Abstract
Growth of malignant cells in solid tumors induces changes to the tumor microenvironment (TME). These changes result in promotion of tumor growth, invasion, and metastasis, but also in tumor resistance to drugs and radiotherapy. The enhanced permeability and retention (EPR) effect in neo-angiogenic tumor tissue enables the transport of therapeutic molecules from the circulation into the tumor, but studies show that further diffusion of these agents is often not sufficient for efficient tumor eradication. Despite the hyperpermeable vasculature facilitating the delivery of drugs and tracers, the high density of stromal cells and matrix proteins, in combination with the elevated interstitial fluid pressure in the microenvironment of solid tumors, presents a barrier which limits the delivery of compounds to the core of the tumor. Reversing the cancer-cell-induced changes to the microenvironment as well as novel nanoparticle strategies to circumvent tumor-induced stromal changes have therefore been suggested as potential methods to improve the delivery of therapeutic molecules and drug efficacy. Strategies to modulate the TME, i.e., normalization of tumor vasculature and depletion of excessive stromal proteins and cells, show promising results in enhancing delivery of therapeutic compounds. Modulation of the TME may therefore enhance the efficacy of current cancer treatments and facilitate the development of novel treatment methods as an alternative for invasive resection procedures.
1. Introduction
Solid tumors consist of a mass of malignant cancer cells (CCs) without cysts or liquid, formed by rapid and uncontrolled cell growth. These tumors are heterogeneous in nature and are composed of CCs, stromal cells, abnormal vasculature, and abundant extracellular matrix proteins. The rapid growth of CCs and tumor-associated cells causes the development of several unique characteristics in tumor-associated blood vessels and the tumor microenvironment (TME), which are both beneficial and detrimental to the delivery of systemically administered drugs. Due to misaligned, leaking capillaries with wide fenestrae and low lymphatic drainage, small particles and cells tend to aggregate in tumor tissue, which is known as the enhanced permeability and retention (EPR) effect (Figure 1). Deviating cell growth and aberrant vasculature also result in abnormal fluid dynamics and an increase in the distances that drugs need to diffuse through in the interstitial space, as CCs are physically further away from blood vessels. An abundant TME, as particularly present in breast cancer and pancreatic ductal adenocarcinoma, promotes stromal cell growth and produces high levels of extracellular matrix (ECM) proteins such as interstitial collagens type 1 and 3, laminin, and fibrin; this is called the desmoplastic reaction. Consequently, CCs in the core of these solid tumors are surrounded by a dense layer of stromal cells and ECM proteins. Due to this layer, the interstitial fluid pressure (IFP) in tumor tissues is higher than in surrounding tissues, further diminishing the diffusion of therapeutic molecules via the stroma into the core of the tumor, enhancing the tumor’s insensitivity to treatment [1].
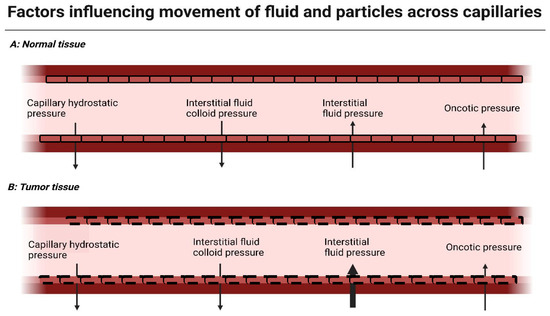
Figure 1.
Factors influencing flow of fluid and particles across capillaries. Capillary hydrostatic pressure and interstitial fluid colloid pressure are forces driving fluid/particles out of the circulation. Interstitial fluid pressure and oncotic pressure are forces driving the flow of fluid/particles into capillaries. Situation (A) resembles these forces in normal tissue. In tumor tissue, depicted in (B), the tissue interstitial fluid pressure is elevated (indicated by the enlarged arrow), preventing fluid flow into the capillary and decreasing local drug delivery. Created with BioRender.com, 5 January 2024.
Instead of surgical resection, a novel emerging field of cancer therapy focuses on the improvement of the penetration and delivery of drugs into tumors by modulating or normalizing the TME to enhance the effect of anti-cancer therapeutics. Several different avenues of research have been identified to achieve reversal of an abnormal TME. These include normalization of the tumor vasculature, depletion of tumor stromal proteins, and depletion of tumor stromal cells. In this context, novel, nanoparticle (NP)-based strategies are being developed, which specifically target the TME, either to circumvent tumor-induced changes to the TME in order to increase the yield of delivery of therapeutic molecules, or to stimulate modulation of the TME itself. Novel NP-based strategies will be discussed in this review in conjunction with related TME-modulating strategies, as both methods are often used simultaneously to achieve optimal drug delivery. The aim of this review is to assess and review the advances that have been made in improving drug delivery into solid tumor cores by modulation or normalization of the TME and associated nanoparticle strategies.
2. Modulating the Tumor Vasculature to Improve Perfusion and Drug Delivery
Elevated IFP is found in many solid tumors. Poor perfusion, abnormal blood vessel organization, and leaking blood vessels all contribute to an elevated IFP. An elevated IFP reduces the accumulation of particles and cells into the tumor core [2]. An increase in perfusion would ensure that more of a systemic drug can be delivered to the tumor region via the EPR effect, whilst simultaneously normalizing the IFP, thereby increasing the diffusion of NPs into the tumor core. Perfusion in tumor cores can be modulated by constricting or dilating blood vessels, facilitated by vasoconstricting and vasodilating factors, respectively. Contraction or dilation of blood vessels is mediated by the contractility of smooth muscle cells lining blood vessel walls which are influenced by extracellular and intracellular signals. Contributing to the abnormal vasculature in tumor tissues is dysregulation of the octapeptide receptor angiotensin-2. Angiotensin-2 (AT-2), mainly through its antiotensin-1 receptor (AT-1), promotes the proliferation of smooth muscle cells and is a regulator of vasoconstriction. Enhanced AT-2 levels have been associated with cancer progression and angiogenesis [3]. Losartan is an AT-2 antagonist with vasodilatory properties and is clinically used as antihypertension medication. Losartan treatment in tumor tissue in animal models reduced IFP intratumorally and increased vascular perfusion, which was facilitated by a reduction in collagen and hyaluronan production [4]. A reduction in collagen and hyaluronan content in tumor tissue is thought to decompress blood vessels and increase vessel diameter and perfusion; see Figure 1.
