Cell Salvage in Oncological Surgery, Peripartum Haemorrhage and Trauma
Abstract
1. Introduction
2. Current Recommendations and Guidelines
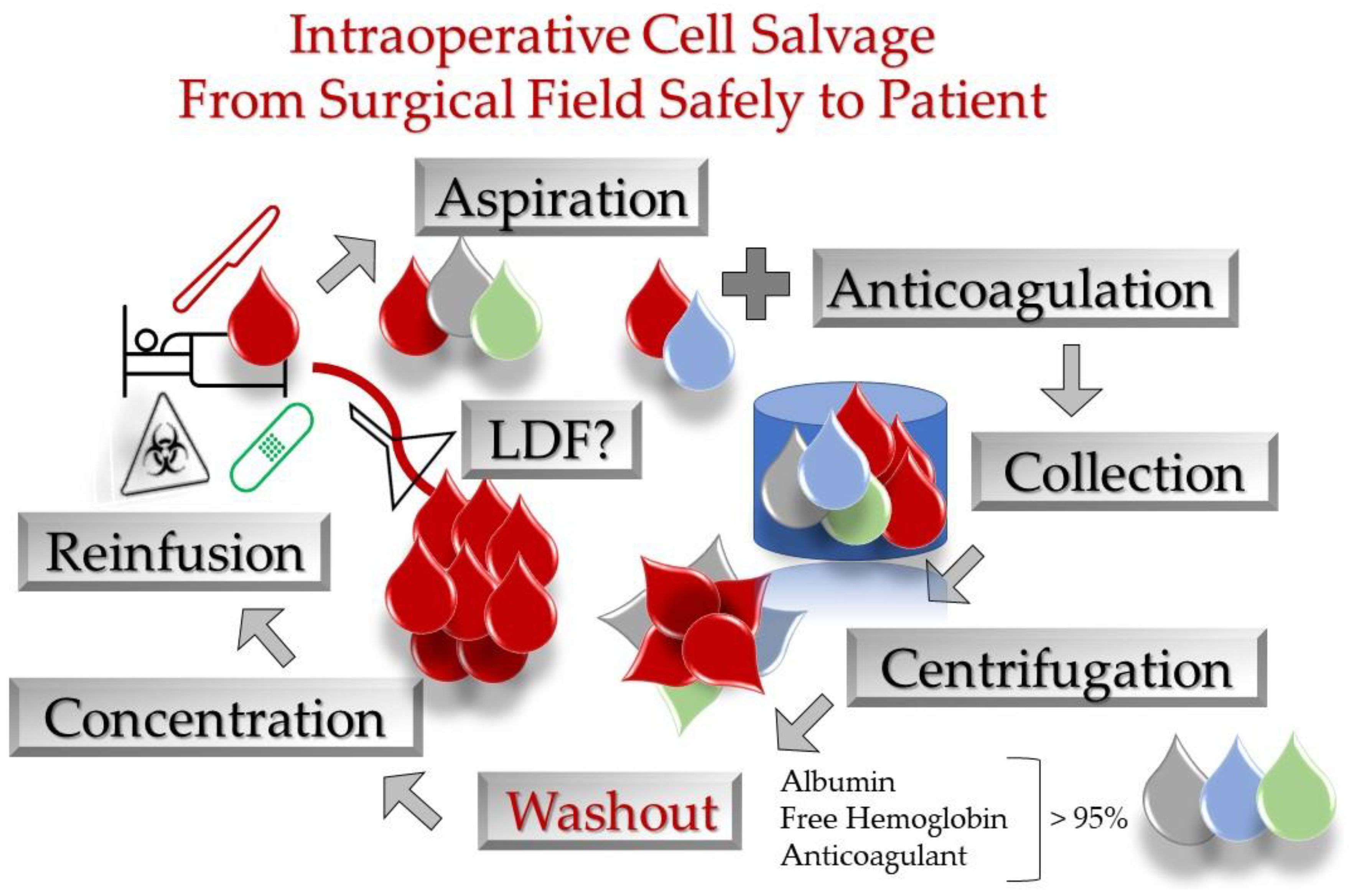
3. Criteria Determining RBC Volume to Re-Transfuse and Technical Aspects
- i.
- Viable and efficient RBC recovery of 90%;
- ii.
- Washout of 90%;
- iii.
- Haematocrit 55–80%;
- iv.
- Free haemoglobin clearance of 95%;
- v.
- Albumin clearance of 96%.
