Multidisciplinary Management of an Atypical Gigantic Sciatic Nerve Schwannoma: Case Presentation and Systematic Review
Abstract
1. Introduction
2. Illustrative Case
2.1. Histological Examination
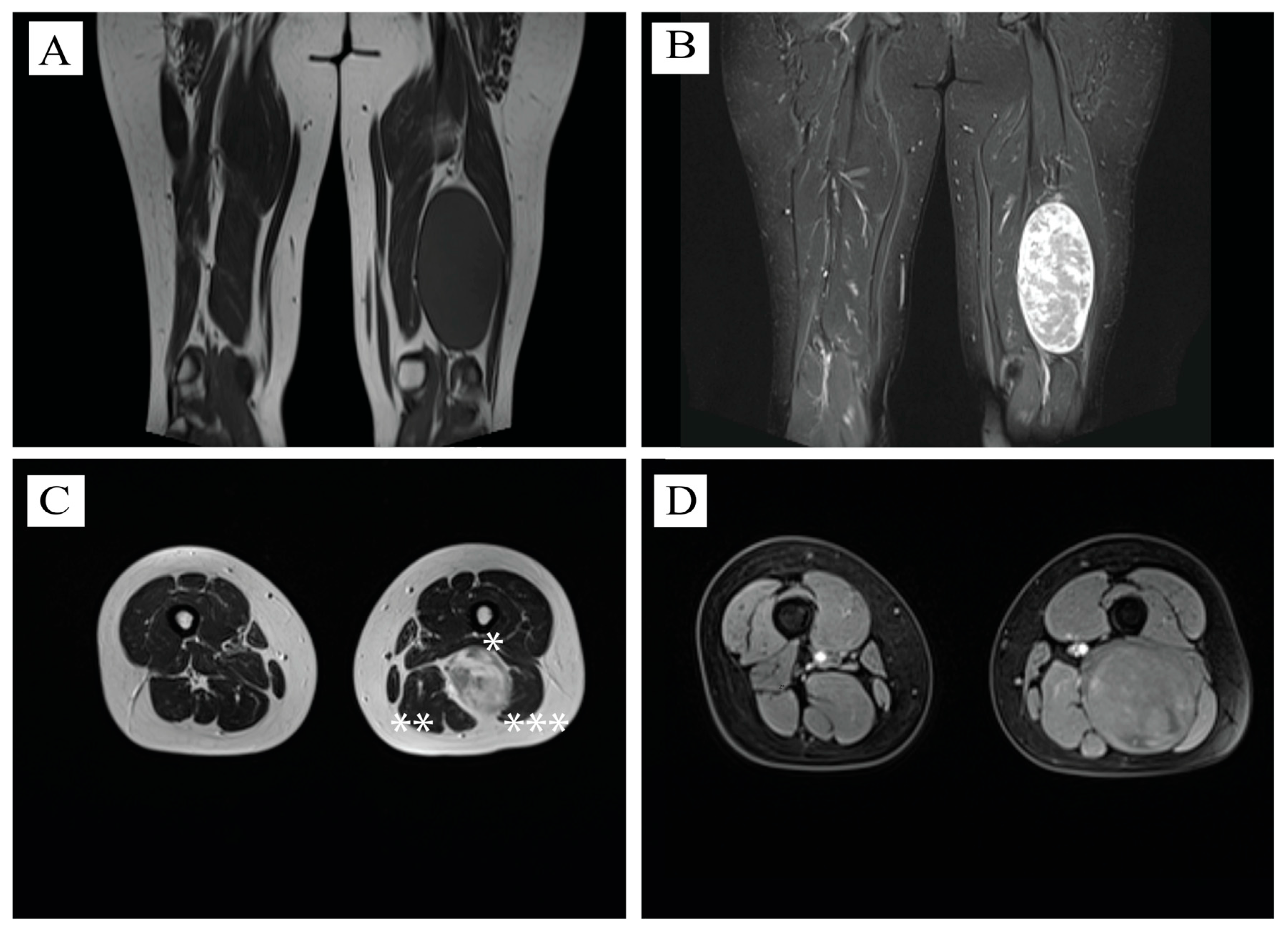
2.2. MRI Aspect
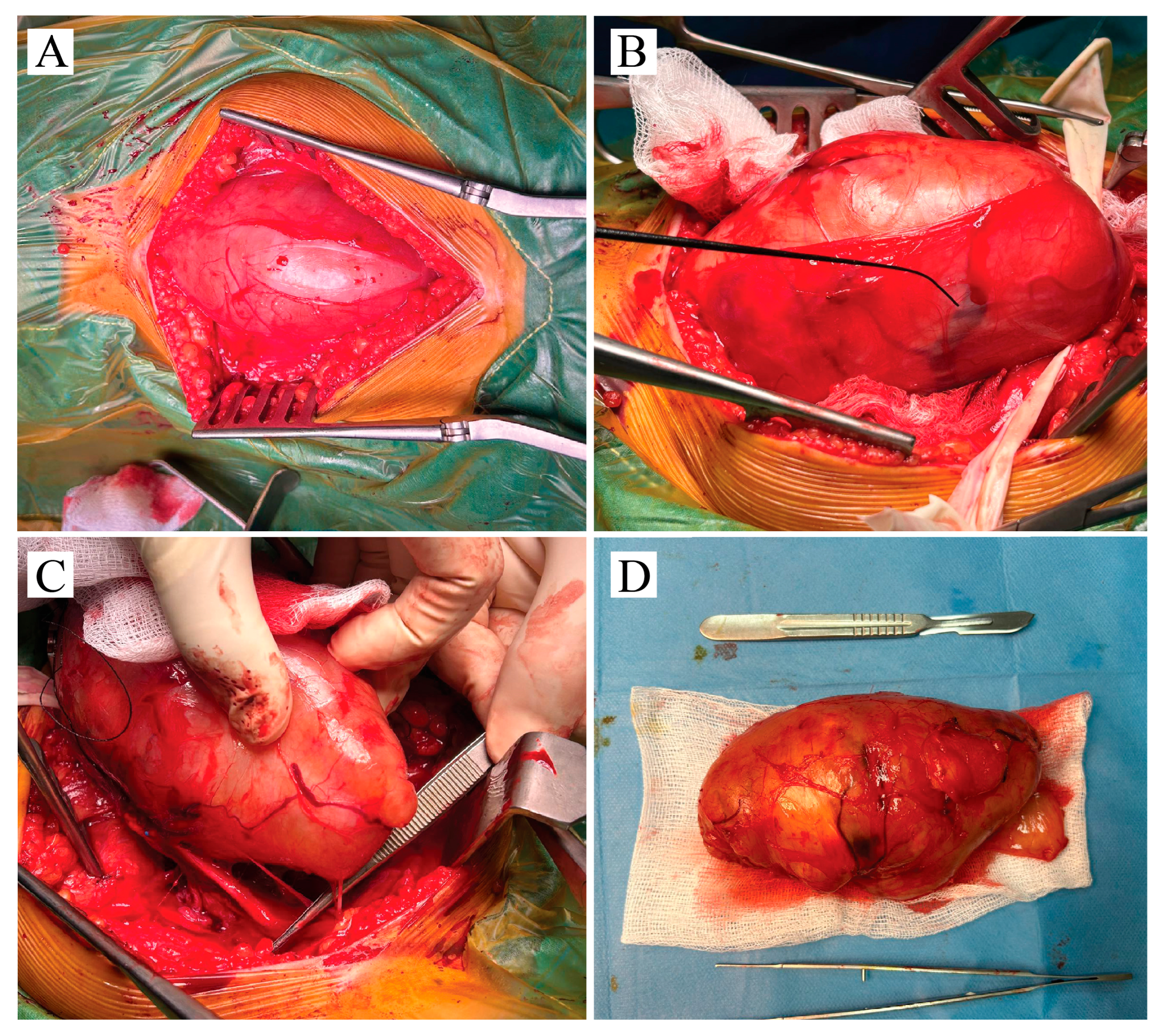
2.3. Operative Course
2.4. Consent
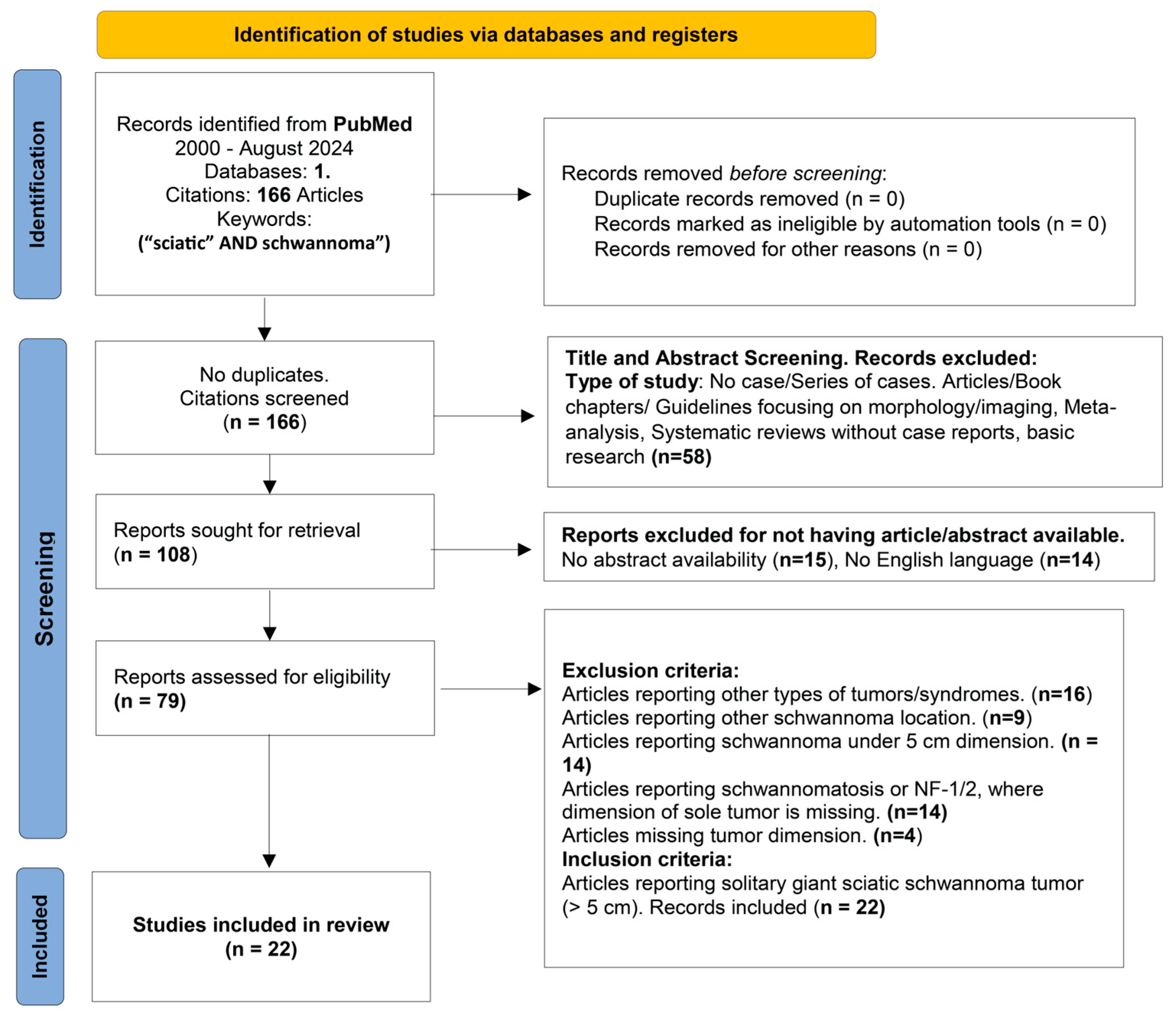
3. Materials and Methods
4. Discussions
4.1. Demographics
4.2. Clinical Presentation
4.3. Tumor Morphology and Behavior
4.4. Imaging and Biopsy
4.5. Treatment, Operative Course, Intraoperative Neuromonitoring, Postoperative Evolution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NF-2 | Neurofibromatosis type 2 |
| TS | Turner syndrome |
| T2WI | T2-weighted images |
| INM | Intraoperative neurophysiological monitoring |
| MPNST | Malignant peripheral sheath tumors |
| GD | Gadolinium |
| FS | Fat saturation |
| N/A | Not available |
| bFGF | Basic fibroblast growth factor |
| MISS | Membrane-initiated steroid signaling |
| M-CSF | Macrophage colony stimulating factor |
| sEMG | Spontaneous electromyography |
| SSEPs | Somatosensory evoked potentials |
| NS-RADS | Neuropathy Score Reporting and Data System |
Appendix A
| Article Name | Age, Gender | Symptoms | Physical Aspect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic | Pain | Tingling | Decreased Sensation | Motor Deficit | Palpable | Not-Palpable | ||||
| Radicular | Local | |||||||||
| Present case | 35, F | X | X | |||||||
| 1 | Xiong Y et al., 2024 [5] | 40, M | X | X | ||||||
| 2 | Jelti et al., 2023 [6] | 47, F | X | X | X | |||||
| 3 | Yan J et al., 2023 [7] | 43, F | X | X | X | X | ||||
| 4 | Lakomkin et al., 2022 [8] | 70, F | X | X | ||||||
| 5 | Yi et al., 2021 [9] | 37, F | X | X | ||||||
| 6 | Utomo et al., 2021 [10] | 35, F | X | X | X | |||||
| 7 | Maes et al., 2020 [11] | 50, F | X | X | ||||||
| 8 | Telera et al., 2019 [2] | 79, F | X | X | X | X | ||||
| 9 | As-Sultany et al., 2017 [1] | 39, F | X | X | ||||||
| 10 | Rosario et al., 2016 [12] | 64, M | X | X | ||||||
| 11 | Hidaka et al., 2016 [13] | 48, F | X | X | ||||||
| 12 | Godkin et al., 2016 [14] | 40, F | X | X | X | |||||
