Abstract
Introduction: Traumatic brain injury (TBI) involves a diverse group of head blunt and/or penetrating injuries and is a leading cause of death in the U.S., accounting for one-third of all injury-related deaths. A post-injury hyperglycemic state may commonly impact TBI prognosis and strongly correlate with injury severity. Diabetes mellitus (DM) may also be a source of concomitant hyperglycemia that can worsen prognosis, with previous literature suggesting that DM could be an independent predictor of poor outcome and mortality after TBI. Methods: Using the multi-center, national TriNetX database, we performed a propensity score-matched analysis of severe TBI patients with (DM) and without DM (NDM) from 2014 to 2024. We examined the risk of mortality and complications, including sepsis, cerebral infarction, and pulmonary embolism. We also performed a sub-group analysis comparing the risk of mortality and complications between patients with either insulin-dependent or insulin-independent forms of DM. Results: A total of 26,019 patients were included (4604 DM vs. 21,415 NDM). After propensity score matching, patients with DM had a significantly lower risk of mortality (RR: 0.815; 95% CI: 0.771–0.861; p < 0.05) and ventilator dependency (RR: 0.902; 95% CI: 0.844–0.963; p < 0.05) compared to NDM patients. However, patients with DM had a significantly higher risk of cerebral infarctions, seizures, pneumonia, and sepsis (p < 0.05). Sub-group analysis found no significant difference in mortality or complications between insulin-dependent and insulin-independent forms of DM. Conclusion: Our results suggest that hyperglycemia secondary to DM plays a complicated role in the outcomes after severe TBI. Unexpectedly, we identified both increased and decreased complications in patients with DM. These results reflect the current challenges in the literature surrounding pre-existing DM in patients’ outcomes, the impact of diabetic medications on patient outcomes, and the changing role of aggressive glucose management in critical care patients.
1. Introduction
Traumatic brain injury (TBI) is a leading cause of death in the United States, accounting for approximately one-third of all injury-related deaths [1]. TBI encompasses a broad spectrum of injuries resulting from external forces applied to the head, ranging from mild concussions to severe diffuse axonal injuries. The severity of TBI is commonly classified using the Glasgow Coma Scale (GCS), which assesses motor response, verbal response, and eye-opening ability [2,3]. Despite advancements in acute trauma care, the aftermath of severe TBI frequently includes long-term neurological, cognitive, and physical impairments, imposing substantial societal and economic burdens [4].
The prognosis of TBI is often influenced by various systemic factors, one of which is post-injury hyperglycemia. The metabolic response to brain injury includes an acute surge in stress hormones such as cortisol and catecholamines, driving hepatic gluconeogenesis and insulin resistance. Prisco et al. and others have demonstrated that an early hyperglycemic state correlates with other factors responsible for secondary injury and serves as a marker of inflammatory reaction responsible for early cardiovascular and respiratory impairment, thereby associating hyperglycemia with increased mortality, prolonged hospital stays, and worsened functional outcomes [5,6,7]. Several mechanisms contribute to the deleterious effects of hyperglycemia, including oxidative stress, endothelial dysfunction, disruption of the blood–brain barrier, and exacerbation of neuroinflammation [8]. However, the management of hyperglycemia in critically ill patients is complex. While intensive glucose control may mitigate hyperglycemia-associated damage, it also increases the risk of hypoglycemia, which itself can lead to neuronal energy failure and worsen brain injury outcomes [9]. Considering that approximately 1 in 10 Americans has diabetes mellitus (DM) [10], the interplay between DM and TBI warrants close examination. Patients with pre-existing DM exhibit baseline vascular dysfunction, heightened systemic inflammation, and impaired immune responses, all of which may exacerbate secondary brain injury mechanisms post-TBI. Additionally, DM is associated with chronic cerebral microvascular disease, which can impair neuroplasticity and hinder post-injury recovery [11]. Thus, quantifying the impact of DM on TBI outcomes is essential for refining clinical management strategies and optimizing rehabilitation approaches for this vulnerable population.
Despite the biological plausibility of DM influencing TBI prognosis, there is a paucity of large-scale epidemiological studies investigating this relationship, particularly in the United States. Prior research has largely been limited to small, single-center studies with heterogeneous populations, limiting generalizability. To address this gap, we leveraged the TriNetX database, a global federated research network that aggregates de-identified electronic health records (EHRs) from multiple healthcare institutions. This database allows for robust, real-world data analysis across diverse patient populations, providing an opportunity to evaluate associations between DM and TBI outcomes with greater statistical power and external validity. Specifically, we examined key clinical outcomes, including in-hospital mortality and the incidence of secondary complications (such as infections and seizures), to clarify whether DM represents an independent risk factor for poor TBI prognosis or whether its effects are mediated through other comorbidities, such as hypertension and obesity, which frequently coexist with DM.
2. Materials and Methods
2.1. Data Source
The multi-center, national TriNetX Research Network database was accessed through a retrospective query of the US Collaborative Network dataset. This platform aggregates de-identified clinical data, such as demographics, diagnoses, procedures, and medication prescriptions, from 70 healthcare organizations and over 121 million patients across the United States. Data from January 2014 to December 2024 were included in this study, while records before January 2014 were excluded from the analysis. Institutional Board Review exemption was approved through the Drexel College of Medicine.
