1. Introduction
Thyroid nodules are common, but thyroid carcinoma is a rare entity, compared to other solid tumors. Diagnosis is seldom made preoperatively, thus, the indication for surgery is generally the suspicion that a nodule might be malignant. Since most nodules turn out to be benign, diagnostic workflow and the interdisciplinary judgement of thyroid nodules, for example in an interdisciplinary endocrine board, are pivotal for reducing the number of “unnecessary surgeries”.
The standard diagnostic work up of thyroid nodules includes pertechnetate scintigraphy and sonography. Based on ultrasound features such as echogenity, “taller-than-wide” shape, irregular margins and microcalcifications, the TIRADS classification is used to assess the risk of malignancy of hypofunctional thyroid nodules [
1,
2].
Fine-needle aspiration cytology (FNAC) is an invasive diagnostic tool generally used either if the nodule needs to be proven benign for interventional, non-surgical treatment and conservative management or if ultrasound is not clearly suspicious. In case of a clearly malignant appearance in ultrasound (TIRADS 5), upfront surgery is often chosen. FNAC has been shown to produce hemorrhaging, siderophagia, granulation tissue, papillary hyperplasia, fibrosis, calcification, capsular distortion, cholesterol clefts and vascular thrombosis [
3]. Some of these alterations can even lead to misdiagnosis in favor of carcinoma [
3].
In addition to causing histologic changes, which might reduce its role in future guidelines in favor of ultrasound, in 20% of cases, FNAC results are not helpful, because cytological features lack specific characteristics needed for a definitive diagnosis [
4]. The Bethesda System for Reporting Thyroid Cytopathology [
5,
6] includes two common categories of indeterminate cytology: atypical or follicular lesion of undetermined significance (Bethesda category III) and follicular neoplasm/suspicious for follicular (or Hürthle cell) neoplasm (Bethesda category IV). The observed rates of malignancy in these categories are 6–48% for Bethesda III and 14–34% for Bethesda IV, varying widely among institutions [
4]. The reason for this is that intraobserver and interobserver bias can be high [
4].
Molecular testing of FNAC was developed over the past decade for improving diagnostic precision in these categories. Despite being technically feasible (they can be performed on stained FNA smears) and commercially available, molecular tests such as BRAFV600E, normalized concentrations of HMGA2 mRNA, microRNAs and mitochondrial/nuclear DNA ratio analyses are not yet established for the routine setting in most centers in Germany [
7]. Moreover, FNAC material is often not sufficient for molecular testing in our experience.
Tc-99m-methoxyisobutylisonitrile (MIBI) scintigraphy is less expensive than molecular testing [
8] and more easily available, but it is not currently included in guidelines and the algorithm for the management of indeterminate nodules. In this study, we aimed to review the use and the impact of MIBI scintigraphy for the management of indeterminate nodules at our German interdisciplinary university center. Secondly, we aimed to determine whether a MIBI match might obviate the need for surgery in the indeterminate thyroid nodules Bethesda III and IV at our institution.
2. Materials and Methods
Electronic and paper records of the endocrine tumor board and Departments of Nuclear Medicine, Endocrinology, Surgery and Pathology, beside the records of the Endocrine Board, were screened to find patients with hypofunctional nodules, undergoing both thyroid fine needle aspiration (FNA) and Tc 99m-MIBI-scintigraphy (MIBI) before surgery during a period of four years (1 January 2015 until 31 December 2018).
The diagnostic workflow at our institution includes ultrasound and pertechnetate scintigraphy for all nodules. All clearly malignant nodules 5 and some TIRADS 4b nodules are referred directly to surgery, generally without further examination, if patients agree with the recommendation. Undetermined TIRADS 4a or growing TIRADS 3 hypofunctional nodules, in addition to some TIRADS 4b nodules in patients unwilling to be operated on, usually undergo MIBI-scintigraphy and/or FNA, in order to increase diagnostic precision. There is no standard order, however, and sometimes MIBI is performed before and sometimes after FNA, depending on the judgment of the examining physician and the preferences and wishes of patients. In many cases, it is performed as a subsequent diagnostic step after sonography and pertechnetate scintigraphy, before FNA, because it is noninvasive.
