Abstract
Background: The craniovertebral junction is a complex region, connecting the occiput, the atlas, the axis, and the containing vital neural and vascular structures. There is a great variability in diseases involving the craniovertebral junction, of different nature, each rare in frequency. Methods: We conducted a retrospective chart review of the patients diagnosed with extremely rare pathologies of the craniovertebral junction that we have operated in the last 5 years. Results: After excluding the relatively more frequent pathologies, we identified nine cases of rare craniovertebral junction pathologies. Six were operated using an endoscopic endonasal approach, two using a far lateral transcranial approach, and one underwent a C1 hemilaminectomy. Conclusions: Diagnosis and management of the rare pathologies of the craniovertebral junction are challenging. A multidisciplinary approach is recommended for the proper management of these patients.
1. Introduction
The craniovertebral junction (CVJ) is a sticking point, connecting different anatomical compartments. Superiorly, it is delimited by the clivus in the anterior part and by the occipital bone, and inferiorly by the C1–C2 complex. It is situated at the midpoint of the skull base (SB), acting as a dividing line between structures inside the cranium and the regions of the face and neck [1].
It is also the region where the brain and the spinal cord are contiguous. It is surrounded by noble and vital structures, like cranial nerves and vessels such as the internal carotid artery and internal jugular vein. It represents the deepest point of the SB; therefore, it was clinically impossible to explore the CVJ before the advent of CT and MRI imaging, and impossible to surgically approach this area properly before the use of microscopes and endoscopes. Due to the difficult position, the anatomical and pathological knowledge of this area was gained quite recently.
The CVJ presents the characteristics of the border zone also for the pathologies that arise here, requiring the multidisciplinary expertise of otorhinolaryngologists, neurosurgeons, neuroradiologists, pathologists, radiotherapists, and oncologists to undertake the appropriate treatment. Comprehending the anatomy of this area is crucial for grasping various pathological conditions and preparing for surgical interventions. The craniovertebral junction (CVJ) is affected by a wide range of diseases, each of them infrequent and diverse in nature. This diversity adds to the complexity of managing such cases, making it even more demanding. In this article, we present a compilation of nine exceptionally rare pathologies that we have encountered and managed in the craniovertebral junction region over the past 5 years. These cases represent unique and challenging clinical scenarios that required specialized care and multidisciplinary collaboration. By sharing our experiences with these rare conditions, we aim to contribute to the existing knowledge and foster better understanding and management of such complex cases in the medical community. Our hope is that this article will serve as a valuable resource for clinicians and researchers dealing with rare craniovertebral junction pathologies and ultimately improve patient outcomes.
2. Materials and Methods
According to Goel and colleagues, the most “common” pathologies in this region include intradural meningiomas, neurofibromas, lung or breast metastatic tumors, chordomas, chondrosarcomas, and nasopharyngeal carcinoma. [2]. We retrospectively reviewed all the records of patients with rare pathologies of the CVJ region—all except those mentioned above—referred to the Neurosurgery Unit of the Fondazione IRCCS Policlinico San Matteo-Pavia from January 2017 to December 2021.
We collected and reviewed for each patient all the clinical and pathological reports and imaging studies available.
We acquired informed consent from both the patient and the parents of participants below 18 years of age to utilize their personal data for scientific purposes. Moreover, since our study involved non-experimental data and adhered to standard clinical methods outlined in the literature, it did not necessitate any specific authorizations from our Ethics Committee.
3. Results
In total, 9 patients (5 males and 4 females, mean age 52, range 7–78 years old) were included in this study.
In six patients, the lesions were approached using an endoscopic endonasal technique (6/9–67%), while in three patients, the far lateral transcranial approach was the preferred choice (33%).
Table 1 provides a concise overview of the key patient characteristics, technical data, and histopathological findings.

Table 1.
Demographic and clinical features of the 9 patients included in this study.
3.1. Case 1—Clival Osteoradionecrosis
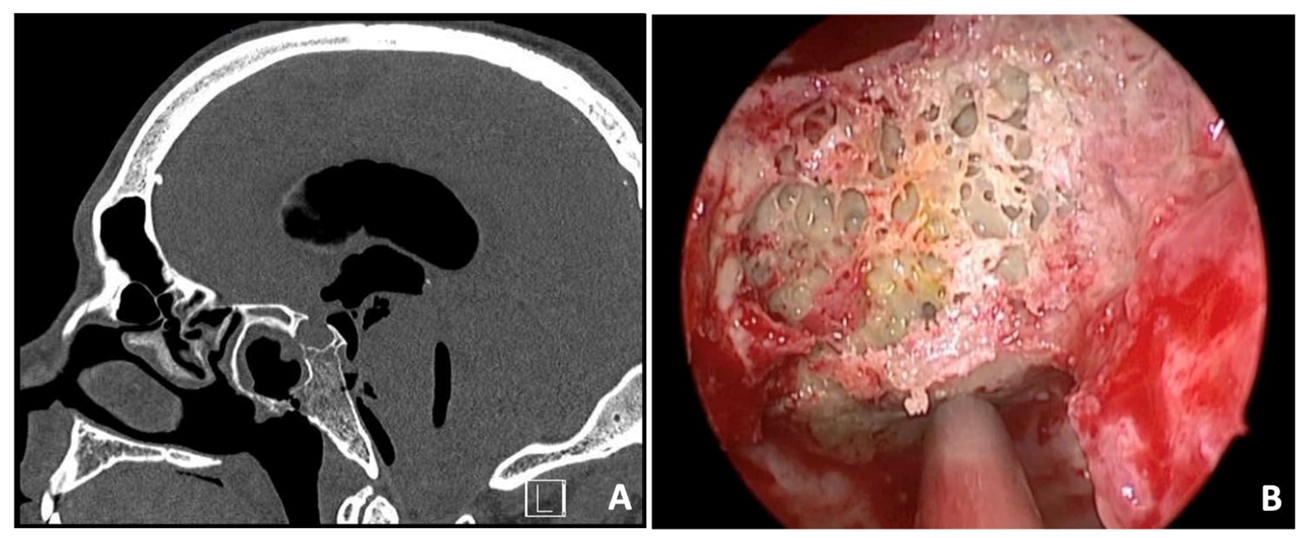
A 36-year-old man presented at the Emergency Department (ED) with symptoms of disorientation, drowsiness, headache, fever, and watery discharge from the nose. Four years earlier, he had undergone radical neck dissection and chemoradiotherapy to treat an undifferentiated nasopharyngeal carcinoma. Due to a relapse of the disease in the clivus and sphenoidal bone, a second-line stereotactic radiotherapy was performed two years later. Upon examination with nasal endoscopy, necrotic bone was discovered in the clival region, specifically in the paramedian area of the nasopharynx, along with active cerebrospinal fluid rhinorrhea. A CT scan revealed a significant tension pneumocephalus, with erosion observed in the infero-posterior parts of the sphenoidal sinus, and a potential case of radionecrosis in the clivus (Figure 1A). The patient underwent an endoscopic endonasal repair of the clival fistula using a pedunculated nasoseptal flap (Figure 1B). Pathological examination results indicated radionecrosis with signs of infiltration by undifferentiated non-keratinizing squamocellular carcinoma. Follow-up CT and MRI scans of the brain showed progressive absorption of the pneumocephalus without any signs of infection. The patient was then presented at the tumor board to determine the next course of oncological treatments. Unfortunately, the patient succumbed to the disease after three years.
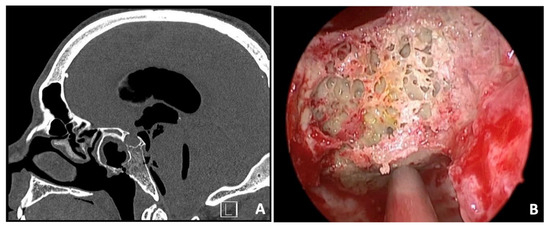
Figure 1.
Sagittal preoperative CT scan showing a massive tension pneumocephalus with erosion of infero-posterior parts of sphenoidal sinus and possible radionecrosis of the clivus (A). Intraoperative view of the necrotic clival bone (B).
