Isolated Tricuspid Regurgitation: Insights into Pathophysiology, Advanced Diagnostics, and Emerging Therapeutic Strategies
Abstract
1. Introduction
2. Etiology and Pathophysiology of Isolated TR
3. Clinical Evaluation of Isolated TR
4. Multimodality Imaging Evaluation
5. Risk Scores of Isolated TR
6. Medical Management of Isolated TR
7. Surgical Management of Isolated TR
8. Transcatheter Interventions for Isolated TR
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Welle, G.A.; Hahn, R.T.; Lindenfeld, J.; Lin, G.; Nkomo, V.T.; Hausleiter, J.; Lurz, P.C.; Pislaru, S.V.; Davidson, C.J.; Eleid, M.F. New Approaches to Assessment and Management of Tricuspid Regurgitation Before Intervention. JACC Cardiovasc. Interv. 2024, 17, 837–858. [Google Scholar] [CrossRef]
- Sala, A.; Lorusso, R.; Alfieri, O. Isolated tricuspid regurgitation: A plea for early correction. Int. J. Cardiol. 2022, 353, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Fender, E.A.; Zack, C.J.; Nishimura, R.A. Isolated tricuspid regurgitation: Outcomes and therapeutic interventions. Heart 2018, 104, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Moyssakis, I.; Tektonidou, M.G.; Vasilliou, V.A.; Samarkos, M.; Votteas, V.; Moutsopoulos, H.M. Libman-Sacks endocarditis in systemic lupus erythematosus: Prevalence, associations, and evolution. Am. J. Med. 2007, 120, 636–642. [Google Scholar] [CrossRef]
- Iftikhar, S.F.; Alahmadi, M.H.; Ahmad, F. Tricuspid Valve Endocarditis; StatPearls: Tampa, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538423/ (accessed on 13 April 2025).
- Sala, A.; Hahn, R.T.; Kodali, S.K.; Mack, M.J.; Maisano, F. Tricuspid Valve Regurgitation: Current Understanding and Novel Treatment Options. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101041. [Google Scholar] [CrossRef] [PubMed]
- Addetia, K.; Harb, S.C.; Hahn, R.T.; Kapadia, S.; Lang, R.M. Cardiac Implantable Electronic Device Lead-Induced Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2019, 12, 622–636. [Google Scholar] [CrossRef]
- Neumann, S.; Rüffer, A.; Sachweh, J.; Biermann, D.; Herrmann, J.; Jerosch-Herold, M.; Hazekamp, M.; Sinning, C.; Zengin, E.; Blankenberg, S. Narrative review of Ebstein’s anomaly beyond childhood: Imaging, surgery, and future perspectives. Cardiovasc. Diagn. Ther. 2021, 11, 1310. [Google Scholar] [CrossRef]
- Mas, P.T.; Rodríguez-Palomares, J.F.; Antunes, M.J. Secondary tricuspid valve regurgitation: A forgotten entity. Heart 2015, 101, 1840–1848. [Google Scholar]
- Wang, T.K.M.; Unai, S.; Xu, B. Contemporary review in the multi-modality imaging evaluation and management of tricuspid regurgitation. Cardiovasc. Diagn. Ther. 2021, 11, 804–817. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Akyuz, K.; Mentias, A.; Kirincich, J.; Duran Crane, A.; Xu, S.; Popovic, Z.B.; Xu, B.; Gillinov, A.M.; Pettersson, G.B. Contemporary Etiologies, Outcomes, and Novel Risk Score for Isolated Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2022, 15, 731–744. [Google Scholar] [CrossRef]
- Vieitez, J.M.; Monteagudo, J.M.; Mahia, P.; Perez, L.; Lopez, T.; Marco, I.; Perone, F.; González, T.; Sitges, M.; Bouzas, A. New insights of tricuspid regurgitation: A large-scale prospective cohort study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Topilsky, Y.; Maltais, S.; Medina Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, E72–E227. [Google Scholar]
- Izumi, C.; Miyake, M.; Takahashi, S.; Matsutani, H.; Hashiwada, S.; Kuwano, K.; Hayashi, H.; Nakajima, S.; Nishiga, M.; Hanazawa, K.; et al. Progression of isolated tricuspid regurgitation late after left-sided valve surgery. Clinical features and mechanisms. Circ. J. 2011, 75, 2902–2907. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Song, J.M.; Park, J.P.; Lee, J.W.; Kang, D.H.; Song, J.K. Long-term prognosis of isolated significant tricuspid regurgitation. Circ. J. 2010, 74, 375–380. [Google Scholar] [CrossRef]
