Necessary Harmony Between Anesthesia and Neurosurgery During Extracranial–Intracranial Bypass: A Review of Neuroanesthesia Strategies and Perioperative Insights
Abstract
1. Introduction
2. Indications for Cerebral Bypass Surgery
3. Technical Considerations
3.1. High and Low Flow Bypass
3.2. Determinant of Cerebral Perfusion
3.2.1. Mean Arterial Pressure
3.2.2. Arterial Blood Gases
3.2.3. Cerebral Metabolic Rate of Oxygen
3.2.4. Anesthetic Pharmacodynamics
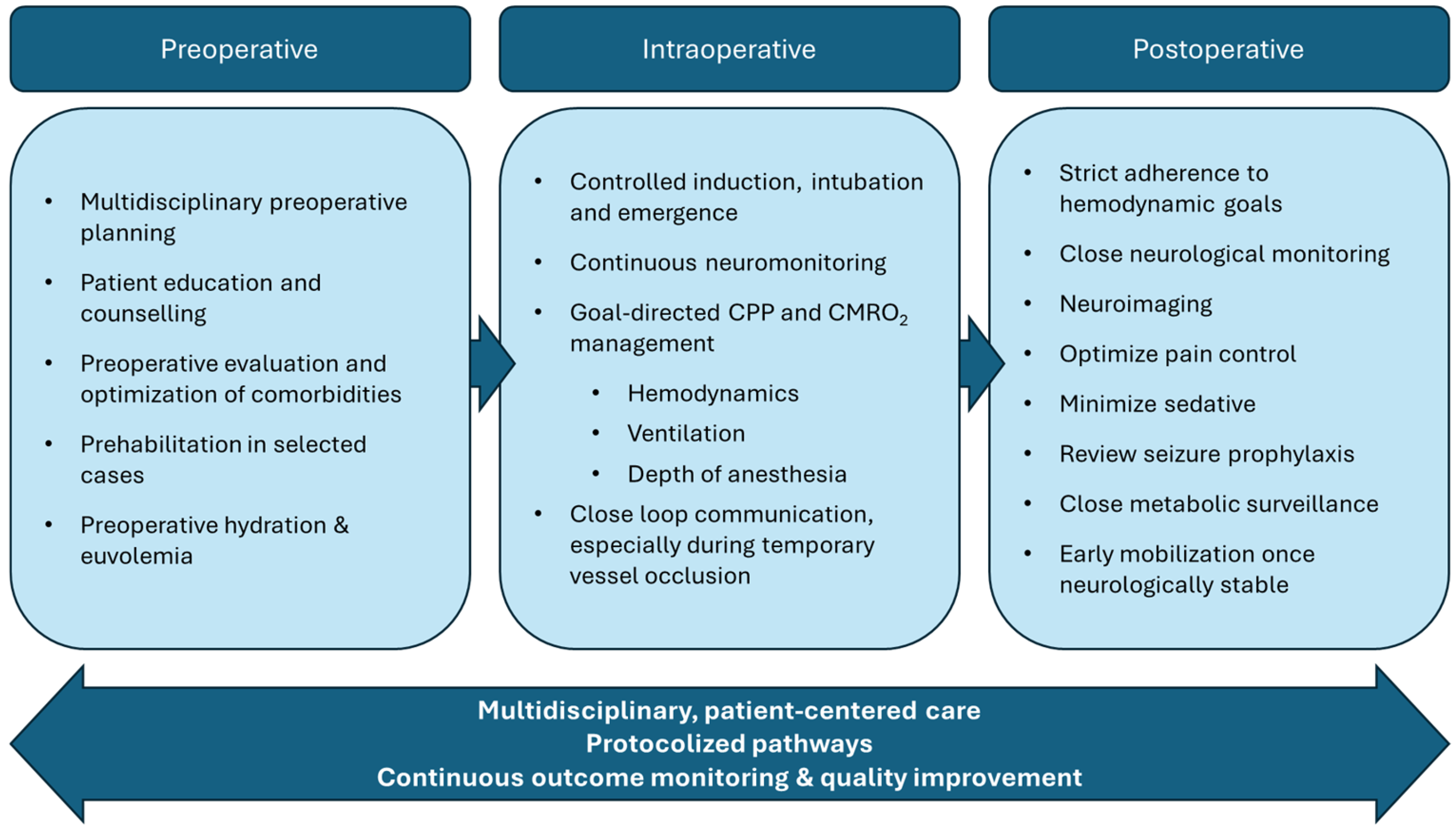
4. Perioperative Considerations
4.1. Preoperative Considerations
4.1.1. Patient Selection
4.1.2. Multidisciplinary Planning
4.1.3. Presurgical Optimization
4.2. Intraoperative Considerations
4.2.1. Fluid Management
4.2.2. Anesthetic Agent Selection
4.2.3. Hemodynamic Goals
4.2.4. Burst Suppression
4.2.5. Emergence
4.3. Postoperative Considerations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists (classification) |
| BIS | Bispectral Index |
| CBF | Cerebral blood flow |
| CBV | Cerebral blood volume |
| CMRO2 | Cerebral metabolic rate of oxygen consumption |
| CPP | Cerebral perfusion pressure |
| CSF | Cerebrospinal fluid |
| CT | Computed tomography |
| EC–IC | Extracranial–intracranial |
| ICA | Internal carotid artery |
| ICP | Intracranial pressure |
| MAC | Minimum alveolar concentration |
| MAP | Mean arterial pressure |
| MCA | Middle cerebral artery |
| MLCK | Myosin light chain kinase |
| MMD | Moyamoya disease |
| MTT | Mean transit time |
| NCCU | Neurocritical care unit |
| NO | Nitric oxide |
| PaCO2 | Partial pressure of carbon dioxide in cerebral arteries |
| PaO2 | Partial pressure of oxygen in cerebral arteries |
| SSEP | Somatosensory evoked potential |
| STA | Superficial temporal artery |
| TIVA | Total intravenous anesthesia |
References
- Fisher, M. OCCLUSION OF THE INTERNAL CAROTID ARTERY. Arch. Neurol. Psychiatry 1951, 65, 346–377. [Google Scholar] [CrossRef]
- Yasagril, M.G. Reconstructive and Constructive Surgery of the Cerebral Arteries in Man. Microsurgery applied to neurosurgery. In Microsurgery: Applied to Neurosurgery; Georg Thieme: Stuttgart, Germany, 1969; pp. 82–150. [Google Scholar]
- Doherty, R.J.; Caird, J.; Crimmins, D.; Kelly, P.; Murphy, S.; McGuigan, C.; Tubridy, N.; King, M.D.; Lynch, B.; Webb, D.; et al. Moyamoya disease and moyamoya syndrome in Ireland: Patient demographics, mode of presentation and outcomes of EC–IC bypass surgery. Ir. J. Med Sci. (1971-) 2020, 190, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Muroi, C.; Khan, N.; Bellut, D.; Fujioka, M.; Yonekawa, Y. Extracranial–intracranial bypass in atherosclerotic cerebrovascular disease: Report of a single centre experience. Br. J. Neurosurg. 2011, 25, 357–362. [Google Scholar] [CrossRef][Green Version]
