Abstract
Treatment of basilar artery trunk aneurysms is still challenging today, although numerous approaches and modalities of treatment exist. The authors present a case of a patient with a partially thrombosed, ruptured basilar trunk artery aneurysm successfully treated by clipping occlusion of the rupture point and multilayered wrapping of the aneurysmal dome. A 49-year-old man presented to our emergency room with a chief complaint of altered mental status. The patient was diagnosed with subarachnoid hemorrhage (SAH). No apparent bleeding point was identified on initial 3-dimension computerized tomography (CT) angiography and digital subtraction angiography (DSA). Follow-up DSA revealed a partially thrombosed saccular aneurysm emerging from the basilar trunk. We decided to treat the aneurysm surgically with partial clipping including the bleb and wrapping via the anterior transpetrosal approach. The surgery was performed successfully without any complications, and the residual blood flow within the aneurysm diminished remarkably over time. Although direct clipping and wrapping for basilar trunk artery aneurysms is one of the most challenging operations, it is a highly effective treatment for complex aneurysms, especially if other treatments are not available.
1. Introduction
Ruptured basilar trunk aneurysms can lead to devastating outcomes, even in the modern era. The aneurysms are close to the critical structures and direct surgery for the lesion requires complex skull base procedures [1]. In addition, in certain cases with large aneurysm size, and with the location of the aneurysm protruding to the lateral side, the proximal vascular control is complicated [2]. The indication of the surgery is limited as the operation is highly complex and alternate treatments exist. The endovascular treatment for cerebral aneurysms has developed drastically in recent years [3].
The use of a flow diverter and stent-assisted coil embolization have been reported for treatment of basilar aneurysms [4,5]. However, serious complications are known to occur, such as brainstem infarction. The treatment modality for this particular lesion should be well considered [6].
When complete occlusion of the aneurysm is difficult, wrapping of the aneurysm is an additional or alternate treatment option. The optimal material to wrap the aneurysm has not yet been determined.
