Abstract
Meralgia paresthetica is a compressive neuropathy of the lateral femoral cutaneous nerve. Surgery is the gold standard for severe cases. However, no high-quality evidence exists on which strategy is best: decompression or neurectomy. Data of a consecutive series of 52 patients treated for meralgia paresthetica over 25 years (1997–2022) were retrospectively collected from medical records and telephone interviews. In total, 27 women and 25 men were operated on; 11 patients had iatrogenic meralgia paresthetica. Decompression was performed on 47 patients, and neurectomy in 8 cases (5 primary neurectomies plus 3 failed neurolysis). Out of the patients who underwent decompression, 41 (87.2%) benefited from the treatment; 3 had pain relief, but no benefit on paresthesia; and 3 reported pain persistence. The latter required neurectomy to resolve symptoms. The eight patients who underwent neurectomy experienced symptom relief but had an obvious anesthetic area persisting over years. Complications were rare (3.8%): a groin hematoma in the post-operative course and an inguinal herniation 6 months after surgery. Surgery, be it neurolysis or neurectomy, offers excellent results with low risks. Decompression has been proven to be adequate in almost all patients, avoiding the side effects of neurectomy. The latter should be confined to failed decompression or to iatrogenic meralgia.
1. Introduction
Meralgia paresthetica (MP), also known as Bernardt–Roth syndrome [1,2], is a neuropathy due to the lesion or entrapment of the lateral femoral cutaneous nerve (LFCN).
The core of this clinical condition is aptly summarized by the Greek etymology of the term itself: meralgia (μηρός = thigh and ἄλγος = pain) paresthetica (πara = similar and αἴσθησις = sensation).
Indeed, the symptoms include pain and altered sensation in the anterolateral part of the thigh, often associated with alopecia in the involved area.
MP is classified into spontaneous and iatrogenic forms depending on the cause of the nerve injury or compression. According to the literature, spontaneous forms may be due to diabetes mellitus, lead poisoning, alcoholism, hypothyroidism, direct compression from seat belts or close-fitting clothes, or increased intra-abdominal pressure resulting from obesity, pregnancy, or tumors. Most of the cases, however, are idiopathic: the cause of LFCN entrapment is simply related to the extremely variable anatomical course of the nerve, with some variants being more at risk than others [3,4].
On the other hand, iatrogenic forms follow a surgical act causing direct injury to the nerve. In rare occasions, they may be due to patient positioning (prone) during the procedure. In addition, MP cases have been reported after prone ventilation in the ICU [3].
The anatomical course of the LFCN is highly variable. It arises from the union of the posterior branches of the L2 and L3 roots, and then emerges from the lateral edge of the Psoas muscle and crosses the Iliacus muscle obliquely, toward the Anterior Superior Iliac Spine (ASIS). At this point, it enters the thigh, generally passing under the inguinal ligament and finally divides into anterior and posterior branches that perforate the fascia lata.
The most frequently reported site of compression is between the fascia lata and the inguinal ligament [5].
Seven anatomical variants of the LFCN course, in its passage from the abdomen to the lower limb, have been described. Notably, in 90% of cases, it passes below or through the inguinal ligament (variants 1 and 2) medial to the sartorius muscle and the ASIS. The other variants, where the nerve passes above the inguinal ligament or above/laterally/through the ASIS, or through the sartorius cannot be responsible for MP [6,7,8].
The first diagnosis is essentially clinical and is based on the presence of typical sensory neurological impairment in the anterolateral part of the thigh with no motor involvement. Such a diagnosis is often delayed, if not misrecognized or mistaken for L2 or L3 radiculopathies, which are more common. Nevertheless, in patients with normal lumbar spinal MRI, clinical suspicion should be high. The Pelvic Compression Test is among the most sensitive and specific maneuvers for diagnosing MP. This test is based on the premise that, as the LFCN is compressed by the inguinal ligament, alleviating the ligament’s tension should reduce the pressure on the nerve and temporarily alleviate the symptoms. In fact, the test consists of exerting downward pressure for 45 s on the pelvis of the patient positioned in lateral decubitus with the symptomatic side facing upward. If the symptoms subside, the test is considered positive. According to the study published by Nouraei et al. in 2007, this examination is a simple and highly useful tool for the diagnosis of MP, particularly when a differential diagnosis between MP and spinal pathologies is required. The authors suggest that this test also allows for an early understanding of whether nerve decompression may indeed lead to a clinical benefit [9]. Electrodiagnostic findings may not be specific when nerve conduction from the contralateral LFCN cannot be measured. Conversely, if there is a clear difference between the two sides, it is a valuable diagnostic adjunct. At times, it is also used to rule out radiculopathy or plexopathy of a different nature. The use of ultrasound has been proposed as an alternative method for diagnosing MP. Indeed, it not only allows the confirmation of nerve entrapment but also is of great help in identifying a neuroma or other possible underlying causes. Furthermore, given the variability of the anatomical course of the nerve, ultrasound can be extremely useful in guiding local injections.