All findings (sonography, scintigraphy, MIBI and/or FNA, if available) are presented and discussed in the interdisciplinary endocrine board, including nuclear medicine, endocrinology, pathology and surgery, for generating interdisciplinary official recommendations, conforming to the current guidelines but also taking into account, age, comorbidities and the wishes of patients.
All FNAs included in the present study were performed in our Nuclear Medicine Department and were analyzed in our Department for Pathology. Bethesda categories were defined as follows: Category I = nondiagnostic, Category II = benign, Category III = atypia of undetermined significance, Category IV = suspicious for follicular neoplasm, Category V = suspicious for malignancy, and Category VI = malignant. All cases in which FNA was not performed (in cases when the patients refused it or requested upfront surgery, mostly because of symptoms) were excluded. BRAF mutation analysis was not yet routinely performed between 2015 and 2018 at our institution (only in selected cases).
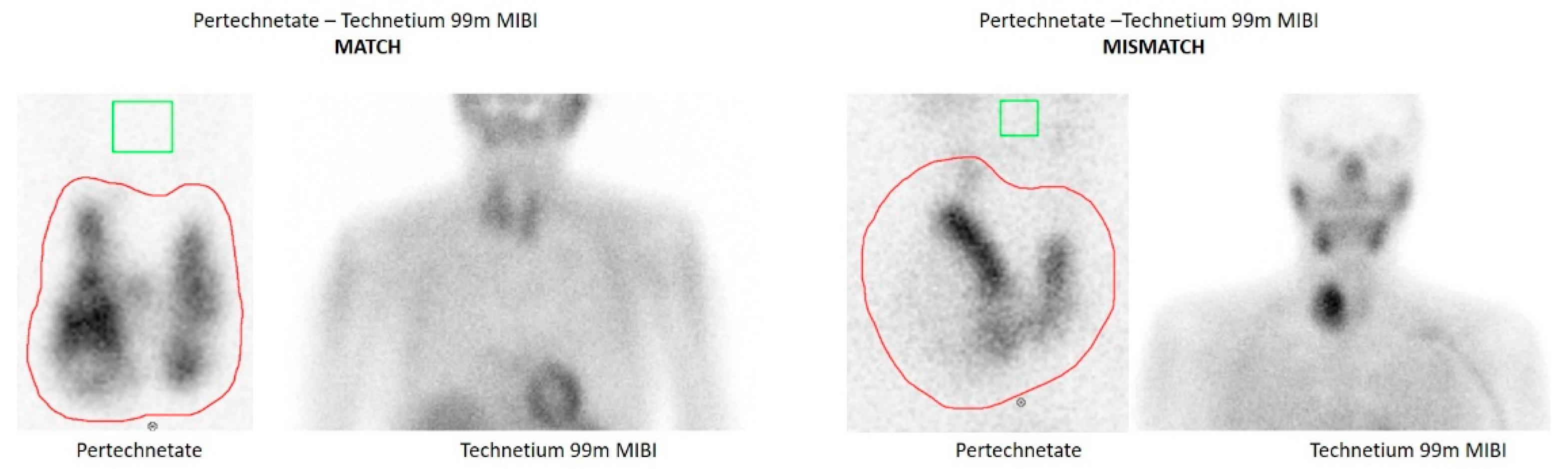
MIBI examinations were performed in our Nuclear Medicine Department. Otherwise MIBI scans were demonstrated and discussed in the endocrine board, when available, in order to minimize subjectivity of their evaluation. Tc-99m-MIBI images were classified as a “match” (no uptake compared to normal thyroid tissue), as an intermediate finding (isointense uptake to normal thyroid tissue), or a “mismatch” (significant uptake compared to normal thyroid tissue and in contrast to the lacking uptake in pertechnetate scintigraphy).
Figure 1 depicts two examples of MIBI match and mismatch results.
