Neuroprotective Role of Cyclic AMP Signaling in Dopaminergic Degeneration Induced by a Parkinson’s Disease Toxin, Rotenone
Abstract
1. Introduction
2. Materials and Methods
3. Results
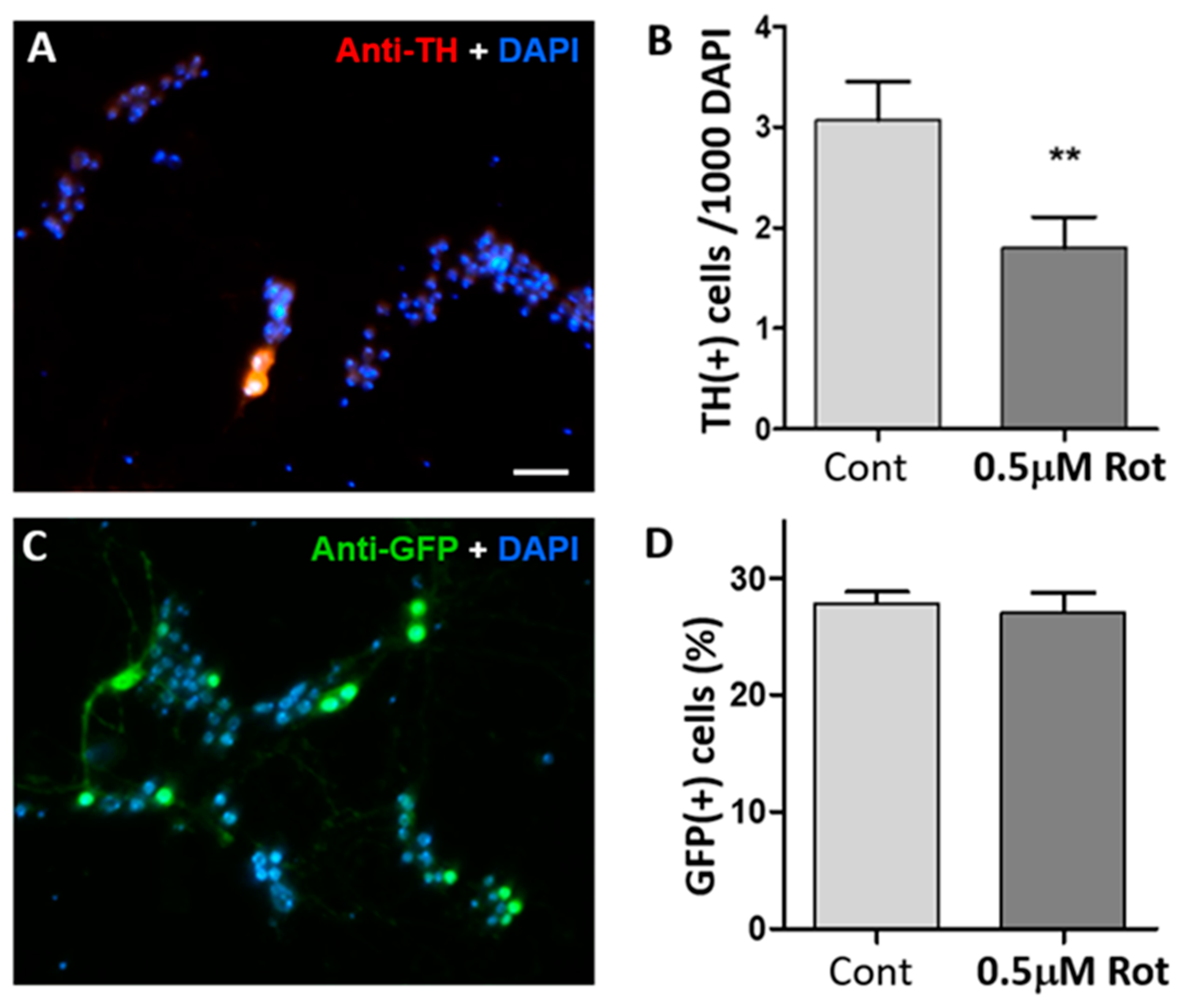
3.1. Rotenone Selectively Degenerates Drosophila Dopaminergic Neurons in Culture
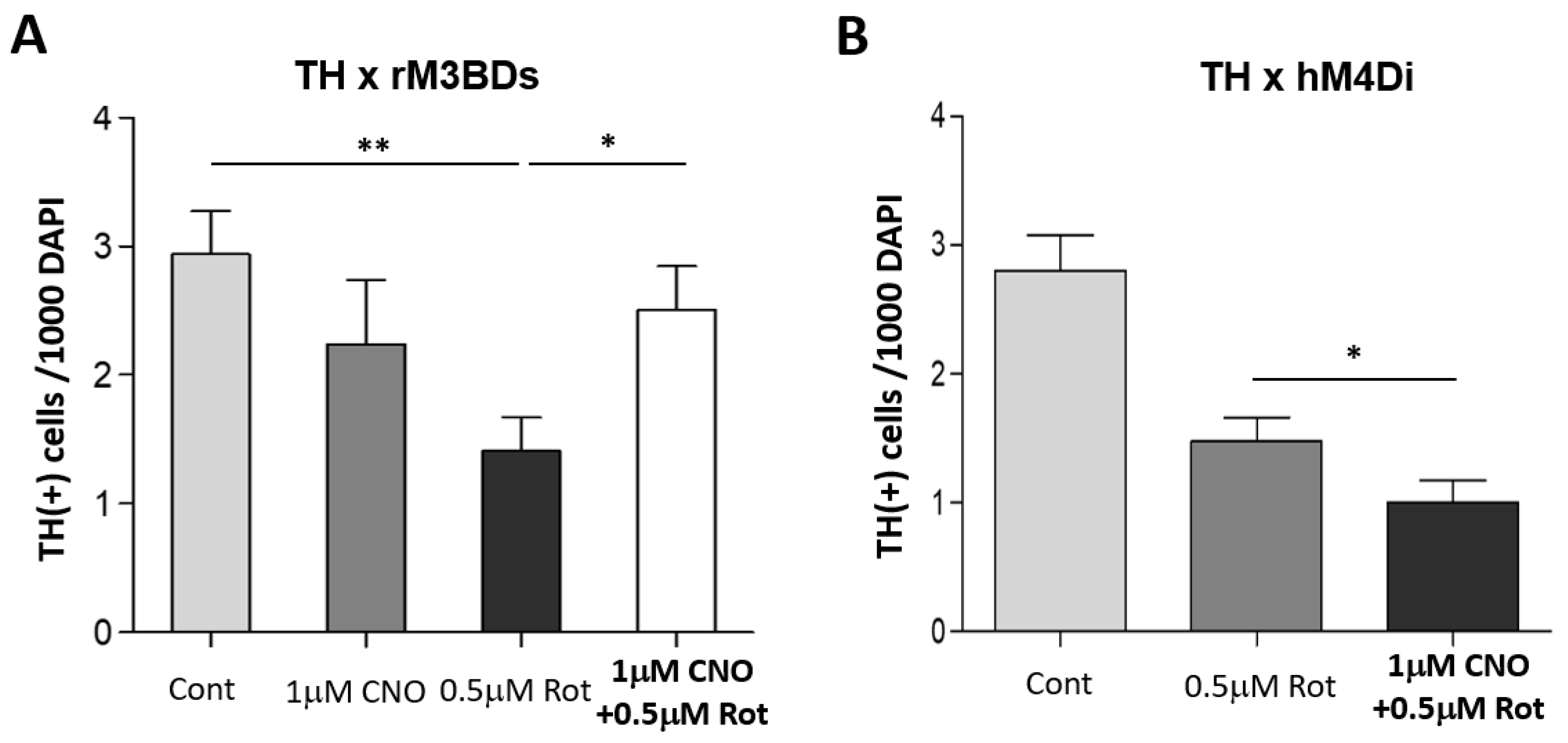
3.2. DREADD Activation Modulates Sensitivity of DA Neurons to Rotenone
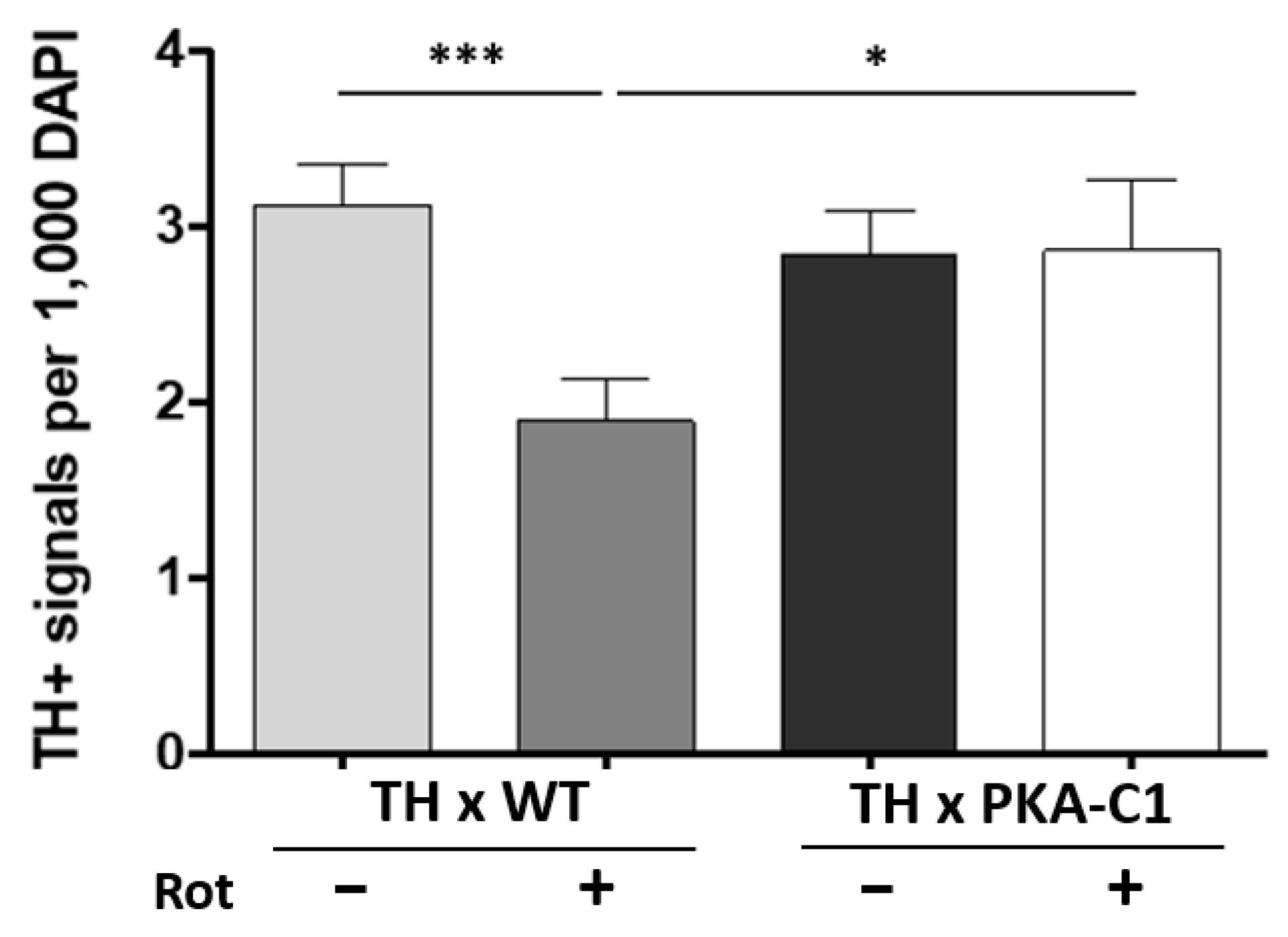
3.3. PKA-C1 Overexpression Protects Against DA Neurodegeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Lau, L.M.L.; Breteler, M.M.B. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Giguère, N.; Burke Nanni, S.; Trudeau, L.-E. On cell loss and selective vulnerability of neuronal populations in Parkinson’s disease. Front. Neurol. 2018, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Mari, M.J.; Vallldeoriola, F.; Molinuevo, J.L. History of levodopa and dopamine agonists in Parkinson’s disease treatment. Neurology 1998, 50 (Suppl. 6), S2–S10. [Google Scholar] [CrossRef]
- Kitamura, Y.; Taniguchi, T.; Shimohama, S.; Akaike, A.; Nomura, Y. Neuroprotective Mechanisms of Antiparkinsonian Dopamine D2-Receptor Subfamily Agonists. Neurochem. Res. 2003, 28, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Morgante, F.; Merola, A.; Fasano, A.; Marsili, L.; Fox, S.H.; Bezard, E.; Picconi, B.; Calabresi, P.; Lang, A.E. Levodopa-induced dyskinesia in Parkinson disease: Current and evolving concepts. Ann. Neurol. 2018, 84, 797–811. [Google Scholar] [CrossRef]
- Dagda, R.K.; Das Banerjee, T. Role of protein kinase A in regulating mitochondrial function and neuronal development: Implications to neurodegenerative diseases. Rev. Neurosci. 2015, 26, 359–370. [Google Scholar] [CrossRef]