3.2. Case 2—Ecchordosis Physaliphora
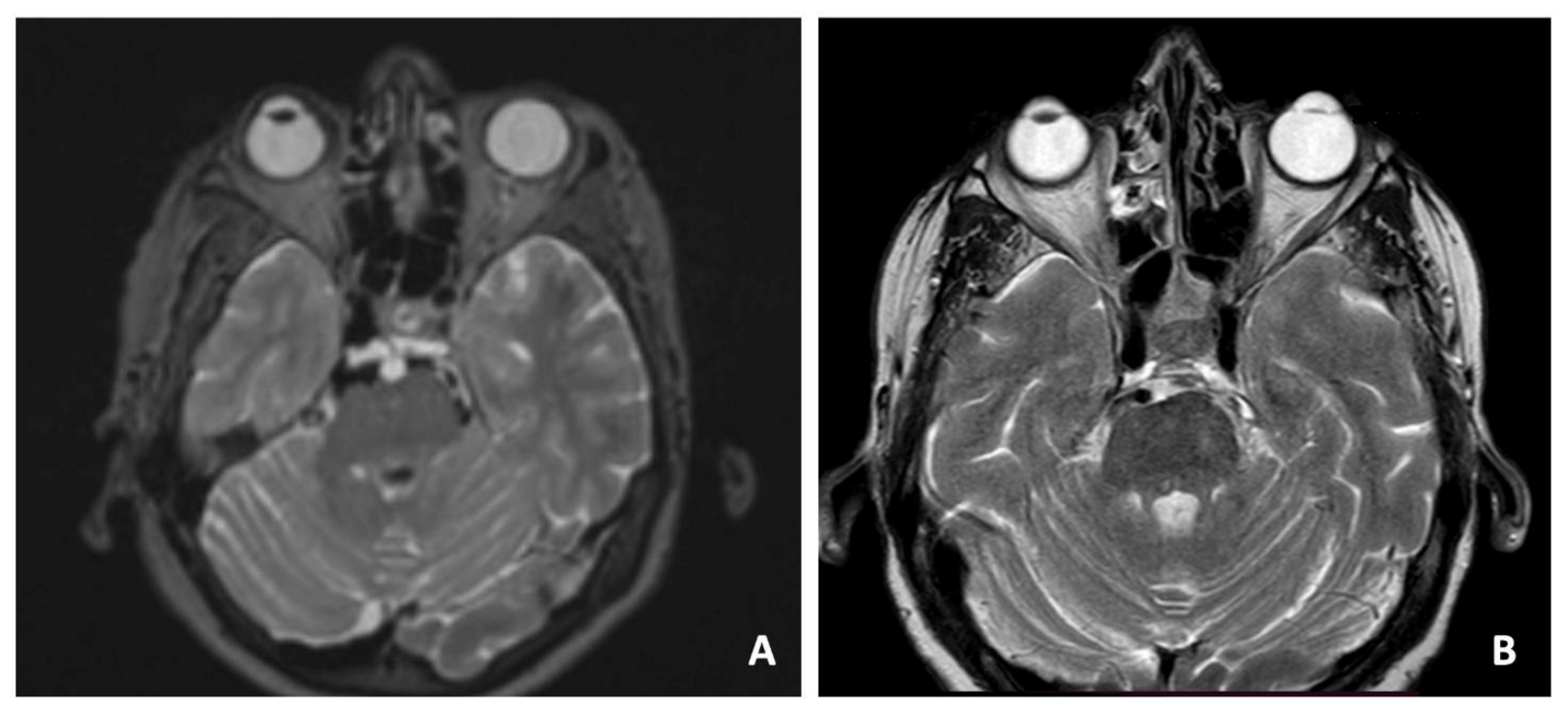
A 69-year-old woman with cephalea and aqueous rhinorrhea underwent a cerebral MRI scan with the finding of a clival lesion with bony erosion (Figure 2A). An endoscopic endonasal transsphenoidal approach to the clival area was performed to remove the lesion, with an SB repair using the three-layer technique. The pathological examination revealed ecchordosis physaliphora. The postoperative MRI showed a complete removal of the lesion, and the patients underwent clinical/radiological follow-up (Figure 2B).
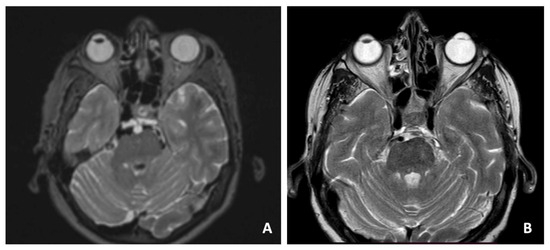
Figure 2.
Preoperative T1-weighted axial MRI scan showing the clival lesion (A). Postoperative MRI (B).
3.3. Case 3—Hepatocellular Carcinoma Metastasis
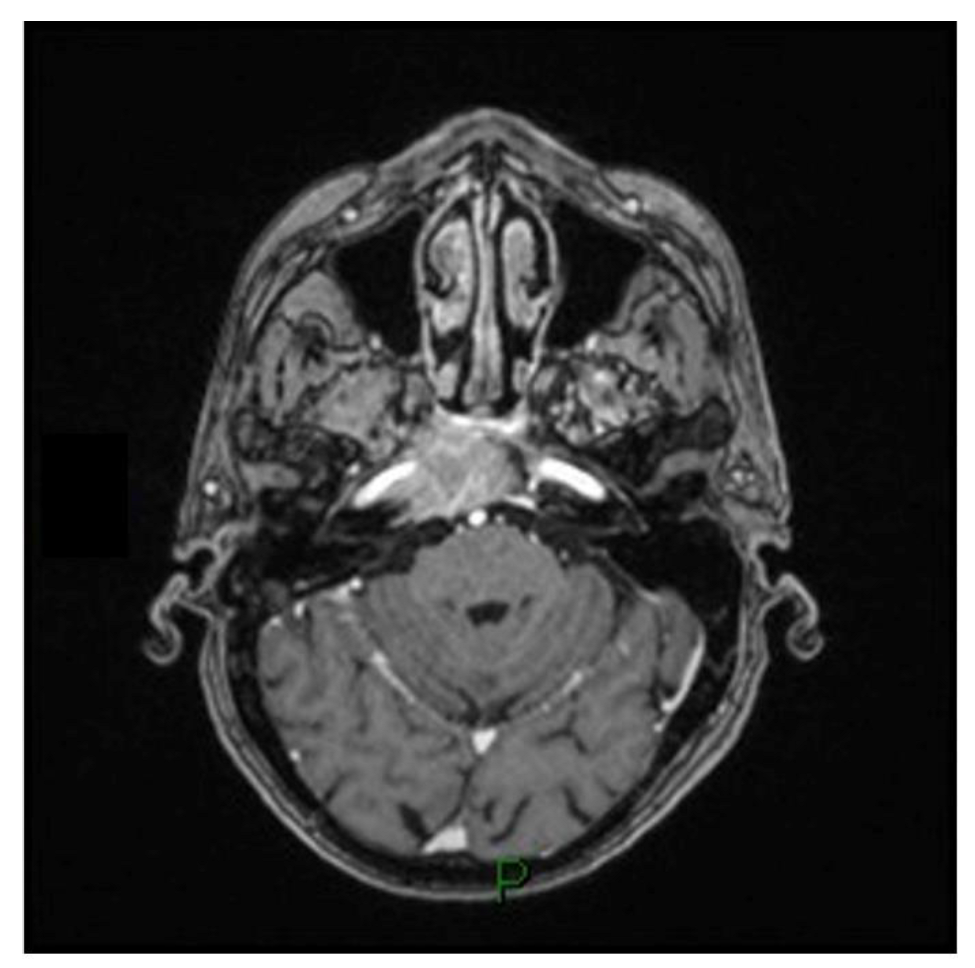
A 79-year-old man with a history of HCV-related cirrhosis presented with progressive diplopia due to a VI cranial nerve impairment. An MRI scan revealed an expansive process in the clival region encompassing the right carotid siphon and infiltrating the ipsilateral cavernous sinus (Figure 3). The patient underwent a minimally invasive endoscopic endonasal transsphenoidal approach to the clival region. The aim of this procedure was to perform a biopsy, debulk the affected area, and achieve decompression of the cavernous sinus. The morphologic and immunohistochemical examination suggested a clival localization of a carcinoma of hepatic origin. The total body CT scan revealed multiple metastatic localizations and the patient underwent tumor board evaluation and scheduled for palliate chemotherapy and died after three months.
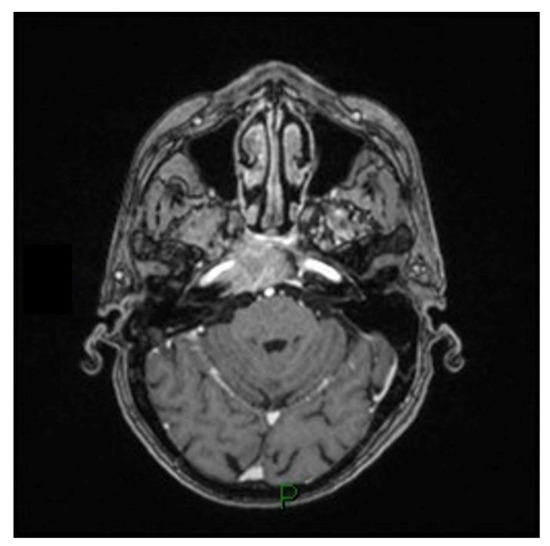
Figure 3.
