Toward Biology-Driven Diagnosis of Atypical Parkinsonian Disorders
Abstract
1. Introduction
2. Progressive Supranuclear Palsy (PSP)
2.1. Aetiology, Genetics, and Pathobiology
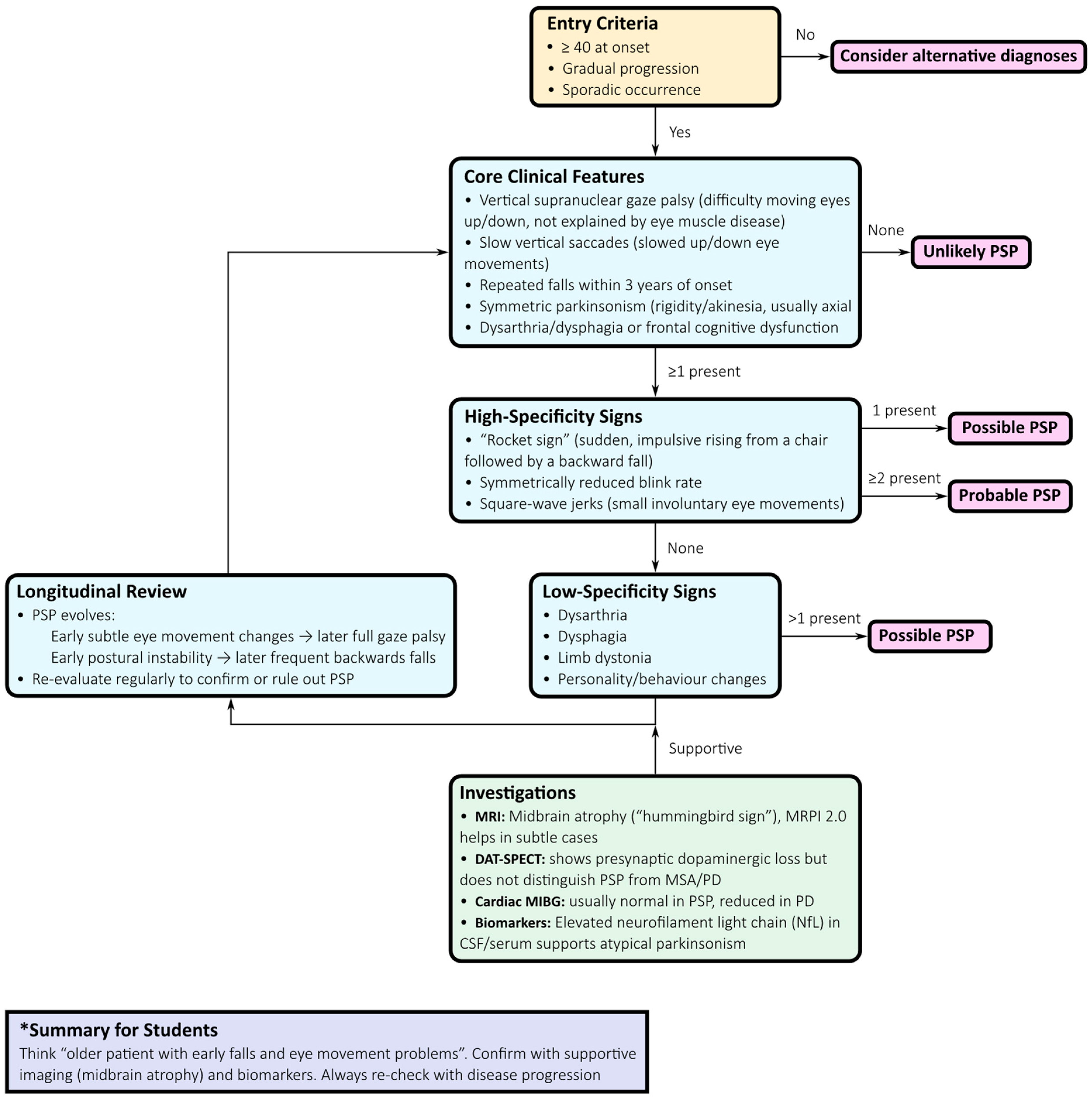
2.2. Diagnostic Approach: From Clinical Suspicion to Biology-Driven Certainty
- Clinical evaluation: Symmetric axial-predominant Parkinsonism, vertical gaze impairment, pseudobulbar features, and dysexecutive cognitive profile should prompt suspicion of PSP.
- Ocular motor assessment: Slowing of vertical saccades progressing to supranuclear gaze palsy, with square-wave jerks, provides high specificity.
- Phenotypic classification: The MDS criteria support early recognition of variant phenotypes, including PSP-P, PSP with predominant gait freezing (PSP-PGF), PSP–corticobasal syndrome (PSP-CBS), PSP–frontotemporal dementia (PSP-FTD), and language-predominant variants.
- Neuroimaging: Midbrain atrophy with relative pontine sparing—the “hummingbird sign”—is a supportive feature. Quantitative measures such as the MRPI 2.0, increasingly available via automated pipelines, improve diagnostic accuracy, especially in distinguishing PSP-P from PD and MSA [7].
- Supportive imaging: Dopamine transporter SPECT (DAT-SPECT) confirms presynaptic nigrostriatal degeneration but lacks nosological specificity. Cardiac 123I-MIBG scintigraphy, typically normal in PSP but reduced in Lewy body disorders, aids differential diagnosis, particularly with protocols incorporating salivary gland uptake [9].
- Fluid biomarkers: NfL concentrations in cerebrospinal fluid or plasma are significantly higher in PSP than in PD and correlate with disease progression. Combined with MRI markers, NfL improves triage and prognostication [10].
- Longitudinal reassessment: Regular re-evaluation is crucial, as phenotypes evolve and diagnostic certainty increases over time.