- Dagda, R.K.; Gusdon, A.M.; Pien, I.; Strack, S.; Green, S.; Li, C.; Van Houten, B.; Cherra, S.J.; Chu, C.T. Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson’s disease. Cell Death Differ. 2011, 18, 1914–1923. [Google Scholar] [CrossRef]
- Dagda, R.K.; Pien, I.; Wang, R.; Zhu, J.; Wang, K.Z.; Callio, J.; Banerjee, T.D.; Dagda, R.Y.; Chu, C.T. Beyond the mitochondrion: Cytosolic PINK1 remodels dendrites through protein kinase A. J. Neurochem. 2014, 128, 864–877. [Google Scholar] [CrossRef]
- Rubin, G.M.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar]
- Whitworth, A.J.; Wes, P.D.; Pallanck, L.J. Drosophila models pioneer a new approach to drug discovery for Parkinson’s disease. Drug Discov. Today 2006, 11, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Jakes, R.; Spillantini, M.G. The synucleinopathies: Twenty Years On. J. Parkinsons Dis. 2017, 7, S53–S71. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Bowling, K.; Funderburk, C.; Lawal, H.; Inamdar, A.; Wang, Z.; O’Donnell, J.M. Interaction of genetic and environmental factors in a Drosophila Parkinsonism model. J. Neurosci. 2007, 27, 2457–2467. [Google Scholar] [CrossRef]
- Coulom, H.; Birman, S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 2004, 24, 10993–10998. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.-D.; Wiemerslage, L.; LaBreck, C.J.; Khan, M.; Kannan, K.; Wang, X.; Zhu, X.; Lee, D.; Fridell, Y.-W.C. The neuroprotective effect of human uncoupling protein 2 (hUCP2) requires cAMP-dependent protein kinase in a toxin model of Parkinson’s disease. Neurobiol. Dis. 2014, 69, 180–191. [Google Scholar] [CrossRef]
