STING Signaling Deficiency Exacerbates Demyelination and Immune Infiltration in Focal EAE Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Focal EAE Lesion
2.3. Propidium Iodide
2.4. Tissue Processing
2.5. Histology
2.6. Flow Cytometry
2.7. RNA Sequencing
2.8. BioMark, Fluidigm Analysis
2.9. Quantification of Pathology
2.10. Statistics
3. Results
3.1. Focal EAE Pathology in Corpus Callosum Is Influenced by Type I IFN Signaling
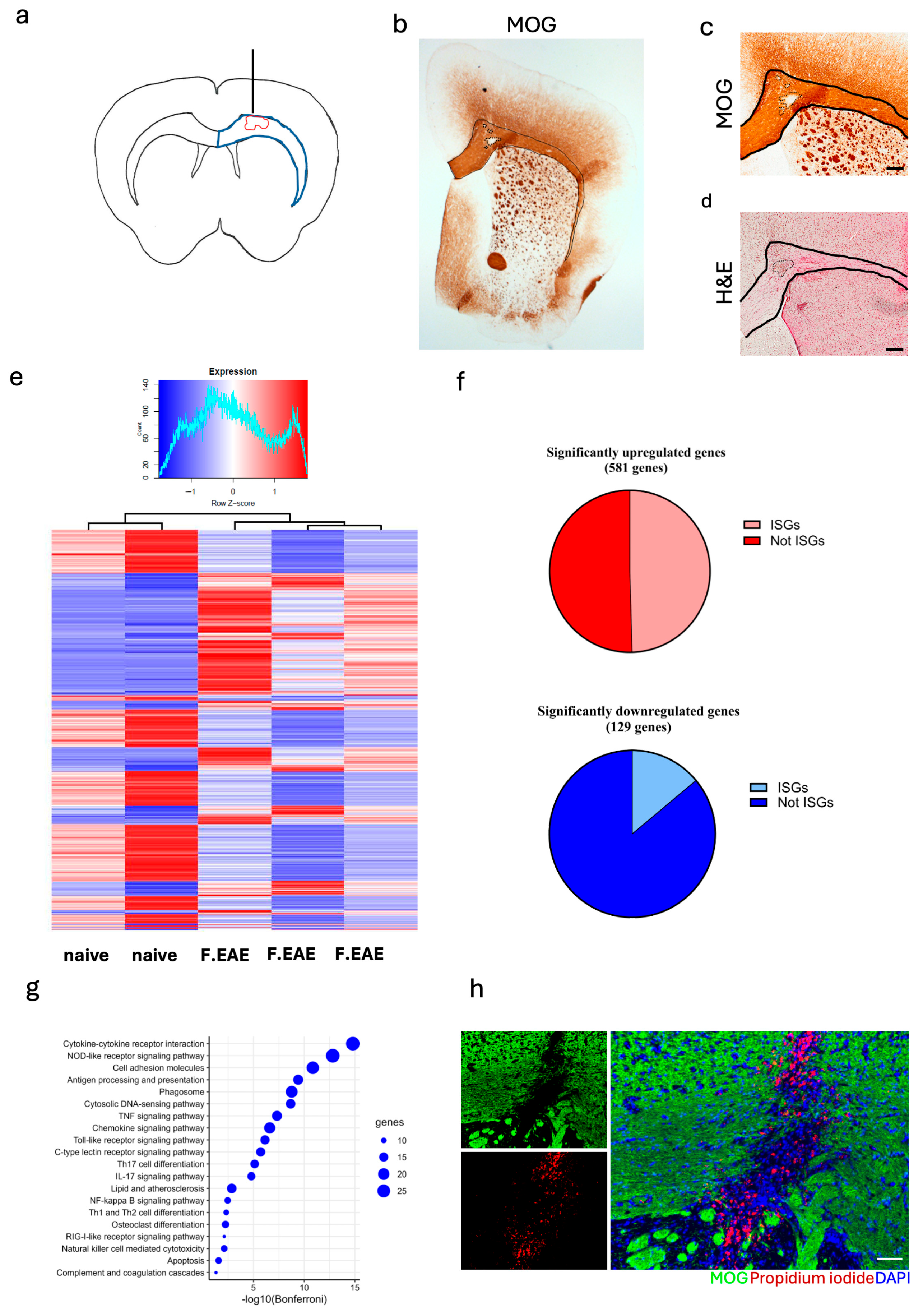
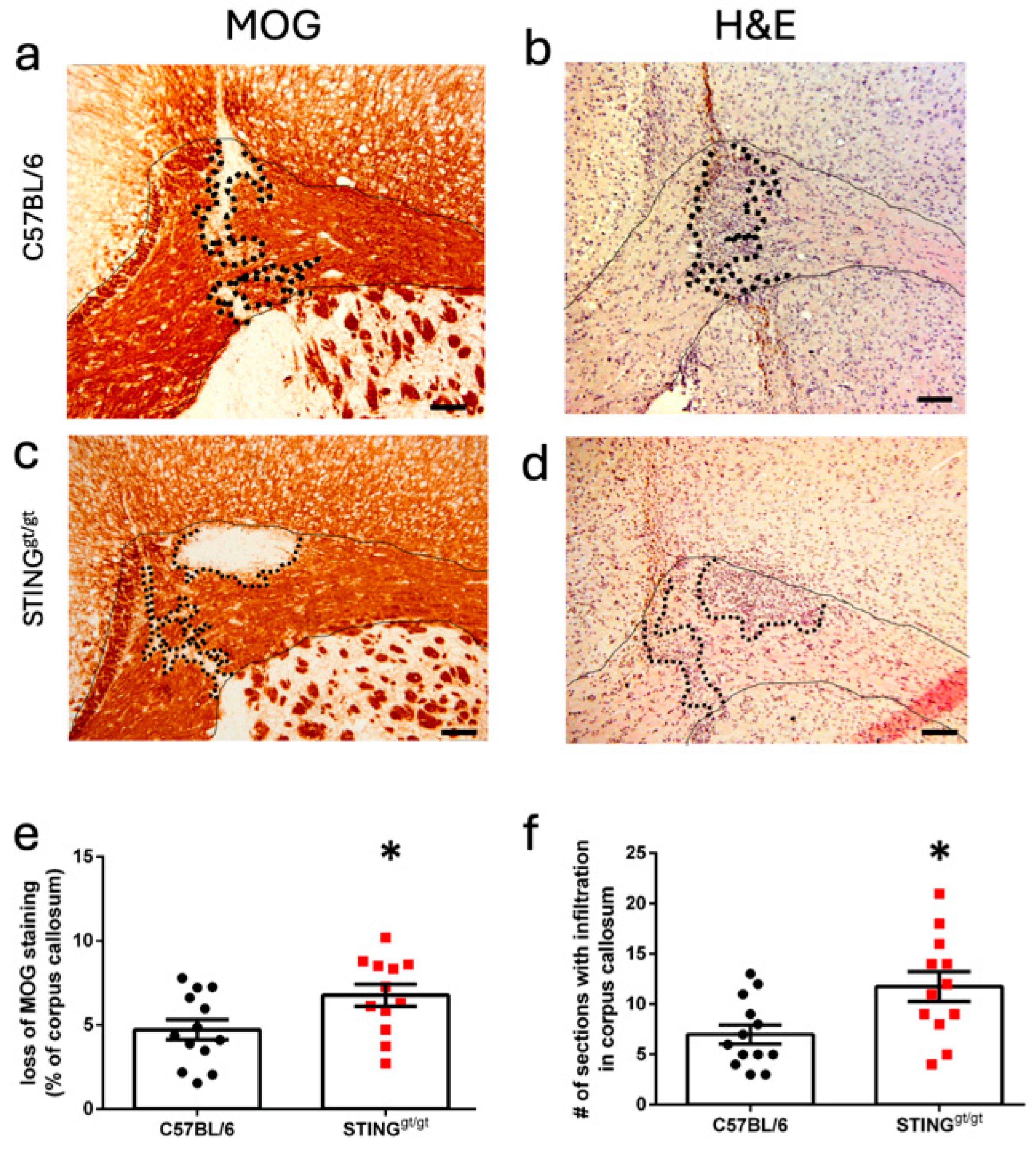
3.2. STING Deficiency Increased Focal Lesions in Female Mice
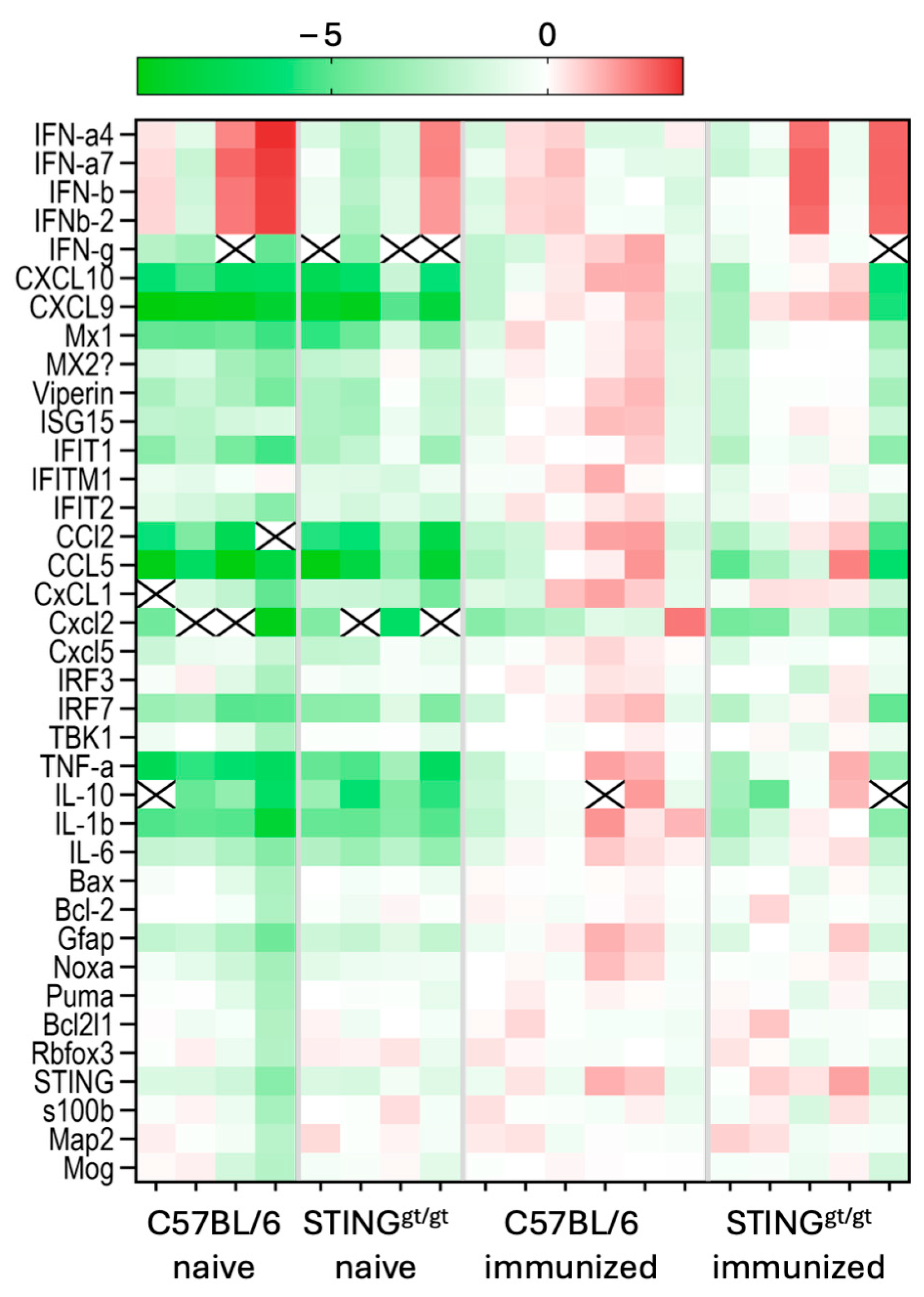
3.3. Lowered ISG Expression Was Associated with Focal Lesions in STING-Deficient Mice
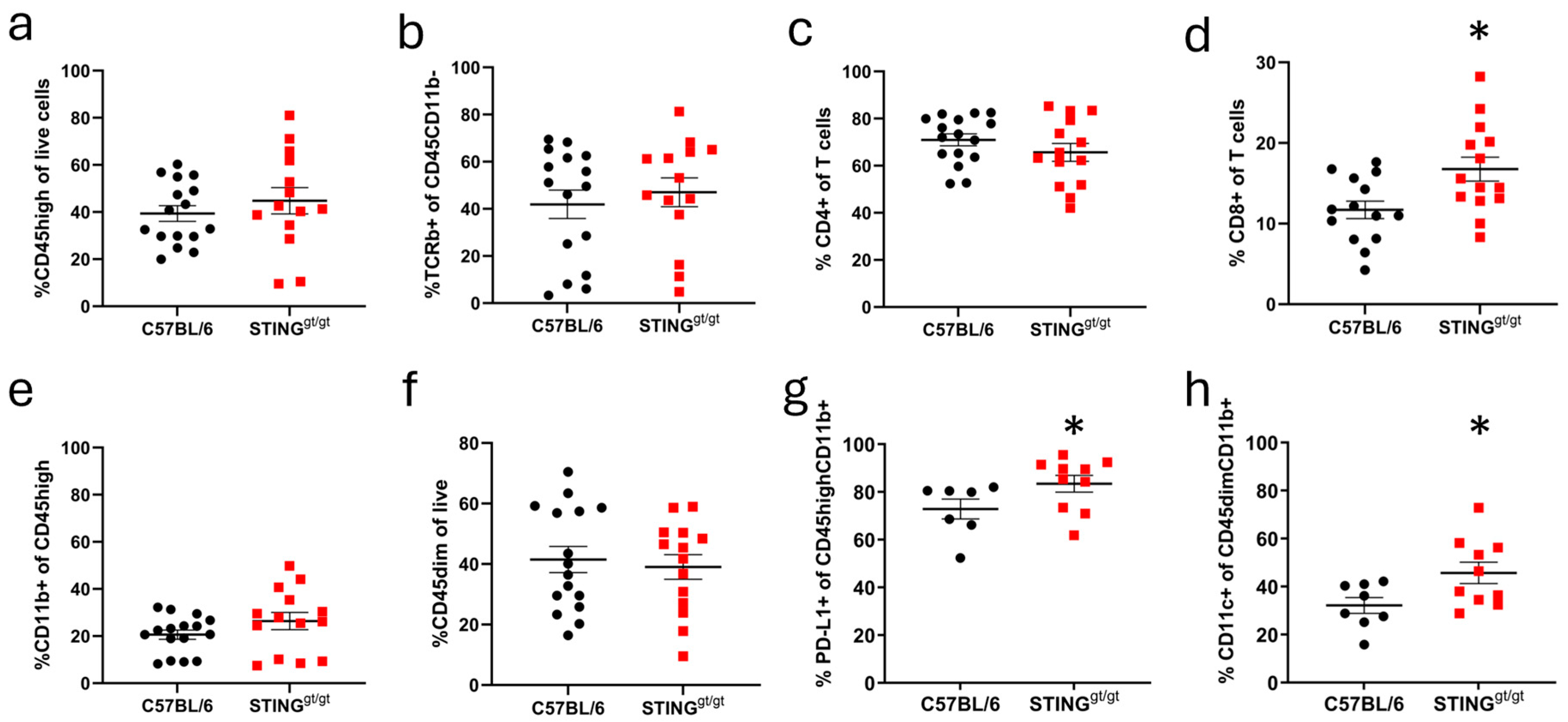
3.4. STING/Deficient Mice Show Altered Infiltration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPR | Pattern recognition receptor |
| IFN | Interferon |
| EAE | Experimental autoimmune encephalomyelitis |
| MS | Multiple sclerosis |
| STING | stimulator of IFN genes |
| CNS | Central nervous system |
| ISGs | Interferon stimulated genes |
| TLR | Toll-like receptor |
| WT | Wild type |
| CC | Corpus callosum |
| MOG | Myelin oligodendrocyte glycoprotein |
| µl | Microliter |
| H&E | Hematoxylin and eosin |
| FC | Fold change |
References
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Paludan, S.R.; Bowie, A.G. Immune sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Touil, T.; Fitzgerald, D.; Zhang, G.X.; Rostami, A.; Gran, B. Cutting Edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-beta. J. Immunol. 2006, 177, 7505–7509. [Google Scholar] [CrossRef]