2.3. Phenotypic Spectrum and Evolution
2.4. Investigations and Biomarkers
2.4.1. MRI
2.4.2. Tau PET
2.4.3. Dopaminergic Imaging and Autonomic Tracers
2.4.4. Fluid Biomarkers
2.4.5. Neurophysiology
2.5. Neuropathology
2.6. Treatment and Management
2.7. Future Directions
3. Corticobasal Degeneration (CBD)
3.1. Etiology and Genetics
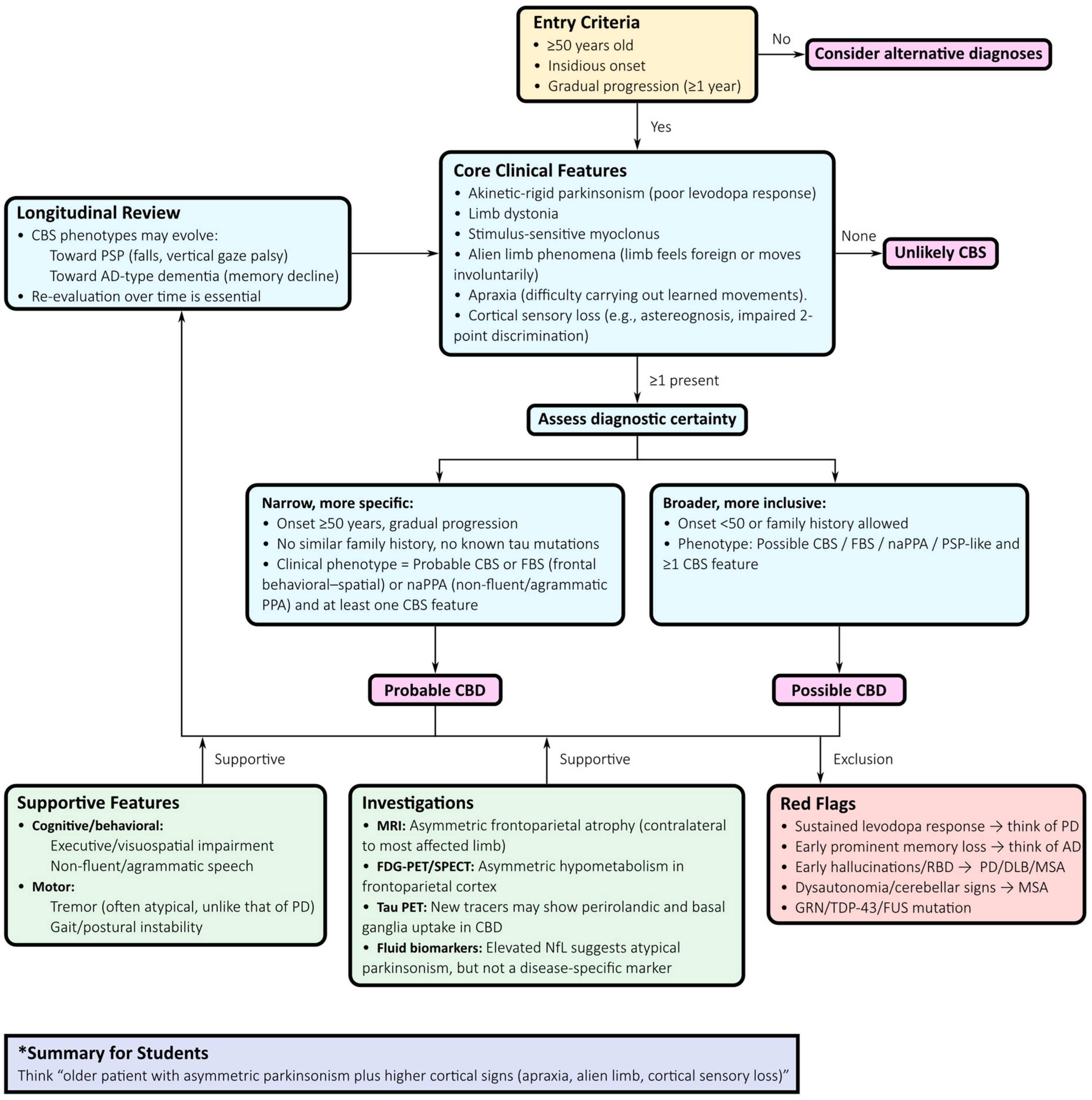
3.2. Diagnostic Approach
- Application of Armstrong criteria: Distinguishing between “probable” and “possible” CBD offers a standardized clinical framework. Although sensitivity and specificity are limited, they provide an initial scaffold for structured evaluation [51].
- Neuropsychological evaluation: Comprehensive testing of executive, visuospatial, and language domains is essential. A non-fluent or agrammatic primary progressive aphasia profile favours CBD, whereas early episodic memory impairment points toward AD-related CBS [56].
- MRI assessment: CBD classically presents with asymmetric frontoparietal atrophy contralateral to the most affected limb, sometimes involving the basal ganglia. While not diagnostic, these findings strengthen the clinicopathological correlation. Advanced morphometric methods, such as voxel-based morphometry or cortical thickness mapping, can detect early changes but lack individual-level specificity [6,58].
- Functional imaging: FDG-PET and perfusion SPECT typically demonstrate asymmetric hypometabolism or hypoperfusion in the frontoparietal cortex and basal ganglia, often extending into the supplementary motor area, with relative sparing of the midbrain and cerebellum. These features distinguish CBD from PSP and MSA but overlap with AD-related CBS [59].
- Longitudinal reassessment: Phenotypic evolution is frequent, with progression toward PSP-like Richardson’s syndrome, behavioural variant FTD, non-fluent aphasia, or posterior cortical atrophy. Ongoing clinical review is essential, as early labels often evolve with disease progression.
3.3. Phenotypic Spectrum and Evolution
3.4. Investigations and Biomarkers
3.4.1. MRI
3.4.2. FDG-PET and Perfusion SPECT
3.4.3. Tau PET
3.4.4. Fluid Biomarkers
3.4.5. Neurophysiology
3.5. Pathology
3.6. Treatment and Management
3.7. Future Perspectives
4. Multiple System Atrophy (MSA)
4.1. Etiology and Genetics
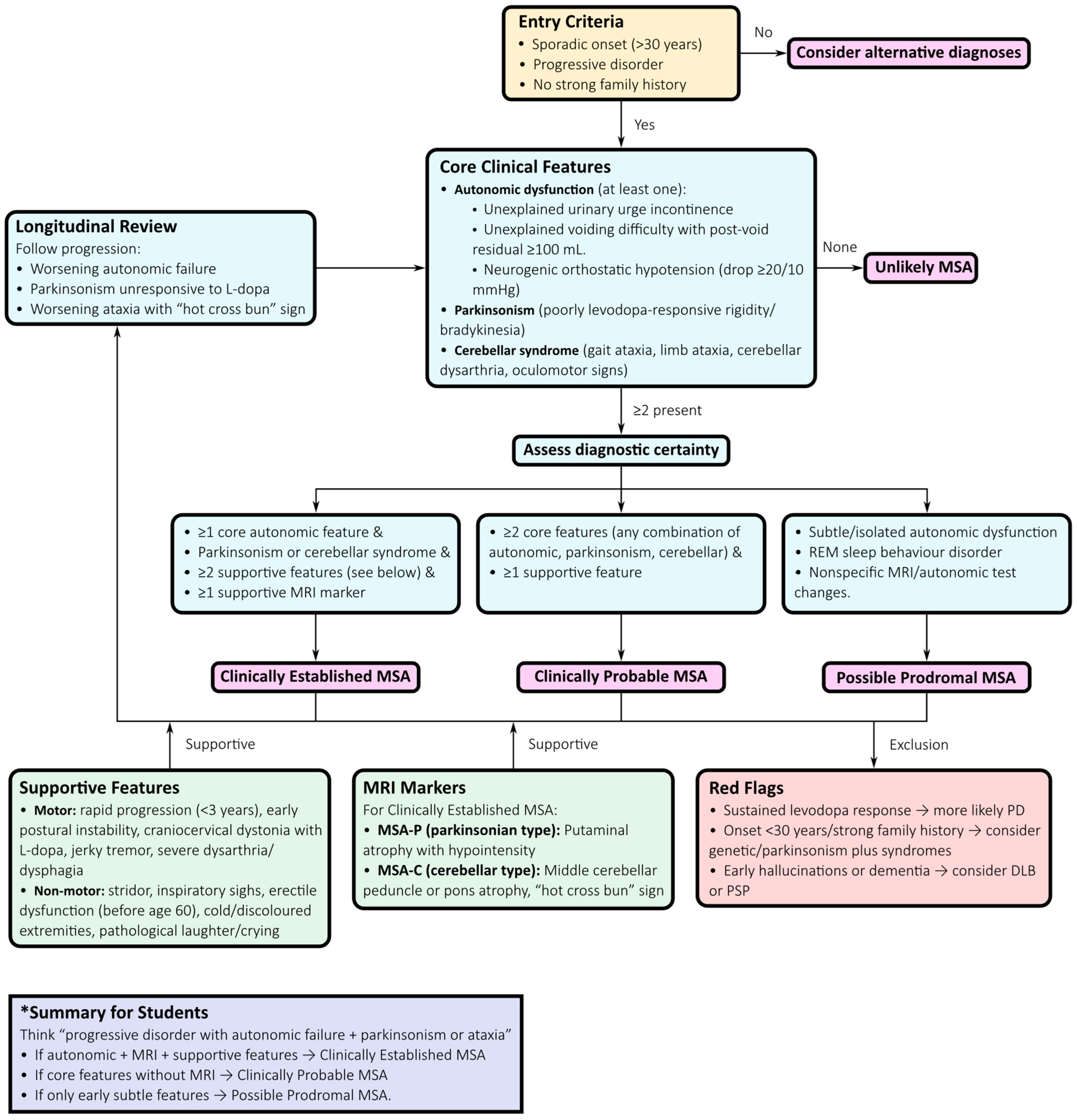
4.2. Diagnostic Approach
- Initial presentation: Suspect MSA when symmetric Parkinsonism (MSA-P) or cerebellar ataxia (MSA-C) occurs alongside autonomic failure. Autonomic symptoms frequently precede motor signs and should trigger early diagnostic consideration.