We herein present a case of a patient with a partially thrombosed, ruptured basilar trunk artery aneurysm treated via the anterior transpetrosal approach utilizing the skull base technique. Clipping occlusion of the rupture point and wrapping of the aneurysmal dome was successfully achieved.
2. Case Report
A 49-year-old man presented to our emergency room with a chief complaint of altered mental status. The patient’s Glasgow Coma Scale (GCS) score was 10 points and he had no apparent motor deficit on examination. The patient had no family whom we could contact in order to obtain his medical and social background. His baseline modified Rankin Scale (mRS) was 0. A plain computerized tomography (CT) of the head showed diffuse subarachnoid hemorrhage (SAH) (Figure 1a,b). Modified Fisher Scale was 3. 3-dimension CT angiography (3D-CTA) and subsequent digital subtraction angiography (DSA) revealed the pearl and string sign of the right vertebral artery (VA).
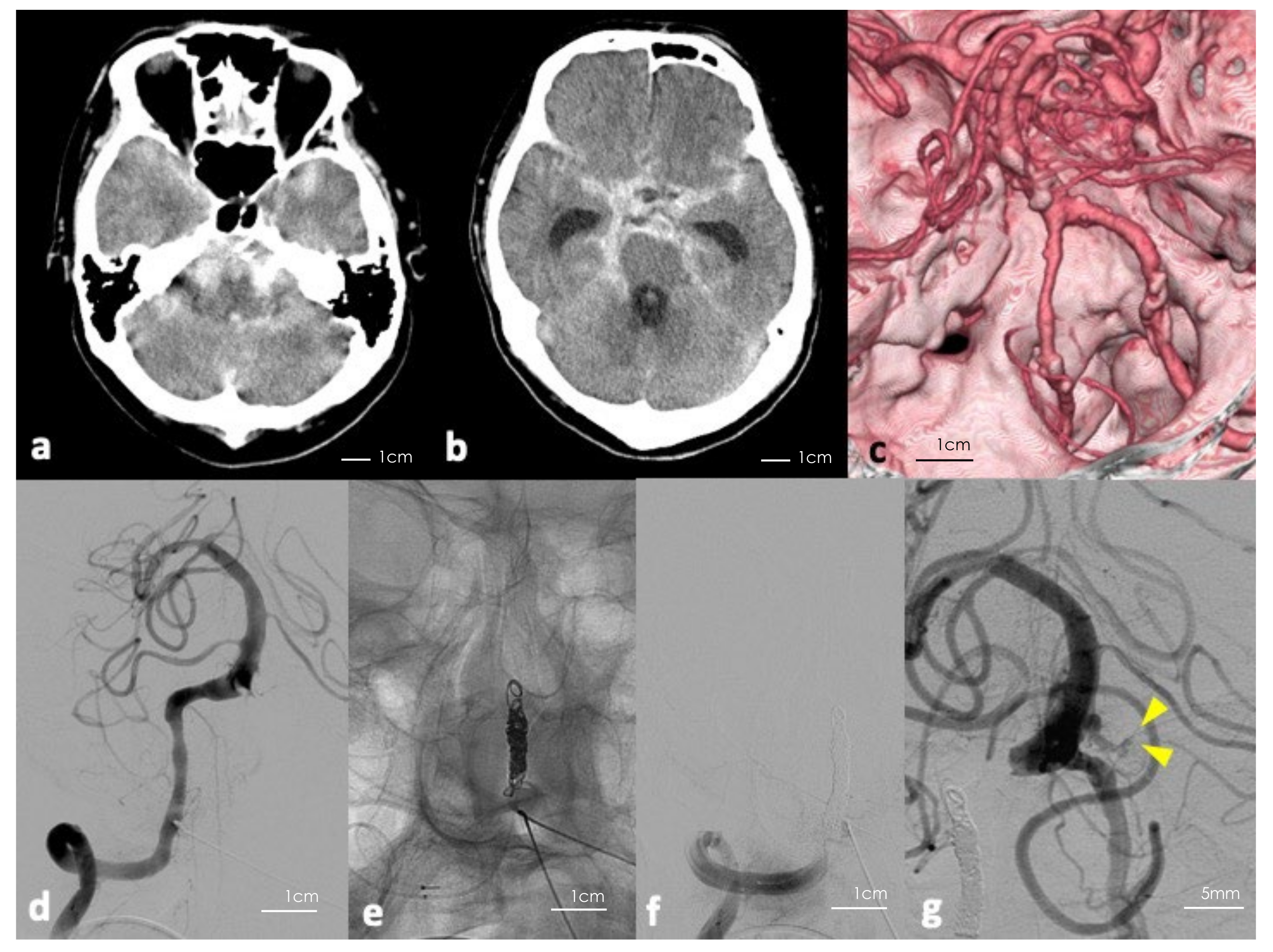
Figure 1.
(a,b) Initial computerized tomography (CT) of the head showing massive subarachnoid hemorrhage. (c) Three-dimension reconstructed CT angiography of the head showing no apparent saccular aneurysm. (d–f) Digital subtraction angiography (DSA) of the right vertebral artery before and after parental artery occlusion using coils. (g) Follow-up DSA showing blood flow in the aneurysm including the bleb (yellow arrowheads indicating the bleb).
Based on Figure 1c, from the distribution of the hematoma and findings on DSA, we assumed that the rupture point could be caused by the dissection of the right VA. Thus, we performed a parent artery embolization (Figure 1d–f). However, follow-up DSA revealed a previously undetected partially thrombosed saccular aneurysm at the vertebrobasilar junction or the lower section basilar trunk (Figure 1g). The neck was 2.4 mm × 2.2 mm, the aneurysmal dome was approximately 8 mm × 6 mm × 6 mm, and the lumen was 4 mm in a longitudinal direction. To prevent re-rupture of the aneurysm, we performed microsurgical clipping and wrapping. Following parent artery embolization, the patient’s mental status recovered to GCS 14 points, but deteriorated gradually due to hydrocephalus. His preoperative GCS was 8 points and modified Rankin Scale (mRS) score was 5.
