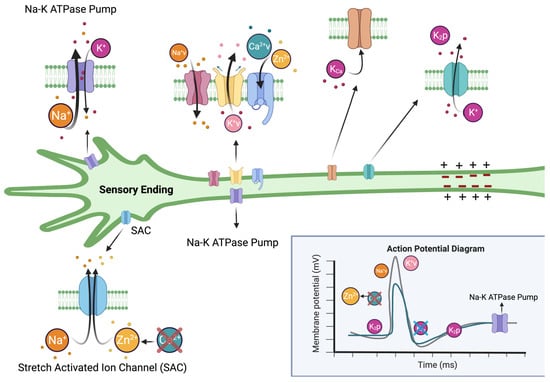
The Effects of Zinc on Proprioceptive Sensory Function and Nerve Conduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
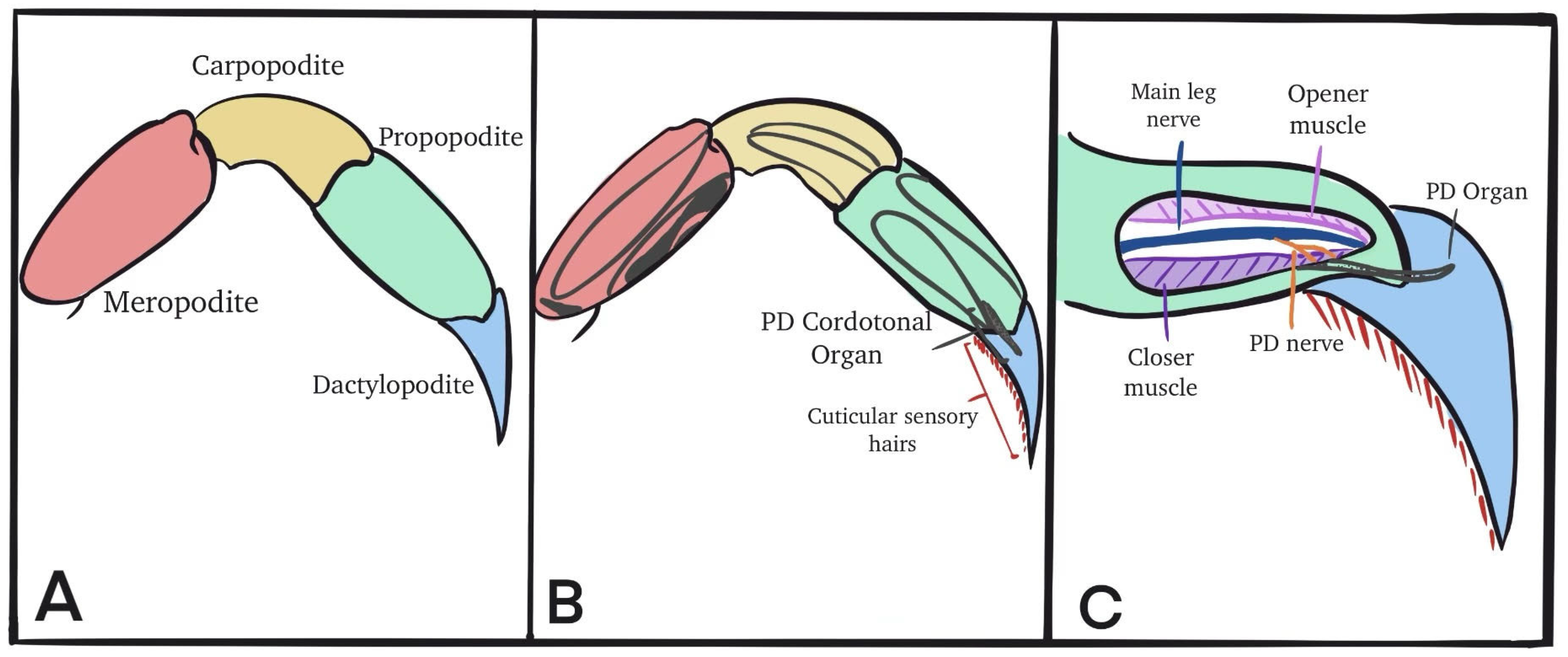
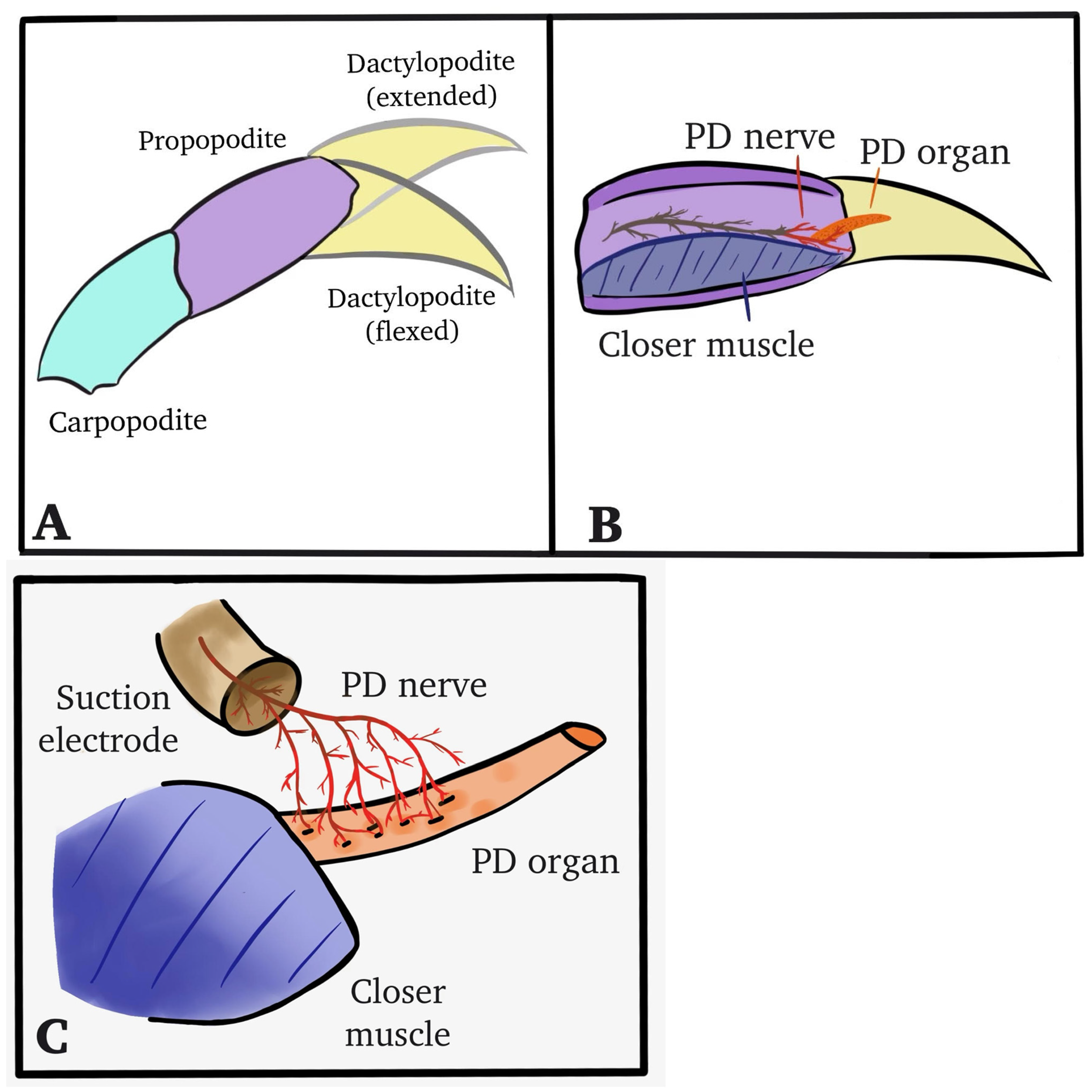
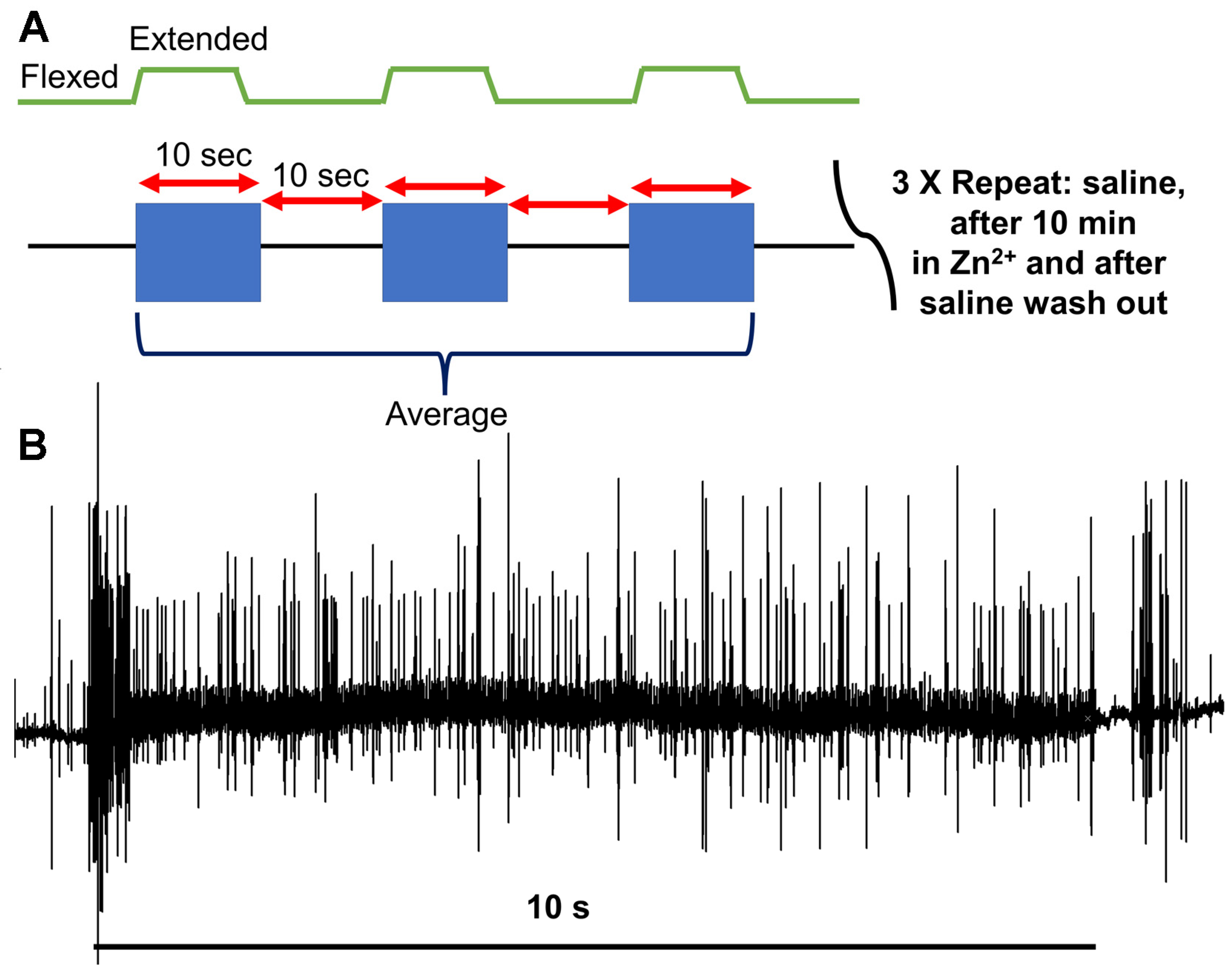
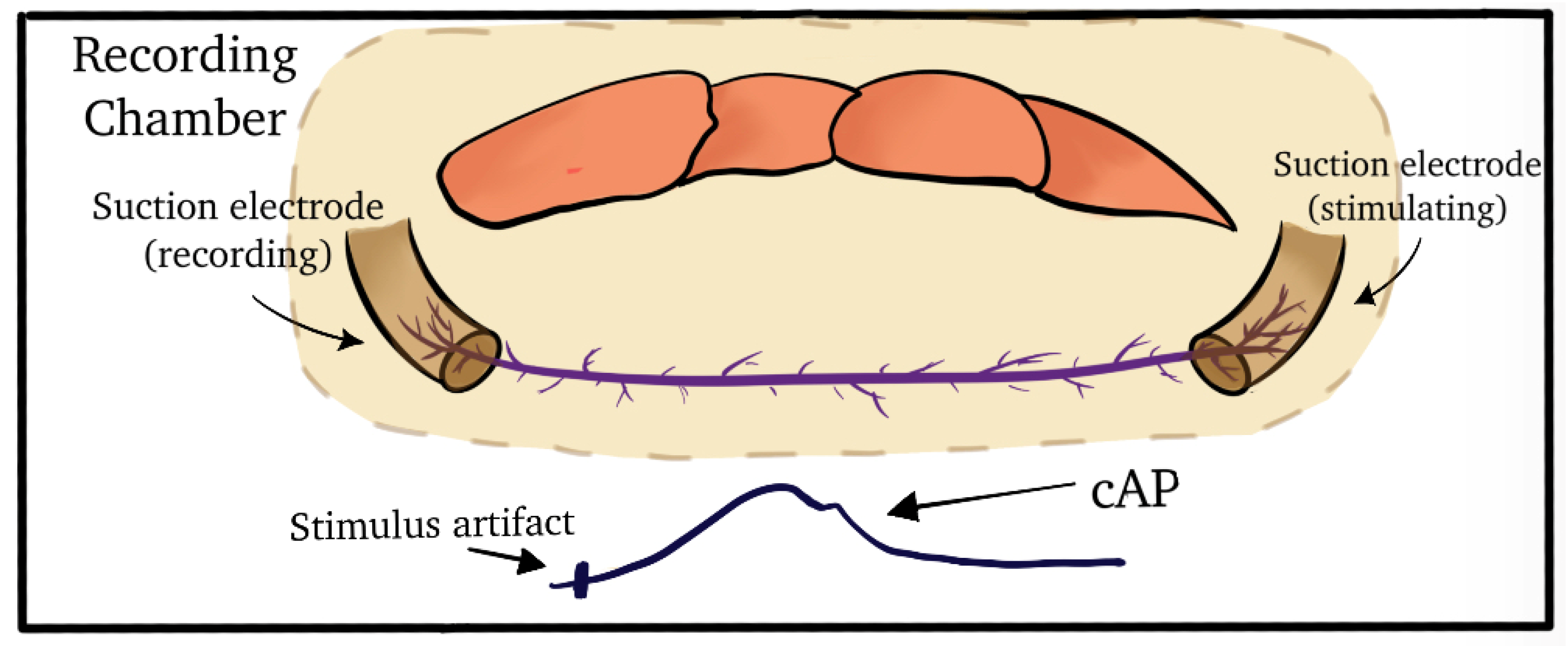
2.2. Dissection and Physiology
2.3. Statistical Analysis
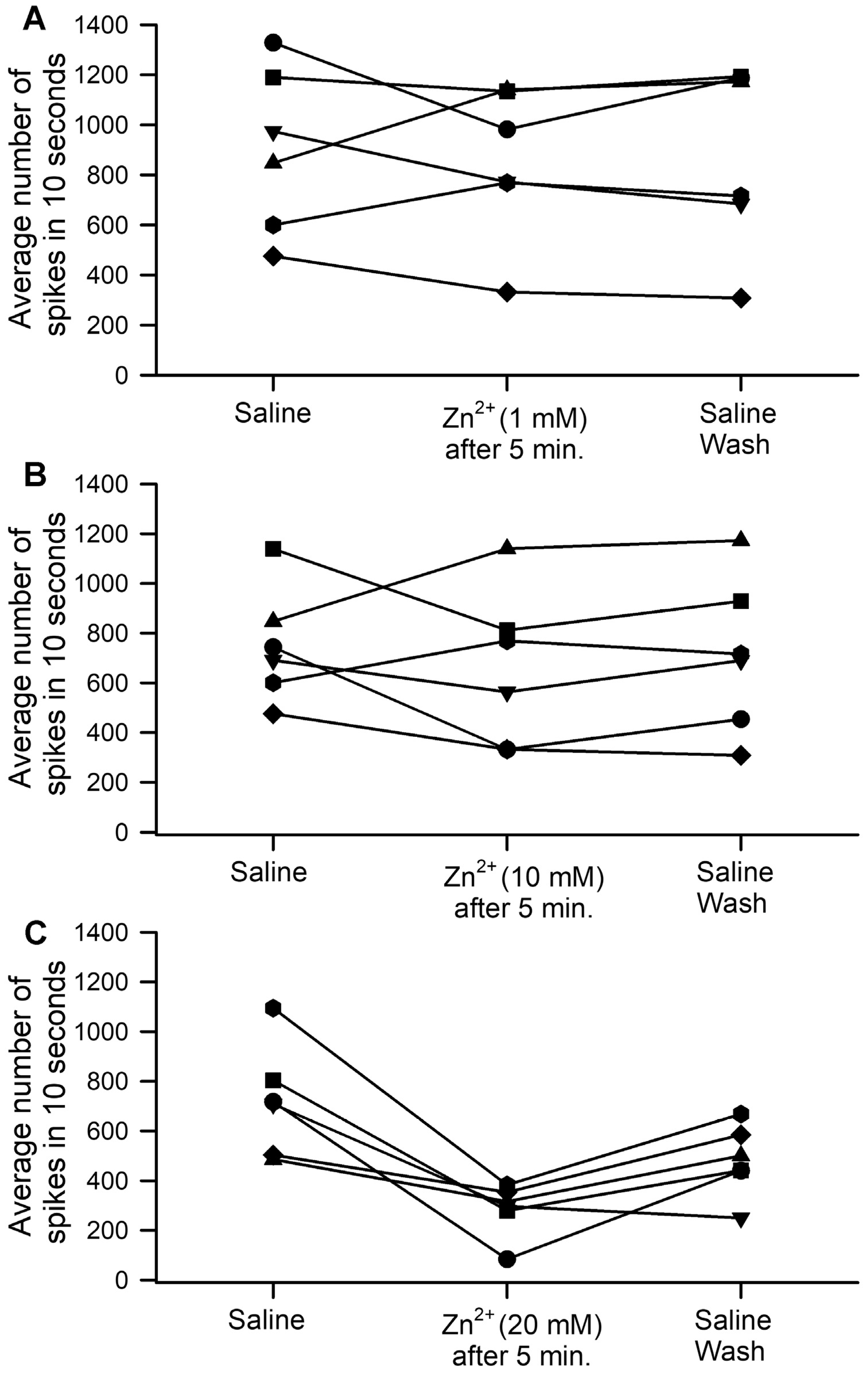
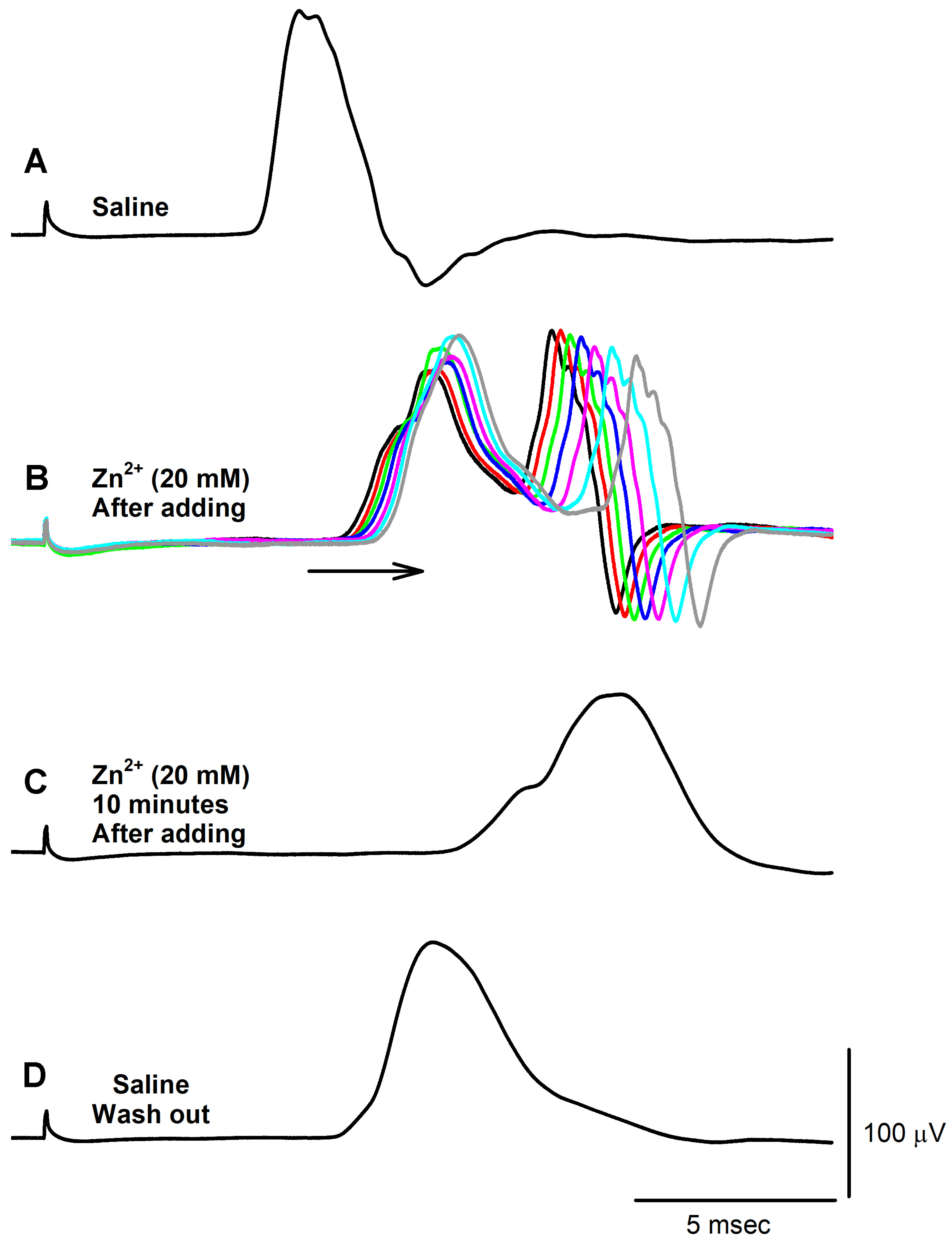
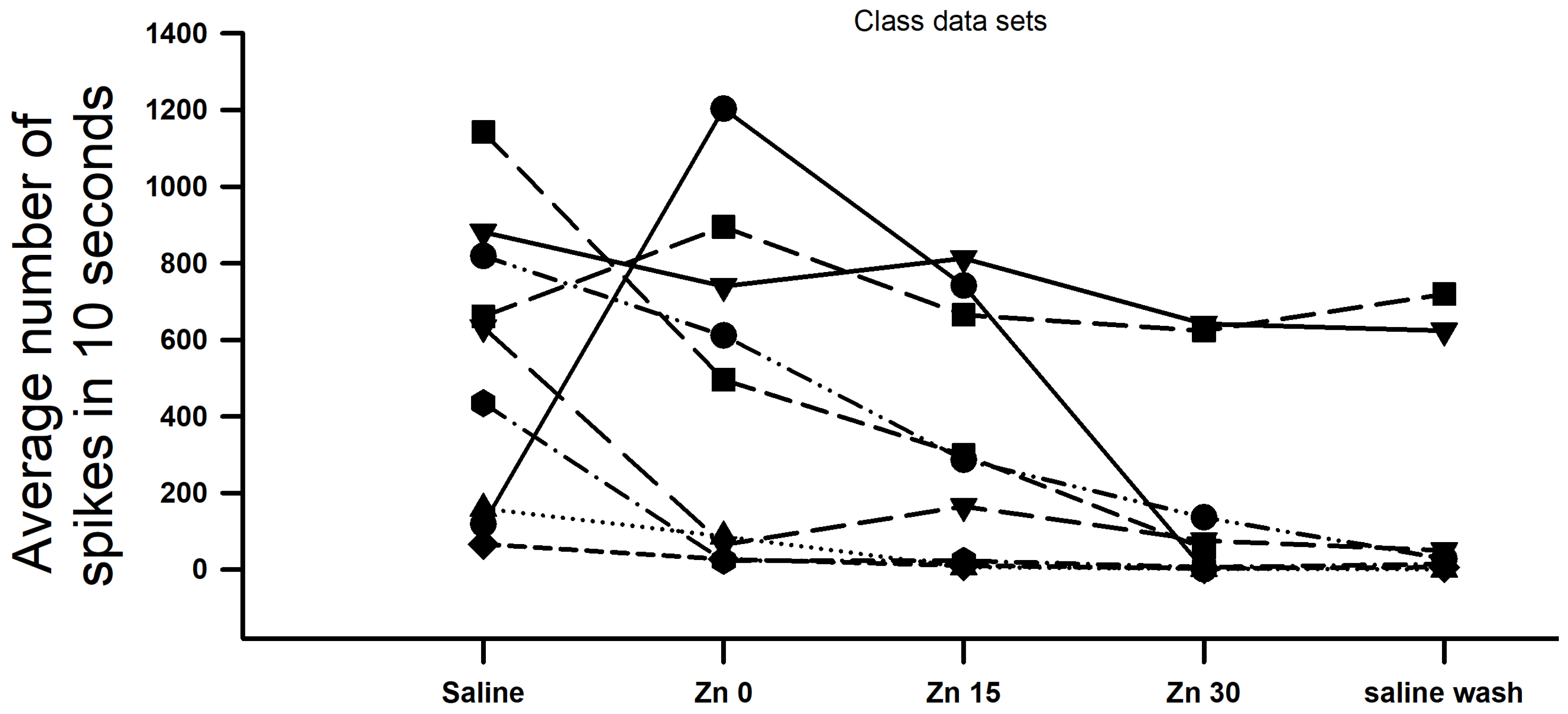
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Parkin, G. Synthetic analogues relevant to the structure and function of zinc enzymes. Chem. Rev. 2004, 104, 699–767. [Google Scholar] [CrossRef]
- Ryu, M.-S.; Aydemir, T.B. Zinc. In Present Knowledge in Nutrition, 11th ed.; Marriott, B.P., Birt, D.F., Stallings, V.A., Yates, A.A., Eds.; Wiley-Blackwell: Cambridge, MA, USA, 2020; pp. 393–408. [Google Scholar]
- Brown, M.A.; Thom, J.V.; Orth, G.L.; Cova, P.; Juarez, J. Food posing involving zinc contamination. Arch. Environ. Health 1964, 8, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Brewer, G.J.; Schoomaker, E.B.; Rabbani, P. Hypocupremia induced by zinc therapy in adults. JAMA 1978, 240, 2166–2168. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.G.; McMaster, D.; Elmes, M.E.; Love, A.H. Anaemia and low serum-copper during zinc therapy. Lancet 1977, 2, 774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Seydi, E.; Soltani, M.; Ramazani, M.; Zarei, M.H.; Pourahmad, J. Occupational exposure in lead and zinc mines induces oxidative stress in miners lymphocytes: Role of mitochondrial/lysosomal damage. Main Group Metal. Chem. 2020, 43, 154–163. [Google Scholar] [CrossRef]