- Autonomic assessment: Comprehensive autonomic testing—including tilt-table testing, Valsalva manoeuvre, and quantitative sudomotor axon reflex testing—documents cardiovascular and sudomotor involvement. Urodynamic studies can reveal detrusor overactivity with impaired contractility or detrusor–sphincter dyssynergia, providing strong diagnostic support [76,77,78].
- Motor examination: Poor or transient levodopa response, jerky cortical myoclonus, or focal dystonia favour MSA over PD, where sustained levodopa responsiveness and a classic rest tremor are typical [72].
- MRI assessment: Structural MRI findings such as the “hot cross bun” sign in the pons, putaminal atrophy and hypointensity, and middle cerebellar peduncle (MCP) hyperintensity or atrophy support the diagnosis. Quantitative morphometric measures, including MCP width and pons-to-MCP area ratios, further improve early diagnostic accuracy [6,79,80].
- FDG-PET: Patterns of putaminal, pontine, and cerebellar hypometabolism with relative cortical sparing support MSA and assist in distinguishing it from PSP and PD [83].
- Longitudinal reassessment: Because MSA phenotypes evolve over time, serial evaluations are essential. Patients initially presenting with isolated autonomic failure may later develop parkinsonian or cerebellar features, clarifying the diagnosis and informing prognosis and clinical trial eligibility [72,76,85].
4.3. Phenotypic Spectrum
4.4. Investigations and Biomarkers
4.4.1. MRI
4.4.2. Functional Imaging
4.4.3. Autonomic Testing
4.4.4. Fluid and Molecular Biomarkers
4.4.5. Neurophysiology
4.5. Pathology
4.6. Treatment and Management
4.7. Future Perspectives
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-syn | Alpha-synuclein |
| 123I-IBZM | [123I]Iodobenzamide |
| 123I-MIBG | [123I]Metaiodobenzylguanidine |
| 4R-tau | 4-repeat tauopathy |
| AD | Alzheimer’s disease |
| APD | Atypical parkinsonian disorder |
| AUC | Area under the receiver operating characteristic curve |
| CBS | Corticobasal syndrome |
| CBD | Corticobasal degeneration |
| CSF | Cerebrospinal fluid |
| DAT-SPECT | Dopamine transporter single-photon emission computed tomography |
| FDG-PET | [18F]Fluorodeoxyglucose positron emission tomography |
| FTD | Frontotemporal dementia |
| FTLD | Frontotemporal lobar degeneration |
| FTLD-TDP | FTLD with TPD-43-immunoreactive pathology |
| GSK-3β | Glycogen synthase kinase-3 beta |
| MCP | Middle cerebellar peduncle |
| MDS | Movement Disorder Society |
| MRI | Magnetic resonance imaging |
| MRPI 2.0 | Magnetic Resonance Parkinsonism Index 2.0 |
| MSA | Multiple system atrophy |
| MSA-C | Multiple system atrophy, cerebellar phenotype |
| MSA-P | Multiple system atrophy, parkinsonian phenotype |
| NfL | Neurofilament light chain |
| PD | Parkinson’s disease |
| PET | Positron emission tomography |
| PSP | Progressive supranuclear palsy |
| PSP-CBS | PSP with corticobasal syndrome |
| PSP-FTD | PSP with frontotemporal dementia |
| PSP-P | PSP–Parkinsonism |
| PSP-PGF | PSP with predominant gait freezing |
| PSP-RS | PSP–Richardson’s syndrome |
References
- Hoglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Muller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Wenning, G.K.; Stankovic, I.; Vignatelli, L.; Fanciulli, A.; Calandra-Buonaura, G.; Seppi, K.; Palma, J.A.; Meissner, W.G.; Krismer, F.; Berg, D.; et al. The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov. Disord. 2022, 37, 1131–1148. [Google Scholar] [CrossRef]
- Iankova, V.; Respondek, G.; Saranza, G.; Painous, C.; Camara, A.; Compta, Y.; Aiba, I.; Balint, B.; Giagkou, N.; Josephs, K.A.; et al. Video-tutorial for the Movement Disorder Society criteria for progressive supranuclear palsy. Park. Relat. Disord. 2020, 78, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Le Heron, C.; Anderson, T. Corticobasal syndrome: A practical guide. Pract. Neurol. 2021, 21, 276–285. [Google Scholar] [CrossRef]
- Nouh, C.D.; Younes, K. Diagnosis and Management of Progressive Corticobasal Syndrome. Curr. Treat. Options Neurol. 2024, 26, 319–338. [Google Scholar] [CrossRef]
- Ortega-Robles, E.; de Celis Alonso, B.; Cantillo-Negrete, J.; Carino-Escobar, R.I.; Arias-Carrion, O. Advanced Magnetic Resonance Imaging for Early Diagnosis and Monitoring of Movement Disorders. Brain Sci. 2025, 15, 79. [Google Scholar] [CrossRef]
- Quattrone, A.; Bianco, M.G.; Antonini, A.; Vaillancourt, D.E.; Seppi, K.; Ceravolo, R.; Strafella, A.P.; Tedeschi, G.; Tessitore, A.; Cilia, R.; et al. Development and Validation of Automated Magnetic Resonance Parkinsonism Index 2.0 to Distinguish Progressive Supranuclear Palsy-Parkinsonism From Parkinson’s Disease. Mov. Disord. 2022, 37, 1272–1281. [Google Scholar] [CrossRef]
- Quattrone, A.; Morelli, M.; Nigro, S.; Quattrone, A.; Vescio, B.; Arabia, G.; Nicoletti, G.; Nistico, R.; Salsone, M.; Novellino, F.; et al. A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson’s disease. Park. Relat. Disord. 2018, 54, 3–8. [Google Scholar] [CrossRef]
- Catalan, M.; Dore, F.; Polverino, P.; Bertolotti, C.; Sartori, A.; Antonutti, L.; Cucca, A.; Furlanis, G.; Capitanio, S.; Manganotti, P. (123)I-Metaiodobenzylguanidine Myocardial Scintigraphy in Discriminating Degenerative Parkinsonisms. Mov. Disord. Clin. Pract. 2021, 8, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Demiri, S.; Veltsista, D.; Siokas, V.; Spiliopoulos, K.C.; Tsika, A.; Stamati, P.; Chroni, E.; Dardiotis, E.; Liampas, I. Neurofilament Light Chain in Cerebrospinal Fluid and Blood in Multiple System Atrophy: A Systematic Review and Meta-Analysis. Brain Sci. 2025, 15, 241. [Google Scholar] [CrossRef]
