Hamid, R.; Harding, C.; Parkinson, R.; Reid, S.; Thiruchelvam, N. British Association of Urological Surgeons (BAUS) consensus document: Management of bladder and ureteric injury [Review of British Association of Urological Surgeons (BAUS) consensus document: Management of bladder and ureteric injury]. BJU Int. 2021, 128, 539. [Google Scholar] [CrossRef]
- Mills, J.T.; Burris, M.B.; Warburton, D.J.; Conaway, M.R.; Schenkman, N.S.; Krupski, T.L. Positioning injuries associated with robotic assisted urological surgery. J. Urol. 2013, 190, 580–584. [Google Scholar] [CrossRef] [PubMed]
- La Riva, A.; Sayegh, A.S.; Perez, L.C.; Poncel, J.; Medina, L.G.; Adamic, B.; Powers, R.; Cacciamani, G.E.; Aron, M.; Gill, I.; et al. Obturator Nerve Injury in Robotic Pelvic Surgery: Scenarios and Management Strategies. Eur. Urol. 2023, 83, 361–368. [Google Scholar] [CrossRef]
- Eberlin, K.R.; Safa, B.; Buntic, R.; Rekant, M.S.; Richard, M.J.; Styron, J.F.; Bendale, G.; Isaacs, J. Usability of Nerve Tape: A Novel Sutureless Nerve Coaptation Device. J. Hand Surg. Am. 2024, 49, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.C.; Sayegh, A.S.; La Riva, A.; Medina, L.G.; Adamic, B.; Powers, R.; Gill, I.S.; Sotelo, R. V11-01 Obturator nerve injury during robotic pelvic surgery: How to approach it? J. Urol. 2022, 207 (Suppl. S5), e921. [Google Scholar] [CrossRef]
- Tinelli, R.; Uccella, S.; Nappi, L.; D’amato, G.; Cicinelli, E.; Angioni, S. Obturator nerve injury in a chemo and radio-resistant patient with a locally-advanced cervical cancer after two previous uterine artery embolizations for severe vaginal bleeding: Case report and review of literature. Eur. J. Obs. Gynecol. Reprod. Biol. 2020, 252, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Göçmen, A.; Şanlıkan, F. Immediate repair of an incompletely transected obturator nerve during robotic-assisted pelvic lymphadenectomy. J. Minim. Invasive Gynecol. 2015, 22, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.; Markovich, B. Anatomy, abdomen and pelvis, obturator nerve. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gözen, A.S.; Aktoz, T.; Akin, Y.; Klein, J.; Rieker, P.; Rassweiler, J. Is it possible to draw a risk map for obturator nerve injury during pelvic lymph node dissection? The Heilbronn experience and a review of the literature. J. Laparoendosc. Adv. Surg. Tech. A 2015, 25, 826–832. [Google Scholar] [CrossRef]
- Sotelo, R.; Arriaga, J.; Aron, M. (Eds.) Complications in Robotic Urologic Surgery; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Aydogmus, S.; Kelekci, S.; Aydogmus, H.; Ekmekci, E.; Secil, Y.; Ture, S. Obturator Nerve Injury: An Infrequent Complication of TOT Procedure. Case Rep. Obs. Gynecol. 2014, 2014, 290382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moinzadeh, A.; DeLong, J.; Tuerk, I.A.; Sorcini, A. Obturator nerve injury during robotic assisted radical cystectomy: Recognition of injury and robotic repair. J. Urol. 2009, 181, 602–603. [Google Scholar] [CrossRef]
- Bohrer, J.C.; Walters, M.D.; Park, A.; Polston, D.; Barber, M.D. Pelvic nerve injury following gynecologic surgery: A prospective cohort study. Am. J. Obs. Gynecol. 2009, 201, 531.e1–531.e7. [Google Scholar] [CrossRef] [PubMed]
- Forte, F.; De Santis, E.; Introini, C.; Asimakopoulos, A.; Cirocchi, R.; Palmieri, M.; Serraino, A.; Cofone, L.; Artico, M.; Galassi, F.M. Genitofemoral nerve course and branching variations: What we see during laparoscopic extended pelvic lymph-node dissection in radical prostatectomy for prostate cancer and how to avoid intraoperative lesions? A retrospective analysis. Folia Morphol. 2025. [CrossRef] [PubMed]
