Radial Artery Used as Conduit for Coronary Artery Bypass Grafting
Abstract
1. Introduction and Historic Context
2. Technical Consideration
2.1. Harvesting and Preparation of the Radial Artery
2.2. Risk for Hand Ischaemia
3. Contraindications to Radial Artery Conduit Coronary Grafting
4. Grafting of the Radial Artery: Clinical Results
4.1. Early Clinical Results
4.2. Late Clinical Results
5. Complication
6. The Destiny of Radial Artery Grafts
Determinants of RA Patency
7. Radial Artery Versus Other Grafts
8. Comment
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hillis, L.D.; Smith, P.K.; Anderson, J.L.; Bittl, J.A.; Briges, C.R.; Byrne, J.G.; Cigarroa, J.E.; Disesa, V.J.; Hiratzka, L.F.; Hutter, A.M., Jr.; et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2011, 58, e123–e210. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Uva, M.; Neumann, F.J.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. J. Cardiothorac. Surg. 2018, 55, 4–90. [Google Scholar] [CrossRef]
- Epstein, A.J.; Polsky, D.; Yang, F.; Yang, L.; Groeneveld, P.W. Coronary revascularization trends in the United States, 2001–2008. JAMA 2011, 305, 1769–1776. [Google Scholar] [CrossRef]
- Hannan, E.L.; Racz, M.J.; Gold, J.; Cozzens, K.; Stamato, N.J.; Powell, T.; Hibberd, M.; Walford, G.; American College of Cardiology; American Heart Association. Adherence of catheterization laboratory cardiologists to American College of Cardiology/American Heart Association guidelines for percutaneous coronary interventions and coronary artery bypass graft surgery: What happens in actual practice? Circulation 2010, 121, 267–275. [Google Scholar] [CrossRef]
- Head, S.J.; Kaul, S.; Mack, M.J.; Serruys, P.W.; Taggart, D.P.; Holmes, D.R., Jr.; Leon, M.P.; Marco, J.; Bogers, A.J.J.C.; Kappetein, A.P. The rationale for Heart Team decision-making for patients with stable, complex coronary artery disease. Eur. Heart J. 2013, 34, 2510–2518. [Google Scholar] [CrossRef]
- Frutkin, A.D.; Lindsey, J.B.; Mehta, S.K.; House, J.A.; Spertus, J.A.; Cohen, D.J.; Rumsfeld, J.S.; Marso, S.P.; NCDR. Drug-eluting stents and the use of percutaneous coronary intervention among patients with class I indications for coronary artery bypass surgery undergoing index revascularization: Analysis from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc. Interv. 2009, 2, 614–621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carpentier, A.; Guermonprez, J.L.; Deloche, A.; Frechette, C.; Dubost, C. The aorta-to-coronary radial bypass graft: A technique avoiding pathological changes in grafts. Ann. Thorac. Surg. 1973, 16, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Geha, A.S.; Krone, R.J.; McCormick, J.R.; Baue, A.E. Selection of coronary bypass: Anatomic, physiological and angiographic considerations of vein and mammary artery grafts. J. Thorac. Cardiovasc. Surg. 1975, 70, 414–431. [Google Scholar] [CrossRef]
- Acar, C.; Jebara, V.A.; Portoghese, M.; Beyssen, B.; Pagny, J.Y.; Grare, P.; Chachques, J.C.; Fabiani, J.N.; Deloche, A.; Guermonprez, J.L.; et al. Revival of the radial artery for coronary bypass grafting. Ann. Thorac. Surg. 1992, 54, 652–660. [Google Scholar] [CrossRef]
- Acar, C.; Ramsheyi, A.; Pagny, J.Y.; Barrier, P.; Fabiani, J.N.; Deloche, A.; Guermonprez, J.L.; Carpentier, A. The radial artery for coronary artery bypass grafting: Clinical and angiographic results at five years. J. Thorac. Cardiovasc. Surg. 1998, 116, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Achouh, P.; Boutekadjirt, R.; Toledano, D.; Hammoudi, N.; Pagny, J.Y.; Goube, P.; Ould Isselmou, K.; Lancelin, B.; Fouquet, R.; Acar, C. Long term (5-to-20 year) patency of the radial artery for coronary bypass grafting. J. Thorac. Cardiovasc. Surg. 2010, 140, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Achouh, P.; Ould Isselmou, K.; Boutekadjirt, R.; D’Alessandro, C.; Pagny, J.Y.; Fouquet, R.; Fabiani, J.N.; Acar, C. Reappraisal of a twenty-year experience with the radial artery as a conduit for coronary bypass grafting. Eur. J. Cardiothorac. Surg. 2012, 41, 87–92. [Google Scholar] [CrossRef]
- Taggart, D.P.; Altman, D.G.; Gray, A.M.; Lees, B.; Gerry, S.; Benedetto, U.; Flather, M.; ART Investigators. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. N. Engl. J. Med. 2016, 375, 2540–2549. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.P.; Benedetto, U.; Gerry, S.; Altman, D.G.; Gray, A.M.; Lees, B.; Gaudino, M.; Zamvar, V.; Bochenek, A.; Buxton, B.; et al. Arterial Revascularization Trial Investigators. Bilateral versus Single Internal-Thoracic-Artery Grafts at 10 Years. N. Engl. J. Med. 2019, 380, 437–446. [Google Scholar] [CrossRef]
- Gaudino, M.; Benedetto, U.; Fremes, S.; Biondi-Zoccai, G.; Sedrakyan, A.; Puskas, J.D.; Angelini, G.D.; Buxton, B.; Frati, G.; Hare, D.L.; et al. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N. Engl. J. Med. 2018, 378, 2069–2077. [Google Scholar] [CrossRef]
- Collins, P.; Webb, C.M.; Chong, C.F.; Moat, N.E.; RSVP Investigators. Radial artery versus saphenous vein patency randomized trial: Five-year angiographic follow-up. Circulation 2008, 117, 2859. [Google Scholar] [CrossRef] [PubMed]
- Buxton, B.F.; Raman, J.S.; Ruengsakulrach, P.; Gordon, I.; Rosalion, A.; Bellomo, R.; Horrigan, M.; Hare, D.L. Radial artery patency and clinical outcomes: Five-year interim results of a randomized trial. J. Thorac. Cardiovasc. Surg. 2003, 125, 1363–1371. [Google Scholar] [CrossRef]
- Petrovic, I.; Nezic, D.; Peric, M.; Milojevic, P.; Djokic, O.; Kosevic, D.; Tasic, N.; Djukanovic, B.; Otasevic, P. Radial artery vs. saphenous vein graft used asthe second conduit for surgical myocardial revascularization: Long-term clinical follow-up. J. Cardiothorac. Surg. 2015, 10, 127. [Google Scholar] [CrossRef]
- Deb, S.; Cohen, E.A.; Singh, S.K.; Une, D.; Laupacis, A.; Fremes, S.E. Radial artery and saphenous vein patency more than 5 years after coronary artery bypass surgery: Results from RAPS (Radial Artery Patency Study). J. Am. Coll. Cardiol. 2012, 60, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Sethi, G.K.; Holman, W.; Thai, H.; McFalls, E.; Ward, H.B.; Kelly, R.F.; Rhenman, B.; Tobler, G.H.; Bakaeen, F.G.; et al. Radial artery grafts vs. saphenous vein grafts in coronary artery bypass surgery: A randomized trial. JAMA 2011, 305, 167–174. [Google Scholar] [CrossRef]
- Royse, A.G.; Brennan, A.P.; Ou-Young, J.; Pawanis, Z.; Canty, D.J.; Royse, C.F. 21 Year Survival of Left Internal Mammary Artery-Radial Artery-Y Graft. J. Am. Coll. Cardiol. 2018, 72, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Sutherland, F.W.; Al-Attar, N.; Spadaccio, C. Incomplete Revascularization in PCI and CABG: When Two Plus Two Does Not Make Four. J. Am. Coll. Cardiol. 2016, 68, 877–878. [Google Scholar] [CrossRef]
- Loop, F.D.; Lytle, B.W.; Cosgrove, D.M.; Stewart, R.W.; Goormastic, M.; Williams, G.W.; Golding, L.A.; Gill, C.C.; Taylor, P.C.; Sheldon, W.C.; et al. Influence of the internal-mammary artery graft on 10-year survival and other cardiac events. N. Engl. J. Med. 1986, 314, 1–6. [Google Scholar] [CrossRef]
- Lytle, B.W.; Blackstone, E.H.; Loop, F.D.; Houghtaling, P.L.; Arnold, J.H.; Akhrass, R.; McCarthy, P.M.; Cosgrove, D.M. Two internal thoracic artery grafts are better than one. J. Thorac. Cardiovasc. Surg. 1999, 117, 855–872. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Uva, M.; Gaudino, M.; Schwann, T.; Acar, C.; Nappi, F.; Benedetto, U.; Ruel, M. Corrigendum to ‘Radial artery as a conduit for coronary artery bypass grafting: A state-of-the-art primer’. Eur. J. Cardiothorac. Surg. 2018, 54, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Mehta, R.H.; Hafley, G.E.; Williams, J.B.; Mack, M.J.; Peterson, E.D.; Allen, K.B.; Harrington, R.A.; Gibson, C.M.; Califf, R.M.; et al. Relationship between vein graft failure and subsequent clinical outcomes after coronary artery bypass surgery. Circulation 2012, 125, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Tatoulis, J.; Buxton, B.F.; Fuller, J.A. The right internal thoracic artery: Is it underutilized? Curr. Opin. Cardiol. 2011, 26, 528–535. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018, 40, 87–165. [Google Scholar] [CrossRef]
- ElBardissi, A.W.; Aranki, S.F.; Sheng, S.; O’Brien, S.M.; Greenberg, C.C.; Gammie, J.S. Trends in isolated coronary artery bypass grafting: An analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J. Thorac. Cardiovasc. Surg. 2012, 143, 273–281. [Google Scholar] [CrossRef]
- Hlatky, M.A.; Boothroyd, D.B.; Reitz, B.A.; Shilane, D.A.; Baker, L.C.; Go, A.S. Adoption and effectiveness of internal mammary artery grafting in coronary artery bypass surgery among Medicare beneficiaries. J. Am. Coll. Cardiol. 2014, 63, 33–39. [Google Scholar] [CrossRef]
- Tabata, M.; Grab, J.D.; Khalpey, Z.; Edwards, F.H.; O’Brien, S.M.; Cohn, L.H.; Morton Bolman, R., 3rd. Prevalence and variability of internal mammary artery graft use in contemporary multivessel coronary artery bypass graft surgery: Analysis of the Society of Thoracic Surgeons National Cardiac Database. Circulation 2009, 120, 935–940. [Google Scholar] [CrossRef]
- Alexander, J.H.; Hafley, G.; Harrington, R.A.; Peterson, E.D.; Ferguson, T.B., Jr.; Lorenz, T.J.; Goyal, A.; Gibson, M.; Mack, M.J.; Gennevois, D.; et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: A randomized controlled trial. JAMA 2005, 294, 2446–2454. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.; Cheng, D.; Cohn, W.; Connoly, M.; Edgerton, J.; Falk, V.; Martin, J.; Ohtsuka, T.; Vitali, R. Endoscopic vascular harvest in coronary artery bypass grafting surgery: A consensus statement of the International Society of Minimally Invasive Cardiothoracic Surgery (ISMICS) 2005. Innovations 2005, 1, 51–60. [Google Scholar] [CrossRef]
- Kelly, R.; Buth, K.J.; Légaré, J.F. Bilateral internal thoracic artery grafting is superior to other forms of multiple arterial grafting in providing survival benefit after coronary bypass surgery. J. Thorac. Cardiovasc. Surg. 2012, 144, 1408–1415. [Google Scholar] [CrossRef]
- Suma, H.; Tanabe, H.; Takahashi, A.; Horii, T.; Isomura, T.; Hirose, H.; Amano, A. Twenty years experience with the gastroepiploic artery graft for CABG. Circulation 2007, 116 (Suppl. 11), I188–I191. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.D.; Cohen, E.A.; Naylor, C.D.; Fremes, S.E.; Radial Artery Patency Study Investigators. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N. Engl. J. Med. 2004, 351, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Calafiore, A.M.; Di Giammarco, G.; Luciani, N.; Maddestra, N.; Di Nardo, E.; Angelini, R. Composite arterial conduits for a wider arterial myocardial revascularization. Ann. Thorac. Surg. 1994, 58, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Royse, A.; Eccleston, D.; Royse, C.; iGRAFT Collaborators. Bilateral versus Single Internal-Thoracic-Artery Grafts. N. Engl. J. Med. 2017, 376, e37. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute. What to Expect After Coronary Artery Bypass Grafting NIH CABG; National Heart, Lung, and Blood Institute: Bethesda, MD, USA.