2.2. Cohort Selection
Patients of all ages diagnosed with severe TBI were included in this retrospective cohort analysis (Figure 1). We used codes from the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), and Logical Observation Identifiers Names and Codes (LOINC) codes to identify patients with their first incidence of TBI. A severe TBI diagnosis was made with at least one of the following ICD-10-CM codes: Intracranial injury (S06), Fracture of skull and facial bones (S02), or Other and unspecified injuries of head (S09), along with either a Glasgow coma scale score of 3–8 (LOINC 9269-2), Glasgow coma scale score of 3–8 (ICD-10-CM R40.243), Glasgow coma scale score of 3–8, unspecified time (ICD-10-CM R40.2430), Glasgow coma scale score of 3–8, at hospital admission (ICD-10-CM R40.2433), Glasgow coma scale score of 3–8, at arrival to emergency department (ICD-10-CM R40.2432), Glasgow coma scale score of 3–8, in the field [EMT or ambulance] (ICD-10-CM R40.2431), or a Glasgow coma scale score of 3–9, 24 h or more after hospital admission (ICD-10-CM R40.2434) within 1 month on or after any instance of the TBI. Case cohorts were diagnosed with DM (ICD-10-CM E08-E13). Control cohorts were individuals who suffered a severe TBI but were not previously diagnosed with DM. For sub-group analysis, individuals with insulin-dependent DM were identified from the cohort of DM patients using the following ICD-10-CM code: E10. Likewise, individuals with insulin-independent DM were identified from the cohort of DM patients using the following ICD-10-CM code: E11.
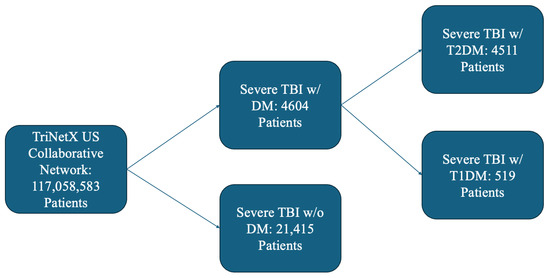
Figure 1.
Outline of Patient Selection Criteria from TriNetX.
2.3. Variables
All variables used for propensity score matching were measured at the index date (date of TBI) and any time before. Demographics included age at TBI, sex, and race. Chronic medical conditions included hypertension (I10–I16), dyslipidemia (E78), heart failure (I50), ischemic heart disease (I20–I25), liver fibrosis or cirrhosis (K74), obesity (E65–E68), acute kidney failure and chronic kidney disease (N17–N19), personal history of nicotine dependence (Z87.891), nicotine dependence (F17), alcohol abuse and dependence (F10.1–F10.2), chronic lower respiratory diseases (J40–J47), atrial fibrillation and flutter (I48), and other peripheral vascular diseases (I73). Medications included cardiovascular medications (VA CV000), and blood products/modifiers/volume expanders (VA BL000).
2.4. Outcome Measures
The primary outcome assessed was the one-month risk of mortality. Secondary outcomes included the one-month risk of occurrence of pulmonary embolism (PE), deep vein thrombosis (DVT), cerebral infarction, myocardial infarction (MI), ventilator dependency, surgical site infections, craniotomy/craniectomy procedures, seizures, pneumonia, percutaneous endoscopic gastrostomy (PEG) tube dependency, and sepsis. These secondary outcomes were chosen to examine the potential links between diabetes mellitus and clinical outcomes associated with the sequelae of traumatic brain injury (TBI). For instance, patients with TBI may require PEG tube placement due to facial trauma, swallowing difficulties, or altered consciousness, all of which can impair the ability to ingest food orally [12]. Similarly, mechanical ventilation and tracheostomy may be necessary in TBI patients with compromised respiratory function, lack of airway reflexes, or difficulties in clearing secretions [13]. Additionally, craniotomy/craniectomy procedures are often performed in TBI patients to address elevated intracranial pressure or brain herniation resulting from cerebral edema or hemorrhage. Moreover, MIs, cerebral infarctions, DVT, and PEs are common outcomes related to TBI-induced coagulopathy [14].
2.5. Statistical Analysis
Descriptive statistics were used to analyze demographic and baseline clinical data. Risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using the TriNetX analytical platform. Propensity score matching was performed, using the greedy nearest neighbor algorithm, based on demographics and mortality risk factors, including chronic medical conditions, as factors that may indicate or influence TBI severity.
3. Results
3.1. Diabetic vs. Non-Diabetic Cohorts
A total of 26,019 patients met the inclusion criteria, comprising 4604 patients with DM and 21,415 non-DM (NDM) patients. Following propensity score matching, baseline demographic and clinical characteristics were similar between the groups (Table 1).

Table 1.
Patient Demographics for DM vs. NDM Cohorts.
Patients with DM demonstrated a significantly lower risk of in-hospital mortality (RR: 0.815; 95% CI: 0.771–0.861; p < 0.05) and ventilatory dependence (RR: 0.902; 95% CI: 0.844–0.963; p < 0.05) compared to non-DM patients (Table 2). However, despite lower mortality rates, DM patients experienced a higher risk of several complications including sepsis (RR: 1.321; 95% CI: 1.163–1.500; p < 0.05), pneumonia (RR: 1.200; 95% CI: 1.088–1.322; p < 0.05), and seizures (RR: 1.174; 95% CI: 1.069–1.289; p < 0.05). Furthermore, patients with DM faced an increased risk of requiring a tracheostomy (RR: 1.278; 95% CI: 1.137–1.436; p < 0.05) and percutaneous endoscopic gastrostomy (PEG) (RR: 1.265; 95% CI: 1.122–1.428; p < 0.05).

Table 2.
Risk Ratios for DM vs. NDM Cohorts.
3.2. Insulin-Dependent Diabetes vs. Insulin-Independent Diabetes Cohorts
A total of 5030 patients contained data regarding DM treatment, comprising 519 patients with insulin-dependent DM (IDDM) and 4511 patients with insulin-independent DM (NIDDM). After propensity score matching, baseline demographic and clinical characteristics were comparable between the groups (Table 3).