2.1. The Role of Vasoconstriction and Angiogenic Factors in Tumor Tissue
Interestingly, increasing vasoconstriction factor levels has also been investigated as a method to increase blood flow into tumor tissues. Vasoconstrictors like AT-2 facilitate vasoconstriction through contraction of smooth muscle cells. Tumor tissues, however, have impaired muscular structure due to abnormal growth, so application of endogenous vasoconstrictors has been shown to have little effect on vasoconstriction in tumors. Normal blood vessels outside the tumor are affected by AT-2; therefore, application of endogenous vasoconstrictors will increase blood flow towards tumor tissues, whilst having no significant effect on tumor tissue. Still, one study increased AT-2 levels for 15–20 min in patients with several tumor types and found significantly increased drug delivery in tumors and a subsequent therapeutical response.
De novo formation of vasculature in healthy tissues is facilitated by a tightly regulated balance between pro- and anti-angiogenic factors. This balance is disrupted in the formation of tumor tissues, leading to an emphasis on angiogenesis and the excessive formation of, often abnormal, vasculature. Modulation of anti-angiogenic factors is therefore a viable option to normalize tumor vasculature [5]. Treating tumor tissue with anti-angiogenic drugs would reduce the formation of blood vessels and reduce perfusion overall, potentially reducing drug delivery to tumor tissue. Several anti-angiogenic drugs, however, have a positive effect on the delivery of drugs into the tumor core. Anti-angiogenic drugs normalize the remaining vasculature, restoring its functionality whilst retaining permeability and thereby increasing the efficacy of the EPR effect [5]. Vascular endothelial growth factor (VEGF) is the key pro-angiogenic mediator secreted by tumor tissues. Its secretion is induced by the growth factors and hypoxia present in tumor tissues. Production of VEGF induces the angiogenic switch, which causes excessive production of the aforementioned often abnormal vasculature in tumor tissues [6].
Bevacizumab, the first anti-angiogenic cancer drug developed, was introduced in 2004. Bevacizumab is an antibody binding to VEGF-A, thereby preventing interaction with the VEGF receptor. Studies have shown that bevacizumab normalizes tumor vasculature and inhibits the formation of new blood vessels and even removes newly formed vessels in tumor tissues [7]. As bevacizumab targets angiogenesis, treatment with this drug has been focused mainly on the treatment of solid tumors with abnormal vasculature. Bevacizumab treatment has been shown to be effective and remains part of current cancer treatment in the clinic for a variety of cancer types. Other drugs targeting VEGF have been developed and are currently undergoing clinical trials, such as cediranib. Cediranib/recentin is a competitive inhibitor of all three VEGF receptors, preventing the binding and action of VEGF [8]. In breast cancer animal models, treatment with cediranib increased vascular permeability and oxygenation, but decreased micro vessel density, indicating a normalization of vasculature [9]. Currently, cediranib is under development for the treatment of cervical, ovaria, and peritoneal cancer, as a coated tablet for oral administration.
Interestingly, treatment with paclitaxel (PTX), an anti-proliferative drug used against several solid tumors, induces results similar to treatment with anti-angiogenic factors [10]. Aside from its anti-proliferative properties against CCs, it has several anti-angiogenic properties, like blocking the proliferation of endothelial cells and the formation of new blood vessels induced by VEGF [11]. Treatment with NPs containing PTX increased tumor vascular permeability and decreased IFP for 7 days in animal models. Additionally, treatment with PTX NPs significantly increased the presence of smooth muscle cells and pericyte coverage on tumor vasculature, inducing a shift to normalized vasculature. A lack of smooth muscle cells and pericytes is a hallmark of abnormal tumor vasculature [5,12].
2.2. Utilizing Growth Factors to Modulate Tumor Stroma
Other important regulators of tumor vasculature are several growth factors and their respective receptors. Growth factors function as agents to signal initiation of proliferation or formation of certain tissues. Abnormal levels of growth factors are known to influence cancer progression and can promote ECM protein production [13]. Platelet-derived growth factor (PDGF) is a regulator of blood vessel formation and mitogenesis [14]. Blocking platelet-derived growth factor receptor-β (PDGFR-β) resulted in a reduction in IFP and increased the density of blood vessels and perfusion in tumor tissue in animal models [15]. PDGFR-β blocking with imatinib mesylate induced normalization of lung tumor blood vessels in animal models, indicated by an increase in pericytes near vessels and an increase in perfusion [16]. PDGFR-β is also a regulator of ECM protein production [17]. Blocking PDGFR-β receptors contributes to depletion of ECM proteins, thereby reducing mechanical stress on tumor blood vessels and increasing perfusion [10].
Epidermal growth factor receptor (EGFR) is a transmembrane receptor for epidermal growth factor, which promotes tumor cell proliferation, invasion, and metastasis. EGFR levels are enhanced or constitutively active in many different cancer types [18]. Increased activation of EGFR leads to enhanced levels of VEGF, which in turn leads to the abnormal growth of vessels found in many solid tumors [19,20]. Blocking EGFR with erlotinib has been shown to normalize tumor blood vessels in colorectal and breast cancer animal models. The perfusion in the mentioned tumor types was significantly increased, facilitated by an increased density of blood vessels and the normalization of vessels already present in tumor tissue. Priming with erlotinib and subsequent treatment with drug-carrying NPs induced a significant increase in drug efficacy compared to treatment without erlotinib [21]. Recently, Darabi et al. used the abundance of EGFR on tumor cells to achieve increased tumor targeting of drug-carrying nanoparticles. They synthesized a solid lipid nanoparticle, which encapsulated doxorubicin (DOX), and was coated with CD44- and EGFR-binding aptamers. CD44 and EGFR are receptors abundantly present on the outside of triple-negative breast cancer cells. Incorporating aptamers for both CD44 and EGFR on the solid nanoparticle therefore has the potential to significantly increase the tumor targeting of these nanoparticles, especially for late-stage heterogenic cancers. This dual targeting method showed promising results in vitro, and more preclinical research is currently being conducted [22].