4. Safety and Quality Considerations of ICS
5. Cell Salvage in Oncological Surgery
5.1. Tumour Cells in Salvaged Blood Considered with Non-Metastatic Potential
5.2. Metastatic Spinal Tumour Surgery
5.3. Gastrointestinal and Urogenital Cancer
6. The Role of Cell Salvage in Peripartum Haemorrhage (PPH)
6.1. Major Concerns in Obstetrics
6.2. Controversial Use of LDF in PPH
6.3. Clinical Indications for ICS in Obstetrics
6.4. Safety and Cost-Effectiveness of ICS in PPH
7. Cell Salvage in Acute Trauma and Haemorrhagic Shock
7.1. The Risk of Blood Re-Transfusion in Trauma Bleeding
7.2. Weak Evidence of Cell Salvage in Civilian Trauma Bleeding
7.3. Combat Injury, Combat Blood
7.4. Cell Salvage in Specific Injuries
7.4.1. Pelvis Fractures
7.4.2. Haemothorax
7.5. ‘Cross-Talking’ Inflammation-Coagulation-Immunity in Trauma
7.6. Overall Considerations for ICS in Trauma Haemorrhage
8. Measures to Improve ICS Implementation in PPH and Trauma
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carless, P.A.; Henry, D.A.; Moxey, A.J.; O’connell, D.L.; Brown, T.; Fergusson, D.A. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst. Rev. 2006, CD001888. [Google Scholar] [CrossRef]
- Liumbruno, G.M.; Liumbruno, C.; Rafanelli, D. Autologous blood in obstetrics: Where are we going now? Blood Transfus. 2012, 10, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Manuel, M.; Slappendel, R.; Dafydd, T. Laboratory characteristics and clinical utility of post-operative cell salvage: Washed or unwashed blood transfusion? Blood Transfus. 2011, 9, 248–261. [Google Scholar] [CrossRef]
- Brinke, M.J.T.; Weerwind, P.W.; Teerenstra, S.; Feron, J.C.M.; Van Der Meer, W.; Brouwer, M.H.J. Leukocyte removal efficiency of cell-washed and unwashed whole blood: An in vitro study. Perfusion 2005, 20, 335–341. [Google Scholar] [CrossRef]
- Klein, A.A.; Bailey, C.R.; Charlton, A.J.; Evans, E.; Guckian-Fisher, M.; McCrossan, R.; Nimmo, A.F.; Payne, S.; Shreeve, K.; Smith, J.; et al. Association of Anaesthetists guidelines: Cell salvage for peri-operative blood conservation 2018. Anaesthesia 2018, 73, 1141–1150. [Google Scholar] [CrossRef]
- Frank, S.M.; Sikorski, R.A.; Konig, G.; Tsilimigras, D.I.; Hartmann, J.; Popovsky, M.A.; Pawlik, T.M.; Waters, J.H. Clinical Utility of Autologous Salvaged Blood: A Review. J. Gastrointest. Surg. 2019, 24, 464–472. [Google Scholar] [CrossRef]
- Sikorski, R.A.; Rizkalla, N.A.; Yang, W.W.; Frank, S.M. Autologous blood salvage in the era of patient blood management. Vox Sang. 2017, 112, 499–510. [Google Scholar] [CrossRef]
- Kozek-Langenecker, S.A.; Ahmed, A.B.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.; Fries, D.; et al. Management of severe perioperative bleeding. Eur. J. Anaesthesiol. 2017, 34, 332–395. [Google Scholar] [CrossRef]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef]
- Gum, J.L.; Carreon, L.Y.; Kelly, M.P.; Hostin, R.; Robinson, C.; Burton, D.C.; Polly, D.W.; Shaffrey, C.I.; Lafage, V.; Schwab, F.J.; et al. Cell Saver for Adult Spinal Deformity Surgery Reduces Cost. Spine Deform. 2017, 5, 272–276. [Google Scholar] [CrossRef]
- Kelly, P.D.; Parker, S.L.; Mendenhall, S.K.; Bible, J.E.; Sivasubramaniam, P.; Shau, D.N.; McGirt, M.J.; Devin, C.J. Cost-Effectiveness of Cell Saver in Short-segment Lumbar Laminectomy and Fusion (≤3 Levels). Spine 2015, 40, E978–E985. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.H.; Dyga, R.M.; Waters, J.F.; Yazer, M.H. The volume of returned red blood cells in a large blood salvage program: Where does it all go? Transfusion 2011, 51, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.M. Who benefits from red blood cell salvage? Utility and value of intraoperative autologous transfusion. Transfusion 2011, 51, 2058–2060. [Google Scholar] [CrossRef] [PubMed]
- Beiderlinden, M.; Brau, C.; Di Grazia, S.; Wehmeier, M.; Treschan, T.A. Argatroban for anticoagulation of a blood salvage system —An ex-vivo study. BMC Anesthesiol. 2015, 16, 1–7. [Google Scholar] [CrossRef]
- Yazer, M.H.; Waters, J.H.; Elkin, K.R.; Rohrbaugh, M.E.; Kameneva, M.V. A comparison of hemolysis and red cell mechanical fragility in blood collected with different cell salvage suction devices. Transfusion 2008, 48, 1188–1191. [Google Scholar] [CrossRef]