Table 3.
Patient Demographics for IDDM vs. NIDDM Cohorts.
No significant differences in mortality or complication rates were observed between IDDM and NIDDM patients (Table 4). For graphical representations of the risk ratios for the DM vs. NDM cohorts and IDDM vs. NIDDM cohorts, please see the Figures S1 and S2 in Supplementary Materials.

Table 4.
Risk Ratios for IDDM vs. NIDDM Cohorts.
4. Discussion
Our findings provide a nuanced perspective on the interplay between diabetes mellitus (DM) and traumatic brain injury (TBI), challenging traditional expectations regarding DM’s impact on critical illness outcomes. While DM patients exhibited lower in-hospital mortality and reduced ventilator dependence, they were significantly more likely to develop sepsis, pneumonia, and seizures, as well as require tracheostomy and percutaneous endoscopic gastrostomy (PEG) tube placement. A comparison of insulin-dependent and non-dependent DM revealed no differences in demographics or outcomes. This paradox underscores the complex physiological interactions between chronic metabolic dysfunction and acute neurological trauma, where our results suggest that while DM may confer certain survival advantages, it also predisposes patients to prolonged hospitalization and greater morbidity.
4.1. Impact of DM on Outcomes
One of the most striking findings of this study is the lower in-hospital mortality observed in TBI patients with DM. This contrasts with existing literature suggesting that DM generally exacerbates outcomes in critically ill populations resulting in an increased risk of in-hospital mortality, as was found in the study conducted by Lustenberger et al. [15]. Moreover, one study in Taiwan supported the adverse impact of DM on outcomes in the TBI patient population [16]. Specifically, Tseng et al. found that patients with DM had more craniotomies, longer hospital stays, and longer ICU stays; these results were tied to a transient hyperglycemic state that was also determined to be strongly associated with a higher in-hospital mortality rate. The paradoxical results observed in our study may be explained by several potential mechanisms, including the Preconditioning Hypothesis. This hypothesis suggests that chronic hyperglycemia induces adaptive cellular mechanisms that enhance resilience to injury [17]. Such adaptations may involve modifications in metabolic pathways, upregulation of antioxidant defenses, or cellular stress responses that mitigate acute TBI-induced damage [18]. Furthermore, the presence of DM often prompts more intensive medical management, including early glucose control, closer monitoring, and prophylactic measures against complications, which could inadvertently improve survival outcomes [19].
Another plausible explanation is the role of anti-diabetic medications. Metformin, a commonly prescribed drug for NIDDM, has demonstrated neuroprotective effects in both preclinical and clinical settings. It modulates mitochondrial function, reduces oxidative stress, and exerts anti-inflammatory properties that may mitigate secondary brain injury mechanisms. Additionally, metformin has been linked to improved endothelial function and enhanced blood–brain barrier integrity, which could reduce the extent of cerebral edema and secondary neuronal damage following TBI [20]. Similarly, GLP-1 receptor agonists, which are increasingly used in DM management, have been shown to promote neurogenesis and reduce neuroinflammation, potentially contributing to improved survival in TBI patients. These medications enhance insulin sensitivity while also exerting direct effects on the central nervous system, including improved synaptic plasticity and neuronal survival. Animal studies have demonstrated that GLP-1 receptor agonists can reduce brain lesion size and improve functional outcomes after experimental TBI, suggesting a potential therapeutic benefit in human patients [21,22]. Beyond metformin and GLP-1 receptor agonists, other anti-diabetic medications may also play a role. Sodium-glucose cotransporter-2 (SGLT2) inhibitors, for instance, have been implicated in reducing systemic inflammation and oxidative stress, which could influence post-TBI recovery [23]. Some evidence suggests that these agents promote ketone body production, providing an alternative energy substrate for injured neurons and potentially supporting cognitive function during the recovery phase [24]. The cumulative impact of these medications suggests that certain pharmacological interventions used to manage DM may confer unintended neuroprotective benefits in the setting of TBI.
Despite the apparent survival benefit of DM, the increased risk of complications in diabetic TBI patients remains a significant concern. Hyperglycemia-induced immune dysfunction, microvascular damage, and delayed wound healing are well-documented consequences of uncontrolled DM, which may elevate susceptibility to infections, hinder recovery, and extend hospital stays [25,26]. In our study, pneumonia, a major cause of morbidity in TBI patients, was significantly more frequent in the DM cohort. This may be due to altered lung mechanics and the loss of glycemic control secondary to acute stress [27,28]. The heightened requirement for tracheostomy and PEG tube placement further supports this, as these interventions are often necessitated by prolonged respiratory compromise and swallowing dysfunction [29]. Additionally, the increased seizure risk in diabetic TBI patients is another critical finding. While the exact pathophysiology remains unclear, hyperglycemia-induced neuronal excitability and inflammation-driven alterations in neurotransmitter balance are plausible contributing factors [30,31]. Additionally, hypoglycemic episodes, which are more common in patients receiving insulin therapy, may exacerbate neuronal instability and predispose individuals to post-traumatic seizures [32]. Ultimately, our findings also support the close management of DM in critically ill TBI patients to minimize these potential adverse effects.