T1-weighted axial MRI scan showing the expansive process in the clival region encompassing the right carotid siphon and infiltrating the ipsilateral cavernous sinus.
3.4. Case 4—Capillary Hemangioma
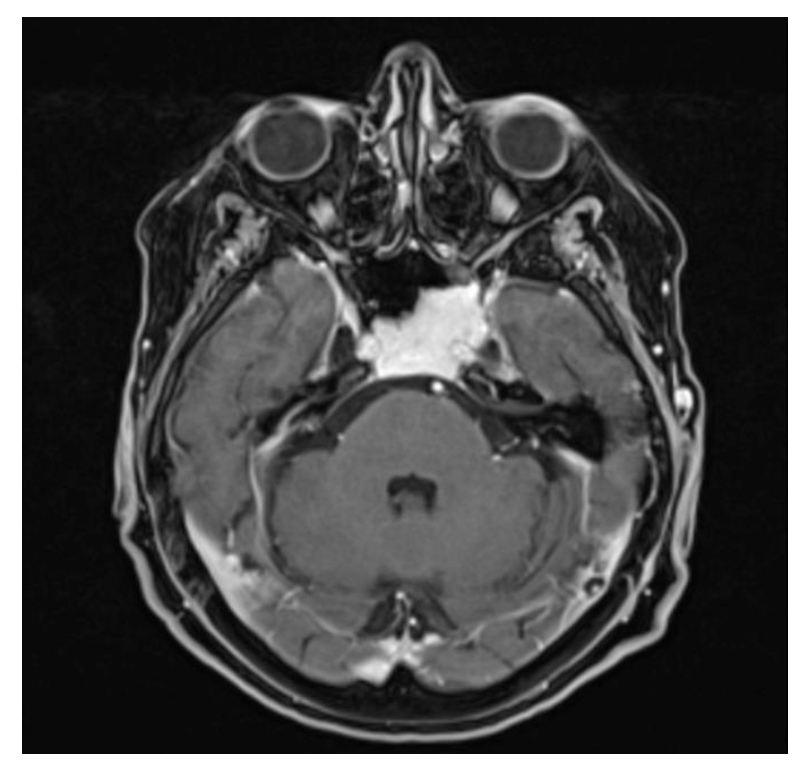
A 77-year-old woman underwent cerebral MRI for cognitive impairment. The images showed a clival expanding lesion, involving the left cavernous sinus (Figure 4). The patient underwent a transsphenoidal biopsy using the endoscopic technique and the pathological examination revealed a capillary hemangioma. The patient tolerated the biopsy well and no further surgical intervention was required. The follow-up at 5 years showed no progression and the patient remained asymptomatic for the lesion.
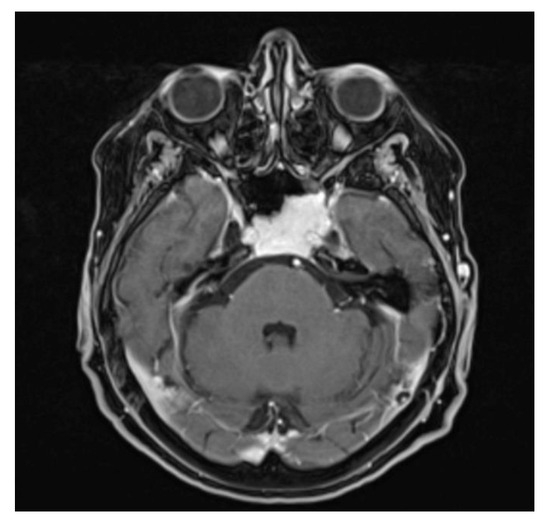
Figure 4.
Contrast-enhanced T1-weighted axial MRI scan: the capillary hemangioma extends to the clivus and the left cavernous sinus, with intense homogeneous signal lesion enhancement.
3.5. Case 5—Embryonal Rhabdomyosarcoma
A 7-year-old boy presented with VI and VII right cranial nerve palsy, unresponsive to corticosteroid therapy. CT and MRI were performed with the evidence of right otomastoiditis with SB erosion and replacement with the enhancing lesion enveloping the intrapetrous carotid artery, the acoustic-facial bundle, the VI cranial nerve, the jugular bulb, and the trigeminal nerve. The patient underwent a trans clival biopsy with the pathological finding of an embryonal rhabdomyosarcoma. The patient underwent chemo-radiotherapy treatment and, at the time of writing, is on maintenance chemotherapy.
3.6. Case 6—Cholesterol Granuloma
A 46-year-old man presented with symptoms of headache and photophobia. An MRI scan revealed a growing lesion in the spheno-clival region, but there were no indications of infiltration into surrounding structures. A basal CT scan showed osteolysis of the posterior sphenoidal wall. To address the issue, an endoscopic transsphenoidal approach was employed to access the posterior sphenoidal wall. During the procedure, the lesion, which exhibited a cystic appearance, was drained. No sign of CSF leak was found. The patient was dismissed after two days without complications. The pathological examination revealed a cholesterol granuloma. No further treatment was necessary, and the patient is now on follow-up.
3.7. Case 7—Extradural Meningioma
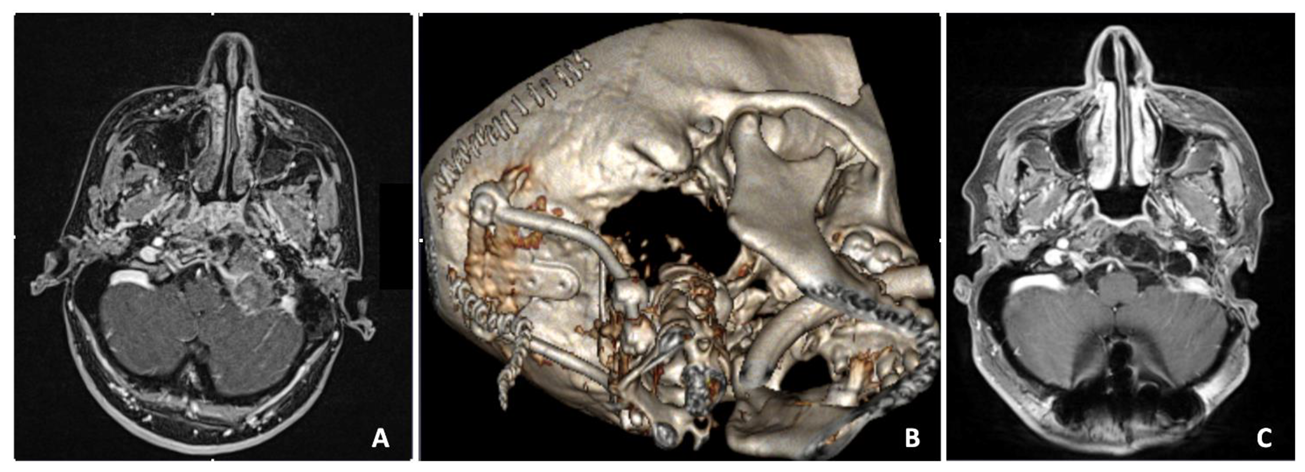
A 36-year-old female suffered from intense cervical pain. MRI disclosed the presence of a voluminous mass replacing the left condyle and eroding part of the lower clivus (Figure 5A). The mass was completely extradural and showed mixed contrast enhancement due to the presence of multiple cysts intermingled with a more parenchymatous tissue. Due to the unusual imaging features, a transsphenoidal biopsy was first performed. Histology revealed a meningioma. Hence, the patient underwent a left far lateral approach with complete resection of the mass. An occipito-cervical instrumentation was then placed to stabilize the CVJ (Figure 5B). Postoperatively, the patient showed left hypoglossal nerve palsy and rhinolalia, which resolved completely after two months. The last MRI follow-up at 5 years showed no relapse (Figure 5C).
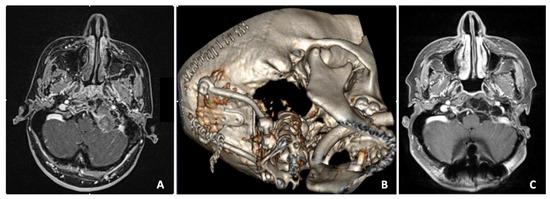
Figure 5.