- Morris, D.R.; Levenson, C.W. Ion channels and zinc: Mechanisms of neurotoxicity and neurodegeneration. J. Toxicol. 2012, 2012, 785647. [Google Scholar] [CrossRef]
- Fischer Walker, C.; Black, R.E. Zinc and the risk for infectious disease. Annu. Rev. Nutr. 2004, 24, 255–275. [Google Scholar] [CrossRef]
- Black, R. Micronutrient deficiency: An underlying cause of morbidity and mortality. Bull World Health Org. 2003, 81, 79–89. [Google Scholar]
- Lee, J.Y.; Cole, T.B.; Palmiter, R.D.; Koh, J.Y. Accumulation of zinc in degenerating hippocampal neurons of ZnT3-null mice after seizures: Evidence against synaptic vesicle origin. J. Neurosci. 2000, 20, RC79. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.L.; Ton-That, D.; Sullivan, P.G.; Jonas, E.A.; Gee, K.R.; Kaczmarek, L.K.; Weiss, J.H. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc. Natl. Acad. Sci. USA 2003, 100, 6157–6162. [Google Scholar] [CrossRef] [PubMed]
- Aras, M.A.; Aizenman, E. Redox regulation of intracellular zinc: Molecular signaling in the life and death of neurons. Antioxid. Redox Signal. 2011, 15, 2249–2263. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.M.; Gershoff, S.N. Effects of zinc deficiency on the oxidation of retinol and ethanol in rats. J. Nutr. 1975, 105, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.A.; Russel, R.M.; Carney, E.A.; Oaks, E.V. Reversal of night blindness in cirrhotics with zinc sulfat (Abstract). Am. J. Clin. Nutr. 1977, 30, 612. [Google Scholar]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Cancalon, P. Degeneration and regeneration of olfactory cells induced by ZnSO4 and other chemicals. Tissue Cell 1982, 14, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Matulionis, D.H. Light and electron microscopic study of the effects of ZnSO4 on mouse nasal respiratory epithelium and subsequent responses. Anat. Rec. 1975, 183, 63–82. [Google Scholar] [CrossRef]
- Hentig, J.T.; Byrd-Jacobs, C.A. Exposure to zinc sulfate results in differential effects on olfactory sensory neuron subtypes in adult zebrafish. Int. J. Mol. Sci. 2016, 17, 1445. [Google Scholar] [CrossRef]
- Gu, Q.; Lin, R.L. Heavy metals zinc, cadmium, and copper stimulate pulmonary sensory neurons via direct activation of TRPA1. J. Appl. Physiol. 1985, 108, 891–897. [Google Scholar] [CrossRef]
- Bush, B.M.H. Proprioceptive reflexes in the legs of Carcinus meanas. J. Exp. Biol. 1962, 39, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Bush, B.M.H. Proprioception by chordotonal organs in the mero-carpopodite and carpo-propodite joints of Carcinus maenas legs. Comp. Biochem. Physiol. 1965, 14, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Bush, B.M.H. Proprioception by the coxo-basal chordotonal organ, CB, in legs of the crab, Carcinus maenas. J. Exp. Biol. 1965, 42, 285–297. [Google Scholar] [CrossRef]
