Clinical Impact of Preoperative Anemia in Patients Undergoing Peripheral Vascular Interventions: A Systematic Review
Abstract
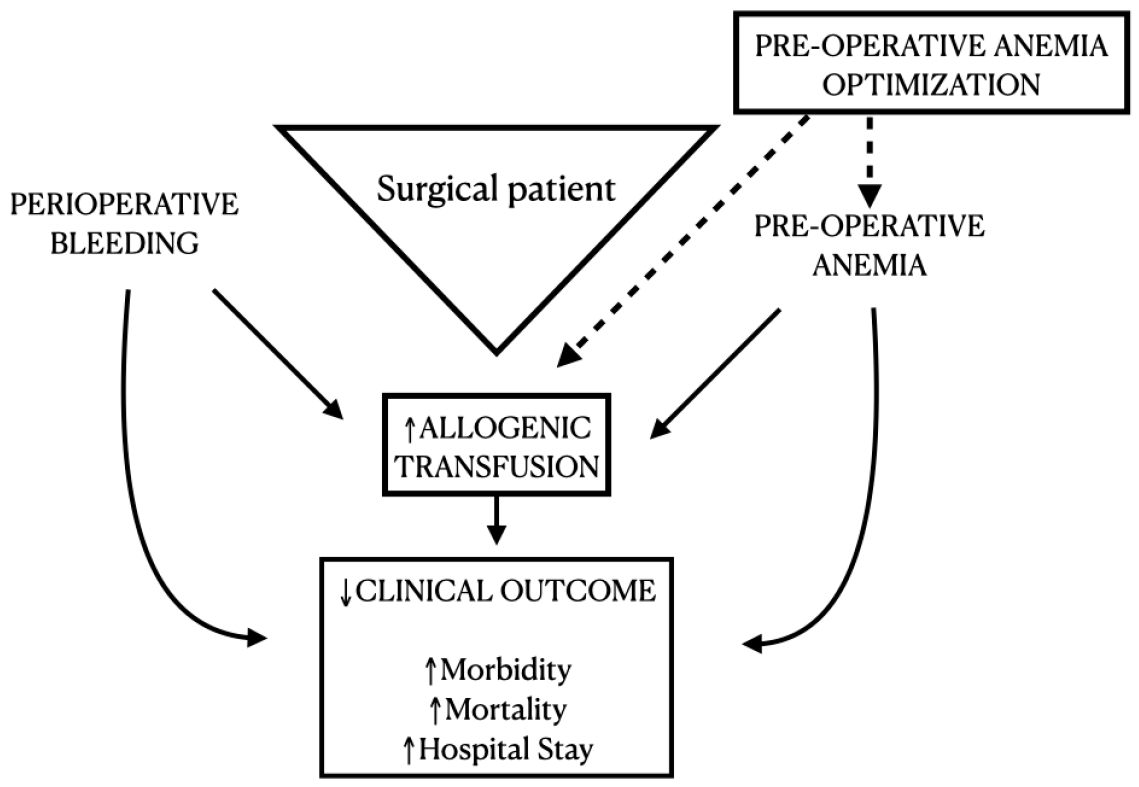
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Data Source and Searches
2.3. Selection of Studies
2.4. Data Extraction, the Risk of Bias Assessment, and Certainty of Evidence
3. Results
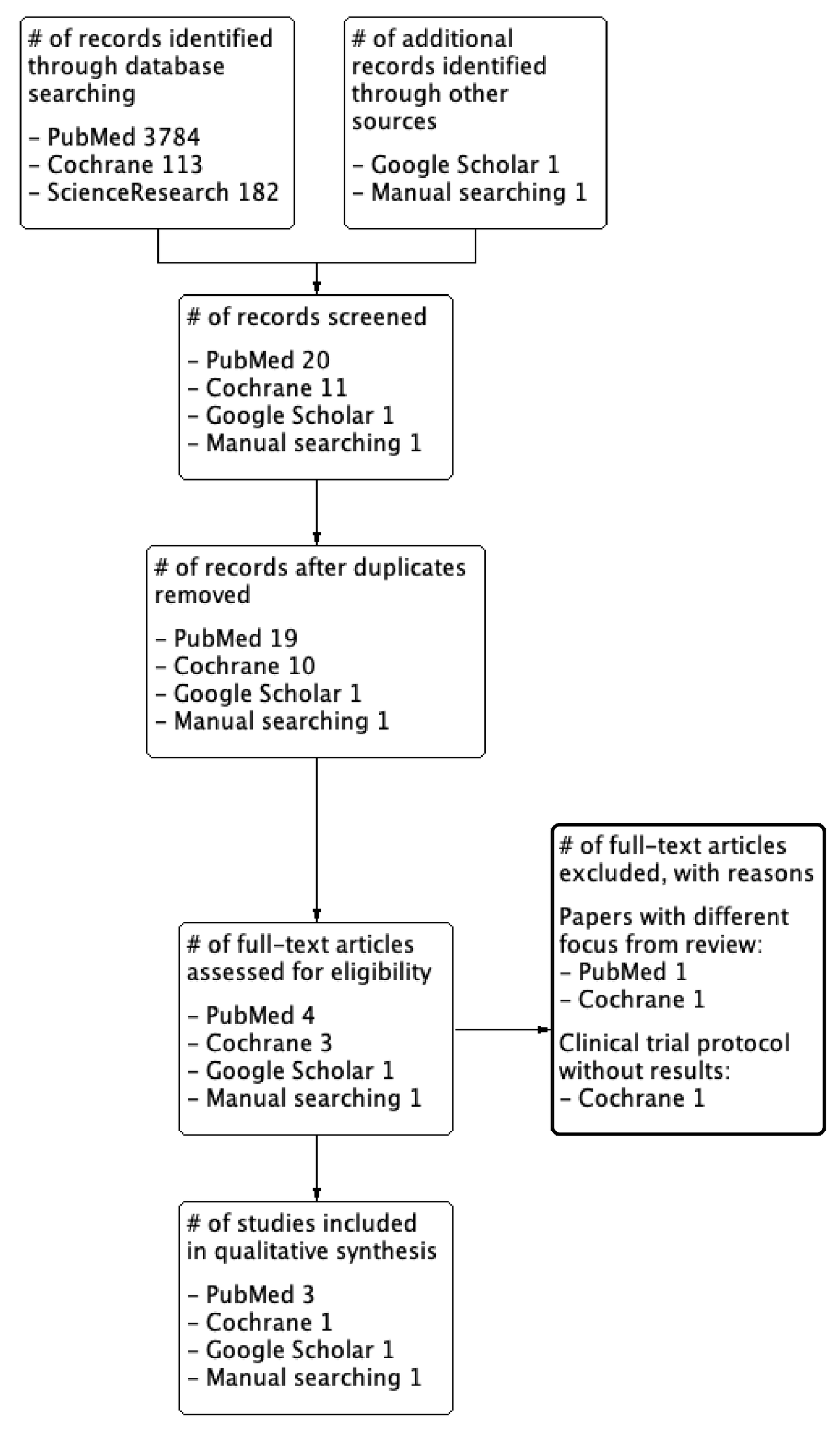
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Observational Prospective Studies
3.4. Observational Retrospective Studies
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esteban, C.; Rodríguez, P.; Escudero, J.R.; Clarà, A.; Fernández, A.; Fernández, S.; Agúndez, I. Anaemia in patients who underwent vascular surgery: A significant predictor of amputation and death. Med. Clín. 2019, 152, 6–12. [Google Scholar] [CrossRef]
- Muñoz, M.; Acheson, A.G.; Auerbach, M.; Besser, M.; Habler, O.; Kehlet, H.; Liumbruno, G.M.; Lasocki, S.; Meybohm, P.; Baikady, R.R.; et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2016, 72, 233–247. [Google Scholar] [CrossRef]
- Desormais, I.; Aboyans, V.; Bura, A.; Constans, J.; Cambou, J.-P.; Messas, E.; Labrunie, A.; Lacroix, P. Anemia, an Independent Predictive Factor for Amputation and Mortality in Patients Hospitalized for Peripheral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 202–207. [Google Scholar] [CrossRef]
- Leal-Noval, S.R.; Muñoz-Gómez, M.; Jiménez-Sánchez, M.; Cayuela, A.; Leal-Romero, M.; Puppo-Moreno, A.; Enamorado, J.; Arellano-Orden, V. Red blood cell transfusion in non-bleeding critically ill patients with moderate anemia: Is there a benefit? Intensive Care Med. 2013, 39, 445–453. [Google Scholar] [CrossRef]
- Muñoz, M.; Gómez-Ramírez, S.; Campos, A.; Ruiz, J.; Liumbruno, G.M. Pre-operative anaemia: Prevalence, consequences and approaches to management. Blood Transfus. 2015, 13, 370–379. [Google Scholar]
- Klein, A.J.; Ross, C.B. Endovascular treatment of lower extremity peripheral arterial disease. Trends Cardiovasc. Med. 2016, 26, 495–512. [Google Scholar] [CrossRef]