- McCormack, L.J.; Cauldwell, E.W.; Anson, B.J. Brachial and antebrachial arterial patterns; a study of 750 extremities. Surg. Gyn. Obst. 1953, 96, 43–54. [Google Scholar] [PubMed]
- Acar, C.; Jebara, V.; Portoghese, M.; Fontaliran, F.; Dervanian, P.; Chachques, J.C.; Meininger, V.; Carpentier, A. Comparative anatomy and histology of the radial artery and the internal thoracic artery: Implication for coronary artery bypass. Surg. Radiol. Anat. 1991, 13, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Cikirikcioglu, M.; Yasa, M.; Kerry, Z.; Posacioglu, H.; Boga, M.; Yagdi, T.; Topçuoglu, N.; Büket, S.; Hamulu, A. The effects of the harmonic scalpel on the vasoreactivity and endothelial integrity of the radial artery: A comparison of two different techniques. J. Thorac. Cardiovasc. Surg. 2001, 122, 624–626. [Google Scholar] [CrossRef][Green Version]
- Galadja, Z.; Peterffy, A. Minimally invasive harvesting of the radial artery as a coronary artery bypass graft. Ann. Thorac. Surg. 2001, 72, 291–293. [Google Scholar] [CrossRef]
- Ronan, J.W.; Perry, L.A.; Barner, H.B.; Sundt, T.M., 3rd. Radial artery harvest: Comparison of ultrasonic dissection with standard technique. Ann. Thorac. Surg. 2000, 69, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.V. Thromboangiitis obliterans: Methods of diagnosis of chronic occlusive arterial lesions distal to the wrist with illustrative cases. Am. J. Med. Sci. 1929, 178, 237. [Google Scholar] [CrossRef]
- Kaminski, R.W.; Barnes, R.W. Critique of the Allen test for continuity of the palmar arch assessed by Doppler ultrasound. Surg. Gyn. Obst. 1976, 142, 861–864. [Google Scholar]
- Manabe, S.; Tabuchi, N.; Toyama, M.; Yoshizaki, T.; Kato, M.; Wu, H.; Kotani, M.; Sunamori, M. Oxygen pressure measurement during grip exercise reveals exercise intolerance after radial harvest. Ann. Thorac. Surg. 2004, 77, 2066–2070. [Google Scholar] [CrossRef] [PubMed]
- Starnes, S.L.; Wolk, S.W.; Lampman, R.M.; Shanley, C.J.; Prager, R.L.; Kong, B.K.; Fowler, J.J.; Page, J.M.; Babcock, S.L.; Lange, L.A.; et al. Non-invasive evaluation of hand circulation before radial artery harvest for coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 1999, 117, 261–266. [Google Scholar] [CrossRef]
- Pola, P.; Serricchio, M.; Flore, R.; Manasse, E.; Favuzzi, A.; Possati, G.F. Safe removal of the radial artery for myocardial revascularization. J. Thorac. Cardiovasc. Surg. 1996, 112, 737–744. [Google Scholar] [CrossRef]
- Jarvis, M.A.; Jarvis, C.L.; Jones, P.R.; Spyt, T.J. Reliability of Allen’s test in selection of patients for radial artery harvest. Ann. Thorac. Surg. 2000, 70, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Abu-Omar, Y.; Mussa, S.; Anastasiadis, K.; Steel, S.; Hands, L.; Taggart, D.P. Duplex ultrasonography predicts safety of radial artery harvest in the presence of an abnormal Allen test. Ann. Thorac. Surg. 2004, 77, 116–119. [Google Scholar] [CrossRef]
- Royse, A.G.; Royse, C.F.; Maleskar, A.; Garg, A. Harvest of the radial artery for coronary artery surgery preserves maximal blood flow of the forearm. Ann. Thorac. Surg. 2004, 78, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Ormont, M.L.; Lambert, E.H.; Needleman, L.; Halpern, E.J.; Diehl, J.T.; Edie, R.N.; Mannion, J.D. The role of preoperative radial artery ultrasound and digital plethysmography prior to coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2001, 19, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Dumanian, G.A.; Segelman, K.; Mispireta, L.A.; Walsh, J.A.; Hendrickson, M.F.; Wilgis, E.F. Radial artery use in bypass grafting does not change digital blood flow or hand function. Ann. Thorac. Surg. 1998, 65, 1284–1287. [Google Scholar] [CrossRef]
- Chong, W.C.; Ong, P.J.; Hayward, C.S.; Collins, P.; Moat, N.E. Effects of radial artery harvesting on forarm function and blood flow. Ann. Thorac. Surg. 2003, 75, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Chang, B.-C.; Heo, Y.J. Digital blood flow after radial artery harvest for coronary artery bypass grafting. Ann. Thorac. Surg. 2004, 77, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Meharwal, Z.S.; Trehan, N. Functional status of the hand after radial artery harvesting: Results in 3977 cases. Ann. Thorac. Surg. 2001, 72, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Muneretto, C.; Bisleri, G.; Negri, A.; Manfredi, J.; Carone, E.; Morgan, J.A.; Metra, M.; Dei Cas, L. Left internal thoracic artery-radial artery composite grafts as the technique of choice for myocardial revascularization in elderly patients: A prospective randomized evaluation. J. Thorac. Cardiovasc. Surg. 2004, 127, 179–184. [Google Scholar] [CrossRef]
- Brodman, R.F.; Hirsh, L.E.; Frame, R. Effect of radial artery harvest on collateral forearm blood flow and digital perfusion. J. Thorac. Cardiovasc. Surg. 2002, 123, 512–516. [Google Scholar] [CrossRef]
- Lo, T.S.; Nolan, J.; Fountzopoulos, E.; Behan, M.; Butler, R.; Hetherington, S.L.; Vijayalakshmi, K.; Rajagopal, R.; Fraser, D.; Zaman, A.; et al. Radial artery anomaly and its influence on transradial coronary procedural outcome. Heart 2009, 95, 410–415. [Google Scholar] [CrossRef]
- Chamberlain, M.H.; Taggart, D.P. Bifurcating Radial Artery: A Useful Anatomic Variation for Coronary artery bypass grafting. Ann. Thorac. Surg. 2001, 72, 1399. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, B.L.; Kambara, A.M.; Rossi, F.H.; Moreira, S.M.; Silva Jordao de Oliveira, E.; de Carvalho Linhares Filho, F.A.; Bastos Metzger, P.; Zampieri Passalacqua, A. Left subclavian artery stenting: An option for the treatment of the coronary-subclavian steal syndrome. Rev. Bras. Cir. Cardiovasc. 2014, 29, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Tondi, P.; Serricchio, M.; Spatuzza, P.; Santoliquido, A.; Flora, R.; Girola, F.; Nasso, G.; Pola, P.; Possati, G. Atherosclerotic involvement of the radial artery in patients with coronary artery disease and its relation with midterm radial artery graft patency and endothelial function. J. Thorac. Cardiovasc. Surg. 2003, 126, 1968–1971. [Google Scholar] [CrossRef]
- Valsecchi, O.; Vassileva, A.; Musumeci, G.; Rossini, R.; Tespili, M.; Guagliumi, G.; Mihalcsik, L.; Gavazzi, A.; Ferrazzi, P. Failure of transradial approach during coronary interventions: Anatomic considerations. Catheter. Cardiovasc. Interv. 2006, 67, 870–878. [Google Scholar] [CrossRef]
- Mounsey, C.A.; Mawhinney, J.A.; Werner, R.S.; Taggart, D.P. Does previous transradial catheterization preclude use of the radial artery as a conduit in coronary artery bypass surgery? Circulation 2016, 134, 681–688. [Google Scholar] [CrossRef]
- Staniloae, C.S.; Mody, K.P.; Sanghvi, K.; Mindrescu, C.; Coppola, J.T.; Antonescu, C.R.; Shah, S.; Patel, T. Histopathologic changes of the radial artery wall secondary to transradial catheterization. Vasc. Health Risk Manag. 2009, 5, 527–532. [Google Scholar] [CrossRef]
- Rashid, M.; Kwok, C.S.; Pancholy, S.; Chugh, S.; Kedev, S.A.; Bernat, I.; Ratib, K.; Large, A.; Fraser, D.; Nolan, J.; et al. Radial artery occlusion after transradial interventions: A systematic review and meta-analysis. J. Am. Heart Assoc. 2016, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.R. Radial pseudoaneurysm following trans-radial coronary angiography. J. Assoc. Physicians India. 2013, 61, 282–283. [Google Scholar]
- Pulikal, G.A.; Cox, I.A.; Talwar, S. Radial arteriovenous fistula after cardiac catheterization. Circulation 2005, 111, e99. [Google Scholar] [CrossRef]
- Kamiya, H.; Ushijima, T.; Kanamori, T.; Ikeda, C.; Nakagaki, C.; Ueyama, K.; Watanabe, G. Use of the radial artery graft after transradial catheterization: Is it suitable as a bypass conduit? Ann. Thorac Surg. 2003, 76, 1505–1509. [Google Scholar] [CrossRef]
- Edwards, F.H.; Clark, R.E.; Schwartz, M. Coronary artery bypass grafting: The Society of Thoracic Surgeons National Database experience. Ann. Thorac. Surg. 1994, 57, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Michel, P.; Gauducheau, E.; Lemeshow, S.; Salamon, R. European system for cardiac operative risk evaluation (EuroSCORE). Eur. J. Cardiothorac. Surg. 1999, 16, 9–13. [Google Scholar] [CrossRef]
- Shroyer, A.L.; Coombs, L.P.; Peterson, E.D.; Eiken, M.C.; DeLong, E.R.; Chen, A.; Ferguson, T.B., Jr.; Grover, F.L.; Edwards, F.H.; Society of Thoracic Surgeon. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann. Thorac. Surg. 2003, 75, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Online STS Adult Cardiac Surgery Risk Calculator (STS Short-Term/Operative Risk Calculator). Available online: https://acsdriskcalc.research.sts.org/ (accessed on 30 October 2024).