- Dann, A.; Poeck, H.; Croxford, A.L.; Gaupp, S.; Kierdorf, K.; Knust, M.; Pfeifer, D.; Maihoefer, C.; Endres, S.; Kalinke, U.; et al. Cytosolic RIG-I-like helicases act as negative regulators of sterile inflammation in the CNS. Nat. Neurosci. 2011, 15, 98–106. [Google Scholar] [CrossRef]
- Khorooshi, R.; Morch, M.T.; Holm, T.H.; Berg, C.T.; Dieu, R.T.; Draeby, D.; Issazadeh-Navikas, S.; Weiss, S.; Lienenklaus, S.; Owens, T. Induction of endogenous Type I interferon within the central nervous system plays a protective role in experimental autoimmune encephalomyelitis. Acta Neuropathol. 2015, 130, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Dubik, M.; Marczynska, J.; Morch, M.T.; Webster, G.; Jensen, K.N.; Wlodarczyk, A.; Khorooshi, R.; Owens, T. Innate Signaling in the CNS Prevents Demyelination in a Focal EAE Model. Front. Neurosci. 2021, 15, 682451. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Knight, P.H.; Ren, Y.; Ren, H.; Zheng, J.; Wu, X.; Ren, J.; Sawyer, R.G. The emerging role of stimulator of interferons genes signaling in sepsis: Inflammation, autophagy, and cell death. Acta Physiol. 2019, 225, e13194. [Google Scholar] [CrossRef] [PubMed]