- Brenner, L.B.; Sousa, M.P.; Andreão, F.F.; Prestes, M.Z.; Palavani, L.B.; Batista, S.; Koester, S.W.; Rabelo, N.N.; Bertani, R.; Welling, L.C.; et al. Clinical and Technical Outcomes of Intracranial-Intracranial Bypass for Treating Complex Intracranial Aneurysms: An Analysis of 255 Patients. World Neurosurg. 2024, 187, 223–235.e4. [Google Scholar] [CrossRef] [PubMed]
- Amin-Hanjani, S.; Butler, W.E.; Ogilvy, C.S.; Carter, B.S.; Barker, F.G. Extracranial—intracranial bypass in the treatment of occlusive cerebrovascular disease and intracranial aneurysms in the United States between 1992 and 2001: A population-based study. J. Neurosurg. 2005, 103, 794–804. [Google Scholar] [CrossRef]
- Awano, T.; Sakatani, K.; Yokose, N.; Kondo, Y.; Igarashi, T.; Hoshino, T.; Nakamura, S.; Fujiwara, N.; Murata, Y.; Katayama, Y.; et al. Intraoperative EC–IC Bypass Blood Flow Assessment With Indocyanine Green Angiography in Moyamoya and Non-Moyamoya Ischemic Stroke. World Neurosurg. 2010, 73, 668–674. [Google Scholar] [CrossRef]
- Dengler, J.; Cabraja, M.; Faust, K.; Picht, T.; Kombos, T.; Vajkoczy, P. Intraoperative neurophysiological monitoring of extracranial-intracranial bypass procedures. J. Neurosurg. 2013, 119, 207–214. [Google Scholar] [CrossRef]
- Grüter, B.E.; Tosic, L.; Voglis, S.; Vasella, F.; Mutschler, V.; Bichsel, O.; Scherrer, N.; Regli, L.; Esposito, G. Trends in Literature on Cerebral Bypass Surgery: A Systematic Review. Cerebrovasc. Dis. 2021, 51, 102–113. [Google Scholar] [CrossRef]
- Meling, T.R.; Patet, G. The role of EC–IC bypass in ICA blood blister aneurysms—a systematic review. Neurosurg. Rev. 2020, 44, 905–914. [Google Scholar] [CrossRef]
- Schaller, B. Extracranial-Intracranial Bypass to Reduce the Risk of Ischemic Stroke in Intracranial Aneurysms of the Anterior Cerebral Circulation: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2008, 17, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.-K.; Lawton, M.T. Practice Trends in Intracranial Bypass Surgery in a 21-Year Experience. World Neurosurg. 2019, 125, e717–e722. [Google Scholar] [CrossRef]
- Sanai, N.; Zador, Z.; Lawton, M.T. Bypass surgery for complex brain aneurysms. Neurosurgery 2009, 65, 670–683. [Google Scholar] [CrossRef]
- Wolfswinkel, E.M.; Landau, M.J.; Ravina, K.; Kokot, N.C.; Russin, J.J.; Carey, J.N. EC-IC bypass for cerebral revascularization following skull base tumor resection: Current practices and innovations. J. Surg. Oncol. 2018, 118, 815–825. [Google Scholar] [CrossRef]
- Wessels, L.; Hecht, N.; Vajkoczy, P. Bypass in neurosurgery—indications and techniques. Neurosurg. Rev. 2018, 42, 389–393. [Google Scholar] [CrossRef]
