1. Introduction
Adjacent segment problems after cervical spine instrumentation are widely reported in the literature, with an incidence of 12% to 38% [
1,
2]. Both the proximal and the distal junction adjacent to the fixed levels can undergo degenerative changes, and these can range from adjacent segment degeneration (ASD), which is asymptomatic and noted only on radiographs, to adjacent segment disease and even adjacent segment instability, which typically require surgery [
3]. Studies have investigated both proximal and distal junctional ASD, and some studies focused mainly on the distal junction in terms of distal junctional kyphosis [
4,
5]. However, the majority of the available studies focused on ASD after anterior spine surgery [
2,
3,
6,
7], with few papers considering posterior cervical surgery [
8]. ASD from posterior spine surgery is unique in that, typically, in posterior spine surgery, longer segments are fused, and the decompression of neural elements may be more extensive, spanning longer segments or removing additional bony and ligamentous structures [
9,
10].
Another question that still remains about instrumentation in the posterior cervical spine is how much of this risk of ASD can be attributed to the surgical procedure rather than to a natural age-related degeneration. To date, no study has proven that a single risk factor correlates directly with this pathology, and we intended to identify the risk factors associated with adjacent segment problems. In addition, the highly mobile nature of the cervical spine brings with it challenges at every level. Previous studies have shown that stopping an instrumentation at C4 and C5, which is considered the apex of cervical lordosis, with highly mobile adjacent segments would cause problems [
11]. Biomechanical studies have also shown increased translation and rotational mobility at C2/3 and C7/T1; hence, the concern would be that should the upper or lower instrumented level stop at these levels, this would be a risk factor for the occurrence of adjacent segment degeneration [
12]. Our study aimed to retrospectively look at all the factors involved, including pre-, intra- and post-operative factors, and present data on the incidence and risk factors of ASD and on its progression to symptomatic adjacent segment disease after posterior cervical fusion surgery.
2. Materials and Methods
A retrospective analysis was conducted to assess the prognosis of patients who had undergone posterior instrumentation of the cervical spine between January 2014 and December 2017 at our institute. This study with reference number 2019/00021 was approved by the National Healthcare Group (NHG) Domain Specific Review Board (DSRB), Singapore, and a waiver of informed consent was granted. Based on the inclusion criteria, we recruited all individuals who underwent posterior instrumentation of the cervical spine and were subjected to relevant radiological investigations (either X-rays or computed tomography [CT] scans) pre- and post-operatively at 1, 3, 6, 12, 24 months. The follow-up duration was 24 months, and the patients must have satisfied a minimum follow-up of 6 months to be included in the study. As for the exclusion criteria, we did not consider patients with underlying inflammatory conditions such as rheumatoid arthritis, as well as patients who had multi-level disease and underwent instrumentation which clearly did not address the upper/lower levels. The demographic data recorded for each participant at the beginning of the study included age, gender, body mass index (BMI), Charlson comorbidity index, and smoking history.
2.1. Pre- and Intra-Operative Variables
We recorded each patient’s preoperative cervical lordosis, C2–7 sagittal vertical axis (SVA), disc heights above and below the levels planned for instrumentation, and the segmental angle of the instrumented vertebrae. We also recorded the number and location of the levels operated on, the type of implant, the extent of decompression performed, the levels of fusion, the technique of placing screws (freehand or navigated), and the type of screws (lateral mass, pedicle, pars, etc.) placed.
2.2. Outcome Variables
We recorded the outcome variables at 1, 3, 6, 12, 24 months post-operatively. Two separate blinded consultants analyzed plain X-rays or CT images looking for evidence of adjacent segment degeneration or signs of adjacent segment instability. We recorded post-operative cervical lordosis, post operative C2–7 SVA, and disc heights above and below the levels of fusion. The disc height measurements were given importance as important pathologies such as kyphosis or instability directly affect the disc height and are reflected in the measurements. As such, the T1 slope, which is a variable of interest correlating to distal junctional kyphosis, was not included into the analysis due to a lack of data. Apart from these, we took note of complications that occurred within the 2 years of follow-up, which included implant loosening noted on plain films and revision surgery resulting from implant-related complications.