- Blank, T.; Prinz, M. Type I interferon pathway in CNS homeostasis and neurological disorders. Glia 2017, 65, 1397–1406. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Cao, X. Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. J. Autoimmun. 2017, 83, 1–11. [Google Scholar] [CrossRef]
- Farooqi, N.; Gran, B.; Constantinescu, C.S. Are current disease-modifying therapeutics in multiple sclerosis justified on the basis of studies in experimental autoimmune encephalomyelitis? J. Neurochem. 2010, 115, 829–844. [Google Scholar] [CrossRef]
- Prinz, M.; Schmidt, H.; Mildner, A.; Knobeloch, K.P.; Hanisch, U.K.; Raasch, J.; Merkler, D.; Detje, C.; Gutcher, I.; Mages, J.; et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 2008, 28, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Teige, I.; Treschow, A.; Teige, A.; Mattsson, R.; Navikas, V.; Leanderson, T.; Holmdahl, R.; Issazadeh-Navikas, S. IFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J. Immunol. 2003, 170, 4776–4784. [Google Scholar] [CrossRef] [PubMed]
- Reinert, L.S.; Lopusna, K.; Winther, H.; Sun, C.; Thomsen, M.K.; Nandakumar, R.; Mogensen, T.H.; Meyer, M.; Vaegter, C.; Nyengaard, J.R.; et al. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat. Commun. 2016, 7, 13348. [Google Scholar] [CrossRef]
- Ferecsko, A.S.; Smallwood, M.J.; Moore, A.; Liddle, C.; Newcombe, J.; Holley, J.; Whatmore, J.; Gutowski, N.J.; Eggleton, P. STING-Triggered CNS Inflammation in Human Neurodegenerative Diseases. Biomedicines 2023, 11, 1375. [Google Scholar] [CrossRef]
