Carbamoylated Erythropoietin Rescues Autism-Relevant Social Deficits in BALB/cJ Mice
Abstract
1. Introduction
2. Methods and Materials
2.1. Animals
2.2. Drug Administration
2.3. Behavioral Timeline
2.4. Three-Chamber Social Approach Task
2.5. Open Field
2.6. Elevated Plus Maze
2.7. Forced Swim
2.8. Statistics
3. Results
3.1. Weights
3.2. Three-Chamber Social Approach Task
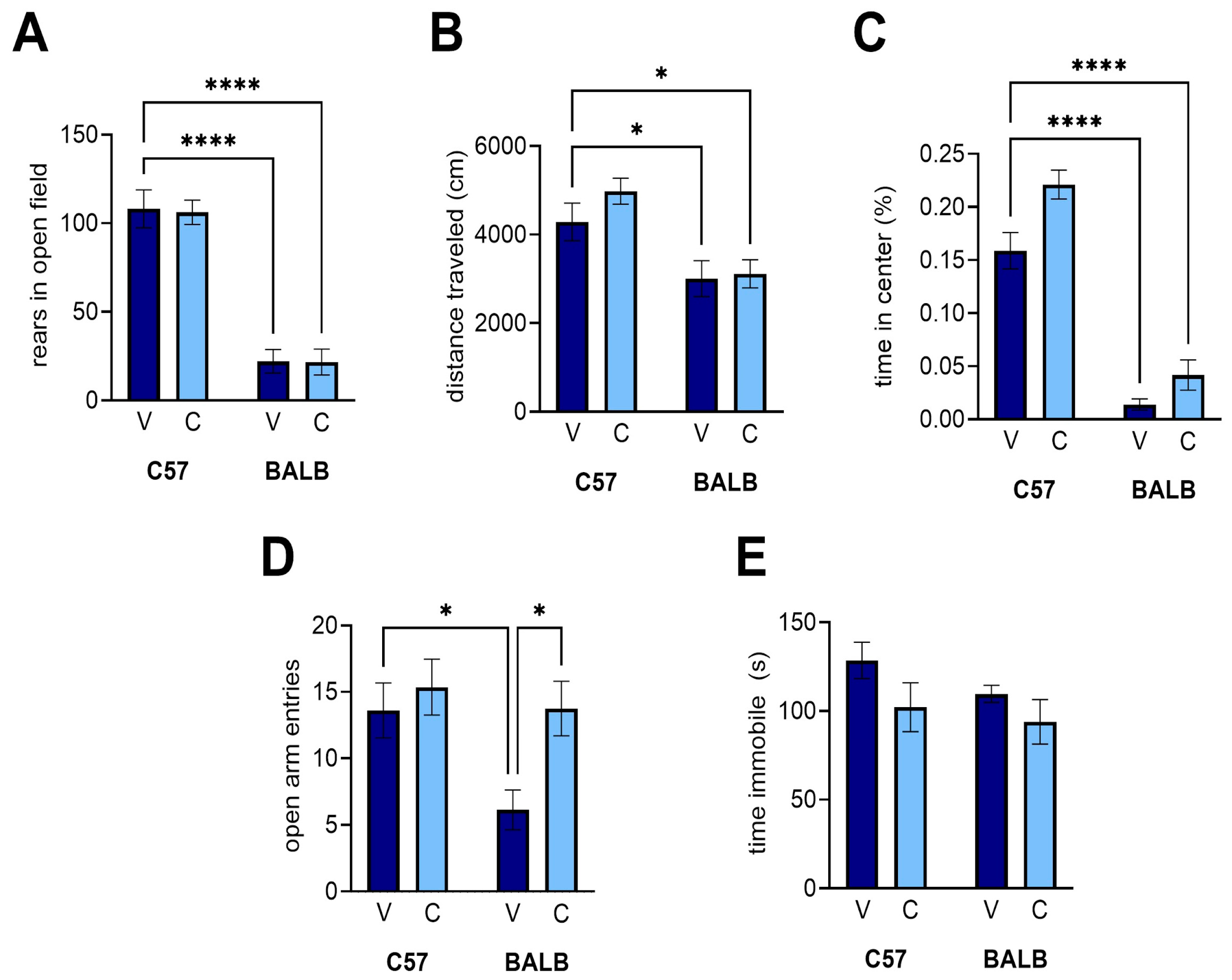
3.3. Open Field Task
3.4. Elevated Plus Maze
3.5. Porsolt’s Forced Swim Task
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism Spectrum Disorder. Nat. Rev. Dis. Primer 2020, 6, 5. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2021, 70, 1–16. [Google Scholar] [CrossRef]
- Cakir, J.; Frye, R.E.; Walker, S.J. The Lifetime Social Cost of Autism: 1990–2029. Res. Autism Spectr. Disord. 2020, 72, 101502. [Google Scholar] [CrossRef]
- Gandal, M.J.; Leppa, V.; Won, H.; Parikshak, N.N.; Geschwind, D.H. The Road to Precision Psychiatry: Translating Genetics into Disease Mechanisms. Nat. Neurosci. 2016, 19, 1397–1407. [Google Scholar] [CrossRef]
- Manoli, D.S.; State, M.W. Autism Spectrum Disorder Genetics and the Search for Pathological Mechanisms. Am. J. Psychiatry 2021, 178, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sztainberg, Y.; Zoghbi, H.Y. Lessons Learned from Studying Syndromic Autism Spectrum Disorders. Nat. Neurosci. 2016, 19, 1408–1417. [Google Scholar] [CrossRef]
- Aishworiya, R.; Valica, T.; Hagerman, R.; Restrepo, B. An Update on Psychopharmacological Treatment of Autism Spectrum Disorder. Neurotherapeutics 2022, 19, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Banker, S.M.; Gu, X.; Schiller, D.; Foss-Feig, J.H. Hippocampal Contributions to Social and Cognitive Deficits in Autism Spectrum Disorder. Trends Neurosci. 2021, 44, 793–807. [Google Scholar] [CrossRef]
- Hu, C.; Li, H.; Li, J.; Luo, X.; Hao, Y. Microglia: Synaptic Modulator in Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 958661. [Google Scholar] [CrossRef]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-Inhibition Balance as a Framework for Investigating Mechanisms in Neuropsychiatric Disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef]
- Zoghbi, H.Y.; Bear, M.F. Synaptic Dysfunction in Neurodevelopmental Disorders Associated with Autism and Intellectual Disabilities. Cold Spring Harb. Perspect. Biol. 2012, 4, a009886. [Google Scholar] [CrossRef]
- Camuso, S.; La Rosa, P.; Fiorenza, M.T.; Canterini, S. Pleiotropic Effects of BDNF on the Cerebellum and Hippocampus: Implications for Neurodevelopmental Disorders. Neurobiol. Dis. 2022, 163, 105606. [Google Scholar] [CrossRef] [PubMed]