- Cooper, R.L. Proprioceptive neurons of chordotonal organs in the crab, Cancer magister Dana (Decapoda, Brachyura). Crustaceana 2008, 81, 447–475. [Google Scholar] [CrossRef]
- Cooper, R.L.; Hartman, H.B. Quantification of responses from proprioceptive neurons in the limbs of the crab, Cancer magister. J. Exp. Zool. 1999, 284, 629–636. [Google Scholar] [CrossRef]
- Hartman, H.B.; Boettiger, E.G. The functional organization of the propus-dactylus organ in Cancer irroratus Say. Comp. Biochem. Physiol. 1967, 22, 651–663. [Google Scholar] [CrossRef]
- Atkins, D.E.; Bosh, K.L.; Breakfield, G.W.; Daniels, S.E.; Devore, M.J.; Fite, H.E.; Guo, L.Z.; Henry, D.K.J.; Kaffenberger, A.K.; Manning, K.S.; et al. The effect of calcium ions on mechanosensation and neuronal activity in proprioceptive neurons. NeuroSci 2021, 2, 353–371. [Google Scholar] [CrossRef]
- Brock, K.E.; Elliott, E.R.; Taul, A.C.; Asadipooya, A.; Bocook, D.; Burnette, T.; Chauhan, I.V.; Chhadh, B.; Crane, R.; Glover, A.; et al. The Effects of Lithium on Proprioceptive Sensory Function and Nerve Conduction. NeuroSci 2023, 4, 280–295. [Google Scholar] [CrossRef]
- Dayaram, V.; Malloy, C.; Martha, S.; Alvarez, B.; Chukwudolue, I.; Dabbain, N.; Mahmood, D.; Goleva, S.; Hickey, T.; Ho, A.; et al. The effect of CO2, intracellular pH and extracellular pH on mechanosensory proprioceptor responses in crayfish and crab. Am. J. Undergrad. Res. 2017, 14, 85–99. [Google Scholar] [CrossRef]