Our results align with emerging research suggesting that the impact of DM on outcomes in critical care settings is more nuanced than previously recognized. Published literature has reported a similar dichotomy of protective and adverse outcomes in diabetic patients admitted to intensive care units (ICUs). Specifically, a study by Hermanides et al. identified that intensive glycemic control showed a borderline significant reduction in poor neurological outcomes when compared to standard management. However, there was no significant reduction in the risk of mortality and a significant increase in the risk of hypoglycemia [33]. These results were partially corroborated by a more recent study from Garcia-Ballestas, who also found that intensive glycemic control significantly reduced poor neurological outcomes and increased good outcomes but was not correlated with a significant reduction in the risk of mortality [34]. However, this study found no relationship between worsening outcomes and hypoglycemia from intensive glycemic management. Moreover, a systematic review by Hanafy et al. examined the impact of various comorbidities on functional outcomes post-TBI [35]. Amongst various comorbid disorders, including cancer, hepatic disease, and hypertension, the review found inconsistent findings regarding the association between DM and functional outcomes. Specifically, while DM was associated with poor three-month functional independence on the Modified Rankin Scale, it was not associated with discharge, three-month functional impairment, or six-month functional impairment on the Glasgow Outcome Scale or twelve-month functional independence on the Modified Rankin Scale. This suggests that the relationship between DM and critical illness outcomes may not be straightforward but influenced by various factors such as glycemic control, the use of anti-diabetic therapies, and the specific nature of the underlying injury or illness. Discrepancies in the literature can likely be attributed to differences in study designs, variations in glucose management protocols, and the heterogeneity of patient populations included in these studies [36].
Other studies, including systematic reviews and meta-analyses on glycemic control as part of a TBI treatment protocol, further support the notion of there being unclear guidelines on glycemic management, highlighting that while intensive glycemic control aims to mitigate hyperglycemia-related complications, it may not improve—and could potentially worsen—outcomes in TBI patients. Notably, The NICE-SUGAR trial demonstrated that intensive glucose control amongst patients in the intensive care unit did not improve mortality in critically ill patients and, in some cases, increased the risk of hypoglycemia-related complications [37]. More specifically, this large, international, randomized trial found that intensive glucose control increased the absolute risk of death at ninety days by 2.6 percentage points compared to conventional glucose control. Strikingly, the results from the NICE-SUGAR trial contrast the landmark study by Van den Berghe et al. that originally recommended intensive glucose control using insulin therapy [9]. However, the NICE-SUGAR trial was unable to delineate whether the negative effects of tight glycemic management resulted from the reduced blood glucose level, increased administration of insulin, or occurrence of hypoglycemia among others. Thus, these findings support our assertion that aggressive glucose management in TBI cases may not yield uniformly positive outcomes and should be carefully individualized as there are multiple potential confounding variables tied to the treatment protocol.
Aside from the NICE-SUGAR trial, a systematic review and meta-analysis of published randomized controlled trials by Zhu et al. examined DM in critically ill patients and found a similar paradox: while intensive glycemic control resulted in a decreased infection rate and length of stay in the intensive care unit, it was also associated with a higher risk of hypoglycemia and the associated complications of acute hypoglycemic episodes. Importantly, the study found that intensive glycemic control has no significant impact on the mortality of patients who suffered a TBI [38]. The study highlighted the dual nature of DM’s impact on critical illness—providing metabolic preconditioning while simultaneously impairing immune defenses. This aligns closely with our findings, reinforcing the idea that diabetes may not be an outright risk factor for mortality in TBI, but rather a modifier of disease trajectory by prolonging the recovery process and necessitating more aggressive management of secondary complications. Similarly, research by Vespa et al. emphasized the importance of glycemic variability over absolute glycemic control in determining outcomes. Specifically, the study found that TBI patients displayed an increase in global glucose metabolism under tight glycemic control in a time-dependent fashion, thereby resulting in an increased risk for cerebral metabolic crisis after TBI with the strict maintenance of a limited glucose supply, supporting the notion of providing increased glucose delivery through mild hyperglycemia instead of glucose restriction as part of the treatment protocol [39]. These studies underscore the need for a more nuanced approach to glucose control in TBIs, avoiding both extremes of hyperglycemia and hypoglycemia.
The results from these studies may contribute to the more liberal regulation of DM in critically ill TBI patients and highlight the complexity of improving outcomes in these patients. Importantly, these findings have significant clinical implications, suggesting that while DM may not be an outright negative prognostic factor for TBI survival, its presence necessitates tailored management strategies. The increased risk of pneumonia, sepsis, and other complications indicates a need for aggressive infection prevention measures, respiratory support, and neurological surveillance in diabetic TBI patients. Future clinical guidelines should consider balancing glycemic control strategies to mitigate the risk of hyperglycemia-induced complications while avoiding the detrimental effects of hypoglycemia. Furthermore, the increased incidence of seizures in diabetic TBI patients highlights the importance of early seizure prophylaxis and monitoring in this population. Identifying patients at the highest risk for post-traumatic epilepsy could help guide personalized treatment approaches and improve long-term neurological outcomes [40]. Beyond acute care, rehabilitation strategies for diabetic TBI survivors should be adapted to address the unique challenges posed by metabolic dysfunction. Given the potential impact of chronic hyperglycemia on neuroplasticity and cognitive recovery, rehabilitation programs should incorporate tailored interventions that optimize glycemic control while promoting functional recovery [41]. Multidisciplinary rehabilitation teams should collaborate to integrate strategies that address both metabolic and neurological factors influencing recovery trajectories in diabetic TBI patients.
This study’s findings also underscore the need for long-term follow-up studies to evaluate the sustained impact of DM on post-TBI outcomes. While our analysis focused primarily on short-term in-hospital outcomes, future research should examine the long-term cognitive, functional, and psychosocial consequences of TBI in diabetic patients. Understanding the prolonged impact of DM on neurocognitive recovery, quality of life, and disability status could inform more effective rehabilitation strategies and long-term care planning.
