Blockade of Catecholamine Reuptake in the Prelimbic Cortex Decreases Top-Down Attentional Control in Response to Novel, but not Familiar Appetitive Distracters, within a Timing Paradigm
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Apparatus
2.3. Behavioral Procedures
2.3.1. Interval Timing–Left Lever
2.3.2. Appetitive Noise Training–Right Lever
2.4. Surgery
2.5. Drugs
2.6. Local Infusions
2.7. Testing with and without Noise Distracters
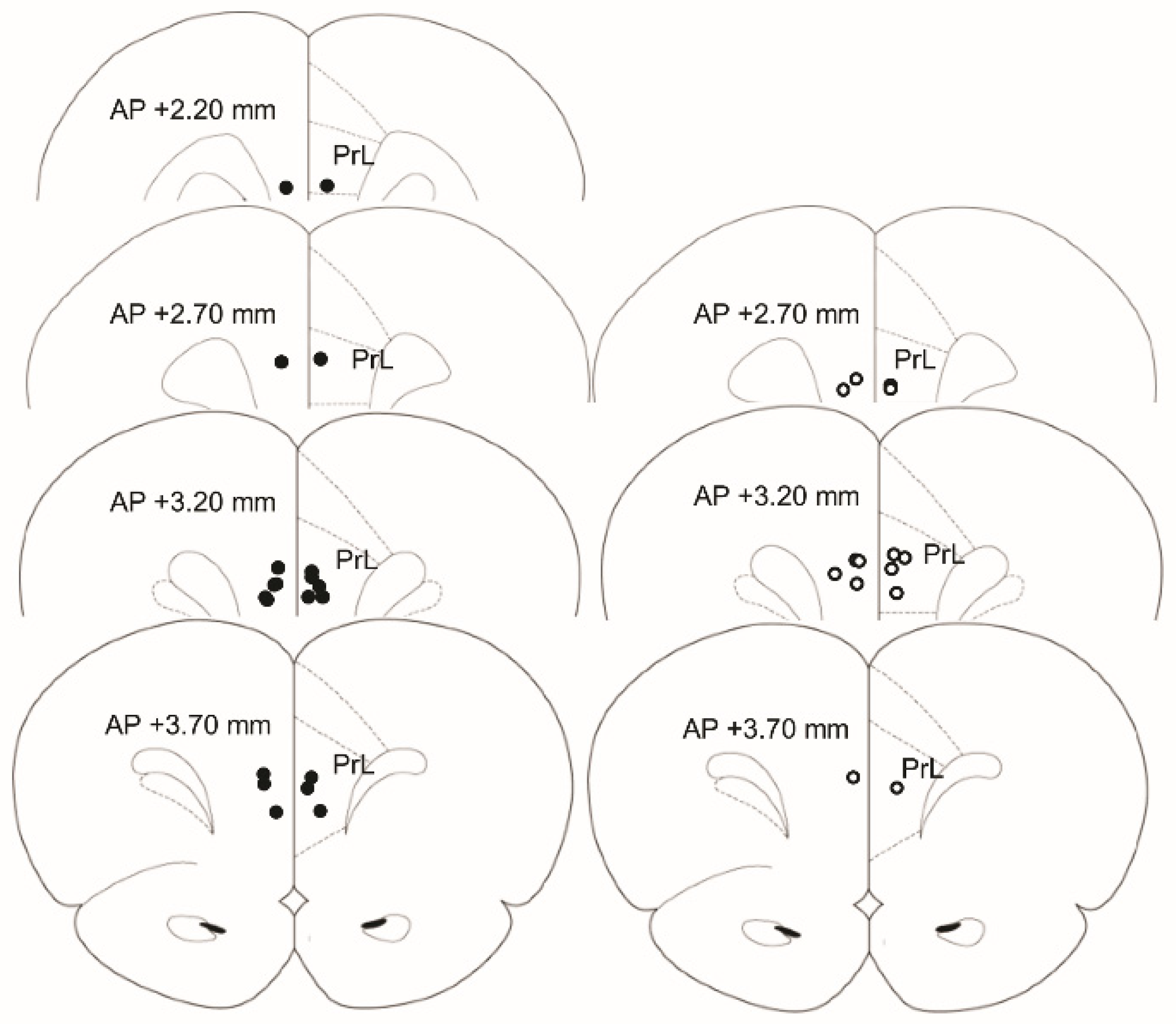
2.8. Histology
2.9. Data Collection and Analysis
2.10. Statistical Analyses
3. Results
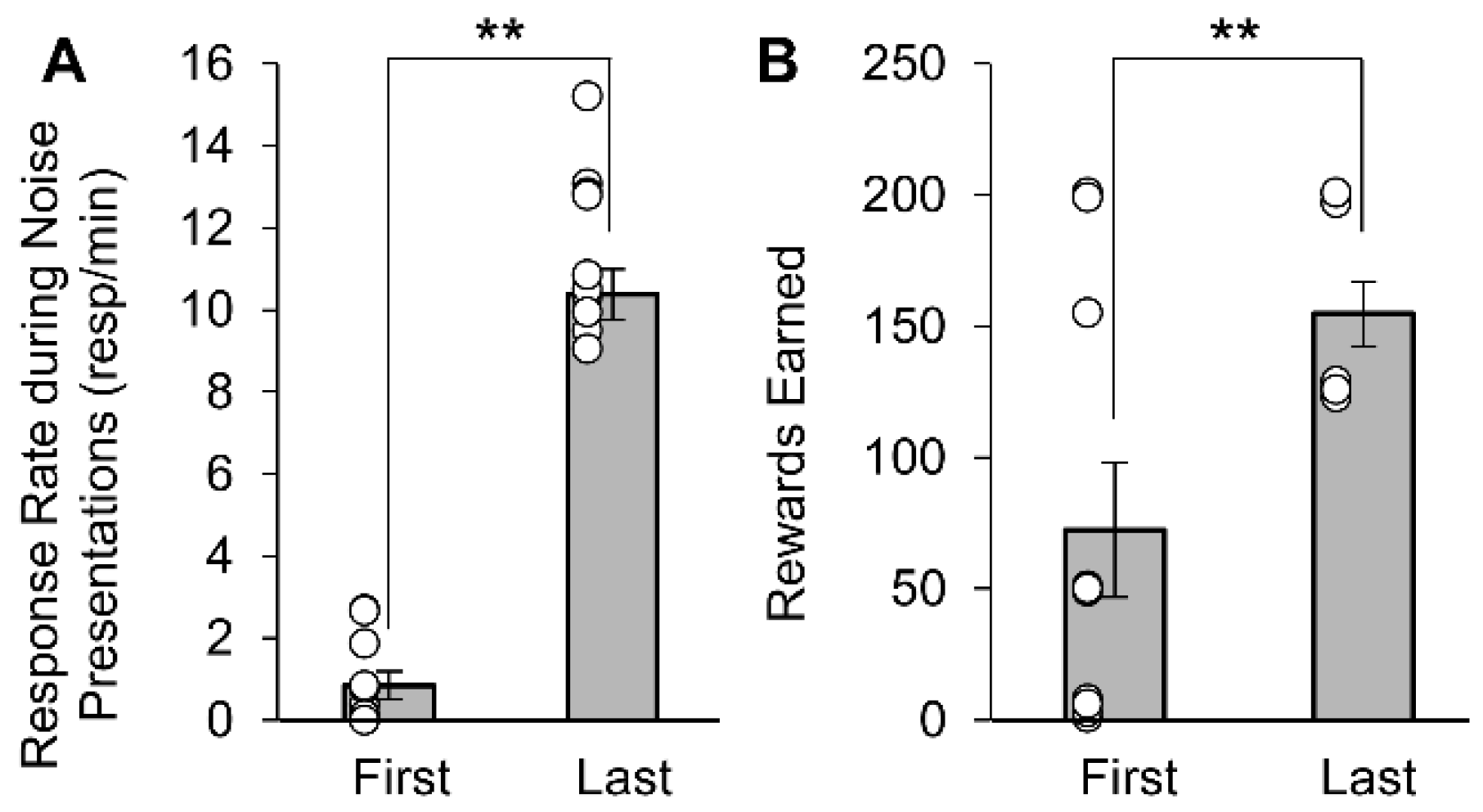
3.1. Appetitive Rats Conditioned to the Noise Stimuli
3.2. PrL Blockade of Catecholamine Reuptake Did Not Alter Timing Accuracy in Trials without Distracters
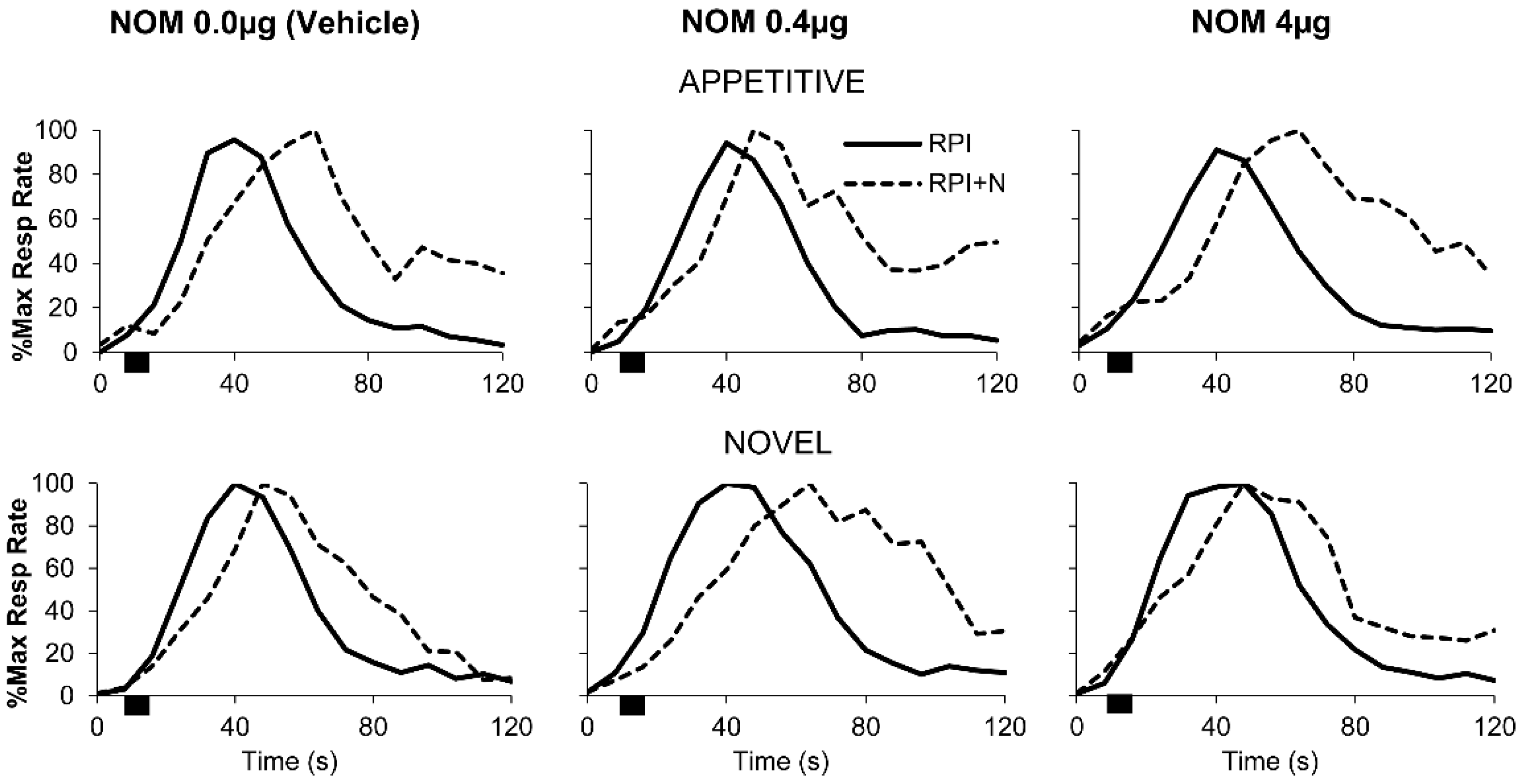
3.3. PrL Blockade of Catecholamine Reuptake Significantly Modulated Responding after Novel Distracters in an Inverted-U, Dose-Dependent Manner but Not after Familiar Appetitive Distracters
3.4. PrL Blockade of Catecholamine Reuptake did not Significantly Change the Width of the Response Functions
3.5. PrL Blockade of Catecholamine Reuptake did not Significantly Change the Coefficients of Determination (r2) of the Response Functions
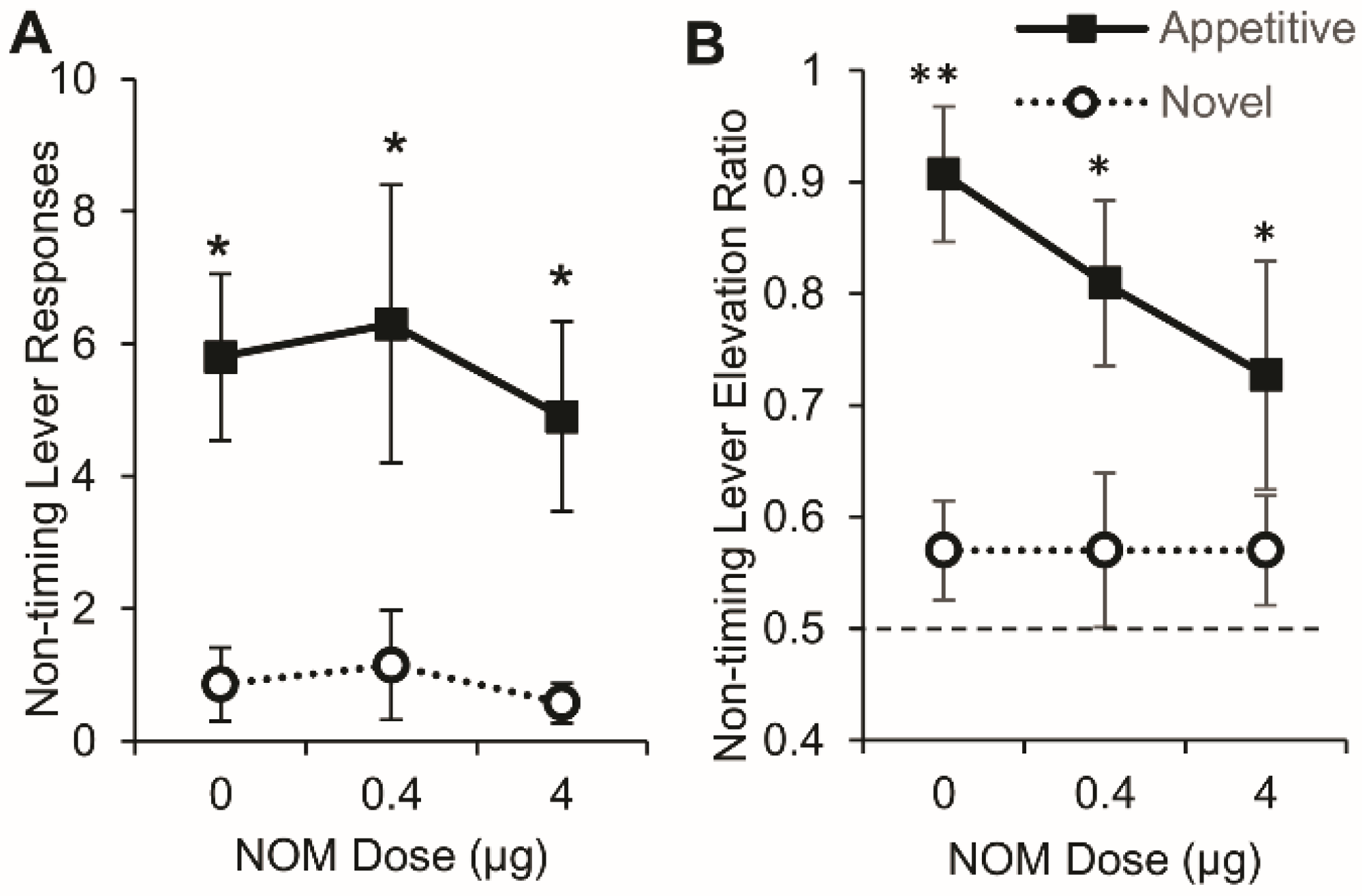