- Arias-Carrion, O.; Guerra-Crespo, M.; Padilla-Godinez, F.J.; Soto-Rojas, L.O.; Manjarrez, E. alpha-Synuclein Pathology in Synucleinopathies: Mechanisms, Biomarkers, and Therapeutic Challenges. Int. J. Mol. Sci. 2025, 26, 5405. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Gomes, B.; Farris, C.M.; Ma, Y.; Concha-Marambio, L.; Lebovitz, R.; Nellgard, B.; Dalla, K.; Constantinescu, J.; Constantinescu, R.; Gobom, J.; et al. Alpha-Synuclein seed amplification assay as a diagnostic tool for parkinsonian disorders. Park. Relat. Disord. 2023, 117, 105807. [Google Scholar] [CrossRef]
- Jellinger, K.A. Comorbid pathologies and their impact on multiple system atrophy: Current view. J. Neural Transm. 2025. [Google Scholar] [CrossRef]
- Jellinger, K.A. Comorbid pathologies and their impact on progressive supranuclear palsy: Current view. J. Neural Transm. 2025. [Google Scholar] [CrossRef]
- Dunalska, A.; Pikul, J.; Schok, K.; Wiejak, K.A.; Alster, P. The Significance of Vascular Pathogenesis in the Examination of Corticobasal Syndrome. Front. Aging Neurosci. 2021, 13, 668614. [Google Scholar] [CrossRef]
- Alster, P.; Otto-Ślusarczyk, D.; Szlufik, S.; Duszyńska-Wąs, K.; Drzewińska, A.; Wiercińska-Drapało, A.; Struga, M.; Kutyłowski, M.; Friedman, A.; Madetko-Alster, N. The significance of glial cell line-derived neurotrophic factor analysis in Progressive Supranuclear Palsy. Sci. Rep. 2024, 14, 2805. [Google Scholar] [CrossRef]
- Alster, P.; Otto-Ślusarczyk, D.; Wiercińska-Drapało, A.; Struga, M.; Madetko-Alster, N. The potential significance of hepcidin evaluation in progressive supranuclear palsy. Brain Behav. 2024, 14, e3552. [Google Scholar] [CrossRef]
- Alster, P.; Otto-Ślusarczyk, D.; Kutyłowski, M.; Migda, B.; Wiercińska-Drapało, A.; Jabłońska, J.; Struga, M.; Madetko-Alster, N. The associations between common neuroimaging parameters of Progressive Supranuclear Palsy in magnetic resonance imaging and non-specific inflammatory factors—Pilot study. Front. Immunol. 2024, 15, 1458713. [Google Scholar] [CrossRef] [PubMed]
- Mena, A.M.; Strafella, A.P. Imaging pathological tau in atypical parkinsonisms: A review. Clin. Park. Relat. Disord. 2022, 7, 100155. [Google Scholar] [CrossRef]
- Stamelou, M.; Respondek, G.; Giagkou, N.; Whitwell, J.L.; Kovacs, G.G.; Hoglinger, G.U. Evolving concepts in progressive supranuclear palsy and other 4-repeat tauopathies. Nat. Rev. Neurol. 2021, 17, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Barer, Y.; Chodick, G.; Cohen, R.; Grabarnik-John, M.; Ye, X.; Zamudio, J.; Gurevich, T. Epidemiology of Progressive Supranuclear Palsy: Real World Data from the Second Largest Health Plan in Israel. Brain Sci. 2022, 12, 1126. [Google Scholar] [CrossRef]
- Lyons, S.; Trepel, D.; Lynch, T.; Walsh, R.; O’Dowd, S. The prevalence and incidence of progressive supranuclear palsy and corticobasal syndrome: A systematic review and meta-analysis. J. Neurol. 2023, 270, 4451–4465. [Google Scholar] [CrossRef]
- Street, D.; Bevan-Jones, W.R.; Malpetti, M.; Jones, P.S.; Passamonti, L.; Ghosh, B.C.; Rittman, T.; Coyle-Gilchrist, I.T.; Allinson, K.; Dawson, C.E.; et al. Structural correlates of survival in progressive supranuclear palsy. Park. Relat. Disord. 2023, 116, 105866. [Google Scholar] [CrossRef] [PubMed]
- Nysetvold, E.; Lopez, L.N.; Cogell, A.N.; Fryk, H.; Pace, N.D.; Taylor, S.S.; Rhoden, J.; Nichols, C.A.; Pillas, D.; Klein, A.; et al. Progressive Supranuclear palsy (PSP) disease progression, management, and healthcare resource utilization: A retrospective observational study in the US and Canada. Orphanet J. Rare Dis. 2024, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.A.; Chen, Z.; Won, H.; Huang, A.Y.; Lowe, J.K.; Wojta, K.; Yokoyama, J.S.; Bensimon, G.; Leigh, P.N.; Payan, C.; et al. Joint genome-wide association study of progressive supranuclear palsy identifies novel susceptibility loci and genetic correlation to neurodegenerative diseases. Mol. Neurodegener. 2018, 13, 41. [Google Scholar] [CrossRef]
- Farrell, K.; Humphrey, J.; Chang, T.; Zhao, Y.; Leung, Y.Y.; Kuksa, P.P.; Patil, V.; Lee, W.P.; Kuzma, A.B.; Valladares, O.; et al. Genetic, transcriptomic, histological, and biochemical analysis of progressive supranuclear palsy implicates glial activation and novel risk genes. Nat. Commun. 2024, 15, 7880. [Google Scholar] [CrossRef] [PubMed]
- Hoglinger, G.U.; Melhem, N.M.; Dickson, D.W.; Sleiman, P.M.; Wang, L.S.; Klei, L.; Rademakers, R.; de Silva, R.; Litvan, I.; Riley, D.E.; et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 2011, 43, 699–705. [Google Scholar] [CrossRef]
- Sanchez-Contreras, M.Y.; Kouri, N.; Cook, C.N.; Serie, D.J.; Heckman, M.G.; Finch, N.A.; Caselli, R.J.; Uitti, R.J.; Wszolek, Z.K.; Graff-Radford, N.; et al. Replication of progressive supranuclear palsy genome-wide association study identifies SLCO1A2 and DUSP10 as new susceptibility loci. Mol. Neurodegener. 2018, 13, 37. [Google Scholar] [CrossRef]
- Wang, H.; Chang, T.S.; Dombroski, B.A.; Cheng, P.L.; Patil, V.; Valiente-Banuet, L.; Farrell, K.; McLean, C.; Molina-Porcel, L.; Rajput, A.; et al. Whole-genome sequencing analysis reveals new susceptibility loci and structural variants associated with progressive supranuclear palsy. Mol. Neurodegener. 2024, 19, 61. [Google Scholar] [CrossRef]
- Cullinane, P.W.; Fumi, R.; Theilmann Jensen, M.; Jabbari, E.; Warner, T.T.; Revesz, T.; Morris, H.R.; Rohrer, J.D.; Jaunmuktane, Z. MAPT-Associated Familial Progressive Supranuclear Palsy with Typical Corticobasal Degeneration Neuropathology: A Clinicopathological Report. Mov. Disord. Clin. Pract. 2023, 10, 691–694. [Google Scholar] [CrossRef]