- McCubbin, S.; Jeoung, A.; Waterbury, C.; Cooper, R.L. Pharmacological profiling of stretch activated channels in proprioceptive neuron. Comp. Biochem. Physiol. C 2020, 233, 108765. [Google Scholar] [CrossRef]
- Stanley, C.E.; Adams, R.; Nadolski, J.; Amrit, E.; Barrett, M.; Bohnett, C.; Campbell, K.; Deweese, K.; Dhar, S.; Gillis, B.; et al. The effects of tricaine mesylate on arthropods: Crayfish, crab and Drosophila. Iinvertebr. Neurosci. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Pankau, C.; Nadolski, J.; Tanner, H.; Cryer, C.; Di Girolamo, J.; Haddad, C.; Lanning, M.; Miller, M.; Neely, D.; Wilson, R.; et al. Examining the effect of manganese on physiological processes: Invertebrate models. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 251, 109209. [Google Scholar] [CrossRef]
- Tanner, H.N.; Atkins, D.E.; Bosh, K.L.; Breakfield, G.W.; Daniels, S.E.; Devore, M.J.; Fite, H.E.; Guo, L.; Henry, D.; Kaffenberger, A.; et al. The effect of TEA, 4-AP and in combination on primary sensory neurons in a marine crustacean model. J. Pharmacol. Toxicol. 2022, 17, 14–27. [Google Scholar] [CrossRef]
- Ison, B.J.; Abul-Khoudoud, M.O.; Ahmed, S.; Alhamdani, A.W.; Ashley, C.; Bidros, P.C.; Bledsoe, C.O.; Bolton, K.E.; Capili, J.G.; Henning, J.N.; et al. The Effect of Doxapram on Proprioceptive Neurons: Invertebrate Model. NeuroSci 2022, 3, 566–588. [Google Scholar] [CrossRef]