2.3. Definition of the Cervical Parameters
Cervical lordosis was defined as the cobb angle between the lower endplates measured from C2 to C7 (
Figure 1a) [
13]. C2–7 SVA was the distance from the posterosuperior corner of C7 to a vertical line from the center of the C2 vertebrae (
Figure 1b) [
13]. The disc height was measured at the midpoint of two adjacent endplates (
Figure 1c) [
14]. In cases where the X-rays were not clear (predominantly, for lower cervical and upper thoracic region), the disc heights were measured on CT scans.
Adjacent segment degeneration was defined by new degenerative changes at a spinal level adjacent to a surgically treated level. We referred to Alhashash et al.’s interpretation of adjacent segment degeneration as the presence of loss of disc height, foraminal stenosis, or osteophyte formation [
11]. Any of these changes being present would indicate adjacent segment degeneration. The loss of disc height was defined by a greater than 25% loss of height compared with pre-operative parameters, as per the modified Matsumoto’s classification [
15].
We also looked for adjacent segment instability. As described by Alizada et al. [
14], it was defined as any increase in the cervical segmental curvature index (difference between the heights of the anterior and posterior intervertebral spaces divided by the height of the inferior adjacent vertebral body) or the presence of an angular or horizontal displacement on adjacent levels.
2.4. Statistical Analysis
Statistical analysis was performed using Stata 13 (StataCorp, College Station, TX, USA). Categorical variables are presented as numbers and percentage, while continuous variables are presented as mean ± SD (standard deviation). We used the 2 × 2 Fischer’s exact test to determine the statistical significance for discontinuous variables between the two groups, while the t-test was used to determine the significance for values following a normal distribution. A p-value of <0.05 was deemed as statistically significant.
3. Results
A total of 147 patients met the initial criteria. After exclusion, 87 patients were recruited. The majority of those excluded were not considered due to a lack of adequate follow-up data. Of the 87 patients included, 26 (29.9%) were found to have signs of ASD. One patient required revision surgery for symptomatic adjacent segment disease with cord compression. No instability was noted in any patient.
Figure 2 shows a flowchart of the patient recruitment and follow-up.
3.1. Patient Demographics
There were 21 females (24.2%) and 66 males (75.8%). The mean age was 65.4 years for the group with ASD and 62.7 for the group without ASD. The Charlson comorbidity index was 3 for the group with ASD and 2.5 for the group without ASD. The mean body mass index (BMI) of both groups was comparable, at 25.7. The demographic information of our study population is summarized in
Table 1.
3.2. Pre-Operative Parameters: Pre-Operative Cervical Lordosis and Indication for Surgery
The pre-operative cervical lordosis was 3.6 ± 12.1 degrees for patients with signs of ASD and 10.5 ± 12.8 degrees for patients without ASD. This result was statistically significant, with
p = 0.02. We also further stratified the patients according to the indication for surgery and its relationship with patients developing ASD. Seventy-three patients underwent posterior cervical spine surgery for degenerative conditions, eleven patients for trauma-related reasons, and three patients for instability-related conditions. None of the patients who underwent surgery for trauma- or instability-related conditions developed ASD. We found that 26 patients (35.6%) who underwent surgery for degenerative conditions developed ASD, while 47 (64.4%) patients in this sub-group did not develop ASD. The pre-operative parameters are presented in
Table 2.
3.3. Surgical Parameters: Does the Technique of Screw Placement or the Extent of Decompression Affect the Development of ASD?
In the cohort examined, 55 patients underwent surgery with screws placed via free-hand techniques using intraoperative imaging X-rays, while 32 patients had screws placed using O-arm navigation, performed with an intraoperative computerized tomography scan of the cervical spine. We found that 16 of the 55 (29.1%) patients with free-hand screws developed ASD, and 10 of the 22 (45.5%) patients with navigated screws developed ASD. The technique of screw placement did not significantly affect the development of ASD. There was also no significant difference between the number of levels fused and the extent of decompression when comparing the patients with ASD and those without ASD. The mean number of levels fused for the patients with ASD was 4.1 ± 0.9, while that for the patients without ASD was 4.5 ± 1.6. The mean number of levels of decompression performed for the patients with ASD was 3.8 ± 1.1, while that for the patients without ASD was 3.9 ± 1.5. These results are summarized in
Table 3.