- Varga, S.J.; Qi, C.; Podolsky, E.; Lee, D. A new Drosophila model to study the interaction between genetic and environmental factors in Parkinson’s disease. Brain Res. 2014, 1583, 277–286. [Google Scholar] [CrossRef]
- Spivey, A. Rotenone and paraquat linked to Parkinson’s disease: Human exposure study supports years of animal studies. Environ. Health Perspect. 2011, 119, A259. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, M.A.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Perier, C.; Bové, J.; Vila, M.; Przedborski, S. The rotenone model of Parkinson’s disease. Trends Neurosci. 2003, 26, 345–346. [Google Scholar] [CrossRef]
- Panov, A.; Dikalov, S.; Shalbuyeva, N.; Taylor, G.; Sherer, T.; Greenamyre, J.T. Rotenone model of Parkinson disease: Multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J. Biol. Chem. 2005, 280, 42026–42035. [Google Scholar] [CrossRef]
- Goldman, S.M. Environmental toxins and Parkinson’s disease. Ann. Rev. Pharm. Toxicol. 2014, 54, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Becnel, J.; Johnson, O.; Majeed, Z.R.; Tran, V.; Yu, B.; Roth, B.L.; Cooper, R.L.; Kerut, E.K.; Nichols, C.D. DREADDs in Drosophila: A Pharmacogenetic Approach for Controlling Behavior, Neuronal Signaling, and Physiology in the Fly. Cell Rep. 2013, 4, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Friggi-Grelin, F.; Coulom, H.; Meller, M.; Gomez, D.; Hirsh, J.; Birman, S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 2003, 54, 618–627. [Google Scholar] [CrossRef]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Lee, D. Suppression of inhibitory GABAergic transmission by cAMP signaling pathway: Alterations in learning and memory mutants. Eur. J. Neurosci. 2013, 37, 1383–1393. [Google Scholar] [CrossRef]