- Dua, A.; Lee, C.J. Epidemiology of Peripheral Arterial Disease and Critical Limb Ischemia. Tech. Vasc. Interv. Radiol. 2016, 19, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ambulgekar, N.V.; Grey, S.F.; Rosman, H.S.; Othman, H.; Davis, T.P.; Nypaver, T.J.; Schreiber, T.; Yamasaki, H.; Lalonde, T.; Henke, P.K.; et al. Association of Anemia with Outcomes in Patients Undergoing Percutaneous Peripheral Vascular Intervention: Insights From the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2 VIC). J. Invasive Cardiol. 2018, 30, 35–42. [Google Scholar] [PubMed]
- Althoff, F.C.; Neb, H.; Herrmann, E.; Trentino, K.M.; Vernich, L.; Füllenbach, C.; Freedman, J.; Waters, J.H.; Farmer, S.; Leahy, M.F.; et al. Multimodal Patient Blood Management Program Based on a Three-pillar Strategy: A Systematic Review and Me-ta-analysis. Ann. Surg. 2019, 269, 794–804. [Google Scholar] [CrossRef]
- Toor, I.S.; Jaumdally, R.J.; Moss, M.S.; Babu, S.B. Preprocedural hemoglobin predicts outcome in peripheral vascular disease patients undergoing percutaneous transluminal angioplasty. J. Vasc. Surg. 2009, 50, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Li, X.-Q.; Zhou, Q.-H.; Moher, D.; Ling, C.; Yu, W.-F. From QUOROM to PRISMA: A Survey of High-Impact Medical Journals’ Instructions to Authors and a Review of Systematic Reviews in Anesthesia Literature. PLoS ONE 2011, 6, e27611. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Montori, V.; Akl, E.A.; Djulbegovic, B.; Falck-Ytter, Y.; et al. GRADE guidelines: 4. Rating the quality of evidence—Study limitations (risk of bias). J. Clin. Epidemiol. 2011, 64, 407–415. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Rind, D.; Devereaux, P.J.; Montori, V.M.; Freyschuss, B.; Vist, G.; et al. GRADE guidelines: 6. Rating the quality of evidence—Imprecision. J. Clin. Epidemiol. 2011, 64, 1283–1293. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE Working Group. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Falck-Ytter, Y.; Jaeschke, R.; Vist, G.; et al. GRADE Working Group. GRADE guidelines: 8. Rating the quality of evidence—Indirectness. J. Clin. Epidemiol. 2011, 64, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Montori, V.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Djulbegovic, B.; Atkins, D.; Falck-Ytter, Y.; et al. GRADE guidelines: 5. Rating the quality of evidence—Indirectness. J. Clin. Epidemiol. 2011, 64, 1277–1282. [Google Scholar] [CrossRef]
- Mensel, C.; Juhl-Olsen, P.; Guldbrand, V.; Eivindson, M.; Eldrup, N. Preoperative Optimisation in Vascular Patients to Reduce Peri- and Postoperative Blood Transfusions and Postoperative Complications—A Proof of Concept Study. Eur. J. Vasc. Endovasc. Surg. 2019, 58, e752. [Google Scholar] [CrossRef]
- Chau, M.; Richards, T.; Evans, C.; Butcher, A.; Collier, T.; Klein, A. The UK Cardiac and Vascular Surgery Interventional Anaemia Response (CAVIAR) Study: Protocol for an observational cohort study to determine the impact and effect of pre-operative anaemia management in cardiac and vascular surgical patients. BMJ Open 2017, 7, e014872. [Google Scholar] [CrossRef]
- Henke, P.K.; Park, Y.J.; Hans, S.; Bove, P.; Cuff, R.; Kazmers, A.; Schreiber, T.; Gurm, H.S.; Grossman, P.M. The Association of Peri-Procedural Blood Transfusion with Morbidity and Mortality in Patients Undergoing Percutaneous Lower Extremity Vascular Interventions: Insights from BMC2 VIC. PLoS ONE 2016, 11, e0165796. [Google Scholar] [CrossRef]
- Kolte, D.; Kennedy, K.; Shishehbor, M.; Abbott, J.; Khera, S.; Soukas, P. Thirty-Day Readmissions After Endovascular or Surgical Therapy for Critical Limb Ischemia: Analysis of the 2013 to 2014 Nationwide Readmissions Databases. J. Vasc. Surg. 2017, 66, 1911. [Google Scholar] [CrossRef][Green Version]