| 13 | Chikkanna et al., 2016 [15] | 40, M | X | X | X | |||||
| 14 | Naik et al., 2015 [16] | 26, M | X | X | ||||||
| 15 | Eroglu et al., 2013 [17] | 40, F | X | X | ||||||
| 16 | Hamdi et al., 2009 [18] | 45, M | X | X | X | |||||
| 17 | Omezzine et al., 2009 [19] | 42, F | X | X | X | |||||
| 18 | Yang et al., 2007 [20] | 67, M | X | X | ||||||
| 19 | Lee et al., 2007 [21] | 73, M | X | X | ||||||
| 20 | Consales et al., 2006 [22] | 39, F | X | X | ||||||
| 21 | Rekha et al., 2004 [4] | 60, M | X | X | ||||||
| 22 | Maini et al., 2003 [23] | 21, F | X | X | X | X | ||||
| Article Name | Dominant Imaging Aspect Reported | Maximum Diameter (cm) | |
|---|---|---|---|
| Present case | Hypointense T1WI, Hyperintense T2WI, solid mass | 14 × 7 cm | |
| 1 | Xiong Y et al., 2024 [5] | Homogeneous oval mass | 5 cm |
| 2 | Jelti et al., 2023 [6] | Hyperintense T2WI, heterogeneous | 7.2 cm |
| 3 | Yan J et al., 2023 [7] | Hypointense T1WI, Isointense T2WI, heterogeneous | 5 cm |
| 4 | Lakomkin et al., 2022 [8] | Angiosarcoma transformation. | 7.9 cm |
| 5 | Yi et al., 2021 [9] | Heterogeneous signal | 7 cm |
| 6 | Utomo et al., 2021 [10] | Hypointense T1WI, Hyperintense T2WI, cystic | 13.9 cm |
| 7 | Maes et al., 2020 [11] | Hyperintense T2WI, heterogeneous mass | 7 cm |
| 8 | Telera et al., 2019 [2] | Hyperintense T1WI, heterogeneousT2WI, solid mass | 7 cm |
| 9 | As-Sultany et al., 2017 [1] | 2 Tumors. Homogeneous isointense T1WI | 11 cm and 6 cm |
| 10 | Rosario et al., 2016 [12] | Hyperintense T2WI, cystic homogeneous mass | 11 cm |
| 11 | Hidaka et al., 2016 [13] | Heterogeneous solid mass | 6.5 cm |
| 12 | Godkin et al., 2016 [14] | Multi-loculated mass | 9 cm |
| 13 | Chikkanna et al., 2016 [15] | Hyperintense T2WI | 5 cm |
| 14 | Naik et al., 2015 [16] | Cystic mass | 25 cm |
| 15 | Eroglu et al., 2013 [17] | Isointense T1WI, homogeneous fusiform mass | 6 cm |
| 16 | Hamdi et al., 2009 [18] | Isointense T1WI, Hyperintense T2WI | 7.8 cm |
| 17 | Omezzine et al., 2009 [19] | Isointense T1WI, Hyperintense T2WI. Heterogeneous mass | 5 cm |
| 18 | Yang et al., 2007 [20] | Low-density on CT, homogeneous oval mass | 5.1 cm |
| 19 | Lee et al., 2007 [21] | Partial Hyperintensity T1WI, large multilobulated | 30 cm |