Treatment with VEGF-production-blocking small interfering RNA (siRNA) obtains similar results in renal cell carcinomas. After treatment with siRNA, perfusion and the efficacy of the EPR effect were significantly increased in renal cell carcinoma animal models [23]. The efficacy of blocking VEGF signaling, however, appears to be dependent on the tumor type. In tumors where blood vessels are clustered around tumor cells, like renal and hepatocellular carcinomas, VEGF blocking induces a beneficial effect on the perfusion and efficacy of the EPR effect. In tumors where vessels are clustered around stromal cells, like colorectal cancer, detrimental effects are observed after VEGF blocking, with increased collagen levels and decreased intratumoral accumulation of NPs [24]. The reasons for the discrepancy between tumor types could be the different composition of the TME in these tumors. The tumor types where a beneficial effect was observed are possibly hypervascularized, whereas detrimental effects will be observed in hypovascularized tumor types; see Figure 2.
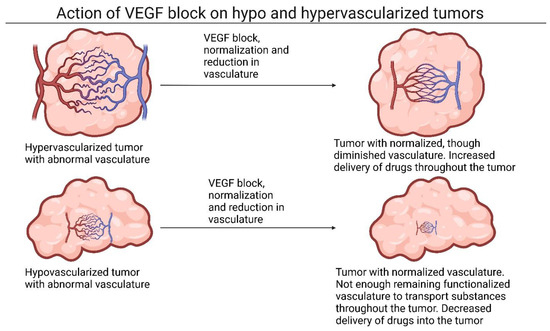
Figure 2.
Action of VEGF blockers on hypo- and hypervascularized tumors. On the left, hyper- and hypovascularized tumors are presented. After both tumors have VEGF signaling blocked, different results appear. Hypervascularized tumors likely have diminished but normalized vasculature, which increases delivery of subsequently administered drugs. Hypovascularized tumors likely have a normalized vasculature as well, though lack enough vasculature to benefit from normalization of vasculature. Instead, delivery of subsequently administered drugs is decreased Abbreviation: VEGF, vascular endothelial growth factor. Created with BioRender.com, 5 January 2024.
Normalization of vasculature includes a reduction in vessel density, but a functionalization of the remaining vasculature. Hypervascularized tumors may have sufficient remaining functionalized vasculature to benefit from this treatment, whereas hypovascularized tumors may lack sufficient remaining vasculature to benefit from the advantages of NP delivery, though more research is needed to confirm this theory.
3. Modulating Stromal Proteins Reduces Interstitial Fluid Pressure (IFP) and Improves Diffusion of Drugs into the Tumor Core
A hallmark of most solid tumors is an overproduction of ECM proteins. The increased density of ECM proteins in tumor tissue contributes to the elevated IFP found in tumors, which, as discussed previously, complicates drug delivery into tumor cores. The abnormal fluid dynamics and diffusion in tumors can therefore cause tumor drug resistance. In this section, we will discuss developments in halting the production of ECM proteins or directly removing them to increase the delivery of drug-carrying NPs to tumor cores. The anionic glycosaminoglycan hyaluronan is one of the main components of the ECM and is overproduced in several solid tumors. A potential agent to reduce the amount of hyaluronan in tumor tissue is hyaluronidase (HAase). HAase has several subtypes, of which HAase-1 and HAase-2 have the highest enzymatic activity in mammals. HAase directly digests hyaluronan. Research indicates that HAase treatment is effective in lowering the interstitial pressure in tumor tissue by normalizing the amount of hyaluronan in tumor tissue in animal models [25,26]. Additionally, normalization of hyaluronan induces an increase in the dilation of blood vessels and the density of blood vessels in animal models (41), most likely due to a reduction in mechanical pressure in blood vessels. Furthermore, priming of the TME with HAase results in an increase in delivery of drug-carrying NPs into the tumor core in animal models [26]; see Figure 3A.
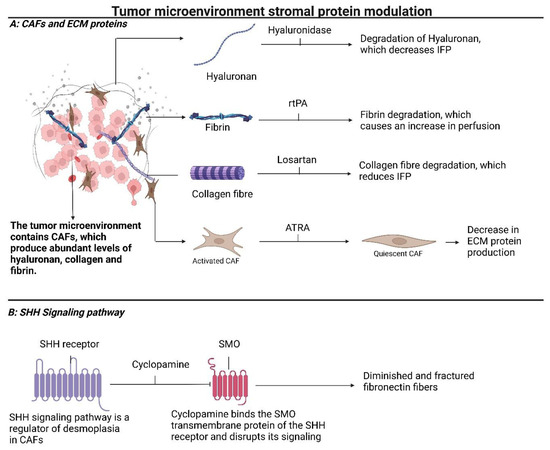
Figure 3.
Tumor microenvironment stromal protein modulation: (A) displays a cluster of CCs with a TME consisting of cancer-associated fibroblasts and overexpressed proteins, like hyaluronan, fibrin, and collagens. Possibilities for therapeutic intervention are indicated. (B) shows stroma downregulation based on interference in the SHH signaling pathway. Administration of cyclopamine results in degradation of fibronectin fibers and downsizing of the ECM within the desmoplasia. Abbreviations: CAF, cancer-associated fibroblast; ECM, extracellular matrix; ATRA, all-trans retinoic acid; IFP, interstitial fluid pressure; SHH, sonic hedgehog; SMO, smoothened; rtPA, recombinant tissue type plasminogen activator. Created with BioRender.com, 5 January 2024.
Fibrin is another abundant component of the ECM which is involved in the coagulation response. Fibrin is overexpressed in many solid tumors and is predominantly present near tumor vessels [27]. Due to its proximity to blood vessels, an overabundance of fibrin can mechanically compress and constrict blood vessels, even more so than hyaluronan. The fibrin content of tumor tissues can be modulated by the use of recombinant tissue type plasminogen activator (rtPA), a thrombolytic drug; see Figure 3A. The use of rtPA in animal models indicates that normalization of fibrin improves tumor tissue perfusion and consequently improves the delivery of drug-carrying NPs to the tumor core [28]. Further reductions in the protein content of the tumor ECM might be achieved through the depletion of collagen fibers. Collagen is spread throughout most tumor tissues and is overproduced in many solid tumors [29].
