Status Epilepticus and Neurosyphilis: A Case Report and a Narrative Review
Abstract
1. Introduction
- -
- asymptomatic form: CSF abnormalities are found in a patient with serological evidence of syphilis but without any clinical neurological symptom/sign. This form could appear weeks after infection;
- -
- meningeal form: characterized by diffuse meninges inflammation that could lead to headache, nausea, vomiting, neck stiffness, photophobia, cranial nerve deficits (including deafness, vertigo and blindness), confusion, lethargy and seizures. This form could appear weeks/months after infection.
- -
- meningovascular form: inflammation of the meninges and a vasculitis of small and medium arteries happen, leading to thrombosis, causing strokes as well as myelopathy if the spinal cord vessels are involved.
- -
- general paralysis of the insane/paralytic dementia: caused by a chronic meningoencephalitis leading to diffuse cerebral atrophy. Clinically it could be characterized by the appearance of mood disturbances, personality changes, psychosis, mania, dementia with memory and executive function impairment, confusion, seizures, tremors, dysarthria and pupillary abnormalities (known as Argyll Robertson pupils);
- -
- tabe dorsalis: caused by the degeneration of the dorsal columns and roots of the spinal cord, leading to sensory ataxia and crisis of acute pain, but also bladder dysfunction and pupillary and ocular palsies.
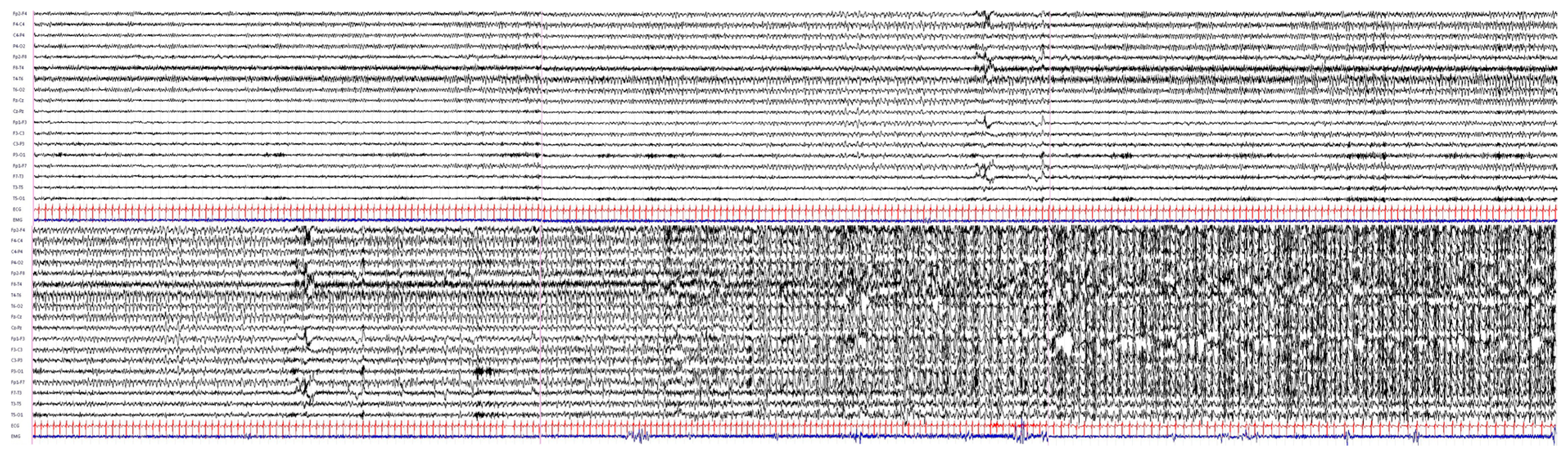
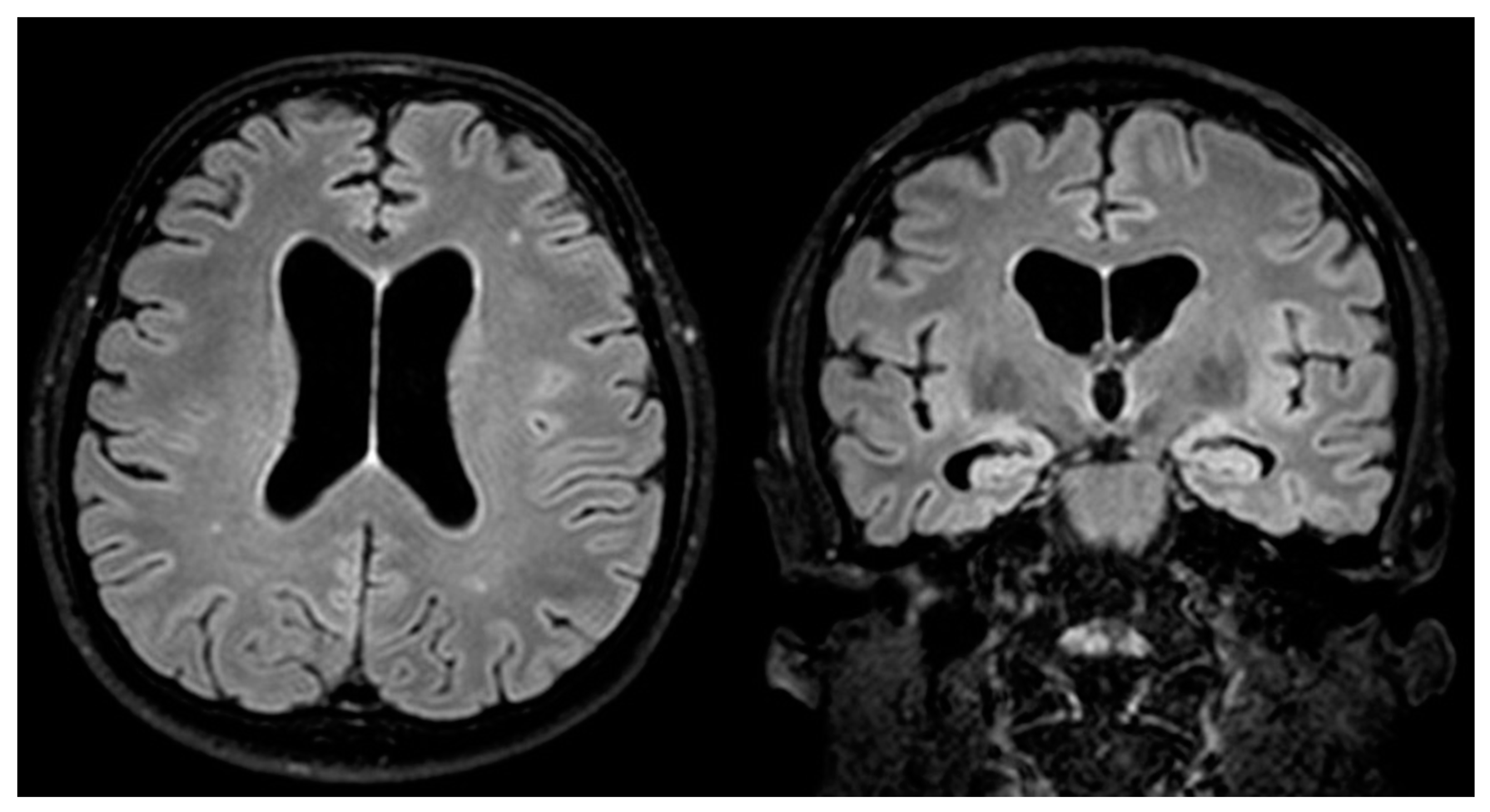
2. Case Report
3. Literature Review
3.1. Material and Methods: Literature Search Strategy and Study Selection Process
- Definite diagnosis of status epilepticus.
- Definite diagnosis of syphilis.
- Articles written in the English language or papers written in other languages for which a detailed and clear English abstract was available.
- Papers published in a peer-reviewed journal.
- Studies conducted on animals or in vitro models.
- Reviews, books.
- A previous diagnosis of symptomatic epilepsy or idiopathic/genetic epilepsy.