- O’Neil, A.S.; Krall, R.M.; Vascassenno, R.; Cooper, R.L. Exploring Mechanisms in a Medical Treatment for a Disease: A Teaching/Learning Module. Adv. Biol. Lab. Educ. Assoc. Biol. Lab. Edu. 2023, 43, 35. [Google Scholar]
- Majeed, Z.R.; Titlow, J.; Hartman, H.B.; Cooper, R.L. Proprioception and tension receptors in crab limbs: Student laboratory exercises. J. Vis. Exp. 2013, 80, e51050. [Google Scholar]
- Geffeney, S.L.; Goodman, M.B. How we feel: Ion channel partnerships that detect mechanical inputs and give rise to touch and pain perception. Neuron 2012, 74, 609–619. [Google Scholar] [CrossRef]
- Whitear, M. Chordotonal organs in Crustacea. Nature 1960, 187, 522–523. [Google Scholar] [CrossRef]
- Kamalanathan, S.; Balachandran, K.; Parthan, G.; Hamide, A. Chvostek’s sign: A video demonstration. BMJ Case Rep. 2012, 2012, bcr2012007098. [Google Scholar] [CrossRef]
- Patel, M.; Hu, E.W. Trousseau Sign. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Mackie, G.O.; Meech, R. Separate sodium and calcium spikes in the same axon. Nat. Cell Biol. 1985, 313, 791–793. [Google Scholar] [CrossRef]
- Jirounek, P.; Chardonness, E.; Brunet, P.C. Afterpotentials in nonmyelinated nerve fibers. J. Neurophysiol. 1991, 65, 860–873. [Google Scholar] [CrossRef]
- Robert, A.; Jirounek, P. Uptake of potassium by nonmyelinating Schwann cells induced by axonal activity. J. Neurophysiol. 1994, 72, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Mert, T.; Gunes, Y.; Guven, M.; Günay, I.; Ozcengiz, D. Effects of calcium and magnesium on peripheral nerve conduction. Pol. J. Pharmacol. 2003, 55, 25–30. [Google Scholar] [PubMed]