The patient underwent clipping to prevent the re-rupture of the aneurysm. We chose to perform the surgery via the anterior transpetrosal approach with intraoperative DSA. Our operative strategy was based on a paper by Kawase et al. [7]. The craniotomy was centered low over the petrous ridge and the lower part of the squamous temporal bone was removed. We coagulated and cut the middle meningeal artery to manipulate near the foramen spinosum, with the greater superficial petrosal nerve carefully preserved when peeling the dura of the middle fossa base from the skull. The anterior petrosal ridge was then approached with a drill to create a 2 × 1 cm groove in the anterior part of the pyramidal bone, known as Kawase’s Triangle. Small dural incisions were made above and below the superior petrosal sinus, and after ligating the sinus with a surgical thread. We then carefully cut the tentorium, with special care not to damage the fourth cranial nerve running along the free edge, and exposed the fifth cranial nerve.
Since achieving proximal vascular control was expected to be difficult through the surgical corridor, we used a temporary balloon catheter. The balloon catheter was introduced coaxially through the angiography catheter and placed in the atlantic portion (V3) of the left VA. Because the right VA was occluded in advance, expansion of the balloon within the left VA was sufficient for proximal control. We expanded the balloon tentatively to evaluate the appropriate inflation volume, then the balloon catheter was withdrawn back into the angiography catheter to prevent thrombus formation. Left temporal craniotomy and extensive anterior pyramidal bone removal was performed.
We dissected the aneurysm from the fifth cranial nerve and surrounding anatomical structures. The basilar artery and superior cerebellar artery and fifth cranial nerve were dissected from the aneurysm, and a full view of the dome was obtained.
The aneurysm had a broad neck and the basilar artery was located medially, deeper than the aneurysmal dome, and was partially thrombosed. Due to the aneurysm being large and its stiffness, complete occlusion of the aneurysmal neck could cause neck laceration and intraoperative re-rupture. To maximize safety, we applied the clip to the aneurysmal dome, including the rupture point.