The management of MP includes conservative attempts, e.g., patient education about avoiding tight clothing, weight loss, medications (e.g., NSAIDs, topical capsaicin, lidocaine, gabapentin, phenytoin, carbamazepine) and a trial of physical therapy.
Indeed, sometimes a conservative attitude or simply waiting can lead to a spontaneous resolution of the symptomatology.
The literature also reports cases of symptom improvement after pulsed radiofrequency ablation of the LFCN, electroacupuncture, and kinesiologic taping [10].
However, if symptoms persist despite conservative therapies, the gold standard of treatment is surgery. Spinal cord stimulation has also been considered, in cases of refractory pain, when surgical or other conservative treatment methods fail [3].
There is no high-level evidence on which is the best treatment to offer to MP patients. This makes the debate on this issue still open. In particular, as far as surgical treatment is concerned, it is not yet proven whether it is better to perform neurolysis or neurectomy. The terms decompression and neurolysis are used in this text as synonymous, although they have two subtly different meanings. Here, both terms are used to refer to the release of the nerve from compression, regardless of whether it is scar adhesion or any other type of anatomical structure (muscle, tendon, ligament, and so on).
The purpose of the present study is to share our experience with long-term follow-up in the surgical treatment of MP in 52 patients over 25 years of age operated on at our department.
2. Materials and Methods
2.1. Patients and Methods
Sixty-one consecutive patients were treated for MP at our department from January 1997 to January 2022. Fifty-two of them were available for follow-up recordings. The following data were retrospectively collected from medical records and telephone interviews: gender, age at surgery, presentation symptoms, type of MP (spontaneous or iatrogenic), comorbidities (diabetes mellitus, alcohol abuse, hypertension, mixed anxiety–depressive disorder, abdominal neoplasms), diagnostic work-up, operative management (neurolysis or neurectomy), clinical outcome, and complications.
The diagnosis of MP was made based on clinical symptoms and signs: the Pelvic Compression Test and Tinel at the ASIS were positive in all the patients included in the study. In cases of suspected L2 radiculopathy, patients underwent electromyography and MRI of the lumbosacral spine to rule out causes related to the spine. Ultrasound of the LFCN was performed in some patients to confirm the presence of nerve compression and/or the presence of a neuroma or any other injury (Figure 1 and Figure 2).
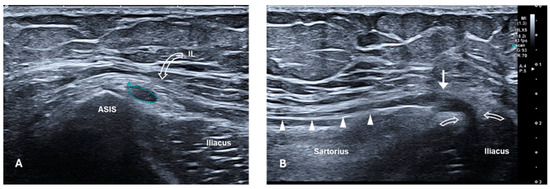
Figure 1.
High-resolution ultrasound of the LFCN with an 18 Mhz transducer in a patient with symptoms of meralgia paraesthetica. (A) Short-axis ultrasound at the level of the ASIS. The LFCN (outlined), located medial to the ASIS and deeper than the IL (curved arrow), appears hypoechoic and swollen with loss of the normal fascicular pattern (CSA = 8.8 mm2, normal range = 1–3 mm2). (B) Long-axis sonogram provides a panoramic view of the nerve course from the IL (curved arrows) to the proximal thigh, over the sartorius muscle. US confirms the neuromatous appearance of the nerve (white arrow), compressed at the level of the splitting of the IL (curved arrows). Distally to the neuroma, the nerve is normal (white arrowheads). ASIS, anterior superior iliac spine; IL, inguinal ligament; LFCN, lateral femoral cutaneous nerve; CSA, cross-sectional area; US, ultrasound.
