3D Printing for Medical Applications: Current State of the Art and Perspectives during the COVID-19 Crisis
Abstract
:1. Introduction
2. The 3D Printing Process and Medical Applications
2.1. 3D Printing in Medicine
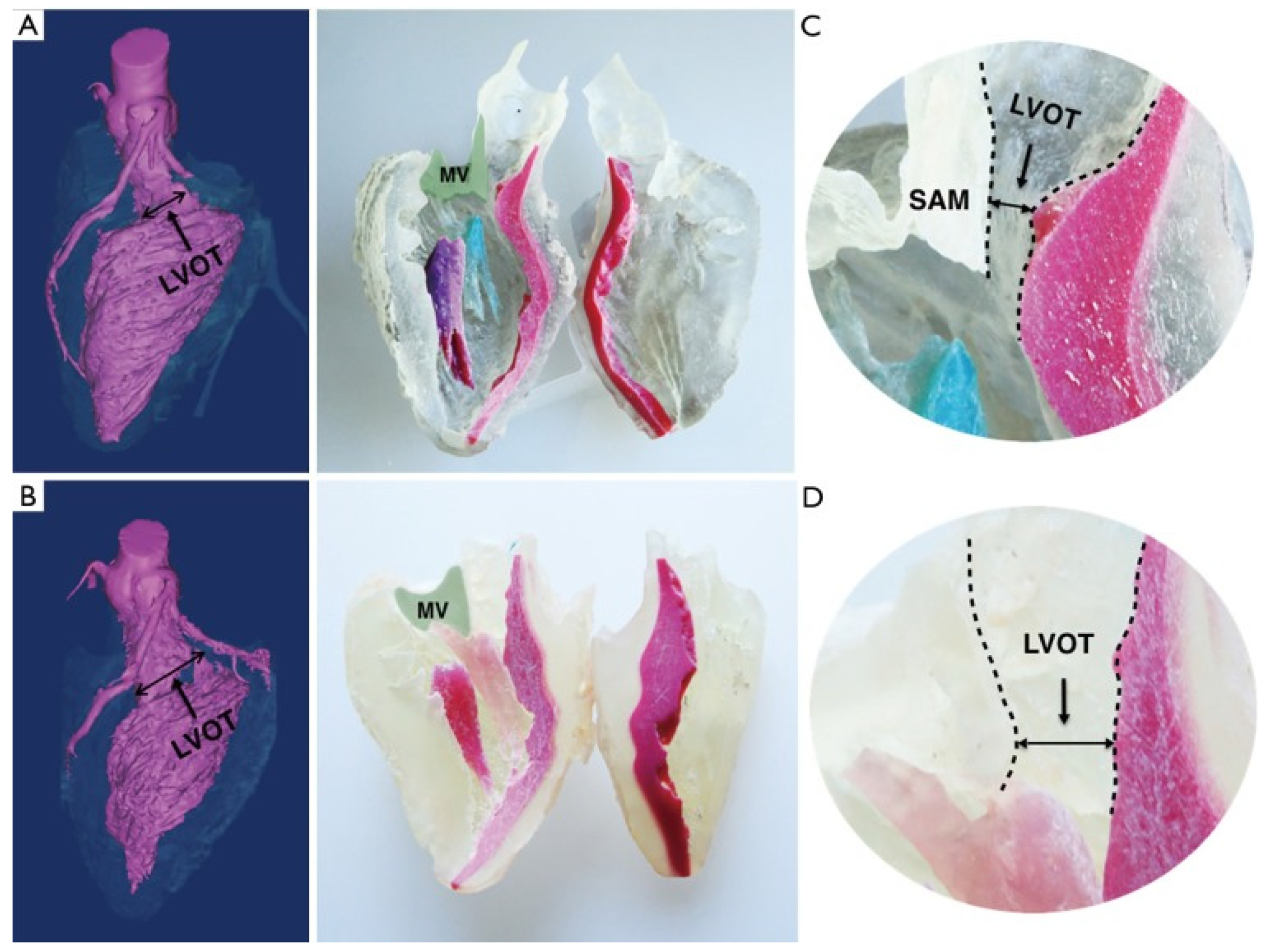
2.1.1. 3D Printing for Heart Surgery
2.1.2. 3D Printing for Lung Surgery
2.1.3. 3D Bioprinting in Vaccine Testing and Drug Delivery
