Return to Sport after Pediatric Osteochondral Lesions: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Study Creation and Initial Search
2.2. Inclusion and Exclusion Criteria
2.3. Article Screening Process
2.4. Study Definitions
2.5. Data Extraction
2.6. Article Quality Grading
2.7. Statistical Analysis
3. Results
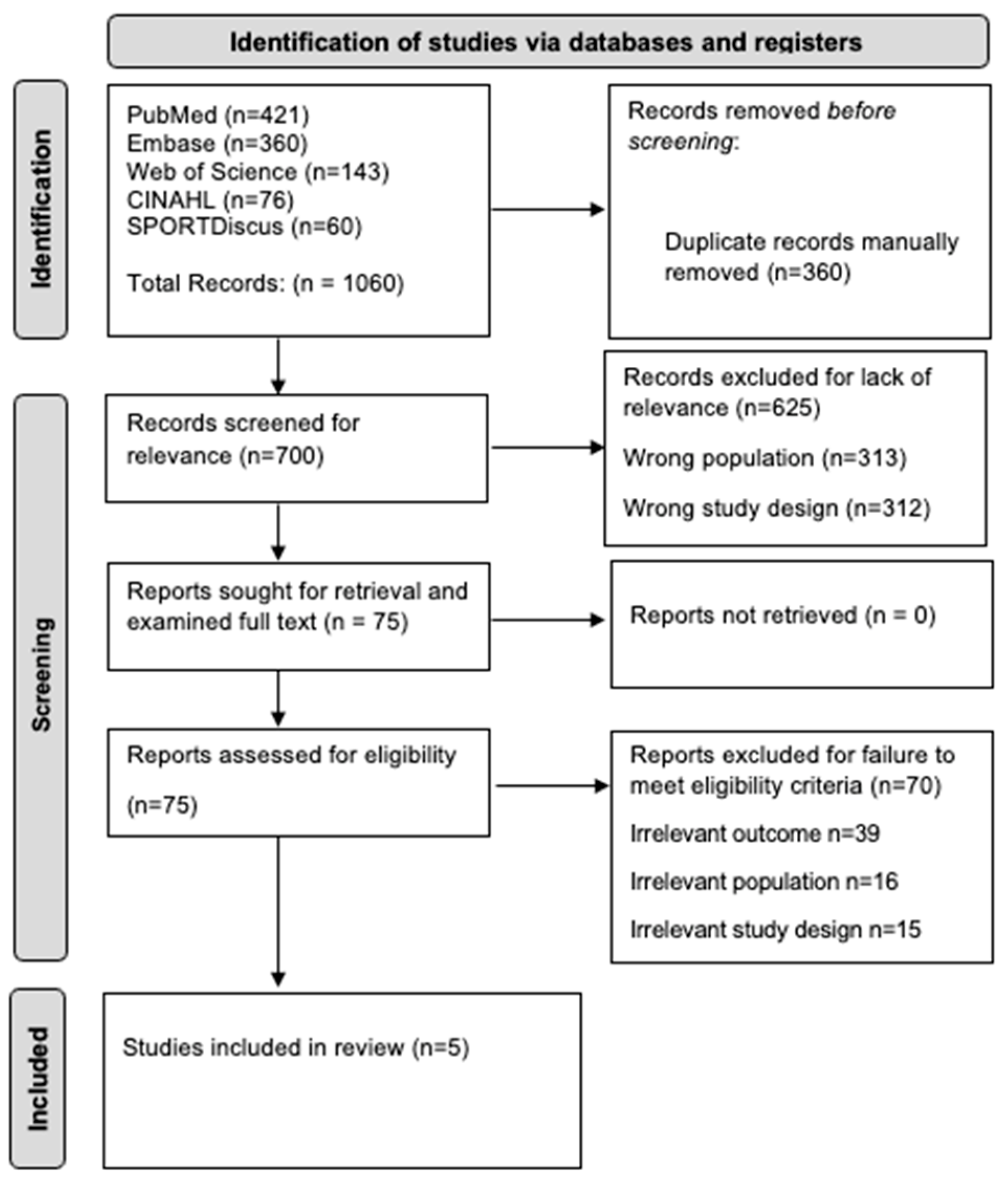
3.1. Initial Search Results
3.2. Article Quality Results
3.3. Patient and Study Characteristics
3.4. RTS Achievement and Criteria
3.5. Clinical Outcomes Following Surgery Using Time-Based RTS Criteria
3.6. Clinical Outcomes Following Surgery Using Milestone-Based RTS Criteria
3.7. Clinical Outcomes Following Surgery Using Mixed RTS Criteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bae, S.; Lee, H.K.; Lee, K.; Lim, S.; Rim, N.-J.; Kim, J.-S.; Cho, J. Comparison of arthroscopic and magnetic resonance imaging findings in osteochondral lesions of the talus. Foot Ankle Int. 2012, 33, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Griggs, R.; Hall, T.; Motamedi, K.; Boechat, M.I.; Ghahremani, S. Osteochondral Lesions in Pediatric and Adolescent Patients. Semin. Musculoskelet. Radiol. 2014, 18, 505–512. [Google Scholar] [CrossRef]
- Anastasio, A.T.; Bagheri, K.; Peairs, E.M.; Grant, C.; Adams, S.B. Juvenile Osteochondral Lesions of the Talus: Current Concepts Review and an Update on the Literature. Children 2023, 10, 884. [Google Scholar] [CrossRef]
- Bauer, K.L. Osteochondral Injuries of the Knee in Pediatric Patients. J. Knee Surg. 2018, 31, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Bruns, J.; Habermann, C.; Werner, M. Osteochondral Lesions of the Talus: A Review on Talus Osteochondral Injuries, Including Osteochondritis Dissecans. Cartilage 2021, 13 (Suppl. S1), 1380S–1401S. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.B.; da Cruz, I.A.N.; Nery, C.; Silva, F.D.; Filho, A.G.O.; Carneiro, B.C.; Nico, M.A.C. Osteochondral lesions of the talar dome: An up-to-date approach to multimodality imaging and surgical techniques. Skelet. Radiol. 2021, 50, 2151–2168. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.J.; Ho, J.; Allen, C.R. Evaluation and management of osteochondral lesions of the knee. Physician Sportsmed. 2011, 39, 60–69. [Google Scholar] [CrossRef]
- Buck, T.M.F.; Lauf, K.; Dahmen, J.; Altink, J.N.; Stufkens, S.A.S.; Kerkhoffs, G.M.M.J. Non-operative management for osteochondral lesions of the talus: A systematic review of treatment modalities, clinical- and radiological outcomes. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 3517–3527. [Google Scholar] [CrossRef]