- Liu, F.T.; Lu, J.Y.; Li, X.Y.; Liang, X.N.; Jiao, F.Y.; Ge, J.J.; Wu, P.; Li, G.; Shen, B.; Wu, B.; et al. (18)F-Florzolotau PET imaging captures the distribution patterns and regional vulnerability of tau pathology in progressive supranuclear palsy. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1395–1405. [Google Scholar] [CrossRef]
- Chung, D.C.; Roemer, S.; Petrucelli, L.; Dickson, D.W. Cellular and pathological heterogeneity of primary tauopathies. Mol. Neurodegener. 2021, 16, 57. [Google Scholar] [CrossRef]
- Ichikawa-Escamilla, E.; Velasco-Martinez, R.A.; Adalid-Peralta, L. Progressive Supranuclear Palsy Syndrome: An Overview. IBRO Neurosci. Rep. 2024, 16, 598–608. [Google Scholar] [CrossRef]
- Chovatiya, H.; Pillai, K.; Reddy, C.; Thalakkattu, A.; Avarachan, A.; Chacko, M.; Kishore, A. Video-Oculography for Enhancing the Diagnostic Accuracy of Early Oculomotor Dysfunction in Progressive Supranuclear Palsy. J. Mov. Disord. 2025, 18, 77–86. [Google Scholar] [CrossRef]
- Facchin, A.; Buonocore, J.; Crasa, M.; Quattrone, A.; Quattrone, A. Systematic assessment of square-wave jerks in progressive supranuclear palsy: A video-oculographic study. J. Neurol. 2024, 271, 6639–6646. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, J.; Behler, A.; Dreyhaupt, J.; Ludolph, A.C.; Pinkhardt, E.H.; Kassubek, J. Diagnostic value of video-oculography in progressive supranuclear palsy: A controlled study in 100 patients. J. Neurol. 2021, 268, 3467–3475. [Google Scholar] [CrossRef] [PubMed]
- Brendel, M.; Barthel, H.; van Eimeren, T.; Marek, K.; Beyer, L.; Song, M.; Palleis, C.; Gehmeyr, M.; Fietzek, U.; Respondek, G.; et al. Assessment of 18F-PI-2620 as a Biomarker in Progressive Supranuclear Palsy. JAMA Neurol. 2020, 77, 1408–1419. [Google Scholar] [CrossRef]
- Hong, J.; Lu, J.; Liu, F.; Wang, M.; Li, X.; Clement, C.; Lopes, L.; Brendel, M.; Rominger, A.; Yen, T.C.; et al. Uncovering distinct progression patterns of tau deposition in progressive supranuclear palsy using [(18)F]Florzolotau PET imaging and subtype/stage inference algorithm. eBioMedicine 2023, 97, 104835. [Google Scholar] [CrossRef]
- Messerschmidt, K.; Barthel, H.; Brendel, M.; Scherlach, C.; Hoffmann, K.T.; Rauchmann, B.S.; Rullmann, M.; Marek, K.; Villemagne, V.L.; Rumpf, J.J.; et al. (18)F-PI-2620 Tau PET Improves the Imaging Diagnosis of Progressive Supranuclear Palsy. J. Nucl. Med. 2022, 63, 1754–1760. [Google Scholar] [CrossRef]
- Wallert, E.D.; van de Giessen, E.; Knol, R.J.J.; Beudel, M.; de Bie, R.M.A.; Booij, J. Imaging Dopaminergic Neurotransmission in Neurodegenerative Disorders. J. Nucl. Med. 2022, 63, 27S–32S. [Google Scholar] [CrossRef] [PubMed]
- Ebina, J.; Mizumura, S.; Shibukawa, M.; Morioka, H.; Nagasawa, J.; Yanagihashi, M.; Hirayama, T.; Ishii, N.; Kobayashi, Y.; Inaba, A.; et al. Comparison of MIBG uptake in the major salivary glands between Lewy body disease and progressive supranuclear palsy. Clin. Park. Relat. Disord. 2024, 11, 100287. [Google Scholar] [CrossRef] [PubMed]
- Pitton Rissardo, J.; Fornari Caprara, A.L. Cardiac 123I-Metaiodobenzylguanidine (MIBG) Scintigraphy in Parkinson’s Disease: A Comprehensive Review. Brain Sci. 2023, 13, 1471. [Google Scholar] [CrossRef] [PubMed]
- Baiardi, S.; Rossi, M.; Giannini, G.; Mammana, A.; Polischi, B.; Sambati, L.; Mastrangelo, A.; Magliocchetti, F.; Cortelli, P.; Capellari, S.; et al. Head-to-head comparison of four cerebrospinal fluid and three plasma neurofilament light chain assays in Parkinsonism. npj Park. Dis. 2025, 11, 98. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, W.; Xu, W.; Li, J.Q.; Hou, X.H.; Ou, Y.N.; Yu, J.T.; Tan, L. Neurofilament Light Chain in Cerebrospinal Fluid and Blood as a Biomarker for Neurodegenerative Diseases: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2019, 72, 1353–1361. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, J.; Cai, T.; He, C.; Li, Y.; Li, X.; Chen, Z.; Wang, L.; Zhang, Y. Quantitative susceptibility mapping and blood neurofilament light chain differentiate between parkinsonian disorders. Front. Aging Neurosci. 2022, 14, 909552. [Google Scholar] [CrossRef]
- Suppa, A.; Asci, F.; Kamble, N.; Chen, K.-H.; Sciacca, G.; Merchant, S.H.I.; Tijssen, M.A.J.; Chen, R.; Hallett, M.; Pal, P.K. Neurophysiology of Atypical Parkinsonian Syndromes: A Study Group Position Paper. Mov. Disord. 2025, 40, 1451–1510. [Google Scholar] [CrossRef]
- Apetauerova, D.; Scala, S.A.; Hamill, R.W.; Simon, D.K.; Pathak, S.; Ruthazer, R.; Standaert, D.G.; Yacoubian, T.A. CoQ10 in progressive supranuclear palsy: A randomized, placebo-controlled, double-blind trial. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e266. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Litvan, I.; Hoglinger, G.U.; Burn, D.; Lees, A.; Andres, M.V.; Gomez-Carrillo, B.; Leon, T.; Del Ser, T.; Investigators, T. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov. Disord. 2014, 29, 470–478. [Google Scholar] [CrossRef]
- Hoglinger, G.U.; Litvan, I.; Mendonca, N.; Wang, D.; Zheng, H.; Rendenbach-Mueller, B.; Lon, H.K.; Jin, Z.; Fisseha, N.; Budur, K.; et al. Safety and efficacy of tilavonemab in progressive supranuclear palsy: A phase 2, randomised, placebo-controlled trial. Lancet Neurol. 2021, 20, 182–192. [Google Scholar] [CrossRef]
- Rowe, J.B.; Holland, N.; Rittman, T. Progressive supranuclear palsy: Diagnosis and management. Pract. Neurol. 2021, 21, 376–383. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef]
- Lee, S.E.; Rabinovici, G.D.; Mayo, M.C.; Wilson, S.M.; Seeley, W.W.; DeArmond, S.J.; Huang, E.J.; Trojanowski, J.Q.; Growdon, M.E.; Jang, J.Y.; et al. Clinicopathological correlations in corticobasal degeneration. Ann. Neurol. 2011, 70, 327–340. [Google Scholar] [CrossRef]