A growing body of evidence suggests that metabolic dysfunction plays a key role in influencing neuroinflammatory responses post-TBI [42]. Chronic hyperglycemia has been associated with exacerbated inflammatory cascades, leading to prolonged neuroinflammation and secondary neuronal injury. Future studies should explore the potential of anti-inflammatory therapies in mitigating these effects and improving neurological outcomes in diabetic TBI patients. Targeting inflammatory pathways through pharmacological and lifestyle interventions could be a promising approach to enhancing post-TBI recovery. Moreover, research examining the impact of insulin resistance on cerebral metabolism post-TBI could provide valuable insights into the mechanisms driving cognitive decline and functional impairments. Insulin signaling plays a crucial role in neuronal survival and synaptic plasticity, and disruptions in this pathway may contribute to poor outcomes in diabetic TBI patients [43]. Investigating the role of insulin-sensitizing agents in restoring cerebral metabolic function post-TBI could pave the way for novel therapeutic strategies.
From a public health perspective, the rising prevalence of both DM and TBI necessitates a proactive approach to optimizing outcomes in this high-risk population. In high-volume trauma centers, managing glycemic control presents significant challenges. Magro et al. discuss the difficulties faced by a tertiary orthopedic hospital during the pandemic, including managing patient flows, reallocating staffing, and maintaining urgent care services [44]. Moreover, as highlighted by Mackersie and Dicker, common pitfalls in trauma management, such as inadequate assessment, delayed interventions, and miscommunication, can adversely affect patient outcomes [45]. Fortunately, tight glucose control (TGC) protocols have shown promise in improving patient outcomes, where maintaining consistent glucose levels and minimizing variability were associated with better survival rates [46]. Thus, to address these multifaceted challenges and incorporate necessary revisions to hospital practices, educational initiatives aimed at increasing awareness of the bidirectional relationship between metabolic health and brain injury could encourage better pre-injury glycemic control and post-injury management [47]. Healthcare systems should implement integrated care models that facilitate seamless coordination between endocrinologists, neurologists, and rehabilitation specialists to ensure comprehensive management of diabetic TBI patients.
The potential influence of sex differences on TBI outcomes in diabetic patients is another area warranting further exploration. Emerging evidence suggests that hormonal variations and sex-specific metabolic responses may modulate post-TBI recovery trajectories [48]. Investigating how sex-related differences in glucose metabolism and neuroinflammation affect outcomes in diabetic TBI patients could provide valuable insights into personalized treatment approaches. Additionally, examining genetic predispositions that influence both metabolic and neuroinflammatory responses could uncover new risk stratification methods for diabetic TBI patients. Genetic polymorphisms affecting insulin signaling, oxidative stress regulation, and inflammatory responses may contribute to inter-individual variations in post-TBI recovery [49]. Future studies incorporating genomic analyses could help identify biomarkers predictive of outcomes in diabetic TBI patients, facilitating the development of precision medicine approaches.
Lastly, the socioeconomic burden of managing diabetic TBI patients warrants attention. The increased risk of prolonged hospital stays, readmissions, and long-term disability in this population has significant economic implications. Policymakers and healthcare institutions should prioritize resource allocation to optimize post-TBI care in diabetic patients, ensuring equitable access to rehabilitation services and support systems. Implementing cost-effective strategies, such as telemedicine-based follow-ups and community-based rehabilitation programs, could improve long-term outcomes while reducing healthcare expenditures.
4.2. Limitations
The retrospective design of this study inherently carries the risk of confounding, as unmeasured variables that could not be accounted for due to intrinsic limitations of the TriNetX software may have influenced the results. Specifically, the lack of detailed data on glucose levels to indicate the level of glycemic management in this study’s patients, medication usage, adherence to treatment plans, and long-term outcomes limits our ability to understand the mechanisms underlying the observed associations fully. Moreover, long-term functional outcomes were not assessed, leaving gaps in understanding how DM affects post-hospital recovery trajectories. Regarding comorbidities, this study’s patients were not stratified by the level of care received regarding the trauma rating of the hospital they were treated at (Level I, II, III, etc.). This is a crucial limitation, which may have influenced the paradoxical results between increased morbidity and reduced mortality, since treatment in a dedicated neurotrauma service is associated with improved outcomes [50]. Additionally, since not all severe TBIs are the same, the type of injury, which the patients were not stratified by, such as penetrating vs. non-penetrating injury or space-occupying vs. brain swelling or polytrauma vs. isolated head injury, may have introduced biases in the analysis. Furthermore, each of the complications included as outcome measures can also increase glycemic levels in non-DM patients [51,52], although this limitation is compensated for by the dichotomized patients by their DM status and DM sub-groups. Nonetheless, this study benefits from several strengths that enhance its validity and generalizability. The use of a large, multi-center dataset allows for a broad representation of the diabetic TBI population, providing robust insights into the interplay between DM and TBI outcomes. Rigorous statistical techniques, including propensity score matching, were employed to adjust for potential confounders, thus strengthening the credibility of the findings. Future prospective studies with more comprehensive data collection on these factors would be beneficial in elucidating the full scope of DM’s impact on TBI recovery and survival.
5. Conclusions
This study highlights the complex and somewhat paradoxical role of diabetes mellitus (DM) in the context of severe traumatic brain injury (TBI), suggesting a potential protective association with in-hospital mortality while simultaneously conferring an increased risk for secondary complications such as infections and seizures. However, these findings must be interpreted with caution, as our analysis is inherently limited by the lack of data on glycemic control, treatment adherence, and the presence of other comorbidities that may influence TBI outcomes. Given these unmeasured confounders, it remains unclear whether the observed protective effect reflects a true physiological phenomenon or is instead a result of selection biases and unaccounted clinical variables. Nonetheless, our study challenges the traditional notion of DM as an unequivocally detrimental comorbidity in critical illness and underscores the need for a more nuanced approach to managing diabetic patients with severe TBI. Future research should incorporate detailed metabolic profiling, real-time glycemic monitoring, and individualized treatment strategies to better delineate the impact of DM on neurotrauma outcomes. By advancing our understanding of the interplay between chronic metabolic dysfunction and acute neurological injury, we may refine therapeutic strategies and ultimately improve care for this vulnerable patient population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/surgeries6020038/s1, Figure S1: Complications Risk for DM vs. NDM; Figure S2: Complications Risk for T1DM vs. T2DM Cohorts.