- Waters, J.H.; Williams, B.; Yazer, M.H.; Kameneva, M.V. Modification of Suction-Induced Hemolysis During Cell Salvage. Anesthesia Analg. 2007, 104, 684–687. [Google Scholar] [CrossRef]
- Hoetink, A.; Scherphof, S.F.; Mooi, F.J.; Westers, P.; Van Dijk, J.; Van De Leur, S.J.; Nierich, A.P. An In Vitro Pilot Study Comparing the Novel HemoClear Gravity-Driven Microfiltration Cell Salvage System with the Conventional Centrifugal XTRA™ Autotransfusion Device. Anesthesiol. Res. Pr. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Coggins, A.; Santos, A.D.L.; Zaklama, R.; Murphy, M. Interdisciplinary clinical debriefing in the emergency department: An observational study of learning topics and outcomes. BMC Emerg. Med. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Seyfried, T.F.; Gruber, M.; Pawlik, M.T.; Kasper, S.; Mandle, R.J.; Hansen, E. A new approach for fat removal in a discontinuous autotransfusion device-concept and evaluation. Vox Sang. 2017, 112, 759–766. [Google Scholar] [CrossRef]
- Seyfried, T.F.; Haas, L.; Gruber, M.; Breu, A.; Loibl, M.; Hansen, E. Fat removal during cell salvage: A comparison of four different cell salvage devices. Transfusion 2015, 55, 1637–1643. [Google Scholar] [CrossRef]
- Seyfried, T.; Hansen, E. Maschinelle Autotransfusion: Wissenschaftliche Evidenz, klinische Praxis und rechtliche Rah-menbedingungen [Cell salvage: Scientific evidence, clinical practice and legal framework]. Der Anaesthesist 2019, 68, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, T.M.; Burnside, J.L.; Hodge, A.B.; Naguib, A.N.; Gomez, D. The Impact of Three Different Wash Solutions on Autotransfusion Products. J. Extra-Corporeal Technol. 2018, 50, 113–116. [Google Scholar]
- Hinson, W.D.; Rogovskyy, A.S.; Lawhon, S.D.; Mankin, K.M.T. Influence of a cell salvage washing system and leukocyte reduction filtration on bacterial contamination of canine whole blood ex vivo. Vet. Surg. 2020, 49, 989–996. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Peiro-Garcia, A.; Ferri-De-Barros, F.; Parsons, D. Hemolysis Following Intraoperative Cell Salvage Replacement in a Scoliosis Patient With Sickle Cell Trait. Spine 2017, 42, E1331–E1333. [Google Scholar] [CrossRef]
- Schott, N.J.; Yazer, M.H.; Krohner, R.; Waters, J.H. Failure of Intraoperative Red Cell Salvage: A Patient with Sickle Cell Disease and HELLP (Hemolysis, Elevated Liver enzymes and Low Platelets) Syndrome. J. Extra-Corporeal Technol. 2014, 46, 314–316. [Google Scholar]
- Caliste, X.A.; McArthur, K.A.; Sava, J.A. Autotransfusion in emergent operative trauma resuscitation. Eur. J. Trauma Emerg. Surg. 2014, 40, 541–545. [Google Scholar] [CrossRef]
- Stachura, A.; Poplawski, T.; Michalik, D.; Pomianowski, S.; Jacobsson, M.; Król, R.; Åberg, M.; Bengtsson, A. Transfusion of intra-operative autologous whole blood: Influence on complement activation and interleukin formation. Vox Sang. 2010, 100, 239–246. [Google Scholar] [CrossRef]
- Sloan, T.B.; Myers, G.; Janik, D.J.; Burger, E.M.; Patel, V.V.; Jameson, L.C. Intraoperative Autologous Transfusion of Hemolyzed Blood. Anesthesia Analg. 2009, 109, 38–42. [Google Scholar] [CrossRef]
- Minkara, A.A.; Lin, A.Y.; Vitale, M.G.; Roye, D.P. Acute Kidney Injury Secondary to Cell Saver in Posterior Spinal Fusion. Spine Deform. 2017, 5, 430–434. [Google Scholar] [CrossRef]
- Santise, G.; Maselli, D.; Malanga, D.; Di Vito, A.; Mandarino, N.; Boccadamo, G.; Zeppa, P.; Amorosi, A.; Viglietto, G.; Rizzuto, A.; et al. Identification of mesothelial cells in in-traoperative blood salvage. Am. J. Transl. Res. 2019, 11, 1771–1779. [Google Scholar] [PubMed]
- Sreelakshmi, T.R.; Eldridge, J. Acute hypotension associated with leucocyte depletion filters during cell salvaged blood transfusion. Anaesthesia 2009, 65, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Kessack, L.K.; Hawkins, N. Severe hypotension related to cell salvaged blood transfusion in obstetrics. Anaesthesia 2010, 65, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Gabel, J.; Malm, C.J.; Radulovic, V.; Hakimi, C.S.; Westerberg, M.; Jeppsson, A. Cell saver processing mitigates the negative effects of wound blood on platelet function. Acta Anaesthesiol. Scand. 2016, 60, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Pescatori, M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst. Rev. 2006, 2006, CD005033. [Google Scholar] [CrossRef]