- Ferguson, T.B., Jr.; Coombs, L.P.; Peterson, E.D. Preoperative beta-blocker use and mortality and morbidity following CABG surgery in North America. JAMA 2002, 287, 2221–2227. [Google Scholar] [CrossRef]
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2014, 64, 1929–1949. [Google Scholar] [CrossRef] [PubMed]
- da Costa, F.D.; da Costa, I.A.; Poffo, R.; Abuchim, D.; Gaspar, R.; Garcia, L.; Faraco, D.L. Myocardial revascularization with the radial artery: A clinical and angiographic study. Ann. Thorac. Surg. 1996, 62, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Dietl, C.A.; Benoit, C.H. Radial artery graft for coronary revascularization; technical considerations. Ann. Thorac. Surg. 1995, 60, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Manasse, E.; Sperti, G.; Suma, H.; Canosa, C.; Kol, A.; Martinelli, L.; Schiavello, R.; Crea, F.; Maseri, A.; Possati, G. Use of the radial artery for myocardial revascularization. Ann. Thorac. Surg. 1996, 62, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Fazel, S.; Mallidi, H.R.; Pelletier, M.P.; Sever, J.Y.; Christakis, G.T.; Goldman, B.S.; Fremes, S.E. Radial artery use is safe in patients with moderate to severe left ventricular dysfunction. Ann. Thorac. Surg. 2003, 75, 1414–1421. [Google Scholar] [CrossRef]
- Tatoulis, J.; Buxton, B.F.; Fuller, J.A. The radial artery in coronary reoperations. Eur. J. Cardiothorac. Surg. 2001, 19, 266–273. [Google Scholar] [CrossRef]
- Royse, A.G.; Royse, C.F.; Tatoulis, J. Total arterial revascularization and factors influencing in-hospital mortality. Eur. J. Cardiothorac. Surg. 1999, 16, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Lusini, M.; Spadaccio, C.; Nenna, A.; Covino, E.; Acar, C.; Chello, M. Papillary Muscle Approximation Versus Restrictive Annuloplasty Alone for Severe Ischemic Mitral Regurgitation. J. Am. Coll. Cardiol. 2016, 67, 2334–2346. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Nenna, A.; Lusini, M.; Fraldi, M.; Acar, C.; Chello, M. Is subvalvular repair worthwhile in severe ischemic mitral regurgitation? Subanalysis of the Papillary Muscle Approximation trial. J. Thorac. Cardiovasc. Surg. 2017, 153, 286–295.e2. [Google Scholar] [CrossRef]
- Rama, A.; Nappi, F.; Praschker, B.G.; Gandjbakhch, I. Papillary muscle approximation for ischemic mitral valve regurgitation. J. Card. Surg. 2008, 23, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Nenna, A.; Spadaccio, C.; Lusini, M.; Chello, M.; Fraldi, M.; Acar, C. Predictive factors of long-term results following valve repair in ischemic mitral valve prolapse. Int. J. Cardiol. 2016, 204, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.M.; Thomas, M.R. Severe spasm of a radial artery coronary bypass graft during coronary intervention. Catheter. Cardiovasc. Interv. 1999, 47, 331–335. [Google Scholar] [CrossRef]
- Macina, A.; Torosoff, M.; Millar, R.D. Recurrent spasm of radial artery graft mimicking fixed stenosis. J. Invasive. Cardiol. 2022, 14, 640–641. [Google Scholar]
- He, G.W.; Fan, K.Y.; Chiu, S.W.; Chow, W.H. Injection of vasodilators into arterial grafts through cardiac catheter to relieve spasm. Ann. Thorac. Surg. 2000, 69, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Gabe, E.D.; Figal, J.C.; Wisner, J.N.; Laguens, R. Radial artery graft vasospasm. Eur. J. Cardiothorac. Surg. 2001, 19, 102–104. [Google Scholar] [CrossRef]
- Moran, S.V.; Baeza, R.; Guarda, E.; Zalaquett, R.; Irrazaval, M.J.; Marchant, E.; Deck, C. Predictors of radial artery patency for coronary bypass operations. Ann. Thorac. Surg. 2001, 72, 1552–1556. [Google Scholar] [CrossRef]
- Gaudino, M.; Luciani, N.; Nasso, G.; Salica, A.; Canosa, C.; Possati, G. Is postoperative calcium channel blocker therapy needed in patients with radial artery grafts? J. Thorac. Cardiovasc. Surg. 2005, 129, 532–535 . [Google Scholar] [CrossRef]
- Patel, A.; Asopa, S.; Dunning, J. Should patients receiving a radial artery conduit have post-operative calcium channel blockers? Interact. Cardiovasc. Thorac. Surg. 2006, 5, 251–257. [Google Scholar] [CrossRef][Green Version]
- Hata, M.; Raman, J.; Matalanis, G.; Rosalion, A.; Storer, M.; Hare, D.; Buxton, B.F. Post harvest wound infection and patient’s perception: Comparative study between radial artery and saphenous vein harvest sites. Ann. Thorac. Cardiovasc. Surg. 2002, 8, 97–101. [Google Scholar] [PubMed]
- Saeed, I.; Anyanwu, A.C.; Yacoub, M.H.; Amrani, M. Subjective patient outcome following coronary artery bypass using the radial artery: Results of a cross-sectional survey of harvest site complications and quality of life. Eur. J. Cardiothorac. Surg. 2001, 20, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Modline, T.; Al-Ruzzeh, S.; Mazrani, W.; Azeem, F.; Bustami, M.; Ilsley, C.; Amrani, M. Use of radial artery graft reduces the morbidity of coronary artery bypass graft surgery in patients aged 65 years and older. Ann. Thorac. Surg. 2002, 74, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Reeves, B.; Marchetto, G.; Mehesh, B.; Lim, K.; Angelini, G.D. Radial versus right internal thoracic artery as a second arterial conduit for coronary surgery: Early and midterm outcomes. J. Thorac. Cardiovasc. Surg. 2003, 126, 39–47. [Google Scholar] [CrossRef]
- Lemma, M.; Gelpi, G.; Mangini, A.; Vanelli, P.; Carro, C.; Condemi, A.; Antona, C. Myocardial revascularization with multiple arterial grafts: Comparison between the radial artery and the right internal thoracic artery. Ann. Thorac. Surg. 2001, 71, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Borger, M.A.; Cohen, G.; Buth, K.J.; Rao, V.; Bozinovski, J.; Liaghati-Nasseri, N.; Mallidi, H.; Feder-Elituy, R.; Sever, J.; Christakis, G.T.; et al. Multiple arterial grafts. Radial versus right internal thoracic arteries. Circulation 1998, 98, II7–II13; discussion II13–II14. [Google Scholar]
- Dai, C.; Lu, Z.; Zhu, H.; Xue, S.; Lian, F. Bilateral internal mammary artery grafting and risk of sternal wound infection: Evidence from observational studies. Ann. Thorac. Surg. 2013, 95, 1938–1945. [Google Scholar] [CrossRef]
- Puskas, J.D.; Sadiq, A.; Vassiliades, T.A.; Kilgo, P.D.; Lattouf, O.M. Bilateral internal thoracic artery grafting is associated with significantly improved long-term survival, even among diabetic patients. Ann. Thorac. Surg. 2012, 94, 710–715; discussion 715–716. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.V.; Shah, I.K.; Dunlay, S.M.; Erwin, P.J.; Locker, C.; Altarabsheh, S.E.; Boislon, B.A.; Park, S.J.; Joyce, L.D. Bilateral internal thoracic artery harvest and deep sternal wound infection in diabetic patients. Ann. Thorac. Surg. 2013, 95, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Galadja, Z.; Szentkiralyi, I.; Peterffy, A. Neurologic complications after radial artery harvesting. J. Thorac. Cardiovasc. Surg. 2002, 123, 194–195. [Google Scholar] [CrossRef]
- Ikizler, M.; Ozkan, S.; Dernek, S.; Ozdemir, C.; Erdinc, O.O.; Sevin, B.; Ozdemir, G.; KuralWe, T. Does radial artery harvesting for coronary revascularization cause neurological injury in the forearm and hand? Eur. J. Cardiothor. Surg. 2005, 28, 420–424. [Google Scholar] [CrossRef][Green Version]