- Haywood, N.; Mehaffey, J.H.; Chancellor, W.Z.; Beller, J.P.; Speir, A.; Quader, M.; Yarboro, L.T.; Teman, N.R.; Ailawadi, G. Burden of Tricuspid Regurgitation in Patients Undergoing Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2021, 111, 44–50. [Google Scholar] [CrossRef]
- Luo, Y.; Leng, J.; Shi, R.; Jiang, Y.; Chen, D.; Wu, Q.; Tie, H.; Wang, Z.; Zhao, Y.; Li, X.; et al. Concomitant tricuspid valve surgery in patients undergoing left ventricular assist device: A systematic review and meta-analysis. Int. J. Surg. 2024, 110, 3039–3049. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation 2014, 129, 2440–2492. [Google Scholar] [CrossRef]
- Maeder, M.T.; Holst, D.P.; Kaye, D.M. Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J. Card. Fail. 2008, 14, 824–830. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Zaidi, A.; Oxborough, D.; Augustine, D.X.; Bedair, R.; Harkness, A.; Rana, B.; Robinson, S.; Badano, L.P.; Education Committee of the British Society of Echocardiography. Echocardiographic assessment of the tricuspid and pulmonary valves: A practical guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G95–G122. [Google Scholar] [CrossRef] [PubMed]
- Peri, Y.; Sadeh, B.; Sherez, C.; Hochstadt, A.; Biner, S.; Aviram, G.; Ingbir, M.; Nachmany, I.; Topaz, G.; Flint, N.; et al. Quantitative assessment of effective regurgitant orifice: Impact on risk stratification, and cut-off for severe and torrential tricuspid regurgitation grade. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Francis, J.M.; Myerson, S.; Selvanayagam, J.B.; Neubauer, S. The role of cardiovascular magnetic resonance imaging in heart failure. JACC 2009, 54, 1407–1424. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Akyuz, K.; Reyaldeen, R.; Griffin, B.P.; Popovic, Z.B.; Pettersson, G.B.; Gillinov, A.M.; Flamm, S.D.; Xu, B.; Desai, M.Y.; et al. Prognostic Value of Complementary Echocardiography and Magnetic Resonance Imaging Quantitative Evaluation for Isolated Tricuspid Regurgitation. Circ. Cardiovasc. Imaging 2021, 14, E012211. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Luxford, J.; Bassin, L.; D’Ambra, M. Echocardiography of the tricuspid valve: Acknowledgements. Ann. Cardiothorac. Surg. 2017, 6, 223. [Google Scholar] [CrossRef]
- Puchalski, M.D.; Lui, G.K.; Miller-Hance, W.C.; Brook, M.M.; Young, L.T.; Bhat, A.; Roberson, D.A.; Mercer-Rosa, L.; Miller, O.I.; Parra, D.A.; et al. Guidelines for Performing a Comprehensive Transesophageal Echocardiographic: Examination in Children and All Patients with Congenital Heart Disease: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 173–215. [Google Scholar] [CrossRef]
- Jost, Z.T.; Nooli, N.P.; Ali, A.E.; Jaganathan, V.; Nanda, N.C. Three-dimensional echocardiography of the tricuspid valve. Front. Cardiovasc. Med. 2023, 10, 1114715. [Google Scholar] [CrossRef]
- Hahn, R.T. State-of-the-Art Review of Echocardiographic Imaging in the Evaluation and Treatment of Functional Tricuspid Regurgitation. Circ. Cardiovasc. Imaging 2016, 9, e005332. [Google Scholar] [CrossRef]
- Ahn, Y.; Koo, H.J.; Kang, J.W.; Yang, D.H. Tricuspid Valve Imaging and Right Ventricular Function Analysis Using Cardiac CT and MRI. Korean J. Radiol. 2021, 22, 1946–1963. [Google Scholar] [CrossRef] [PubMed]
- Winkel, M.G.; Brugger, N.; Khalique, O.K.; Gräni, C.; Huber, A.; Pilgrim, T.; Billinger, M.; Windecker, S.; Hahn, R.T.; Praz, F.; et al. Imaging and Patient Selection for Transcatheter Tricuspid Valve Interventions. Front. Cardiovasc. Med. 2020, 7, 511948. [Google Scholar] [CrossRef]