As expected, based on the preoperative imaging studies, we found the bleb, which we presumed to be the rupture point, on the lateral side of the aneurysm (Figure 2a). Careful inspection revealed that there was no perforator originating from the aneurysm. We applied an L-shaped titanium clip (Sugita #22, blade length 10 mm) to the rupture point. The blade was aligned parallel to the basilar artery, avoiding the fifth cranial nerve (Figure 2b). Finally, we applied DuraGen® (Integra Life Sciences Corp., Princeton, NJ, USA) to the remaining dome and covered the area with fascia so that the wrapping was multilayered. The layers were fixed with fibrin glue (Figure 2c,d). Occlusion of the rupture point and the patency of the parent artery and branches was confirmed with Intraoperative Doppler sonography, DSA, and Indocyanine Green video angiography.
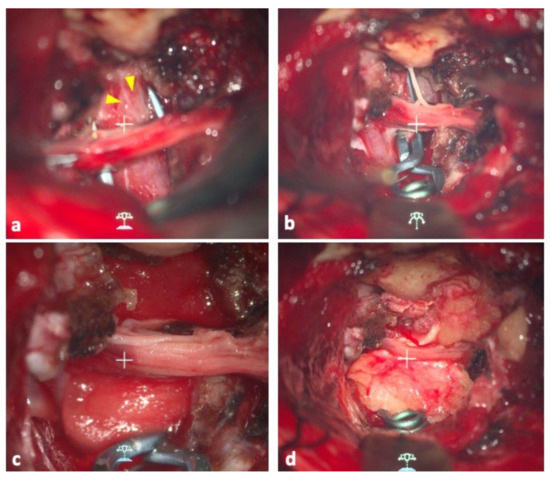
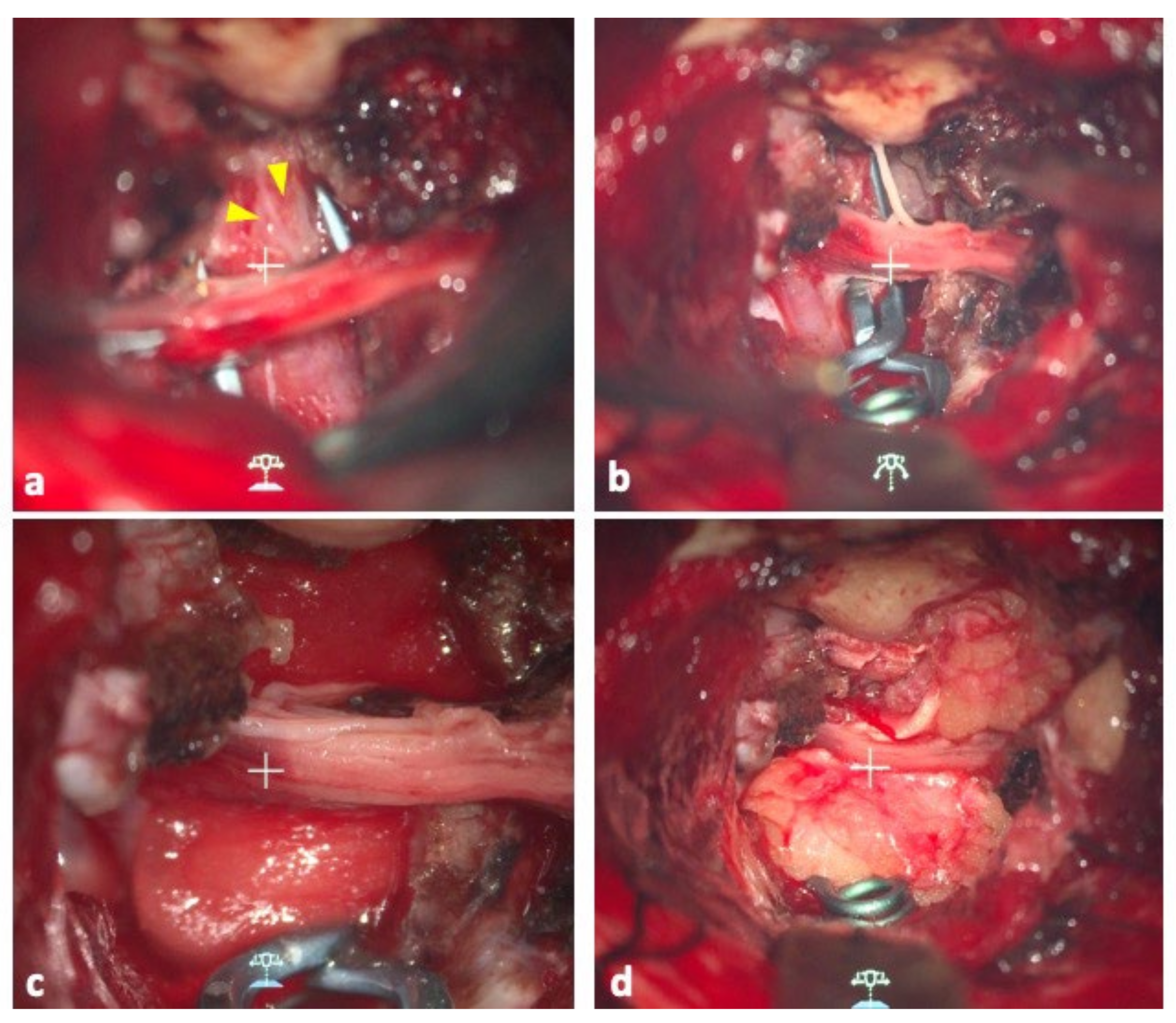
Figure 2.
(a) Operative view of the aneurysm. Yellow arrowheads indicate the location of the bleb. (b) The clip blade was aligned parallel to the basilar artery, avoiding the fifth cranial nerve. (c) Operative view after DuraGen was applied to the remaining dome. (d) The same area was covered with the patient’s fascia to make the wrapping multilayered. The layers were fixed with fibrin glue.
The patient’s postoperative plain CT showed no additional hemorrhages, and DSA revealed a disappearance of the bleb of the aneurysm (Figure 3a). Although he did not have any new neurological deficit, MRI showed a DWI high lesion in the left posterior inferior cerebellar artery (PICA) territory. No arterial sacrifice was made during the surgery. Thus, the cause of the infarction could be due to thrombus formation around the inserted catheter. After undergoing ventriculoperitoneal shunting, his consciousness disturbance level gradually improved during the course without re-rupture of the aneurysm. On the 26th day after the surgery, follow-up DSA revealed disappearance of the bleb and a remarkable shrinkage of the residual blood flow inside the aneurysm (Figure 3b,c). On the 72nd day after the onset of SAH, the patient was transferred to a long-term care hospital. His final follow-up mRS was 5.