- Masanneck, L.; Eichler, S.; Vogelsang, A.; Korsen, M.; Wiendl, H.; Budde, T.; Meuth, S.G. The STING-IFN-beta-Dependent Axis Is Markedly Low in Patients with Relapsing-Remitting Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 9249. [Google Scholar] [CrossRef]
- Lemos, H.; Huang, L.; McGaha, T.; Mellor, A.L. STING, nanoparticles, autoimmune disease and cancer: A novel paradigm for immunotherapy? Expert. Rev. Clin. Immunol. 2015, 11, 155–165. [Google Scholar] [CrossRef]
- Mathur, V.; Burai, R.; Vest, R.T.; Bonanno, L.N.; Lehallier, B.; Zardeneta, M.E.; Mistry, K.N.; Do, D.; Marsh, S.E.; Abud, E.M.; et al. Activation of the STING-Dependent Type I Interferon Response Reduces Microglial Reactivity and Neuroinflammation. Neuron 2017, 96, 1290–1302. [Google Scholar] [CrossRef]
- Johnson, B.M.; Uchimura, T.; Gallovic, M.D.; Thamilarasan, M.; Chou, W.C.; Gibson, S.A.; Deng, M.; Tam, J.W.; Batty, C.J.; Williams, J.; et al. STING Agonist Mitigates Experimental Autoimmune Encephalomyelitis by Stimulating Type I IFN-Dependent and -Independent Immune-Regulatory Pathways. J. Immunol. 2021, 206, 2015–2028. [Google Scholar] [CrossRef]
- Sauer, J.D.; Sotelo-Troha, K.; von Moltke, J.; Monroe, K.M.; Rae, C.S.; Brubaker, S.W.; Hyodo, M.; Hayakawa, Y.; Woodward, J.J.; Portnoy, D.A.; et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 2011, 79, 688–694. [Google Scholar] [CrossRef]