3.4. Surgical Parameters: Does the Extent of Fusion Affect the Development of ASD?
Seventy-eight patients had cervical-only fusions (instrumentation limited up to C7), while nine patients had their instrumentation extending down to the cervico-thoracic region. We found that 25 of the 78 (47.2%) patients with cervical-only fusions developed ASD, while 1 of the 9 (12.5%) patients with cervico-thoracic fusions developed ASD.
When analyzing the individual levels of cervical fusion, 19 patients had their upper or lower fusion segment ending at C4 or C5. Of these 19 patients, 8 developed ASD (42.1%). The remaining 68 patients had a cervical instrumentation and fusion that spanned across the apex of the normal cervical lordosis (i.e., the instrumentation extended to C2, C6, or C7). We found that 18 of the remaining 68 patients (26.5%) with their upper or lower fusion ending at C3, C6, or C7 developed ASD. However, this result was not statistically significant, with a p value of 0.25.
3.5. Post-Operative Parameters: C2–7 SVA and Disc Heights
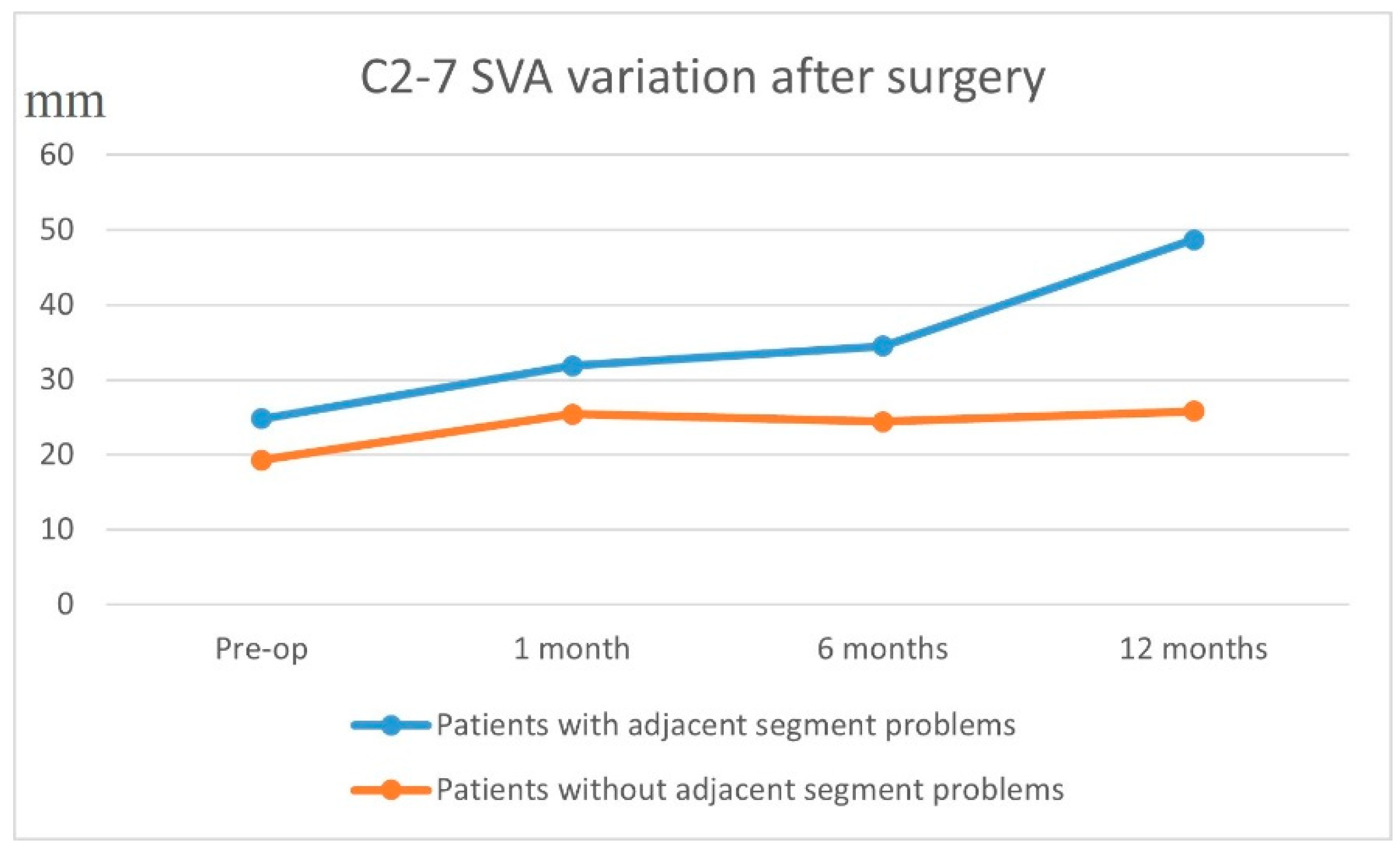
We analyzed the preoperative vs. postoperative C2–7 SVA and disc heights to see if changes in these parameters were correlated with a higher chance of adjacent segment disease. Patients with ASD had a higher pre-op C2–7 SVA of 24.8 ± 15 mm, while those without ASD had a pre-op C2–7 SVA of 19.3 ± 14.6 mm (
Figure 3). At 1-year post-op, the patients with ASD had a C2–7 SVA of 48.7 ± 68.8 mm, whilst those without ASD had a C2–7 SVA of 25.8 ± 15.3 mm (
p = 0.01).
Pre-op disc height changes above and below the fusion levels were comparable between the two groups, with an average disc height above the fusion level of 6.1 ± 1.1 mm for the patients with ASD and of 5.9 ± 1.0 mm for those without ASD, and a disc height below the fusion level of 5.2 ± 1.5 mm for the patients with ASD and of 5.7 ± 1.3 mm for those without ASD. At 1-year post-op, the disc height above fusion level for the patients with ASD was 5.7 ± 1.1 mm, and that for those without ASD remained at 5.9 ± 1.0 mm. Similarly, the disc height below the fusion level for the patients with ASD showed a minimal drop to 4.8 ± 1.3 mm, and that for those without ASD remained at 5.7 ± 1.3 mm. These changes were non-significant in comparison to the values measured preoperatively. The surgical and post-operative parameters are presented in