| 20 | Consales et al., 2006 [22] | Pear-shaped, solid mass | 5.5 cm |
| 21 | Rekha et al., 2004 [4] | Isointense T1WI | 15 cm |
| 22 | Maini et al., 2003 [23] | Heterogeneous mass | 7.6 cm |
| Item | Value | ||
|---|---|---|---|
| Multidisciplinary team involvement | 5/22 (22.7%) | ||
| Tumor Location | |||
| Upper thigh | Middle thigh | Lower thigh | Pelvic region |
| 4/22 (18.1%) | 2/22 (9.1%) | 5/22 (22.7%) | 9/22 (40.9%) |
| Extent of resection | |||
| Complete removal | Partial Removal | N/A | |
| 16/22 (72.7%) | 4/22 (18.2%) | 2/22 (9.1%) | |
| Follow-up | |||
| <6 months | 6–12 months | >12 months | N/A |
| 3/22 (13.6%) | 6/22 (27.2%) | 6/22 (27.2%) | 7/22 (31.8%) |
| Recurrence | |||
| No (15/22—68.1%) | Yes (1/22—4.5%) | N/A (6/22—27.2%) | |
| Postoperative deficit | |||
| No deficit | With deficit | N/A | |
| 16/22 (72.7%) | 5/22 (22.7%) | 1/22 (4.5%) | |
References
- As-Sultany, M.; Ben-Ghashir, N.; Mistry, A.; Chandrasekar, C. Giant schwannomas of the sciatic nerve. BMJ Case Rep. 2017, 2017, bcr2016218466. [Google Scholar] [CrossRef]
- Telera, S.; Raus, L.; Vietti, V.; Pace, A.; Villani, V.; Galié, E.; Freda, N.; Carosi, M.; Costantini, M. Schwannomas of the sciatic nerve: A rare and neglected diagnosis. A review of the literature with two illustrative cases. Clin. Neurol. Neurosurg. 2020, 195, 105889. [Google Scholar] [CrossRef]
- Owens, T.C.; de Lomba, W.C.; Schroeder, C.B.; Mingrino, J.; Fridley, J.; Oyelese, A.A.; Miner, T.J.; Liu, P.Y.; Woo, A.S.; Gokaslan, Z.L.; et al. Excision of a supergiant spinal schwannoma: Illustrative case. J. Neurosurg. Case Lessons 2024, 8, CASE24224. [Google Scholar] [CrossRef] [PubMed]
- Rekha, A.; Ravi, A. Sciatic nerve schwannoma. Int. J. Low. Extrem. Wounds 2004, 3, 165–167. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, J.; Yang, H.J. Concomitant treatment of ureteral calculi and ipsilateral pelvic sciatic nerve schwannoma with transperitoneal laparoscopic approach: A case report. World J. Clin. Cases 2024, 12, 1947–1953. [Google Scholar] [CrossRef]