Collagen depositions can be depleted through the use of losartan which binds the angiotensin-2 type 1 receptor and causes downstream downregulation of tumor transforming growth factor-β1 (TGF-β1), which affects collagen production; see Figure 3A. Losartan induces a significant reduction in the hyaluronan and collagen present in tumor tissues, reduces IFP, and enhances drug delivery post-losartan treatment to tumor cores in animal models [4,30,31]. Collagen, hyaluronan, and other ECM proteins are produced by perpetually activated cancer-associated fibroblasts (CAFs) [32]. Inducing quiescence in CAFs might therefore normalize ECM protein production in tumor tissue. All-trans retinoic acid (ATRA) is an inducer of quiescence of CAFs. Treatment with ATRA induces quiescence and subsequent ECM remodeling, and normalizes cytokine secretion in pancreatic cancer animal models [33]. A more direct method to deplete collagen content in the TME is through the use of collagenase, a collagen-degrading enzyme. Collagenase has been shown to be effective in reducing collagen levels in cancer tissue, but can be harmful to healthy tissue. Yang et al. therefore used a collagenase-carrying NP, which released its content only when irradiated with near-infrared light. This method has the potential to limit damage to healthy tissue, as only the irradiated tissues are exposed to collagenase. Additionally, these NPs were composed of gold, which has the intrinsic ability to increase oxygen levels under irradiation with near-infrared light for a photodynamic therapy effect. This multifunctional therapy resulted in decreased levels of ECM proteins, deeper penetration into the tumor, and a subsequent increase in drug yield in PDAC tissue. Furthermore, gold nanoparticles have a high X-ray attenuation rate, making this therapy a potential tool for treatment monitoring via CT imaging [34].
Recently, it was found that besides volume and matrix density, the specific composition of the collagen matrix also contributes to cancer progression. Collagen type 7 binds to and regulates the spatial organization of collagen type 1. Collagen type 7 is primarily secreted by CAFs, and contributes to modulating the TME to support metastasis. Furthermore, collagen type 7 was found to be a predictor for cancer progression and survival, making it a likely target for future research [35].
Han et al. combined ATRA treatment with siRNA-targeting heat shock protein 47 (HSP47), a chaperone protein necessary for the proper folding of collagen proteins. Their research indicates that the combined treatment of heat shock protein 47 (HSP47) with ATRA significantly decreased the amount of activated CAFs and depleted the amount of collagen and fibronectin in tumor tissue in animal models. Furthermore, the combination treatment of ATRA and HSP47 siRNA also facilitated an increase in perfusion and subsequent drug penetration and delivery to tumor cores in animal models [36]. An overproduction of ECM proteins by CAFs can also be mitigated by blocking the sonic hedgehog (SHH) signaling pathway, which is a regulator of desmoplasia in solid tumors. Blocking the SHH signaling pathway can be achieved by administration of cyclopamine, which binds to smoothened (SMO), a transmembrane receptor involved in cell–cell communication; see Figure 3B. Cyclopamine treatment induced diminished and fractured fibronectin fibers and increased perfusion in tumor tissue in animal models. Moreover, combination therapy of cyclopamine with chemotherapy drugs showed increased anti-tumoral efficacy, likely due to the ECM-disruption-mediated increased penetration of chemotherapy drugs into the core of the tumor [36].
4. Modulating Stromal Cells to Induce Normalization, Quiescence, or Cell Death
Activated stromal cells in tumor tissue are known to contribute to cancer progression, ECM protein production, tumor drug resistance, and elevated IFP [37]. This section of the review will therefore briefly discuss novel strategies in normalizing or modulating tumor stroma cells to promote drug tumor penetration. Modulating stromal-cancer-associated fibroblasts, engaged in crosstalk with CCs or the ECM, can downregulate the perpetual activation of excessive stroma formation in most cancer types [38,39]. Unmodified gold nanoparticles (AuNPs) have been reported to intrinsically affect crosstalk between CAFs and CCs by blocking activation of the mitogen-activated protein kinase (MAPK) signaling pathway and induce a reversal of epithelial-to-mesenchymal transition (EMT) by downregulating several growth factors such as PDGF, transforming growth factor β (TGF-β), and hepatocyte growth factor [38,40]; see Figure 4A. Treatment with AuNPs induces quiescence in CAFs, and reduces the expression of major ECM proteins collagen 1 and 3, fibronectin, several key antiangiogenic proteins, fibroblast-activating protein, and α smooth muscle actin (α-SMA). This is facilitated by an inhibition of the p42/44 and p38 MAPK signaling pathways. Additionally, AuNPs disrupt the bi-directional crosstalk between CCs and CAFs due to alterations in the cell secretome in these cells [41].
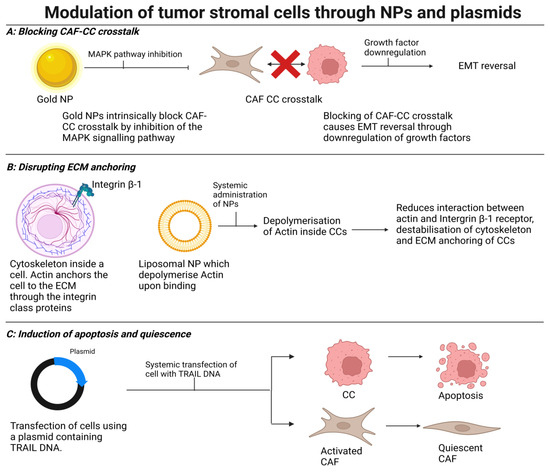
Figure 4.
Modulation of tumor stromal cells through NPs and plasmids: (A) An unmodified gold NP blocks crosstalk between CCs and CAFs. Blockage of the MAPK signaling pathway downregulates growth factor expression, leading to reversal of EMT. (B) Disruption of CC anchoring within TME by administration of a liposomal NP which depolymerizes actin, leading to reduced interaction between CC integrins with the surrounding extracellular matrix. (C) Induction of apoptosis of CCs and de-activation of CAFs via systemic administration of plasmids coding for TRAIL (NF-related apoptosis-inducing ligand).