- Kumar, N.; Zaw, A.S.; Kantharajanna, S.B.; Khoo, B.L.; Lim, C.T.; Thiery, J.P. Metastatic efficiency of tumour cells can be impaired by intraoperative cell salvage process: Truth or conjecture? Transfus. Med. 2017, 27, 327–334. [Google Scholar] [CrossRef]
- Cata, J.; Wang, H.; Gottumukkala, V.; Reuben, J.; Sessler, D. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br. J. Anaesth. 2013, 110, 690–701. [Google Scholar] [CrossRef]
- Poruk, K.E.; Valero, V.; Saunders, T.; Blackford, A.L.; Griffin, J.; Poling, J.; Hruban, R.H.; Anders, R.A.; Herman, J.; Zheng, L.; et al. Circulating Tumor Cell Phenotype Predicts Recurrence and Survival in Pancreatic Adenocarcinoma. Ann. Surg. 2016, 264, 1073–1081. [Google Scholar] [CrossRef]
- Zaw, A.S.; Kantharajanna, S.B.; Kumar, N. Is Autologous Salvaged Blood a Viable Option for Patient Blood Management in Oncologic Surgery? Transfus. Med. Rev. 2016, 31, 56–61. [Google Scholar] [CrossRef]
- Waters, J.H.; Yazer, M.; Chen, Y.-F.; Kloke, J. Blood salvage and cancer surgery: A meta-analysis of available studies. Transfusion 2012, 52, 2167–2173. [Google Scholar] [CrossRef]
- Hansen, E. Is intraoperative blood salvage really safe in cancer surgery? Transfusion 2012, 52, 2723–2724. [Google Scholar] [CrossRef]
- Catling, S.; Williams, S.; Freites, O.; Rees, M.; Davies, C.; Hopkins, L. Use of a leucocyte filter to remove tumour cells from intra-operative cell salvage blood. Anaesthesia 2008, 63, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Beck-Schimmer, B.; Romero, B.; Booy, C.; Joch, H.; Haller, U.; Pasch, T.; Spahn, D.R. Release of inflammatory mediators in irradiated cell salvage blood and their biological consequences in human beings following transfusion. Eur. J. Anaesthesiol. 1999, 21, 46–52. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, L.; He, C.; Tai, S.; Ma, C.; Yang, T.; Wang, D. Evaluation of riboflavin photochemical treatment for inactivation of HCT116 tumor cells mixed in simulative intraoperative salvage blood. Transfusion 2019, 59, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Catling, S.J.; Thornton, C.A.; Russell, I.T. Bradykinin and cysteinyl leukotriene concentrations in cell-salvaged blood before and after passage through negatively charged filters during clinical use in cancer patients: A pilot study. Anaesthesia 2015, 70, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Elmalky, M.; Yasin, N.; Rodrigues-Pinto, R.; Stephenson, J.; Carroll, C.; Smurthwaite, G.; Verma, R.; Mohammad, S.; Siddique, I. The safety, efficacy, and cost-effectiveness of intraoperative cell salvage in metastatic spine tumor surgery. Spine J. 2017, 17, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Chen, Y.; Zaw, A.S.; Nayak, D.; Ahmed, Q.; Soong, R.; Wong, H.K. Use of intraoperative cell-salvage for autologous blood transfusions in metastatic spine tumour surgery: A systematic review. Lancet Oncol. 2014, 15, e33–e41. [Google Scholar] [CrossRef]
- Kumar, N.; Ravikumar, N.; Tan, J.Y.H.; Akbary, K.; Patel, R.S.; Kannan, R. Current Status of the Use of Salvaged Blood in Metastatic Spine Tumour Surgery. Neurospine 2018, 15, 206–215. [Google Scholar] [CrossRef]
- Kang, R.; Seath, B.E.; Huang, V.; Barth, R.J.; Kang, R.; Seath, B.E.; Huang, V.; Barth, R.J. Impact of Autologous Blood Transfusion on Survival and Recurrence among Patients Undergoing Partial Hepatectomy for Colorectal Cancer Liver Metastases. J. Am. Coll. Surg. 2018, 228, 902–908. [Google Scholar] [CrossRef]
- Araujo, R.; Pantanali, L.H.S.; Haddad, L.; Filho, J.A.R.; D’Albuquerque, L.A.C.; Andraus, W. Does autologous blood transfusion during liver transplantation for hepatocellular carcinoma increase risk of recurrence? World J. Gastrointest. Surg. 2016, 8, 161–168. [Google Scholar] [CrossRef]
- Pinto, M.A.; Chedid, M.F.; Sekine, L.; Schmidt, A.P.; Capra, R.P.; Prediger, C.; Prediger, J.E.; Grezzana-Filho, T.J.; Kruel, C.R. Intraoperative cell salvage with autologous transfusion in liver transplantation. World J. Gastrointest. Surg. 2019, 11, 11–18. [Google Scholar] [CrossRef]
- Pinto, M.A.; Grezzana-Filho, T.J.M.; Chedid, A.D.; Leipnitz, I.; Prediger, J.E.; Alvares-Da-Silva, M.R.; de Araújo, A.; Zahler, S.; Lopes, B.B.; Giampaoli, Z.D.; et al. Impact of intraoperative blood salvage and autologous transfusion during liver transplantation for hepatocellular carcinoma. Langenbeck’s Arch. Surg. 2020, 406, 67–74. [Google Scholar] [CrossRef]