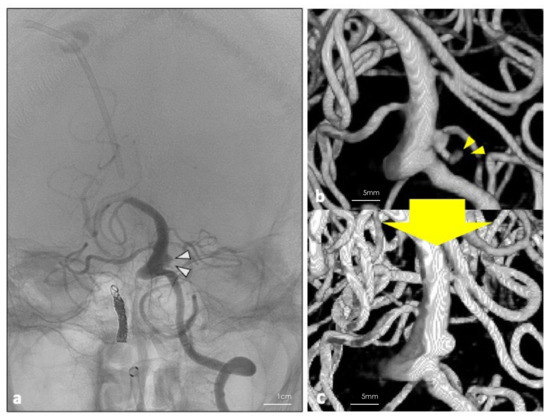
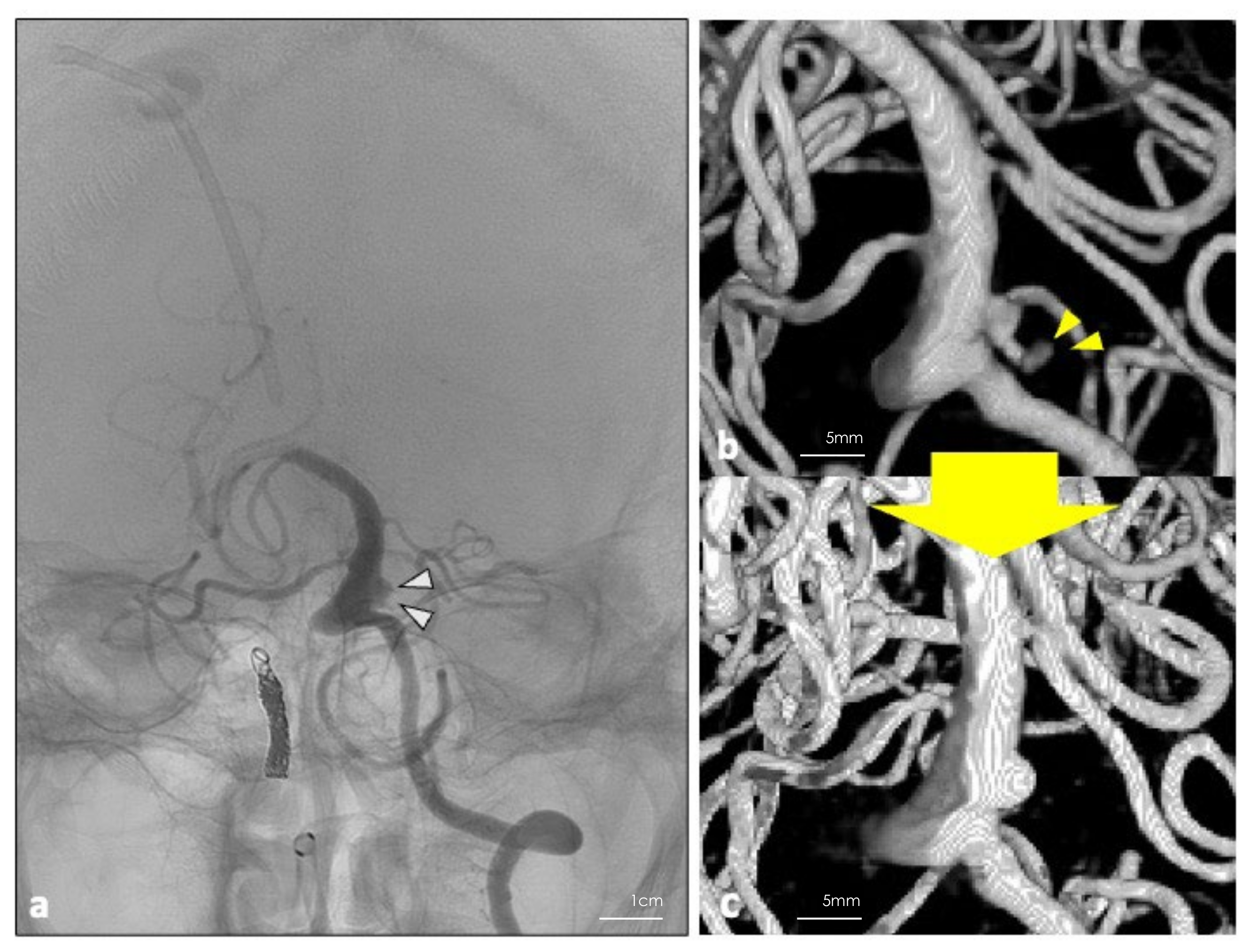
Figure 3.