Further disruption of crosstalk between CCs and the ECM can be facilitated by destabilizing the cytoskeleton of CCs. The actin cytoskeleton of these cells binds to and communicates with the ECM through various integrins on their cell membrane; see Figure 4B. Suzuki et al. developed liposomal NPs modified with acidic pH-sensitive peptide (SAPSp-lipo), which selectively activate in the acidic environment of tumor tissues. Once activated, the liposomal NPs depolymerize actin in CCs. Treatment with SAPSp-lipo reduces the interaction between integrin β1 and the ECM, thereby destabilizing the cytoskeleton and disrupting communication of tumor cells with the ECM in animal models. They also reported that after priming with SAPSp-lipo, subsequent drug delivery was significantly increased in animal model tumor regions where the actin cytoskeleton was destabilized, compared to regions where it was not [42]. Reducing crosstalk between CAFs, CCs, and the ECM is therefore a promising area for further research, but directly affecting CAF growth and functioning is possible as well using the antioxidant and anti-inflammatory compound quercetin.
Quercetin (QC) is a flavonoid found in fruits and vegetables that are consumed daily [43]. QC is currently under investigation for its properties in reverting chemotherapy resistance [44], but studies into the effect of QC on tumor drug resistance also found that QC inhibited CAF growth and collagen deposition [45]. Subsequent research confirmed that priming tumors with QC remodels the TME and significantly increases the uptake and penetration of chemotherapy drugs into the core of tumors. QC blocks a part of the Wnt family signaling pathway, specifically wnt16 [46]. Knockdown of wnt16 expression causes a reduction in α-SMA expression, fibronectin, and consequently collagen content and crosslinking, which is facilitated by fibronectin, in tumor fibroblasts in animal models. The knockdown of wnt16, however, also induced decreased angiogenesis. Whilst decreased angiogenesis could logically contribute to decreased delivery of nanoparticles, research indicates that the knockdown of wnt16 increases the accumulation and penetration of accompanying chemotherapy compounds [47]. This is likely due to normalization of the remaining blood vessel structures, diminished though they are. Further research is necessary to confirm this mechanism.
PYCR1 has recently been indicated as a major regulator of increased stromal protein production in CAFs. PYCR1 is an enzyme for proline production, which is a highly abundant amino acid used for collagen formation. PYCR1 levels have been found to be elevated in many human cancer types. It was found that reducing PYCR1 levels in CAFs resulted in a reduction in collagen production, tumor growth, and metastasis [48]. During the application of systemically applied chemotherapy specifically targeting CCs, it was observed that large doses of these drugs tended to be internalized by stromal CAFs in tumor tissue in what is known as off-site distribution [49]. One research group utilized this mechanism to “reprogram” the CAFs to secrete cytotoxic cytokines. They sought to induce the production of TNF-related apoptosis-inducing ligand (TRAIL) in CAFs in animal models by injection of a plasmid which codes for this protein; see Figure 4C. Treatment with TRAIL plasmids induced apoptosis in CCs near CAFs but not fibroblasts. Additionally, treatment with TRAIL plasmids shifted activated CAFs to a quiescent state. Furthermore, levels of collagen and fibronectin in the TME in tumor animal models dropped significantly after treatment with TRAIL plasmids. TRAIL plasmid treatment also increased tumor blood vessel diameter and blood vessel lumen size, likely due to the previously discussed diminished mechanical compression of ECM proteins on blood vessels. Combination treatment of systemically applied chemotherapy drugs and TRAIL plasmids increased the uptake of chemotherapy drugs into tumor cores 2-fold in animal models [49]. The previously mentioned SHH signaling pathway is also involved in perpetual CAF activation and proliferation [50]. SHH signaling can be blocked using vismodegib, an SHH knockdown agent. Vismodegib treatment remodeled the TME, blocked CAF proliferation, reduced mechanical stress, and improved perfusion by reducing collagen and hyaluronan levels in animal tumor models [51].
Another method to induce quiescence in CAFs is using calcipotriol (Cal). Cal is a vitamin D receptor ligand which transcriptionally deactivates CAFs, inducing quiescence. One research group managed to combine the use of Cal with photothermal therapy (PTT) by using tumor cell microparticles as a delivery vehicle. Cell microparticles are small vesicles, in the 100 to 1000 nm range, which can be derived from tumor cells [52]. Combination therapy of Cal and PTT resulted in a reduction in ECM protein density, which lead to an elevated PTT efficacy. The combination therapy also resulted in increased CD8+ T-cell infiltration and anti-tumor immunity [52,53].
5. Application in Medicine
Thus far, we have only discussed how to achieve increased penetration of drugs into tumor cores. But what are the practical applications of this effect? Most treatment regiments currently in use to treat cancer could possibly benefit from the research conducted to improve drug penetration into tumor cores, as increased delivery of drugs to all regions of the tumor is likely to enhance treatment efficacy. One of the drawbacks discussed in this article, however, is that enhancing access to the tumor core can have the unfortunate side effect of increasing metastasis in patients. Specific drug/treatment combinations therefore need to be researched thoroughly to ascertain whether they are actually beneficial to the treatment before being applied to patients.
Another application is using these methods to enhance the efficacy of surgical techniques. Certain surgical techniques are dependent on specific compounds penetrating deep into tumor tissue in order to visualize the tumor. Image-guided surgery, for instance, depends on fluorophores to selectively bind tumor tissue, thereby exclusively illuminating it [54]. The application of these techniques can be less successful in desmoplastic tumors as the fluorophores can have difficulty penetrating deep into the cores in these tumors [55]. The techniques discussed in this paper could therefore potentially be used to increase the delivery of compounds in highly desmoplastic tumors, thereby increasing the efficacy of these types of surgeries.