- Lyon, T.D.; Ferroni, M.C.; Turner, R.M.; Jones, C.; Jacobs, B.L.; Davies, B.J. Short-term Outcomes of Intraoperative Cell Saver Transfusion During Open Partial Nephrectomy. Urology 2015, 86, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Raval, J.S.; Nelson, J.B.; Woldemichael, E.; Triulzi, D.J. Intraoperative cell salvage in radical prostatectomy does not appear to increase long-term biochemical recurrence, metastases, or mortality. Transfusion 2012, 52, 2590–2593. [Google Scholar] [CrossRef] [PubMed]
- Rogers, W.K.; Wernimont, S.A.; Kumar, G.C.; Bennett, E.; Chestnut, D.H. Acute Hypotension Associated with Intraoperative Cell Salvage Using a Leukocyte Depletion Filter During Management of Obstetric Hemorrhage Due to Amniotic Fluid Embolism. Anesthesia Analg. 2013, 117, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, I.J.; Ralph, C.J. Obstetric intra-operative cell salvage: A review of an established cell salvage service with 1170 re-infused cases. Anaesthesia 2019, 74, 976–983. [Google Scholar] [CrossRef]
- Peacock, L.; Clark, V. Cell Salvage in obstetrics: A review of data from the 2007 Scottish Confidential Audit of Severe Maternal Morbidity. Int. J. Obstet. Anesthesia 2011, 20, 196–198. [Google Scholar] [CrossRef]
- Peacock, L.; Clark, V.; Catling, S. Recent developments in the obstetric use of cell salvage. Transfus. Altern. Transfus. Med. 2012, 12, 66–71. [Google Scholar] [CrossRef]
- Ralph, C.J.; Sullivan, I.; Faulds, J. Intraoperative cell salvaged blood as part of a blood conservation strategy in Caesarean section: Is fetal red cell contamination important? Br. J. Anaesth. 2011, 107, 404–408. [Google Scholar] [CrossRef]
- Sullivan, I.; Faulds, J.; Ralph, C. Contamination of salvaged maternal blood by amniotic fluid and fetal red cells during elective Caesarean section. Br. J. Anaesth. 2008, 101, 225–229. [Google Scholar] [CrossRef][Green Version]
- Geoghegan, J.; Daniels, J.; Moore, P.; Thompson, P.; Khan, K.; Gülmezoglu, A. Cell salvage at caesarean section: The need for an evidence-based approach. Int. J. Obstet. Gynaecol. 2009, 116, 743–747. [Google Scholar] [CrossRef]
- Campbell, J.P.; MacKenzie, M.J.; Yentis, S.M.; Sooranna, S.R.; Johnson, M.R. An evaluation of the ability of leucocyte depletion filters to remove components of amniotic fluid. Anaesthesia 2012, 67, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.H.; Biscotti, C.; Potter, P.S.; Phillipson, E. Amniotic Fluid Removal during Cell Salvage in the Cesarean Section Patient. Anesthesiology 2000, 92, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, I.; Ichikawa, Y.; Nakajima, Y.; Kasahara, M.; Hattori, M.; Nemoto, T. Efficiency of leukocyte depletion filters and micro-aggregate filters following intra-operative cell salvage during cesarean delivery. Int. J. Obstet. Anesthesia. 2019, 41, 59–64. [Google Scholar] [CrossRef]
- Goucher, H.; Wong, C.A.; Patel, S.K.; Toledo, P. Cell Salvage in Obstetrics. Anesthesia Analg. 2015, 121, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Ahn, W.S.; Lee, J.M.; Jeon, J.K.; Kim, H.C.; Lim, Y.J.; Sim, J.Y. A laboratory study of the effects of processing blood through a cell salvage device and leucocyte depletion filter on levels of pro-inflammatory cytokines and bradykinin. Anaesthesia 2013, 68, 1259–1265. [Google Scholar] [CrossRef]
- Dhariwal, S.K.; Khan, K.S.; Allard, S.; Wilson, M.; Moore, P. Does current evidence support the use of intraoperative cell salvage in reducing the need for blood transfusion in caesarean section? Curr. Opin. Obstet. Gynecol. 2014, 26, 425–430. [Google Scholar] [CrossRef]
- Milne, M.E.; Yazer, M.H.; Waters, J.H. Red Blood Cell Salvage During Obstetric Hemorrhage. Obstet. Gynecol. 2015, 125, 919–923. [Google Scholar] [CrossRef]
- Yan, H.; Hu, L.-Q.; Wu, Y.; Fan, Q.; Wong, C.A.; McCarthy, R.J. The Association of Targeted Cell Salvage Blood Transfusion During Cesarean Delivery With Allogeneic Packed Red Blood Cell Transfusions in a Maternity Hospital in China. Anesthesia Analg. 2018, 127, 706–713. [Google Scholar] [CrossRef]
- Zeng, K.; Huang, W.; Yu, C.; Wang, R. How about “The effect of intraoperative cell salvage on allogeneic blood transfusion for patients with placenta accreta”?: An observational study. Medicine 2018, 97, e10942. [Google Scholar] [CrossRef]