- Kougias, P.; Sharath, S.; Barshes, N.R.; Chen, M.; Mills, J.L. Effect of postoperative anemia and baseline cardiac risk on serious adverse outcomes after major vascular interventions. J. Vasc. Surg. 2017, 66, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R. Anemia and patient blood management in hip and knee surgery: A systematic review of the literature. Anesthesiology 2010, 113, 482–495. [Google Scholar] [CrossRef]
- Morton, L.J.; Konrad, K.L.; Xu, T.J.; Lightfoot, N.J. The interaction between pre-operative anaemia and peri-operative blood transfusion on patient outcomes following general surgical procedure: A retrospective review. N. Z. Med. J. 2019, 132, 13–24. [Google Scholar]
- Clevenger, B.; Richards, T. Pre-operative anaemia. Anaesthesia 2014, 70, 20-e8. [Google Scholar] [CrossRef] [PubMed]
- Kozek-Langenecker, S.A.; Ahmed, A.B.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology: First update 2016. Eur. J. Anaesthesiol. 2017, 34, 332–395. [Google Scholar] [CrossRef]
- Oshin, O.A.; Torella, F. Low Hemoglobin Concentration Is Associated with Poor Outcome after Peripheral Arterial Surgery. Vasc. Endovasc. Surg. 2013, 47, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Burton, B.; A’Court, A.M.; Brovman, E.Y.; Scott, M.J.; Urman, R.D.; Gabriel, R.A. Optimizing Preoperative Anemia to Improve Patient Outcomes. Anesthesiol. Clin. 2018, 36, 701–713. [Google Scholar] [CrossRef] [PubMed]
- De Céniga, M.V.; Bravo, E.; Izagirre, M.; Casco, C.; Estallo, L.; Esteban, M.; Barba, A. Anaemia, Iron and Vitamin Deficits in Patients with Peripheral Arterial Disease. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 828–830. [Google Scholar] [CrossRef]
- Girelli, D.; Ugolini, S.; Busti, F.; Marchi, G.; Castagna, A. Modern iron replacement therapy: Clinical and pathophysiological insights. Int. J. Hematol. 2017, 107, 16–30. [Google Scholar] [CrossRef]
- Diez, A.A. Impact of Intravenous Iron Treatment of Preoperative Anemia in Patients with LEAD (IRONPAD). Available online: https://clinicaltrials.gov/ct2/show/NCT04083755 (accessed on 18 April 2020).