- Schmid, C.; Tjan, T.D.; Scheld, H.H. Severe complex regional pain syndrome type II after radial artery harvesting. Ann. Thorac. Surg. 2002, 74, 1250–1251. [Google Scholar] [CrossRef]
- Serruys, P.W.; Morice, M.-C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; van den Brand, M.; Bass, E.J.; et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.F.; Kirchner, J.L.; Phillips-Bute, B.; Gaver, V.; Grocott, H.; Jones, R.H.; Mark, D.B.; Rêves, J.G.; Blumenthal, J.A.; Neurological Outcome Research Group and the Cardiothoracic Anesthesiology Research Endeavors Investigators. Longitudinal assessment of neurocognitive function after coronary artery bypass surgery. N. Engl. J. Med. 2001, 344, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Roach, G.W.; Kanchuger, M.; Mangano, C.M.; Newman, M.; Nussmeier, N.; Wolman, R.; Aggarwal, A.; Marschall, K.; Graham, S.H.; Ley, C. Adverse cerebral outcomes after coronary bypass surgery. N. Engl. J. Med. 1996, 335, 1857–1863. [Google Scholar] [CrossRef]
- Kouchoukos, N.T.; Barzilai, B.; DavilaRomán, V.G. Adverse cerebral outcomes after coronary bypass surgery. N. Engl. J. Med. 1997, 336, 1605–1607. [Google Scholar] [CrossRef]
- Shroyer, A.L.; Grover, F.L.; Hattler, B.; Collins, J.F.; McDonald, G.O.; Kozora, E.; Lucke, J.C.; Baltz, J.H.; Novitzky, D.; Veterans Affairs Randomized On/Off Bypass (ROOBY) Study Group. On-pump versus off-pump coronary-artery bypass surgery. N. Engl. J. Med. 2009, 361, 1827–1837. [Google Scholar] [CrossRef]
- Hlatky, M.A.; Bacon, C.; Boothroyd, D.; Mahanna, E.; Rêves, J.G.; Newman, M.F.; Johnstone, I.; Winston, C.; Brooks, M.M.; Rosen, A.; et al. Cognitive function 5 years after randomization to coronary angioplasty or coronary artery bypass graft surgery. Circulation 1997, 96 (Suppl. 9), II-11–II-15. [Google Scholar]
- Währborg, P.; Booth, J.E.; Clayton, T.; Nugara, F.; Pepper, J.; Weintraub, W.S.; Sigwart, U.; Stables, R.H.; SoS Neuropsychology Substudy Investigators. Neuropsychological outcome after percutaneous coronary intervention or coronary artery bypass grafting: Results from the Stent or Surgery (SoS) Trial. Circulation 2004, 110, 3411–3417. [Google Scholar] [CrossRef]
- Selnes, O.A.; Gottesman, R.F.; Grega, M.A.; Baumgartner, W.A.; Zeger, S.L.; McKhann, G.M. Cognitive and neurologic outcomes after coronary-artery bypass surgery. N. Engl. J. Med. 2012, 366, 250–257. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, D.L.; Berger, M.; Mathew, J.P.; Graffagnino, C.; Milano, C.A.; Newman, M.F. Neurological complications of cardiac surgery. Lancet Neurol. 2014, 13, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Rahimtoola, S.H.; Fessler, C.L.; Grunkemeier, G.L.; Starr, A. Survival 15 to 20 years after coronary bypass surgery for angina. J. Am. Coll. Cardiol. 1993, 21, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbon, G.M.; Kafka, H.P.; Leach, A.J.; Keon, W.J.; Hooper, G.D.; Burton, J.R. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5065 grafts related to survival and reoperation in 1388 patients during 25 years. J. Am. Coll. Cardiol. 1996, 28, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Iaco, A.L.; Teodori, G.; Di Giammarco, G.; Di Mauro, M.; Storto, L.; Mazzei, V.; Vitolla, G.; Mostafa, B.; Calafiore, A.M. Radial artery for myocardial revascularization: Long term clinical and angiographic results. Ann. Thorac. Surg. 2001, 72, 464–469. [Google Scholar] [CrossRef]
- Tranbaugh, R.F.; Dimitrova, K.R.; Friedmann, P.; Geller, C.M.; Harris, L.J.; Stelzer, P.; Cohen, B.; Hoffman, D.M. Radial artery conduits improve long-term survival after coronary artery bypass grafting. Ann. Thorac. Surg. 2010, 90, 1165–1172. [Google Scholar] [CrossRef]
- Zacharias, A.; Schwann, T.A.; Riordan, C.J.; Durham, S.J.; Shah, A.S.; Habib, R.H. Late Results of Conventional Versus All-Arterial Revascularization Based on Internal Thoracic and Radial Artery Grafting. Ann. Thorac. Surg. 2009, 87, 19–26. [Google Scholar] [CrossRef] [PubMed]
- BARI Investigators. The final 10-year follow-up results from the BARI randomized trial. J. Am. Coll. Cardiol. 2007, 49, 1600–1606. [Google Scholar] [CrossRef]
- Cohen, G.; Tamariz, M.G.; Sever, J.Y.; Liaghati, N.; Guru, V.; Christakis, G.T.; Bhatnagar, G.; Cutrara, C.; Abouzahr, L.; Goldman, B.S.; et al. The radial artery versus the saphenous vein graft in contemporary CABG: A case-matched study. Ann. Thorac. Surg. 2001, 71, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, A.; Habib, R.H.; Schwann, T.A.; Riordan, C.J.; Durham, S.J.; Shah, A. Improved survival with radial artery versus vein conduits in coronary bypass surgery with left internal thoracic artery to left anterior descending artery grafting. Circulation 2004, 109, 1489–1496. [Google Scholar] [CrossRef]
- Nasso, G.; Coppola, R.; Bonifazi, R.; Piancone, F.; Bozzetti, G.; Speziale, G. Arterial revascularization in primary coronary artery bypass grafting: Direct comparison of 4 strategies-results of the Stand-in-Y Mammary Study. J. Thorac. Cardiovasc. Surg. 2009, 137, 1093–10100. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Barner, H.B.; Bailey, M.S.; Guthrie, T.J.; Moazami, N.; Pasque, M.K.; Moon, M.R.; Damiano, R.J., Jr. Radial artery grafts in women: Utilization and results. Ann. Thorac. Surg. 2005, 80, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Schwann, T.A.; Zacharias, A.; Riordan, C.J.; Durham, S.J.; Shah, A.S.; Habib, R.H. Survival and graft patency after coronary artery bypass grafting with coronary endarterectomy: Role of arterial versus vein conduits. Ann. Thorac. Surg. 2007, 84, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Schwann, T.A.; Zacharias, A.; Riordan, C.J.; Durham, S.J.; Shah, A.S.; Habib, R.H. Sequential radial artery grafts for multivessel coronary artery bypass graft surgery: 10-year survival and angiography results. Ann. Thorac. Surg. 2009, 88, 31–39. [Google Scholar] [CrossRef]
- Zacharias, A.; Schwann, T.A.; Riordan, C.J.; Durham, S.J.; Shah, A.S.; Engoren, M.; Habib, R.H. Late outcomes after radial artery versus saphenous vein grafting during reoperative coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 2010, 139, 1511–1518. [Google Scholar] [CrossRef][Green Version]
- Schwann, T.A.; Zacharias, A.; Riordan, C.J.; Durham, S.J.; Shah, A.S.; Habib, R.H. Does radial use as a second arterial conduit for coronary artery bypass grafting improve long-term outcomes in diabetics? Eur. J. Cardiothorac. Surg. 2008, 33, 914–923. [Google Scholar] [CrossRef]
- Hayward, P.A.; Hare, D.L.; Gordon, I.; Buxton, B.F. Effect of radial artery or saphenous vein conduit for the second graft on 6-year clinical outcome after coronary artery bypass grafting. Results of a randomised trial. Eur. J. Cardiothorac. Surg. 2008, 34, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Hadinata, I.E.; Hayward, P.A.; Hare, D.L.; Matalanis, G.S.; Seevanayagam, S.; Rosalion, A.; Buxton, B.F. Choice of conduit for the right coronary system: 8-year analysis of Radial Artery Patency and Clinical Outcomes trial. Ann. Thorac. Surg. 2009, 88, 1404–1409. [Google Scholar] [CrossRef]