- Sicaeros, B.; O’Dowd, D.K. Preparation of neuronal cultures from midgastrula stage Drosophila embryos. J. Vis. Exp. 2007, 2007, 226. [Google Scholar]
- O’Dowd, D.K. Voltage-gated currents and firing properties of embryonic Drosophila neurons grown in a chemically defined medium. J. Neurobiol. 1995, 27, 113–126. [Google Scholar] [CrossRef]
- Darya, K.; Ganguly, A.; Lee, D. Quantitative analysis of synaptic boutons in cultured Drosophila neurons. Brain Res. 2009, 1280, 1–12. [Google Scholar] [CrossRef]
- Lee, H.-M.; Giguere, P.M.; Roth, B.L. DREADDs: Novel tools for drug discovery and development. Drug Discov. Today 2014, 19, 469–473. [Google Scholar] [CrossRef]
- Kalderon, D.; Rubin, G.M. Isolation and characterization of Drosophila cAMP-dependent protein kinase genes. Genes Dev. 1988, 2, 1539–1556. [Google Scholar] [CrossRef]
- Cassar, M.; Sunderhaus, E.; Wentzell, J.S.; Kuntz, S.; Strauss, R.; Kretzschmar, D. The PKA-C3 catalytic subunit is required in two pairs of interneurons for successful mating of Drosophila. Sci. Rep. 2018, 8, 2458. [Google Scholar] [CrossRef] [PubMed]
- Ismael, S.; Colvin, R.A.; Lee, D. Activation of cyclic AMP signaling pathway in dopaminergic neurons rescues locomotion defects in a Drosophila larval model of Parkinson’s disease. Brain Res. 2024, 1822, 148641. [Google Scholar] [CrossRef]
- Parisiadou, L.; Yu, J.; Sgobio, C.; Xie, C.; Liu, G.; Sun, L.; Gu, X.L.; Lin, X.; Crowley, N.A.; Lovinger, D.M.; et al. LRRK2 regulates synaptogenesis and dopamine receptor activation through modulation of PKA activity. Nat. Neurosci. 2014, 17, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Helmich, R.C.; Lehéricy, S. Dying-back of ascending noradrenergic projections in Parkinson’s disease. Brain 2021, 144, 2562–2564. [Google Scholar] [CrossRef]