3. 3D Printing PPE in Response to COVID-19
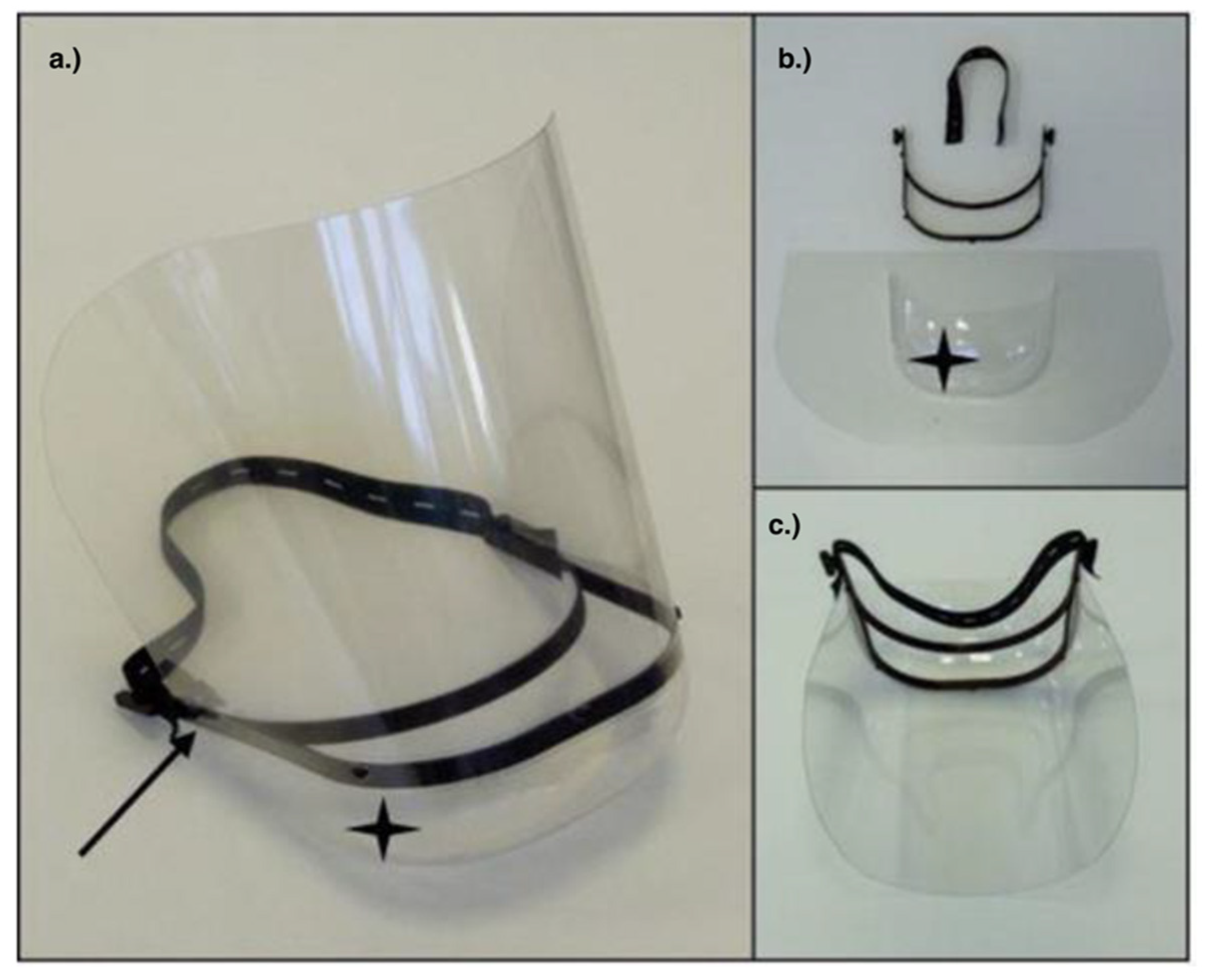
3.1. Face Shields
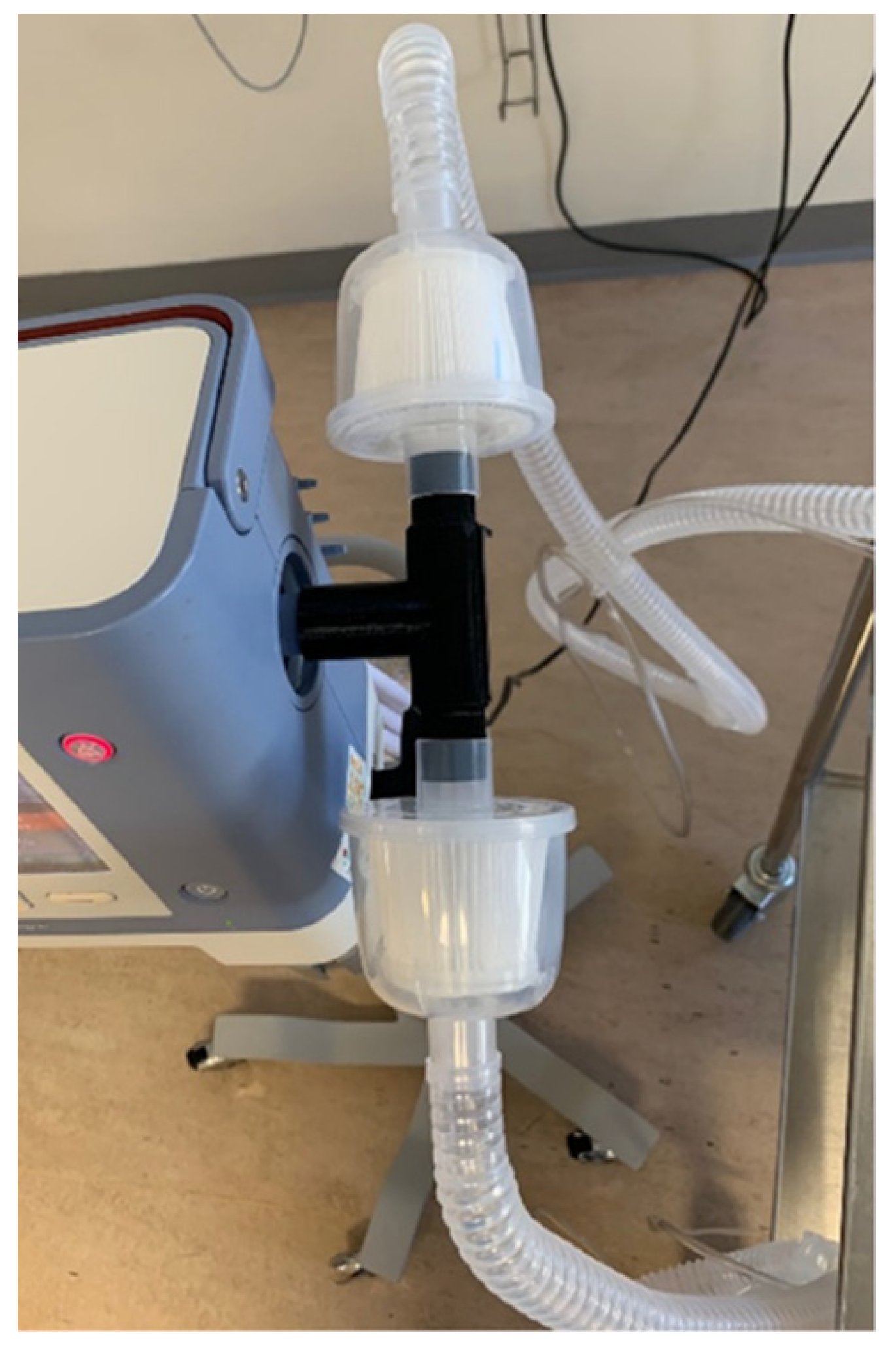
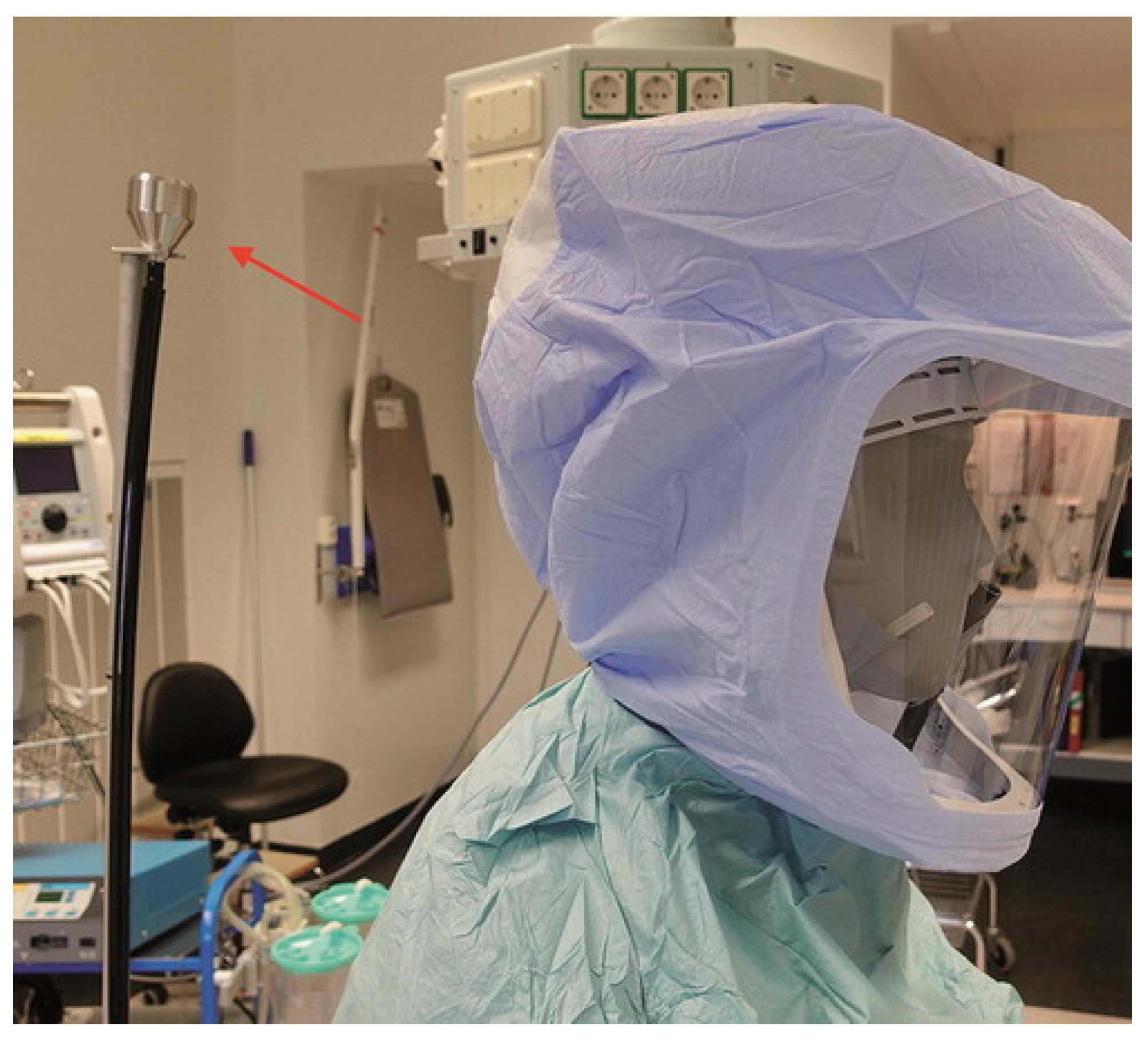
3.2. PAPR
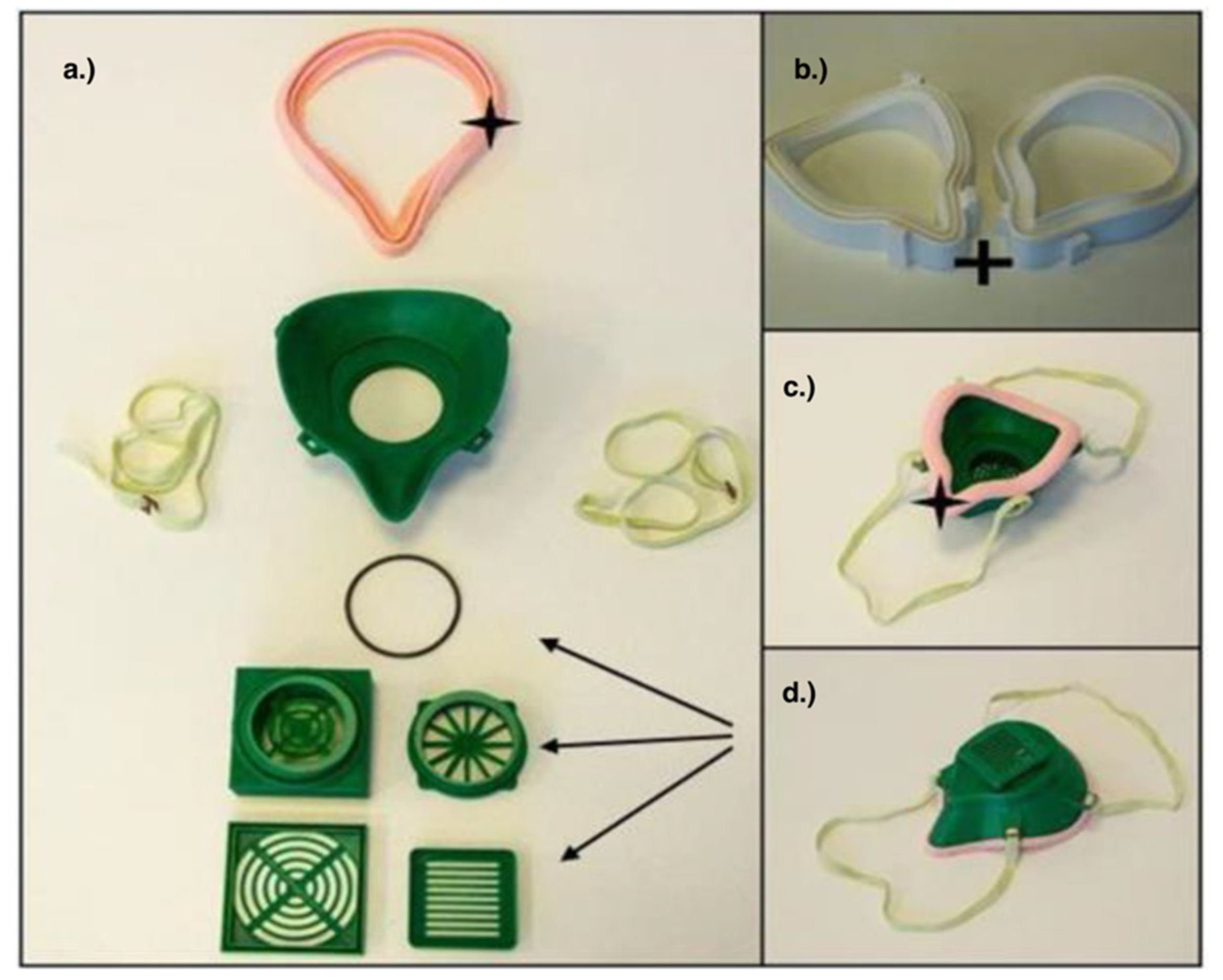
3.3. Respirators
4. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Coronavirus Disease (COVID-19) Situation Report 141; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Novel Coronavirus (2019-nCoV) Situtation Report 1; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Khachfe, H.H.; Chahrour, M.; Sammouri, J.; Salhab, H.A.; Makki, B.E.; Fares, M.Y. An Epidemiological Study on COVID-19: A Rapidly Spreading Disease. Cureus 2020, 12, e7313. [Google Scholar] [CrossRef] [Green Version]
- WHO. Novel Coronavirus (2019-nCoV) Situation Report—7; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Novel Coronavirus (2019-nCoV) Situation Report—9; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Rafiq, D.; Batool, A.; Bazaz, M.A. Three months of COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, e2113. [Google Scholar] [CrossRef] [PubMed]
- CDC Infographic on COVID-19 Symptoms. Available online: covid19-infographic-symptoms-final.pdf (accessed on 20 March 2021).