(a) DSA was performed 26 days post operation. White arrowheads show the residual blood flow in the aneurysm. (b,c) Three-dimension reconstruction of the digital subtraction angiography performed 7 days before and 26 days after the operation. Disappearance of the bleb (yellow arrowheads) and shrinkage of the residual blood flow inside the aneurysm were achieved.
3. Discussion
Basilar artery trunk aneurysms are a rare entity with a prevalence report of 2.1%, and treating the lesion could pose significant challenges to the treating physician. According to Saliou et al. “their cause is diverse, their natural history is poorly understood, and their treatment is challenging” [8]. In addition, the aneurysm in our case was completely thrombosed at the initial time of presentation, making the diagnosis and treatment more complex.
The rarity of the disease makes it difficult to establish standard treatment for vertebrobasilar artery aneurysms. However, endovascular treatment (EVT) has been increasingly selected for the treatment modality for basilar aneurysms [3]. In EVT, patients are given simple coiling, conventional low-metal-coverage stent-assisted EVT. Though the actual numbers differ greatly depending on the literature, the risks of both ischemic and hemorrhagic complications are not low [3,4,8].
In recent years, flow diversion (FD) for basilar artery aneurysms has shown to have a satisfactory result with the long-term aneurysm occlusion rate when used in carefully selected patients [8,9]. However, serious complications have been reported, including acute occlusion of the basilar artery and brain stem infarction post treatment [5,6]. In the acute phase of SAH, the patients are in a hypercoagulable state [10] which could make them susceptible to ischemic complications. FD treatment for basilar artery aneurysms can carry ischemic risks, and should be limited to lesions which are difficult to treat any other way [6]. In our case, the patient had a brainstem infarction at the time of presentation, which therefore made it less likely for us to choose FD treatment as the initial therapy. The patient underwent his endovascular intervention on the first day he was admitted.
Neither a stent nor flow diverter was available for the patient in our country since both devices are not approved for ruptured aneurysm patients in their acute stage. Direct surgical treatment was the only possible approach for the aneurysm to prevent re-rupture.
The anterior transpetrosal approach for the lower basilar artery has been described as early as 1985 by Kawase et al. [7]. Even though new techniques have been introduced [8,9], making the surgical procedure safer and simpler, direct surgery for basilar artery aneurysms is still challenging today.
It was reported by Winkler et al. that “rates of morbidities and mortalities of microsurgical treatment for basilar aneurysms do not differ from that of endovascular treatment, and leveraging strengths of both techniques, equivalent clinical outcomes, and technical proficiency may be achieved with both modalities” [11]. In our case, postoperative DSA showed remarkable shrinkage of the residual blood flow inside the aneurysm. Though we need to follow up closely, partial clipping might be a safe and efficient treatment option for thrombosed basilar aneurysms. The limitation of this approach is that the surgical corridor is narrow and it can only be applied to aneurysms of adequate size and location.
We chose to treat the aneurysm with a combined technique using a balloon catheter for proximal control, which has been reported in the literature and has been shown to be effective [2,12].