- Soares-Aquino, C.; Vasconcelos-Castro, S.; Campos, J.M.; Soares-Oliveira, M. 15-Year varicocelectomy outcomes in pediatric age: Beware of genitofemoral nerve injury. J. Pediatr. Urol. 2021, 17, 537.e1–537.e5. [Google Scholar] [CrossRef] [PubMed]
- Gibelli, F.; Ricci, G.; Sirignano, A.; Bailo, P.; De Leo, D. Iatrogenic femoral nerve injuries: Analysis of medico-legal issues through a scoping review approach. Ann. Med. Surg. 2021, 72, 103055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandford, R.; Nicholson, M.L. Genito-femoral nerve entrapment: A complication of stapling the ureter during laparoscopic live donor nephrectomy. Nephrol. Dial. Transplant. 2001, 16, 2090–2091. [Google Scholar] [CrossRef]
- Burnett, A.L.; Brendler, C.B. Femoral neuropathy following major pelvic surgery: Etiology and prevention. J. Urol. 1994, 151, 163–165. [Google Scholar] [CrossRef]
- Kesikburun, B.; Ekşioğlu, E.; Akyüz, E.Ü.; Demirel, F.; Çakcı, A. Femoral nerve injury after nephrectomy: A case report. J. Back. Musculoskelet. Rehabil. 2015, 28, 873–875. [Google Scholar] [CrossRef]
- Cardosi, R.J.; Cox, C.S.; Hoffman, M.S. Postoperative neuropathies after major pelvic surgery. Obs. Gynecol. 2002, 100, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Manetta, A. Prevention of femoral nerve injuries in gynecologic surgery. Am. J. Obs. Gynecol. 2002, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahlering, T.E.; Skarecky, D.W.; Lee, D.I.; Clayman, R.V. Nerve-sparing robot-assisted radical prostatectomy: Optimal surgical approach to maximize potency and continence. Urology 2011, 78, 273–278. [Google Scholar]
- van Ramshorst, G.H.; Kleinrensink, G.-J.; Hermans, J.J.; Terkivatan, T.; Lange, J.F. Abdominal wall paresis as a complication of laparoscopic surgery. Hernia 2009, 13, 539–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hawksworth, D.J.; Dellon, A.L.; Herati, A.S. Ilioinguinal and iliohypogastric neuralgia as an etiology of bladder pain syndrome. Urol. Case Rep. 2019, 28, 101056. [Google Scholar] [CrossRef]
- North, C.; Ali-Ross, N.; Smith, A.; Reid, F. A prospective study of laparoscopic sacrocolpopexy for the management of pelvic organ prolapse. BJOG 2009, 116, 1251–1257. [Google Scholar] [CrossRef]
- Ganatra, A.M.; Rozet, F.; Sanchez-Salas, R.; Barret, E.; Galiano, M.; Cathelineau, X.; Vallancien, G. The current status of laparoscopic sacrocolpopexy: A review. Eur. Urol. 2009, 55, 1089–1103. [Google Scholar] [CrossRef]
- Jiang, N.; Wu, C.; Zhou, X.; Zhai, G.; Wu, J.; Wu, Y. Cavernous Nerve Injury Resulted Erectile Dysfunction and Regeneration. J. Immunol. Res. 2021, 2021, 5353785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asimakopoulos, A.D.; Campagna, A.; Gakis, G.; Corona Montes, V.E.; Piechaud, T.; Hoepffner, J.L.; Mugnier, C.; Gaston, R. Nerve Sparing, Robot-Assisted Radical Cystectomy with Intracorporeal Bladder Substitution in the Male. J. Urol. 2016, 196, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Giulioni, C.; Asimakopoulos, A.D.; Annino, F.; Garelli, G.; Riviere, J.; Piechaud-Kressmann, J.; Vuong, N.S.; Lopez, L.H.; Roche, J.B.; Rouffilange, J.; et al. First case-series of robot-assisted pudendal nerve release: Technique and outcomes. Surg. Endosc. 2023, 37, 5708–5713. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.H.; Kim, S.C. Changes in sexual function in benign prostatic hyperplasia patients taking dutasteride: 1-year follow-up results. Korean J. Urol. 2011, 52, 632–636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kenney, P.A.; Tuerk, I.A. Complications of laparoscopic retroperitoneal lymph node dissection in testicular cancer. World J. Urol. 2008, 26, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Stening, S.G.; Bourne, S. Supracostal percutaneous nephrolithotomy for upper pole caliceal calculi. J. Endourol. 1998, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Polyzois, I.; Tsitskaris, K.; Oussedik, S. Pudendal nerve palsy in trauma and elective orthopaedic surgery. Injury 2013, 44, 1721–1724. [Google Scholar] [CrossRef] [PubMed]
- Gillitzer, R.; Hampel, C.; Wiesner, C.; Pahernik, S.; Melchior, S.W.; Thüroff, J.W. Pudendal nerve branch injury during radical perineal prostatectomy. Urology. 2006, 67, e1–e423. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, A.; Gainsburg, D.M.; Stock, J.A. Complications associated with patient positioning in urologic surgery. Urology 2010, 76, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Hohenfellner, R. Nerve injuries in urological surgery. Georgian Med. News 2007, 143, 7–11. [Google Scholar] [PubMed]
- Fuchs, E.F.; Forsyth, M.J. Supracostal approach for percutaneous ultrasonic lithotripsy. Urol. Clin. N. Am. 1990, 17, 99. [Google Scholar] [CrossRef]
- Cascella, M.; Quarto, G.; Grimaldi, G.; Izzo, A.; Muscariello, R.; Castaldo, L.; Di Caprio, B.; Bimonte, S.; Del Prete, P.; Cuomo, A.; et al. Neuropathic painful complications due to endopelvic nerve lesions after robot-assisted laparoscopic prostatectomy: Three case reports. Medicine 2019, 98, e18011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nasseh, H.; Pourreza, F.; Saberi, A.; Kazemnejad, E.; Kalantari, B.B.; Falahatkar, S. Focal neuropathies following percutaneous nephrolithotomy (PCNL)--preliminary study. Ger. Med. Sci. 2013, 11, Doc07. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McAllister, M.; Lim, K.; Torrey, R.; Chenoweth, J.; Barker, B.; Baldwin, D.D. Intercostal vessels and nerves are at risk for injury during supracostal percutaneous nephrostolithotomy. J. Urol. 2011, 185, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Masata, J.; Hubka, P.; Martan, A. Pudendal neuralgia following transobturator inside-out tape procedure (TVT-O)--case report and anatomical study. Int. Urogynecol J. 2012, 23, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Sholklapper, T.; Dell’OGlio, P.; Rocco, B.; Annino, F.; Antonelli, A.; Amenta, M.; Borghesi, M.; Bove, P.; Bozzini, G.; et al. The Intraoperative Complications Assessment and Reporting with Universal Standards (ICARUS) Global Surgical Collaboration project: Development of criteria for reporting adverse events during surgical procedures and evaluating their impact on the postoperative course. Eur. Urol. Focus. 2022, 8, 1847–1858. [Google Scholar] [PubMed]
- Sayegh, A.S.; Eppler, M.; Ballon, J.; Hemal, S.; Goldenberg, M.; Sotelo, R.; Cacciamani, G.E. Strategies for improving the standardization of perioperative adverse events in surgery and anesthesiology: “the long road from assessment to collection, grading and reporting”. J. Clin. Med. 2022, 11, 5115. [Google Scholar] [CrossRef] [PubMed]