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Rational Use of Personal Protective Equipment (PPE) for Coronavirus Disease 2019 (COVID-19); WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical Supply Shortages—The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef] [PubMed]
- Kobokovich, A. Ventilator Stockpiling and Availability in the US; Center of Health Security: Baltimore, MD, USA, 2020; Available online: https://www.centerforhealthsecurity.org/resources/COVID-19/COVID-19-fact-sheets/200214-VentilatorAvailability-factsheet.pdf (accessed on 22 July 2021).
- Flanagan, S.T.; Ballard, D.H. 3D Printed Face Shields: A Community Response to the COVID-19 Global Pandemic. Acad. Radiol. 2020, 27, 905–906. [Google Scholar] [CrossRef] [PubMed]
- Ishack, S.; Lipner, S.R. Applications of 3D Printing Technology to Address COVID-19−Related Supply Shortages. Am. J. Med. 2020, 133, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Noorani, R. 3D Printing Technology, Applications, and Selection, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Milewski, J.O. Origins of 3D Metal Printing. Superconductivity 2017, 258, 119–129. [Google Scholar] [CrossRef]
- Savini, A.; Savini, G.G. A short history of 3D printing, a technological revolution just started. In Proceedings of the 2015 ICOHTEC/IEEE International History of High-Technologies and their Socio-Cultural Contexts Conference (HISTELCON), Tel-Aviv, Israel, 18–19 August 2015. [Google Scholar] [CrossRef]
- Horvath, J.; Cameron, R. Mastering 3D Printing, 2nd ed.; Technology in Action; Apress: New York, NY, USA, 2020. [Google Scholar]
- Rendeki, S.; Nagy, B.; Bene, M.; Pentek, A.; Toth, L.; Szanto, Z.; Told, R.; Maroti, P. An overview on personal protective equipment (PPE) fabricated with additive manufacturing technologies in the era of COVID-19 pandemic. Polymers 2020, 12, 2703. [Google Scholar] [CrossRef]
- Remondino, F.; El-hakim, S. Image-based 3D Modelling: A Review. Photogramm. Rec. 2006, 21, 269–291. [Google Scholar] [CrossRef]
- Lee, V. Medical applications for 3D printing: Current and projected uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Health Eng. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, J.S.; Morris, J.M.; Foley, T.A.; Williamson, E.E.; Leng, S.; McGee, K.P.; Kuhlmann, J.L.; Nesberg, L.E.; Vrtiska, T.J. Three-dimensional physical modeling: Applications and experience at mayo clinic. Radiographics 2015, 35, 1989–2006. [Google Scholar] [CrossRef]
- Mitsouras, D.; Liacouras, P.; Imanzadeh, A.; Giannopoulos, A.A.; Cai, T.; Kumamaru, K.K.; George, E.; Wake, N.; Caterson, E.J.; Pomahac, B.; et al. Medical 3D printing for the radiologist. Radiographics 2015, 35, 1965–1988. [Google Scholar] [CrossRef] [PubMed]
- Witowski, J.S.; Pędziwiatr, M.; Major, P.; Budzyński, A. Cost-effective, personalized, 3D-printed liver model for preoperative planning before laparoscopic liver hemihepatectomy for colorectal cancer metastases. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 2047–2054. [Google Scholar] [CrossRef] [Green Version]
- Paul, G.M.; Rezaienia, A.; Wen, P.; Condoor, S.; Parkar, N.; King, W.; Korakianitis, T. Medical Applications for 3D Printing: Recent Developments. Mo. Med. 2018, 115, 75–81. [Google Scholar]
- Mohammed, M.I.; Fitzpatrick, A.P.; Gibson, I. Customised design of a patient specific 3D printed whole mandible implant. KnE Eng. 2017, 2, 104–111. [Google Scholar] [CrossRef]
- Capelli, C.; Schievano, S. Computational analyses and 3D printed models: A combined approach for patient-specific studies. 3D Print. Med. 2017, 73–90. [Google Scholar] [CrossRef]
- Mommaerts, M.Y.; Büttner, M.; Vercruysse, H.; Wauters, L.; Beerens, M. Orbital Wall Reconstruction with Two-Piece Puzzle 3D Printed Implants: Technical Note. Craniomaxillofac. Trauma Reconstr. 2016, 9, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Dodziuk, H. Applications of 3D printing in healthcare. Pol. J. Cardio Thoracic Surg. 2016, 3, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef] [PubMed]
- López-León, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- Goha, A.; Mezue, K.; Edwards, P.; Nunura, F.; Baugh, D.; Madu, E. COVID-19 and the heart: An update for clinicians. Clin. Cardiol. 2020, 43, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Werlein, C.; Länger, F.; Kühnel, M.P.; Jonigk, D.D. COVID-19: Auswirkungen auf Lunge und Herz. Pathologe 2021, 42, 164–171. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Zhu, K.; Wang, C. Patient-specific three-dimensional printing for Kommerell’s diverticulum. Int. J. Cardiol. 2018, 255, 184–187. [Google Scholar] [CrossRef]