- Mastroiacovo, G.; Bonomi, A.; Ludergnani, M.; Franchi, M.; Maragna, R.; Pirola, S.; Baggiano, A.; Caglio, A.; Pontone, G.; Polvani, G.; et al. Is EuroSCORE II still a reliable predictor for cardiac surgery mortality in 2022? A retrospective study study. Eur. J. Cardio-Thoracic Surg. 2023, 64, ezad294. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, J.; Galloo, X.; Taramasso, M.; Heitzinger, G.; Benfari, G.; Kresoja, K.P.; Juarez-Casso, F.; Omran, H.; Bohbot, Y.; Iliadis, C.; et al. TRI-SCORE and benefit of intervention in patients with severe tricuspid regurgitation. Eur. Heart J. 2024, 45, 586–597. [Google Scholar] [CrossRef]
- Dreyfus, J.; Audureau, E.; Bohbot, Y.; Coisne, A.; Lavie-Badie, Y.; Bouchery, M.; Flagiello, M.; Bazire, B.; Eggenspieler, F.; Viau, F.; et al. TRI-SCORE: A new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. Eur. Heart J. 2022, 43, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.E.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef]
- Hahn, R.T.; Lawlor, M.K.; Davidson, C.J.; Badhwar, V.; Sannino, A.; Spitzer, E.; Lurz, P.; Lindman, B.R.; Topilsky, Y.; Baron, S.J.; et al. Tricuspid Valve Academic Research Consortium Definitions for Tricuspid Regurgitation and Trial Endpoints. JACC 2023, 82, 1711–1735. [Google Scholar] [CrossRef]
- Ricci, F.; Bufano, G.; Galusko, V.; Sekar, B.; Benedetto, U.; Awad, W.I.; Dreyfus, G.D.; Dreyfus, X.; Van den Eynde, J.; De Bonis, M.; et al. Tricuspid regurgitation management: A systematic review of clinical practice guidelines and recommendations. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 8, 238–248. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Griffin, B.P.; Miyasaka, R.; Xu, B.; Popovic, Z.B.; Pettersson, G.B.; Gillinov, A.M.; Desai, M.Y. Isolated surgical tricuspid repair versus replacement: Meta-analysis of 15 069 patients. Open Heart 2020, 7, e001227. [Google Scholar] [CrossRef]
- Vassileva, C.M.; Shabosky, J.; Boley, T.; Markwell, S.; Hazelrigg, S. Tricuspid valve surgery: The past 10 years from the Nationwide Inpatient Sample (NIS) database. J. Thorac. Cardiovasc. Surg. 2012, 143, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Jang, M.J.; Kim, K.H.; Hwang, H.Y. Repair versus replacement for the surgical correction of tricuspid regurgitation: A meta-analysis. European Eur. J. Cardio-Thoracic Surg. 2018, 53, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Treatment Options for Severe Functional Tricuspid Regurgitation. Available online: https://www.congenitalcardiologytoday.com/post/treatment-options-for-severe-functional-tricuspid-regurgitation-indications-techniques-and-current (accessed on 16 March 2025).
- Pfannmüller, B.; Misfeld, M.; Borger, M.A.; Etz, C.D.; Funkat, A.K.; Garbade, J.; Mohr, F.W. Isolated reoperative minimally invasive tricuspid valve operations. Ann. Thorac. Surg. 2012, 94, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.J.; Zhang, B.G.; Liu, J.S.; Qian, Y.J.; Guo, Y.Q. Outcomes of tricuspid annuloplasty with and without prosthetic rings: A retrospective follow-up study. J. Cardiothorac. Surg. 2015, 10, 81. [Google Scholar] [CrossRef][Green Version]
- Pfannmüller, B.; Doenst, T.; Eberhardt, K.; Seeburger, J.; Borger, M.A.; Mohr, F.W. Increased risk of dehiscence after tricuspid valve repair with rigid annuloplasty rings. J. Thorac. Cardiovasc. Surg. 2012, 143, 1050–1055. [Google Scholar] [CrossRef]
- Lee, J. Tensile Viscoelastic Properties of Bioprosthetic Heart Valve Materials And of The Pericardium. Ph.D. Thesis, Western University, London, ON, Canada, 1982. [Google Scholar]
- Patlolla, S.H.; Saran, N.; Schaff, H.V.; Crestanello, J.; Pochettino, A.; Stulak, J.M.; Greason, K.L.; King, K.S.; Lee, A.T.; Daly, R.C.; et al. Prosthesis choice for tricuspid valve replacement: Comparison of clinical and echocardiographic outcomes. J. Thorac. Cardiovasc. Surg. 2024, 167, 668–679.e2. [Google Scholar] [CrossRef]
- Cardiopulmonary Bypass—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482190/ (accessed on 16 March 2025).