- Gazyakan, E.M.; Lee, C.-Y.; Wu, C.-T.; Tsao, C.-K.; Craft, R.; Henry, S.L.; Cheng, M.-H.M.; Lee, S.-T. Indications and Outcomes of Prophylactic and Therapeutic Extracranial-to-intracranial Arterial Bypass for Cerebral Revascularization. Plast. Reconstr. Surg.–Glob. Open 2015, 3, e372. [Google Scholar] [CrossRef]
- Joshi, G.; Yamada, Y.; Thavara, B.D.; Tanaka, R.; Miyatini, K.; Nakao, K.; Kawase, T.; Takizava, K.; Kato, Y. EC–IC Bypass; Our experience of cerebral revascularization with intraoperative Dual-Image Video Angiography (Diva). Asian J. Neurosurg. 2020, 15, 499–506. [Google Scholar] [CrossRef]
- Matsukawa, H.; Tanikawa, R.; Kamiyama, H.; Tsuboi, T.; Noda, K.; Ota, N.; Miyata, S.; Tokuda, S. The Valveless Saphenous Vein Graft Technique for EC–IC High-Flow Bypass: Technical Note. World Neurosurg. 2016, 87, 35–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Sia, S.; Morgan, M.; Qian, Y. Flow resistance analysis of extracranial-to-intracranial (EC–IC) vein bypass. J. Biomech. 2012, 45, 1400–1405. [Google Scholar] [CrossRef]
- Dubovoy, A.V.; Ovsyannikov, K.S.; Guzhin, V.E.; Cherepanov, A.V.; Galaktionov, D.M.; Perfil’eV, A.M.; Sosnov, A.O. The use of high-flow extracranial-intracranial artery bypass in pathology of the cerebral and brachiocephalic arteries: Technical features and surgical outcomes. Vopr. neirokhirurgii Im. N.N. Burdenko 2017, 81, 5–21. [Google Scholar] [CrossRef]
- Herzig, R.; Hluštík, P.; Urbánek, K.; Vaverka, M.; Buřval, S.; Macháč, J.; Vlachová, I.; Křupka, B.; Bártková, A.; Šaňák, D.; et al. Can We Identify Patients With Carotid Occlusion Who Would Benefit From Ec/ic Bypass? Review. Biomed. Pap. 2004, 148, 119–122. [Google Scholar] [CrossRef]
- Patel, H.C.; Mcnamara, I.R.; Al-Rawi, P.G.; Kirkpatrick, P.J. Improved cerebrovascular reactivity following low flow EC/IC bypass in patients with occlusive carotid disease. Br. J. Neurosurg. 2010, 24, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Sia, S.F.; Morgan, M.K. High flow extracranial-to-intracranial brain bypass surgery. J. Clin. Neurosci. 2013, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, J.; van der Zwan, A.; Ramos, L.M.; van Osch, M.J.; Golay, X.; Tulleken, C.A.; van der Grond, J. Altered Flow Territories after Extracranial-Intracranial Bypass Surgery. Neurosurgery 2005, 57, 486–494. [Google Scholar] [CrossRef]
- Vu, E.L.; Brown, C.H.; Brady, K.M.; Hogue, C.W. Monitoring of cerebral blood flow autoregulation: Physiologic basis, measurement, and clinical implications. Br. J. Anaesth. 2024, 132, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Smith, M. Cerebral perfusion pressure. Br. J. Anaesth. 2015, 115, 488–490. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.-S.; Hill, C.; Armstrong, K.; Tarumi, T.; Hodics, T.; Hynan, L.S.; Zhang, R. Cerebral Autoregulation of Blood Velocity and Volumetric Flow During Steady-State Changes in Arterial Pressure. Hypertension 2013, 62, 973–979. [Google Scholar] [CrossRef]