- Han, P.; Trinidad, B.J.; Shi, J. Hypocalcemia-induced seizure: Demystifying the calcium paradox. ASN Neuro 2015, 7, 1759091415578050. [Google Scholar] [CrossRef]
- Bostock, H.; Sears, T.A.; Sherratt, R.M. The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J. Physiol. 1981, 313, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.R.; Nadolski, J.; McIntosh, R.D.; Datta, M.; Crawford, D.; Hirtle, J.; Leach, A.B.; Roemer, K.A.; Sotingeanu, L.C.; Taul, A.; et al. Investigation regarding the physiological effects of zinc on Drosophila and crawfish cardiac, neural, synaptic, and behavioral processes. J. Pharmacol. Toxicol. 2023, 18, 166–189. [Google Scholar] [CrossRef]
- Gruss, M.; Mathie, A.; Lieb, W.R.; Franks, N.P. Thetwo-pore-domain K+ channels TREK-1 and TASK-3 are differentially modulated by copper and zinc. Mol. Pharmacol. 2004, 66, 530–537. [Google Scholar]
- Malloy, C.; Dayaram, V.; Martha, S.; Alvarez, B.; Chukwudolue, I.; Dabbain, N.; Mahmood, D.D.; Goleva, S.; Hickey, T.; Ho, A.; et al. The effects of potassium and muscle homogenate on proprioceptive responses in crayfish and crab. J. Exp. Zool. A Ecol. Integr. Physiol. 2017, 327, 366–379. [Google Scholar] [CrossRef]

| Prep | Amplitude of CAP | Neuronal Recruitment | Conduction Time (ms) | Recovery in Saline Rinse |
|---|---|---|---|---|
| 1 | ↑ 44% then ↓ 88% | + | ↓ 17.97 ms | + |
| 2 | ↑ 11% then ↓ 30% | + | ↓ 7.55 ms | + |
| 3 | ↑ 3% then ↓ 39% | + | ↓ 8.04 ms | + |