- Roberts, A.; Duncan, E.C.; Hargrave, P.; Kingery, D.R.; Barnes, J.; Horstemeyer, D.L.; Stahl, R.F. Complications of Cardiopulmonary Bypass From an Anesthesia Perspective: A Clinical Review. HCA Healthc. J. Med. 2023, 4, 3–21. [Google Scholar] [CrossRef]
- Gaudino, M.; Angelini, G.D.; Antoniades, C.; Bakaeen, F.; Benedetto, U.; Calafiore, A.M.; Di Franco, A.; Di Mauro, M.; Fremes, S.E.; Girardi, L.N.; et al. Off-Pump Coronary Artery Bypass Grafting: 30 Years of Debate. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2018, 7, e009934. [Google Scholar] [CrossRef]
- Sauvé, J.A.; Wu, Y.S.; Ghatanatti, R.; Zacharias, J. Minimal Access Tricuspid Valve Surgery. J. Cardiovasc. Dev. Dis. 2023, 10, 118. [Google Scholar] [CrossRef]
- Saran, N.; Dearani, J.A.; Said, S.M.; Greason, K.L.; Pochettino, A.; Stulak, J.M.; Maltais, S.; Cicek, S.; Crestanello, J.; Daly, R.C.; et al. Long-term outcomes of patients undergoing tricuspid valve surgery†. Eur. J. Cardio-Thoracic Surg. 2019, 56, 950–958. [Google Scholar] [CrossRef]
- Andreas, M.; Burri, H.; Praz, F.; Soliman, O.; Badano, L.; Barreiro, M.; Cavalcante, J.L.; De Potter, T.; Doenst, T.; Friedrichs, K.; et al. Tricuspid valve disease and cardiac implantable electronic devices. Eur. Heart J. 2023, 45, 346–365. [Google Scholar] [CrossRef] [PubMed]
- Gabriels, J.K.; Schaller, R.D.; Koss, E.; Rutkin, B.J.; Carrillo, R.G.; Epstein, L.M. Lead management in patients undergoing percutaneous tricuspid valve replacement or repair: A ‘heart team’ approach. Europace 2023, 25, euad300. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Elmariah, S. Transcatheter Tricuspid Valve Therapy. Curr. Treat Options Cardiovasc. Med. 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- TRIGISTRY: Benefit of Isolated Surgical Valve Repair or Replacement for Functional Tricuspid Regurgitation and Long-Term Outcomes Stratified by the TRI-SCORE. Available online: https://www.pcronline.com/News/Whats-new-on-PCRonline/2024/ESC/TRIGISTRY-Benefit-of-isolated-surgical-valve-repair-or-replacement-for-functional-tricuspid-regurgitation-and-long-term-outcomes-stratified-by-the-TRI-SCORE (accessed on 13 April 2025).
- Orban, M.; Besler, C.; Braun, D.; Nabauer, M.; Zimmer, M.; Noack, T.; Mehilli, J.; Hagl, C.; Seeburger, J.; Borger, M.; et al. Six-month outcome after transcatheter edge-to-edge repair of severe tricuspid regurgitation in patients with heart failure. Eur. J. Heart Fail. 2018, 20, 1055–1062. [Google Scholar] [CrossRef]
- Orban, M.; Rommel, K.P.; Ho, E.C.; Unterhuber, M.; Pozzoli, A.; Connelly, K.A.; Deseive, S.; Besler, C.; Ong, G.; Braun, D.; et al. Transcatheter Edge-to-Edge Tricuspid Repair for Severe Tricuspid Regurgitation Reduces Hospitalizations for Heart Failure. JACC Heart Fail. 2020, 8, 265–276. [Google Scholar] [CrossRef]
- Kodali, S.K.; Hahn, R.T.; Davidson, C.J.; Narang, A.; Greenbaum, A.; Gleason, P.; Zahr, F.; Chadderdon, S.; Smith, R.; Grayburn, P.A.; et al. 1-Year Outcomes of Transcatheter Tricuspid Valve Repair. JACC 2023, 81, 1766–1776. [Google Scholar] [CrossRef]
- Bocchino, P.P.; Angelini, F.; Vairo, A.; Andreis, A.; Fortuni, F.; Franchin, L.; Frea, S.; Raineri, C.; Pidello, S.; Conrotto, F.; et al. Clinical Outcomes Following Isolated Transcatheter Tricuspid Valve Repair: A Meta-Analysis and Meta-Regression Study. JACC Cardiovasc. Interv. 2021, 14, 2285–2295. [Google Scholar] [CrossRef]
- Edwards EVOQUE Tricuspid Valve Replacement System—P230013|FDA. Available online: https://www.fda.gov/medical-devices/recently-approved-devices/edwards-evoque-tricuspid-valve-replacement-system-p230013 (accessed on 5 March 2025).