- Rossi, J.L.; Todd, T.; Bazan, N.G.; Belayev, L. Inhibition of Myosin Light-Chain Kinase Attenuates Cerebral Edema after Traumatic Brain Injury in Postnatal Mice. J. Neurotrauma 2013, 30, 1672–1679. [Google Scholar] [CrossRef]
- White, H.; Venkatesh, B. Cerebral Perfusion Pressure in Neurotrauma: A Review. Anesthesia Analg. 2008, 107, 979–988. [Google Scholar] [CrossRef]
- Lidington, D.; Wan, H.; Bolz, S.-S. Cerebral Autoregulation in Subarachnoid Hemorrhage. Front. Neurol. 2021, 12, 688362. [Google Scholar] [CrossRef]
- Iadecola, C.; Davisson, R.L. Hypertension and Cerebrovascular Dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef]
- Ruland, S.; Aiyagari, V. Cerebral Autoregulation and Blood Pressure Lowering. Hypertension 2007, 49, 977–978. [Google Scholar] [CrossRef]
- Yoon, S.; Zuccarello, M.; Rapoport, R.M. pCO2 and pH regulation of cerebral blood flow. Front. Physiol. 2012, 3, 365. [Google Scholar] [CrossRef]
- Yoshihara, M.; Bandoh, K.; Marmarou, A. Cerebrovascular carbon dioxide reactivity assessed by intracranial pressure dynamics in severely head injured patients. J. Neurosurg. 1995, 82, 386–393. [Google Scholar] [CrossRef]
- Duffin, J.; Mikulis, D.J.; Fisher, J.A. Control of Cerebral Blood Flow by Blood Gases. Front. Physiol. 2021, 12, 640075. [Google Scholar] [CrossRef]
- Freeman, R.D.; Li, B. Neural–metabolic coupling in the central visual pathway. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150357. [Google Scholar] [CrossRef]
- Bao, L.; Xu, F. Fundamental research progress of mild hypothermia in cerebral protection. SpringerPlus 2013, 2, 306. [Google Scholar] [CrossRef]
- Oshima, T.; Karasawa, F.; Satoh, T. Effects of propofol on cerebral blood flow and the metabolic rate of oxygen in humans. Acta Anaesthesiol. Scand. 2002, 46, 831–835. [Google Scholar] [CrossRef]
- Renou, A.M.; Vernhiet, J.; Macrez, P.; Constant, P.; Billeerey, J.; Khadaroo, M.; Caillé, J. Cerebral blood flow and metabolism during etomidate anaesthesia in man. Br. J. Anaesth. 1978, 50, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, A.N.; Mejia, V.A.; Maxwell, R.A.; Smith, P.W.; Dart, B.W.; Barker, D.E. Adrenal Suppression Following a Single Dose of Etomidate For Rapid Sequence Induction: A Prospective Randomized Study. J. Trauma Inj. Infect. Crit. Care 2008, 65, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Gregers, M.C.T.; Mikkelsen, S.; Lindvig, K.P.; Brøchner, A.C. Ketamine as an Anesthetic for Patients with Acute Brain Injury: A Systematic Review. Neurocritical Care 2020, 33, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Preethi, J.; Bidkar, P.U.; Cherian, A.; Dey, A.; Srinivasan, S.; Adinarayanan, S.; Ramesh, A.S. Comparison of total intravenous anesthesia vs. inhalational anesthesia on brain relaxation, intracranial pressure, and hemodynamics in patients with acute subdural hematoma undergoing emergency craniotomy: A randomized control trial. Eur. J. Trauma Emerg. Surg. 2019, 47, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Jiao, S.; Zhang, Y.; Wang, L.; Yao, Y.; Yuan, F.; Chen, R.; Zhou, Q. Effects of desflurane and sevoflurane on somatosensory-evoked and motor-evoked potential monitoring during neurosurgery: A randomized controlled trial. BMC Anesthesiol. 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McDowell, F.; Flamm, E.S. EC/IC Bypass Study. Stroke 1986, 17, 1–2. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Wang, T.; Sun, X.; Lu, G.; Xu, X.; Yang, R.; Luo, J.; Bai, X.; Tong, X.; et al. Determining the Optimal Age for Extracranial-Intracranial Bypass Surgery: A Post Hoc Analysis of the CMOSS Randomized Trial. Stroke 2025, 56, 362–370. [Google Scholar] [CrossRef]
- Gobble, R.M.; Hoang, H.; Jafar, J.; Adelman, M. Extracranial-intracranial bypass: Resurrection of a nearly extinct operation. J. Vasc. Surg. 2012, 56, 1303–1307. [Google Scholar] [CrossRef]
- Li, C.; Cao, X.; Ma, Z.; Sun, X.; Hu, F.; Wang, L. Effect of pre-surgery assessments on the prognosis of patients received extracranial-intracranial bypass surgery. Restor. Neurol. Neurosci. 2018, 36, 593–604. [Google Scholar] [CrossRef]
- Kato, N.; Kan, I.; Abe, Y.; Otani, K.; Narikiyo, M.; Nagayama, G.; Nishimura, K.; Mori, R.; Kodama, T.; Ishibashi, T.; et al. Visualization of extracranial-intracranial bypass in moyamoya patients using intraoperative three-dimensional digital subtraction angiography with intravenous contrast injection and robotic C-arm: Patient series. J. Neurosurg. Case Lessons 2021, 1. [Google Scholar] [CrossRef] [PubMed]
- Anokwute, M.C.; Preda, V.; Di Ieva, A. Determining Contemporary Barriers to Effective Multidisciplinary Team Meetings in Neurological Surgery: A Review of the Literature. World Neurosurg. 2023, 172, 73–80. [Google Scholar] [CrossRef]
- Wessels, L.; Hecht, N.; Vajkoczy, P. Patients receiving extracranial to intracranial bypass for atherosclerotic vessel occlusion today differ significantly from the COSS population. Stroke 2021, 52, e599–e604. [Google Scholar] [CrossRef]
- Bush, R.L.; Zhan, H.T.; Purcell, S.T. Preoperative optimization of the vascular surgery patient. Vasc. Heal. Risk Manag. 2015, 11, 379–385. [Google Scholar] [CrossRef]
- Tew, G.A.; Caisley, K.; Danjoux, G. Preoperative exercise training for adults undergoing elective major vascular surgery: A systematic review. PLoS ONE 2022, 17, e0263090. [Google Scholar] [CrossRef]