- Itha, R.; Vaishya, R.; Vaish, A.; Migliorini, F. Management of chondral and osteochondral lesions of the hip: A comprehensive review. Orthopadie 2024, 53, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, L.; Laux, C.J.; Urbanschitz, L.; Espinosa, N.; Klammer, G.; Götschi, T.; Wirth, S.H. Long-term Prognosis After Successful Nonoperative Treatment of Osteochondral Lesions of the Talus: An Observational 14-Year Follow-up Study. Orthop. J. Sports Med. 2020, 8, 2325967120924183. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, S.; Yoshimura, I.; Hagio, T.; Ishimatsu, T.; Sugino, Y.; Fukagawa, R.; Taniguchi, Y.; Yamamoto, T. Return to Sports Activity After Microfracture for Osteochondral Lesion of the Talus in Skeletally Immature Children. Foot Ankle Int. 2024, 45, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Corr, D.; Raikin, J.; O’Neil, J.; Raikin, S. Long-term Outcomes of Microfracture for Treatment of Osteochondral Lesions of the Talus. Foot Ankle Int. 2021, 42, 833–840. [Google Scholar] [CrossRef]
- Georgiannos, D.; Bisbinas, I.; Badekas, A. Osteochondral transplantation of autologous graft for the treatment of osteochondral lesions of talus: 5- to 7-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3722–3729. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.T.; Dowd, T.C.; Giza, E. Surgical Treatment for Osteochondral Lesions of the Talus. Arthroscopy 2021, 37, 3393–3396. [Google Scholar] [CrossRef]
- Vijayan, S.; Bartlett, W.; Bentley, G.; Carrington, R.W.J.; Skinner, J.A.; Pollock, R.C.; Alorjani, M.; Briggs, T.W.R. Autologous chondrocyte implantation for osteochondral lesions in the knee using a bilayer collagen membrane and bone graft: A two- to eight-year follow-up study. J. Bone Jt. Surg. Br. 2012, 94, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.; Amouyel, T.; Benoist, J.; De L’escalopier, N.; Cordier, G.; Freychet, B.; Baudrier, N.; Ferrière, V.D.; Wackenheim, F.L.; Mainard, D.; et al. Return to sport after surgery for osteochondral lesions of the talar dome. Results of a multicenter prospective study on 58 patients. Orthop. Traumatol. Surg. Res. 2023, 109, 103675. [Google Scholar] [CrossRef]
- Seow, D.; Shimozono, Y.; Gianakos, A.L.; Chiarello, E.; Mercer, N.; Hurley, E.T.; Kennedy, J.G. Autologous osteochondral transplantation for osteochondral lesions of the talus: High rate of return to play in the athletic population. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Cognetti, D.J.; Defoor, M.T.; Yuan, T.T.; Sheean, A.J. Knee Joint Preservation in Tactical Athletes: A Comprehensive Approach Based upon Lesion Location and Restoration of the Osteochondral Unit. Bioengineering 2024, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.K.; Ebert, J.R.; Joss, B.; Ackland, T.; Annear, P.; Buelow, J.-U.; Hewitt, B. Patient Characteristics and Predictors of Return to Sport at 12 Months After Anterior Cruciate Ligament Reconstruction: The Importance of Patient Age and Postoperative Rehabilitation. Orthop. J. Sports Med. 2018, 6, 2325967118797575. [Google Scholar] [CrossRef]
- Han, F.; Banerjee, A.; Shen, L.; Krishna, L. Increased Compliance with Supervised Rehabilitation Improves Functional Outcome and Return to Sport after Anterior Cruciate Ligament Reconstruction in Recreational Athletes. Orthop. J. Sports Med. 2015, 3, 2325967115620770. [Google Scholar] [CrossRef] [PubMed]