- Jelti, O.; El Alaoui, O.; Lachkar, A.; Abdeljaouad, N.; Yacoubi, H. An Unusual Case of a Giant Schwannoma of the Sciatic Nerve: A Case Report with a Review of Literature. Cureus 2023, 15, e51155. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhou, R.; Liu, B.; Xu, Y.; Cao, X. Plexiform schwannomas of the sciatic nerve: A case report and review of the literature. Heliyon 2023, 9, e18020. [Google Scholar] [CrossRef]
- Lakomkin, N.; Torres-Mora, J.; Dozois, E.J.; Spinner, R.J. Angiosarcoma arising in a schwannoma of the peripheral nervous system: Illustrative case. J. Neurosurg. Case Lessons 2022, 4, CASE22452. [Google Scholar] [CrossRef]
- Yi, S.; Jin, X.; Xiong, Y.; Fu, B. Permanent neurological deficit caused by misdiagnosis of a giant intrapelvic sciatic schwannoma: A case report. J. Obstet. Gynaecol. Res. 2021, 47, 3413–3417. [Google Scholar] [CrossRef] [PubMed]
- Utomo, S.A.; Bajamal, A.H.; Faris, M.; Ardiansyah, D.; Lunardhi, J.H. Long Completely Cystic Sciatic Schwannoma: A Rare Case. Case Rep. Oncol. 2021, 14, 561–567. [Google Scholar] [CrossRef]
- Maes, R.; Ledoux, P.; de Brouckere, G. A rare cause of sciatica: Sciatic nerve schwannoma—Report of one case with long subclinical course and misleading presentation. SICOT J. 2020, 6, 16. [Google Scholar] [CrossRef]
- Rosario, M.S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Miwa, S.; Inatani, H.; Higuchi, T.; Tsuchiya, H. A case of infected schwannoma mimicking malignant tumor. World J. Surg. Oncol. 2016, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, E.; Ishiyama, Y.; Maeda, C.; Nakahara, K.; Shimada, S.; Mukai, S.; Sawada, N.; Ishida, F.; Kudo, S.E. Laparoscopic Extirpation of a Schwannoma in the Lateral Pelvic Space. Case Rep. Surg. 2016, 2016, 1351282. [Google Scholar] [CrossRef]
- Godkin, O.; Ellanti, P.; O’Toole, G. Large schwannoma of the sciatic nerve. BMJ Case Rep. 2016, 2016, bcr2016217717. [Google Scholar] [CrossRef]
- Chikkanna, J.K.; Gopal, S.; Sampath, D. Mystery of Sciatica Resolved—A Rare Case Report. J. Clin. Diagn. Res. 2016, 10, RD04–RD05. [Google Scholar] [CrossRef]
- Naik, T.; Premkumar, A.; Naik, C.S.; Abhishekh, H.A. Rare case of sciatic schwannoma. Asian J. Neurosurg. 2015, 10, 234–235. [Google Scholar] [CrossRef][Green Version]