- FDA Approves First Transcatheter Tricuspid Valve Replacement Device|tctmd.com. Available online: https://www.tctmd.com/news/fda-approves-first-transcatheter-tricuspid-valve-replacement-device (accessed on 5 March 2025).
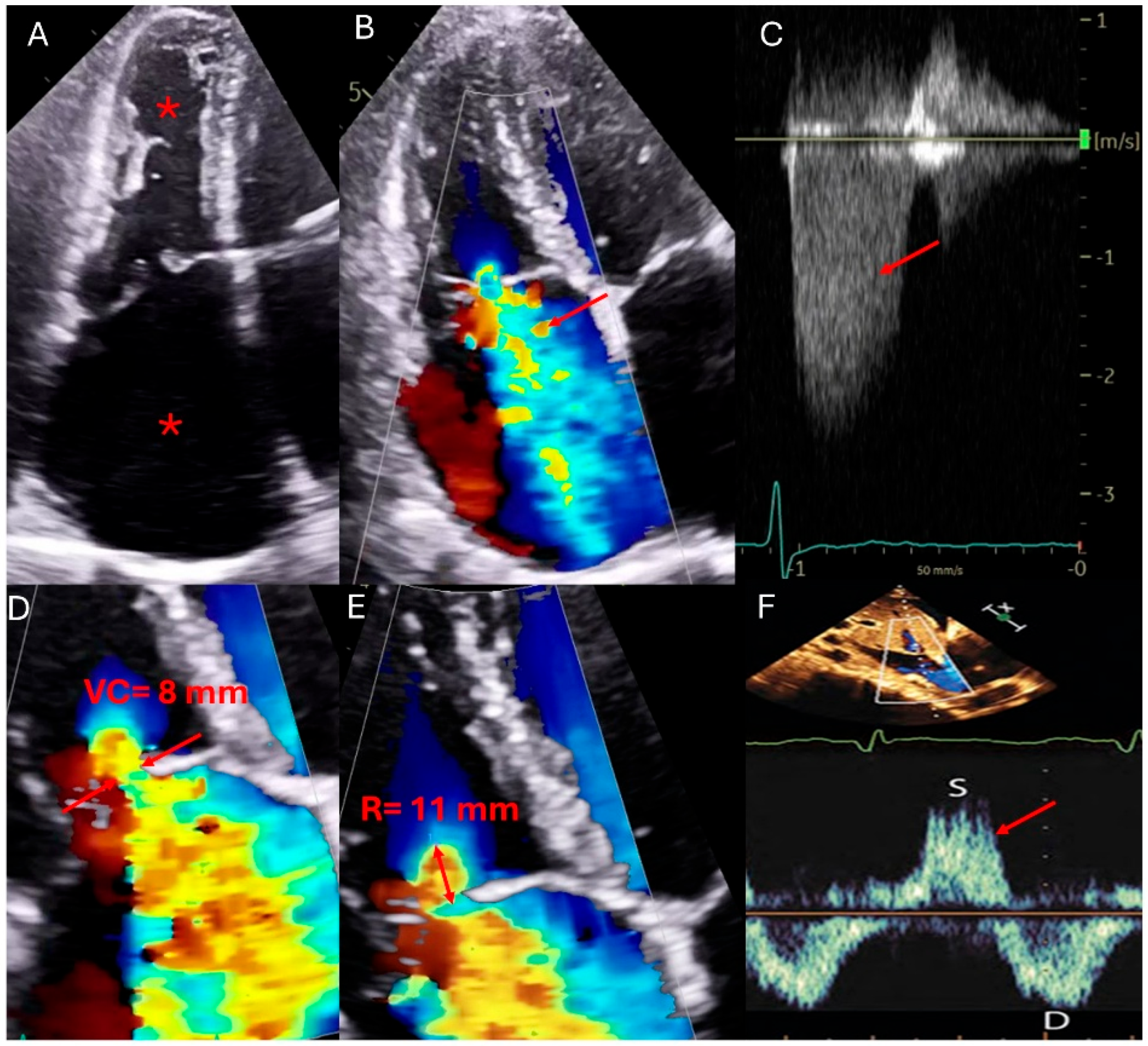
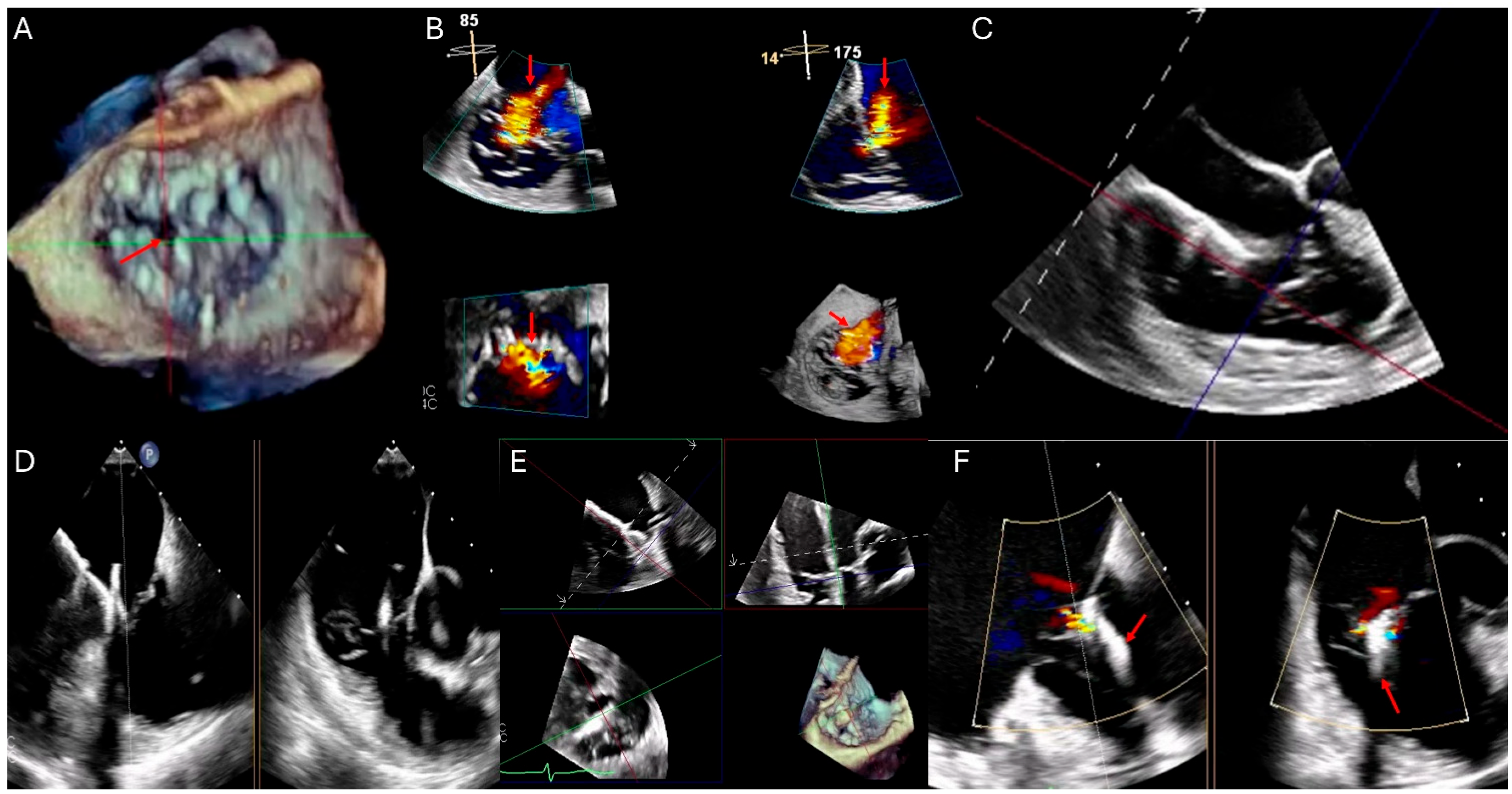
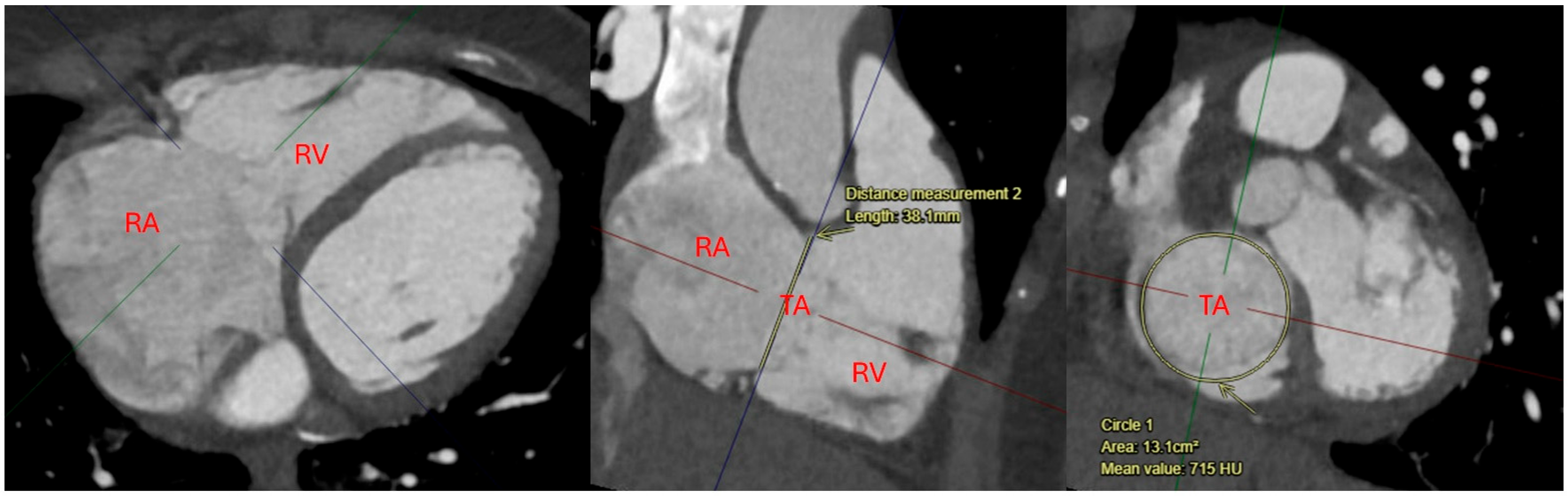

| Modality | Indications | Strengths | Limitations |
|---|---|---|---|
| Transthoracic Echocardiography (TTE) | First-line imaging modality for TR assessment evaluates TR severity, right ventricular function, pulmonary pressures, and inferior vena cava (IVC) size and collapsibility [14,21]. | Widely available, non-invasive, cost-effective. Provides real-time hemodynamic assessment, including TR jet velocity, pressure gradients, and right atrial pressure estimation [14,21]. | Poor acoustic windows in patients with obesity, chronic lung disease, or post-cardiac surgery. Dependent on operator expertise. Subject to interobserver variability [14,21]. |
| Transesophageal Echocardiography (TEE) | Enhanced visualization of the tricuspid valve in cases with poor TTE windows. Useful for assessing leaflet morphology, tricuspid annulus, chordae tendineae, and device-related TR, particularly in patients with cardiac implantable electronic devices (CIEDs) [22,23]. | Superior resolution of valve anatomy and leaflet morphology compared to TTE. Useful for guiding interventions such as transcatheter tricuspid valve repair or replacement. Allows better visualization of prosthetic valve function [22,23]. | Semi-invasive procedure requiring sedation. Limited field of view for evaluating right ventricular function and dependent on transducer positioning for optimal imaging. Potential for esophageal injury or discomfort [22,23]. |