Preoperative axial contrast-enhanced T1-weighted MRI scan showing an extradural voluminous mass with mixed contrast enhancement replacing the left condyle and eroding part of the lower clivus (A). Postoperative CT 3D reconstruction (B). Five years postoperative axial T1 RMI with contrast enhancement, showing the complete removal of the lesion (C).
3.8. Case 8—Ganglion Cyst
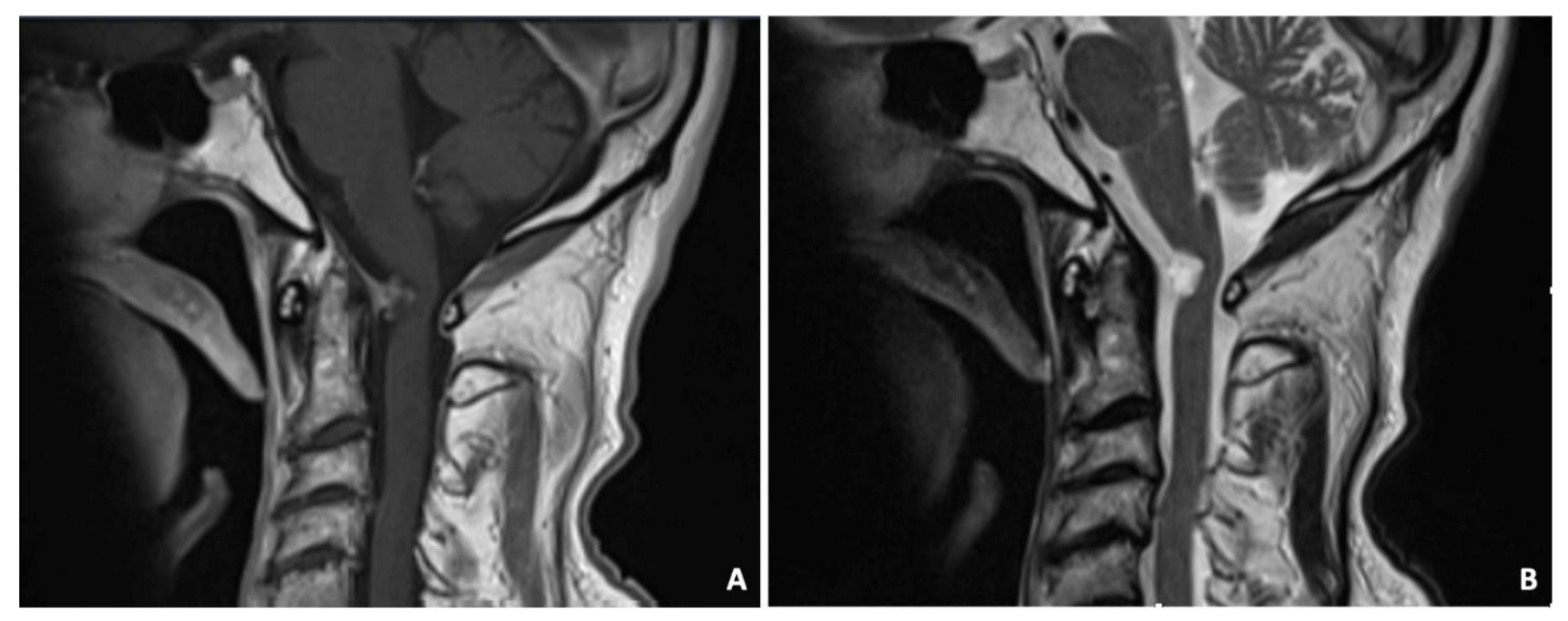
A 75-year-old male was admitted to the ED following a car accident. A plane CT scan showed a retro-odontoid mass. The MRI demonstrated an intradural, in-homogeneously contrast-enhanced cystic mass arising from the dura mater behind the odontoid (Figure 6). The T2 sequences showed a hyperintense signal. The mass compressed and displaced the spinal cord. Clinical examination showed slight right upper limb weakness. Due to the compression and the need to obtain diagnosis, the patient underwent a C1 right hemilaminectomy with complete resection of the mass. Histology confirmed a ganglion cyst. At the 3 years follow-up, the patient was asymptomatic with no signs of relapse.
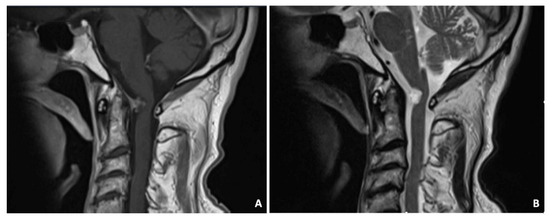
Figure 6.
Sagittal T1-weighted MRI demonstrated the intradural, inhomogeneously contrast-enhanced cystic mass arising from the dura mater behind the odontoid (A). The T2-weighted image showed a hyperintense signal (B).
3.9. Case 9—Histiocytic Sarcoma
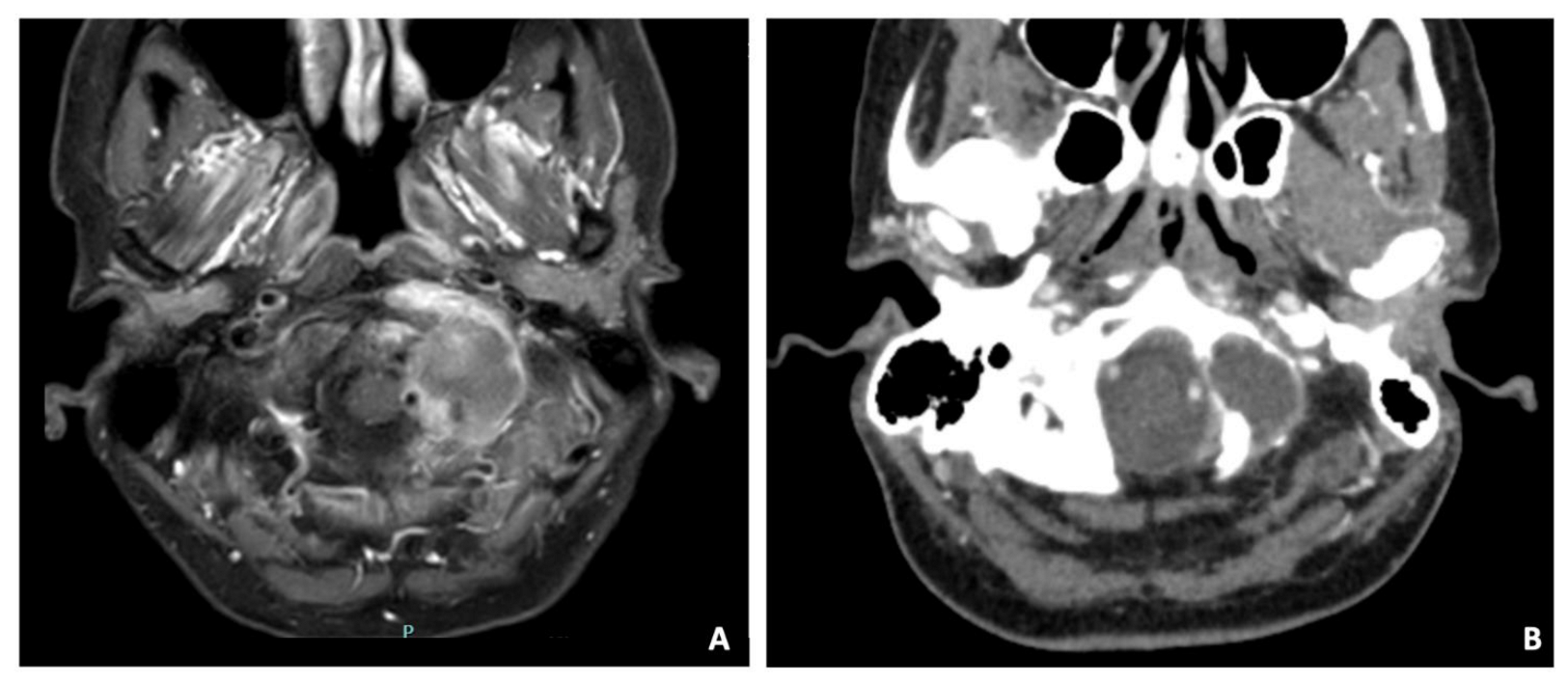
A 47-year-old female suffering from neck pain lasting approximately for five months underwent a CT and subsequently a contrast-enhanced MRI, which detected a mass infiltrating and destroying the left occipital condyle as well as the lateral mass of C1 and the arch (Figure 7). The tumor had a necrotic core surrounded by an intense contrast enhancement. The CT scan demonstrated erosion of the occipital condyle, the articular facet of C1, and of the ipsilateral posterior arch of C1. The vertebral artery was in close contact with the mass and pushed backward. Surgery was proposed with the aim to resect the tumor and stabilizing the CVJ. A left far lateral approach was performed with an uneventful postoperative evolution and the patient was discharged ten days after. The histology demonstrated a histiocytic sarcoma. The patient was then admitted to chemo and radiotherapy, ongoing at the time of writing.