- Khorooshi, R.; Marczynska, J.; Dieu, R.S.; Wais, V.; Hansen, C.R.; Kavan, S.; Thomassen, M.; Burton, M.; Kruse, T.; Webster, G.A.; et al. Innate signaling within the central nervous system recruits protective neutrophils. Acta Neuropathol. Commun. 2020, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Rusinova, I.; Forster, S.; Yu, S.; Kannan, A.; Masse, M.; Cumming, H.; Chapman, R.; Hertzog, P.J. Interferome v2.0: An updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2013, 41, D1040–D1046. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Lemos, H.; Huang, L.; Chandler, P.R.; Mohamed, E.; Souza, G.R.; Li, L.; Pacholczyk, G.; Barber, G.N.; Hayakawa, Y.; Munn, D.H.; et al. Activation of the STING adaptor attenuates experimental autoimmune encephalitis. J. Immunol. 2014, 192, 5571–5578. [Google Scholar] [CrossRef]
- Marta, M.; Andersson, A.; Isaksson, M.; Kampe, O.; Lobell, A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2008, 38, 565–575. [Google Scholar] [CrossRef]
- Miranda-Hernandez, S.; Gerlach, N.; Fletcher, J.M.; Biros, E.; Mack, M.; Korner, H.; Baxter, A.G. Role for MyD88, TLR2 and TLR9 but not TLR1, TLR4 or TLR6 in experimental autoimmune encephalomyelitis. J. Immunol. 2011, 187, 791–804. [Google Scholar] [CrossRef]
- Kerfoot, S.M.; Long, E.M.; Hickey, M.J.; Andonegui, G.; Lapointe, B.M.; Zanardo, R.C.; Bonder, C.; James, W.G.; Robbins, S.M.; Kubes, P. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J. Immunol. 2004, 173, 7070–7077. [Google Scholar] [CrossRef]