- Sergeant, P.; Blackstone, E.; Meyns, B. Is return of angina after coronary artery bypass grafting immutable, can it be delayed, and is it important? J. Thorac. Cardiovasc. Surg. 1998, 116, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Lytle, B.W. Radial versus right internal thoracic artery as a second arterial conduit for coronary surgery: Early and midterm outcomes. J. Thorac. Cardiovasc. Surg. 2003, 126, 5–6. [Google Scholar] [CrossRef]
- Buxton, B.F.; Bellomo, R.; Gordon, I.; Hare, D.L. Radial versus right internal thoracic artery for myocardial revascularization. J. Thorac. Cardiovasc. Surg. 2004, 127, 893–894. [Google Scholar] [CrossRef]
- Di Lazzaro, D.; Ragni, T.; Di Manici, G.; Bardelli, G.; Da Col, U.; Grasselli, F.; Antoniella, A.; Papa, W.; Crusco, F.; Giovagnoni, A. Noninvasive midterm follow-up of radial artery bypass grafts with 16-slice computed tomography. Ann. Thorac. Surg. 2006, 82, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ropers, D.; Pohle, F.K.; Kuettner, A.; Pflederer, T.; Anders, K.; Daniel, W.G.; Bautz, W.; Baum, U.; Achenbach, S. Diagnostic accuracy of noninvasive coronary angiography in patients after bypass surgery using 64-slice spiral computed tomography with 330-ms gantry rotation. Circulation 2006, 114, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
- Feuchtner, G.M.; Schachner, T.; Bonatti, J.; Friedrich, G.J.; Soegner, P.; Klauser, A.; zur Nedden, D. Diagnostic performance of 64-slice computed tomography in evaluation of coronary artery bypass grafts. Am. J. Roentgenol. 2007, 189, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Achouh, P.; Acar, C. Twenty-year fate of the radial artery graft. Ann Cardiothorac Surg. 2013, 2, 481–484. [Google Scholar]
- Chen, A.H.; Nakao, T.; Brodman, R.F.; Greenberg, M.; Charney, R.; Menegus, M.; Johnson, M.; Grose, R.; Frame, R.; Hu, E.C.; et al. Early postoperative angiographic assessment of radial grafts used for coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 1996, 111, 1208–1212. [Google Scholar] [CrossRef]
- Wendler, O.; Hennen, B.; Demertzis, S.; Markwirth, T.; Tscholl, D.; Lausberg, H.; Huang, Q.; Dübener, L.F.; Langer, F.; Schäfers, H.J. Complete arterial revascularization in multivessel coronary artery disease with 2 conduits (skeletonized grafts and T grafts). Circulation 2000, 102 (Suppl. 3), III79–III83. [Google Scholar] [CrossRef] [PubMed]
- Royse, A.G.; Royse, C.F.; Tatoulis, J.; Grigg, L.E.; Shah, P.; Hunt, D.; Better, N.; Marasco, S.F. Postoperative radial artery angiography for coronary artery bypass surgery. Eur. J. Cardiothorac. Surg. 2000, 17, 294–304. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amano, A.; Hirose, H.; Takahashi, A.; Nagano, N. Coronary artery bypass grafting using the radial artery: Midterm results in a Japanese institute. Ann. Thorac. Surg. 2001, 72, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Tatoulis, J.; Royse, A.G.; Buxton, B.F.; Fuller, J.A.; Skillington, P.D.; Goldblatt, J.C.; Brown, R.P.; Rowland, M.A. The radial artery in coronary surgery: A 5 year experience—Clinical and angiographic results. Ann. Thorac. Surg. 2002, 73, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.; Trivedi, S.; Stafford, G.; Bett, J.H. Five-year angiographic patency of radial artery bypass grafts. Circulation 2004, 110 (Suppl. 1), II23–II26. [Google Scholar] [CrossRef]
- Possati, G.; Gaudino, M.; Prati, F.; Alessandrini, F.; Trani, C.; Glieca, F.; Mazzari, M.A.; Luciani, N.; Schiavoni, G. Long term results of the radial artery used for myocardial revascularization. Circulation 2003, 108, 1350–1354. [Google Scholar] [CrossRef]
- Khot, U.N.; Friedman, D.T.; Pettersson, G.; Smedira, N.G.; Li, J.; Ellis, S.G. Radial artery bypass grafts have an increased occurrence of angiographically severe stenosis and occlusion compared with left internal mammary arteries and saphenous vein grafts. Circulation 2004, 109, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Desai, N.; Koyama, T.; Chan, E.; Cohen, E.A.; Fremes, S.E.; Radial Artery Patency Study Investigators. Radial artery angiographic string sign: Clinical consequences and the role of pharmacologic therapy. Ann. Thorac. Surg. 2006, 81, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Terzi, A.; Tespili, M.; Ferrazzi, P. Reversal of radial artery « string sign » at 6 months follow-up. Eur. J. Cardiothorac. Surg. 2003, 23, 432–434. [Google Scholar] [CrossRef][Green Version]
- Manabe, S.; Fukui, T.; Shimokawa, T.; Tabata, M.; Katayama, Y.; Morita, S.; Takanashi, S. Increased graft occlusion or string sign in composite arterial grafting for mildly stenosed target vessels. Ann. Thorac. Surg. 2010, 89, 683–687. [Google Scholar] [CrossRef]
- Gaudino, M.; Prati, F.; Caradonna, E.; Trani, C.; Burzotta, F.; Schiavoni, G.; Glieca, F.; Possati, G. Implantation in the coronary circulation induces morphofunctional transformation of radial grafts from muscular to elastomuscular. Circulation 2005, 112 (Suppl. 9), I208–11. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G.; Fremes, S.E. Prevention of radial artery graft spasm: A survey of Canadian surgical centres. Can. J. Cardiol. 2003, 19, 677–681. [Google Scholar] [PubMed]
- Goube, P.; Hammoudi, N.; Pagny, J.Y.; Boutekadjirt, R.; Toledano, D.; Achouh, P.; Acar, C. Radial artery graft stenosis treated by percutaneous intervention. Eur. J. Cardiothorac. Surg. 2010, 37, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Yonetsu, T.; Kakuta, T.; Lee, T.; Takayama, K.; Kakita, K.; Iwamoto, T.; Kawaguchi, N.; Takahashi, K.; Yamamoto, G.; Iesaka, Y.; et al. Assessment of acute injuries and chronic intimal thickening of the radial artery after transradial coronary intervention by optical coherence tomography. Eur. Heart J. 2010, 31, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Ajani, A.E.; Garg, N.; GebreEyesus, A.; Varghese, J.; Pinnow, E.; Waksman, R.; Pichard, A.D.; Lindsay, J. Percutaneous interventions in radial artery grafts: Clinical and angiographic outcomes. Catheter. Cardiovasc. Interv. 2003, 59, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Lefkovits, J.; Holmes, D.R.; Califf, R.M.; Safian, R.D.; Pieper, K.; Keeler, G.; Topol, E.J. Predictors and sequelae of distal embolization during saphenous vein graft intervention from the CAVEAT-II trial: Coronary Angioplasty Versus Excisional Atherectomy Trial. Circulation 1995, 92, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Wendler, O.; Hennen, B.; Markwirth, T.; Nikoloudakis, N.; Graeter, T.; Schäfers, H.J. Complete arterial revascularization in the diabetic patient-early postoperative results. Thorac. Cardiovasc. Surg. 2001, 49, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.D.; Naylor, C.D.; Kiss, A.; Cohen, E.A.; Feder-Elituv, R.; Miwa, S.; Radhakrishnan, S.; Dubbin, J.; Schwartz, L.; Fremes, S.E.; et al. Impact of patient and target-vessel characteristics on arterial and venous bypass graft patency: Insight from a randomized trial. Circulation 2007, 115, 684–691. [Google Scholar] [CrossRef]
- Singh, S.K.; Desai, N.D.; Petroff, S.D.; Deb, S.; Cohen, E.A.; Radhakrishnan, S.; Schwartz, L.; Dubbin, J.; Fremes, S.E.; Radial Artery Patency Study Investigators. The impact of diabetic status on coronary artery bypass graft patency: Insights from the radial artery patency study. Circulation 2008, 118 (Suppl. 14), S222–S225. [Google Scholar] [CrossRef] [PubMed]