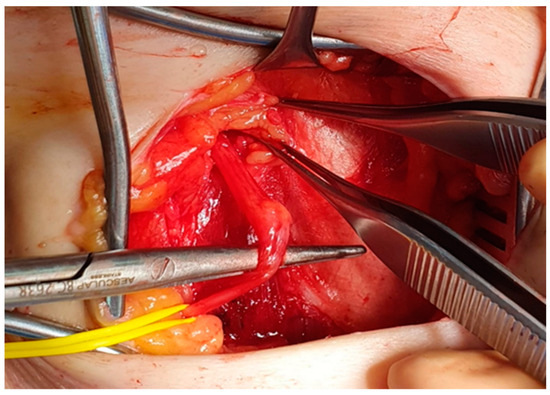
Figure 2.
Intraoperative image. A neuroma of the LFCN is identified in a patient affected by iatrogenic meralgia paresthetica. LFCN, lateral femoral cutaneous nerve.
Surgery was proposed to patients with a diagnosis suggestive of MP who had not improved after conservative therapies or when pain, which on occasions was really excruciating, demanded a prompt definitive treatment.
Exclusion criteria were patients with atypical symptomatology (e.g., symptoms more suggestive of radiculopathy, or extending beyond the anterolateral part of the thigh), missing data, or incomplete follow-up.
Given these criteria, 9 patients were excluded from the study.
The surgical outcome was assessed as “Good” if both pain and paresthesia/dysesthesia disappeared, “Fair” if pain was relieved but paresthesia/dysesthesia remained, or “Poor” in the case of no or only slight improvement.
2.2. Surgical Technique
Neurolysis was the first-line strategy, while neurectomy was reserved for cases of decompression failure or in the case of an already damaged LFCN (usually iatrogenic).
The following is a description of the surgical technique used in our department.
A transverse incision of about 3 cm is made inferomedially to the ASIS, usually below the inguinal ligament line (Figure 3).
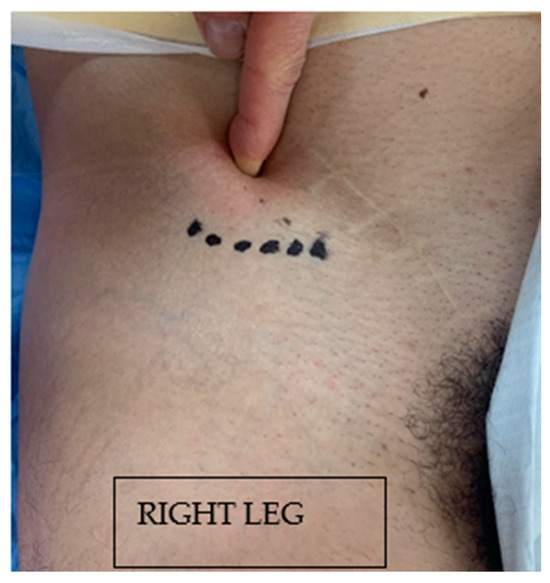
Figure 3.
Surgical incision. A transverse incision of about 3 cm is made inferomedially to the ASIS, usually below the inguinal ligament line. ASIS, anterior superior iliac spine.
The basic idea is to decompress and intercept the main trunk of the nerve which is best identifiable where it enters the abdomen medially to the ASIS. Like a river receiving all the creeks from the surrounding valleys, we must intercept the very final mouth to be sure to treat the culprit of the syndrome. There is no point in isolating the distal branches. The interstitium between the sartorius muscle and the tensor of the fascia lata is explored until the LFCN is identified (Figure 4).
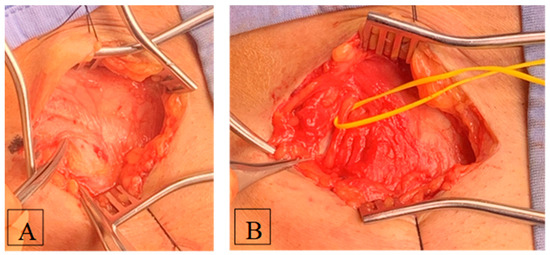
Figure 4.
Intraoperative images. (A) The interstitium between the sartorius muscle and the tensor of the fascia lata is explored until the LFCN is identified. (B) The direction of the nerve from proximal to distal must never point medially. Once identified and charged into a vessel loop, the nerve is followed to its entry into the abdomen, usually below the inguinal ligament. At this point, the inferior lamina of the inguinal ligament is cut to decompress the nerve. LFCN, lateral femoral cutaneous nerve.