- Eroglu, U.; Bozkurt, M.; Ozates, O.; Akturk, S.; Tuna, H. Sciatic nerve schwannoma: Case report. Turk. Neurosurg. 2014, 24, 120–122. [Google Scholar] [CrossRef]
- Hamdi, M.F.; Aloui, I.; Ennouri, K. Sciatica secondary to sciatic nerve schwannoma. Neurol. India 2009, 57, 685–686. [Google Scholar] [CrossRef] [PubMed]
- Omezzine, S.J.; Zaara, B.; Ben Ali, M.; Abid, F.; Sassi, N.; Hamza, H.A. A rare cause of non discal sciatica: Schwannoma of the sciatic nerve. Orthop. Traumatol. Surg. Res. 2009, 95, 543–546. [Google Scholar] [CrossRef]
- Yang, C.C.; Chen, H.C.; Chen, C.M. Endoscopic resection of a presacral schwannoma. Case Rep. J. Neurosurg. Spine 2007, 7, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Wen, M.C.; Wang, J. Epithelioid angiosarcoma arising in a deep-seated plexiform schwannoma: A case report and literature review. Hum. Pathol. 2007, 38, 1096–1101. [Google Scholar] [CrossRef]
- Consales, A.; Poppi, M.; Stumpo, M. Sciatic schwannoma spanning the sciatic notch: Removal by an anterior, trans-abdominal approach. Br. J. Neurosurg. 2006, 20, 46–48. [Google Scholar] [CrossRef]
- Maini, A.; Tripathi, M.; Shekar, N.C.; Malhotra, A. Sciatic schwannoma of the thigh detected on bone scan: A case report. Clin. Imaging 2003, 27, 191–193. [Google Scholar] [CrossRef]
- Sarmiento, M.E.; Espinoza, C.F.; López, C.L.; Fuentes-Rocabado, N.; Chaurasia, B. Giant sciatic nerve schwannoma: A rare case report and literature review. Ann. Med. Surg. 2024, 86, 4921–4926. [Google Scholar] [CrossRef] [PubMed]
- Gokkus, K.; Saylik, M.; Birtay, T.; Sahin, M.S. A case report of giant malignant schwannoma of the sciatic nerve associated with neurofibromatosis-1: A CARE-compliant article. Medicine 2023, 102, e36358. [Google Scholar] [CrossRef]
- Grübel, N.; Antoniadis, G.; Uerschels, A.K.; Gembruch, O.; Marschal, V.; Deininger, S.; König, R.; Pala, A.; Bremer, J.; Dengler, N.F.; et al. Peripheral Nerve Tumor Study. Collection of Rare Peripheral Nerve Tumors—Insights from the German Registry. Cancers 2024, 16, 2599. [Google Scholar] [CrossRef]
- Pellerino, A.; Verdijk, R.M.; Nichelli, L.; Andratschke, N.H.; Idbaih, A.; Goldbrunner, R. Diagnosis and Treatment of Peripheral and Cranial Nerve Tumors with Expert Recommendations: An EUropean Network for RAre CANcers (EURACAN) Initiative. Cancers 2023, 15, 1930. [Google Scholar] [CrossRef]