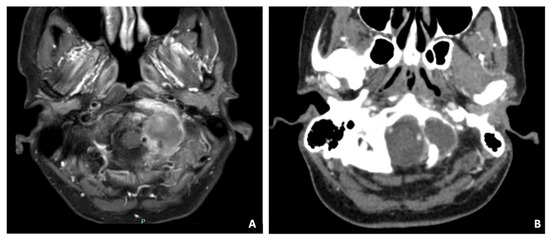
Figure 7.
Axial T1-weighted MRI (A) and CT scan (B) detecting a mass with necrotic core surrounded by an intense contrast enhancement, infiltrating and destroying the left occipital condyle and the lateral mass of C1 and the arch. The CT scan demonstrated erosion of the occipital condyle, the articular facet of C1, and of the ipsilateral posterior arch of C1. Vertebral artery was in close contact with the mass and pushed backward.
4. Discussion
The CVJ, defined as the transition point between the cranium and the upper cervical spine, is considered the most complex region of the axial skeleton [3].
It can be affected by congenital malformation, inflammatory, traumatic, degenerative, and neoplastic disease.
Besides the congenital anomalies that may involve the occiput, the atlas, or the axis, the CVJ is the possible location of various acquired conditions. Among the inflammatory and degenerative pathologies, rheumatoid arthritis is the most common one, followed by psoriasis and osteoarthritis.
Tumors affecting the CVJ are infrequent and can pose challenges in diagnosis due to the wide array of symptoms they may manifest. Often, medical professionals may overlook this region while considering potential diagnoses [2]. Among the tumors that preferentially develop at the CVJ, meningiomas are the most commonly encountered benign tumors in this area, followed by neurofibromas. Metastatic tumors, particularly originating from the lung and breast, are more common than primary tumors. Malignant neoplasms that can arise in this region include chordomas, chondrosarcomas, and nasopharyngeal carcinoma.
Searching in the literature, there are only few records of the effects of radiotherapy on the SB and the CVJ in particular. We found 11 cases of delayed cerebrospinal fluid (CSF) leak and 9 cases of SB osteoradionecrosis (ORN) after radiotherapy and proton therapy [4,5,6,7,8,9,10].
Certain authors have hypothesized that delayed cerebrospinal fluid (CSF) leaks following radiotherapy could be attributed to radiation-induced weakening or necrosis of the mucosal membrane [5]. Radiation therapy (RT) not only affects tumor cells but also damages healthy cells, including those in the vascular endothelium. This leads to a combination of conditions known as the triad of hypoxia, hypocellularity, and hypovascularity [11]. As the natural cycle of collagen tissue regeneration continues, the damaged tissue becomes unable to repair itself. While RT can cause osteoradionecrosis (ORN) in any bone, bones with inherently limited blood supply, like the mandible, tend to be more vulnerable. Bones of the skull base, including the temporal bone, basisphenoid–basiocciput, ethmoid, and maxillary facial bones, are also potential targets for ORN. Certain risk factors, such as Type II diabetes mellitus, smoking, and vascular diseases, may contribute to the development of ORN. Among head and neck malignancies, RT for nasopharyngeal carcinoma is a significant factor responsible for the occurrence of ORN. On the other hand, some researchers have suggested a potential link between CSF leaks after stereotactic radiosurgery and empty sella syndrome [6,9]. Usually, ORN has a delayed and subtle presentation, and it must be distinguished from recurrent disease or radiation-induced malignancies [12]. In our case report, the pathological examination revealed ORN with malignancy recurrency and the patient underwent oncological treatment.
Ecchordosis physaliphora (EP) is a congenital and benign lesion characterized by nodules of gelatinous tissue, believed to be ectopic remnants of the notochord along the craniospinal axis. It is most commonly found at the clivus and sacrum levels. To suspect EP, radiological findings should be considered, with typical features being hypointensity in T1-weighted and hyperintensity in T2-weighted MRI scans. Crucially, EP does not show contrast enhancement after the administration of gadolinium. CT images may reveal an osseous pedicle or stalk-like projection connecting the EP lesion to the dorsal aspect of the clivus, which can be an incidental finding. Regarding location, EP is mainly observed along the midline and intradurally, but it can be found in various combinations of the extradural, subdural, and subarachnoid spaces. Due to its variable presentation and location, careful evaluation of imaging findings is essential for the accurate diagnosis of ecchordosis physaliphora. Symptomatic EP is an exceptionally uncommon lesion that originates from residual notochordal remnants. It can occur at various points along the craniospinal axis, but its most frequent location is on the dorsal surface of the clivus. Typically, EP follows a benign and slow-growing course, often requiring only radiological monitoring. However, in rare instances, it can manifest with clinical symptoms, necessitating therapeutic intervention. Up to now, the literature has documented only 40 cases of symptomatic EP that underwent surgical treatment [13,14,15,16,17,18]. The complete removal of the lesion and the SB repair allowed the complete resolution of the symptoms in our patient.
SB and CVJ are possible sites of metastasis from lung, breast, and prostate cancer. The most common primary sites for metastases to the skull base include the prostate, kidney, liver (hepatocellular carcinoma), thyroid, gastrointestinal tract (adenocarcinoma), breast, lung, melanoma, and lymphoma. Typical clinical presentations in patients with skull base metastases include isolated sixth nerve palsy, diplopia, multiple nerve palsies, or headache. Unfortunately, patients with skull base metastases tend to have a poor prognosis, with a median survival of approximately 10 months. On imaging, metastases may appear as low-signal areas on T2-weighted MRI, which can potentially help differentiate them from other skull base tumors like chordomas and chondrosarcomas. This imaging characteristic is useful for narrowing down the differential diagnosis and guiding further investigations and treatment decisions.
Hepatocellular carcinoma (HCC) is a highly aggressive tumor, and the majority of patients experience a rapid decline, succumbing to the disease within a few months of being diagnosed. Because of its aggressive nature, distant metastases to the central nervous system (CNS) and skull base (SB) are exceedingly uncommon. Reviewing the literature, only 15 cases of HCC with SB involvement had been reported [19,20,21,22,23,24,25,26,27,28,29,30]. Due to the aggressiveness and the multiple metastatic localizations, our patient died after a few months of our intervention.
Capillary hemangioma (CH) is a benign vascular tumor that differs from venous and cavernous types of hemangioma from the histopathological findings of tightly coordinated capillary-like canals [31]. CHs may arise within the head and neck region, predominantly targeting the skin and mucosa surfaces of female infants, such as the nasal cavity and the tongue [32]. CHs affecting the SB are rarely reported. Searching in the literature, only seven cases of skull base CH are described, mainly in the pediatric population [33,34,35,36,37]. For symptomatic capillary hemangiomas, total resection is the gold standard. In the case of asymptomatic lesions, as the case we reported, once a definite histological evaluation is obtained, the literature regarding the optimal management is limited. Our patient underwent a radiological follow-up and, in 5 years, is still asymptomatic, and images showed no progression.
Rhabdomyosarcoma (RMS) is an aggressive and uncommon high-grade malignant tumor, characterized by small round blue cells resembling skeletal muscle [38]. It constitutes about 3 to 5% of all childhood malignancies and is the most prevalent soft-tissue sarcoma in children. The embryonal subtype is the most common histological variant, accounting for around 70% of RMS cases [38]. In childhood, RMS often occurs in the head and neck region, making up approximately 40% of cases. Among head and neck RMSs, about 40% to 50% affect parameningeal sites such as the nasal cavity, paranasal sinuses, nasopharynx, middle ear, mastoid region, pterygopalatine fossa, and infratemporal fossa [39]. The paranasal sinuses are the most frequently affected site, followed by the nasopharynx, nasal cavity, and external nose [40,41]. RMSs arising in the temporal bone and petrous apex are particularly rare, with only 9 and 2 reported cases, respectively [42,43,44]. Definitive standardized treatment sequences for RMS are controversial. Various combinations of surgery, radiotherapy, and chemotherapy are described. The patient in our case series responded to a bimodal therapy and is, at the time of writing, on maintenance chemotherapy.