- Oak, S.R.; Klein, B.; Verma, N.N.; Kerzner, B.; Fortier, L.M.; Chava, N.S.; Reinold, M.M.; Bedi, A. Rehabilitation and Return to Play of the Athlete after an Upper Extremity Injury. Arthrosc. Sports Med. Rehabilit. 2022, 4, e163–e173. [Google Scholar] [CrossRef] [PubMed]
- Lorange, J.-P.; Senécal, L.; Moisan, P.; Nault, M.-L. Return to Sport after Pediatric Anterior Cruciate Ligament Reconstruction: A Systematic Review of the Criteria. Am. J. Sports Med. 2024, 52, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.; Joseph, A.; Patel, A.; Ahluwalia, R.; Ray, R. Modified Brostrom repair with suture tape augmentation for lateral ankle instability: A systematic review. Foot Ankle Surg. 2021, 27, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Ishimatsu, T.; Yoshimura, I.; Kanazawa, K.; Hagio, T.; Yamamoto, T. Return to sporting activity after osteochondral autograft transplantation for Freiberg disease in young athletes. Arch. Orthop. Trauma Surg. 2017, 137, 959–965. [Google Scholar] [CrossRef]
- Kramer, D.E.; Glotzbecker, M.P.; Shore, B.J.; Zurakowski, D.; Yen, Y.-M.; Kocher, M.S.; Micheli, L.J. Results of Surgical Management of Osteochondritis Dissecans of the Ankle in the Pediatric and Adolescent Population. J. Pediatr. Orthop. 2015, 35, 725–733. [Google Scholar] [CrossRef][Green Version]
- Pallamar, M.; Eder, T.; Ganger, R.; Farr, S. Surgical treatment of atraumatic osteochondrosis dissecans of the immature talus-Clinical results and prevalence of radiographic joint degeneration after a median follow-up of 72.5 months. Foot Ankle Surg. 2022, 28, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Vasukutty, N.V.; Akrawi, H.; Theruvil, B.; Uglow, M. Ankle arthroscopy in children. Ann. R. Coll. Surg. Engl. 2011, 93, 232–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ardern, C.L.; Taylor, N.F.; Feller, J.A.; Whitehead, T.S.; Webster, K.E. Psychological responses matter in returning to preinjury level of sport after anterior cruciate ligament reconstruction surgery. Am. J. Sports Med. 2013, 41, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.L.; Tanaka, M.J.; Cosgarea, A.J.; Ginsburg, R.D.; Dreher, G.M. How Mental Health Affects Injury Risk and Outcomes in Athletes. Sports Health 2024, 16, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Dumont, G.D.; Hogue, G.D.; Padalecki, J.R.; Okoro, N.; Wilson, P.L. Meniscal and chondral injuries associated with pediatric anterior cruciate ligament tears: Relationship of treatment time and patient-specific factors. Am. J. Sports Med. 2012, 40, 2128–2133. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liu, S.; Yan, J.; Wang, L.; Lei, X.; Wu, H.; Zhu, Y.; Zhang, Y. The Time-Effect Relationship between Time to Surgery and In-Hospital Postoperative Pneumonia in Older Patients with Hip Fracture. Gerontology 2024, 70, 155–164. [Google Scholar] [CrossRef]
