Uterine Smooth Muscle Tumor of Uncertain Malignant Potential: A Retrospective, Monocentric Cohort Study
Abstract
1. Introduction
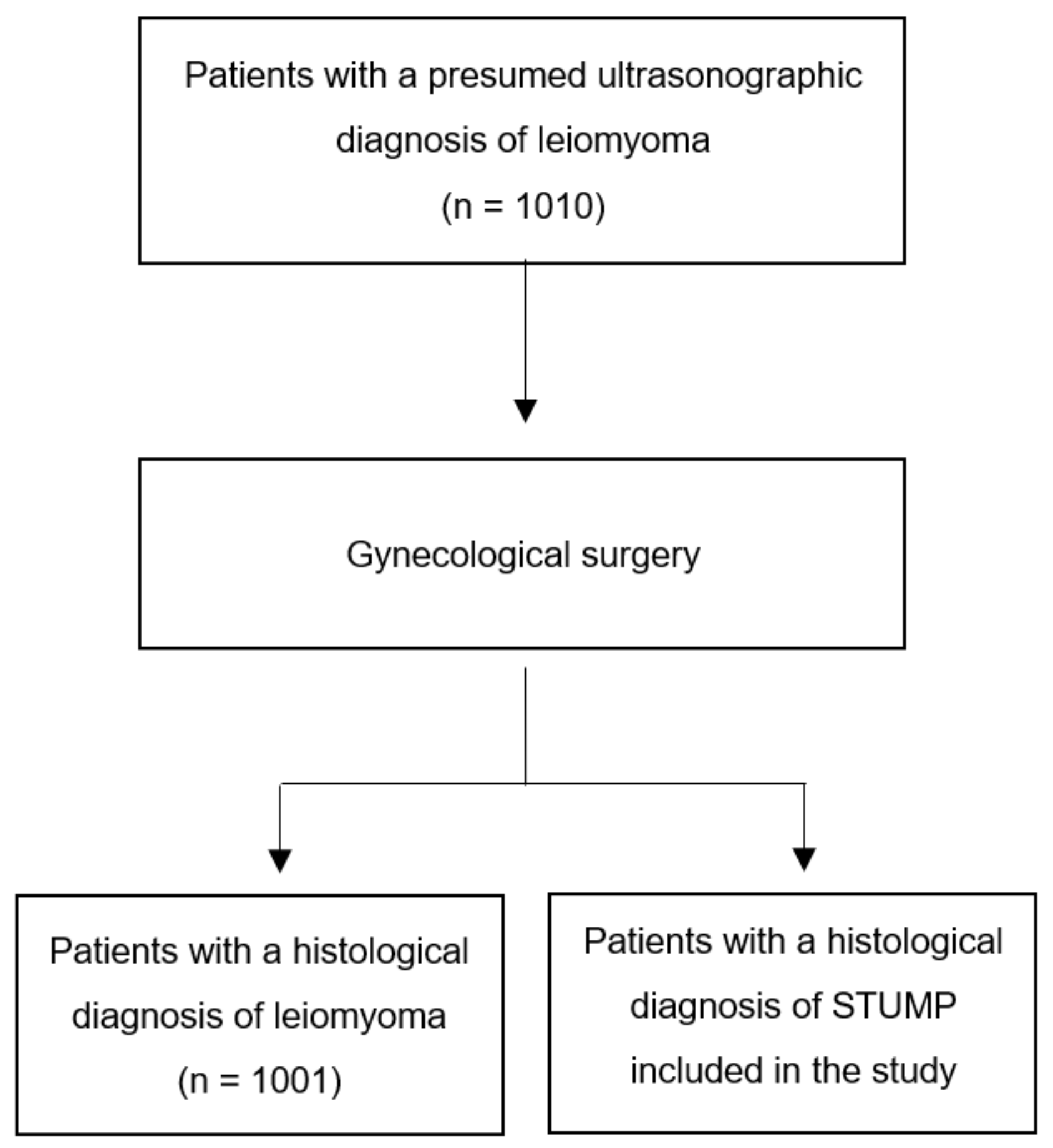
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dall’Asta, A.; Gizzo, S.; Musarò, A.; Quaranta, M.; Noventa, M.; Migliavacca, C.; Sozzi, G.; Monica, M.; Mautone, D.; Berretta, R. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): Pathology, follow-up, and recurrence. Int. J. Clin. Exp. Pathol. 2014, 7, 8136–8142. [Google Scholar] [PubMed]
- Croce, S.; Chibon, F. MED12 and uterine smooth muscle oncogenesis: State of the art and perspectives. Eur. J. Cancer 2015, 51, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Ura, B.; Monasta, L.; Arrigoni, G.; Battisti, I.; Licastro, D.; Di Lorenzo, G.; Romano, F.; Aloisio, M.; Peterlunger, I.; Stabile, G.; et al. Phosphoproteins Involved in the Inhibition of Apoptosis and in Cell Survival in the Leiomyoma. J. Clin. Med. 2019, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Gökaslan, H.; Türkeri, L.; Kavak, Z.N.; Eren, F.; Şişmanoğlu, A.; İlvan, Ş.; Durmuşoğlu, F. Differential diagnosis of smooth muscle tumors utilizing p53, pTEN and Ki-67 expression with estrogen and progesterone receptors. Gynecol. Obstet. Investig. 2005, 59, 36–40. [Google Scholar] [CrossRef]
- Bodner, K.; Bodner-Adler, B.; Kimberger, O.; Czerwenka, K.; Mayerhofer, K. Estrogen and progesterone receptor expression in patients with uterine smooth muscle tumors. Fertil. Steril. 2004, 81, 1062–1066. [Google Scholar] [CrossRef]
- Ciebiera, M.; Włodarczyk, M.; Wrzosek, M.; Męczekalski, B.; Nowicka, G.; Łukaszuk, K.; Ciebiera, M.; Słabuszewska-Jóźwiak, A.; Jakiel, G. Role of Transforming Growth Factor β in Uterine Fibroid Biology. Int. J. Mol. Sci. 2017, 18, 2435. [Google Scholar] [CrossRef]
- Uluer, E.T.; Inan, S.; Ozbilgin, K.; Karaca, F.; Dicle, N.; Sancı, M. The role of hypoxia related angiogenesis in uterine smooth muscle tumors. Biotech. Histochem. 2015, 90, 102–110. [Google Scholar] [CrossRef]
- Plewka, A.; Madej, P.; Plewka, D.; Kowalczyk, A.; Miskiewicz, A.; Wittek, P.; Leks, T.; Bilski, R. Immunohistochemical localization of selected pro-inflammatory factors in uterine myomas and myometrium in women of various ages. Folia Histochem. Cytobiol. 2013, 51, 73–83. [Google Scholar] [CrossRef]
- Conconi, D.; Redaelli, S.; Lissoni, A.A.; Cilibrasi, C.; Perego, P.; Gautiero, E.; Sala, E.; Paderno, M.; Dalprà, L.; Landoni, F.; et al. Genomic and Epigenomic Profile of Uterine Smooth Muscle Tumors of Uncertain Malignant Potential (STUMPs) Revealed Similarities and Differences with Leiomyomas and Leiomyosarcomas. Int. J. Mol. Sci. 2021, 22, 1580. [Google Scholar] [CrossRef]