Indication for surgery was based on all available information, including patient age, symptoms (e.g., neck discomfort caused by the thyroid nodule), findings of cervical ultrasound and other imaging results such as pertechnetate and Tc-99m-MIBI scintigraphy and results of FNA. Most surgeries were performed as hemithyroidectomies and thyroidectomies by two experienced and certified Fellows of the European Board of Surgery (FEBS) for endocrine surgery.
Data were analyzed using IBM SPSS Statistics for Windows, Version 25.0 (Armonk, NY, USA).
This study is based solely on a retrospective analysis of anonymized routine patient data, who underwent common treatment at our university hospital. Data were collected by medical staff and analyzed in an anonymized fashion, according to the ethical code of our university.
3. Results
In 128 (32.6%) of 392 cases of hypofunctional thyroid nodules (HTN) presented for surgical treatment in the endocrine board, a Tc-99m-methoxyisobutylisonitrile (MIBI) scintigraphy result was taken into account, in addition to ultrasound and pertechnetate scintigraphy, which are routinely performed at our institution.
In 78 (19.9%) of these 128 cases, invasive diagnostic in the form of FNA was additionally performed in order to further increase diagnostic precision. However, in 49 (62.8%) patients, FNA delivered indeterminate (Bethesda III and IV) cytological diagnoses, in addition to 2 (2.56%) non-diagnostic Bethesda I, 23 (29.5%) Bethesda II and 4 (5.1%) Bethesda V diagnoses.
The present study focused on these 49 cytological indeterminate cases, in order to determine whether the MIBI result might be helpful in risk stratifying these categories with malignancy rates of 6–48% and 14–34%, varying widely among institutions [
2].
Table 1 summarizes the characteristics of the 49 patients with indeterminate cytology included in the present study.
99mcTc MIBI scintigraphy delivered match results in 8 (21.1%) of the 38 Bethesda III tumors, mismatch in 22 (57.9%), and intermediary results 8 (21%). In the Bethesda IV group (
n = 11), there were two (18.2%) match and nine (81.8%) mismatch results (
Table 2).
Table 2 summarizes the results of MIBI scintigraphy in indeterminate Bethesda III and IV nodules.
A differentiated thyroid cancer (DTC) was diagnosed in three (7.7%) of the Bethesda III patients, all displaying mismatch or intermediary MIBI results. In only one case, a match result turned out to be a papillary microcarcinoma.
Only one Bethesda IV patient (9%) with a MIBI mismatch result had two papillary microcarcinomas of 2 and 9 mm, respectively (
Table 3).
Table 3 summarizes the malignancy rates of MIBI match and non-match Bethesda III and IV nodules, respectively.
Tc99m-MIBI scintigraphy had a negative predictive value of 90% (95% CI 58.7–98.3%) and a positive predictive value of 10.3% (95% CI 6.7–15.4%) in indeterminate Bethesda III and IV nodules. Sensitivity was 80% (95% CI 28.4–99.5%) and specificity was 20.4% (95% CI 9.8–35.3%). If the microcarcinomas are excluded, the NPV turns out to be 100%, the PPV 7.69% (95% CI 6.68–8.85%), the sensitivity 100.00% (95% CI 29.24–100.00%), and the specificity 21.74% (95% 10.95–36.36%).
Relying on the negative MIBI scintigraphy results might have spared 10 (20.4%) surgeries and missed one papillary microcarcinoma.
Thirty-five (71.4%) patients with indeterminate cytological results and non-match (mismatch and intermediary) results in the MIBI scan underwent surgery but turned out to have benign nodules.