- Buxton, B.F.; Durairaj, M.; Hare, D.L.; Gordon, I.; Moten, S.; Orford, V.; Seevanayagam, S. Do angiographic results from symptom-directed studies reflect true graft patency? Ann. Thorac. Surg. 2005, 80, 896–901. [Google Scholar] [CrossRef]
- Tatoulis, J.; Buxton, B.F.; Fuller, J.A.; Meswani, M.; Theodore, S.; Powar, N.; Wynne, R. Long-term patency of 1108 radial arterial-coronary angiograms over 10 years. Ann. Thorac. Surg. 2009, 88, 23–30. [Google Scholar] [CrossRef]
- Paz, M.A.; Lupon, J.; Bosch, X.; Pomar, J.L.; Sanz, G. Predictors of early saphenous vein aortocoronary bypass graft occlusion. Ann. Thorac. Surg. 1993, 56, 1101–1106. [Google Scholar] [CrossRef]
- Chow, M.S.; Sim, E.; Orszulak, T.A.; Schaff, H.V. Patency of internal thoracic artery grafts: Comparison of right versus left and importance of vessel grafted. Circulation 1994, 90 Pt 2, II129–II132. [Google Scholar] [PubMed]
- Maniar, H.S.; Sundt, T.M.; Barner, H.B.; Prasad, S.M.; Peterson, L.; Absi, T.; Moustakidis, P. Effect of target stenosis and location on radial artery graft patency. J. Thorac. Cardiovasc. Surg. 2002, 123, 45–52. [Google Scholar] [CrossRef]
- Gaudino, M.; Alessandrini, F.; Pragiola, C.; Cellini, C.; Glieac, F.; Luciani, N.; Giroal, F.; Possati, G. Effect of target artery location and severity of stenosis on long term patency of aorta-anastomosed vs. internal thoracic artery anastomosed radial artery grafts. Eur. J. Cardiothorac. Surg. 2004, 25, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Yie, K.; Na, C.Y.; Oh, S.S.; Kim, J.H.; Shinn, S.H.; Seo, H.J. Angiographic results of the radial artery graft patency according to the degree of native coronary stenosis. Eur. J. Cardiothorac. Surg. 2008, 33, 341–348. [Google Scholar] [CrossRef]
- Calafiore, A.M.; Di Giammarco, G.; Teodori, G.; D’Annunzio, E.; Vitolla, G.; Fino, C.; Maddestra, N. Radial artery and inferior epigastric artery in composite grafts: Improved midterm angiographic results. Ann Thorac Surg. 1995, 60, 517–523; discussion 523–524. [Google Scholar] [CrossRef] [PubMed]
- Weinschelbaum, E.E.; Macchia, A.; Caramutti, V.M.; Machain, H.A.; Raffaelli, H.A.; Favaloro, M.R.; Favaloro, R.R.; Dulbecco, E.A.; Abud, J.A.; De Laurentiis, M.; et al. Myocardial revascularization with radial and mammary arteries: Initial and mid-termresults. Ann. Thorac. Surg. 2000, 70, 1378–1383. [Google Scholar] [CrossRef]
- Calafiore, A.M.; Di Mauro, M.; D’Alessandro, S.; Teodori, G.; Vitolla, G.; Contini, M.; Iaco, A.L.; Spira, G. Revascularization of the lateral wall: Long termangiographic and clinical results of radial artery versus right internal thoracic artery grafting. J. Thorac. Cardiovasc. Surg. 2002, 123, 225–231. [Google Scholar] [CrossRef][Green Version]
- Muneretto, C.; Bisleri, G.; Negri, A.; Manfredi, J.; Metra, M.; Nodari, S.; Culot, L.; Dei Cas, L. Total arterial myocardial revascularization with composite grafts improves results of coronary surgery in elderly: A prospective randomized comparison with conventional coronary artery bypass surgery. Circulation 2003, 108 (Suppl 1), II29–II33. [Google Scholar] [CrossRef] [PubMed]
- Lemma, M.; Mangini, A.; Gelpi, G.; Innorta, A.; Spina, A.; Antona, C. Is it better to use the radial artery as a composite graft? Clinical and angiographic results of aorto-coronary versus Y-graft. Eur. J. Cardiothorac. Surg. 2004, 26, 110–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jung, S.H.; Song, H.; Choo, S.J.; Je, H.G.; Chung, C.H.; Kang, J.W.; Lee, J.W. Comparison of radial artery patency according to proximal anastomosis site: Direct aorta to radial artery anastomosis is superior to radial artery composite grafting. J. Thorac. Cardiovasc. Surg. 2009, 138, 76–83. [Google Scholar] [CrossRef]
- Kulik, A.; Ruel, M.; Jneid, H.; Ferguson, B.; Hiratzka, L.F.; Ikonomidis, J.S.; Lopez-Jimenez, F.; McNallan, S.; Patel, M.; Roger, V.L.; et al. Secondary prevention after coronary artery bypass graft surgery: A scientific statement from the American Heart Association. Circulation 2015, 131, 927–964. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.B.; Lopes, R.D.; Hafley, G.E.; Ferguson, T.B.; Mack, M.J.; Gibson, C.M.; Harrington, R.A.; Peterson, E.D.; Smith, P.K.; Mehta, R.H.; et al. Relationship between postoperative clopidogrel use and subsequent angiographic and clinical outcomes following coronary artery bypass grafting. J. Thromb. Thrombolysis 2013, 36, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zheng, Z.; Pi, Y.; Lu, B.; Lu, J.; Hu, S. Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery a single-center, randomized, controlled trial. J. Am. Coll. Cardiol. 2010, 56, 1639–1643. [Google Scholar] [CrossRef]
- Deo, S.V.; Dunlay, S.M.; Shah, I.K.; Altarabsheh, S.A.; Erwin, P.J.; Boislon, B.A.; Park, S.J.; Joyce, L.D. Dual anti-platelet therapy after coronary artery bypass grafting: Is there any benefit? A systematic review and meta-analysis. J. Card. Surg. 2013, 28, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, A.G.; Achenbach, S.; Taylor, A.J. Meta-analysis of effect of single versus dual antiplatelet therapy on early patency of bypass conduits after coronary artery bypass grafting. Am. J. Cardiol. 2013, 112, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.; Tjomsland, O.; Halvorsen, D.; Wiseth, R.; Wahba, A.; Karevold, A.; Haaverstad, R. Effect of clopidogrel on midterm graft patency following off-pump coronary revascularization surgery. Heart Surg. Forum 2006, 9, E581–E856. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Wong, G.C.; Mayo, J.; Bernstein, V.; Mancini, G.B.J.; Ye, J.; Skarsgard, P.; Starovoytov, A.; Cairns, J. Ticagrelor and aspirin for the prevention of cardiovascular events after coronary artery bypass graft surgery. Heart 2016, 102, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, Y.; Xu, Z.; Cheng, Z.; Mei, J.; Chen, X.; Wang, X. Effect of ticagrelor plus aspirin, ticagrelor alone, or aspirin alone on saphenous vein graft patency 1 year after coronary artery bypass grafting: A randomized clinical trial. JAMA 2018, 319, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.M.; Janssen, P.W.A.; Peper, J.; Soliman-Hamad, M.A.; van Straten, A.H.M.; Klein, P.; Hackeng, C.M.; Sonker, U.; Bekker, M.W.A.; von Birgelen, C.; et al. Effect of adding ticagrelor to standard aspirin on saphenous vein graft patency in Patients Undergoing Coronary Artery Bypass Grafting POPular CABG): A randomized, double-blind, placebo-controlled trial. Circulation 2020, 142, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Kulik, A.; Abreu, A.M.; Boronat, V.; Kouchoukos, N.T.; Ruel, M. Ticagrelor versus aspirin and vein graft patency after coronary bypass: A randomized trial. J. Card. Surg. 2022, 37, 563–570. [Google Scholar] [CrossRef]