- George, J.; Sharma, V.; Farooque, K.; Mittal, S.; Trikha, V.; Malhotra, R. The Impact of Surgical Timing of Hip Fracture on Mortality: Do the Cause and Duration of Delay Matter? Hip Pelvis 2023, 35, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qiang, L.; Yang, Q.; Fan, L.; Wang, J.; Yang, Y.; Shi, Z.; Li, T. Delayed surgery is associated with adverse outcomes in patients with hip fracture undergoing hip arthroplasty. BMC Musculoskelet. Disord. 2023, 24, 286. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, O.S.; Lee, S.H. Return to Sports After Athletes Undergo Meniscal Surgery: A Systematic Review. Clin. J. Sport Med. 2019, 29, 29–36. [Google Scholar] [CrossRef]
- Gill, L.E.; Klingele, K.E. Management of foot and ankle injuries in pediatric and adolescent athletes: A narrative review. Orthop. Res. Rev. 2018, 10, 19–30. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Vannini, F.; Di Martino, A.; Andriolo, L.; Sessa, A.; Perdisa, F.; Balboni, F.; Filardo, G.; The ESSKA U45 Committee. Low rate of return to pre-injury sport level in athletes after cartilage surgery: A 10-year follow-up study. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2502–2510. [Google Scholar]
- Davies, G.J.; McCarty, E.; Provencher, M.; Manske, R.C. ACL Return to Sport Guidelines and Criteria. Curr. Rev. Musculoskelet. Med. 2017, 10, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Doege, J.; Ayres, J.M.; Mackay, M.J.; Tarakemeh, A.; Brown, S.M.; Vopat, B.G.; Mulcahey, M.K. Defining Return to Sport: A Systematic Review. Orthop. J. Sports Med. 2021, 9, 23259671211009589. [Google Scholar] [CrossRef] [PubMed]
- Paterno, M.V.; Rauh, M.J.; Thomas, S.; Hewett, T.E.; Schmitt, L.C. Return-to-Sport Criteria After Anterior Cruciate Ligament Reconstruction Fail to Identify the Risk of Second Anterior Cruciate Ligament Injury. J. Athl. Train. 2022, 57, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Casp, A.J.; Bodkin, S.G.M.; Kew, M.E.; Noona, S.W.B.; Lesevic, M.B.; Hart, J.M.P.; Diduch, D. Quadriceps Strength Is Influenced by Skeletal Maturity in Adolescents Recovering From Anterior Cruciate Ligament Reconstruction. J. Pediatr. Orthop. 2021, 41, e141–e146. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.; Antunes, A.; Thomis, M.; Silva, R.; Marques, G.; Silva, A.; Nunes, R.; Delgado, M.; Jardim, P.; Xixaro, R.; et al. Interrelationships among skeletal age, growth status and motor performances in female athletes 10–15 years. Ann. Hum. Biol. 2024, 51, 2297733. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.W.; Guo, X.; Fong, S.S.; Mak, K.-K.; Pang, M.Y. Activity participation intensity is associated with skeletal development in pre-pubertal children with developmental coordination disorder. Res. Dev. Disabil. 2012, 33, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
| First Author (Year) | Study Type | Total MINORS Score | Clearly Stated Aim | Inclusion of Consecutive Patients | Prospective Collection of Data | End Points Appropriate to Study Aim | Unbiased Assessment of Study End Point | What Follow-Up Period Appropriate to Study Aim | Less than 5% Lost to Follow Up | Prospective Calculation of the Study Size | Adequate Control Group | Contemporary Groups | Baseline Equivalence of Groups | Adequate Statistical Analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kramer et al. (2015) [27] | Non-comparative | 9 (low) | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 1 | - | - | - | - |