One must not enter the muscle tissue. If this happens, it is the wrong place. In fact, the surgical approach needs to be performed in the correct position and working direction. Generally, the difficulty in identifying LFCN immediately can be due to a too medial position of the surgical access. The direction of the nerve from proximal to distal must never point medially. Once identified and charged into a vessel loop, the nerve is followed to its entry into the abdomen, usually below the inguinal ligament. At this point, the inferior lamina of the inguinal ligament is cut to decompress the nerve. The tips of a pair of Metzenbaum scissors must be able to clearly enter the extraperitoneal abdominal space. Then, one is sure that the nerve is free. The opening is limited laterally by the conjoint tendon terminating with its medial insertion to the ASIS and must be limited in width lest an inguinal herniation finds its way. Then, the superficial layers are closed as usual.
In the case of the neurectomy procedure, the LFCN is first coagulated and then cut, and the proximal stump is then embedded into the abdomen. In the latter case, the conjoint tendon and deep fascia may be sutured to protect against inguinal herniation.
3. Results
The series consisted of 52 patients (27 women and 25 men); 11 of those had iatrogenic MP. The latter were related to complications from an anterior approach in prosthetic hip replacement (five cases), inguinal hernioplasties (three patients), back surgery (two cases), and abdominal–pelvic surgery (two patients).
The mean age at the time of surgery was 49.9 ± 16.6 years (range, 14–82). The affected side was the right in 27 patients, and the left in the remaining 25. No bilateral surgeries were performed.
Regarding comorbidities, 2 out of 52 patients were suffering from type II diabetes mellitus, 6 from hypertension, and 3 from mixed anxiety–depressive disorder. None had a history of alcohol abuse or abdominal tumors. Furthermore, no correlation between the onset of symptoms and a previous pregnancy was found in any case. The mean BMI was 23.5 ± 4.2 kg/m2 (range, 19.6–36), 7 patients had a BMI ≥ 30 kg/m2, and the remaining 45 had a BMI < 30 kg/m2.
All surgical procedures were performed by the senior author (S.F.).
Neurolysis was performed in 47 patients, while neurectomy in 8 cases, of whom 3 had undergone a previous decompression. Out of the patients who underwent neurolysis, 41 (87.2%) fully improved after the treatment (outcome: Good); 3 (6.4%) reported pain relief, but no improvement in paresthesia (outcome: Fair); and the remaining 3 patients (6.4%) reported persistence of pain (outcome: Poor). The latter three patients agreed to neurectomy. All eight patients who underwent neurectomy experienced symptom relief but had an obvious anesthetic area persisting over years (outcome: Good).
Complications were rare (3.8%): one groin hematoma in the post-operative course and an inguinal herniation 6 months after surgery.
In patients who benefited from surgery, significant clinical improvements were achieved in the immediate post-operative period and were maintained at follow-up.
4. Discussion
Meralgia paresthetica is a condition that, despite clinically apparent criteria (anterolateral pain in the thigh without any modification of the knee reflex), may be not so easily diagnosed, especially as a first clinical hypothesis. The usual cause is the lack of specific expertise in the field of peripheral nervous system disorders, which is present in the vast majority of neurosurgical institutions. Indeed, one of the main aims of this study was to promote and deepen the knowledge of MP to include it in the differential diagnosis of all those patients suffering from pain and dysesthesia in the anterolateral region of the thigh.
The literature extensively discusses [3,5,11] the risk factors of idiopathic forms of MP. These include tight clothing, metabolic alterations, alcoholism, obesity, pregnancy, or tumors. More recently, it has been realized that the greatest risk probably depends on the wide anatomical variability of the nerve course, with some anatomical variants being at greater risk of compression than others [6,7].
Actually, a glance at the results of our study does not reveal an alleged role of the above-mentioned risk factors in the development of MP. Type II diabetes mellitus was found in only two patients (1%), while mixed anxiety–depressive disorder, in three cases (1.6%). No cancer or alcohol abuse was found in the history of any patient. Neither was any correlation found between pregnancy and the onset of symptoms.
Another important finding emerging from our results is the lack of correlation between obesity and the increased risk of developing MP. In fact, the average BMI was 23.5 ± 4.2 kg/m2 (range, 19.6–36), with only 7 out of 52 patients having a BMI ≥ 30 kg/m2. As with other syndromes, we must accept for the final cause of MP the term “idiopathic”, which means that we do not know why it happens.