| Computed Tomography (CT) | Primarily used for anatomical assessment of the tricuspid valve and surrounding structures. Useful in pre-procedural planning for transcatheter interventions, identifying lead-associated complications, and assessing right atrial and ventricular size [21,23]. | High spatial resolution for anatomical evaluation of the tricuspid valve, right heart chambers, and adjacent structures. Useful for identifying lead placement issues, fibrosis, and pre-procedural planning for transcatheter valve interventions [21,23]. | Exposure to ionizing radiation and contrast agents. Limited ability to assess hemodynamics in real-time. Not ideal for repeated follow-up imaging due to cumulative radiation exposure [21,23]. |
| Cardiac Magnetic Resonance (CMR) | Gold standard for right ventricular function and volume assessment. Provides accurate quantification of TR severity, regurgitant volume, and right ventricular ejection fraction (RVEF). Useful for assessing myocardial fibrosis and tissue characterization [24,25]. | Highly accurate and reproducible quantification of TR severity, right ventricular volumes, and function. Provides detailed tissue characterization, enabling assessment of myocardial fibrosis, which is critical in advanced heart failure patients [24,25]. | Limited availability, high cost, and longer acquisition time. Requires patient cooperation and breath-holding. Contraindicated in patients with certain metallic implants or severe claustrophobia [24,25]. |