Author Contributions
Conceptualization, K.S., S.R., R.R. and M.K.; data curation, K.S.; formal analysis, K.S., S.R. and M.K.; investigation, K.S. and S.R.; methodology, K.S., S.R., R.R. and M.K.; project administration, K.S. and M.K.; supervision, R.R. and M.K.; validation, K.S., S.R., R.R. and M.K.; visualization, K.S., S.R., R.R. and M.K.; writing—original draft, K.S. and M.K.; writing—review and editing, K.S., S.R., R.R. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because any data displayed on the TriNetX Platform in aggregate form, or any patient-level data provided in a data set generated by the TriNetX Platform, only contains de-identified data as per the de-identification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. This formal determination by a qualified expert, refreshed in December 2020, supersedes the need for TriNetX’s previous waiver from the Western Institutional Review Board (IRB). This means that analysis on the TriNetX Platform or offline analysis of downloaded datasets is not considered “human subjects research” and is exempt from IRB review. Furthermore, TriNetX analytics only display data in the form of aggregated and statistical summaries of de-identified information. Therefore, no PHI or Personal Data is made available to users on the platform.
Informed Consent Statement
This retrospective study is exempt from informed consent. The data reviewed is a secondary analysis of existing data, does not involve intervention or interaction with human subjects, and is de-identified per the de-identification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. The process by which the data is de-identified is attested to through a formal determination by a qualified expert as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule. This formal determination by a qualified expert refreshed on December 2020.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from TriNetX, LLC and are available from the authors with the permission of TriNetX, LLC.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CI | Confidence interval |
| LOINC | Logical Observation Identifiers Names and Codes |
| DM | Diabetes mellitus |
| DVT | Deep vein thrombosis |
| ICD-10-CM | International Classification of Diseases, Tenth Revision, Clinical Modification |
| IDDM | Insulin-dependent diabetes mellitus |
| MI | Myocardial infarction |
| NIDDM | Non-insulin dependent diabetes mellitus |
| PE | Pulmonary embolism |
| PEG | Percutaneous endoscopic gastrostomy |
| RR | Risk ratio |
| SMD | Standardized mean difference |
| TBI | Traumatic brain injury |
References
- Wong, V.S.; Langley, B. Epigenetic changes following traumatic brain injury and their implications for outcome, recovery and therapy. Neurosci. Lett. 2016, 625, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Rovlias, A.; Kotsou, S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery 2000, 46, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.; Scaife, E.R.; Hansen, K.W.; Downey, E.C. Hyperglycemia and outcomes from pediatric traumatic brain injury. J. Trauma-Inj. Infect. Crit. Care 2003, 55, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Bürki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Jeremitsky, E.; Omert, L.A.; Dunham, C.M.; Wilberger, J.; Rodriguez, A. The impact of hyperglycemia on patients with severe brain injury. J. Trauma-Inj. Infect. Crit. Care 2005, 58, 47–50. [Google Scholar] [CrossRef]
- Prisco, L.; Iscra, F.; Ganau, M.; Berlot, G. Early predictive factors on mortality in head injured patients: A retrospective analysis of 112 traumatic brain injured patients. J. Neurosurg. Sci. 2012, 56, 131–136. [Google Scholar]
- Chong, S.L.; Harjanto, S.; Testoni, D.; Ng, Z.M.; Low, C.Y.D.; Lee, K.P.; Lee, J.H. Early Hyperglycemia in Pediatric Traumatic Brain Injury Predicts for Mortality, Prolonged Duration of Mechanical Ventilation, and Intensive Care Stay. Int. J. Endocrinol. 2015, 2015, 719476. [Google Scholar] [CrossRef]
- Shi, J.; Dong, B.; Mao, Y.M.; Guan, W.; Cao, J.C.; Zhu, R.X.; Wang, S.N. Review: Traumatic brain injury and hyperglycemia, a potentially modifiable risk factor. Oncotarget 2016, 7, 71052–71061. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef]
- Type 2 Diabetes. Available online: https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html (accessed on 18 April 2023).