Table 3.
Of the 26 patients with adjacent segment degeneration, 1 patient underwent a revision operation. The revision operation was performed in a patient with previous instrumentation from C3 to T2, who subsequently developed symptomatic adjacent level disease at C1/C2 with cord compression. The instrumentation was revised up to C1.
3.6. Multivariate Binary Logistic Regression Analysis
Upon performing a multivariate binary logistic regression with the dependent variable being the outcome of ASD and the covariates being age, gender, Charlson comorbidity index, indication of spondylosis, BMI, pre-op cervical lordosis, C2–7 SVA, no of levels decompression, number of levels fused, and disc height above and below the fusion levels, it was found that none of these variables were independent risk factors of ASD (
Table 4).
Even though reduced preoperative cervical lordosis and the indication of degenerative spondylosis may have shown an influence over occurrence of ASD according to our univariate analysis, the multivariate regression analysis did not identify them as independent risk factors.
4. Discussion
This study aimed to identify the risk factors for ASD following the posterior instrumentation of the cervical spine. One interesting finding of this study is that patients with a smaller degree of preoperative cervical lordosis were associated with a higher incidence of ASD. This is in line with the evidence highlighted by Scheer et al. [
16] and Zhang et al. [
17], who concluded that a pre-existing loss of normal cervical lordosis is a risk factor for developing ASD. This was observed in patients who underwent anterior surgery. No comparative studies on ASD after posterior cervical spine surgery were found. In our two groups of patients with and without ASD, a relatively minor difference of 6.9° in cervical lordosis yielded a significant outcome, whether or not they developed ASD (3.6° vs. 10.5°), which suggests the importance of each degree of restoration of cervical lordosis to minimize ASD. However, this must be interpreted with caution, since our multivariate regression did not identify any independent risk factors.