- Reim, D.; Schmeisser, M.J. Neurotrophic Factors in Mouse Models of Autism Spectrum Disorder: Focus on BDNF and IGF-1. Adv. Anat. Embryol. Cell Biol. 2017, 224, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Bukatova, S.; Bacova, Z.; Osacka, J.; Bakos, J. Mini Review of Molecules Involved in Altered Postnatal Neurogenesis in Autism. Int. J. Neurosci. 2023, 134, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, J.; Gong, H.; Liu, T.; Li, X.; Fan, X. Implication of Hippocampal Neurogenesis in Autism Spectrum Disorder: Pathogenesis and Therapeutic Implications. Curr. Neuropharmacol. 2023, 21, 2266–2282. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, L.; Zhang, Y.-W. Microglial Dysfunction in Autism Spectrum Disorder. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2024, 30, 10738584241252576. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, J.; Li, Y. Microglia and Astrocytes Underlie Neuroinflammation and Synaptic Susceptibility in Autism Spectrum Disorder. Front. Neurosci. 2023, 17, 1125428. [Google Scholar] [CrossRef]
- Newton, S.S.; Sathyanesan, M. Erythropoietin and Non-Erythropoietic Derivatives in Cognition. Front. Pharmacol. 2021, 12, 728725. [Google Scholar] [CrossRef]
- Vittori, D.C.; Chamorro, M.E.; Hernández, Y.V.; Maltaneri, R.E.; Nesse, A.B. Erythropoietin and Derivatives: Potential Beneficial Effects on the Brain. J. Neurochem. 2021, 158, 1032–1057. [Google Scholar] [CrossRef]
- Hassouna, I.; Ott, C.; Wüstefeld, L.; Offen, N.; Neher, R.A.; Mitkovski, M.; Winkler, D.; Sperling, S.; Fries, L.; Goebbels, S.; et al. Revisiting Adult Neurogenesis and the Role of Erythropoietin for Neuronal and Oligodendroglial Differentiation in the Hippocampus. Mol. Psychiatry 2016, 21, 1752–1767. [Google Scholar] [CrossRef]
- Leconte, C.; Bihel, E.; Lepelletier, F.-X.; Bouët, V.; Saulnier, R.; Petit, E.; Boulouard, M.; Bernaudin, M.; Schumann-Bard, P. Comparison of the Effects of Erythropoietin and Its Carbamylated Derivative on Behaviour and Hippocampal Neurogenesis in Mice. Neuropharmacology 2011, 60, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Sirén, A.-L.; Faßhauer, T.; Bartels, C.; Ehrenreich, H. Therapeutic Potential of Erythropoietin and Its Structural or Functional Variants in the Nervous System. Neurotherapeutics 2009, 6, 108–127. [Google Scholar] [CrossRef]
- Leist, M.; Ghezzi, P.; Grasso, G.; Bianchi, R.; Villa, P.; Fratelli, M.; Savino, C.; Bianchi, M.; Nielsen, J.; Gerwien, J.; et al. Derivatives of Erythropoietin That Are Tissue Protective but Not Erythropoietic. Science 2004, 305, 239–242. [Google Scholar] [CrossRef]