- Machado-Lopez, A.; Simón, C.; Mas, A. Molecular and Cellular Insights into the Development of Uterine Fibroids. Int. J. Mol. Sci. 2021, 22, 8483. [Google Scholar] [CrossRef]
- Styer, A.K.; Rueda, B.R. The Epidemiology and Genetics of Uterine Leiomyoma. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 3–12. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, M.S.; Buchanan, E.M. Uterine Fibroids: Diagnosis and Treatment. Am. Fam. Physician 2017, 95, 100–107. [Google Scholar] [PubMed]
- Giuntoli, R.L., II; Metzinger, D.S.; DiMarco, C.S.; Cha, S.S.; Sloan, J.A.; Keeney, G.L.; Gostout, B.S. Retrospective review of 208 patients with leiomyosarcoma of the uterus: Prognostic indicators, surgical management, and adjuvant therapy. Gynecol. Oncol. 2003, 89, 460–469. [Google Scholar] [CrossRef]
- Abeler, V.M.; Røyne, O.; Thoresen, S.; Danielsen, H.E.; Nesland, J.M.; Kristensen, G.B. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 2009, 54, 355–364. [Google Scholar] [CrossRef]
- Devaud, N.; Vornicova, O.; Razak, A.R.A.; Khalili, K.; Demicco, E.G.; Mitric, C.; Bernardini, M.Q.; Gladdy, R.A. Leiomyosarcoma: Current Clinical Management and Future Horizons. Surg. Oncol. Clin. N. Am. 2022, 31, 527–546. [Google Scholar] [CrossRef]
- Bell, S.W.; Kempson, R.L.; Hendrickson, M.R. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am. J. Surg. Pathol. 1994, 18, 535–558. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of Female Reproductive Organs; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Bonneau, C.; Thomassin-Naggara, I.; Dechoux, S.; Cortez, A.; Darai, E.; Rouzier, R. Value of ultrasonography and magnetic resonance imaging for the characterization of uterine mesenchymal tumors. Acta Obstet. Gynecol. Scand. 2014, 93, 261–268. [Google Scholar] [CrossRef]
- Lin, G.; Yang, L.-Y.; Huang, Y.-T.; Ng, K.-K.; Ng, S.-H.; Ueng, S.-H.; Chao, A.; Yen, T.-C.; Chang, T.-C.; Lai, C.-H. Comparison of the diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted MRI in the differentiation between uterine leiomyosarcoma/smooth muscle tumor with uncertain malignant potential and benign leiomyoma. J. Magn. Reson. Imaging 2016, 43, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.C.; Di Legge, A.; Bonatti, M.; Manfredi, R.; Scambia, G. Imaging techniques for evaluation of uterine myomas. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.S.; Han, A.; Chew, S.H.; Low, J. A clinicopathologic study of uterine smooth muscle tumours of uncertain malignant potential (STUMP). Ann. Acad. Med. Singap. 2010, 39, 625–628. [Google Scholar] [CrossRef]