4. Discussion
Tc-99m-methoxyisobutylisonitrile (MIBI) is a lipophilic, monovalent, cationic complex, which is taken up by mitochondria. The uptake by parathyroid tumors was first published in 1989 by Coakley et al. [
9]. The pathophysiologic explanation for Tc99m MIBI retention is the presence of mitochondria-rich oxyphil cells, for example, in parathyroid tissue, causing a slower wash-out than in mitochondria-poorer tissue [
10]. Routine use of Tc99mMIBI for preoperative localization of parathyroid adenomas sometimes leads to the identification of MIBI-positive thyroid hypofunctional nodules. Sixty-one percent of the incidentally diagnosed thyroid carcinomas were MIBI-positive in the study of Greilsamer et al. [
11], leading the authors to suggest that thyroid nodules incidentally discovered on MIBI in hyperparathyroidism patients should be resected. In their study, the sensitivity, specificity, positive predictive value, and negative predictive value of MIBI were 61, 78, 50, and 85%, respectively [
11].
Whether Tc99m MIBI scintigraphy should be routinely used alongside sonography and FNA for the diagnostic work up of hypofunctional thyroid nodules is still a matter of discussion. MIBI scintigraphy does not currently play a role in the guidelines or algorithms of diagnostic work-up of thyroid nodules, although more than ten studies demonstrated a high negative predictive value of ≥97% for ruling out malignancy in the case of a match between pertechnetate and MIBI scintigraphy [
9]. In contrast, a positive Tc-99m-MIBI does not seem to sufficiently differentiate between a benign and a malignant nodule. In Germany, three studies revealed a positive predictive value of thyroid malignancy in the case of a mismatch between 15 and 20% [
12,
13,
14]. A recent meta-analysis, however, showed both low sensitivity and specificity [
15].
The first finding of the present study is that MIBI scintigraphy, in addition to FNAC, was only performed in 19.9% of patients with suspicious sonographic hypofunctional nodules, and it is therefore still underused at our institution. It is likely that patients with symptoms were directly referred to surgery without MIBI, thus explaining the low number of examinations. On the other hand, patients with MIBI match might have been treated conservatively, without discussion by the tumor board, and are therefore not included in the present analysis.
Secondly, we observed that Tc-99m-MIBI scintigraphy had a high negative predictive value of 90–100% (95% CI 58.7–98.3%), with only one carcinoma diagnosis of a 9 mm pT1a microcarcinoma missed. These results are concordant with most literature [
11]. Thus, relying on a negative MIBI match despite an indeterminate cytological result and choosing conservative treatment might have obviated the need for 10 (20.4%) surgeries. However, one 9 mm papillary microcarcinoma would have been missed. The possible delay in the case diagnosis that was made during follow up, however, would probably cause no significant consequences for the oncologic outcome of the patient.
The reason why some benign thyroid nodules retain MIBI is still unclear. Some parathyroid adenomas are negative in the MIBI scan, preventing the pathologist from finding a significant histopathologic correlation for MIBI retention. The volume of the adenomas, the incidence of cystic adenomas, cell-type dominance (oxyphilic cell), percent fat, and the Ki-67 ratio were not found to be significantly different in MIBI-positive and -negative parathyroid adenomas in a recent study [
16], as opposed to an older study [
17]. The rate of hyalinization, however, was 13% in MIBI-positive and 28% in MIBI-negative subjects, the difference being statistically significant (
p = 0.04) [
16]. The expression of proliferative cell nuclear antigen (PCNA) was greater in parathyroid glands with high MIBI scores compared to those with low scores in another study [
18]. PCNA is a key protein of abnormal cell proliferation. Its gene is highly expressed in many tumors, including breast, lung, gastric, liver, colon and bladder cancer [
19]. Two studies revealed a remarkably higher positive rate of PCNA in thyroid carcinoma tissue in contrast to normal thyroid tissue [
19,
20,
21]. The PCNA expression was also found to correlate with echo, calcification, and blood flow in thyroid ultrasound [
19]. According to a number of reports, thyroid cancer development follows a slow process. Ultrasound proves that an irregular or blurry nodule edge as well as vascular pattern and microcalcifications inside the nodule are closely associated with malignant changes of the tumor. Thus, it might be possible that MIBI-positive nodules, which turn out to be benign at the time of surgery, have a higher risk of progressing into malignant nodules. This might be a subject for future research.