- Sandner, S.; Redfors, B.; Angiolillo, D.J.; Audisio, K.; Fremes, S.E.; Janssen, P.W.A.; Kulik, A.; Mehran, R.; Peper, J.; Ruel, M.; et al. Association of Dual Antiplatelet Therapy with Ticagrelor with Vein Graft Failure After Coronary Artery Bypass Graft Surgery: A Systematic Review and Meta-analysis. JAMA 2022, 328, 554–562. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129 (Suppl. 2), S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J. 2018 American Heart Association/American College of Cardiology Multisociety Guideline on the Management of Blood Cholesterol: Primary Prevention. JAMA Cardiol. 2019, 4, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, S.; Wetterslev, J.; Lund, J.T.; Lilleør, N.B.; Perko, M.J.; Kelbaek, H.; Madsen, J.K.; Steinbrüchel, D.A. One-year results of total arterial revascularization vs. conventional coronary surgery: CARRPO trial. Eur. Heart J. 2009, 30, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Motwani, J.G.; Topol, E.J. Aortocoronary saphenous vein graft disease: Pathogenesis, predisposition, and prevention. Circulation 1998, 97, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Tatoulis, J.; Buxton, B.F.; Fuller, J.A. Patencies of 2127 arterial to coronary conduits over 15 years. Ann. Thorac. Surg. 2004, 77, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Cellini, C.; Pragliola, C.; Trani, C.; Burzotta, F.; Schiavoni, G.; Nasso, G.; Possati, G. Arterial versus venous bypass grafts in patients with in-stent restenosis. Circulation 2005, 112, I265–I269. [Google Scholar] [CrossRef]
- Hayward, P.A.; Gordon, I.R.; Hare, D.L.; Matalanis, G.; Horrigan, M.L.; Rosalion, A.; Buxton, B.F. Comparable patencies of the radial artery and right internal thoracic artery or saphenous vein beyond 5 years: Results from the Radial Artery Patency and Clinical Outcomes trial. J. Thorac. Cardiovasc. Surg. 2010, 139, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.J.; Zhao, S.; Tian, D.H.; Taggart, D.P.; Yan, T.D. A meta-analysis comparing bilateral internal mammary artery with left internal mammary artery for coronary artery bypass grafting. Ann. Cardiothorac. Surg. 2013, 2, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Shine, B.; Rehman, S.M.; Altman, D.G.; Taggart, D.P. Effect of bilateral internal mammary artery on long-term survival: A meta-analysis approach. Circulation 2014, 130, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Buxton, B.F.; Ruensakulrach, P.; Fuller, J.; Rosalion, A.; Reid, C.M.; Tatoulis, J. The right internal thoracic artery graft-benefits of grafting the left coronary system and native vessels with a high-grade stenosis. Eur. J. Cardiothorac. Surg. 2000, 18, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.J.; Durairaj, M.; Gordon, I.; Fuller, J.; Rosalion, A.; Seevanayagam, S.; Tatoulis, J.; Buxton, B.F. Factors affecting patency of internal thoracic artery graft: Clinical and angiographic study in 1434 symptomatic patients operated between 1982 and 2002. Eur. J. Cardiothorac. Surg. 2004, 26, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Hirose, H.; Amano, A.; Takahashi, A. Triple arterial coronary revascularization using the radial artery and bilateral internal mammary arteries versus the gastroepiploic artery and bilateral internal mammary arteries. Circ. J. 2002, 66, 544–548. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glineur, D.; D’hoore, W.; Price, J.; Dormeus, S.; de Kerchove, L.; Dion, R.; Noirhomme, P.; El Khoury, G. Survival benefit of multiple arterial grafting in a 25-year single-institutional experience: The importance of the third arterial graft. Eur. J. Cardiothorac. Surg. 2012, 42, 284–290; discussion 290–291. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Asai, T.; Matsubayashi, K.; Kambara, A.; Kinoshita, T.; Takashima, N.; Hosoba, S. In offpump surgery, skeletonized gastroepiploic artery is superior to saphenous vein in patients with bilateral internal thoracic arterial grafts. Ann. Thorac. Surg. 2011, 91, 1159–1164. [Google Scholar] [CrossRef]
- Di Mauro, M.; Lorusso, R.; Di Franco, A.; Foschi, M.; Rahouma, M.; Soletti, G.; Calafiore, A.M.; Gaudino, M. What is the best graft to supplement the bilateral internal thoracic artery to the left coronary system? A meta-analysis. Eur. J. Cardiothorac. Surg. 2019, 56, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Pevni, D.; Uretzky, G.; Yosef, P.; Gal Yanay, B.; Shapira, I.; Nesher, N.; Braunshtein, R.; Mohr, R. Revascularization of the right coronary artery in bilateral internal thoracic artery grafting. Ann. Thorac. Surg. 2005, 79, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.Y.; Cho, K.R.; Kim, K.B. Equivalency of right internal thoracic artery and right gastroepiploic artery composite grafts: Five-year outcomes. Ann. Thorac. Surg. 2013, 96, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Glineur, D.; D’hoore, W.; de Kerchove, L.; Noirhomme, P.; Price, J.; Hanet, C.; El Khoury, G. Angiographic predictors of 3-year patency of bypass grafts implanted on the right coronary artery system: A prospective randomized comparison of gastroepiploic artery, saphenous vein, and right internal thoracic artery grafts. J. Thorac. Cardiovasc. Surg. 2011, 142, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, M.; Contini, M.; Iacò, A.; Bivona, A.; Gagliardi, M.; Varone, E.; Bosco, P.; Calafiore, A.M. Bilateral internal thoracic artery on the left side: A propensity score- matched study of impact of the third conduit on the right side. J. Thorac. Cardiovasc. Surg. 2009, 137, 869–874. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.A. Radial artery for coronary artery bypass grafting: 23-year patency. Ann. Thorac. Surg. 1999, 68, 2390–2391. [Google Scholar] [CrossRef]
- Buxton, B.F.; Shi, W.Y.; Tatoulis, J.; Fuller, J.A.; Rosalion, A.; Hayward, P.A. Total arterial revascularization with internal thoracic and radial artery grafts in triple-vessel coronary artery disease is associated with improved survival. J. Thorac. Cardiovasc. Surg. 2014, 148, 1238–1243; discussion 1243–1244. [Google Scholar] [CrossRef]
- Raja, S.G.; Benedetto, U.; Jothidasan, A.; Jujjavarapu, R.K.; Ukwu, U.F.; De Robertis, F.; Bahrami, T.; Gaer, J.A.; Amrani, M. For the Harefield Cardiac Outcomes Research Group. Right internal mammary artery versus radial artery as second arterial conduit in coronary artery bypass grafting: A case-control study of 1526 patients. Int. J. Surg. 2015, 16, 183–189. [Google Scholar] [CrossRef]
- Ruttmann, E.; Fischler, N.; Sakic, A.; Chevtchik, O.; Alber, H.; Schistek, R.; Ulmer, H.; Grimm, M. Second internal thoracic artery versus radial artery in coronary artery bypass grafting: A long-term, propensity score-matched follow-up study. Circulation 2011, 124, 1321–1329. [Google Scholar] [CrossRef] [PubMed]

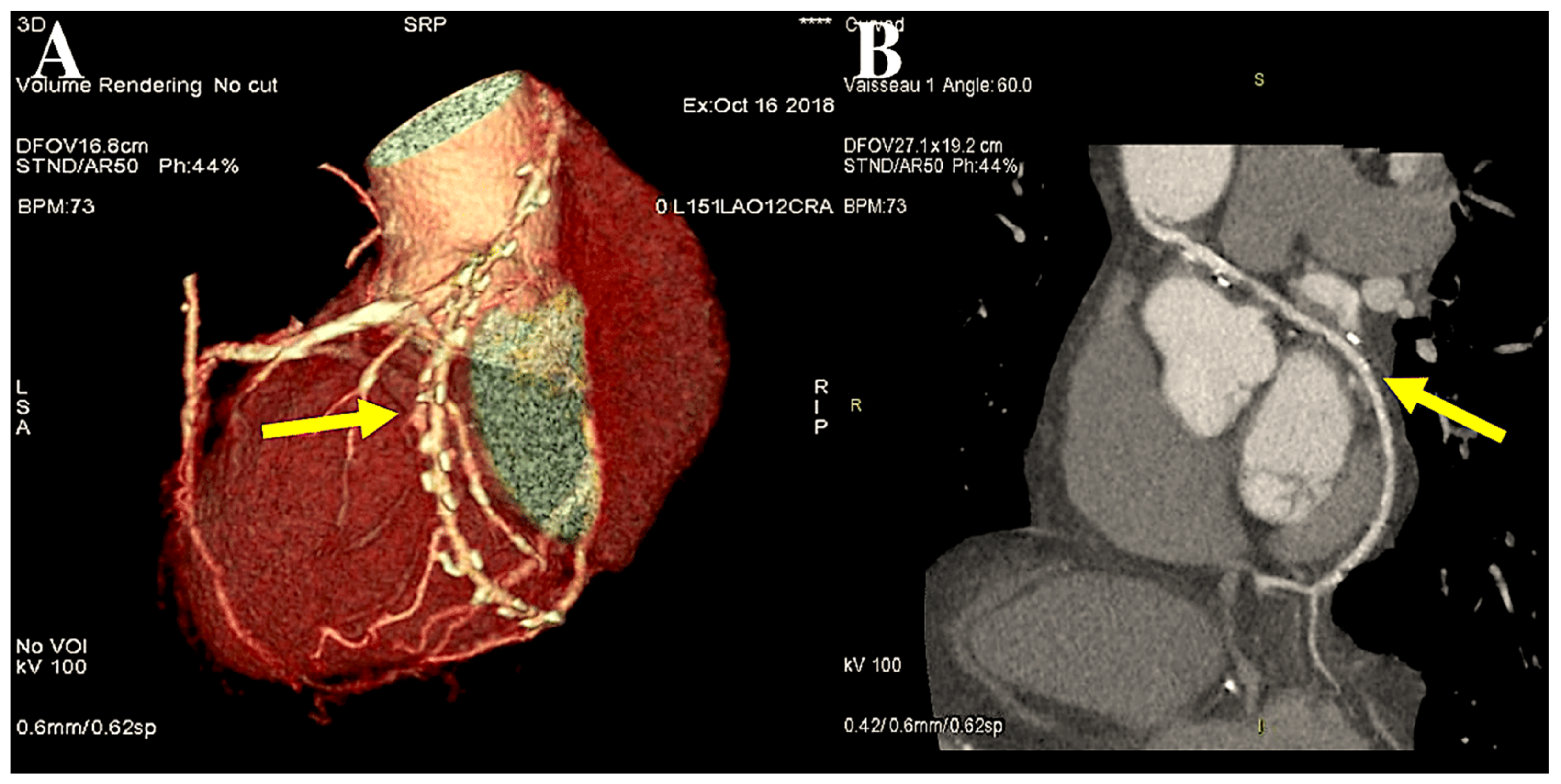
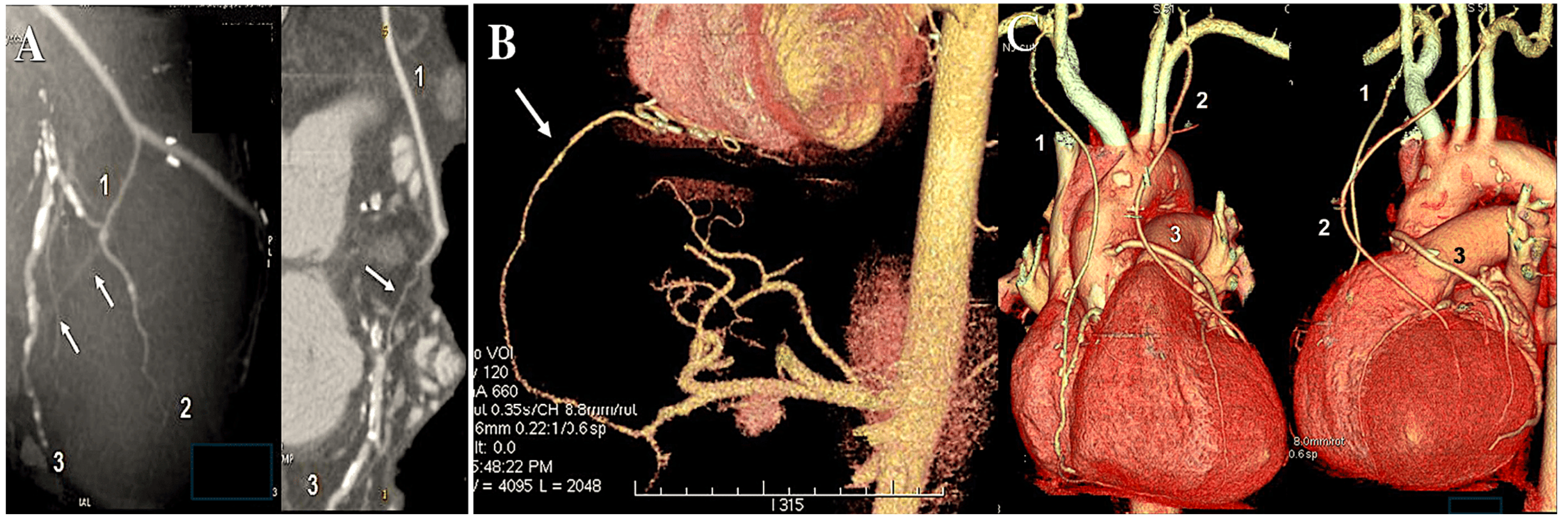
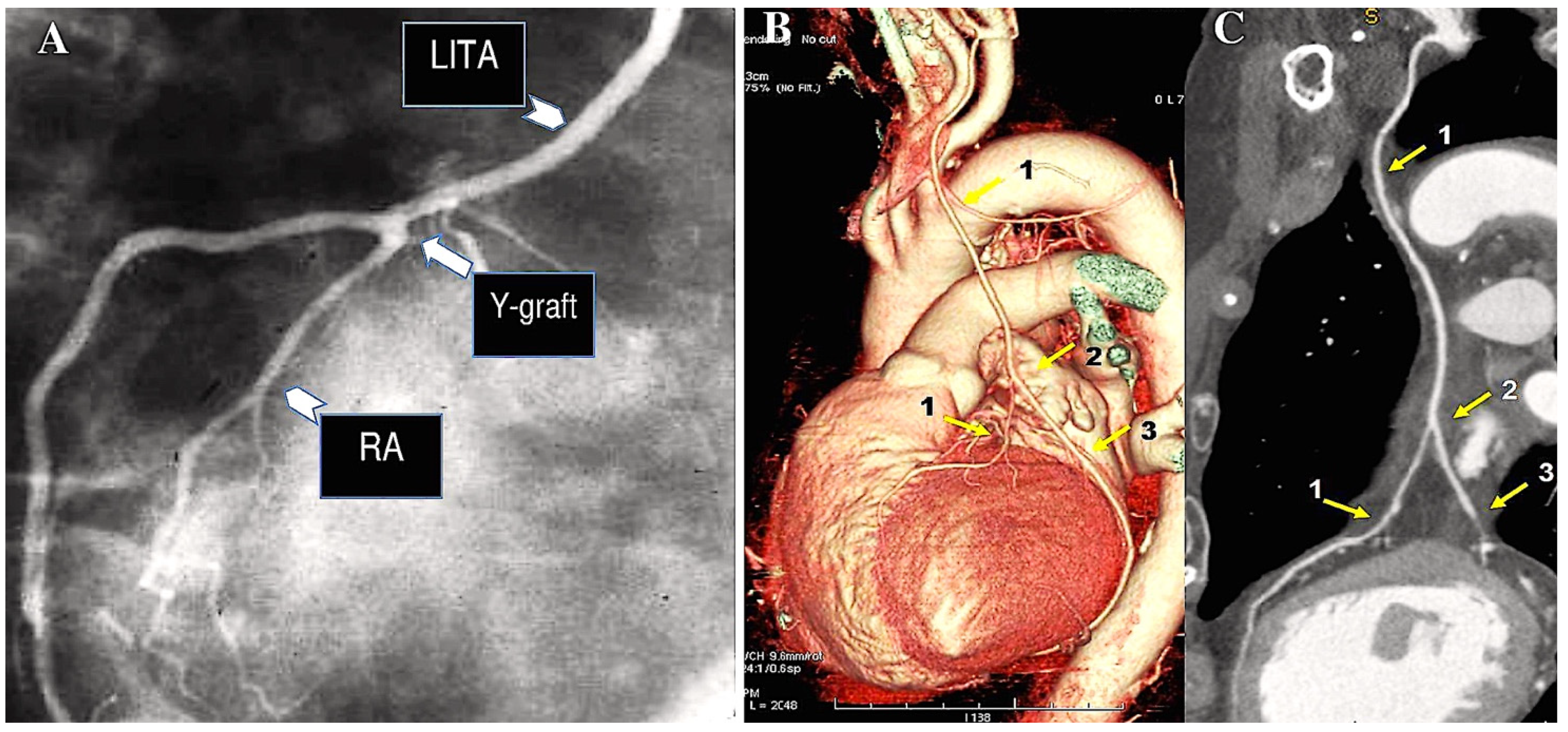
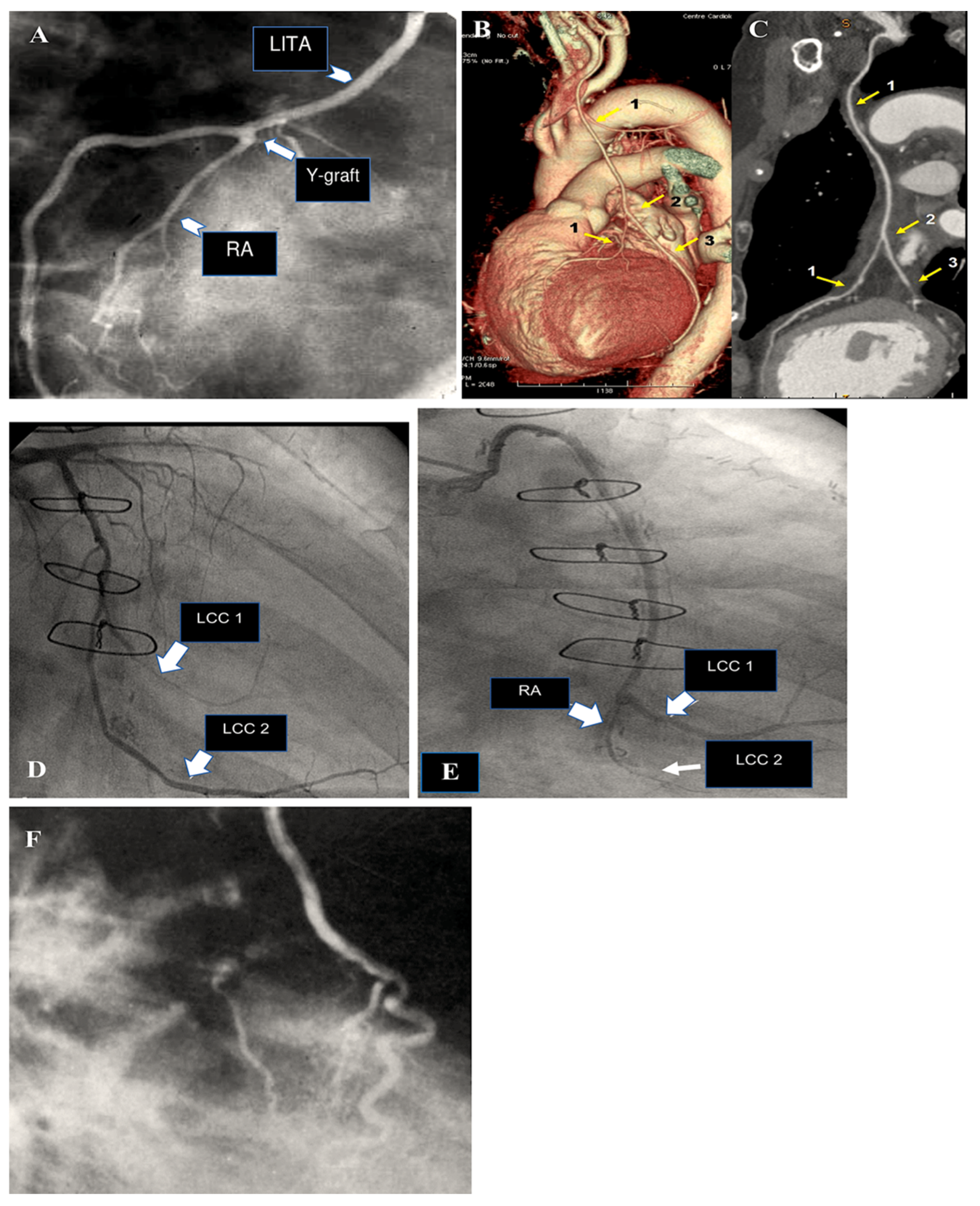
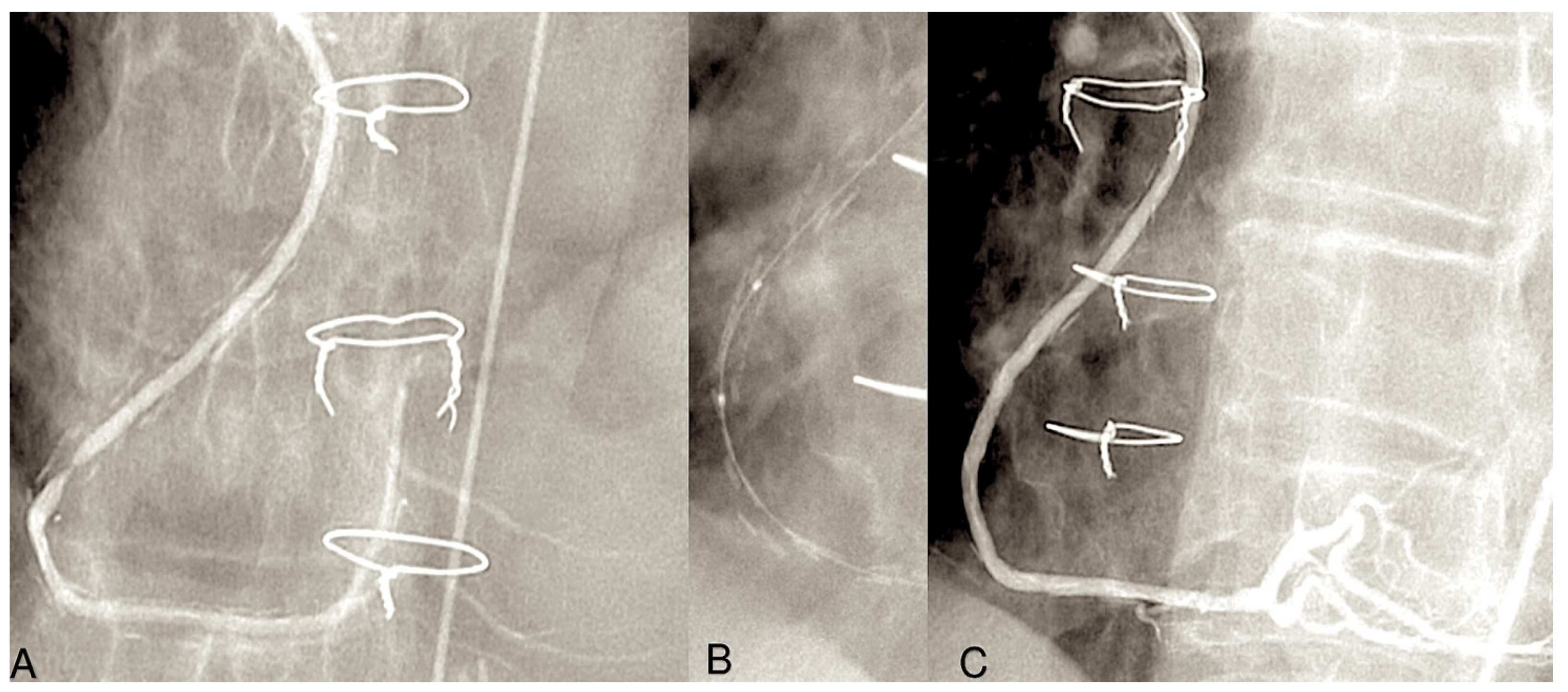
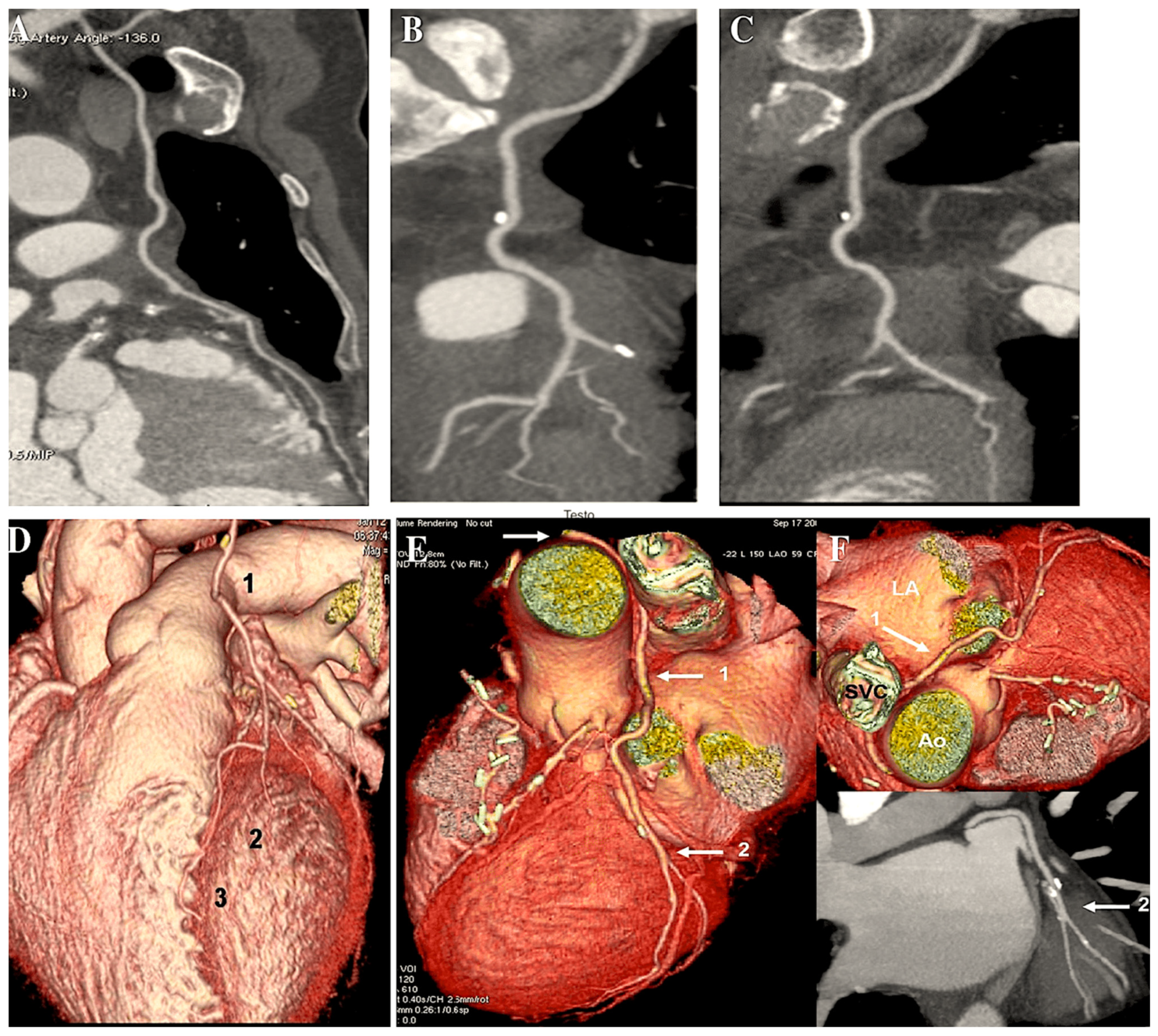
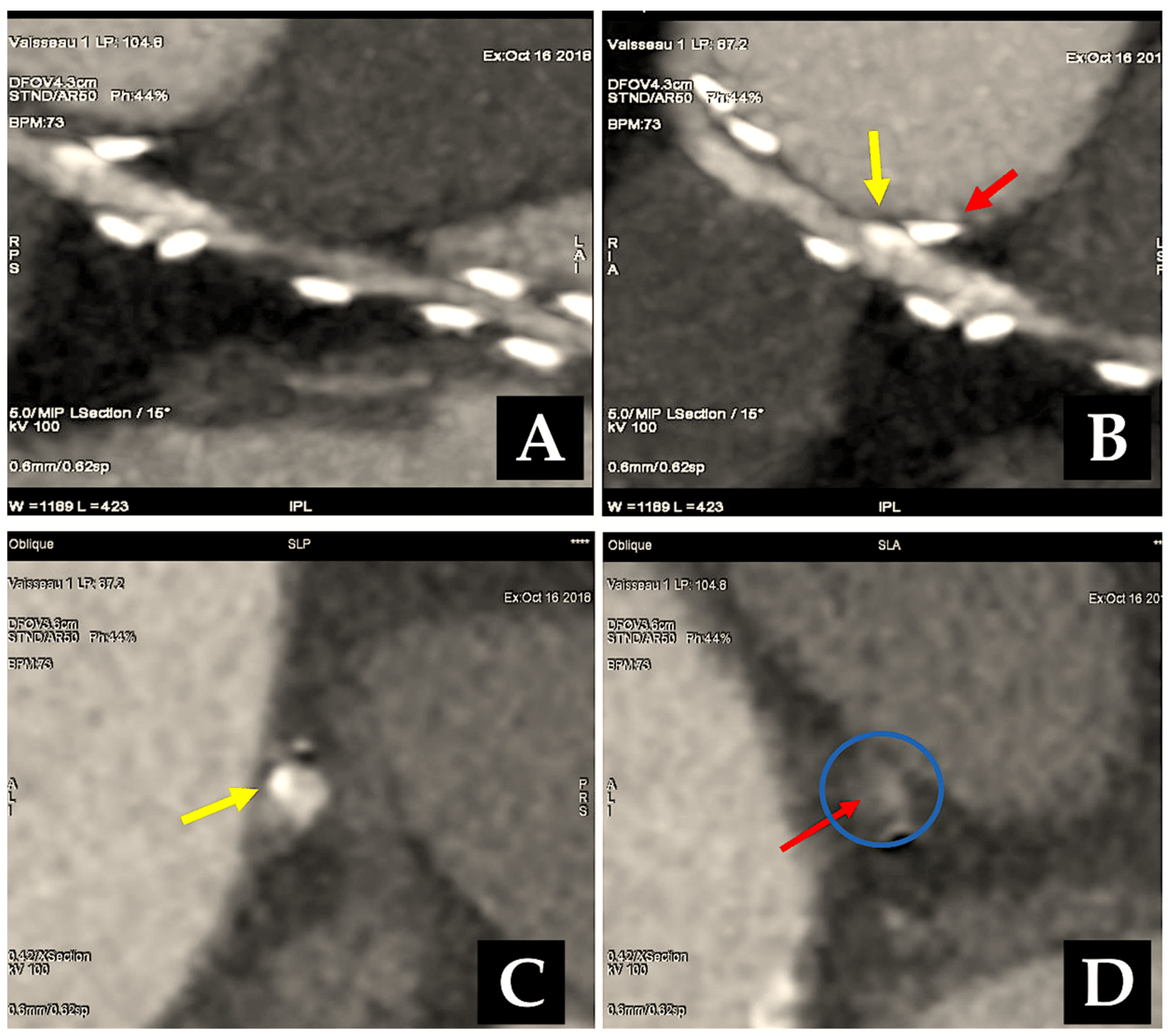
| 1971 | Carpentier was the first to utilise the radial artery as a conduit for bypassing the coronary arteries. A series of 30 patients underwent surgical procedures utilising the radial artery [7]. |