- Guntupalli, S.R.; Ramirez, P.T.; Anderson, M.L.; Milam, M.R.; Bodurka, D.C.; Malpica, A. Uterine smooth muscle tumor of uncertain malignant potential: A retrospective analysis. Gynecol. Oncol. 2009, 113, 324–326. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Prat, J. Uterine sarcomas: A review. Gynecol. Oncol. 2010, 116, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Incognito, G.G.; D’Urso, G.; Incognito, D.; Lello, C.; Miceli, A.; Palumbo, M. Management of a giant uterine smooth muscle tumor of uncertain malignant potential in a 32-year-old woman: Case report and review of the literature. Minerva Obstet. Gynecol. 2022, 74, 466–470. [Google Scholar] [PubMed]
- Ip, P.P.; Cheung, A.N.; Clement, P.B. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): A clinicopathologic analysis of 16 cases. Am. J. Surg. Pathol. 2009, 33, 992–1005. [Google Scholar] [PubMed]
- White, M.P.; Rahimi, S.; Garely, A.; Buhl, A.; Dean, R.M. Uterine smooth Muscle tumors of Uncertain Malignant Potential (STUMP): Review of Pathophysiology, Classification, diagnosis, treatment, and surveillance. J. Healthc. Commun. 2017, 2, 40. [Google Scholar]
- Hughes, L.; Roex, A.; Parange, A. STUMP, a surprise finding in a large fibroid uterus in a 20-year-old woman. Int. J. Women’s Health 2018, 10, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Vilos, G.A.; Marks, J.; Ettler, H.C.; Vilos, A.G.; Prefontaine, M.; Abu-Rafea, B. Uterine smooth muscle tumors of uncertain malignant potential: Diagnostic challenges and therapeutic dilemmas. Report of 2 cases and review of the literature. J. Minim. Invasive Gynecol. 2012, 19, 288–295. [Google Scholar]
- Macciò, A.; Chiappe, G.; Kotsonis, P.; Lavra, F.; Serra, M.; Demontis, R.; Madeddu, C. Abdominal leiomyosarcomatosis after surgery with external morcellation for occult smooth muscle tumors of uncertain malignant potential: A case report. Int. J. Surg. Case Rep. 2017, 38, 107–110. [Google Scholar]
- Rizzo, A.; Ricci, A.D.; Saponara, M.; De Leo, A.; Perrone, A.M.; De Iaco, P.; Nannini, M.A.P.A.M. Recurrent Uterine Smooth-Muscle Tumors of Uncertain Malignant Potential (STUMP): State of The Art. Anticancer Res. 2020, 40, 1229–1238. [Google Scholar]
- Canzonieri, V.; D’Amore, E.S.; Bartoloni, G.; Piazza, M.; Blandamura, S.; Carbone, A. Leiomyomatosis with vascular invasion. A unified pathogenesis regarding leiomyoma with vascular microinvasion, benign metastasizing leiomyoma and intravenous leiomyomatosis. Virchows Arch. 1994, 425, 541–545. [Google Scholar] [CrossRef]
- Han, A.K.W.; Hong, K.; Kim, M.; Kim, M.K.; Kim, M.L.; Jung, Y.W.; Yun, B.S.; Seong, S.J. Unexpected uterine smooth muscle tumor of uncertain malignant potential and sarcoma: A single center cohort study in South Korea. Taiwan. J. Obstet. Gynecol. 2020, 59, 275–281. [Google Scholar] [PubMed]