4.1. Patient Demographics and Incidence of ASD after Posterior Cervical Spine Surgery
Our study found an incidence of ASD associated with posterior cervical spine surgery of about 30% at 2 years. This is slightly lower than the incidence determined in other studies which examined ASD after anterior cervical spine surgery, with Matsumoto et al. [
15] reporting a prevalence of ASD of 42.9–45.3%, and Kim et al. [
18] showing a prevalence of ASD of 40.74%. However, we do know that adjacent segment disease occurs in anterior cervical procedures at an average rate of 2.9% per year [
19], and our study followed up patients only up to 2 years. It is likely that a longer follow-up may have given a clearer picture regarding the exact incidence. Other demographic parameters such as age, Charlson comorbidity index, and patient’s body mass index did not have any significant impact on the development of adjacent segment disease.
Another important finding of our study is that all cases of adjacent segment degeneration were patients who underwent surgery for degenerative conditions such as cervical spondylosis. Patients who underwent posterior cervical fusion for trauma-related conditions such as fractures or ligamentous injuries did not develop adjacent segment disease. This is in agreement with other studies that showed that ASD develops as a consequence of the natural history of progression of degenerative changes combined with the loss of mobility of the fused and instrumented levels [
11]. It is possible that these patients had already undergone degenerative changes at the adjacent levels, which were not apparent on imaging, and surgery accelerated the degenerative process.
4.2. C2–7 SVA and ASD
When looking at the surgical parameters that may affect adjacent segment degeneration, post-op C2–7 SVA is an important marker that tells a clinician the sagittal alignment after fusion and instrumentation. We found that patients with signs of ASD had a greater (more lordotic) post-op C2–7 SVA (48.7 ± 68.8 mm) at 1 year compared to the group with ASD (25.8 ± 15.3 mm). Many papers have emphasized the importance of restoring the sagittal alignment in patients after posterior cervical fusion [
20,
21], and having a lower C2–7 SVA prevents cervical spine decompensation, which minimizes the development of ASD. On the basis of our data, we believe that an adequate restoration of the C2–7 SVA is essential and that aiming for a lower C2–7 SVA, which needs to be maintained throughout the follow-up, would be protective against developing ASD.
4.3. Levels of Fusion and ASD
Surgical factors that were previously identified to influence ASD (mean number of segments fused, cervical-only fusions versus cervico-thoracic fusions, stopping the fusion at the level C5) did not turn out to be significant risk factors in our cohort [
1]. Though the result was not significant, we did notice a trend toward fewer cases of ASD in the group with cervico-thoracic fusions or fusions extending longer (to C2, C6 or C7), and this was likely due to the extension of the instrumentation and fusion across longer segments and the avoidance of more mobile segments at C4 or C5. In addition, the placement of cervical screws via the free-hand or the navigated technique did not make any significant difference with respect to the development of adjacent segment degeneration.
4.4. Is the Upper or the Lower Segment More Affected by ASD?
We further stratified the patients according to the levels of adjacent segment degeneration to determine if which level would be more affected. On comparing the 1-year disc heights between patients with and without ASD, statistical significance was noted. However, given the minimal loss found in comparison to that measured in the pre-operative status, the clinical importance of this observation is questionable. One additional factor that was not measured in this study is junctional kyphosis, both proximal and distal. This measurement might have provided a clearer picture to answer the question of whether the upper or the lower segment is more affected; hence, this should be considered as a limitation of this study.
4.5. Adjacent Segment Degeneration Versus Adjacent Segment Disease
A distinction should be made between adjacent segment degeneration (asymptomatic, radiographic findings only) and symptomatic adjacent segment disease. In our study, one case required revision surgery to address the problem of adjacent segment disease. The majority of our patients were either asymptomatic or had minor symptoms; thus, even if there were radiographic features of ASD, this would likely not progress to symptomatic adjacent segment disease in the short time.
4.6. Limitations
There are several limitations to this study. Firstly, adjacent segment disease is known to occur even many years after fusion surgery, and hence, our follow up results at 24 months may not have captured the full spectrum of patients who would develop ASD after posterior instrumentation of the cervical spine [
22]. In addition, ideally, we should have performed a post-operative magnetic resonance imaging at each follow-up for a better evaluation of the patients’ ASD, as this would have allowed us to determine the extent of disc degeneration and disc protrusion and the presence of foraminal stenosis as outlined by Matsumoto et al. [
15]. Regarding the measurements, we considered C2–C7 SVA and cervical lordosis measured at one month as the post-operative measurement. In both these measurements, we noted there was not much change in the measured angles. With this data, we are not able to directly answer if excessive or under-corrected cervical lordosis or SVA is a definite risk factor for ASD. Lastly, we did not analyze implant-related factors such as length of the screws, types of screws, and the use of biologics to augment fusion, which may have influenced the occurrence of ASD.