6. Future Prospects
Most of the articles referenced in this study are investigating novel methods which are in the early stages of development. Recently, some of the methods on modulation of the stromal components and immune response in the TME have undergone clinical trials. PEGPH20, a recombinant hyaluronidase, was used to deplete hyaluronan in the TME of metastatic PDAC. In an open-label phase 2 clinical trial, improvements in progression-free survival were observed. Of note in this specific trial was that patients at risk of thromboembolic events were excluded after an imbalance in thromboembolic events was observed in the PEGPH20 group [56]. Due to the positive results, a subsequent phase 3 trial was performed. In this phase 3 trial, however, no benefit on progression-free survival and overall survival increase were found. As such, research into this compound has halted [57]. Another clinical trial targeting PDAC used ibrutinib. Ibrutinib is an inhibitor of Bruton’s tyrosine kinase. Monotherapy with ibrutinib modulated the immune response in the TME towards an activated T-cell, monocyte, and DC phenotype. Interestingly, combination therapy of ibrutinib with a standard treatment of gemcitabine and nab-paclitaxel did not exhibit this effect [58]. As mentioned previously, CD40 is a prominent target for modulation in the TME of PDAC patients. A clinical trial where selicrelumab, a CD40 agonistic monoclonal antibody, was evaluated reported modulations to the immune response and stromal components. In the selicrelumab arm of the trial, T-cell activity was boosted, tumor fibrosis was reduced, and M2 macrophage numbers were diminished, accompanied by a decrease in inflammatory cytokines. This resulted in an increased progression-free survival in participating patients. These results prompted a subsequent phase 2 clinical trial [59]. Isatuximab is a monoclonal antibody targeting CD38, which blocks the action of ATRA in the TME. In a phase 1/2 open-label study, isatuximab was combined with atezolizumab, a PD-1/PD-L1-targeting antibody, to assess whether CD38 inhibition could modulate the immune response. In this study, tumor-infiltrating CD38+ cells were reduced, but no modulation of T-regs or PD-L1 expression was found. A phase 3 study on this subject will therefore not be conducted [60]. Another clinical trial assessed whether it was possible to modulate TAM activity through inhibition of leukemia inhibitory factor (LIF). LIF activity leads to changes in the immune response in the TME. The monoclonal antibody AZD0171 binds to LIF, thereby modulating TAM activity to an anti-tumor phenotype. In a phase 1 trial, a slight prolonged progression-free survival was observed along with an M2 to M1 macrophage shift. Although ani-tumor activity was relatively small, the TME modulation observed in patients with previously treated PDAC was promising, prompting a further phase 2 clinical trial [61].
The results of these clinical trials are promising, with several subsequent trials currently in progress. The focus of most of these, however, is on immune modulation. It would be interesting to see whether the other methods discussed in this article could achieve similar results in the future.
7. Conclusions
Despite decades of research and development in novel cancer therapies, cancer remains a leading cause of death among populations worldwide. A significant barrier to the therapeutic efficiency of cancer treatment that we are yet to overcome is the limited penetration of currently used cancer therapies into tumor tissue. This review provides an overview of the challenges and advances in modulating the TME in order to enhance tumor drug penetration. Tumors develop dysfunctional vasculature, dense concentrations of ECM proteins, and abnormal stromal cells. These developments all influence the penetration of drugs into the tumor microenvironment, and we have addressed several potential solutions to overcome the changes tumors make to the TME. Great achievements in increasing the penetration of drugs into tumor tissue have been made, but, as discussed in this review, most remain untested in human patients. It is currently unknown how certain changes made to the TME as discussed here would affect a human host. Changes to the vasculature and ECM are as likely to promote tumor growth or metastasis as increasing drug delivery to tumor tissue without further research, since both greatly benefit from a normalization of vasculature and an opening up of the dense ECM barrier surrounding tumors. As seen in the results of VEGF blocking in different types of tumors, certain treatments can be beneficial in one tumor type and detrimental in another. Further research and application in human studies are therefore of vital importance to discern which changes to the TME in specific tumor types could enhance cancer therapy efficacy.
Author Contributions
Conceptualization, H.H., C.F.M.S. and F.A.V.; writing—draft, H.H.; writing—review and editing, H.H., C.F.M.S., A.L.V. and F.A.V.; visualization, H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was sponsored by the Dutch Surgical Society for Medical Students (DSSMS-VCMS).
Acknowledgments
We would like to thank J.A. Sier, D. Donato, and B. Hogers (Leiden University) for their invaluable support during the literature research and writing of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thomas, D.; Radhakrishnan, P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol. Cancer 2019, 18, 14. [Google Scholar] [CrossRef]
- Heldin, C.H.; Rubin, K.; Pietras, K.; Ostman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef]
- Ishikane, S.; Takahashi-Yanaga, F. The role of angiotensin II in cancer metastasis: Potential of renin-angiotensin system blockade as a treatment for cancer metastasis. Biochem. Pharmacol. 2018, 151, 96–103. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013, 4, 2516. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69 (Suppl. S3), 4–10. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Dietrich, J.; Wang, D.; Batchelor, T.T. Cediranib: Profile of a novel anti-angiogenic agent in patients with glioblastoma. Expert. Opin. Investig. Drugs 2009, 18, 1549–1557. [Google Scholar] [CrossRef]