Since we had assumed preoperatively that complete occlusion of the aneurysm with a clip would be of high risk, we wrapped the aneurysmal dome using the patient’s fascia and DuraGen® (an artificial dural substitute) in a multilayered fashion after applying the clip to the rupture point. As far as we are aware, this is the first study to use DuraGen® as a wrapping material. Several agents and wrapping techniques have been used to treat aneurysms [13,14,15]; however, there is no clear consensus on what the ideal material is. In addition, adverse events related to the material used for wrapping (such as gauze and muslin) are also reported [16,17,18,19,20,21,22]. DuraGen® is made of collagen, is replaced within six-to-eight weeks, and is resorbed and replaced by dura [23]. Because the material itself will be replaced in several weeks, we used the patient’s fascia in combination to make it multilayered. No foreign reaction has been reported in clinical use; therefore, inflammation and granuloma formation are unlikely to occur. Furthermore, DuraGen® promotes fibroblast migration, which could make it suitable for wrapping material. In cases where the surgical field is deep, it is technically difficult to wrap the aneurysm tightly along the dome. In such cases, DuraGen® is useful for its soft and smooth texture and can be easily applied to the aneurysmal dome. Although we cannot tell in the long term and more data are warranted to prove its efficacy, there is a possibility that DuraGen® could be used as a safer substitute for wrapping material.
4. Conclusions
We present a case with a partially thrombosed ruptured basilar trunk artery aneurysm. The lesion was successfully treated by combining partial clipping of the bleb and multilayered wrapping of the aneurysmal dome using a novel material.
As in this case, a direct microsurgical approach for basilar trunk artery aneurysms is known to be one of the most challenging operations. However, it is an effective treatment modality for complex aneurysms, in combination with a wrapping technique if other options are not available.
Author Contributions
Conceptualization, R.T. and T.S.; data curation, T.S. and M.K.; writing—original draft preparation, T.S.; writing—review and editing, M.K., Y.K., R.T.; supervision, R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Kawasaki Municipal Hospital (Kawasaki, Japan) (protocol code 1373, approved on 9 November 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. We obtained informed consent for publication from the patient.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Higa, T.; Ujiie, H.; Kato, K.; Kamiyama, H.; Hori, T. Basilar artery trunk saccular aneurysms: Morphological characteristics and management. Neurosurg. Rev. 2009, 32, 181–191, discussion 191. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, K.; Yoshimoto, T.; Takahashi, A.; Ogawa, A. Direct clipping of basilar trunk aneurysms using temporary balloon occlusion. J. Neurosurg. 1994, 80, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, K.; Song, J.; Yu, J. Endovascular Therapy for Basilar Arterial Trunk Aneurysms. Front. Neurol. 2021, 12, 625909. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Shojima, M.; Iijima, A.; Oya, S.; Matsui, T.; Yoshikawa, G.; Tsutsumi, K.; Nakatomi, H.; Saito, N. Stent-assisted Coiling for Ruptured Basilar Artery Dissecting Aneurysms: An Initial Experience of Four Cases. Neurol. Med. Chir. 2016, 56, 43–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.-B.; Shi, W.-W.; Zhang, G.-X.; Lu, H.-C.; Ma, J. Flow diverter treatment of posterior circulation aneurysms. A meta-analysis. Neuroradiology 2016, 58, 391–400. [Google Scholar] [CrossRef]
- Kulcsár, Z.; Ernemann, U.; Wetzel, S.G.; Bock, A.; Goericke, S.; Panagiotopoulos, V.; Forsting, M.; Ruefenacht, D.A.; Wanke, I. High-profile flow diverter (silk) implantation in the basilar artery: Efficacy in the treatment of aneurysms and the role of the perforators. Stroke 2010, 41, 1690–1696. [Google Scholar] [CrossRef]