Cholesterol granulomas (CG) are chronic inflammatory lesions mainly found in the apex of the petrous part of the temporal bone. These are non-cancerous, tumor-like formations characterized by a cystic cavity filled with fluid containing cholesterol crystals, red blood cells, their breakdown products, multinucleated giant cells, and hemosiderin. These elements are encased by a fibrous capsule [45,46].
To date, 27 cases of sellar and 2 cases of clival CG have been reported [47,48,49]. The exact origin of cholesterol granulomas remains uncertain and is likely to be diverse. One hypothesis suggests that these granulomas could potentially arise as a degenerative form of Rathke’s cleft cysts. However, the understanding of the underlying mechanisms and definitive etiology of cholesterol granulomas requires further investigation and research [49]. On CT scans, cholesterol granulomas appear as smoothly marginated, expansile lesions with the same density as brain tissue and do not enhance with contrast. Differentiating cholesterol granulomas from petrous bone cholesteatomas on MRI is usually feasible since cholesterol granulomas typically exhibit a characteristic hyperintense signal on T1-weighted MRI images. This imaging feature aids in distinguishing them from other similar lesions. Treatment consists in drainage of the CH, and, as in the case described, after the surgical approach, no further interventions are required.
Primary extradural meningiomas (PEMs) are uncommon tumors. The pathogenesis of PEMs is not fully understood, and several hypotheses have been proposed. One possibility is that they originate from the nerve root segment where the arachnoid (the delicate membrane covering the brain and spinal cord) contacts the dura mater (the tough outer layer of the meninges). Another hypothesis suggests that PEMs might arise from aberrant arachnoid islets located in the epidural space. Alternatively, they may develop from the external surface of the dura mater and extend into the extradural space. Further research is needed to gain a deeper understanding of the exact mechanisms responsible for the development of PEMs [50,51]. A review of the literature showed only 11 cases of PEMs in the spheno-occipital region [51]. Meningioma is a surgically treated disease.
The optimal treatment for meningiomas is total resection with wide margins. In our case, the far lateral approach allowed the complete resection with no recurrence at the 5 years follow-up.
Ganglion cysts (GCs) are cystic formations that can be either single or multilocular. They form due to the degeneration and cystic softening of the connective tissue found in joint capsules and tendon sheaths. This degenerative process can occur as a result of either degenerative disease or trauma to the affected area [52]. Generally, GCs originate from the wrist and knuckles, but occasionally they occur in the spinal canal mainly in lumbar and sacral vertebrae. Odontoid localization is extremely rare, with about 50 cases reported so far [53,54]. For diagnosis and spinal decompression, surgery is mandatory. In our case, the complete resection through hemilaminectomy resulted in good postoperative neurological recovery and the patient was asymptomatic with no signs of relapse.
Histiocytic sarcoma (HS) is an extremely uncommon malignancy originating from hematopoietic cells, exhibiting both morphologic and immunophenotypic evidence of histiocytic differentiation [55]. This rare cancer predominantly occurs in lymph nodes, and its incidence in extranodal sites, such as the gastrointestinal tract, spleen, and soft tissues, is relatively low [56]. Involvement of the primary skull base is exceedingly rare, and to date, only one case of HS in the craniovertebral junction (CVJ), specifically the spheno-clival region, has been reported [57]. HS is an invasive tumor that has shown poor response to treatments, and there is currently no widely accepted effective therapy available for this condition [58].
In the literature, most reported cases of histiocytic sarcoma have undergone some combination of surgical intervention, radiation therapy, and/or chemotherapy as part of their treatment regimen. The specific treatment approach may vary depending on factors such as the tumor’s location, size, stage, and the patient’s overall health. However, given the rarity of HS and the limited data available, there is no standardized treatment protocol, and management decisions are often made on a case-by-case basis [58]. Treatment for our patient consisted of surgery followed by concurrent chemoradiation, ongoing at the time of writing.
As far as the surgical approaches to CVJ are concerned, the access to this region is still challenging due to its characteristics. Historically considered as a nobody’s land, the CVJ with its combination of noble and vital structures requires experienced surgeons and dedicated surgical instruments. The interdisciplinary alliance between otorhinolaryngologists and neurosurgeons is crucial to properly address CVJ pathologies [59].
Various surgical approaches have been proposed for the management of lesions in different regions of the craniovertebral junction (CVJ). The choice of approach depends on the specific location of the lesion [2,60].
For lesions on the ventral aspect of the CVJ, several approaches have been utilized, including the endoscopic endonasal approach (EEA), transoral approach (TOA), and submandibular approach (SMA) [60].
Lesions on the anterolateral aspect of the CVJ can be accessed through the posterior suboccipital approach (SOA) and the postero-lateral or far lateral approach (FLA). Extradural lesions in the same region may be addressed using an anterolateral or extreme lateral approach (ELA) [60]. Each of these surgical approaches provides access to different areas of the CVJ, allowing surgeons to tailor their approach based on the characteristics and location of the lesion. Finally, anterior odontoidectomy and ventral decompression of the craniocervical junction can be achieved through three different operative corridors: the transoral, endonasal, and transcervical approaches. Over the last decade, there has been increased interest in the endoscopic endonasal transclival transodontoid approach to the craniovertebral junction. This approach offers a minimally invasive surgical alternative, especially in cases of irreducible basilar invagination located above the palatine line or lower-lying, thanks to angled endoscopes and instrumentation. By avoiding the oral cavity, the endonasal route has the advantage of reducing complications such as tongue swelling, prolonged intubation, velopharyngeal insufficiency, and dysphagia, leading to a faster postoperative recovery.
When planning surgery, various factors must be considered, including the specific location of the clivus involved (superior, middle, or inferior part), the histology of the pathology, its lateral extension, and the structures involved. Additionally, the patient’s anatomy, previous treatments, need for follow-up therapies, and comorbidities should be taken into account. Therefore, the surgical approach should be tailored to each patient’s individual needs rather than chosen arbitrarily. Using multiple surgical corridors around the relevant anatomy allows the surgeon to avoid dissection passing through the plane of cranial nerves, which optimizes the resection and reduces morbidity. This patient-centered approach, combined with a comprehensive evaluation of various factors, ensures a more successful and tailored surgical outcome for each individual case.
The present paper confirms that due to the anatomic complexities of this region and the concrete possibility of unusual pathologies, not only is the cooperation between otorhinolaryngologists and neurosurgeons mandatory, but so is the presence of the multidisciplinary expertise of neuroradiologists, pathologists, radiotherapists, and oncologists to set up the appropriate treatment.
Besides some of the most common diseases of the CVJ, there are rare pathologies for which there are not many reports in the literature, and our experience, which cannot be exhaustive, may be useful to provide a perspective.
5. Conclusions
The CVJ can be the host of rare pathologies and, due to the presence of bony, muscular, vascular, and neural structures in narrow spaces, the approach to this area is a complex challenge. The multidisciplinary coworking is fundamental to offer the patient the best treatment.
The results obtained from this study cohort represent the collective experience of our institution, showcasing the collaborative efforts of our multidisciplinary team in managing patients undergoing craniovertebral junction (CVJ) surgery. The successful outcomes and insights gained from this approach highlight the importance of teamwork and coordinated efforts in providing optimal care for these patients.