Besides the small number of patients included, and the selection bias associated with a surgical collective, hypofunctional nodules which were clearly malignant by ultrasound or FNAC (Bethesda V and VI) most probably did not even undergo MIBI scintigraphy but upfront surgery, and are therefore not part of this collective. This explains the low positive predictive value of MIBI scintigraphy in this study. These limitations must be taken into account in the interpretation of the present study. However, the negative predictive value of 90–100% has been reported in several other studies [
11] and deserves more clinical regard in our opinion, especially considering that most thyroid carcinomas have an indolent development and an excellent prognosis. The following workflow might be tested in future clinical studies (
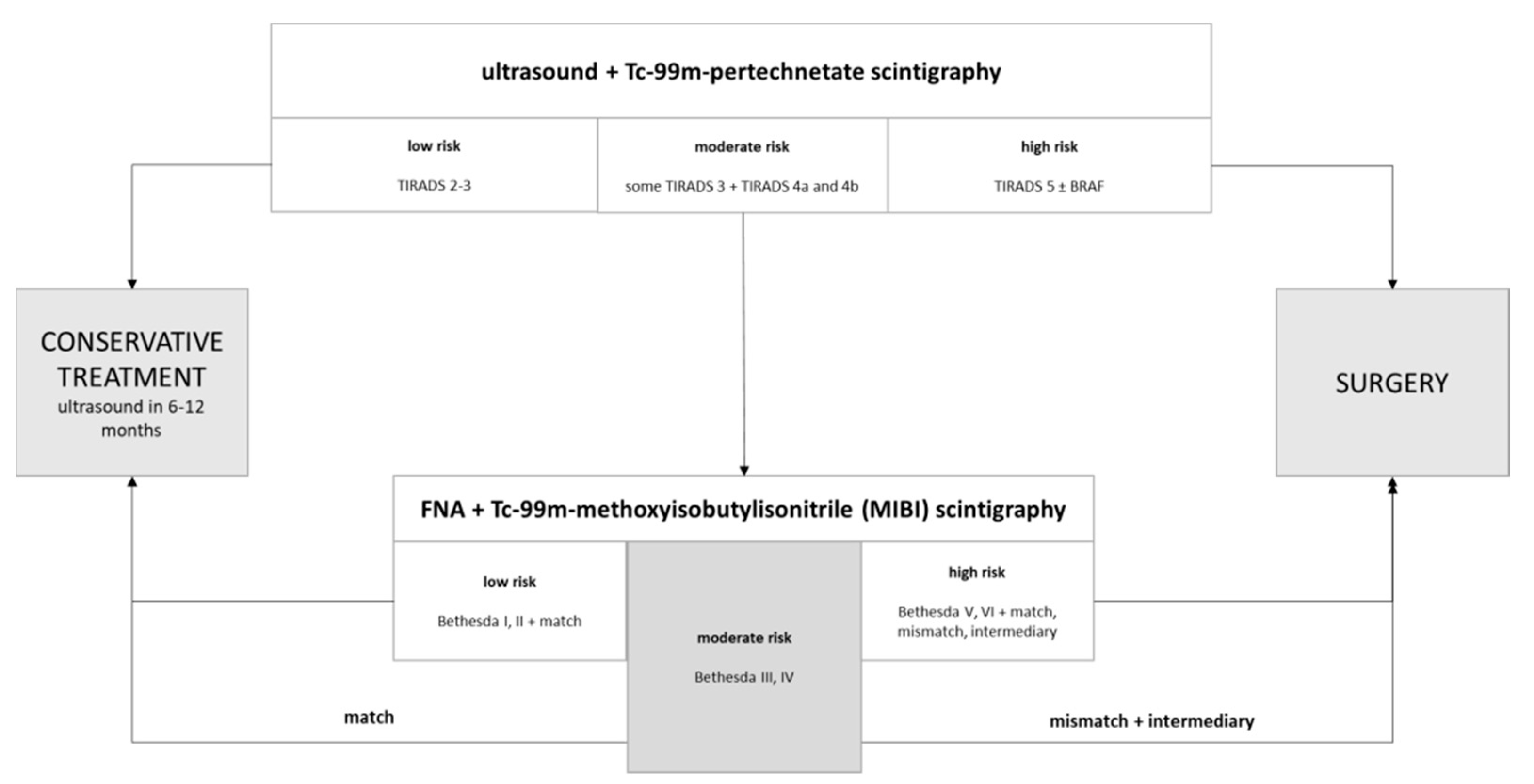
Figure 2) with larger patient sample collectives and in a prospective manner.