| 4 | ↑ 134% then ↓ 14% | + | ↓ 14.02 ms | + |
| 5 | ↑ 20 then ↓ 29% | + | ↓ 3.8 ms | + |
| 6 | ↑ 303% then ↓ 50% | + | ↓ 17.97 ms | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elliott, E.R.; Brock, K.E.; Taul, A.C.; Asadipooya, A.; Bocook, D.; Burnette, T.; Chauhan, I.V.; Chhadh, B.; Crane, R.; Glover, A.; et al. The Effects of Zinc on Proprioceptive Sensory Function and Nerve Conduction. NeuroSci 2023, 4, 305-318. https://doi.org/10.3390/neurosci4040025
Elliott ER, Brock KE, Taul AC, Asadipooya A, Bocook D, Burnette T, Chauhan IV, Chhadh B, Crane R, Glover A, et al. The Effects of Zinc on Proprioceptive Sensory Function and Nerve Conduction. NeuroSci. 2023; 4(4):305-318. https://doi.org/10.3390/neurosci4040025
Chicago/Turabian StyleElliott, Elizabeth R., Kaitlyn E. Brock, Alaina C. Taul, Artin Asadipooya, Devin Bocook, Tessa Burnette, Isha V. Chauhan, Bilal Chhadh, Ryan Crane, Ashley Glover, and et al. 2023. "The Effects of Zinc on Proprioceptive Sensory Function and Nerve Conduction" NeuroSci 4, no. 4: 305-318. https://doi.org/10.3390/neurosci4040025
APA StyleElliott, E. R., Brock, K. E., Taul, A. C., Asadipooya, A., Bocook, D., Burnette, T., Chauhan, I. V., Chhadh, B., Crane, R., Glover, A., Griffith, J., Hudson, J. A., Kashif, H., Nwadialo, S. O., Neely, D. M., Nukic, A., Patel, D. R., Ruschman, G. L., Sales, J. C., ... Cooper, R. L. (2023). The Effects of Zinc on Proprioceptive Sensory Function and Nerve Conduction. NeuroSci, 4(4), 305-318. https://doi.org/10.3390/neurosci4040025