3.6. PrL Blockade of Catecholamine Reuptake did not Affect Lever Pressing on either the Timing or Nontiming Levers
3.6.1. Timing Lever
3.6.2. Nontiming Lever
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buhusi, C.V.; Meck, W.H. (Eds.) Timing Behavior; Springer: Berlin, Germany, 2010; pp. 1319–1323. [Google Scholar]
- Buhusi, C.V.; Meck, W.H. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 2005, 6, 755–765. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Oprisan, S.A.; Buhusi, M. Biological and Cognitive Frameworks for a Mental Timeline. Front. Neurosci. 2018, 12, 377. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Oprisan, S.A.; Buhusi, M. Clocks within Clocks: Timing by Coincidence Detection. Curr. Opin. Behav. Sci. 2016, 8, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.L.; Richer, P.; Doyere, V. The effect of an intruded event on peak-interval timing in rats: Isolation of a postcue effect. Behav. Process. 2007, 74, 300–310. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Meck, W.H. Time sharing in rats: A peak-interval procedure with gaps and distracters. Behav. Process. 2006, 71, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.R.; He, O.H.; Buhusi, M.; Buhusi, C.V. Dissociation of the role of the prelimbic cortex in interval timing and resource allocation: Beneficial effect of norepinephrine and dopamine reuptake inhibitor nomifensine on anxiety-inducing distraction. Front Integr. Neurosci. 