- Wu, W.T.; Chang, K.V.; Hsu, Y.C.; Yang, Y.C.; Hsu, P.C. Ultrasound Imaging for a Rare Cause of Sciatica: A Schwannoma of the Sciatic Nerve. Cureus 2020, 12, e8214. [Google Scholar] [CrossRef] [PubMed]
- Abreu, E.; Aubert, S.; Wavreille, G.; Gheno, R.; Canella, C.; Cotten, A. Peripheral Tumor and Tumor-Like Neurogenic Lesions. Eur. J. Radiol. 2013, 82, 38–50. [Google Scholar] [CrossRef]
- Zipfel, J.; Al-Hariri, M.; Gugel, I.; Grimm, A.; Steger, V.; Ladurner, R.; Krimmel, M.; Tatagiba, M.; Schuhmann, M.U. Surgical Management of Sporadic Peripheral Nerve Schwannomas in Adults: Indications and Outcome in a Single Center Cohort. Cancers 2021, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, G.; Xu, X.; Wang, K.; Zhu, Y.; Qi, X.; Lv, J.; Wang, Y.; Shao, S. Surgical Strategies for Peripheral Nerve Schwannoma Based on the Intraoperative Neurophysiological Monitoring. Lap. Endosc. Robot. Surg. 2019, 2, 65–69. [Google Scholar] [CrossRef]
- Sasaki, H.; Nagano, S.; Yokouchi, M.; Setoguchi, T.; Shimada, H.; Yamamoto, T.; Ishidou, Y.; Komiya, S. Utility of Intraoperative Monitoring with Motor-Evoked Potential during the Surgical Enucleation of Peripheral Nerve Schwannoma. Oncol. Lett. 2018, 15, 9327–9332. [Google Scholar] [CrossRef]
- Thirumala, P.D.; Mohanraj, S.K.; Habeych, M.; Wichman, K.; Chang, Y.F.; Gardner, P.; Snyderman, C.; Crammond, D.J.; Balzer, J. Value of Free-Run Electromyographic Monitoring of Lower Cranial Nerves in Endoscopic Endonasal Approach to Skull Base Surgeries. J. Neurol. Surg. B Skull Base 2012, 73, 236–244. [Google Scholar] [CrossRef][Green Version]
- Quimby, A.E.; Lui, J.; Chen, J. Predictive Ability of Direct Electrical Stimulation on Facial Nerve Function Following Vestibular Schwannoma Surgery: A Systematic Review and Meta-Analysis. Otol. Neurotol. 2021, 42, 493–504. [Google Scholar] [CrossRef]
- Toleikis, J.R.; Pace, C.; Jahangiri, F.R.; Hemmer, L.B.; Toleikis, S.C. Intraoperative Somatosensory Evoked Potential (SEP) Monitoring: An Updated Position Statement by the American Society of Neurophysiological Monitoring. J. Clin. Monit. Comput. 2024, 38, 1003–1042. [Google Scholar] [CrossRef]