| Parameter | Mild TR | Moderate TR | Severe TR | |
|---|---|---|---|---|
| A-Qualitative | Leaflet flail or prolapse | Usually, not present | More prevalent | |
| TR jet shape | Central | Central or eccentric | Central or eccentric | |
| Doppler signal density | Faint | Dense and parabolic | Early peaking triangle | |
| RV dilatation, IVC collapsibility | NA | Maybe | Present | |
| Systolic hepatic venous flow | Dominance | Blunting | Reversal | |
| B-Quantitative | TR jet area/RA area | Less than 20% | 20–40% | More than 40% |
| Radius of proximal isovelocity surface area (PISA) | Less than 0.6 | 0.6–0.9 | More than or equal 0.9 | |
| Vena contracta | Less than 0.3 cm | 0.3–0.69 cm | More than or equal 0.7 cm | |
| Effective regurgitant orifice area (EROA) | Less than 0.2 cm2 | 0.2–0.39 cm2 | More than or equal 0.4 cm2 | |
| Regurgitant volume | Less than 30 mL/cycle | 30–44 mL/cycle | More than or equal 45 mL/cycle | |
| 2020 ACC/AHA Guidelines [14] | 2021 ESC/EACTS Guidelines [21] | ||
|---|---|---|---|
| I (B) | Severe TR during left-sided valve surgery [14]. | I (B) | Moderate TR with annular dilation (>40 mm or >21 mm/m2) during left-sided valve surgery [21]. |
| IIa (B) | Symptomatic severe TR without severe RV dysfunction or pulmonary hypertension [14]. | I (C) | Symptomatic severe TR despite medical therapy, without left-sided valve disease or severe RV dysfunction [21]. |
| IIa (B) | Progressive TR (stage B), undergoing left side valve surgery if tricuspid annulus end-diastolic diameter more than 4 cm or symptomatic [14]. | IIa (C) | Asymptomatic severe TR with progressive RV dilation or dysfunction [21]. |