Author Contributions
Conceptualization, C.Z. and E.M.; methodology, G.S.; validation, C.Z., F.S. and P.D.-M.; formal analysis, E.M.; writing—original draft preparation, E.M.; writing—review and editing, C.Z. and F.P.; visualization, A.M., E.P. and C.G.; supervision, C.Z. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study since it involved non-experimental data and adhered to standard clinical methods outlined in the literature.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kimura, F.; Kim, K.S.; Friedman, H.; Russell, E.J.; Breit, R.; Kimura, K.S.K.F.; Chaljub, G.; Van Fleet, R.; Guinto, F.C.; Crow, W.N.; et al. MR imaging of the normal and abnormal clivus. Am. J. Roentgenol. 1990, 155, 1285–1291. [Google Scholar] [CrossRef]
- Goel, A.; Cacciola, F. The Craniovertebral Junction, Diagnosis, Pathology, Surgical Techniques, 1st ed.; Thieme Medical Publishers, Inc.: Leipzig, Germany, 2011. [Google Scholar]
- Menezes, A.H. Decision Making for Management of Craniovertebral Junction Pathology. Oper. Tech. Neurosurg. 2005, 8, 125–130. [Google Scholar] [CrossRef]
- Tang, R.; Mao, S.; Li, D.; Ye, H.; Zhang, W. Treatment and Outcomes of Iatrogenic Cerebrospinal Fluid Leak Caused by Different Surgical Procedures. World Neurosurg. 2020, 143, e667–e675. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, H.Y.; Dhong, H.-J.; Chung, S.-K.; Kong, D.-S.; Nam, D.-H.; So, Y.K.; Hong, S.D. Delayed Cerebrospinal Fluid Leakage After Treatment of Skull Base Tumors: Case Series of 9 Patients. World Neurosurg. 2019, 132, e591–e598. [Google Scholar] [CrossRef]
- Ogawa, Y.; Tominaga, T. Delayed Cerebrospinal Fluid Leakage 10 Years after Transsphenoidal Surgery and Gamma Knife Surgery. Neurol. Med.-Chir. 2007, 47, 483–485. [Google Scholar] [CrossRef]
- Adel, M.; Chang, K.-P. Using a nasoseptal flap for the reconstruction of osteoradionecrosis in nasopharyngeal carcinoma: A case report. J. Otolaryngol.-Head Neck Surg. 2016, 45, 27. [Google Scholar] [CrossRef]
- Korchi, A.M.; Garibotto, V.; Lovblad, K.-O.; Haller, S.; Weber, D.C. Radiologic Patterns of Necrosis After Proton Therapy of Skull Base Tumors. Can. J. Neurol. Sci./J. Can. Sci. Neurol. 2013, 40, 800–806. [Google Scholar] [CrossRef][Green Version]
- Hongmei, Y.; Zhe, W.; Jing, W.; Daokui, W.; Peicheng, C.; Yongjie, L. Delayed cerebrospinal fluid rhinorrhea after gamma knife surgery in a patient with a growth hormone-secreting adenoma. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2012, 19, 900–902. [Google Scholar] [CrossRef]
- Risso, A.; Zoia, C.; Gianformaggio, C.; Pagella, F.; Pusateri, A.; Benazzo, M.; Gaetani, P. Tension pneumocephalus secondary to osteoradionecrosis of the clivus. Rep. Pract. Oncol. Radiother. J. Greatpoland Cancer Cent. Pozn. Pol. Soc. Radiat. Oncol. 2016, 21, 71–75. [Google Scholar] [CrossRef]
- Marx, R.E. Osteoradionecrosis: A new concept of its pathophysiology. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1983, 41, 283–288. [Google Scholar] [CrossRef]
- Wood, J.; Halen, J.V.; Samant, S.; Florendo, N. Radiation-induced sarcoma masquerading as osteoradionecrosis: Case report and literature review. J. Laryngol. Otol. 2015, 129, 279–282. [Google Scholar] [CrossRef]
- Veiceschi, P.; Arosio, A.D.; Agosti, E.; Bignami, M.; Pistochini, A.; Cerati, M.; Castelnuovo, P.; Locatelli, D. Symptomatic ecchordosis physaliphora of the upper clivus: An exceedingly rare entity. Acta Neurochir. 2021, 163, 2475–2486. [Google Scholar] [CrossRef]
- Vilela, M.D.; Pedrosa, H.A.; Filho, M.A.D. A Hemorrhagic Clival Chordoma with a Long Progression-Free Survival. World Neurosurg. 2017, 105, 1042.e1–1042.e4. [Google Scholar] [CrossRef]
- Ahn, S.S.; Han, J. Ecchordosis physaliphora presenting with abducens nerve palsy. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2016, 20, 266–268. [Google Scholar] [CrossRef]
- Ghimire, P.; Shapey, J.; Bodi, I.; Connor, S.; Thomas, N.; Barkas, K. Spontaneous tension pneumocephalus and pneumoventricle in ecchordosis physaliphora: Case report of a rare presentation and review of the literature. Br. J. Neurosurg. 2019, 34, 537–542. [Google Scholar] [CrossRef]
- Derakhshani, A.; Livingston, S.; William, C.; Lieberman, S.; Young, M.; Pacione, D.; Dehkharghani, S. Spontaneous, Intrasphenoidal Rupture of Ecchordosis Physaliphora with Pneumocephalus Captured During Serial Imaging and Clinical Follow-Up: Pathoanatomic Features and Management. World Neurosurg. 2020, 141, 85–90. [Google Scholar] [CrossRef]
- Sun, R.; Ajam, Y.; Campbell, G.; Masel, T. A Rare Case of Ecchordosis Physaliphora Presenting With Headache, Abducens Nerve Palsy, and Intracranial Hypertension. Cureus 2020, 12, e8843. [Google Scholar] [CrossRef]
- Otsuka, N.; Fukunaga, M.; Morita, K.; Ono, S.; Nagai, K.; Tomomitsu, T.; Yanagimoto, S.; Mimura, H.; Yamamoto, S.; Hirano, Y. Accumulation of99mTc-HM-PAO in photon deficient areas in bone scan of bone metastasis from hepatocellular carcinoma. Ann. Nucl. Med. 1992, 6, 215–220. [Google Scholar] [CrossRef]
- Aung, T.H.; Po, Y.C.; Wong, W.K. Hepatocellular carcinoma with metastasis to the skull base, pituitary gland, sphenoid sinus, and cavernous sinus. Hong Kong Med. J. Xianggang Yi Xue Za Zhi 2002, 8, 48. [Google Scholar]
- Escarda, A.; Vaquer, P.; Bonet, L.; Miralbés, S.; Gómez, C.; Obrador, A. Clivus metastasis from hepatocarcinoma associated with transarterial hepatic chemoembolization]. Gastroenterol. Y Hepatol. 2006, 29, 401–404. [Google Scholar] [CrossRef][Green Version]
- Kim, S.R.; Kanda, F.; Kobessho, H.; Sugimoto, K.; Matsuoka, T.; Kudo, M.; Hayashi, Y. Hepatocellular carcinoma metastasizing to the skull base involving multiple cranial nerves. World J. Gastroenterol. 2006, 12, 6727–6729. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.J.; Lee, H.W.; Choi, C.H.; Kim, J.U.; Do, J.H.; Kim, J.K.; Chang, S.K. Hepatocellular carcinoma with metastasis to the cavernous sinus of skull base causing pto-sis. Korean J. Gastroenterol. = Taehan Sohwagi Hakhoe Chi 2008, 52, 389–393. [Google Scholar] [PubMed]
- Isaka, T.; Nakagawa, H.; Suzuki, T.; Yamada, J.; Wada, K.; Kadota, T. Successful Removal of a Giant Skull Base Metastasis from Hepatocellular Carcinoma after Direct Ethanol Injection: Case Report. J. Neurol. Surg. Part B Skull Base 2000, 10, 081–086. [Google Scholar] [CrossRef][Green Version]
- Pallini, R.; Sabatino, G.; Doglietto, F.; Lauretti, L.; Fernandez, E.; Maira, G. Clivus metastases: Report of seven patients and literature review. Acta Neurochir. 2009, 151, 291–296. [Google Scholar] [CrossRef]
- Trivedi, P.; Gupta, A.; Pasricha, S.; Agrawal, G.; Shah, M. Isolated Skull Base Metastasis as the First Manifestation of Hepatocellular Carcinoma—A Rare Case Report with Review of Literature. J. Gastrointest. Cancer 2009, 40, 10–14. [Google Scholar] [CrossRef]
- Tamura, T.; Kawamura, Y.; Ikeda, K.; Seko, Y.; Fukushima, T.; Kumada, H.; Yamada, S.; Matumaru, Y. Hepatocellular carcinoma metastasis to the brain mimicking primary pituitary tumor around the sella turcica. Clin. J. Gastroenterol. 2013, 6, 319–325. [Google Scholar] [CrossRef]
- Cathel, A.; Khan, Y.R.; Blais, D.; Mahato, B.; Mahato, D. Metastatic Disease to Clivus: Biopsy or Not? Cureus 2019, 11, e5658. [Google Scholar] [CrossRef]
- Carey, R.A.B.; Nathaniel, S.; Das, S.; Sudhakar, S. Cavernous sinus syndrome due to skull base metastasis: A rare presentation of hepatocellular carcinoma. Neurol. India 2015, 63, 437. [Google Scholar] [CrossRef]