5. Conclusions
ASD was found to be present in about 30% of our selected cohort who underwent posterior instrumentation of the cervical spine. Even though, according to our univariate analysis, reduced pre-operative cervical lordosis and the indication of degenerative spondylosis seemed to significantly influence the occurrence of ASD, multivariate regression analysis did not identify any independent risk factors. However, we noted a worsening of the cervical sagittal profile as indicated by the C2–7 SVA measurements at 1 year follow-up in the patients with ASD. This highlights the importance of restoring the cervical sagittal profile, which needs to be maintained throughout the follow-up to reduce the risk of ASD. We also noted that, even though patients may develop ASD after the instrumented fusion of the cervical spine, this may not necessarily develop into symptomatic adjacent segment disease requiring revision surgery.
Author Contributions
Conceptualization, W.M.Q.Y., L.Q.T., D.D.L.L., A.-K.K.-P., C.P.N. and J.Y.-L.O.; methodology, W.M.Q.Y., L.Q.T., D.D.L.L. and A.-K.K.-P.; formal analysis, W.M.Q.Y., L.Q.T., D.D.L.L. and A.-K.K.-P.; resources, W.M.Q.Y., C.P.N. and J.Y.-L.O.; data curation, W.M.Q.Y., L.Q.T. and D.D.L.L.; writing—original draft preparation, W.M.Q.Y., L.Q.T., D.D.L.L. and A.-K.K.-P.; writing—review and editing, A.-K.K.-P., C.P.N. and J.Y.-L.O.; supervision, C.P.N. and J.Y.-L.O.; project administration, J.Y.-L.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the National Healthcare Group (NHG), Domain Specific Review Board (DSRB), Singapore (2019/00021).
Informed Consent Statement
The requirement for informed consent was waived as this is a retrospective registry-based study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Helgeson, M.D.; Bevevino, A.J.; Hilibrand, A.S. Update on the evidence for adjacent segment degeneration and disease. Spine J. 2013, 13, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Cao, J.; Wang, L.; Shen, Y. Prevalence of adjacent segment disease following cervical spine surgery: A PRISMA-compliant systematic review and meta-analysis. Medicine 2016, 95, e4171. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.D.; McCowin, P.R.; Davis, D.O.; Dina, T.S.; Mark, A.S.; Wiesel, S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J. Bone Jt. Surg. Am. 1990, 72, 1178–1184. [Google Scholar] [CrossRef]
- Lee, J.J.; Park, J.H.; Oh, Y.G.; Shin, H.K.; Park, B.G. Change in the Alignment and Distal Junctional Kyphosis Development after Posterior Cervical Spinal Fusion Surgery for Cervical Spondylotic Myelopathy-Risk Factor Analysis. J. Korean Neurosurg. Soc. 2022, 65, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Passias, P.G.; Vasquez-Montes, D.; Poorman, G.W.; Protopsaltis, T.; Horn, S.R.; Bortz, C.A.; Segreto, F.; Diebo, B.; Ames, C.; Smith, J.; et al. Predictive model for distal junctional kyphosis after cervical deformity surgery. Spine J. 2018, 18, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Riew, K.D. Adjacent segment disease following cervical spine surgery. J. Am. Acad. Orthop. Surg. 2013, 21, 3–11. [Google Scholar] [CrossRef]
- Ishihara, H.; Kanamori, M.; Kawaguchi, Y.; Nakamura, H.; Kimura, T. Adjacent segment disease after anterior cervical interbody fusion. Spine J. 2004, 4, 624–628. [Google Scholar] [CrossRef]
- Badiee, R.K.; Mayer, R.; Pennicooke, B.; Chou, D.; Mummaneni, P.V.; Tan, L.A. Complications following posterior cervical decompression and fusion: A review of incidence, risk factors, and prevention strategies. J. Spine Surg. 2020, 6, 323–333. [Google Scholar] [CrossRef]