Tomaszewski et al. [7] performed an interesting metanalysis on the prevalence of anatomical variations in the LFCN. They included 25 studies with data ranging from 1997 to 2015 and classified seven anatomical variants, based on the course of the nerve at the transition from the abdomen to the lower limb. Type 1 runs medial to the sartorius muscle, below the inguinal ligament, and medial to the ASIS; type 2 runs through the inguinal ligament; type 3 runs above the inguinal ligament; type 4 runs above the ASIS; type 5 runs lateral (or behind) the ASIS; type 6 runs through the ASIS; and finally, type 7 runs through the sartorius muscle.
Their study showed that most nerves exit the pelvis anterior to the ASIS, under the inguinal ligament and medial to the sartorius muscle, with an overall prevalence of 86.8%.
This incredible anatomical variety might well explain why some patients are at greater risk of developing MP during surgical or non-surgical events.
In the context of MP, the modality of the treatment is certainly the most debated topic in the literature.
The quality of the studies does not provide any indication as to which is the best treatment option. In fact, there are only two randomized clinical trials [12,13] and two prospective studies [14,15], all with a limited number of cases. In addition to these, there are also only two meta-analyses [16,17] and some systematic reviews [18,19,20] based on retrospective studies and case series. Therefore, the level of evidence is not higher than 2a.
According to the literature, conservative therapies can have a good outcome in 22–85% of cases [16,21]. When these results are not achieved, surgical options come into play.
There is also much debate about what is the optimal surgical strategy.
According to a recent meta-analysis performed by Lu et al., 85% of patients improved after neurectomy versus only 63% after neurolysis [17]. Khalil et al. [19] found a good outcome in 88% of patients treated with neurolysis, with 94% of those undergoing neurectomy. Other retrospective studies reported a success rate of 60–78% after neurolysis versus 82–100% with neurectomy [22,23]. Payne et al. [20] performed a systematic review and concluded that there is insufficient evidence to recommend one surgical strategy over the other.
On this basis, some surgeons prefer neurectomy because it provides a higher rate of therapeutic success, while others favor neurolysis because it allows the preservation of sensitivity in the innervated area. The lifelong lack of sensitivity in the anterolateral thigh, in fact, may not be so well accepted, as each of us can experience the event of a transient episode of LFCN compression after inappropriate leaning.
The results of our study agree with what is reported in the literature. After neurolysis, 82.2% of the patients recovered completely, and 6.4% were partially healed (which means no more pain but persistent paresthesia). This would be the final result of the surgical treatment based upon neurectomy anyway. As a matter of fact, only the remaining 6.4% (three patients) had a poor result from decompression/neurolysis. All of them later benefited from the repetition of surgery, namely after neurectomy.
In light of these results, it appears inappropriate to submit all the patients presenting with MP to an ablative surgery (neurectomy) as a first-line treatment only to avoid a 6.4% of repetition of an uneventful second surgical procedure.
On the contrary, neurectomy is the only definitive treatment in case of meralgic pain due to inadvertent section of peripheral branches of the LFCN, as it sometimes happens after an anterior approach to the hip joint. The ensuing painful paresthesia is often hastily qualified as MP although, from a strict point of view, the true MP syndrome is spontaneous. The interruption of a distal branching of the LFCN cannot obviously benefit from decompression (the nerve was not a source of concern before the causative surgical agent), and neurectomy, intuitively, is the only procedure that may aspire to be successful.
Recently, the so-called “dynamic decompression” of the LFCN has been proposed to ensure effective and complete freeing of the nerve. The technique consists of intraoperative full flexion/adduction and extension/abduction of the affected lower limb to check whether the decompression of the LFCN has been adequate. If not, other tissue can be immediately removed to completely free the nerve.
In 2019, Malessy et al. published a retrospective study [24] on 17 patients (19 procedures) operated on between June 2013 and December 2017 with this technique. According to the study in question, dynamic tests on all patients identified additional compression exerted by the tissue, especially behind the nerve.
However, the results reported by the authors, although higher, do not differ unequivocally from those described in the literature: 89% of patients had a complete recovery. In our study, for instance, 82.2% of patients healed completely with a decompression performed using the usual technique.
Certainly, it is necessary for the decompression of the LFCN to be complete, regardless of the surgical technique employed.
Indeed, an incomplete nerve decompression, even in light of the above-mentioned mechanism, could be responsible for therapeutic failure and could therefore explain, at least in part, the lower success rate reported in the literature of neurolysis compared to that of neurectomy.