- Kawase, T.; Toya, S.; Shiobara, R.; Mine, T. Transpetrosal approach for aneurysms of the lower basilar artery. J. Neurosurg. 1985, 63, 857–861. [Google Scholar] [CrossRef]
- Saliou, G.; Sacho, R.H.; Power, S.; Kostynskyy, A.; Willinsky, R.A.; Tymianski, M.; Terbrugge, K.G.; Rawal, S.; Krings, T. Natural History and Management of Basilar Trunk Artery Aneurysms. Stroke 2015, 46, 948–953. [Google Scholar] [CrossRef]
- Graziano, F.; Ganau, M.; Iacopino, D.G.; Boccardi, E. Vertebro-Basilar Junction Aneurysms: A Single Centre Experience and Meta-Analysis of Endovascular Treatments. Neuroradiol. J. 2014, 27, 732–741. [Google Scholar] [CrossRef]
- Lauridsen, S.V.; Hvas, C.L.; Sandgaard, E.; Gyldenholm, T.; Mikkelsen, R.; Obbekjær, T.; Sunde, N.; Tønnesen, E.K.; Hvas, A.M. Thromboelastometry Shows Early Hypercoagulation in Patients with Spontaneous Subarachnoid Hemorrhage. World Neurosurg. 2019, 130, e140–e149. [Google Scholar] [CrossRef]
- Winkler, E.A.; Lee, A.; Yue, J.K.; Raygor, K.P.; Rutledge, W.C.; Rubio, R.R.; Josephson, S.A.; Berger, M.S.; Raper, D.M.S.; Abla, A.A. Endovascular embolization versus surgical clipping in a single surgeon series of basilar artery aneurysms: A complementary approach in the endovascular era. Acta Neurochir. 2021, 163, 1527–1540. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Ricci, A.; Gallucci, M.; Zotta, D.; Scogna, A.; Costagliola, C.; Galzio, R. Combined endovascular and microsurgical approach in the treatment of giant paraclinoid and vertebrobasilar aneurysms. J. Neurosurg. Sci. 2005, 49, 1–6. [Google Scholar] [PubMed]
- Deshmukh, V.R.; Kakarla, U.K.; Figueiredo, E.G.; Zabramski, J.M.; Spetzler, R.F. Long-term Clinical and Angiographic Follow-up of Unclippable Wrapped Intracranial Aneurysms. Neurosurgery 2006, 58, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Abbasi, S.; Moron, F.; Sun, H.; Wilson, C.; Frock, B.; Oppenlander, M.E.; Xu, D.S.; Ghafil, C.; Zabramski, J.M.; Spetzler, R.F.; et al. Techniques and Outcomes of Gore-Tex Clip-Wrapping of Ruptured and Unruptured Cerebral Aneurysms. World Neurosurg. 2016, 90, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Pelissou-Guyotat, I.; Deruty, R.; Mottolese, C.; Amat, D. The use of Teflon as wrapping material in aneurysm surgery. Neurol. Res. 1994, 16, 224–227. [Google Scholar] [CrossRef] [PubMed]
- McFadzean, R.M.; Hadley, D.M.; McIlwaine, G.G. Optochiasmal arachnoiditis following muslin wrapping of ruptured anterior communicating artery aneurysms. J. Neurosurg. 1991, 75, 393–396. [Google Scholar] [CrossRef]
- Felsberg, G.J.; Tien, R.D.; Haplea, S.; Osumi, A.K. Muslin-induced optic arachnoiditis (“gauzoma”): Findings on CT and MR. J. Comput. Assist. Tomogr. 1993, 17, 485–487. [Google Scholar] [CrossRef]
- Prabhu, S.S.; Keogh, A.J.; Parekh, H.C.; Perera, S. Optochiasmal arachnoiditis induced by muslin wrapping of intracranial aneurysms. A report of two cases and a review of the literature. Br. J. Neurosurg. 1994, 8, 471–476. [Google Scholar] [CrossRef]
- Kirollos, R.W.; Tyagi, A.K.; Marks, P.V.; Van Hille, P.T. Muslin induced granuloma following wrapping of intracranial aneurysms: The role of infection as an additional precipitating factor. Acta Neurochir. 1997, 139, 411–415. [Google Scholar] [CrossRef]
- Brochert, A.; Reynolds, T.; Baker, R. MRI in a case of muslin-induced granuloma. Neuroradiology 2003, 45, 82–84. [Google Scholar] [CrossRef]
- Goldsberry, D.H.; Ross, I.B.; Dhillon, G.; Corbett, J.J. Visual Dysfunction Caused by Gauze Wrapping of an Intracranial Aneurysm. J. Neuro-Ophthalmol. 2004, 24, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Binning, M.J.; Shanmugam, V.K.; Schmidt, R.H.; Couldwell, W.T.; Meyer, M.; Cupps, T.; Douglas, A.; McGrail, K. Muslin-induced intracranial vasculopathic stenosis: A report of two cases. Clin. Neurol. Neurosurg. 2012, 114, 63–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamura, R.; Kuranari, Y.; Orikasa, H.; Katayama, M. Meningioma Cell Invasion into DuraGen-Derived Dura Mater: A Case Report. Medicines 2022, 9, 30. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).