- Guo, H.C.; Wang, Y.; Dai, J.; Ren, C.W.; Li, J.H.; Lai, Y.Q. Application of 3D printing in the surgical planning of hypertrophic obstructive cardiomyopathy and physician-patient communication: A preliminary study. J. Thorac. Dis. 2018, 10, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Luo, H.; Gao, C.; Xu, Y. Three-dimensional printing model for the postoperative follow-up of atrial septal defect. Int. J. Cardiol. 2016, 222, 891–892. [Google Scholar] [CrossRef]
- Wang, H.; Song, H.; Yang, Y.; Cao, Q.; Hu, Y.; Chen, J.; Guo, J.; Wang, Y.; Jia, D.; Cao, S.; et al. Three-dimensional printing for cardiovascular diseases: From anatomical modeling to dynamic functionality. Biomed. Eng. Online 2020, 19, 1–16. [Google Scholar] [CrossRef]
- Yoo, S.J.; Spray, T.; Austin, E.H.; Yun, T.J.; van Arsdell, G.S. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J. Thorac. Cardiovasc. Surg. 2017, 153, 1530–1540. [Google Scholar] [CrossRef] [Green Version]
- Hermsen, J.L.; Burke, T.M.; Seslar, S.P.; Owens, D.S.; Ripley, B.; Mokadam, N.A.; Verrier, E.D. Scan, plan, print, practice, perform: Development and use of a patient-specific 3-dimensional printed model in adult cardiac surgery. J. Thorac. Cardiovasc. Surg. 2017, 153, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.-R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Birla, R.K.; Williams, S.K. 3D bioprinting and its potential impact on cardiac failure treatment: An industry perspective. APL Bioeng. 2020, 4, 010903. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Ye, X.; Yao, B.; Zhao, M.; Wu, P.; Liu, G.; Zhuang, D.; Jiang, H.; Chen, X.; He, Y.; et al. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact. Mater. 2021, 6, 1388–1401. [Google Scholar] [CrossRef]
- Birla, R.K. Current State of the Art in Ventricle Tissue Engineering. Front. Cardiovasc. Med. 2020, 7, 591581. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Lung Damage|Johns Hopkins Medicine. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/what-coronavirus-does-to-the-lungs (accessed on 16 June 2021).
- Hasan, S.S.; Capstick, T.; Ahmed, R.; Kow, C.S.; Mazhar, F.; a Merchant, H.; Zaidi, S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2020, 14, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Tzotzos, S.J.; Fischer, B.; Fischer, H.; Zeitlinger, M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Crit. Care 2020, 24, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins Engineers Develop 3D-Printed Ventilator Splitters|Hub. Available online: https://hub.jhu.edu/2020/04/02/3d-printed-ventilator-splitters-for-covid-19/ (accessed on 16 June 2021).
- Vaughan, T.J.; Kirrane, F.; Moerman, K.M.; Cahill, T.; O’Regan, A.; O’Keeffe, D.T. A Novel Dual Non-Invasive Ventilator Continuous Positive Airway Pressure Non-Aerosolization Circuit for Emergency Use in the COVID-19 Pandemic. J. Open Hardw. 2020, 4. [Google Scholar] [CrossRef]
- Rezaei, F.S.; Khorshidian, A.; Beram, F.M.; Derakhshani, A.; Esmaeili, J.; Barati, A. 3D printed chitosan/polycaprolactone scaffold for lung tissue engineering: Hope to be useful for COVID-19 studies. RSC Adv. 2021, 11, 19508–19520. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Tschumperlin, D.J.; Stenmark, K.R. Coming to terms with tissue engineering and regenerative medicine in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L625–L638. [Google Scholar] [CrossRef] [Green Version]
- Tebyanian, H.; Karami, A.; Nourani, M.R.; Motavallian, E.; Barkhordari, A.; Yazdanian, M.; Seifalian, A. Lung tissue engineering: An update. J. Cell. Physiol. 2019, 234, 19256–19270. [Google Scholar] [CrossRef] [PubMed]
- Menon, I.; Bagwe, P.; Gomes, K.; Bajaj, L.; Gala, R.; Uddin, M.; D’Souza, M.; Zughaier, S. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Taddio, A.; Ipp, M.; Thivakaran, S.; Jamal, A.; Parikh, C.; Smart, S.; Sovran, J.; Stephens, D.; Katz, J. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine 2012, 30, 4807–4812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Understanding the Unique Distribution Challenges of COVID-19 Vaccines. Available online: https://www.cnacanada.ca/web/guest/cnacanada/about/listofauthors/authorbio/blogdetails/SA-Ruth-Stewart/understanding-the-unique-distribution-challenges-of-covid-19-vaccines (accessed on 13 July 2021).