- Hirai, T.; Kobayashi, H.; Akiyama, T.; Okuma, T.; Oka, H.; Shinoda, Y.; Ikegami, M.; Tsuda, Y.; Fukushima, T.; Ohki, T.; et al. Predictive Factors for Complications after Surgical Treatment for Schwannomas of the Extremities. BMC Musculoskelet. Disord. 2019, 20, 166. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Zhang, H.; Zheng, X.; Wang, J. Epithelioid Angiosarcoma Arising in Schwannoma: Report of Three Chinese Cases with Review of the Literature. Pathol. Int. 2012, 62, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Hussain, R.; Gago, N.; Oudinet, J.P.; Mattern, C.; Ghoumari, A.M. Progesterone Synthesis in the Nervous System: Implications for Myelination and Myelin Repair. Front. Neurosci. 2012, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Overdiek, A.; Winner, U.; Mayatepek, E.; Rosenbaum, T. Schwann Cells from Human Neurofibromas Show Increased Proliferation Rates under the Influence of Progesterone. Pediatr. Res. 2008, 64, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Geller, M.; Mezitis, S.G.; Nunes, F.P.; Ribeiro, M.G.; Araújo, A.P.; Bronstein, M.D.; Siqueira-Batista, R.; Gomes, A.P.; Oliveira, L.; Cunha, K.S. Progesterone and Estrogen Receptors in Neurofibromas of Patients with NF1. Clin. Med. Pathol. 2008, 1, 93–97. [Google Scholar] [CrossRef]
- Cazzador, D.; Astolfi, L.; Daloiso, A.; Tealdo, G.; Simoni, E.; Mazzoni, A.; Zanoletti, E.; Marioni, G. Tumor Microenvironment in Sporadic Vestibular Schwannoma: A Systematic, Narrative Review. Int. J. Mol. Sci. 2023, 24, 6522. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, A.; Dinh, C.T. Updates on Tumor Biology in Vestibular Schwannoma. Otolaryngol. Clin. N. Am. 2023, 56, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Karas, R.H. Emerging Evidence of the Importance of Rapid, Non-Nuclear Estrogen Receptor Signaling in the Cardiovascular System. Steroids 2013, 78, 589–596. [Google Scholar] [CrossRef]
- Lefèvre, N.; Corazza, F.; Valsamis, J.; Delbaere, A.; De Maertelaer, V.; Duchateau, J.; Casimir, G. The Number of X Chromosomes Influences Inflammatory Cytokine Production Following Toll-Like Receptor Stimulation. Front. Immunol. 2019, 10, 1052. [Google Scholar] [CrossRef]