- Brearton, C.; Bhalla, A.; Mallaiah, S.; Barclay, P. The economic benefits of cell salvage in obstetric haemorrhage. Int. J. Obstet. Anesthesia 2012, 21, 329–333. [Google Scholar] [CrossRef]
- Khan, K.S.; Moore, P.A.S.; Wilson, M.J.; Hooper, R.; Allard, S.; Wrench, I.; Beresford, L.; Roberts, T.E.; McLoughlin, C.; Geoghegan, J.; et al. Cell salvage and donor blood transfusion during cesarean section: A pragmatic, multicentre randomised controlled trial (SALVO). PLoS Med. 2017, 14, e1002471. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Moore, P.; Wilson, M.; Hooper, R.; Allard, S.; Wrench, I.; Roberts, T.; McLoughlin, C.; Beresford, L.; Geoghegan, J.; et al. A randomised controlled trial and economic evaluation of intraoperative cell salvage during caesarean section in women at risk of haemorrhage: The SALVO (cell SALVage in Obstetrics) trial. Health Technol. Assess. 2018, 22, 1–88. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Mallaiah, S.; Djabatey, E.; McNamara, H. Association of Anaesthetists guidelines on cell salvage—A backward step for obstetric practice? Anaesthesia 2018, 73, 1574–1575. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Melnyk, M.V.; Facco, M.F.L.; Waters, M.J.H.; Smith, K.J. Cost-effectiveness Analysis of Intraoperative Cell Salvage for Obstetric Hemorrhage. Anesthesiology 2018, 128, 328–337. [Google Scholar] [CrossRef]
- Li, J.; Sun, S.L.; Tian, J.H.; Yang, K.; Liu, R.; Li, J. Cell salvage in emergency trauma surgery. Cochrane Database Syst. Rev. 2015, 1. [Google Scholar] [CrossRef]
- Esper, S.A. Intra-operative cell salvage: A fresh look at the indications and contraindications. Blood Transfus. 2011, 9, 139–147. [Google Scholar] [CrossRef]
- Boyle, G.; Kuffel, A.; Parmar, K.; Gibson, K.; Smith, M.; Grehan, A.; Hunt, B.J.; Chambers, D.J. A comparison of haemostatic biomarkers during low-risk patients undergoing cardiopulmonary bypass using either conventional centrifugal cell salvage or the HemoSep device. Perfusion 2018, 34, 76–83. [Google Scholar] [CrossRef]
- Chung, Y.S.; Kim, H.R.; Kang, H.; Ryu, C.; Park, B.; Hong, J. Fragility of Red Blood Cells Collected Under Different Conditions With a Cell Saver Device. J. Cardiothorac Vasc Anesthesia. 2019, 33, 1224–1229. [Google Scholar] [CrossRef]
- Craig, E.K.; Yazer, M.H.; Waters, J.H. Red blood cell salvage analysis from clotted blood. Blood Transfus. 2019, 17, 1–5. [Google Scholar] [CrossRef]
- Haddaway, K.; Bloch, E.M.; Tobian, A.A.; Frank, S.M.; Sikorski, R.; Cho, B.C.; Zheng, G.; Jani, J.; Lokhandwala, P.M.; Lawrence, C.E.; et al. Hemostatic properties of cold-stored whole blood leukoreduced using a platelet-sparing versus a non–platelet-sparing filter. Transfusion 2019, 59, 1809–1817. [Google Scholar] [CrossRef]
- Thomas, K.A.; Shea, S.; Yazer, M.H.; Spinella, P.C. Effect of leukoreduction and pathogen reduction on the hemostatic function of whole blood. Transfusion 2019, 59, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.V.R. Autologous Blood Transfusion During Emergency Trauma Operations. Arch. Surg. 2010, 145, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Bhangu, A.; Nepogodiev, D.; Doughty, H.; Bowley, D.M. Intraoperative cell salvage in a combat support hospital: A prospective proof of concept study. Transfusion 2012, 53, 805–810. [Google Scholar] [CrossRef]
- Waters, J.H. Intraoperative Blood Recovery. ASAIO J. 2013, 59, 11–17. [Google Scholar] [CrossRef]
- Bowley, D.M.; Barker, P.; Boffard, K. Intraoperative Blood Salvage in Penetrating Abdominal Trauma: A Randomised, Controlled Trial. World J. Surg. 2006, 30, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Gigengack, R.K.; Verhees, V.; Gemert, A.W.K.-V.; Oen, I.M.; Ossewaarde, T.M.; Koopman, S.S.; Loer, S.A.; van der Vlies, C.H. Cell salvage in burn excisional surgery. Burns 2020, 47, 127–132. [Google Scholar] [CrossRef]
- Perez-Ferrer, A.; Gredilla-Díaz, E.; De Vicente-Sánchez, J.; Navarro-Suay, R.; Gilsanz-Rodríguez, F. Vancomycin added to the wash solution of the cell-saver. Effect on bacterial contamination. Rev. Esp. Anestesiol. Reanim. 2017, 64, 185–191. [Google Scholar] [CrossRef]
- Naumann, D.N.; Boulton, A.; Sandhu, A.; Campbell, K.; Charlton, W.; Gurney, J.M.; Martin, M.J.; Scorer, T.; Doughty, H. Fresh whole blood from walking blood banks for patients with traumatic hemorrhagic shock: A systematic review and meta-analysis. J. Trauma Acute Care Surg. 2020, 89, 792–800. [Google Scholar] [CrossRef]