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An emerging transdermal drug delivery system. J. Pharm. Pharmacol. 2011, 64, 11–29. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.; Prausnitz, M.R.; Rouphael, N. Dissolvable Microneedle Patches to Enable Increased Access to Vaccines against SARS-CoV-2 and Future Pandemic Outbreaks. Vaccines 2021, 9, 320. [Google Scholar] [CrossRef]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, J.; Windmill, J.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C 2019, 102, 743–755. [Google Scholar] [CrossRef]
- Barret, A. COVID-19 Vaccine ‘Smart Patch’ Uses Microneedles to Deliver Immunity. BBC Science Focus Magazine. 6 January 2021. Available online: https://www.sciencefocus.com/news/covid-19-vaccine-smart-patch-uk/ (accessed on 14 July 2021).
- A Vaccine Patch Could Someday Be an Ouchless Option. Available online: https://www.nibib.nih.gov/news-events/newsroom/vaccine-patch-could-someday-be-ouchless-option (accessed on 14 July 2021).
- Microneedle Coronavirus Vaccine Triggers Immune Response in Mice|National Institutes of Health (NIH). Available online: https://www.nih.gov/news-events/nih-research-matters/microneedle-coronavirus-vaccine-triggers-immune-response-mice (accessed on 13 July 2021).
- Nishiguchi, A.; Shima, F.; Singh, S.; Akashi, M.; Moeller, M. 3D-Printing of Structure-Controlled Antigen Nanoparticles for Vaccine Delivery. Biomacromolecules 2020, 21, 2043–2048. [Google Scholar] [CrossRef]
- Acevedo, R.; Restaino, M.A.; Yu, D.; Hoag, S.W.; Flank, S.; Sochol, R.D. 3D Nanoprinted Liquid-Core-Shell Microparticles. J. Microelectromech. Syst. 2020, 29, 924–929. [Google Scholar] [CrossRef]
- U.S. FDA. FAQs on 3D Printing of Medical Devices, Accessories, Components, and Parts during the COVID-19 Pandemic. Available online: https://www.fda.gov/medical-devices/3d-printing-medical-devices/faqs-3dprinting-%0Amedical-devices-accessories-components-and-parts-during-covid-19-pandemic (accessed on 28 May 2020).
- 3D Printing and Other Manufacturing of Personal Protective Equipment in Response to COVID-19. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/covid-19-unconventional-manufacturing-personal-protective-equipment.html (accessed on 14 July 2021).
- 3D Printed Face Shields for Medics and Professionals—Prusa3D—3D Printers from Josef Průša. Available online: https://www.prusa3d.com/covid19/ (accessed on 14 July 2021).
- COVID-19 Response|NIH 3D Print Exchange. Available online: https://3dprint.nih.gov/collections/covid-19-response (accessed on 14 July 2021).
- Wang, D.; Zhang, J.; Liu, Q.; Chen, B.; Liang, Y.; Hu, L.; Jiang, G. 3D Printing Challenges in Enabling Rapid Response to Public Health Emergencies. Innovation 2020, 1, 100056. [Google Scholar] [CrossRef]
- Vordos, N.; Gkika, D.A.; Maliaris, G.; Tilkeridis, K.E.; Antoniou, A.; Bandekas, D.V.; Mitropoulos, A.C. How 3D printing and social media tackles the PPE shortage during Covid-19 pandemic. Saf. Sci. 2020, 130, 104870. [Google Scholar] [CrossRef]
- Throckmorton, A.L.; Bass, E.J.; Ferrick, B.; Ramakrishnan, A.; Eichmann, S.; Catucci, N.; Eshelman, B.; McNamara, J.; Sundquist, E.; Beatson, N.; et al. A Cross University-Led COVID-19 Rapid-Response Effort: Design, Build, and Distribute Drexel AJFlex Face Shields. Ann. Biomed. Eng. 2021, 49, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Wesemann, C.; Pieralli, S.; Fretwurst, T.; Nold, J.; Nelson, K.; Schmelzeisen, R.; Hellwig, E.; Spies, B.C. 3-D Printed Protective Equipment during COVID-19 Pandemic. Materials 2020, 13, 1997. [Google Scholar] [CrossRef] [PubMed]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D printed medicines: Which techniques and polymers are more successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oth, O.; Dauchot, C.; Orellana, M.; Glineur, R. How to Sterilize 3D Printed Objects for Surgical Use? An Evaluation of the Volumetric Deformation of 3D-Printed Genioplasty Guide in PLA and PETG after Sterilization by Low-Temperature Hydrogen Peroxide Gas Plasma. Open Dent. J. 2019, 13, 410–417. [Google Scholar] [CrossRef] [Green Version]