| Pallamar et al. (2022) [28] | Comparative | 15 (moderate) | 2 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 2 | 2 | 0 | 2 |
| Vasukutty et al. (2011) [29] | Non-comparative | 10 (moderate) | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | - | - | - | - |
| Tomonaga et al. (2024) [11] | Non-comparative | 11 (moderate) | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 1 | - | - | - | - |
| Ishimatsu et al. (2017) [26] | Non-comparative | 10 (moderate) | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | - | - | - | - |
| Author (Year) | Patients (n) | Age | Males/Females | Follow-Up | Lesion Location |
|---|---|---|---|---|---|
| Kramer (2015) [27] | 100 (109 ankles) | 14.3 ± 2.3 | Male (n = 25) Female (n = 75) | 39.6 months | Medial talus (n = 80) Lateral talus (n = 21) Central talus (n = 5) Distal tibia (n = 2) |
| Pallamar (2022) [28] | 30 (32 ankles) | 14.7 ± 2.2 | Male (n = 15) Female (n = 15) | 72.5 months | Talus; 100% |
| Vasukutty (2011) [29] | 12 | 13.5 | - | 24 months | Location mixed; not specified |
| Tomonaga (2024) [11] | 16 (17 ankles) | 13.2 ± 1.5 | Male (n = 6) Female (n = 10) | 53.5 months | Talus; 100% |
| Ishimatsu (2017) [26] | 10 | 14.8 ± 1.5 | Male (n = 1) Female (n = 9) | 24.6 months | 2nd metatarsal head (n = 7) 3rd metatarsal head (n = 2) 4th metatarsal head (n = 1) |
| Author (Year) | RTS Definition | RTS Criteria | Criteria Type | RTS % (n) | Time to RTS |
|---|---|---|---|---|---|
| Kramer (2015) [27] | Return to their competitive sport play | Formal physical therapy initiated at 6 weeks, focusing on range of motion (ROM) and strengthening. Return to impact sports was allowed at the surgeon’s discretion starting 3 months after surgery based upon clinical symptoms, physical examination, and imaging. | Mixed | 84% (n = 37/44) | Median: 6 months |
| Pallamar (2022) [28] | Perform sports without limitation | Partial weight-bearing for 4 weeks with stepwise progression to full weight-bearing within an additional 2–4 weeks. Physical therapy was prescribed to assist in strengthening, coordination, and functional recovery. | Mixed | 53.1% (n = 17/32) | No time specified |
| Vasukutty (2011) [29] | Return to their competitive sport play | Patients mobilized with partial weight-bearing crutches were allowed to increase to full-weight-bearing as pain allowed. Physiotherapy was utilized to enhance ROM and proprioception when required. | Milestone | 100% (n = 7/7) | No time specified |
| Tomonaga (2024) [11] | Return to same sport and same level of competition | Postoperative non-weight-bearing activity allowed for 3 weeks, followed by partial weight-bearing activity. Full weight-bearing activity allowed at 7 weeks after surgery. ROM exercises commenced without limit. Ankles with repaired lateral ligaments were splinted for 1 week. RTS was allowed 6 months after surgery. | Time-based | 100% (n = 17/17) | Mean: 6 months |