- Fisher, A.; Carius, B.M.; Corley, J.B.; Dodge, P.; Miles, E.; Taylor, A.L. Conducting fresh whole blood transfusion training. J. Trauma Acute Care Surg. 2019, 87, S184–S190. [Google Scholar] [CrossRef]
- Cap, A.P.; Beckett, A.; Benov, A.; Borgman, M.; Chen, J.; Corley, J.B.; Doughty, H.; Fisher, A.; Glassberg, E.; Gonzales, R.; et al. Whole Blood Transfusion. Mil. Med. 2018, 183, 44–51. [Google Scholar] [CrossRef]
- Daniel, Y.; Habas, S.; Malan, L.; Escarment, J.; David, J.-S.; Peyrefitte, S. Tactical damage control resuscitation in austere military environments. J. R. Army Med. Corps. 2016, 162, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Sahloul, M.; Bowley, D.; Kirkman, E.; Doughty, H. Blood salvage technology after combat injury. J. R. Army Med. Corps. 2018, 164, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.; Burlew, C.C. Preperitoneal Pelvic Packing: How and When. Curr. Trauma Rep. 2014, 1, 1–7. [Google Scholar] [CrossRef]
- Cimbanassi, S.; O’Toole, R.; Maegele, M.; Henry, S.; Scalea, T.M.; Bove, F.; Mezzadri, U.; Capitani, D.; Sala, F.; Kanakaris, N.; et al. Orthopedic injuries in patients with multiple injuries: Results of the 11th trauma update international consensus conference Milan, December 11, 2017. J. Trauma Acute Care Surg. 2020, 88, e53–e76. [Google Scholar] [CrossRef]
- Bigsby, E.; Acharya, M.R.; Ward, A.J.; Chesser, T.J. The Use of Blood Cell Salvage in Acetabular Fracture Internal Fixation Surgery. J. Orthop. Trauma. 2013, 27, e230–e233. [Google Scholar] [CrossRef]
- Odak, S.; Raza, A.; Shah, N.; Clayson, A. Clinical efficacy and cost effectiveness of intraoperative cell salvage in pelvic trauma surgery. Ann. R. Coll. Surg. Engl. 2013, 95, 357–360. [Google Scholar] [CrossRef]
- Firoozabadi, R.; Swenson, A.; Kleweno, C.; Routt, M.C. Cell Saver Use in Acetabular Surgery: Does approach matter: Does approach matter? J. Orthop. Trauma 2015, 29, 349–353. [Google Scholar] [CrossRef]
- Jawed, A.; Ahmed, A.; Williams, M.R. Intra-operative cell salvage in pelvic and acetabular fracture surgery: A retrospective comparative study. Int. Orthop. 2018, 43, 1695–1699. [Google Scholar] [CrossRef]
- Rhee, P.; Inaba, K.; Pandit, V.; Khalil, M.; Siboni, S.; Vercruysse, G.; Kulvatunyou, N.; Tang, A.; Asif, A.; O’Keeffe, T.; et al. Early autologous fresh whole blood transfusion leads to less allogeneic transfusions and is safe. J. Trauma Acute Care Surg. 2015, 78, 729–734. [Google Scholar] [CrossRef]
- Salhanick, M.; Corneille, M.; Higgins, R.; Olson, J.; Michalek, J.; Harrison, C.; Stewart, R.; Dent, D. Autotransfusion of hemothorax blood in trauma patients: Is it the same as fresh whole blood? Am. J. Surg. 2011, 202, 817–822. [Google Scholar] [CrossRef]
- DuBose, J.; Inaba, K.; Demetriades, D.; Scalea, T.M.; O’connor, J.; Menaker, J.; Morales, C.; Konstantinidis, A.; Shiflett, A.; Copwood, B. Management of post-traumatic retained hemothorax: A prospective, observational, multicenter AAST study. J. Trauma Acute Care Surg. 2012, 72, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Harrison, H.B.; Smith, W.Z.; Salhanick, M.A.; Higgins, R.A.; Ortiz, A.; Olson, J.D.; Schwacha, M.G.; Harrison, C.R.; Aydelotte, J.D.; Stewart, R.M.; et al. An experimental model of hemothorax autotransfusion: Impact on coagulation. Am. J. Surg. 2014, 208, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.Z.; Harrison, H.B.; Salhanick, M.A.; Higgins, R.A.; Ortiz, A.; Olson, J.D.; Schwacha, M.G.; Harrison, C.R.; Aydelotte, J.D.; Stewart, R.M.; et al. A small amount can make a difference: A prospective human study of the paradoxical coagulation characteristics of hemothorax. Am. J. Surg. 2013, 206, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Suksompong, S.; Tassaneetrithep, B.; Ariyawatkul, T.; Sirivanasandha, B.; Wilartratsami, S.; Wongsa, A.; Von Bormann, B. Allogeneic red cell transfusion and its influence on relevant humoral and cellular immunological parameters. Eur. J. Anaesthesiol. 2019, 36, 814–824. [Google Scholar] [CrossRef]
- Jackman, R.; Utter, G.; Muench, M.; Heitman, J.W.; Munz, M.M.; Jackman, R.W.; Biswas, H.H.; Rivers, R.M.; Tobler, L.H.; Busch, M.P.; et al. Distinct roles of trauma and transfusion in induction of immune modulation after injury. Transfusion 2012, 52, 2533–2550. [Google Scholar] [CrossRef] [PubMed]