Obviously, patient selection is of paramount importance and is the first recommendation. Sometimes, in fact, a therapeutic failure may depend on an incorrect diagnosis. Therefore, while keeping in mind that the diagnosis of MP is first and foremost clinical, it may be worthwhile in doubtful cases to employ imaging and electrophysiology tools [9].
5. Limitations
Due to this long-lasting retrospective study, nine patients (almost 10%) were lost to follow-up (change in address and the vanishing of old records in the institute’s archives), thus limiting the number of examined cases. The assessment of outcomes is based on what patients reported, and it could be different since the clinical condition is mainly subjective. Nevertheless, ultimately, patient satisfaction is the hallmark of the success of a procedure. Another bias is the significant disparity between the number of patients receiving neurolysis and those with neurectomy. This is because we opted, where possible, to preserve the nerve, keeping neurectomy as a last resort, i.e., in the cases of the failure of a simple decompression procedure or when the nerve had been previously damaged. Our general experience makes us reluctant to perform an unnecessary, although voluntary and therefore controlled, section of a nerve. One never knows the attitude of a single patient toward the possible occurrence of deafferentation pain (anesthesia dolorosa) or of a complex regional pain syndrome, sometimes witnessed after inadvertent nerve sections.
6. Conclusions
To date, there is no significant evidence in the existing literature of which is the best treatment for MP.
According to our results, the combination of a carefully performed surgical approach with neurolysis and the decompression of the LFCN should be considered the first choice in MP. It provides excellent results with a very low complication rate. Adequate nerve decompression and accurate patient selection are key factors in achieving a good outcome.
In our experience, neurectomy is the best therapeutic strategy in cases of iatrogenic MP, when the nerve has been already severely damaged, but it should be considered a second-line option in case of failed neurolysis.
Further prospective studies assessing the outcomes of different MP treatments are needed to improve the level of evidence.
Author Contributions
All authors contributed to the study conception and design. Also, all authors were involved in material preparation and data collection. Conceptualization, S.F. and E.C.; methodology, E.C.; validation, S.F., E.B., and L.M.; formal analysis, E.C.; investigation, E.C.; resources, G.Z.; data curation, E.B.; writing—original draft preparation, E.C., and all authors commented on previous versions of the manuscript; writing—review and editing, S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was waived by our local ethics committee in view of the retrospective nature of the study, and all procedures were performed as part of routine care.
Informed Consent Statement
The research data analysis had no effect on the participants or their medical care and did not require additional informed consent.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are confidential, and patients were assured that their raw data would not be shared.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bernhardt, M. Ueber isoliert im Gebiete des N. cutaneus femoris externus vorkommende Paräesthesien. Neurol. Cent. 1895, 14, 242–244. [Google Scholar]
- Roth, W.K. Meralgia Paraesthetica; S.Karger AG: Basel, Switzerland, 1895. [Google Scholar] [CrossRef]
- Solomons, J.N.T.; Sagir, A.; Yazdi, C. Meralgia Paresthetica. Curr. Pain Headache Rep. 2022, 26, 525–531. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, G.C.W.; Wesstein, M.; Vlak, M.H.M. Preoperative Ultrasound in Patients with Meralgia Paresthetica to Detect Anatomical Variations in the Course of the Lateral Femoral Cutaneous Nerve. World Neurosurg. 2021, 149, e29–e35. [Google Scholar] [CrossRef]
- Pearce, J.M. Meralgia paraesthetica (Bernhardt-Roth syndrome). J. Neurol. Neurosurg. Psychiatry 2006, 77, 84. [Google Scholar] [CrossRef] [PubMed]