- Marseglia, A.; Fratiglioni, L.; Kalpouzos, G.; Wang, R.; Bäckman, L.; Xu, W.L. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: A population-based cohort study. Alzheimers Dement. 2019, 15, 25–33. [Google Scholar] [CrossRef]
- Chaudhry, R.; Kukreja, N.; Tse, A.; Pednekar, G.; Mouchli, A.; Young, L.; Didyuk, O.; Wegner, R.C.; Grewal, N.; Williams, G.W. Trends and Outcomes of Early Versus Late Percutaneous Endoscopic Gastrostomy Placement in Patients with Traumatic Brain Injury: Nationwide Population-based Study. J. Neurosurg. Anesthesiol. 2018, 30, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Galimberti, S.; Graziano, F.; Wiegers, E.J.A.; Lingsma, H.F.; Iaquaniello, C.; Stocchetti, N.; Menon, D.; Citerio, G.; Participants, C.-T.I. Tracheostomy practice and timing in traumatic brain-injured patients: A CENTER-TBI study. Intensive Care Med. 2020, 46, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Harhangi, B.S.; Kompanje, E.J.O.; Leebeek, F.W.G.; Maas, A.I.R. Coagulation disorders after traumatic brain injury. Acta Neurochir. 2008, 150, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Lustenberger, T.; Talving, P.; Lam, L.; Inaba, K.; Bass, M.; Plurad, D.; Demetriades, D. Effect of diabetes mellitus on outcome in patients with traumatic brain injury: A national trauma databank analysis. Brain Inj. 2013, 27, 281–285. [Google Scholar] [CrossRef]
- Tseng, C.C.; Huang, Y.C.; Tu, P.H.; Yip, P.K.; Chang, T.W.; Lee, C.C.; Chen, C.C.; Chen, N.Y.; Liu, Z.H. Impact of Diabetic Hyperglycemia on Clinical Outcomes in Patients with Diabetes Mellitus Following Traumatic Brain Injury. Turk. Neurosurg. 2023, 33, 548–555. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Croft, C.B.; Solodushko, V. Cardioprotective effect of chronic hyperglycemia: Effect on hypoxia-induced apoptosis and necrosis. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H1948–H1954. [Google Scholar] [CrossRef]
- Rojas, D.R.; Tegeder, I.; Kuner, R.; Agarwal, N. Hypoxia-inducible factor 1 protects peripheral sensory neurons from diabetic peripheral neuropathy by suppressing accumulation of reactive oxygen species. J. Mol. Med. 2018, 96, 1395–1405. [Google Scholar] [CrossRef]
- Pasquel, F.J.; Lansang, M.C.; Dhatariya, K.; Umpierrez, G.E. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021, 9, 174–188. [Google Scholar] [CrossRef]
- Patil, S.P.; Jain, P.D.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective effect of metformin in mptp-induced parkinson’s disease in mice. Neuroscience 2014, 277, 747–754. [Google Scholar] [CrossRef]
- Cai, X.S.; She, M.Q.; Xu, M.Y.; Chen, H.Y.; Li, J.J.; Chen, X.L.; Zheng, D.P.; Liu, J.; Chen, S.L.; Zhu, J.B.; et al. GLP-1 treatment protects endothelial cells from oxidative stress-induced autophagy and endothelial dysfunction. Int. J. Biol. Sci. 2018, 14, 1696–1708. [Google Scholar] [CrossRef]
- Siddeeque, N.; Hussein, M.H.; Abdelmaksoud, A.; Bishop, J.; Attia, A.S.; Elshazli, R.M.; Fawzy, M.S.; Toraih, E.A. Neuroprotective effects of GLP-1 receptor agonists in neurodegenerative Disorders: A Large-Scale Propensity-Matched cohort study. Int. Immunopharmacol. 2024, 143, 113537. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Gödtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; Antonio, R.S.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Nojima, I.; Eikawa, S.; Tomonobu, N.; Hada, Y.; Kajitani, N.; Teshigawara, S.; Miyamoto, S.; Tone, A.; Uchida, H.A.; Nakatsuka, A.; et al. Dysfunction of CD8+PD-1+T cells in type 2 diabetes caused by the impairment of metabolism-immune axis. Sci. Rep. 2020, 10, 14928. [Google Scholar] [CrossRef]
- Zhao, J.L.; Yang, S.; Shu, B.; Chen, L.; Yang, R.H.; Xu, Y.B.; Xie, J.L.; Liu, X.S.; Qi, S.H. Transient High Glucose Causes Persistent Vascular Dysfunction and Delayed Wound Healing by the DNMT1-Mediated Ang-1/NF-κB Pathway. J. Investig. Dermatol. 2021, 141, 1573–1584. [Google Scholar] [CrossRef]
- Kopf, S.; Groener, J.B.; Kender, Z.; Fleming, T.; Brune, M.; Riedinger, C.; Volk, N.; Herpel, E.; Pesta, D.; Szendrödi, J.; et al. Breathlessness and Restrictive Lung Disease: An Important Diabetes-Related Feature in Patients with Type 2 Diabetes. Respiration 2018, 96, 29–40. [Google Scholar] [CrossRef]
- Cheng, S.J.; Hou, G.J.; Liu, Z.P.; Lu, Y.; Liang, S.C.; Cang, L.; Zhang, X.Y.; Zou, C.L.; Kang, J.; Chen, Y. Risk prediction of in-hospital mortality among patients with type 2 diabetes mellitus and concomitant community-acquired pneumonia. Ann. Pallliat. Med. 2020, 9, 3313–3325. [Google Scholar] [CrossRef]
- Langmore, S.E.; Krisciunas, G.P.; Warner, H.; White, S.D.; Dvorkin, D.; Fink, D.; McNally, E.; Scheel, R.; Higgins, C.; Levitt, J.E.; et al. Abnormalities of Aspiration and Swallowing Function in Survivors of Acute Respiratory Failure. Dysphagia 2021, 36, 831–841. [Google Scholar] [CrossRef]
- Tang, F.; Lane, S.; Korsak, A.; Paton, J.F.R.; Gourine, A.V.; Kasparov, S.; Teschemacher, A.G. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat. Commun. 2014, 5, 3284. [Google Scholar] [CrossRef]