- Ram, A.; Paul, R.; Viswam, V.; Aravind, B. A Trigeminal Neuropathy From an Inactive Hepatocellular Carcinoma. Cureus 2021, 13, e20340. [Google Scholar] [CrossRef]
- Höpfel-Kreiner, I. Histogenesis of Hemangiomas—An Ultrastructural Study on Capillary and Cavernous Hemangiomas of the Skin. Pathol.-Res. Pract. 1980, 170, 70–90. [Google Scholar] [CrossRef]
- Smoller, B.R.; Apfelberg, D.B. Infantile (juvenile) capillary hemangioma: A tumor of heterogeneous cellular elements. J. Cutan. Pathol. 1993, 20, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.I.; Mural, M.; Rubino, F.; Lopez, E.S.; Cervio, A.; Olvi, L. Clivus Hemangioma in a Pediatric Patient: Case Report. World Neurosurg. 2019, 130, 512–515. [Google Scholar] [CrossRef]
- Moravan, M.; Petraglia, A.L.; Almast, J.; Yeaney, G.A.; Miller, M.C.; Vates, G.E. Intraosseous hemangioma of the clivus: A case report and review of the literature. J. Neurosurg. Sci. 2012, 56, 255–259. [Google Scholar] [PubMed]
- Grosu, A.L.; Nieder, C. Stereotactic Fractionated Radiotherapy for Recurrent Capillary Hemangioma of the Cavernous Sinus. Strahlenther. Onkol. Organ Der Dtsch. Rontgenges. 2006, 182, 179–182. [Google Scholar] [CrossRef]
- Tsao, M.N.; Schwartz, M.L.; Bernstein, M.; Halliday, W.C.; Lightstone, A.W.; Hamilton, M.G.; Jaywant, S.; Laperriere, N.; Almaghrabi, N.A.; Almaghrabi, A.; et al. Capillary hemangioma of the cavernous sinus. J. Neurosurg. 2003, 98, 169–174. [Google Scholar] [CrossRef]
- Morace, R.; Marongiu, A.; Vangelista, T.; Galasso, V.; Colonnese, C.; Giangaspero, F.; Innocenzi, G.; Esposito, V.; Cantore, G. Intracranial Capillary Hemangioma: A Description of Four Cases. World Neurosurg. 2012, 78, 191.e15–191.e21. [Google Scholar] [CrossRef]
- Jawad, N.; McHugh, K. The clinical and radiologic features of paediatric rhabdomyosarcoma. Pediatr. Radiol. 2019, 49, 1516–1523. [Google Scholar] [CrossRef]
- Unsal, A.A.; Chung, S.Y.; Unsal, A.B.; Baredes, S.; Eloy, J.A. A Population-Based Analysis of Survival for Sinonasal Rhabdomyosarcoma. Otolaryngol.-Head Neck Surg. 2017, 157, 142–149. [Google Scholar] [CrossRef]
- Larson, J.H.B.; Rutledge, R.P.-C.; Hunnell, L.P.-C.; Choi, D.K.; Kellogg, R.G.; Naran, S. Nasal Embryonal Rhabdomyosarcoma in the Pediatric Population: Literature Review and Report of Midline Presentation. Plast. Reconstr. Surg.-Glob. Open 2021, 9, e3534. [Google Scholar] [CrossRef]
- Li, X.; Peng, J.; Liu, Z.; Cui, Z.; Zhang, P.; Jin, H. Clinical analysis of 35 cases of adult rhabdomyosarcoma of nasal cavity and sinuses. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi = J. Clin. Otorhinolaryngol. Head Neck Surg. 2020, 34, 223–226. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, K.; Wang, J.; Wu, W.; Ma, L.; Huang, D. A Prospective Evaluation of the Combined Helical Tomotherapy and Chemotherapy in Pediatric Patients with Unresectable Rhabdomyosarcoma of the Temporal Bone. Cell Biochem. Biophys. 2014, 70, 103–108. [Google Scholar] [CrossRef]
- Talenti, G.; Picariello, S.; Robson, C.; Mertiri, L.; Russo, C.; Slater, O.; Bisdas, S.; Abate, M.E.; Perrotta, S.; Hewitt, R.; et al. Magnetic resonance features and cranial nerve involvement in pediatric head and neck rhabdomyosarcomas. Neuroradiology 2021, 63, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Baek, H.J.; Yoon, T.M.; Lee, J.K.; Lim, S.C. Endoscopic transpterygoid approach to a mass in a child. Int. J. Pediatr. Otorhinolaryngol. 2018, 105, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Shrirao, N.; Mukherjee, B.; Krishnakumar, S.; Biswas, J. Cholesterol granuloma: A case series & review of literature. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2015, 254, 185–188. [Google Scholar] [CrossRef]
- Isaacson, B. Cholesterol Granuloma and Other Petrous Apex Lesions. Otolaryngol. Clin. N. Am. 2015, 48, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Emanuelli, E.; Ciorba, A.; Bianchini, C.; Bossolesi, P.; Stomeo, F.; Pelucchi, S. Transnasal endoscopic management of petrous apex and clivus selected lesions. Eur. Arch. Oto-Rhino-Laryngol. 2012, 270, 1747–1750. [Google Scholar] [CrossRef]
- Sade, B.; Batra, P.S.; Scharpf, J.; Citardi, M.J.; Lee, J.H. Minimally Invasive Endoscopic Endonasal Management of Skull Base Cholesterol Granulomas. World Neurosurg. 2012, 78, 683–688. [Google Scholar] [CrossRef]
- Hernández-Estrada, R.A.; Kshettry, V.R.; Vogel, A.N.; Curtis, M.T.; Evans, J.J. Cholesterol granulomas presenting as sellar masses: A similar, but clinically distinct entity from craniopharyngioma and Rathke’s cleft cyst. Pituitary 2016, 20, 325–332. [Google Scholar] [CrossRef]
- Lai, C.Y.A.L.; Salkade, P.R.; Chuah, K.L.; Sitoh, Y.Y. Extradural cervical spinal meningioma mimicking malignancy. J. Radiol. Case Rep. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Shao, H.; Wang, C. Primary extradural meningiomas in head: A report of 19 cases and review of literature. Int. J. Clin. Exp. Pathol. 2015, 8, 5624–5632. [Google Scholar] [PubMed]
- Bruder, M.; Cattani, A.; Gessler, F.; Droste, C.; Setzer, M.; Seifert, V.; Marquardt, G. Synovial cysts of the spine: Long-term follow-up after surgical treatment of 141 cases in a single-center series and comprehensive literature review of 2900 degenerative spinal cysts. J. Neurosurg. Spine 2017, 27, 256–267. [Google Scholar] [CrossRef]
- Miyazawa, R.; Miyawaki, S.; Yamada, K.; Amemiya, S.; Ikemura, M.; Hinata, M.; Uchikawa, H.; Shiode, T.; Kin, T.; Takai, K.; et al. Retro-odontoid Pseudotumor: Two Cases of Intradural Ganglion Cysts Arising from the Odontoid Process with Syringobulbia. World Neurosurg. 2020, 144, 148–153. [Google Scholar] [CrossRef]
- Theodotou, C.B.; Urakov, T.M.; Vanni, S. Atlantoaxial Synovial Cyst: Case Report and Literature Review. World Neurosurg. 2016, 92, 588.e7–588.e15. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Nakamura, S. Histiocytic Sarcoma: An Updated Literature Review Based on the 2008 WHO Classification. J. Clin. Exp. Hematop. 2013, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hornick, J.L.; Jaffe, E.S.; Fletcher, C.D.M. Extranodal Histiocytic Sarcoma: Clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am. J. Surg. Pathol. 2004, 28, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Perez, I.; Gokden, M.; Day, J.D.; Yaziji, H.; Pina-Oviedo, S. Primary histiocytic sarcoma of the clivus with focal extension into central nervous system and neurologic manifestations: First description at an unusual site with an overwhelming and rapid progression. Clin. Neuropathol. 2021, 41, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Y.; Wang, W.; Xu, F.-L. Histiocytic sarcoma of the neck: A case report. Mol. Clin. Oncol. 2018, 9, 54–57. [Google Scholar] [CrossRef]
- Lopez, A.J.; Scheer, J.K.; Leibl, K.E.; Smith, Z.A.; Dlouhy, B.J.; Dahdaleh, N.S. Anatomy and biomechanics of the craniovertebral junction. Neurosurg. Focus 2015, 38, E2. [Google Scholar] [CrossRef] [PubMed]
- Visocchi, M.; Signorelli, F.; Parrilla, C.; Paludetti, G.; Rigante, M. Multidisciplinary approach to the craniovertebral junction. Historical insights, current and future perspectives in the neurosurgical and otorhinolaryngological alliance. Acta Otorhinolaryngol. Ital. Organo Uff. Della Soc. Ital. Di Otorinolaringol. E Chir. Cervico-Facciale 2021, 41 (Suppl. S1), S51–S58. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).