- Bargnes, V.; Davidson, S.; Talbot, L.; Jin, Z.; Poppers, J.; Bergese, S.D. Start Strong, Finish Strong: A Review of Prehabilitation in Cardiac Surgery. Life 2024, 14, 832. [Google Scholar] [CrossRef]
- van der Jagt, M. Fluid management of the neurological patient: A concise review. Crit. Care 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Cascella, M.; Bimonte, S.; Di Napoli, R. Delayed Emergence from Anesthesia: What We Know and How We Act. Local Reg. Anesthesia 2020, 13, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, C.; Noto, A.; Crimi, C.; Sanfilippo, F. Remifentanil-induced postoperative hyperalgesia: Current perspectives on mechanisms and therapeutic strategies. Local Reg. Anesth. 2018, 11, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bithal, P. Anaesthetic considerations for evoked potentials monitoring. J. Neuroanaesth. Crit. Care 2014, 01, 002–012. [Google Scholar] [CrossRef]
- Dashdorj, N.; Corrie, K.; Napolitano, A.; Petersen, E.; Mahajan, R.P.; Auer, D.P. Effects of Subanesthetic Dose of Nitrous Oxide on Cerebral Blood Flow and Metabolism. Anesthesiology 2013, 118, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Brull, S.J.; Kopman, A.F.; Hunter, J.M.; Fülesdi, B.; Arkes, H.R.; Elstein, A.; Todd, M.M.; Johnson, K.B. Consensus Statement on Perioperative Use of Neuromuscular Monitoring. Anesthesia Analg. 2018, 127, 71–80. [Google Scholar] [CrossRef]
- Durga, P.; Kinthala, S.; Sahu, B.P.; Panigrahi, M.K.; Mantha, S.; Ramachandran, G. Efficacy and outcomes of perioperative anesthetic management of extracranial to intracranial bypass for complex intracranial aneurysm in the absence of advanced neurological monitoring. J. Anaesthesiol. Clin. Pharmacol. 2014, 30, 345–350. [Google Scholar] [CrossRef]
- Lonjaret, L.; Lairez, O.; Geeraerts, T.; Minville, V. Optimal perioperative management of arterial blood pressure. Integr. Blood Press. Control. 2014, 7, 49–59. [Google Scholar] [CrossRef]
- Michenfelder, J.D. The Interdependency of Cerebral Functional and Metabolic Effects Following Massive Doses of Thiopental in the Dog. Anesthesiology 1974, 41, 231–236. [Google Scholar] [CrossRef]
- Chui, J.; Manninen, P.; Sacho, R.H.; Venkatraghavan, L. Anesthetic Management of Patients Undergoing Intracranial Bypass Procedures. Anesthesia Analg. 2015, 120, 193–203. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Li, J.; Zhang, J.; Chen, J. Cerebral Hyperperfusion Syndrome After Revascularization Surgery in Patients with Moyamoya Disease: Systematic Review and Meta-Analysis. World Neurosurg. 2020, 135, 357–366.e4. [Google Scholar] [CrossRef]