Data Extraction Process
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ha, T.; Tadi, P.; Dubensky, L. Neurosyphilis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Tudor, M.E.; Al Aboud, A.M.; Gossman, W.G. Syphilis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gonzalez, H.; Koralnik, I.J.; Marra, C.M. Neurosyphilis. Semin. Neurol. 2019, 39, 448–455. [Google Scholar] [CrossRef]
- Conde-Sendín, M.Á.; Amela-Peris, R.; Aladro-Benito, Y.; Maroto, A.A.-M. Current Clinical Spectrum of Neurosyphilis in Immunocompetent Patients. Eur. Neurol. 2004, 52, 29–35. [Google Scholar] [CrossRef]
- Hooshmand, H. Seizure Disorders Associated with Neurosyphilis. Dis. Nerv. Syst. 1976, 37, 133–136. [Google Scholar]
- Gürses, C.; Kürtüncü, M.; Jirsch, J.; Yesilot, N.; Hanag, H.; Baykan, B.; Emre, M.; Gökyig, A.; Andermann, F. Neurosyphilis Presenting with Status Epilepticus. Epileptic Disord. 2007, 9, 6. [Google Scholar]
- Ropper, A.H. Neurosyphilis. N. Engl. J. Med. 2019, 381, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Rissardo, J.P.; Caprara, A.L.F.; Silveira, J.O.F. Generalized Convulsive Status Epilepticus Secondary to Jarisch-Herxheimer Reaction in Neurosyphilis: A Case Report and Literature Review. Neurologist 2019, 24, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, N.; Hirsch, L.J.; Sculier, C.; Loddenkemper, T.; van Baalen, A.; Lancrenon, J.; Emmery, M.; Specchio, N.; Farias-Moeller, R.; Wong, N.; et al. New-Onset Refractory Status Epilepticus (NORSE) and Febrile Infection-Related Epilepsy Syndrome (FIRES): State of the Art and Perspectives. Epilepsia 2018, 59, 745–752. [Google Scholar] [CrossRef]
- LoVecchio, F. Neurosyphilis Presenting as Refractory Status Epilepticus. Am. J. Emerg. Med. 1995, 13, 685–686. [Google Scholar] [CrossRef]
- Heald, A.; Connolly, S.; Hudgson, P. Neurosyphilis Presenting as Complex Partial Status Epilepticus. Eur. Neurol. 1996, 36, 111–112. [Google Scholar] [CrossRef]
- Perri, G.; Ciani, M.; Sbrascini, S. Partial-Complex Status during Neurosyphilis: A Case Report. Boll. Lega Ital. Contro Epilessia 1996, 95–96, 311–313. [Google Scholar]
- Suarez, J.I.; Mlakar, D.; Snodgrass, S.M. Cerebral Syphilitic Gumma in an HIV-Negative Patient Presenting as Prolonged Focal Motor Status Epilepticus. N. Engl. J. Med. 1996, 335, 1159–1160. [Google Scholar] [CrossRef]
- Rinkel, G.J.; Brouwers, P.J.; Lambrechts, D.A. Clinical judgment and decision making in clinical practice. A music conductor with epilepsy followed by memory disorders. Ned. Tijdschr. Geneeskd. 1997, 141, 723–726. [Google Scholar]
- Primavera, A.; Solaro, C.; Cocito, L. De Novo Status Epilepticus as the Presenting Sign of Neurosyphilis. Epilepsia 1998, 39, 1367–1369. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Zifkin, B.; Migneco, O.; Lebrun, C.; Darcourt, J.; Andermann, F. Nonconvulsive Status Epilepticus of Frontal Origin. Neurology 1999, 52, 1174. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Erbetta, A.; Pareyson, D.; Sghirlanzoni, A. Parenchymatous Neurosyphilis. Neurol. Sci. 2001, 22, 281–282. [Google Scholar] [CrossRef]
- Camacho-Salas, A.; Martíez-Salio, A.; García-Morales, I.; Villarejo-Galende, A.; de la Peña, P. Periodic lateralised epileptiform discharges as a form of presentation of neurosyphilis. Rev. Neurol. 2002, 35, 734–737. [Google Scholar]
- Jirsch, J.D.; Andermann, F.; Gross, D.W. Status Epilepticus Presenting in a Patient with Neurosyphilis and a Previously Asymptomatic Arachnoid Cyst. Epilepsia 2002, 43, 775–776. [Google Scholar] [CrossRef]