- Aiba, I.; Hayashi, Y.; Shimohata, T.; Yoshida, M.; Saito, Y.; Wakabayashi, K.; Komori, T.; Hasegawa, M.; Ikeuchi, T.; Tokumaru, A.M.; et al. Clinical course of pathologically confirmed corticobasal degeneration and corticobasal syndrome. Brain Commun. 2023, 5, fcad296. [Google Scholar] [CrossRef]
- Valentino, R.R.; Koga, S.; Walton, R.L.; Soto-Beasley, A.I.; Kouri, N.; DeTure, M.A.; Murray, M.E.; Johnson, P.W.; Petersen, R.C.; Boeve, B.F.; et al. MAPT subhaplotypes in corticobasal degeneration: Assessing associations with disease risk, severity of tau pathology, and clinical features. Acta Neuropathol. Commun. 2020, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, J.S.; Karch, C.M.; Fan, C.C.; Bonham, L.W.; Kouri, N.; Ross, O.A.; Rademakers, R.; Kim, J.; Wang, Y.; Hoglinger, G.U.; et al. Shared genetic risk between corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia. Acta Neuropathol. 2017, 133, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, V.C.; Paraskevas, G.P.; Paraskevas, P.G.; Stefanis, L.; Kapaki, E. Corticobasal degeneration and corticobasal syndrome: A review. Clin. Park. Relat. Disord. 2019, 1, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Mimuro, M.; Iwasaki, Y. Age-Related Pathology in Corticobasal Degeneration. Int. J. Mol. Sci. 2024, 25, 2740. [Google Scholar] [CrossRef]
- Boxer, A.L.; Geschwind, M.D.; Belfor, N.; Gorno-Tempini, M.L.; Schauer, G.F.; Miller, B.L.; Weiner, M.W.; Rosen, H.J. Patterns of Brain Atrophy That Differentiate Corticobasal Degeneration Syndrome From Progressive Supranuclear Palsy. Arch. Neurol. 2006, 63, 81–86. [Google Scholar] [CrossRef]
- Pardini, M.; Huey, E.D.; Spina, S.; Kreisl, W.C.; Morbelli, S.; Wassermann, E.M.; Nobili, F.; Ghetti, B.; Grafman, J. FDG-PET patterns associated with underlying pathology in corticobasal syndrome. Neurology 2019, 92, e1121–e1135. [Google Scholar] [CrossRef] [PubMed]
- Palleis, C.; Brendel, M.; Finze, A.; Weidinger, E.; Bötzel, K.; Danek, A.; Beyer, L.; Nitschmann, A.; Kern, M.; Biechele, G.; et al. Cortical [18F]PI-2620 Binding Differentiates Corticobasal Syndrome Subtypes. Mov. Disord. 2021, 36, 2104–2115. [Google Scholar] [CrossRef]
- Brumberg, J.; Schröter, N.; Blazhenets, G.; Omrane, M.A.; Volz, C.; Weiller, C.; Rijntjes, M.; Frings, L.; Hellwig, S.; Jost, W.H.; et al. [18F]Florzolotau PET for the Differential Diagnosis of Parkinsonism in Patients with Suspected 4-Repeat Tauopathies. J. Nucl. Med. 2025, 66, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Endo, H.; Takahata, K.; Tagai, K.; Suzuki, H.; Onaya, M.; Sano, Y.; Yamamoto, Y.; Kurose, S.; Matsuoka, K.; et al. In vivo PET classification of tau pathologies in patients with frontotemporal dementia. Brain Commun. 2024, 6, fcae075. [Google Scholar] [CrossRef]
- Palleis, C.; Bernhardt, A.M.; Weidinger, E.; Fietzek, U.M.; Jäck, A.; Katzdobler, S.; Gnörich, J.; Bauer, T.; Franzmeier, N.; Perneczky, R.; et al. A Biomarker-Based Classification of Corticobasal Syndrome. Mov. Disord. 2025. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, A.; Konitsiotis, S.; Sioka, C. Differentiating Progressive Supranuclear Palsy and Corticobasal Syndrome: Insights from Cerebrospinal Fluid Biomarkers-A Narrative Review. Medicina 2025, 61, 701. [Google Scholar] [CrossRef] [PubMed]
- Bridel, C.; van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; NFL Group. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef]
- Jellinger, K.A. The Spectrum of Cognitive Impairment in Atypical Parkinsonism Syndromes: A Comprehensive Review of Current Understanding and Research. Diseases 2025, 13, 39. [Google Scholar] [CrossRef]
- Mathew, R.; Bak, T.H.; Hodges, J.R. Diagnostic criteria for corticobasal syndrome: A comparative study. J. Neurol. Neurosurg. Psychiatry 2012, 83, 405–410. [Google Scholar] [CrossRef]
- Unti, E.; Mazzucchi, S.; Calabrese, R.; Palermo, G.; Del Prete, E.; Bonuccelli, U.; Ceravolo, R. Botulinum toxin for the treatment of dystonia and pain in corticobasal syndrome. Brain Behav. 2019, 9, e01182. [Google Scholar] [CrossRef]
- Mitsui, J.; Tsuji, S. Plasma Coenzyme Q10 Levels and Multiple System Atrophy-Reply. JAMA Neurol. 2016, 73, 1499–1500. [Google Scholar] [CrossRef]
- Porto, K.J.; Hirano, M.; Mitsui, J.; Chikada, A.; Matsukawa, T.; Ishiura, H.; Japan Multiple System Atrophy Registry, C.; Toda, T.; Kusunoki, S.; Tsuji, S. COQ2 V393A confers high risk susceptibility for multiple system atrophy in East Asian population. J. Neurol. Sci. 2021, 429, 117623. [Google Scholar] [CrossRef]
- Krismer, F.; Wenning, G.K. Multiple system atrophy: Insights into a rare and debilitating movement disorder. Nat. Rev. Neurol. 2017, 13, 232–243. [Google Scholar] [CrossRef]
- Fanciulli, A.; Wenning, G.K. Multiple-system atrophy. N. Engl. J. Med. 2015, 372, 249–263. [Google Scholar] [CrossRef]
- Ciolli, L.; Krismer, F.; Nicoletti, F.; Wenning, G.K. An update on the cerebellar subtype of multiple system atrophy. Cerebellum Ataxias 2014, 1, 14. [Google Scholar] [CrossRef]
- Cortelli, P.; Calandra-Buonaura, G.; Benarroch, E.E.; Giannini, G.; Iranzo, A.; Low, P.A.; Martinelli, P.; Provini, F.; Quinn, N.; Tolosa, E.; et al. Stridor in multiple system atrophy: Consensus statement on diagnosis, prognosis, and treatment. Neurology 2019, 93, 630–639. [Google Scholar] [CrossRef]
- Giannini, G.; Provini, F.; Cani, I.; Cecere, A.; Mignani, F.; Guaraldi, P.; Di Mirto, C.V.F.; Cortelli, P.; Calandra-Buonaura, G. Tracheostomy is associated with increased survival in Multiple System Atrophy patients with stridor. Eur. J. Neurol. 2022, 29, 2232–2240. [Google Scholar] [CrossRef] [PubMed]