| 1975 | The annual meeting of the American Association for Thoracic Surgery was held in New York. Carpentier reported that occlusions occurred in approximately one-third of patients. The occlusion of the arterial conduit was attributed to spasm of the denervated vessel. The use of the radial artery as a graft was discontinued until the physiological issue was resolved [8]. |
| 1989 | The methodology employed in the harvesting and preparation of the radial artery underwent a modification. The artery was dissected in a pedicled fashion with its satellite veins, a procedure known as “en bloc” dissection. The dilatation of the artery was achieved by infusing it with blood and the vasodilator papaverine at low pressure, with the administration of the antispasmodic drug diltiazem. |
| 1992 | A total of 104 patients who underwent radial artery surgery in the early 1970s were successfully managed [9]. |
| First Author Year of Publication. (Ref. Φ) | Years of Enrolment | Number of Patients | Mean Age (yrs) | Male Sex % | Second Conduit | Second Conduit | Arterial Grafts to CCA (%) | Clinical Follow-Up Span (yrs) | Main Findings | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Death % | ≠ Patency % | Death, Myocardial Infarction, or Repeat Revascularisation % | |||||||||||
| ## Gaudino et al. Radial Investigator # [15] | 1996–2004 | 1036 | 66.8 ± 9.55 | 70.1 | RA 534 | SVG 502 | RA 77.7 | 5 | No difference RA 7.5 vs. SVG 8.4 (p = 0.68) | Better patency RA 8.1% vs. SVG 19.9% (p < 0.001) | Better clinical outcome RA 12.5 vs. SVG 18.7 (p = 0.01) | ||
| Petrovic et al. [18] | 2001–2003 | 200 | 56.4 ± 6.1 | 72.5 | RA 100 | SVG 100 | RA 83 | 8 | No difference RA 12 vs. SVG 12 (p = 0.979) | Better patency RA 8% vs. SVG 14% (p = 0.67) | No difference in clinical outcome RA 28 vs. SVG 36 (p = 0.509) | ||
| Deb S et al. (RAPS) # [19] | 1996–2001 | 561 | 60.4 ± 8.0 | 84.8 | RA 269 | SVG 269 | RA 49.8 | 8.4 | No difference RA and SVG Overall mortality † 11.5 | Better patency RA 18% vs. SVG 28% (p = 0.02) | ¥ MACE worse in patients with study graft stenosis MACE was lower for the RA (p < 0.0001) | ||
| Collins et al. (RSVP) # [16] | 1998–2000 | 142 | 58.5 ± 6.7 | 96.5 | RA 82 | SVG 60 | RA 100 | 5.5 | No difference RA and SVG Overall mortality † 5.63 | Better patency RA 1.7% vs. SVG 13.6% (p = 0.04) | No difference between RA and SVG group | ||
| Buxton et al. (RAPCO) # [17] | 1997–2004 | 619 | 72.8 ± 4.7 | 80.9 | RA 198 | RITA 196 | RA 113 | SVG 112 | RA 100 | 6 | No difference RA 10.6 vs. RITA 11.4 (p = 0.06) RA 7.8 vs. SVG 15.2 (p = 0.54) | No difference RA 10.6 vs. RITA 11.4 (p = 0.06) RA 7.8 vs. SVG 15.2. (p = 0.54) | Better clinical outcome RA 10 vs. RITA 18 (p = 0.8) No difference RA 40 vs. SVG 47 (p = 0.53) Lower reitervention RA vs. SVG No difference RA vs. RITA vs. SVG |
| Goldman et al. [20] | 2003–2008 | 757 | 62 ± 8 | 99 | RA 366 | SVG 367 | RA 26.8 | 1 | No difference RA 2 vs. SVG 2 (p = 0.61) | No difference RA 11 vs. SVG 11 (p = 0.82) | No difference in clinical outcome RA 45 vs. SVG 47 (p = 0.31) | ||
| Observational Study | |||||||||||||
| Buxton et al. [202] | 2001–2013 | † 1156 | 61.7 | 99.8 | RA 779 | RITA 747 | RA 37 | 8 | Better survival RITA 4.50 vs. RA 12.1 [HR] 1.9; 95% (CI) 1.2–3.1 (p = 0.008) | Better patency RITA 7.64 vs. RA 16.8 HR 1.5; 95% CI 1.0–2.2. (p = 0.044) | Increased risk of death and repeat revascularisation in diabetic and obese patients with RA | ||
| Tranbaugh et al. [118] | 1995–2009 | † 1344 | 61.6 ± 9.5 | 76.8 | RA 528 | RITA 528 | RA 100 | 9 | Better survival RA 17 vs. RITA 22, (p = 0.025) | Better patency RA 16.1 vs. RITA 12.6 (p = 0.155) | Fewer event RA 7.6% vs. RITA 14.0%. (p = 0.001) [OR] 0.48; 95% CI, 0.30–0.77; p = 0.002) | ||
| Raja et al. [203] | 1995–2010 | 6059 | 68 ± 9.1 | 78 | RA 4325 | RITA 1089 | SVG 786 | RA 45 | 10 | Better survival Ra vs. SVG HR 0.79; 95% CL 0.70–0.90 (p < 0.001) | Better patency RA vs. SVG No difference RA vs. RITA | Higher incidence of sternal wound infection RITA vs. RA | |
| Royce et al. [21] | 1996–2003 | † 6610 | 67.7 ± 9.8 | 77.2 | RA 236 | SVG 236 | RA 332 | LITA 332 | RA 100 | 11.9 | Better survival RA vs. SVG HR 1.3; 95% CL 1.0–1.6 (p 0.038) | Not evaluated | Not evaluated |
| Achouh et al. [12] | 1989–2003 | 819 | 71.2 ± 10.2 | 78.5 | RA 632 | RITA 58 | SVG 180 | RA 60 | 9.8 | Similar survival between RA, RITA, and SVG | No difference RA 17.2 vs. SVG 18,1 (p = 0.704) RA 17,2 vs. RITA 12,1 (p= 0.32) | No difference between RA, RITA, and SVG | |
| Ruttmann et al. [204] | 2001–2010 | † 1001 | 57.2 ± 9.3 | 89.9 | RA 277 | RITA 277 | RA 96.4 | 57 months | Better survival RITA 1.1 vs. RA 7 HR 0.23; 95% CL 0.066–0.81 (p = 0.022) | Better patency RA 37.9 vs. RITA 10.2 (p = 0.001) | Better event free RITA 4.1 vs. RA 17.8 (HR) 0.18; 95% CL 0.08–0.42; (p < 0.001) | ||
| Achouh et al. [11] | 1989–2001 | 711 | 69 ± 9 | 79 | * RA 202 | RITA 30 | SVG 70 | RA 60 | 9.3 | Similar survival between RA, RITA, and SVG | No difference RA 17 vs. SVG 19 (p = 0.50) RA 17 vs. RITA 13 (p = 0.66) | No difference between RA, RITA, and SVG | |
| Di Mauro et al. [200] | 1991–2002 | † 1496 | 62.5 ± 7.7 | 86.9 | RA 87 | RGEA 208 | RA 36 | 8 | Similar survival RA 8.1 vs. RGE 8.3 (p = 0.129) | No difference BITA plus RA and BITA plus RGEA | Similar events RGEA 1.3 vs. RA 3.3 (p = 0.350) | ||
| Caputo et al. [97] | 1996–2001 | 661 | 56.6 ± 7.9 | 75.7 | RA 325 | RITA 336 | RA 58 | 18 months | Better survival RA vs. RITA HR. RA 0.25; 95% CI, 0.06–1.10 (p = 0.07) | Not reported | Better event-free RA vs. RITA HR; RA, 0.37; 95% CI, 0.16–0.84. (p = 0.02) | ||
| Hirose et al. [193] | 1995 -2001 | 219 | 62.1 ± 8.9 | 65 | RA 96 | RGEA 123 | RA 54 | 2.3 | Similar survival RA 9.7 vs. RGEA 17 | No difference RA 10.1 vs. RGEA 7.1 | Similar event-free RA vs. RGEA | ||
| Acar et al. [10] | 1989–1997 | 910 | 67 ± 9 | 80.7 | * RA 122 | SVG 23 | RA 50 | 7 | 91.6% ± 3.11% | 10 RA (10.78%) | Similar event-free RA vs. LITA | ||
| * Acar et al. [9] | 1989–1991 | 104 | 62.2 ± 8 | 80.7 | * RA 122 | SVG 24 | RA 48.3 | 9.2 months | Similar survival RA vs. SVG No death in RA group | 2 RA graft occluded (6.5%) 1 diagonal branch; 1 LAD | All RA were alive and free of symptoms Sternal wound infection in BITA (2.88 %) | ||
| ** Carpentier 1973 [7] | 1971–1975 | 30 | 168 months | Occlusions in about 1/3 of patients | Occlusion of RA was due to spasm of the denervated vessel. In 1989, angiography showed RA patency anastomosed to the LAD at 14 years | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F.; Nassif, A.; Schoell, T.; Acar, C. Radial Artery Used as Conduit for Coronary Artery Bypass Grafting. Surgeries 2025, 6, 6. https://doi.org/10.3390/surgeries6010006
Nappi F, Nassif A, Schoell T, Acar C. Radial Artery Used as Conduit for Coronary Artery Bypass Grafting. Surgeries. 2025; 6(1):6. https://doi.org/10.3390/surgeries6010006
Chicago/Turabian StyleNappi, Francesco, Aubin Nassif, Thibaut Schoell, and Christophe Acar. 2025. "Radial Artery Used as Conduit for Coronary Artery Bypass Grafting" Surgeries 6, no. 1: 6. https://doi.org/10.3390/surgeries6010006
APA StyleNappi, F., Nassif, A., Schoell, T., & Acar, C. (2025). Radial Artery Used as Conduit for Coronary Artery Bypass Grafting. Surgeries, 6(1), 6. https://doi.org/10.3390/surgeries6010006