- Hannan, C.J.; Lewis, D.; O’Leary, C.; Donofrio, C.A.; Evans, D.G.; Roncaroli, F.; Brough, D.; King, A.T.; Coope, D.; Pathmanaban, O.N. The Inflammatory Microenvironment in Vestibular Schwannoma. Neurooncol. Adv. 2020, 2, vdaa023. [Google Scholar] [CrossRef]
- Giatti, S.; Romano, S.; Pesaresi, M.; Cermenati, G.; Mitro, N.; Caruso, D.; Tetel, M.J.; Garcia-Segura, L.M.; Melcangi, R.C. Neuroactive Steroids and the Peripheral Nervous System: An Update. Steroids 2015, 103, 23–30. [Google Scholar] [CrossRef]
- Jaiswal, S.; Agrawal, V.; Jaiswal, A.K.; Pandey, R.; Mahapatra, A.K. Expression of Estrogen and Progesterone Receptors in Vestibular Schwannomas and Their Clinical Significance. J. Negat. Results Biomed. 2009, 8, 9. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirbu, O.-M.; Moreanu, M.-S.; Pogarasteanu, M.-E.; Plesa, A.; Iordache, M.; Mures, T.; Sirbu, A.M.; Moga, M.; Mitrica, M. Multidisciplinary Management of an Atypical Gigantic Sciatic Nerve Schwannoma: Case Presentation and Systematic Review. NeuroSci 2025, 6, 95. https://doi.org/10.3390/neurosci6040095
Sirbu O-M, Moreanu M-S, Pogarasteanu M-E, Plesa A, Iordache M, Mures T, Sirbu AM, Moga M, Mitrica M. Multidisciplinary Management of an Atypical Gigantic Sciatic Nerve Schwannoma: Case Presentation and Systematic Review. NeuroSci. 2025; 6(4):95. https://doi.org/10.3390/neurosci6040095
Chicago/Turabian StyleSirbu, Octavian-Mihai, Mihai-Stelian Moreanu, Mark-Edward Pogarasteanu, Andreea Plesa, Mihaela Iordache, Teofil Mures, Anca Maria Sirbu, Marius Moga, and Marian Mitrica. 2025. "Multidisciplinary Management of an Atypical Gigantic Sciatic Nerve Schwannoma: Case Presentation and Systematic Review" NeuroSci 6, no. 4: 95. https://doi.org/10.3390/neurosci6040095
APA StyleSirbu, O.-M., Moreanu, M.-S., Pogarasteanu, M.-E., Plesa, A., Iordache, M., Mures, T., Sirbu, A. M., Moga, M., & Mitrica, M. (2025). Multidisciplinary Management of an Atypical Gigantic Sciatic Nerve Schwannoma: Case Presentation and Systematic Review. NeuroSci, 6(4), 95. https://doi.org/10.3390/neurosci6040095