- Xing, G.Q.; Ren, M.; O’Neill, J.T.; Verma, A.; Watson, W.D. Controlled cortical impact injury and craniotomy result in divergent alterations of pyruvate metabolizing enzymes in rat brain. Exp. Neurol. 2012, 234, 31–38. [Google Scholar] [CrossRef]
- Reno, C.M.; Skinner, A.; Bayles, J.; Chen, Y.S.; Daphna-Iken, D.; Fisher, S.J. Severe hypoglycemia-induced sudden death is mediated by both cardiac arrhythmias and seizures. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E240–E249. [Google Scholar] [CrossRef] [PubMed]
- Hermanides, J.; Plummer, M.P.; Finnis, M.; Deane, A.M.; Coles, J.P.; Menon, D.K. Glycaemic control targets after traumatic brain injury: A systematic review and meta-analysis. Crit. Care 2018, 22, 11. [Google Scholar] [CrossRef]
- Garcia-Ballestas, E.; Villafañe, J.; Nuñez-Baez, K.; Perdomo, W.A.F.; Duran, M.A.; Janjua, T.; Moscote-Salazar, L.R.; Agrawal, A. A systematic review and meta-analysis on glycemic control in traumatic brain injury. Clin. Neurol. Neurosurg. 2024, 245, 108504. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, S.; Xiong, C.; Chan, V.; Sutton, M.; Escobar, M.; Colantonio, A.; Mollayeva, T. Comorbidity in traumatic brain injury and functional outcomes: A systematic review. Eur. J. Phys. Rehabil. Med. 2021, 57, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Stoudt, K.; Chawla, S. Don’t Sugar Coat It: Glycemic Control in the Intensive Care Unit. J. Intensive Care Med. 2019, 34, 889–896. [Google Scholar] [CrossRef]
- Finfer, S.; Blair, D.; Bellomo, R.; McArthur, C.; Mitchell, I.; Myburgh, J.; Norton, R.; Potter, J.; Chittock, D.; Dhingra, V.; et al. Intensive versus Conventional Glucose Control in Critically Ill Patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef]
- Zhu, C.R.; Chen, J.J.; Pan, J.C.; Qiu, Z.C.; Xu, T. Therapeutic effect of intensive glycemic control therapy in patients with traumatic brain injury: A systematic review and meta-analysis of randomized controlled trials. Medicine 2018, 97, e11671. [Google Scholar] [CrossRef]
- Vespa, P.; McArthur, D.L.; Stein, N.; Huang, S.C.; Shao, W.; Filippou, M.; Etchepare, M.; Glenn, T.; Hovda, D.A. Tight glycemic control increases metabolic distress in traumatic brain injury: A randomized controlled within-subjects trial. Crit. Care Med. 2012, 40, 1923–1929. [Google Scholar] [CrossRef]
- Laing, J.; Gabbe, B.; Chen, Z.B.; Perucca, P.; Kwan, P.; O’Brien, T.J. Risk Factors and Prognosis of Early Posttraumatic Seizures in Moderate to Severe Traumatic Brain Injury. JAMA Neurol. 2022, 79, 334–341. [Google Scholar] [CrossRef]
- Gupta, M.; Pandey, S.; Rumman, M.; Singh, B.; Mahdi, A.A. Molecular mechanisms underlying hyperglycemia associated cognitive decline. IBRO Neurosci. Rep. 2023, 14, 57–63. [Google Scholar] [CrossRef]
- Lai, J.Q.; Shi, Y.C.; Lin, S.; Chen, X.R. Metabolic disorders on cognitive dysfunction after traumatic brain injury. Trends Endocrinol. Metab. 2022, 33, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.L.; Siu, J.J.; Huang, W.; Askwith, C.; Cao, L. Insulin Modulates Excitatory Synaptic Transmission and Synaptic Plasticity in the Mouse Hippocampus. Neuroscience 2019, 411, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Perazzo, P.; Bottinelli, E.; Possenti, F.; Banfi, G. Managing a tertiary orthopedic hospital during the covid-19 epidemic, main challenges and solutions adopted. Int. J. Environ. Res. Public Health 2020, 17, 4818. [Google Scholar] [CrossRef] [PubMed]
- Mackersie, R.C.; Dicker, R.A. Pitfalls in the Evaluation and Management of the Trauma Patient. Curr. Probl. Surg. 2007, 44, 778–833. [Google Scholar] [CrossRef]
- Eriksson, E.A.; Christianson, D.A.; Vanderkolk, W.E.; Bonnell, B.W.; Hoogeboom, J.E.; Ott, M.M. Tight blood glucose control in trauma patients: Who really benefits. J. Emerg. Trauma Shock 2011, 4, 359–364. [Google Scholar] [CrossRef]
- Kurtz, P.; Rocha, E.E.M. Nutrition Therapy, Glucose Control, and Brain Metabolism in Traumatic Brain Injury: A Multimodal Monitoring Approach. Front. Neurosci. 2020, 14, 190. [Google Scholar] [CrossRef]
- Späni, C.B.; Braun, D.J.; Van Eldik, L.J. Sex-related responses after traumatic brain injury: Considerations for preclinical modeling. Front. Neuroendocrinol. 2018, 50, 52–66. [Google Scholar] [CrossRef]
- Lakshmipathy, D.; Rangarajan, S.; Barreau, A.; Lu, J.; Kleinberg, G.; Lucke-Wold, B. Genetic Contributions to Recovery following Brain Trauma: A Narrative Review. Front. Biosci. 2024, 29, 103. [Google Scholar] [CrossRef]
- Dasic, D.; Morgan, L.; Panezai, A.; Syrmos, N.; Ligarotti, G.K.I.; Zaed, I.; Chibbaro, S.; Khan, T.; Prisco, L.; Ganau, M. A scoping review on the challenges, improvement programs, and relevant output metrics for neurotrauma services in major trauma centers. Surg. Neurol. Int. 2022, 13, 171. [Google Scholar] [CrossRef]
- Hiroyuki, H.; Shigeto, O.; Masataka, N. Blood glucose control in patients with severe sepsis and septic shock. World J. Gastroenterol. 2009, 15, 4132. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Diao, L.; Stanojcic, M.; Xiu, F.; Eckardt, K. Stress Hyperglycemia, Insulin Treatment, and Innate Immune Cells. Int. J. Endocrinol. 2014, 2014, 486403. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).