- Casella, G.; Rasouli, J.; Mason, K.; Boehm, A.; Kumar, G.; Hwang, D.; Thome, R.; Ishikawa, L.; Zhang, G.X.; Ciric, B.; et al. A serine protease inhibitor suppresses autoimmune neuroinflammation by activating the STING/IFN-beta axis in macrophages. Cell Mol. Immunol. 2020, 17, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Cheng, J.; Ko, H.; Tang, Y. Cytosolic DNA sensors in neurodegenerative diseases: From physiological defenders to pathological culprits. EMBO Mol. Med. 2024, 16, 678–699. [Google Scholar] [CrossRef] [PubMed]
- Friese, M.A.; Fugger, L. Pathogenic CD8(+) T cells in multiple sclerosis. Ann. Neurol. 2009, 66, 132–141. [Google Scholar] [CrossRef]
- Izquierdo, J.M. cGAS-STING triggers inflammaging-associated neurodegeneration. Mol. Neurodegener. 2023, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, C.; Han, J.; Zhu, J.; Liu, S.; Jin, T. PD-1/PD-L1 Axis as a Potential Therapeutic Target for Multiple Sclerosis: A T Cell Perspective. Front. Cell Neurosci. 2021, 15, 716747. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, F.; Dariychuk, Z.; Zhen, A.; Daugherty, D.J.; Bannerman, P.; Hanson, A.M.; Pleasure, D.; Soulika, A.; Deng, W.; Chechneva, O.V. Reduction in CD11c(+) microglia correlates with clinical progression in chronic experimental autoimmune demyelination. Neurobiol. Dis. 2021, 161, 105556. [Google Scholar] [CrossRef] [PubMed]
| C57BL/6 | STINGgt/gt | p-Value | |
|---|---|---|---|
| Incidence | 24/44 | 12/40 | 0.029 Fisher’s exact test |
| Time of onset | 13.83 ± 0.31 | 13.25 ± 0.35 | 0.216 Mann-Whitney test |
| Maximum severity | 2.71 ± 0.28 | 3.33 ± 0.36 | 0.174 Mann-Whitney test |