- Nandanwar, D.; Truong, D.D. Multiple system atrophy: Diagnostic challenges and a proposed diagnostic algorithm. Clin. Park. Relat. 2024, 11, 100271. [Google Scholar] [CrossRef]
- Kimpinski, K.; Iodice, V.; Burton, D.D.; Camilleri, M.; Mullan, B.P.; Lipp, A.; Sandroni, P.; Gehrking, T.L.; Sletten, D.M.; Ahlskog, J.E.; et al. The role of autonomic testing in the differentiation of Parkinson’s disease from multiple system atrophy. J. Neurol. Sci. 2012, 317, 92–96. [Google Scholar] [CrossRef]
- Pena-Zelayeta, L.; Delgado-Minjares, K.M.; Villegas-Rojas, M.M.; Leon-Arcia, K.; Santiago-Balmaseda, A.; Andrade-Guerrero, J.; Perez-Segura, I.; Ortega-Robles, E.; Soto-Rojas, L.O.; Arias-Carrion, O. Redefining Non-Motor Symptoms in Parkinson’s Disease. J. Pers. Med. 2025, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Pellecchia, M.T.; Barone, P.; Mollica, C.; Salvatore, E.; Ianniciello, M.; Longo, K.; Varrone, A.; Vicidomini, C.; Picillo, M.; De Michele, G.; et al. Diffusion-weighted imaging in multiple system atrophy: A comparison between clinical subtypes. Mov. Disord. 2009, 24, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Rau, A.; Brumberg, J.; Urbach, H.; Meyer, P.T.; Schröter, N. Neuroimaging in multiple system atrophy: Clinical implications and novel developments. J. Neural Transm. 2025. [Google Scholar] [CrossRef]
- Booth, T.C.; Nathan, M.; Waldman, A.D.; Quigley, A.M.; Schapira, A.H.; Buscombe, J. The Role of Functional Dopamine-Transporter SPECT Imaging in Parkinsonian Syndromes, Part 2. Am. J. Neuroradiol. 2015, 36, 236. [Google Scholar] [CrossRef]
- Orimo, S.; Suzuki, M.; Inaba, A.; Mizusawa, H. 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: A systematic review and meta-analysis. Park. Relat. Disord. 2012, 18, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Biafore, F.; Depetris, T.S.; Bastianello, M. Recent Advancement and Clinical Implications of 18FDG-PET in Parkinson’s Disease, Atypical Parkinsonisms, and Other Movement Disorders. Curr. Neurol. Neurosci. Rep. 2019, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A.; Lantos, P.L. Papp–Lantos inclusions and the pathogenesis of multiple system atrophy: An update. Acta Neuropathol. 2010, 119, 657–667. [Google Scholar] [CrossRef]
- Krismer, F.; Fanciulli, A.; Meissner, W.G.; Coon, E.A.; Wenning, G.K. Multiple system atrophy: Advances in pathophysiology, diagnosis, and treatment. Lancet Neurol. 2024, 23, 1252–1266. [Google Scholar] [CrossRef]
- Arnone, A.; Allocca, M.; Di Dato, R.; Puccini, G.; Laghai, I.; Rubino, F.; Nerattini, M.; Ramat, S.; Lombardi, G.; Ferrari, C.; et al. FDG PET in the differential diagnosis of degenerative parkinsonian disorders: Usefulness of voxel-based analysis in clinical practice. Neurol. Sci. 2022, 43, 5333–5341. [Google Scholar] [CrossRef]
- Du, X.; Zhao, H.; Li, Y.; Dai, Y.; Gao, L.; Li, Y.; Fan, K.; Sun, Z.; Zhang, Y. The value of PET/CT in the diagnosis and differential diagnosis of Parkinson’s disease: A dual-tracer study. npj Park. Dis. 2024, 10, 171. [Google Scholar] [CrossRef]
- Di Luca, D.G.; Perlmutter, J.S. Time for Clinical Dopamine Transporter Scans in Parkinsonism?: Not DAT Yet. Neurology 2024, 102, e209558. [Google Scholar] [CrossRef]
- Miyamoto, T.; Miyamoto, M. Reduced cardiac (123)I-MIBG uptake is a robust biomarker of Lewy body disease in isolated rapid eye movement sleep behaviour disorder. Brain Commun. 2024, 6, fcae148. [Google Scholar] [CrossRef] [PubMed]
- Chelban, V.; Nikram, E.; Perez-Soriano, A.; Wilke, C.; Foubert-Samier, A.; Vijiaratnam, N.; Guo, T.; Jabbari, E.; Olufodun, S.; Gonzalez, M.; et al. Neurofilament light levels predict clinical progression and death in multiple system atrophy. Brain 2022, 145, 4398–4408. [Google Scholar] [CrossRef]
- Singer, W.; Schmeichel, A.M.; Sletten, D.M.; Gehrking, T.L.; Gehrking, J.A.; Trejo-Lopez, J.; Suarez, M.D.; Anderson, J.K.; Bass, P.H.; Lesnick, T.G.; et al. Neurofilament light chain in spinal fluid and plasma in multiple system atrophy: A prospective, longitudinal biomarker study. Clin. Auton. Res. 2023, 33, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Wang, N.; Rajan, S.; Kern, D.; Palma, J.A.; Kaufmann, H.; Freeman, R. Cutaneous alpha-Synuclein Signatures in Patients With Multiple System Atrophy and Parkinson Disease. Neurology 2023, 100, e1529–e1539. [Google Scholar] [CrossRef] [PubMed]
- Shahnawaz, M.; Mukherjee, A.; Pritzkow, S.; Mendez, N.; Rabadia, P.; Liu, X.; Hu, B.; Schmeichel, A.; Singer, W.; Wu, G.; et al. Discriminating alpha-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 2020, 578, 273–277. [Google Scholar] [CrossRef]
- Smith, R.; Capotosti, F.; Schain, M.; Ohlsson, T.; Vokali, E.; Molette, J.; Touilloux, T.; Hliva, V.; Dimitrakopoulos, I.K.; Puschmann, A.; et al. The alpha-synuclein PET tracer [18F] ACI-12589 distinguishes multiple system atrophy from other neurodegenerative diseases. Nat. Commun. 2023, 14, 6750. [Google Scholar] [CrossRef]
- Valera, E.; Masliah, E. The neuropathology of multiple system atrophy and its therapeutic implications. Auton. Neurosci. 2018, 211, 1–6. [Google Scholar] [CrossRef]
- Buur, L.; Wiedemann, J.; Larsen, F.; Ben Alaya-Fourati, F.; Kallunki, P.; Ditlevsen, D.K.; Sorensen, M.H.; Meulien, D. Randomized Phase I Trial of the alpha-Synuclein Antibody Lu AF82422. Mov. Disord. 2024, 39, 936–944. [Google Scholar] [CrossRef]
- Alterity Therapeutics. Alterity Therapeutics Reports Positive Topline Data from Open-Label Phase 2 Clinical Trial of ATH434 in Multiple System Atrophy. Available online: https://www.globenewswire.com/news-release/2025/07/28/3122359/0/en/Alterity-Therapeutics-Reports-Positive-Topline-Data-from-Open-Label-Phase-2-Clinical-Trial-of-ATH434-in-Multiple-System-Atrophy.html (accessed on 8 September 2025).