- Vojvodic, N.M.; Sokic, D.V.; Jankovic, S.M.; Delic, S. Isolated Episodes of Status Epilepticus as the Manifestation of Neurosyphilis: A Case Report. Epilepsia 2003, 44, 623. [Google Scholar] [CrossRef]
- Ubogu, E.E.; Sagar, S.M.; Lerner, A.J.; Maddux, B.N.; Suarez, J.I.; Werz, M.A. Ketamine for Refractory Status Epilepticus: A Case of Possible Ketamine-Induced Neurotoxicity. Epilepsy Behav. 2003, 4, 70–75. [Google Scholar] [CrossRef]
- Ances, B.M.; Shellhaus, R.; Brown, M.J.; Rios, O.V.; Herman, S.T.; French, J.A. Neurosyphilis and Status Epilepticus: Case Report and Literature Review. Epilepsy Res. 2004, 59, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Marano, E. Neurosyphilis with Complex Partial Status Epilepticus and Mesiotemporal MRI Abnormalities Mimicking Herpes Simplex Encephalitis. J. Neurol. Neurosurg. Psychiatry 2004, 75, 833. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-P.; Lin, R.-T.; Liu, C.-K.; Chao, H.-L.; Chao, A.-C. Neurosyphilis Presenting With Status Epilepticus. Neurologist 2006, 12, 314–317. [Google Scholar] [CrossRef]
- Li, C.-H.; Lin, R.-T.; Su, C.-L.; Lin, W.-C. Status Epilepticus as an Initial Manifestation of Neurosyphilis: A Case Report. Kaohsiung J. Med. Sci. 2006, 22, 404–409. [Google Scholar] [CrossRef]
- Boursoulian, L.J.; Shihabuddin, B.S. Complex Partial Status Epilepticus as the Presenting Manifestation of Neurosyphilis. Epilepsia 2007, 48, 16–17. [Google Scholar]
- Otto, B.; Hermans, M.; Seifried, C.; Buchkremer, M.; Lanfermann, H.; Sitzer, M. Neurosyphilis: Wichtige Differenzialdiagnose einer Herpesenzephalitis. Nervenarzt 2007, 78, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Amare, A.; Zenebe, G.; Hammack, J.; Davey, G. Status Epilepticus: Clinical Presentation, Cause, Outcome, and Predictors of Death in 119 Ethiopian Patients. Epilepsia 2008, 49, 600–607. [Google Scholar] [CrossRef]
- Sinha, S.; Harish, T.; Taly, A.B.; Murthy, P.; Nagarathna, S.; Chandramuki, A. Symptomatic Seizures in Neurosyphilis: An Experience from a University Hospital in South India. Seizure 2008, 17, 711–716. [Google Scholar] [CrossRef]
- Sesar, A.; Arias, M.; Requena, I.; Pereiro, I. Status Epilepticus Secondary to Luetic Encephalitis: Evolution of Neuroimaging Findings. J. Neurol. 2008, 255, 438–440. [Google Scholar] [CrossRef]
- Hajjaj, I.; Kissani, N. Status Epilepticus Revealing Syphilitic Meningoencephalitis. Acta Neurol. Belg. 2010, 110, 263–267. [Google Scholar]
- Kuppasani, K.; Vadehra, V.K.; Reddi, A.S. New-Onset Status Epilepticus Develops after a Motor Vehicle Collision. J. Am. Acad. Physician Assist. 2010, 23, 31–32. [Google Scholar] [CrossRef]
- Gaud, S.; Sauvée, M.; Muresan, M.; Gospodaru, N.; Foscolo, S.; Debouverie, M. Lésions Mésiotemporales Gauches et Amnésie Antérograde: Un Cas de Neurosyphilis. Rev. Neurol. 2011, 167, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huang, E.; Xie, B.; Cheng, Y. Neurosyphilis Presenting with Psychotic Symptoms and Status Epilepticus. Neurol. Sci. 2012, 33, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Derouich, I.; Messouak, O.; Belahsen, M.F. Syphilitic Limbic Encephalitis Revealed by Status Epilepticus. Case Rep. 2013, 2013, bcr2012008073. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.-L.; Liu, L.-L.; Zeng, Y.-L.; Zhang, H.-L.; Liu, G.-L.; Zheng, W.-H.; Dong, J.; Wu, J.-Y.; Su, Y.-H.; Lin, L.-R.; et al. Laboratory Findings in Neurosyphilis Patients with Epileptic Seizures Alone as the Initial Presenting Symptom. Diagn. Microbiol. Infect. Dis. 2013, 75, 377–380. [Google Scholar] [CrossRef]