| Author Year | Country | Type of Study | Number of Randomized Participants or Observational Participants | Mean Age (Years) Per Group Being Studied | Male Gender per Group | Inclusion Criteria | Exclusion Criteria | Follow-up Time (Days) |
|---|---|---|---|---|---|---|---|---|
| Mensel (2019) [18] | Denmark | Observational intervention study; ongoing | I: NA C: NA | I: NA C: NA | I: NA C: NA | Iron-deficiency anemic patients in the interventional group; Vascular anemic patients; | NA | Perioperative period |
| Chau (2017) [19] | UK | Observational intervention study; ongoing | I: 144 C: 288 | I: NA C: NA | I: NA C: NA | Patients ≥ 18 years old; Undergoing elective cardiac or vascular surgery; Written informed consent | Patients who are pregnant or lactating; Undergoing renal dialysis (current or planned within the next 12 months); Prisoners; Patients with history of learning disabilities; Adults without mental capacity to consent for themselves. | 30 days |
| Henke (2017) [20] | USA | Prospective, multicenter observational | Total of 23,273; cases between 2010 and 2014 at 47 hospitals | No transfusion patients: 68.3 ± 11.4 Transfusion patients: 71.5 ± 12.2 | No transfusion patients: 13,152 (59%) Transfusion patients: 413 (41.9%) | All patients undergoing elective, urgent, or emergent percutaneous vascular interventions | Age < 18 years old; Critical missing variables; Patients under primary renal, mesenteric, and carotid artery stenting; Hybrid open surgical–endoluminal therapy | Measure until hospital discharge (median of 24 h) |
| Esteban (2019) [1] | Spain | Retrospective multicentric observational | A total of 518; cases between 1 February 2014 and 31 March 2014 at 12 vascular surgery units | NA | 414 (80.1%) | Patients under vascular surgery, in 12 vascular surgery units within the 2 months preceding the study | Emergency surgery; carotid surgery; surgery under ischemic stroke; Patients who had been readmitted to the clinic, to avoid duplicate cases | At least 1 year |
| Kolte (2017) [21] | USA | Retrospective observational | 60,998; 2013 to 2014 cases of Nationwide Readmissions Database | 68.9 ± 11.9 | 36,110 (59.2%) | Readmissions after critical limb ischemia hospitalization Between 2013 and 2014 | Discharge month: December; The patient died during the index hospitalization; Carotid surgery; Discharge was unknown or patient left against medical advice; No endovascular or surgical revascularization or amputation was performed during hospitalization; The patient had two hospitalizations within a 30 day period | Readmissions. 30-day readmissions were 20.4% |
| Kougias (2017) [22] | USA | Retrospective observational | 2106 patients; 2508 surgeries | 67 ± 8.1 | NA | Elective open procedures for occlusive vascular disease; Open or endovascular aneurysm repair; From May 2008 to December 2015 | Patients undergoing peripheral endovascular only interventions; Hemodialysis; Venous interventions | Postoperative period |
| Transfusion | No Transfusion | Total | |
|---|---|---|---|
| Anemic | 820 | 8851 | 9671 |
| Nonanemic | 165 | 13,437 | 13,602 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linhares, R.M.; Bersot, C.D.A.; Pereira, J.E.G.; Galhardo, C., Jr.; Lessa, M.A.; Kietaibl, S. Clinical Impact of Preoperative Anemia in Patients Undergoing Peripheral Vascular Interventions: A Systematic Review. Surgeries 2021, 2, 268-277. https://doi.org/10.3390/surgeries2030027
Linhares RM, Bersot CDA, Pereira JEG, Galhardo C Jr., Lessa MA, Kietaibl S. Clinical Impact of Preoperative Anemia in Patients Undergoing Peripheral Vascular Interventions: A Systematic Review. Surgeries. 2021; 2(3):268-277. https://doi.org/10.3390/surgeries2030027
Chicago/Turabian StyleLinhares, Rafael M., Carlos Darcy A. Bersot, José Eduardo G. Pereira, Carlos Galhardo, Jr., Marcos Adriano Lessa, and Sibylle Kietaibl. 2021. "Clinical Impact of Preoperative Anemia in Patients Undergoing Peripheral Vascular Interventions: A Systematic Review" Surgeries 2, no. 3: 268-277. https://doi.org/10.3390/surgeries2030027
APA StyleLinhares, R. M., Bersot, C. D. A., Pereira, J. E. G., Galhardo, C., Jr., Lessa, M. A., & Kietaibl, S. (2021). Clinical Impact of Preoperative Anemia in Patients Undergoing Peripheral Vascular Interventions: A Systematic Review. Surgeries, 2(3), 268-277. https://doi.org/10.3390/surgeries2030027