- Sathyanesan, M.; Watt, M.J.; Haiar, J.M.; Scholl, J.L.; Davies, S.R.; Paulsen, R.T.; Wiederin, J.; Ciborowski, P.; Newton, S.S. Carbamoylated Erythropoietin Modulates Cognitive Outcomes of Social Defeat and Differentially Regulates Gene Expression in the Dorsal and Ventral Hippocampus. Transl. Psychiatry 2018, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, N.; Mohammadi, M.; Manaheji, H.; Maghsoudi, N.; Katinger, H.; Baniasadi, M.; Zaringhalam, J. Carbamylated Erythropoietin Improves Recognition Memory by Modulating Microglia in a Rat Model of Pain. Behav. Brain Res. 2022, 416, 113576. [Google Scholar] [CrossRef]
- Sampath, D.; McWhirt, J.; Sathyanesan, M.; Newton, S.S. Carbamoylated Erythropoietin Produces Antidepressant-like Effects in Male and Female Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 96, 109754. [Google Scholar] [CrossRef]
- Choi, M.; Ko, S.Y.; Lee, I.Y.; Wang, S.E.; Lee, S.H.; Oh, D.H.; Kim, Y.-S.; Son, H. Carbamylated Erythropoietin Promotes Neurite Outgrowth and Neuronal Spine Formation in Association with CBP/P300. Biochem. Biophys. Res. Commun. 2014, 446, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.K.; Sathyanesan, M.; Kumar, V.; Newton, S.S. A Comparative Analysis of Erythropoietin and Carbamoylated Erythropoietin Proteome Profiles. Life 2021, 11, 359. [Google Scholar] [CrossRef]
- Brodkin, E.S. BALB/c Mice: Low Sociability and Other Phenotypes That May Be Relevant to Autism. Behav. Brain Res. 2007, 176, 53–65. [Google Scholar] [CrossRef]
- Tiwari, N.K.; Sathyanesan, M.; Schweinle, W.; Newton, S.S. Carbamoylated Erythropoietin Induces a Neurotrophic Gene Profile in Neuronal Cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 132–141. [Google Scholar] [CrossRef]
- Rein, B.; Ma, K.; Yan, Z. A Standardized Social Preference Protocol for Measuring Social Deficits in Mouse Models of Autism. Nat. Protoc. 2020, 15, 3464–3477. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Silverman, J.L.; Crawley, J.N. Automated Three-Chambered Social Approach Task for Mice. Curr. Protoc. Neurosci. 2011, 56, 8–26. [Google Scholar] [CrossRef]
- Cosgrove, J.A.; Kelly, L.K.; Kiffmeyer, E.A.; Kloth, A.D. Sex-Dependent Influence of Postweaning Environmental Enrichment in Angelman Syndrome Model Mice. Brain Behav. 2022, 12, e2468. [Google Scholar] [CrossRef] [PubMed]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J. Vis. Exp. JoVE 2015, 96, 52434. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The Mouse Forced Swim Test. J. Vis. Exp. JoVE 2012, 59, 3638. [Google Scholar] [CrossRef]
- Sankoorikal, G.M.V.; Kaercher, K.A.; Boon, C.J.; Lee, J.K.; Brodkin, E.S. A Mouse Model System for Genetic Analysis of Sociability: C57BL/6J versus BALB/cJ Inbred Mouse Strains. Biol. Psychiatry 2006, 59, 415–423. [Google Scholar] [CrossRef]
- Russo, A.M.; Lawther, A.J.; Prior, B.M.; Isbel, L.; Somers, W.G.; Lesku, J.A.; Richdale, A.L.; Dissanayake, C.; Kent, S.; Lowry, C.A.; et al. Social Approach, Anxiety, and Altered Tryptophan Hydroxylase 2 Activity in Juvenile BALB/c and C57BL/6J Mice. Behav. Brain Res. 2019, 359, 918–926. [Google Scholar] [CrossRef]