- Merrill, R.A.; Dagda, R.K.; Dickey, A.S.; Cribbs, J.T.; Green, S.H.; Usachev, Y.M.; Strack, S. Mechanism of Neuroprotective Mitochondrial Remodeling by PKA/AKAP1. PLoS Biol. 2011, 9, e1000612. [Google Scholar] [CrossRef]
- Chalovich, E.M.; Zhu, J.; Caltagarone, J.; Bowser, R.; Chu, C.T. Functional Repression of cAMP Response Element in 6-Hydroxydopamine-treated Neuronal Cells. J. Biol. Chem. 2006, 281, 17870–17881. [Google Scholar] [CrossRef] [PubMed]
| Treatment | TH(+) Cells/1000 DAPI | N |
|---|---|---|
| No CNO | 3.09 +/− 0.35 | 57 |
| 1 μM CNO | 3.12 +/− 0.47 | 62 |
| 5 μM CNO | 1.86 +/− 0.30 ** | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismael, S.; Baitamouni, S.; Lee, D. Neuroprotective Role of Cyclic AMP Signaling in Dopaminergic Degeneration Induced by a Parkinson’s Disease Toxin, Rotenone. NeuroSci 2025, 6, 24. https://doi.org/10.3390/neurosci6010024
Ismael S, Baitamouni S, Lee D. Neuroprotective Role of Cyclic AMP Signaling in Dopaminergic Degeneration Induced by a Parkinson’s Disease Toxin, Rotenone. NeuroSci. 2025; 6(1):24. https://doi.org/10.3390/neurosci6010024
Chicago/Turabian StyleIsmael, Sazan, Sarah Baitamouni, and Daewoo Lee. 2025. "Neuroprotective Role of Cyclic AMP Signaling in Dopaminergic Degeneration Induced by a Parkinson’s Disease Toxin, Rotenone" NeuroSci 6, no. 1: 24. https://doi.org/10.3390/neurosci6010024
APA StyleIsmael, S., Baitamouni, S., & Lee, D. (2025). Neuroprotective Role of Cyclic AMP Signaling in Dopaminergic Degeneration Induced by a Parkinson’s Disease Toxin, Rotenone. NeuroSci, 6(1), 24. https://doi.org/10.3390/neurosci6010024