- I Stiver, S.; Ogilvy, C.S. Acute hyperperfusion syndrome complicating EC–IC bypass. J. Neurol. Neurosurg. Psychiatry 2002, 73, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.; Choy, D.K.; Lwin, S.; Ning, C.; Yeo, T.T.; Shen, L.; Chong, V.F.; Teoh, H.L.; Seet, R.C.; Chan, B.P.; et al. Cerebral Hyperperfusion Syndrome After Superficial Temporal Artery-middle Cerebral Artery Bypass for Severe Intracranial Steno-occlusive Disease. Neurosurgery 2013, 72, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Ito, M.; Uchino, H.; Kawabori, M.; Sugiyama, T. Efficacy and Safety of Combined Revascularization Surgery for Moyamoya Disease: Standard Procedure and Perioperative Management. Trends Treat. Cerebrovasc. Dis. 2025, 136, 99–104. [Google Scholar] [CrossRef]
- Nobles, K.; Cunningham, K.; Fecondo, B.; Closs, S.M.; Donovan, K.; Kumar, M.A. Mobilization in Neurocritical Care: Challenges and Opportunities. Curr. Neurol. Neurosci. Rep. 2024, 25, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schubert, G.A.; Biermann, P.; Weiss, C.; Seiz, M.; Vajkoczy, P.; Schmiedek, P.; Thomé, C. Risk Profile In Extracranial/Intracranial Bypass Surgery—The Role of Antiplatelet Agents, Disease Pathology, and Surgical Technique In 168 Direct Revascularization Procedures. World Neurosurg. 2014, 82, 672–677. [Google Scholar] [CrossRef]
- Hurth, H.; Hauser, T.-K.; Haas, P.; Wang, S.; Mengel, A.; Tatagiba, M.; Ernemann, U.; Khan, N.; Roder, C. Early Post-operative CT-Angiography Imaging After EC–IC Bypass Surgery in Moyamoya Patients. Front. Neurol. 2021, 12, 655943. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bargnes, V., III; Andraous, W.; Bitonti, N.; Jin, Z.; Geralemou, S. Necessary Harmony Between Anesthesia and Neurosurgery During Extracranial–Intracranial Bypass: A Review of Neuroanesthesia Strategies and Perioperative Insights. NeuroSci 2025, 6, 96. https://doi.org/10.3390/neurosci6040096
Bargnes V III, Andraous W, Bitonti N, Jin Z, Geralemou S. Necessary Harmony Between Anesthesia and Neurosurgery During Extracranial–Intracranial Bypass: A Review of Neuroanesthesia Strategies and Perioperative Insights. NeuroSci. 2025; 6(4):96. https://doi.org/10.3390/neurosci6040096
Chicago/Turabian StyleBargnes, Vincent, III, Wesam Andraous, Nicholas Bitonti, Zhaosheng Jin, and Sofia Geralemou. 2025. "Necessary Harmony Between Anesthesia and Neurosurgery During Extracranial–Intracranial Bypass: A Review of Neuroanesthesia Strategies and Perioperative Insights" NeuroSci 6, no. 4: 96. https://doi.org/10.3390/neurosci6040096
APA StyleBargnes, V., III, Andraous, W., Bitonti, N., Jin, Z., & Geralemou, S. (2025). Necessary Harmony Between Anesthesia and Neurosurgery During Extracranial–Intracranial Bypass: A Review of Neuroanesthesia Strategies and Perioperative Insights. NeuroSci, 6(4), 96. https://doi.org/10.3390/neurosci6040096