5. Conclusions
This study suggests that relying on the negative predictive value of MIBI match results might have obviated the need for surgery in some cases of indeterminate cytological nodules. MIBI scintigraphy has an underused potential, which deserves more attention in our opinion.
Author Contributions
Conceptualization, C.C., J.M., M.F. and M.S.; methodology, S.A., J.M., M.E., A.M.S.; software, S.A.; validation, S.A., J.M. and C.C.; formal analysis, S.A., J.M. and C.C.; investigation, S.A. and J.M.; data curation, S.A. and J.M.; writing—Original draft preparation, C.C. and S.A.; writing—Review and editing S.A., J.M., M.S., M.F., M.E., A.M.S. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it is based solely on retrospectively analyzed anonymized routine data of patients, who underwent common treatment at our university hospital. Data were collected by medical staff analyzed in an anonymized fashion, according to the ethical code of our university.
Informed Consent Statement
Patient consent was waived because routine data of patients, who underwent common treatment at our university hospital were collected by medical staff and analyzed in an anonymized fashion. No patient can be identified based on the information contained in this study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Acknowledgments
We would like to thank Elizabeth Christodoulou for English language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kwak, J.Y.; Han, K.H.; Yoon, J.H.; Moon, H.J.; Son, E.J.; Park, S.H.; Jung, H.K.; Choi, J.S.; Kim, B.M.; Kim, E.K. Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk. Radiology 2011, 260, 892–899. [Google Scholar] [CrossRef] [Green Version]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Bolat, F.; Kayaselcuk, F.; Nursal, T.Z.; Reyhan, M.; Bal, N.; Yildirim, S.; Tuncer, I. Histopathological changes in thyroid tissue after fine needle aspiration biopsy. Pathol Res. Pr. 2007, 203, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A.; Sipos, J.A.; Figge, J.J.; Mandel, S.; Haugen, B.R.; Burman, K.D.; et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules With Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2019, 5, 204–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BBaloch, Z.W.; LiVolsi, V.A.; Asa, S.L.; Rosai, J.; Merino, M.J.; Randolph, G.; Vielh, P.; DeMay, R.M.; Sidawy, M.K.; Frable, W.J. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008, 36, 425–437. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Titov, S.; Demenkov, P.S.; Lukyanov, S.A.; Sergiyko, S.V.; Katanyan, G.A.; Veryaskina, Y.A.; Ivanov, M.K. Preoperative detection of malignancy in fine-needle aspiration cytology (FNAC) smears with indeterminate cytology (Bethesda III, IV) by a combined molecular classifier. J. Clin. Pathol. 2020, 73, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, A.; Müller, D.; Behrendt, F.F.; Giovanella, L.; Mottaghy, F.M.; Verburg, F.A. Thyroid nodules with indeterminate cytology: Molecular imaging with (9)(9)mTc-methoxyisobutylisonitrile (MIBI) is more cost-effective than the Afirma gene expression classifier. Eur J. Nucl Med. Mol. Imaging 2014, 41, 1497–1500. [Google Scholar] [CrossRef]
- Coakley, A.J.; Kettle, A.G.; Wells, C.P.; O’Doherty, M.J.; Collins, R.E. 99Tcm sestamibi—A new agent for parathyroid imaging. Nucl Med. Commun. 1989, 10, 791–794. [Google Scholar] [CrossRef]