- Lalonde, R.; Strazielle, C. Relations between Open-Field, Elevated plus-Maze, and Emergence Tests as Displayed by C57/BL6J and BALB/c Mice. J. Neurosci. Methods 2008, 171, 48–52. [Google Scholar] [CrossRef]
- Ellegood, J.; Crawley, J.N. Behavioral and Neuroanatomical Phenotypes in Mouse Models of Autism. Neurotherapeutics 2015, 12, 521–533. [Google Scholar] [CrossRef]
- Arakawa, H. Analysis of Social Process in Two Inbred Strains of Male Mice: A Predominance of Contact-Based Investigation in BALB/c Mice. Neuroscience 2018, 369, 124–138. [Google Scholar] [CrossRef]
- Burket, J.A.; Young, C.M.; Green, T.L.; Benson, A.D.; Deutsch, S.I. Characterization of Gait and Olfactory Behaviors in the Balb/c Mouse Model of Autism Spectrum Disorders. Brain Res. Bull. 2016, 122, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Fairless, A.H.; Dow, H.C.; Kreibich, A.S.; Torre, M.; Kuruvilla, M.; Gordon, E.; Morton, E.A.; Tan, J.; Berrettini, W.H.; Li, H.; et al. Sociability and Brain Development in BALB/cJ and C57BL/6J Mice. Behav. Brain Res. 2012, 228, 299–310. [Google Scholar] [CrossRef]
- Kapitsa, I.G.; Ivanova, E.A.; Voronina, T.A.; Seredenin, S.B. Characteristics of the Behavioral Phenotype of BALB/C Mice. Neurosci. Behav. Physiol. 2021, 51, 93–99. [Google Scholar] [CrossRef]
- An, X.-L.; Zou, J.-X.; Wu, R.-Y.; Yang, Y.; Tai, F.-D.; Zeng, S.-Y.; Jia, R.; Zhang, X.; Liu, E.-Q.; Broders, H. Strain and Sex Differences in Anxiety-Like and Social Behaviors in C57BL/6J and BALB/cJ Mice. Exp. Anim. 2011, 60, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Haratizadeh, S.; Ranjbar, M.; Darvishzadeh-Mahani, F.; Basiri, M.; Nozari, M. The Effects of Postnatal Erythropoietin and Nano-Erythropoietin on Behavioral Alterations by Mediating K-Cl Co-Transporter 2 in the Valproic Acid-Induced Rat Model of Autism. Dev. Psychobiol. 2023, 65, e22353. [Google Scholar] [CrossRef]
- Hosny, S.; Abdelmenem, A.; Azouz, T.; Kamar, S.S.; ShamsEldeen, A.M.; El-Shafei, A.A. Beneficial Effect of Erythropoietin on Ameliorating Propionic Acid-Induced Autistic-Like Features in Young Rats. ACTA Histochem. Cytochem. 2023, 56, 77–86. [Google Scholar] [CrossRef]
- Solmaz, V.; Erdoğan, M.A.; Alnak, A.; Meral, A.; Erbaş, O. Erythropoietin Shows Gender Dependent Positive Effects on Social Deficits, Learning/Memory Impairments, Neuronal Loss and Neuroinflammation in the Lipopolysaccharide Induced Rat Model of Autism. Neuropeptides 2020, 83, 102073. [Google Scholar] [CrossRef]