- Lv, Y.; Chu, F.; Meng, H.; Wang, Z.; Cui, L. A Patient with Progressive Cognitive Decline and Periodic Abnormal Waves in EEG: PLEDs of Neurosyphilis or PSDs of Creutzfeldt-Jakob Disease? Clin. EEG Neurosci. 2014, 45, 218–221. [Google Scholar] [CrossRef]
- Gaspard, N.; Foreman, B.P.; Alvarez, V.; Cabrera Kang, C.; Probasco, J.C.; Jongeling, A.C.; Meyers, E.; Espinera, A.; Haas, K.F.; Schmitt, S.E.; et al. New-Onset Refractory Status Epilepticus: Etiology, Clinical Features, and Outcome. Neurology 2015, 85, 1604–1613. [Google Scholar] [CrossRef]
- Kumari, S.; Hayton, T.; Jumaa, P.; McCorry, D. ‘The Great Imitator’: Neurosyphilis and New-Onset Refractory Status Epilepticus (NORSE) Syndrome. Epilepsy Behav. Case Rep. 2015, 3, 33–35. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Mo, H.-H.; Jung, H.-G.; Baek, S.-H.; Park, S.-H. Neurosyphilis Presenting with Non-Convulsive Status Epilepticus. J. Korean Neurol. Assoc. 2016, 34, 243–245. [Google Scholar] [CrossRef]
- Yu, X.; Shao, X.; Sun, H.; Zhong, C.; Cai, J.; Gao, L. Nonconvulsive Status Epilepticus Associated with Periodic Lateralized Epileptiform Discharges in a Patient with Syphilis. Interdiscip. Neurosurg. 2016, 5, 35–37. [Google Scholar] [CrossRef][Green Version]
- Sakai, K.; Yazawa, S.; Sugimoto, A.; Nakao, K.; Tsuruta, K.; Ochiai, E.; Suzuki, Y.; Matsuhashi, M. Electroclinical and Radiological Observation of Dysfunctional Zones in a Patient with Neurosyphilis. Epileptic Disord. 2018, 20, 164–168. [Google Scholar] [CrossRef]
- Toudou-Daouda, M.; Filali-Adib, A.; Slassi, A.; Belahsen, M.-F.; Souirti, Z. Limbic Encephalitis: Experience of a Moroccan Center. Brain Behav. 2019, 9, e01177. [Google Scholar] [CrossRef]
- Zifko, U.; Lindner, K.; Wimberger, D.; Volc, B.; Grisold, W. Jarisch-Herxheimer Reaction in a Patient with Neurosyphilis. J. Neurol. Neurosurg. Psychiatry 1994, 57, 865–867. [Google Scholar] [CrossRef]
- Kojan, S.; Van Ness, P.C.; Diaz-Arrastia, R. Nonconvulsive Status Epilepticus Resulting from Jarisch-Herxheimer Reaction in a Patient with Neurosyphilis. Clin. Electroencephalogr. 2000, 31, 138–140. [Google Scholar] [CrossRef]
- Kobayashi, J.; Nakagawa, Y.; Tobisawa, S.; Isozaki, E.; Koide, R. Deterioration of MRI Findings Related to Jarisch–Herxheimer Reaction in a Patient with Neurosyphilis. J. Neurol. 2011, 258, 699–701. [Google Scholar] [CrossRef]
- Gaspard, N. Unusual Causes of Status Epilepticus. In Status Epilepticus, Current Clinical Neurology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Saunderson, R.B.; Chan, R.C. Mesiotemporal Changes on Magnetic Resonance Imaging in Neurosyphilis: Brief Communication. Intern. Med. J. 2012, 42, 1057–1063. [Google Scholar] [CrossRef]
- Meletti, S.; Monti, G.; Mirandola, L.; Vaudano, A.E.; Giovannini, G. Neuroimaging of Status Epilepticus. Epilepsia 2018, 59 (Suppl. 2), 113–119. [Google Scholar] [CrossRef]
- Galovic, M.; van Dooren, V.Q.H.; Postma, T.; Vos, S.B.; Caciagli, L.; Borzì, G.; Rosillo, J.C.; Vuong, K.A.; de Tisi, J.; Nachev, P.; et al. Progressive Cortical Thinning in Patients With Focal Epilepsy. JAMA Neurol. 2019, 76, 1230–1239. [Google Scholar] [CrossRef]