| Ishimatsu (2017) [26] | Return to the same sporting activity undertaken preoperatively | Return to sporting activity was allowed from 3 months postoperatively. | Time-based | 100% (n = 10/10) | Mean: 3.5 months |
| Author (Year) | Berndt and Harty Stage | PROS | Radiographic Outcomes |
|---|---|---|---|
| Kramer (2015) [27] | I: n = 14, II: n = 50, III: n = 16, IV: n = 3 | Average FAOS score: 77 ± 18; Symptoms FAOS: 73 ± 21; Pain FAOS: 81 ± 21; Function/Sports FAOS: 76 ± 19; ADLs FAOS: 91 ± 15; QOL FAOS: 64 ± 25; Total FAOS: 385 ± 90 | Healed lesion (n = 13, 16%); Improved lesion (n = 51, 64%); Unchanged lesion (n = 14, 18%); Worse lesion (n = 2, 3%) |
| Pallamar (2022) [28] | I: n = 11, II: n = 11, III: n = 6, IV: n = 0 | Berndt: I, NRS 2.2 ± 2.4, AOFAS 93.4 ± 9.4, FFI 7.3 ± 0.1. Berndt: II, NRS 3.5 ± 2.1, AOFAS 86.9 ± 9.8, FFI 15.0 ± 0.1. Berndt: III, NRS 3.3 ± 2.1, AOFAS 90.8 ± 12.2, FFI 13.3 ± 0.1 | Complete OCL restoration (n = 6); Mild signs of joint degeneration (n = 16) |
| Vasukutty (2011) [29] | - | Average FAOS score: 87 with complete relief of symptoms at 3 months | - |
| Tomonaga (2024) [11] | Pre: I: n = 4, II: n = 4, III: n = 7, IV: n = 2. Post: normal: n = 3, I: n = 12, II: n = 2, III: n = 0, IV: n = 0 | JSSF Score Pre: 76.1, Post: 94.9. AAS Score: Pre: 6.5, Post: 6.4 | Radiographic Stage decreased in 13 of 17 ankles. In three ankles, OLT was not detectable. |
| Ishimatsu (2017) [26] | - | Halasi score Preop: 6.5 ± 1.5; Postop: 6.0 ± 1.8 (p = 0.18) | Radiographs at the final follow-up revealed an adequate configuration of the metatarsal head without osteoarthritic changes in the operated MTP joint. In eight patients, MRI at the final follow-up showed consolidation between the transplanted osteochondral autograft and the subchondral bone and smooth configuration of the articular surface of the metatarsal head with presentation of the transplanted cartilage. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaspure, O.S.; Patel, S.; Baumann, A.N.; Lininger, J.; Anastasio, A.T. Return to Sport after Pediatric Osteochondral Lesions: A Systematic Review. Surgeries 2024, 5, 908-919. https://doi.org/10.3390/surgeries5040073
Anaspure OS, Patel S, Baumann AN, Lininger J, Anastasio AT. Return to Sport after Pediatric Osteochondral Lesions: A Systematic Review. Surgeries. 2024; 5(4):908-919. https://doi.org/10.3390/surgeries5040073
Chicago/Turabian StyleAnaspure, Omkar S., Shiv Patel, Anthony N. Baumann, Jake Lininger, and Albert T. Anastasio. 2024. "Return to Sport after Pediatric Osteochondral Lesions: A Systematic Review" Surgeries 5, no. 4: 908-919. https://doi.org/10.3390/surgeries5040073
APA StyleAnaspure, O. S., Patel, S., Baumann, A. N., Lininger, J., & Anastasio, A. T. (2024). Return to Sport after Pediatric Osteochondral Lesions: A Systematic Review. Surgeries, 5(4), 908-919. https://doi.org/10.3390/surgeries5040073