- Xiao, W.; Ruan, S.; Yu, W.; Wang, R.; Hu, C.; Liu, R.; Gao, H. Normalizing Tumor Vessels To Increase the Enzyme-Induced Retention and Targeting of Gold Nanoparticle for Breast Cancer Imaging and Treatment. Mol. Pharm. 2017, 14, 3489–3498. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef]
- Myoung, H.; Hong, S.D.; Kim, Y.Y.; Hong, S.P.; Kim, M.J. Evaluation of the anti-tumor and anti-angiogenic effect of paclitaxel and thalidomide on the xenotransplanted oral squamous cell carcinoma. Cancer Lett. 2001, 163, 191–200. [Google Scholar] [CrossRef]
- Danhier, F.; Danhier, P.; De Saedeleer, C.J.; Fruytier, A.C.; Schleich, N.; des Rieux, A.; Sonveaux, P.; Gallez, B.; Preat, V. Paclitaxel-loaded micelles enhance transvascular permeability and retention of nanomedicines in tumors. Int. J. Pharm. 2015, 479, 399–407. [Google Scholar] [CrossRef]
- Witsch, E.; Sela, M.; Yarden, Y. Roles for growth factors in cancer progression. Physiology 2010, 25, 85–101. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Pietras, K.; Rubin, K.; Sjoblom, T.; Buchdunger, E.; Sjoquist, M.; Heldin, C.H.; Ostman, A. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002, 62, 5476–5484. [Google Scholar] [PubMed]
- Zhang, B.; Shi, W.; Jiang, T.; Wang, L.; Mei, H.; Lu, H.; Hu, Y.; Pang, Z. Optimization of the tumor microenvironment and nanomedicine properties simultaneously to improve tumor therapy. Oncotarget 2016, 7, 62607–62618. [Google Scholar] [CrossRef] [PubMed]
- Olson, P.; Hanahan, D. Cancer. Breaching the cancer fortress. Science 2009, 324, 1400–1401. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Goel, S.; Duda, D.G.; Fukumura, D.; Jain, R.K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Maity, A.; Pore, N.; Lee, J.; Solomon, D.; O’Rourke, D.M. Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3’-kinase and distinct from that induced by hypoxia. Cancer Res. 2000, 60, 5879–5886. [Google Scholar]
- Chen, Q.; Xu, L.; Chen, J.; Yang, Z.; Liang, C.; Yang, Y.; Liu, Z. Tumor vasculature normalization by orally fed erlotinib to modulate the tumor microenvironment for enhanced cancer nanomedicine and immunotherapy. Biomaterials 2017, 148, 69–80. [Google Scholar] [CrossRef]
- Darabi, F.; Saidijam, M.; Nouri, F.; Mahjub, R.; Soleimani, M. Anti-CD44 and EGFR Dual-Targeted Solid Lipid Nanoparticles for Delivery of Doxorubicin to Triple-Negative Breast Cancer Cell Line: Preparation, Statistical Optimization, and In Vitro Characterization. Biomed. Res. Int. 2022, 2022, 6253978. [Google Scholar] [CrossRef]
- Sakurai, Y.; Hada, T.; Yamamoto, S.; Kato, A.; Mizumura, W.; Harashima, H. Remodeling of the Extracellular Matrix by Endothelial Cell-Targeting siRNA Improves the EPR-Based Delivery of 100 nm Particles. Mol. Ther. 2016, 24, 2090–2099. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kato, A.; Sakurai, Y.; Hada, T.; Harashima, H. Modality of tumor endothelial VEGFR2 silencing-mediated improvement in intratumoral distribution of lipid nanoparticles. J. Control. Release 2017, 251, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Chao, Y.; Xiang, J.; Han, X.; Song, G.; Feng, L.; Liu, J.; Yang, G.; Chen, Q.; Liu, Z. Hyaluronidase To Enhance Nanoparticle-Based Photodynamic Tumor Therapy. Nano Lett. 2016, 16, 2512–2521. [Google Scholar] [CrossRef]
- Dai, J.; Han, S.; Ju, F.; Han, M.; Xu, L.; Zhang, R.; Sun, Y. Preparation and evaluation of tumour microenvironment response multistage nanoparticles for epirubicin delivery and deep tumour penetration. Artif. Cells Nanomed. Biotechnol. 2018, 46, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Norberg, S.M.; Shalinsky, D.R.; Hu-Lowe, D.D.; McDonald, D.M. Effect of inhibition of vascular endothelial growth factor signaling on distribution of extravasated antibodies in tumors. Cancer Res. 2006, 66, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiang, T.; She, X.; Shen, S.; Wang, S.; Deng, J.; Shi, W.; Mei, H.; Hu, Y.; Pang, Z.; et al. Fibrin degradation by rtPA enhances the delivery of nanotherapeutics to A549 tumors in nude mice. Biomaterials 2016, 96, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef]
- Lai, Y.H.; Chiang, C.S.; Kao, T.H.; Chen, S.Y. Dual-drug nanomedicine with hydrophilic F127-modified magnetic nanocarriers assembled in amphiphilic gelatin for enhanced penetration and drug delivery in deep tumor tissue. Int. J. Nanomed. 2018, 13, 3011–3026. [Google Scholar] [CrossRef] [PubMed]
- Cun, X.; Ruan, S.; Chen, J.; Zhang, L.; Li, J.; He, Q.; Gao, H. A dual strategy to improve the penetration and treatment of breast cancer by combining shrinking nanoparticles with collagen depletion by losartan. Acta Biomater. 2016, 31, 186–196. [Google Scholar] [CrossRef]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef] [PubMed]
- Froeling, F.E.; Feig, C.; Chelala, C.; Dobson, R.; Mein, C.E.; Tuveson, D.A.; Clevers, H.; Hart, I.R.; Kocher, H.M. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology 2011, 141, 1486–1497.e14. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, J.G.; Zhou, Q.M.; Yu, J.N.; Lu, Y.F.; Wang, X.J.; Zhou, J.P.; Ding, X.F.; Du, Y.Z.; Yu, R.S. Extracellular matrix modulating enzyme functionalized biomimetic Au nanoplatform-mediated enhanced tumor penetration and synergistic antitumor therapy for pancreatic cancer. J. Nanobiotechnol. 2022, 20, 524. [Google Scholar] [CrossRef]
- Papanicolaou, M.; Parker, A.L.; Yam, M.; Filipe, E.C.; Wu, S.Z.; Chitty, J.L.; Wyllie, K.; Tran, E.; Mok, E.; Nadalini, A.; et al. Temporal profiling of the breast tumour microenvironment reveals collagen XII as a driver of metastasis. Nat. Commun. 2022, 13, 4587. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, Y.; Xu, Y.; Zhao, X.; Zhang, Y.; Yang, X.; Wang, Y.; Zhao, R.; Anderson, G.J.; Zhao, Y.; et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat. Commun. 2018, 9, 3390. [Google Scholar] [CrossRef] [PubMed]
- Denton, A.E.; Roberts, E.W.; Fearon, D.T. Stromal Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2018, 1060, 99–114. [Google Scholar] [CrossRef]