- Miettinen, M. Smooth muscle tumors of soft tissue and non-uterine viscera: Biology and prognosis. Mod. Pathol. 2014, 27 (Suppl. 1), S17–S29. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Zannoni, G.F. Uterine smooth muscle tumors of unknown malignant potential: A challenging question. Gynecol. Oncol. 2019, 154, 631–637. [Google Scholar] [PubMed]
- Atkins, K.A.; Arronte, N.; Darus, C.J.; Rice, L.W. The Use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am. J. Surg. Pathol. 2008, 32, 98–102. [Google Scholar] [CrossRef]
- Ip, P.P.; Tse, K.Y.; Tam, K.F. Uterine smooth muscle tumors other than the ordinary leiomyomas and leiomyosarcomas: A review of selected variants with emphasis on recent advances and unusual morphology that may cause concern for malignancy. Adv. Anat. Pathol. 2010, 17, 91–112. [Google Scholar]
- Berretta, R.; Rolla, M.; Merisio, C.; Giordano, G.; Nardelli, G.B. Uterine smooth muscle tumor of uncertain malignant potential: A three-case report. Int. J. Gynecol. Cancer 2008, 18, 1121–1126. [Google Scholar] [CrossRef]
| Patient | Age (Years) | Symptoms | Preoperative Imaging Features | Surgical Management | Histological Features | Medical Treatment | Follow-Up | Relapse |
|---|---|---|---|---|---|---|---|---|
| 1 | 71 | Menometrorrhagia causing fatigue, pelvic pain, bloating, and constipation | Solitary 4 cm round, hypoechoic intramural lesion with a clear whorled pattern in the posterior uterine wall | LPT hysterectomy | Endometrial cancer (pT1 pN0 G2 FIGO Stage IA) and STUMP | No | Normal | No |
| 2 | 32 | Menometrorrhagia causing weakness, pelvic pain, menstrual cramps, and lower abdominal fullness | Isolated 8 cm isoechoic, intramural mass with a whorled appearance in the anterior uterine wall | LPT myomectomy | Moderate atypia, <10 MFs/10 HPFs, no CTCN | No | Normal | No |
| 3 | 57 | Menometrorrhagia causing fatigue, pelvic pain, and painful bowel movements | Single, sizable 13 cm intramural growth, appearing as a round, well-circumscribed hypoechoic mass in the uterine fundus | LPT hysterectomy | <10 MFs/10 HPFs, CTCN, spindle cells = 30% | No | Clinically stable | Well-differentiated leiomyosarcoma at the left pararectal fossa and the right thigh 36 months after surgery |
| 4 | 36 | Menometrorrhagia with prolonged periods, pelvic pain, lower back pain, and bloating | Solitary 6 cm hypoechoic intramural lesion with a distinctive whorled texture in the posterior uterine wall | LPT myomectomy | Atypia, <10 MFs/10 HPFs, no CTCN | No | Normal | No |