- Aszmann, O.C.; Dellon, E.S.; Dellon, A.L. Anatomical course of the lateral femoral cutaneous nerve and its susceptibility to compression and injury. Plast. Reconstr. Surg. 1997, 100, 600–604. [Google Scholar] [CrossRef]
- Tomaszewski, K.A.; Popieluszko, P.; Henry, B.M.; Roy, J.; Sanna, B.; Kijek, M.R.; Walocha, J.A. The surgical anatomy of the lateral femoral cutaneous nerve in the inguinal region: A meta-analysis. Hernia 2016, 20, 649–657. [Google Scholar] [CrossRef]
- Carai, A.; Fenu, G.; Sechi, E.; Crotti, F.M.; Montella, A. Anatomical variability of the lateral femoral cutaneous nerve: Findings from a surgical series. Clin. Anat. 2009, 22, 365–370. [Google Scholar] [CrossRef]
- Nouraei, S.A.; Anand, B.; Spink, G.; O’Neill, K.S. A novel approach to the diagnosis and management of meralgia paresthetica. Neurosurgery 2007, 60, 696–700. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.; Gyorfi, M.J.; Ha, S.P. Lateral Femoral Cutaneous Nerve Radiofrequency Ablation for Long-term Control of Refractory Meralgia Paresthetica. Pain Med. 2020, 21, 1433–1436. [Google Scholar] [CrossRef]
- van Eerten, P.V.; Polder, T.W.; Broere, C.A. Operative treatment of meralgia paresthetica: Transection versus neurolysis. Neurosurgery 1995, 37, 63–65. [Google Scholar] [CrossRef]
- Kiliç, S.; Özkan, F.Ü.; Külcü, D.G.; Öztürk, G.; Akpinar, P.; Aktas, I. Conservative Treatment Versus Ultrasound-Guided Injection in the Management of Meralgia Paresthetica: A Randomized Controlled Trial. Pain Physician 2020, 23, 253–262. [Google Scholar] [PubMed]
- Kloosterziel, M.E.; Tavy, D.L.J.; Arends, S.; Zijdewind, J.M.; van Zwet, E.W.; Wirtz, P.W. Meralgia paresthetica: Nerve stimulator-guided injection with methylprednisolone/lidocaine, a double-blind randomized placebo-controlled study. Muscle Nerve. 2020, 61, 788–791. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, G.C.; Kloet, A. Comparison of effectiveness of different surgical treatments for meralgia paresthetica: Results of a prospective observational study and protocol for a randomized controlled trial. Clin. Neurol. Neurosurg. 2015, 134, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Vered, E.; Volchek, L. Relieving symptoms of meralgia paresthetica using Kinesio taping: A pilot study. Arch. Phys. Med. Rehabil. 2010, 91, 1137–1139. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Torri, L.; Signori, A. Treatment of meralgia paresthetica (Lateral Femoral Cutaneous Neuropathy): A meta-analysis of ultrasound-guided injection versus surgery. Eur. J. Radiol. 2021, 139, 109736. [Google Scholar] [CrossRef]
- Lu, V.M.; Burks, S.S.; Heath, R.N.; Wolde, T.; Spinner, R.J.; Levi, A.D. Meralgia paresthetica treated by injection, decompression, and neurectomy: A systematic review and meta-analysis of pain and operative outcomes. J. Neurosurg. 2021, 135, 912–922. [Google Scholar] [CrossRef]
- Khalil, N.; Nicotra, A.; Rakowicz, W. Treatment for meralgia paraesthetica. Cochrane Database Syst. Rev. 2008, CD004159.pub2. [Google Scholar] [CrossRef]
- Khalil, N.; Nicotra, A.; Rakowicz, W. Treatment for meralgia paraesthetica. Cochrane Database Syst. Rev. 2012, CD004159.pub3. [Google Scholar] [CrossRef]
- Payne, R.; Seaman, S.; Sieg, E.; Langan, S.; Harbaugh, K.; Rizk, E. Evaluating the evidence: Is neurolysis or neurectomy a better treatment for meralgia paresthetica? Acta Neurochir. 2017, 159, 931–936. [Google Scholar] [CrossRef]
- Patijn, J.; Mekhail, N.; Hayek, S.; Lataster, A.; van Kleef, M.; Van Zundert, J. Meralgia Paresthetica. Pain Pract. 2011, 11, 302–308. [Google Scholar] [CrossRef]
- de Ruiter, G.C.; Wurzer, J.A.; Kloet, A. Decision making in the surgical treatment of meralgia paresthetica: Neurolysis versus neurectomy. Acta Neurochir. 2012, 154, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Benezis, I.; Boutaud, B.; Leclerc, J.; Fabre, T.; Durandeau, A. Lateral femoral cutaneous neuropathy and its surgical treatment: A report of 167 cases. Muscle Nerve. 2007, 36, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Malessy, M.J.A.; Eekhof, J.; Pondaag, W. Dynamic decompression of the lateral femoral cutaneous nerve to treat meralgia paresthetica: Technique and results. J. Neurosurg. 2018, 131, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).