- Patel, C.N.; Salahudeen, H.M.; Lansdown, M.; Scarsbrook, A.F. Clinical utility of ultrasound and 99mTc sestamibi SPECT/CT for preoperative localization of parathyroid adenoma in patients with primary hyperparathyroidism. Clin. Radiol. 2010, 65, 278–287. [Google Scholar] [CrossRef]
- Greilsamer, T.; Blanchard, C.; Christou, N.; Drui, D.; Ansquer, C.; Le Bras, M.; Cariou, B.; Caillard, C.; Mourrain-Langlois, E.; Delemazure, A.S.; et al. Langenbecks Management of thyroid nodules incidentally discovered on MIBI scanning for primary hyperparathyroidism. Arch. Surg. 2015, 400, 313–318. [Google Scholar] [CrossRef]
- Schmidt, M.; Schenke, S. Update 2019 zur MIBI-Szintigraphie bei hypofunktionellen Schilddrüsenknoten. Der Nuklearmediziner 2019, 42, 174–182. [Google Scholar] [CrossRef]
- Theissen, P.; Schmidt, M.; Ivanova, T.; Dietlein, M.; Schicha, H. MIBI scintigraphy in hypofunctioning thyroid nodules—Can it predict the dignity of the lesion? Nuklearmedizin 2009, 48, 144–152. [Google Scholar] [CrossRef]
- Leidig-Bruckner, G.; Cichorowski, G.; Sattler, P.; Bruckner, T.; Sattler, B. Evaluation of thyroid nodules—Combined use of (99m)Tc-methylisobutylnitrile scintigraphy and aspiration cytology to assess risk of malignancy and stratify patients for surgical or nonsurgical therapy—A retrospective cohort study. Clin. Endocrinol (Oxf.) 2012, 76, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Schenke, S.; Zimny, M.; Rink, T.; Stahl, U.; Fritzel, M.; Klett, R. 99mTc-MIBI scintigraphy of hypofunctional thyroid nodules. Comparison of planar and SPECT imaging. Nuklearmedizin 2014, 53, 105–110. [Google Scholar] [PubMed]
- Kim, S.J.; Lee, S.W.; Jeong, S.Y.; Pak, K.; Kim, K. Diagnostic Performance of Technetium-99m Methoxy-Isobutyl-Isonitrile for Differentiation of Malignant Thyroid Nodules: A Systematic Review and Meta-Analysis. Thyroid 2018, 28, 1339–1348. [Google Scholar] [CrossRef]
- Ozderya, A.; Temizkan, S.; Gul, A.E.; Ozugur, S.; Cetin, K.; Aydin, K. Biochemical and pathologic factors affecting technetium-99m-methoxyisobutylisonitrile imaging results in patients with primary hyperparathyroidism. Ann. Nucl. Med. 2018, 32, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, S.; Hidai, H.; Chiba, T.; Takagi, Y.; Nagatani, Y.; Matsubara, S. Hyperfunctional parathyroid glands with 99mTc-MIBI scan: Semiquantitative analysis correlated with histologic findings. J. Nucl Med. 1999, 40, 1792–1797. [Google Scholar]
- Custódio, M.R.; Montenegro, F.; Costa, A.F.; dos Reis, L.M.; Buchpiguel, C.A.; Oliveira, S.G.; Noronha, I.L.; Moysés, R.M.; Jorgetti, V. MIBI scintigraphy, indicators of cell proliferation and histology of parathyroid glands in uraemic patients. Nephrol. Dial. Transpl. 2005, 20, 1898–1903. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Yao, X.; Zhou, J.; Zhou, H.; Lu, G.; Wang, Y. Correlations of PCNA expression with thyroid cancer ultrasound and histopathologic features. Int J. Clin. Exp. Pathol. 2019, 12, 1378–1384. [Google Scholar]
- Inoue, A.; Kikuchi, S.; Hishiki, A. A small molecule inhibitor of monoubiquitinated proliferating cell nuclear antigen (PCNA) inhibits repair of interstrand DNA crosslink, enhances DNA double-strand break, and sensitizes cancer cells to cisplatin. J. Biol. Chem. 2014, 289, 7109–7120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).