| 5 | 50 | Menometrorrhagia with irregular menstrual cycle, pelvic pain intensified during periods, and dysuria | Lone, round, 4 cm hypoechoic intramural mass, displaying a characteristic whorled pattern in the anterior uterine wall | LPT hysterectomy | Focal atypia, <10 MFs/10 HPFs | No | Normal | No |
| 6 | 50 | Menometrorrhagia leading to anemia, pelvic pain, sensation of pelvic heaviness, and discomfort during intercourse | Single, well-defined 2 cm intramural lesion of hypoechoic texture with a whorled pattern in the right lateral uterine wall | LPS hysterectomy | Moderate atypia, <10 MFs/10 HPFs, no CTCN, Ki-67 = 5% | No | Normal | No |
| 7 | 28 | Menometrorrhagia causing tiredness and lightheadedness, with pelvic pain, menstrual cramps, and dysuria | Solitary 4.5 cm hypoechoic intramural mass with a clear whorled appearance in the left lateral uterine wall | LPT myomectomy | Moderate atypia, low mitotic count | No | Normal | No |
| 8 | 33 | Menometrorrhagia leading to anemia, pelvic pain, and difficulties emptying the bladder | Lone intramural growth of 7 cm, appearing as a well-circumscribed, hypoechoic mass with a typical whorled pattern in the anterior uterine wall | LPT myomectomy | GnRH agonists for 6 months after surgery | Myometrial lesion 10 months after surgery | Second surgery refused by the patient | |
| 9 | 53 | Menometrorrhagia leading to anemia and lower back discomfort | Solitary, large 10 cm intramural tumor, presenting as a hypoechoic mass with a distinct whorled texture in the uterine fundus | LPT hysterectomy | <5 MFs/10 HPFs | No | Normal | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ettore, C.; Incognito, G.G.; Gulino, F.A.; Russo, E.; Cannone, F.; Ettore, G. Uterine Smooth Muscle Tumor of Uncertain Malignant Potential: A Retrospective, Monocentric Cohort Study. Surgeries 2023, 4, 412-419. https://doi.org/10.3390/surgeries4030041
Ettore C, Incognito GG, Gulino FA, Russo E, Cannone F, Ettore G. Uterine Smooth Muscle Tumor of Uncertain Malignant Potential: A Retrospective, Monocentric Cohort Study. Surgeries. 2023; 4(3):412-419. https://doi.org/10.3390/surgeries4030041
Chicago/Turabian StyleEttore, Carla, Giosuè Giordano Incognito, Ferdinando Antonio Gulino, Emanuele Russo, Francesco Cannone, and Giuseppe Ettore. 2023. "Uterine Smooth Muscle Tumor of Uncertain Malignant Potential: A Retrospective, Monocentric Cohort Study" Surgeries 4, no. 3: 412-419. https://doi.org/10.3390/surgeries4030041
APA StyleEttore, C., Incognito, G. G., Gulino, F. A., Russo, E., Cannone, F., & Ettore, G. (2023). Uterine Smooth Muscle Tumor of Uncertain Malignant Potential: A Retrospective, Monocentric Cohort Study. Surgeries, 4(3), 412-419. https://doi.org/10.3390/surgeries4030041