| Weight change at day 16 post immunization (%) | 104.8 ± 7.1 | 100.7 ± 8.4 | 0.016 Mann-Whitney test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mørch, M.T.; Reinert, L.S.; Benmamar-Badel, A.; Dubik, M.; Burton, M.; Thomassen, M.; Kruse, T.; Asgari, N.; Paludan, S.R.; Owens, T.; et al. STING Signaling Deficiency Exacerbates Demyelination and Immune Infiltration in Focal EAE Lesions. NeuroSci 2025, 6, 106. https://doi.org/10.3390/neurosci6040106
Mørch MT, Reinert LS, Benmamar-Badel A, Dubik M, Burton M, Thomassen M, Kruse T, Asgari N, Paludan SR, Owens T, et al. STING Signaling Deficiency Exacerbates Demyelination and Immune Infiltration in Focal EAE Lesions. NeuroSci. 2025; 6(4):106. https://doi.org/10.3390/neurosci6040106
Chicago/Turabian StyleMørch, Marlene T., Line S. Reinert, Anouk Benmamar-Badel, Magdalena Dubik, Mark Burton, Mads Thomassen, Torben Kruse, Nasrin Asgari, Søren R. Paludan, Trevor Owens, and et al. 2025. "STING Signaling Deficiency Exacerbates Demyelination and Immune Infiltration in Focal EAE Lesions" NeuroSci 6, no. 4: 106. https://doi.org/10.3390/neurosci6040106
APA StyleMørch, M. T., Reinert, L. S., Benmamar-Badel, A., Dubik, M., Burton, M., Thomassen, M., Kruse, T., Asgari, N., Paludan, S. R., Owens, T., & Khorooshi, R. (2025). STING Signaling Deficiency Exacerbates Demyelination and Immune Infiltration in Focal EAE Lesions. NeuroSci, 6(4), 106. https://doi.org/10.3390/neurosci6040106