- Aguado-Maestro, I.; de Frutos-Serna, M.; González-Nava, A.; de Santos, A.B.M.; García-Alonso, M. Are the common sterilization methods completely effective for our in-house 3D printed biomodels and surgical guides? Injury 2021, 52, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-Y.; Kong, Y.-C.; See, J.-J.; Kan, R.K.; Lim, M.P.; Chen, Q.; Lim, B.; Ong, S. Anaesthetic management of patients with COVID-19: Infection prevention and control measures in the operating theatre. Br. J. Anaesth. 2020, 125, e239–e241. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.M.; Richardson, E.S.; Hernandez, N.M.; Bobbert, D.W.; Gall, K.; Fearis, P. Helmet Modification to PPE With 3D Printing During the COVID-19 Pandemic at Duke University Medical Center: A Novel Technique. J. Arthroplast. 2020, 35, S23–S27. [Google Scholar] [CrossRef]
- Stryker. Flyte Personal Protection System Flyte and T4/T5 Togas and Hoods Are Components of Stryker’ s Personal Protection System and Are Intended to Protect the Patient, Healthcare Personnel and Operating Room Personnel against Contamination, Exposure of Inf; Stryker: Kalamazoo, MI, USA, 2020. [Google Scholar]
- Temmesfeld, M.J.; Jakobsen, R.B.; Grant, P. Does a surgical helmet provide protection against aerosol transmitted disease? Acta Orthop. 2020, 91, 538–542. [Google Scholar] [CrossRef]
- Khoo, D.; Yen, C.C.; Chow, W.T.; Jain, P.; Loh, N.-H.W.; Teo, W.W.; Koh, C. Ultra-portable low-cost improvised powered air-purifying respirator: Feasibility study. Br. J. Anaesth. 2020, 125, e264–e266. [Google Scholar] [CrossRef]
- National Institute for Occupational Safety and Health. Determination of Air Flow for Powered Air-Purifying Respirators Standard Testing Procedure (STP); National Institute for Occupational Safety and Health: Washington, DC, USA, 2005. [Google Scholar]
- Provenzano, D.; Rao, Y.J.; Mitic, K.; Obaid, S.N.; Pierce, D.; Huckenpahler, J.; Berger, J.; Goyal, S.; Loew, M.H. Rapid Prototyping of Reusable 3D-Printed N95 Equivalent Respirators at the George Washington University. Preprints 2020. [Google Scholar] [CrossRef] [Green Version]
- National Air Filtration Association. Understanding MERV|NAFA User’s Guide to ANSI/ASHRAE 52.2. 2018. Available online: https://www.nafahq.org/understanding-merv-nafa-users-guide-to-ansi-ashrae-52-2/ (accessed on 28 May 2020).
- Liu, D.C.Y.; Koo, T.H.; Wong, J.K.K.; Wong, Y.H.; Fung, K.S.C.; Chan, Y.; Lim, H.S. Modifying reusable elastomeric respirators to utilise breathing system filters with 3D printed adapters, a safe alternative to N95 during COVID-19. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Yang, L.; Zhu, H.; Wu, L.; Ji, P.; Yang, J.; Gu, Z. Recent advances and challenges in materials for 3D bioprinting. Prog. Nat. Sci. 2020, 30, 618–634. [Google Scholar] [CrossRef]
- How 3D Printing has Changed the Game in the Medical Field. 2019. Available online: https://www.docwirenews.com/future-of-medicine/infographics/how-3d-printing-has-changed-the-game-in-the-medical-field/ (accessed on 20 March 2021).
- Using 3D Printing to Blend Biology and Electronics. Available online: https://www.designnews.com/materials-assembly/using-3d-printing-blend-biology-and-electronics (accessed on 14 July 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagen, A.; Chisling, M.; House, K.; Katz, T.; Abelseth, L.; Fraser, I.; Bradley, S.; Kirsch, R.; Morris, J.; Giles, J.W.; et al. 3D Printing for Medical Applications: Current State of the Art and Perspectives during the COVID-19 Crisis. Surgeries 2021, 2, 244-259. https://doi.org/10.3390/surgeries2030025
Hagen A, Chisling M, House K, Katz T, Abelseth L, Fraser I, Bradley S, Kirsch R, Morris J, Giles JW, et al. 3D Printing for Medical Applications: Current State of the Art and Perspectives during the COVID-19 Crisis. Surgeries. 2021; 2(3):244-259. https://doi.org/10.3390/surgeries2030025
Chicago/Turabian StyleHagen, Andrew, Megan Chisling, Kevin House, Tal Katz, Laila Abelseth, Ian Fraser, Stephen Bradley, Rebecca Kirsch, Jacob Morris, Joshua W. Giles, and et al. 2021. "3D Printing for Medical Applications: Current State of the Art and Perspectives during the COVID-19 Crisis" Surgeries 2, no. 3: 244-259. https://doi.org/10.3390/surgeries2030025
APA StyleHagen, A., Chisling, M., House, K., Katz, T., Abelseth, L., Fraser, I., Bradley, S., Kirsch, R., Morris, J., Giles, J. W., & Willerth, S. M. (2021). 3D Printing for Medical Applications: Current State of the Art and Perspectives during the COVID-19 Crisis. Surgeries, 2(3), 244-259. https://doi.org/10.3390/surgeries2030025