- Caciagli, L.; Xiao, F.; Wandschneider, B.; Koepp, M.J. Imaging Biomarkers of Anti-Epileptic Drug Action: Insights from Magnetic Resonance Imaging. Curr. Pharm. Des. 2017, 23, 5727–5739. [Google Scholar] [CrossRef][Green Version]
- Tondelli, M.; Vaudano, A.E.; Sisodiya, S.M.; Meletti, S. Valproate Use Is Associated With Posterior Cortical Thinning and Ventricular Enlargement in Epilepsy Patients. Front. Neurol. 2020, 11, 622. [Google Scholar] [CrossRef]
- Giovannini, G.; Monti, G.; Polisi, M.M.; Mirandola, L.; Marudi, A.; Pinelli, G.; Valzania, F.; Girardis, M.; Nichelli, P.F.; Meletti, S. A One-Year Prospective Study of Refractory Status Epilepticus in Modena, Italy. Epilepsy Behav. 2015, 49, 141–145. [Google Scholar] [CrossRef]
- Ouwens, I.M.D.; Fiolet, A.T.L.; Thijs, R.D.; Koehler, P.J.; Verhoeven, W.M.A. Neurosyphilis Mimicking Autoimmune Encephalitis: A Case Report and Review of the Literature. Clin. Neuropsychiatry 2020, 17, 175–180. [Google Scholar] [CrossRef]
| Clinical Characteristics | SE as Expression of Neurosyphilis | SE as Expression of JHR in Neurosyphilis | p | |
|---|---|---|---|---|
| N | N 45 (100%) | N 5 (100%) | ||
| Gender | Male | 33 (73%) | 4 (80%) | 0.49 |
| Female | 4 (9%) | 1 (20%) | ||
| Not Reported | 8 (18%) | 0 | ||
| Age at SE presentation (years) | Average/Median/Range | 46/45/29–62 | 48/48/23–71 | 0.762 |
| Immunodeficiency | Yes | 2 (5%) | 0 | 1.00 |
| No | 24 (53%) | 4 (80%) | ||
| Not reported | 19 (42%) | 1 (20%) | ||
| Clinical presentation | NOSE/NORSE | 35 (78%) | 4 (80%) | 1.00 |
| Epilepsy diagnosis before SE | 7 (16%) | 1 (20%) | ||
| Not reported | 3 (7%) | 0 | ||
| Mood disturbances before SE | 8 (18%) | 2 (40%) | 0.26 | |
| Cognitive disturbances before SE | 14 (31%) | 4 (80%) | 0.05 | |
| Syphilis diagnosis | At SE presentation | 38 (85%) | 4 (80%) | 1.00 |
| Already known syphilitic patients | 7 (16%) | 1 (20%) | ||
| Clinical types of SE | Convulsive forms | 21 (47%) | 1 (20%) | 0.35 |
| Non-convulsive forms | 19 (42%) | 4 (80%) | ||
| Not reported | 5 (11%) | 0 | ||
| SE response to treatment | Responsive | 21 (47%) | 3 (60%) | 1.00 |
| Refractory/Super-Refractory | 10 (22%) | 1 (20%) | ||
| Not reported | 14 (31%) | 1 (20%) | ||
| Neuroimage characteristics | Normal | 0 | 1 (20%) | 0.1 |
| Atrophy | 9 (20%) | 3 (60%) | 0.08 | |
| White matter hyperintensities | 20 (44%) | 2 (40%) | 1.00 | |
| Strokes | 2 (5%) | 0 | 1.00 | |
| Gumma | 1 (2%) | 0 | 1.00 | |
| Not reported | 13 (29%) | 0 | 0.31 | |
| Outcome | Recovery | 8 (18%) | 1 (20%) | 1.00 |
| Improvement with sequelae | 16 (36%) | 1 (20%) | 0.65 | |
| Severe disability | 7 (16%) | 3 (60%) | 0.048 | |
| Deceased | 2 (5%) | 0 | 1.00 | |
| Not reported | 12 (25%) | 0 | 0.32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannini, G.; Meletti, S. Status Epilepticus and Neurosyphilis: A Case Report and a Narrative Review. NeuroSci 2021, 2, 416-426. https://doi.org/10.3390/neurosci2040031
Giovannini G, Meletti S. Status Epilepticus and Neurosyphilis: A Case Report and a Narrative Review. NeuroSci. 2021; 2(4):416-426. https://doi.org/10.3390/neurosci2040031
Chicago/Turabian StyleGiovannini, Giada, and Stefano Meletti. 2021. "Status Epilepticus and Neurosyphilis: A Case Report and a Narrative Review" NeuroSci 2, no. 4: 416-426. https://doi.org/10.3390/neurosci2040031
APA StyleGiovannini, G., & Meletti, S. (2021). Status Epilepticus and Neurosyphilis: A Case Report and a Narrative Review. NeuroSci, 2(4), 416-426. https://doi.org/10.3390/neurosci2040031