2012, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Buhusi, C.V. Time-sharing in rats: Effect of distracter intensity and discriminability. J. Exp. Psychol. Anim. Behav. Process. 2012, 38, 30–39. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Meck, W.H. Relative time sharing: New findings and an extension of the resource allocation model of temporal processing. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 1875–1885. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Meck, W.H. Interval timing with gaps and distracters: Evaluation of the ambiguity, switch, and time-sharing hypotheses. J. Exp. Psychol. Anim. Behav. Process. 2006, 32, 329–338. [Google Scholar] [CrossRef]
- Buhusi, C.V. Dopaminergic Mechanisms of Interval Timing and Attention. In Functional and Neural Mechanisms of Interval Timing; Meck, W.H., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 317–338. [Google Scholar]
- Buhusi, M.; Bartlett, M.J.; Buhusi, C.V. Sex differences in interval timing and attention to time in C57Bl/6J mice. Behav. Brain Res. 2017, 324, 96–99. [Google Scholar] [CrossRef]
- Oprisan, S.A.; Dix, S.; Buhusi, C.V. Phase resetting and its implications for interval timing with intruders. Behav. Process. 2014, 101, 146–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aum, S.W.; Brown, B.L.; Hemmes, N.S. The effects of concurrent task and gap events on peak time in the peak procedure. Behav. Process. 2004, 65, 43–56. [Google Scholar] [CrossRef]