- Robinson, S.; Corbett, C.J.; Winer, J.L.; Chan, L.A.S.; Maxwell, J.R.; Anstine, C.V.; Yellowhair, T.R.; Andrews, N.A.; Yang, Y.; Sillerud, L.O.; et al. Neonatal Erythropoietin Mitigates Impaired Gait, Social Interaction and Diffusion Tensor Imaging Abnormalities in a Rat Model of Prenatal Brain Injury. Exp. Neurol. 2018, 302, 1–13. [Google Scholar] [CrossRef]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the Elevated Plus-Maze and Open-Field Tests for the Assessment of Anxiety-Related Behaviour in Inbred Mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Osborn, M.; Rustom, N.; Clarke, M.; Litteljohn, D.; Rudyk, C.; Anisman, H.; Hayley, S. Antidepressant-Like Effects of Erythropoietin: A Focus on Behavioural and Hippocampal Processes. PLoS ONE 2013, 8, e72813. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.; van Beek, J.; Larsen, A.K.; Gerwien, J.; Christensen, S.; Cerami, A.; Brines, M.; Leist, M.; Ghezzi, P.; Torup, L. Reduced Functional Deficits, Neuroinflammation, and Secondary Tissue Damage after Treatment of Stroke by Nonerythropoietic Erythropoietin Derivatives. J. Cereb. Blood Flow Metab. 2007, 27, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, S.I.; Burket, J.A.; Jacome, L.F.; Cannon, W.R.; Herndon, A.L. D-Cycloserine Improves the Impaired Sociability of the Balb/c Mouse. Brain Res. Bull. 2011, 84, 8–11. [Google Scholar] [CrossRef]
- Jacome, L.F.; Burket, J.A.; Herndon, A.L.; Cannon, W.R.; Deutsch, S.I. D-Serine Improves Dimensions of the Sociability Deficit of the Genetically-Inbred Balb/c Mouse Strain. Brain Res. Bull. 2011, 84, 12–16. [Google Scholar] [CrossRef]
- Arakawa, H. Involvement of Serotonin and Oxytocin in Neural Mechanism Regulating Amicable Social Signal in Male Mice: Implication for Impaired Recognition of Amicable Cues in BALB/c Strain. Behav. Neurosci. 2017, 131, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Haratizadeh, S.; Ranjbar, M.; Basiri, M.; Nozari, M. Astrocyte Responses to Postnatal Erythropoietin and Nano-Erythropoietin Treatments in a Valproic Acid-Induced Animal Model of Autism. J. Chem. Neuroanat. 2023, 130, 102257. [Google Scholar] [CrossRef]
- Hagen, E.; Shprung, D.; Minakova, E.; Washington, J.; Kumar, U.; Shin, D.; Sankar, R.; Mazarati, A. Autism-Like Behavior in BTBR Mice Is Improved by Electroconvulsive Therapy. Neurotherapeutics 2015, 12, 657–666. [Google Scholar] [CrossRef]
- Luo, Y.; Lv, K.; Du, Z.; Zhang, D.; Chen, M.; Luo, J.; Wang, L.; Liu, T.; Gong, H.; Fan, X. Minocycline Improves Autism-Related Behaviors by Modulating Microglia Polarization in a Mouse Model of Autism. Int. Immunopharmacol. 2023, 122, 110594. [Google Scholar] [CrossRef]
- Perets, N.; Hertz, S.; London, M.; Offen, D. Intranasal Administration of Exosomes Derived from Mesenchymal Stem Cells Ameliorates Autistic-like Behaviors of BTBR Mice. Mol. Autism 2018, 9, 57. [Google Scholar] [CrossRef]