- Sperb, N.; Tsesmelis, M.; Wirth, T. Crosstalk between Tumor and Stromal Cells in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 5486. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Zou, L.; Zhu, Z. Role of Exosomes in Crosstalk Between Cancer-Associated Fibroblasts and Cancer Cells. Front. Oncol. 2019, 9, 356. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Saha, S.; Wang, E.; Robertson, J.D.; Bhattacharya, R.; Mukherjee, P. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 6700–6705. [Google Scholar] [CrossRef]
- Saha, S.; Xiong, X.; Chakraborty, P.K.; Shameer, K.; Arvizo, R.R.; Kudgus, R.A.; Dwivedi, S.K.; Hossen, M.N.; Gillies, E.M.; Robertson, J.D.; et al. Gold Nanoparticle Reprograms Pancreatic Tumor Microenvironment and Inhibits Tumor Growth. ACS Nano 2016, 10, 10636–10651. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Itakura, S.; Matsui, R.; Nakayama, K.; Nishi, T.; Nishimoto, A.; Hama, S.; Kogure, K. Tumor Microenvironment-Sensitive Liposomes Penetrate Tumor Tissue via Attenuated Interaction of the Extracellular Matrix and Tumor Cells and Accompanying Actin Depolymerization. Biomacromolecules 2017, 18, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaite, E.; Segura-Aguilar, J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, J.; Ji, C. Quercetin: A potential drug to reverse multidrug resistance. Life Sci. 2010, 87, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, S.; Xue, X.; Zhu, X.; Song, S.; Wang, B.; Jiang, L.; Qin, M.; Liang, H.; Gao, L. Quercetin and doxorubicin co-delivery using mesoporous silica nanoparticles enhance the efficacy of gastric carcinoma chemotherapy. Int. J. Nanomed. 2018, 13, 5113–5126. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Miao, L.; Goodwin, T.J.; Li, J.; Liu, Q.; Huang, L. Quercetin Remodels the Tumor Microenvironment To Improve the Permeation, Retention, and Antitumor Effects of Nanoparticles. ACS Nano 2017, 11, 4916–4925. [Google Scholar] [CrossRef]
- Miao, L.; Wang, Y.; Lin, C.M.; Xiong, Y.; Chen, N.; Zhang, L.; Kim, W.Y.; Huang, L. Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin. J. Control. Release 2015, 217, 27–41. [Google Scholar] [CrossRef]
- Kay, E.J.; Paterson, K.; Riera-Domingo, C.; Sumpton, D.; Dabritz, J.H.M.; Tardito, S.; Boldrini, C.; Hernandez-Fernaud, J.R.; Athineos, D.; Dhayade, S.; et al. Cancer-associated fibroblasts require proline synthesis by PYCR1 for the deposition of pro-tumorigenic extracellular matrix. Nat. Metab. 2022, 4, 693–710. [Google Scholar] [CrossRef]
- Miao, L.; Liu, Q.; Lin, C.M.; Luo, C.; Wang, Y.; Liu, L.; Yin, W.; Hu, S.; Kim, W.Y.; Huang, L. Targeting Tumor-Associated Fibroblasts for Therapeutic Delivery in Desmoplastic Tumors. Cancer Res. 2017, 77, 719–731. [Google Scholar] [CrossRef]
- Gieniec, K.A.; Butler, L.M.; Worthley, D.L.; Woods, S.L. Cancer-associated fibroblasts-heroes or villains? Br. J. Cancer 2019, 121, 293–302. [Google Scholar] [CrossRef]
- Mpekris, F.; Papageorgis, P.; Polydorou, C.; Voutouri, C.; Kalli, M.; Pirentis, A.P.; Stylianopoulos, T. Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy. J. Control. Release 2017, 261, 105–112. [Google Scholar] [CrossRef]
- Sier, V.Q.; de Vries, M.R.; van der Vorst, J.R.; Vahrmeijer, A.L.; van Kooten, C.; Cruz, L.J.; de Geus-Oei, L.F.; Ferreira, V.; Sier, C.F.M.; Alves, F.; et al. Cell-Based Tracers as Trojan Horses for Image-Guided Surgery. Int. J. Mol. Sci. 2021, 22, 755. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yong, T.; Wei, Z.; Bie, N.; Zhang, X.; Zhan, G.; Li, J.; Qin, J.; Yu, J.; Zhang, B.; et al. Reversing insufficient photothermal therapy-induced tumor relapse and metastasis by regulating cancer-associated fibroblasts. Nat. Commun. 2022, 13, 2794. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Du, Y.; Zhang, Z.; He, K.; Cheng, Z.; Yin, L.; Dong, D.; Li, C.; Li, W.; Hu, Z.; et al. Fluorescence image-guided tumour surgery. Nat. Rev. Bioeng. 2023, 1, 161–179. [Google Scholar] [CrossRef]
- Vuijk, F.A.; Houvast, R.; Baart, V.M.; van de Velde, C.J.H.; Vahrmeijer, A.L.; Hilling, D.E.; Mieog, J.S.D.; Slingerland, M.; de Geus-Oei, L.F.; Hawinkels, L.J.A.C.; et al. Molecular Imaging of the Tumor Stroma and Beyond. In The Tumor Stroma: Biology and Therapeutics; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2022. [Google Scholar]
- Hingorani, S.R.; Zheng, L.; Bullock, A.J.; Seery, T.E.; Harris, W.P.; Sigal, D.S.; Braiteh, F.; Ritch, P.S.; Zalupski, M.M.; Bahary, N.; et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J. Clin. Oncol. 2018, 36, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; Tempero, M.; Corrie, P.G. HALO-109-301: A Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. 2018, 14, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Betts, C.; Zhang, L.; Griffith, M.J.; Solman, I.; Chen, B.; Liu, E.; Tamaki, W.; Stultz, J.; Marquez, J.; et al. Modulation of myeloid and T cells in vivo by Bruton’s tyrosine kinase inhibitor ibrutinib in patients with metastatic pancreatic ductal adenocarcinoma. J. Immunother. Cancer 2023, 11, e005425. [Google Scholar] [CrossRef]
- Byrne, K.T.; Betts, C.B.; Mick, R.; Sivagnanam, S.; Bajor, D.L.; Laheru, D.A.; Chiorean, E.G.; O’Hara, M.H.; Liudahl, S.M.; Newcomb, C.; et al. Neoadjuvant Selicrelumab, an Agonist CD40 Antibody, Induces Changes in the Tumor Microenvironment in Patients with Resectable Pancreatic Cancer. Clin. Cancer Res. 2021, 27, 4574–4586. [Google Scholar] [CrossRef]
- Simonelli, M.; Garralda, E.; Eskens, F.; Gil-Martin, M.; Yen, C.J.; Obermannova, R.; Chao, Y.; Lonardi, S.; Melichar, B.; Moreno, V.; et al. Isatuximab plus atezolizumab in patients with advanced solid tumors: Results from a phase I/II, open-label, multicenter study. ESMO Open 2022, 7, 100562. [Google Scholar] [CrossRef]
- Borazanci, E.; Schram, A.M.; Garralda, E.; Brana, I.; Vieito Villar, M.; Spreafico, A.; Oliva, M.; Lakhani, N.J.; Hoffman, K.; Hallett, R.M.; et al. Phase I, first-in-human study of MSC-1 (AZD0171), a humanized anti-leukemia inhibitory factor monoclonal antibody, for advanced solid tumors. ESMO Open 2022, 7, 100530. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).