- Dohrmann, G.J.; Hsieh, J.C. Long-term results of anterior versus posterior operations for herniated cervical discs: Analysis of 6000 patients. Med. Princ. Pr. 2014, 23, 70–73. [Google Scholar] [CrossRef]
- Yonenobu, K.; Oda, T. Posterior approach to the degenerative cervical spine. Eur. Spine J. 2003, 12 (Suppl. 2), S195–S201. [Google Scholar] [CrossRef]
- Alhashash, M.; Shousha, M.; Boehm, H. Adjacent Segment Disease After Cervical Spine Fusion: Evaluation of a 70 Patient Long-Term Follow-Up. Spine (Phila Pa 1976) 2018, 43, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Frobin, W.; Leivseth, G.; Biggemann, M.; Brinckmann, P. Sagittal plane segmental motion of the cervical spine. A new precision measurement protocol and normal motion data of healthy adults. Clin. Biomech. 2002, 17, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ames, C.P.; Blondel, B.; Scheer, J.K.; Schwab, F.J.; Le Huec, J.C.; Massicotte, E.M.; Patel, A.A.; Traynelis, V.C.; Kim, H.J.; Shaffrey, C.I.; et al. Cervical radiographical alignment: Comprehensive assessment techniques and potential importance in cervical myelopathy. Spine (Phila Pa 1976) 2013, 38, S149–S160. [Google Scholar] [CrossRef]
- Alizada, M.; Li, R.R.; Hayatullah, G. Cervical instability in cervical spondylosis patients: Significance of the radiographic index method for evaluation. Orthopade 2018, 47, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Okada, E.; Ichihara, D.; Watanabe, K.; Chiba, K.; Toyama, Y.; Fujiwara, H.; Momoshima, S.; Nishiwaki, Y.; Iwanami, A.; et al. Anterior cervical decompression and fusion accelerates adjacent segment degeneration: Comparison with asymptomatic volunteers in a ten-year magnetic resonance imaging follow-up study. Spine (Phila Pa 1976) 2010, 35, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.K.; Tang, J.A.; Smith, J.S.; Acosta, F.L., Jr.; Protopsaltis, T.S.; Blondel, B.; Bess, S.; Shaffrey, C.I.; Deviren, V.; Lafage, V.; et al. Cervical spine alignment, sagittal deformity, and clinical implications: A review. J. Neurosurg. Spine 2013, 19, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, Y.; Liu, H.; Zhang, J.; He, F.; Chen, A.; Yang, H.; Pi, B. Association between sagittal balance and adjacent segment degeneration in anterior cervical surgery: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 430. [Google Scholar] [CrossRef]
- Kim, S.W.; Limson, M.A.; Kim, S.B.; Arbatin, J.J.; Chang, K.Y.; Park, M.S.; Shin, J.H.; Ju, Y.S. Comparison of radiographic changes after ACDF versus Bryan disc arthroplasty in single and bi-level cases. Eur. Spine J. 2009, 18, 218–231. [Google Scholar] [CrossRef]
- Hilibrand, A.S.; Carlson, G.D.; Palumbo, M.A.; Jones, P.K.; Bohlman, H.H. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J. Bone Jt. Surg. Am. 1999, 81, 519–528. [Google Scholar] [CrossRef]
- Katsuura, A.; Hukuda, S.; Saruhashi, Y.; Mori, K. Kyphotic malalignment after anterior cervical fusion is one of the factors promoting the degenerative process in adjacent intervertebral levels. Eur. Spine J. 2001, 10, 320–324. [Google Scholar] [CrossRef]
- Xing, R.; Liu, W.; Li, X.; Jiang, L.; Yishakea, M.; Dong, J. Characteristics of cervical sagittal parameters in healthy cervical spine adults and patients with cervical disc degeneration. BMC Musculoskelet. Disord. 2018, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Litrico, S.; Lonjon, N.; Riouallon, G.; Cogniet, A.; Launay, O.; Beaurain, J.; Blamoutier, A.; Pascal-Mousselard, H.; French Society of Spine, S. Adjacent segment disease after anterior cervical interbody fusion: A multicenter retrospective study of 288 patients with long-term follow-up. Orthop. Traumatol. Surg. Res. 2014, 100, S305–S309. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).