| IIb (C) | Asymptomatic or mildly symptomatic severe TR with progressive RV dilation or dysfunction [14]. | ||
| IIb (B) | Persistent right-side failure signs or symptoms with severe TR, previously underwent left-sided valve surgery, in the absence of pulmonary hypertension, such as RV systolic dysfunction [14]. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaban, M.; El Roumi, J.; Malik, M.A.; Arockiam, A.D.; Haroun, E.; Wang, T.K.M. Isolated Tricuspid Regurgitation: Insights into Pathophysiology, Advanced Diagnostics, and Emerging Therapeutic Strategies. Surgeries 2025, 6, 39. https://doi.org/10.3390/surgeries6020039
Shaban M, El Roumi J, Malik MA, Arockiam AD, Haroun E, Wang TKM. Isolated Tricuspid Regurgitation: Insights into Pathophysiology, Advanced Diagnostics, and Emerging Therapeutic Strategies. Surgeries. 2025; 6(2):39. https://doi.org/10.3390/surgeries6020039
Chicago/Turabian StyleShaban, Mohammed, Joseph El Roumi, Muhammad Ahmed Malik, Aro Daniela Arockiam, Elio Haroun, and Tom Kai Ming Wang. 2025. "Isolated Tricuspid Regurgitation: Insights into Pathophysiology, Advanced Diagnostics, and Emerging Therapeutic Strategies" Surgeries 6, no. 2: 39. https://doi.org/10.3390/surgeries6020039
APA StyleShaban, M., El Roumi, J., Malik, M. A., Arockiam, A. D., Haroun, E., & Wang, T. K. M. (2025). Isolated Tricuspid Regurgitation: Insights into Pathophysiology, Advanced Diagnostics, and Emerging Therapeutic Strategies. Surgeries, 6(2), 39. https://doi.org/10.3390/surgeries6020039