- Godinho, M.; Padim, P.; Evora, P.R.B.; Scarpelini, S. Curbing Inflammation in hemorrhagic trauma: A review. Rev. Col. Bras. Cir. 2015, 42, 273–278. [Google Scholar] [CrossRef]
- Dunne, J.R.; Malone, D.L.; Tracy, J.K.; Napolitano, L.M. Allogenic Blood Transfusion in the First 24 Hours after Trauma Is Associated with Increased Systemic Inflammatory Response Syndrome (SIRS) and Death. Surg. Infect. 2004, 5, 395–404. [Google Scholar] [CrossRef]
- Lannan, K.L.; Sahler, J.; Spinelli, S.L.; Phipps, R.P.; Blumberg, N. Transfusion immunomodulation—The case for leukoreduced and (perhaps) washed transfusions. Blood Cells Mol. Dis. 2012, 50, 61–68. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Lambris, J.; Ward, P.A. Innate immune responses to trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef]
- Fragkou, P.C.; Torrance, H.D.; Pearse, R.M.; Ackland, G.L.; Prowle, J.R.; Owen, H.C.; Hinds, C.J.; O’Dwyer, M.J. Perioperative blood transfusion is associated with a gene transcription profile characteristic of immunosuppression: A prospective cohort study. Crit. Care 2014, 18, 541. [Google Scholar] [CrossRef]
- Hart, S.; Cserti-Gazdewich, C.M.; McCluskey, S.A. Red cell transfusion and the immune system. Anaesthesia 2014, 70, 38-e16. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.L.; Van Stein, D.; Vlaar, A.P.J. Antibody-mediated transfusion-related acute lung injury; from discovery to prevention. Br. J. Haematol. 2015, 170, 597–614. [Google Scholar] [CrossRef] [PubMed]
- West, F.B.; Silliman, C.C. Transfusion-related acute lung injury: Advances in understanding the role of proinflammatory mediators in its genesis. Expert Rev. Hematol. 2013, 6, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Sayah, D.M.; Looney, M.R.; Toy, P. Transfusion Reactions. Crit. Care Clin. 2012, 28, 363–372. [Google Scholar] [CrossRef]
- 115. Tung, J.P.; Chiaretti, S.; Dean, M.M.; Sultana, A.J.; Reade, M.C.; Fung, Y.L. Transfusion-related acute lung injury (TRALI): Potential pathways of development, strategies for prevention and treatment, and future research directions. Blood Rev. 2022, 100926. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Mira, J.C.; Brakenridge, S.C.; Moldawer, L.L.; Moore, F.A. Persistent Inflammation, Immunosuppression and Catabolism Syndrome (PICS) HHS Public Access. Crit. Care Clin. 2017, 33, 245–258. [Google Scholar] [CrossRef]
- Roets, M.; Sturgess, D.J.; Obeysekera, M.P.; Tran, T.V.; Wyssusek, K.H.; Punnasseril, J.E.J.; Da Silva, D.; Van Zundert, A.; Perros, A.J.; Tung, J.P.; et al. Intraoperative Cell Salvage as an Alternative to Allogeneic (Donated) Blood Transfusion: A Prospective Observational Evaluation of the Immune Response Profile. Cell Transplant. 2020, 29. [Google Scholar] [CrossRef]
- Carroll, C.; Young, F. Intraoperative cell salvage. BJA Educ. 2021, 21, 95–101. [Google Scholar] [CrossRef]
| Topical coagulants, drugs | Activated leukocytes and platelets |
| Methyl methacrylate (bone cement) | Bacteria, endotoxins |
| Irrigation solutions (iodized) | Enzymes from cellular disruption |
| Topical antibiotics | Activated complement: C3a, C5a |
| Fat cells, malignant cells, mesothelial cells | Activated fibrinolytic products, plasmin |
| Bone splinters | Fibrin, split products, and D-dimers |
| Smoke from electrocautery (carbon oxide) | Organic fluids: faecal, urine, ascites, gastric acid, bile, amniotic fluid |
|
|
|
|
|
|
|
|
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora Miquel, L.; Manrique Muñoz, S.; Maegele, M. Cell Salvage in Oncological Surgery, Peripartum Haemorrhage and Trauma. Surgeries 2022, 3, 44-63. https://doi.org/10.3390/surgeries3010007
Mora Miquel L, Manrique Muñoz S, Maegele M. Cell Salvage in Oncological Surgery, Peripartum Haemorrhage and Trauma. Surgeries. 2022; 3(1):44-63. https://doi.org/10.3390/surgeries3010007
Chicago/Turabian StyleMora Miquel, Lidia, Susana Manrique Muñoz, and Marc Maegele. 2022. "Cell Salvage in Oncological Surgery, Peripartum Haemorrhage and Trauma" Surgeries 3, no. 1: 44-63. https://doi.org/10.3390/surgeries3010007
APA StyleMora Miquel, L., Manrique Muñoz, S., & Maegele, M. (2022). Cell Salvage in Oncological Surgery, Peripartum Haemorrhage and Trauma. Surgeries, 3(1), 44-63. https://doi.org/10.3390/surgeries3010007