- Eppler, M.; Sayegh, A.S.; Goldenberg, M.; Sholklapper, T.; Hemal, S.; Cacciamani, G.E. If you know them, you avoid them: The imperative need to improve the narrative regarding perioperative adverse events. J. Clin. Med. 2022, 11, 4978. [Google Scholar] [CrossRef]
- Garg, S.P.; Hassan, A.M.; Patel, A.; Ketheeswaran, S.; Galiano, R.D.; Ko, J.H. A systematic review of nerve grafting, end-to-end repair, and nerve transfer for obturator nerve injuries. Int. J. Gynecol. Cancer 2022, 32, 1177–1182. [Google Scholar] [CrossRef]
- Burbano-Luna, J.; Merchán-Jiménez, M.A.; Moreno-Capacho, M.; Pareja Franco, R. Obturator nerve injury and repair during laparoscopic lymphadenectomy. Case Rep. Rev. Lit. ]. Rev. Colomb. Obs. Ginecol. 2019, 70, 115–121. (In Spanish) [Google Scholar]
- Ramani, A.P.; Ryndin, I.; Veetil, R.T.P.; Han, H.; Hendlin, K.; Monga, M. Novel. Technique for removal of misdirected laparoscopic Weck clips. Urology 2007, 70, 168–169. [Google Scholar] [CrossRef]
- Andan, C.; Bakır, M.S.; Sen, S.; Aksin, S. Concurrent primary repair obturator nerve transection during pelvic lymphadenectomy procedure via laparoscopical approach. Int. J. Surg. Case Rep. 2018, 53, 394–396. [Google Scholar] [CrossRef]
- Guzzi, G.; Ricciuti, R.A.; Della Torre, A.; Lo Turco, E.; Lavano, A.; Longhini, F.; La Torre, D. Intraoperative Neurophysiological Monitoring in Neurosurgery. J. Clin. Med. 2024, 13, 2966. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Brzezicki, G. Current techniques and concepts in peripheral nerve repair. Int. Rev. Neurobiol. 2011, 109, 27–46. [Google Scholar]
- Georgiou, M.; Bunting, S.C.; Davies, H.A.; Loughlin, A.J.; Golding, J.P.; Phillips, J.B. Engineered neural tissue for peripheral nerve repair. Biomaterials 2013, 34, 7335–7343. [Google Scholar] [CrossRef]
- Menderes, G.; Vilardo, N.; Schwab, C.L.; Azodi, M. Incidental injury and repair of obturator nerve during laparoscopic pelvic lymphadenectomy. Gynecol. Oncol. 2016, 142, 208. [Google Scholar] [CrossRef]
- Schlomm, T.; Tennstedt, P.; Huxhold, C.; Steuber, T.; Salomon, G.; Michl, U.; Heinzer, H.; Hansen, J.; Budäus, L.; Steurer, S.; et al. Neurovascular structureadjacent frozen-section examination (NeuroSAFE) increases nerve-sparing frequency and reduces positive surgical margins in open and robotassisted laparoscopic radical prostatectomy: Experience after 11,069 consecutive patients. Eur. Urol. 2012, 62, 333–340. [Google Scholar] [CrossRef]
- Van der Slot, M.A.; den Bakker, M.A.; Tan, T.S.C.; Remmers, S.; Busstra, M.B.; Gan, M.; Klaver, S.; Rietbergen, J.B.W.; Kweldam, C.F.; Kliffen, M.; et al. NeuroSAFE in radical prostatectomy increases the rate of nerve-sparing surgery without affecting oncological outcome. BJU Int. 2022, 130, 628–636. [Google Scholar] [CrossRef]
- Samuel, L.T.; Munim, M.A.; Acuña, A.J.; Sultan, A.A.; Kamath, A.F. Modified iliac spine wafer osteotomy for exposure during Bernese periacetabular osteotomy. J. Hip Preserv. Surg. 2019, 6, 421. [Google Scholar] [CrossRef]
- Beloborodov, V.; Vorobev, V.; Golub, I.; Frolov, A.; Kelchevskaya, E.; Tsoktoev, D.; Maksikova, T. A multidisciplinary approach to urinary system iatrogenic injuries. Cent. Eur. J. Urol. 2020, 73, 534–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sungur, U.; Polat, H.; Yılmaz, H.; Güner, E. Multidisciplinary Collaborative Operations of Urology and Other Surgical Specialties: Thirteen Years of Experience at a Single Center. J. Urol. Surg. 2022, 9, 253–259. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).