- Hu, M.T. REM sleep behavior disorder (RBD). Neurobiol. Dis. 2020, 143, 104996. [Google Scholar] [CrossRef]
- Fanciulli, A.; Stankovic, I.; Krismer, F.; Seppi, K.; Levin, J.; Wenning, G.K. Chapter Five—Multiple system atrophy. In International Review of Neurobiology; Stamelou, M., Höglinger, G.U., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 149, pp. 137–192. [Google Scholar]
- Chia, R.; Ray, A.; Shah, Z.; Ding, J.; Ruffo, P.; Fujita, M.; Menon, V.; Saez-Atienzar, S.; Reho, P.; Kaivola, K.; et al. Genome sequence analyses identify novel risk loci for multiple system atrophy. Neuron 2024, 112, 2142–2156.e2145. [Google Scholar] [CrossRef]
- Heras-Garvin, A.; Refolo, V.; Schmidt, C.; Malfertheiner, K.; Wenning, G.K.; Bradbury, M.; Stamler, D.; Stefanova, N. ATH434 Reduces α-Synuclein-Related Neurodegeneration in a Murine Model of Multiple System Atrophy. Mov. Disord. 2021, 36, 2605–2614. [Google Scholar] [CrossRef]
- Kallunki, P.; Sotty, F.; Willén, K.; Lubas, M.; David, L.; Ambjørn, M.; Bergström, A.-L.; Buur, L.; Malik, I.; Nyegaard, S.; et al. Rational selection of the monoclonal α-synuclein antibody amlenetug (Lu AF82422) for the treatment of α-synucleinopathies. npj Park. Dis. 2025, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, I.; Kuijpers, M.; Kaufmann, H. An update on multiple system atrophy. Curr. Opin. Neurol. 2024, 37, 400–408. [Google Scholar] [CrossRef]
- Olfati, N.; Akhoundi, F.H.; Litvan, I. Atypical Parkinsonian Disorders. Neurol. Clin. 2025, 43, 249–277. [Google Scholar] [CrossRef]
- Respondek, G.; Kurz, C.; Arzberger, T.; Compta, Y.; Englund, E.; Ferguson, L.W.; Gelpi, E.; Giese, A.; Irwin, D.J.; Meissner, W.G.; et al. Which ante mortem clinical features predict progressive supranuclear palsy pathology? Mov. Disord. 2017, 32, 995–1005. [Google Scholar] [CrossRef]
- Sengupta, U.; Kayed, R. Amyloid β, Tau, and α-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef] [PubMed]
- Keir, G.; Roytman, M.; Mashriqi, F.; Shahsavarani, S.; Franceschi, A.M. Atypical Parkinsonian Syndromes: Structural, Functional, and Molecular Imaging Features. AJNR Am. J. Neuroradiol. 2024, 45, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.-Y.; Kwon, H.S.; Kwon, M.-S.; Nahm, M.; Jin, H.K.; Bae, J.-s.; Kim, S.H. Biomarkers and therapeutic strategies targeting microglia in neurodegenerative diseases: Current status and future directions. Mol. Neurodegener. 2025, 20, 82. [Google Scholar] [CrossRef]
- Campagnolo, M.; Fiorenzato, E.; Musso, G.; Misenti, V.; Cauzzo, S.; Cagnin, A.; Biundo, R.; Bussè, C.; Fogliano, C.A.; Mozzetta, S.; et al. The role of blood-based biomarkers in Parkinsonian disorders, Alzheimer’s disease and frontotemporal dementia. J. Neurol. Sci. 2025, 476, 123617. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.E.; Barmpoutis, A.; Wu, S.S.; DeSimone, J.C.; Schauder, M.; Chen, R.; Parrish, T.B.; Wang, W.-e.; Molho, E.; Morgan, J.C.; et al. Automated Imaging Differentiation for Parkinsonism. JAMA Neurol. 2025, 82, 495–505. [Google Scholar] [CrossRef]
- Dennis, A.-G.P.; Strafella, A.P. The role of AI and machine learning in the diagnosis of Parkinson’s disease and atypical parkinsonisms. Park. Relat. Disord. 2024, 126, 106986. [Google Scholar] [CrossRef]
| Feature | Progressive Supranuclear Palsy (PSP) | Corticobasal Syndrome (CBS/CBD) | Multiple System Atrophy (MSA) | Parkinson’s Disease (PD) |
|---|---|---|---|---|
| Prevalence | ~7 per 100,000 | ~4 per 100,000 | ~4 per 100,000 | Much higher than atypical Parkinsonisms |
| Onset Age | ~63 years | >60 years, variable | 53–55 years | Typically > 60 years |
| Motor Symptoms | Symmetric Parkinsonism, early axial rigidity, backwards falls | Asymmetric rigidity, dystonia, myoclonus, apraxia | MSA-P: symmetric Parkinsonism (poor levodopa response); MSA-C: cerebellar signs (ataxia, dysarthria) | Asymmetric Parkinsonism, classic rest tremor, shuffling gait |
| Ocular Signs | Vertical supranuclear gaze palsy, slow vertical saccades | Difficulty initiating voluntary saccades, gaze apraxia | Rare, nonspecific ocular signs | Rare ocular involvement |
| Cognitive Profile | Subcortical dementia (executive dysfunction, slowed processing) | Frontal-executive and parietal dysfunction; alien limb; cortical sensory loss | Cognitive impairment may occur, but not early or prominent | Cognitive impairment usually occurs late (dementia in advanced PD) |
| Autonomic Dysfunction | Not prominent early; may appear late | Rare or mild | Prominent: orthostatic hypotension, urinary incontinence/retention, erectile dysfunction, constipation | Mild compared with MSA |
| Key Pathology | 4R-tauopathy (globose tangles, tufted astrocytes) | 4R-tauopathy (astrocytic plaques, ballooned neurons) | α-synucleinopathy (glial cytoplasmic inclusions) | α-synucleinopathy (Lewy bodies) |
| Imaging | Midbrain atrophy (“hummingbird sign”); increased MRPI 2.0 | Asymmetric frontoparietal atrophy (contralateral to the affected limb) | “Hot cross bun” sign (pons), putaminal atrophy/hypointensity | Often, normal or nonspecific changes |
| Levodopa Response | Poor, transient at best | Poor or absent | Poor or transient (rare sustained response) | Good, especially early |
| Other Key Features | Early falls (<3 yrs), pseudobulbar palsy, reduced blink, square-wave jerks | Alien limb, cortical sensory loss, asymmetric apraxia | Early stridor, rapid progression, cold extremities, REM sleep behavior disorder | Classic pill-rolling tremor, clear honeymoon response to levodopa |
| Teaching tip for students | Falls and eye movement problems early | Asymmetric Parkinsonism with cortical signs (apraxia, alien limb phenomenon). | Autonomic failure plus Parkinsonism/ataxia | Asymmetric, tremor-dominant, levodopa-responsive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Carrión, O.; Romero-Gutiérrez, E.; Ortega-Robles, E. Toward Biology-Driven Diagnosis of Atypical Parkinsonian Disorders. NeuroSci 2025, 6, 107. https://doi.org/10.3390/neurosci6040107
Arias-Carrión O, Romero-Gutiérrez E, Ortega-Robles E. Toward Biology-Driven Diagnosis of Atypical Parkinsonian Disorders. NeuroSci. 2025; 6(4):107. https://doi.org/10.3390/neurosci6040107
Chicago/Turabian StyleArias-Carrión, Oscar, Elizabeth Romero-Gutiérrez, and Emmanuel Ortega-Robles. 2025. "Toward Biology-Driven Diagnosis of Atypical Parkinsonian Disorders" NeuroSci 6, no. 4: 107. https://doi.org/10.3390/neurosci6040107
APA StyleArias-Carrión, O., Romero-Gutiérrez, E., & Ortega-Robles, E. (2025). Toward Biology-Driven Diagnosis of Atypical Parkinsonian Disorders. NeuroSci, 6(4), 107. https://doi.org/10.3390/neurosci6040107