- Perets, N.; Segal-Gavish, H.; Gothelf, Y.; Barzilay, R.; Barhum, Y.; Abramov, N.; Hertz, S.; Morozov, D.; London, M.; Offen, D. Long Term Beneficial Effect of Neurotrophic Factors-Secreting Mesenchymal Stem Cells Transplantation in the BTBR Mouse Model of Autism. Behav. Brain Res. 2017, 331, 254–260. [Google Scholar] [CrossRef]
- Segal-Gavish, H.; Karvat, G.; Barak, N.; Barzilay, R.; Ganz, J.; Edry, L.; Aharony, I.; Offen, D.; Kimchi, T. Mesenchymal Stem Cell Transplantation Promotes Neurogenesis and Ameliorates Autism Related Behaviors in BTBR Mice. Autism Res. 2016, 9, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Ellegood, J.; Babineau, B.A.; Henkelman, R.M.; Lerch, J.P.; Crawley, J.N. Neuroanatomical Analysis of the BTBR Mouse Model of Autism Using Magnetic Resonance Imaging and Diffusion Tensor Imaging. NeuroImage 2013, 70, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Thurm, A.; Ethridge, S.B.; Soller, M.M.; Petkova, S.P.; Abel, T.; Bauman, M.D.; Brodkin, E.S.; Harony-Nicolas, H.; Wöhr, M.; et al. Reconsidering Animal Models Used to Study Autism Spectrum Disorder: Current State and Optimizing Future. Genes Brain Behav. 2022, 21, e12803. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Elgersma, Y.; Maffei, L.; Hagerman, R. Treatment of Neurodevelopmental Disorders in Adulthood. J. Neurosci. 2012, 32, 14074–14079. [Google Scholar] [CrossRef]



| BALB (n = 24 Total) | C57 (n = 21 Total) | |
|---|---|---|
| Carbamoylated erythropoietin (CEPO, 40 μg/kg; n = 23 total) | 12 | 11 |
| Vehicle (n = 22 total) | 12 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Street, A.L.; Thakkar, V.P.; Lemke, S.W.; Schoenbeck, L.M.; Schumacher, K.M.; Sathyanesan, M.; Newton, S.S.; Kloth, A.D. Carbamoylated Erythropoietin Rescues Autism-Relevant Social Deficits in BALB/cJ Mice. NeuroSci 2025, 6, 25. https://doi.org/10.3390/neurosci6010025
Street AL, Thakkar VP, Lemke SW, Schoenbeck LM, Schumacher KM, Sathyanesan M, Newton SS, Kloth AD. Carbamoylated Erythropoietin Rescues Autism-Relevant Social Deficits in BALB/cJ Mice. NeuroSci. 2025; 6(1):25. https://doi.org/10.3390/neurosci6010025
Chicago/Turabian StyleStreet, Amaya L., Vedant P. Thakkar, Sean W. Lemke, Liza M. Schoenbeck, Kevin M. Schumacher, Monica Sathyanesan, Samuel S. Newton, and Alexander D. Kloth. 2025. "Carbamoylated Erythropoietin Rescues Autism-Relevant Social Deficits in BALB/cJ Mice" NeuroSci 6, no. 1: 25. https://doi.org/10.3390/neurosci6010025
APA StyleStreet, A. L., Thakkar, V. P., Lemke, S. W., Schoenbeck, L. M., Schumacher, K. M., Sathyanesan, M., Newton, S. S., & Kloth, A. D. (2025). Carbamoylated Erythropoietin Rescues Autism-Relevant Social Deficits in BALB/cJ Mice. NeuroSci, 6(1), 25. https://doi.org/10.3390/neurosci6010025