- Meck, W.H. Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Res. 2006, 1108, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.T.; Cheng, R.K.; Meck, W.H. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 2011, 36, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Merchant, H.; Harrington, D.L.; Meck, W.H. Neural basis of the perception and estimation of time. Annu. Rev. Neurosci. 2013, 36, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Buhusi, C.V.; Reyes, M.B.; Gathers, C.A.; Oprisan, S.A.; Buhusi, M. Inactivation of the Medial-Prefrontal Cortex Impairs Interval Timing Precision, but Not Timing Accuracy or Scalar Timing in a Peak-Interval Procedure in Rats. Front Integr. Neurosci. 2018, 12, 20. [Google Scholar] [CrossRef]
- Tucci, V.; Buhusi, C.V.; Gallistel, R.; Meck, W.H. Towards an integrated understanding of the biology of timing. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014, 369, 20120470. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Euston, D.R.; Gruber, A.J.; McNaughton, B.L. The role of medial prefrontal cortex in memory and decision making. Neuron 2012, 76, 1057–1070. [Google Scholar] [CrossRef]
- Buhusi, M.; Olsen, K.; Buhusi, C.V. Increased temporal discounting after chronic stress in CHL1-deficient mice is reversed by 5-HT2C agonist Ro 60-0175. Neuroscience 2017, 357, 110–118. [Google Scholar] [CrossRef]
- Kim, J.; Jung, A.H.; Byun, J.; Jo, S.; Jung, M.W. Inactivation of medial prefrontal cortex impairs time interval discrimination in rats. Front. Behav. Neurosci. 2009, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.; Droit-Volet, S. Time perception, depression and sadness. Behav. Process. 2009, 80, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.S.; Leikauf, J.E.; Holt-Gosselin, B.; Staveland, B.R.; Williams, L.M. Paying attention to attention in depression. Transl. Psychiatry 2019, 9, 279. [Google Scholar] [CrossRef]
- Koenigs, M.; Grafman, J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009, 201, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.L.; Noudoost, B. The role of prefrontal catecholamines in attention and working memory. Front. Neural Circuits 2014, 8, 33. [Google Scholar] [CrossRef]
- Maricq, A.V.; Roberts, S.; Church, R.M. Methamphetamine and time estimation. J. Exp. Psychol. Anim. Behav. Process. 1981, 7, 18–30. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Meck, W.H. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav. Neurosci. 2002, 116, 291–297. [Google Scholar] [CrossRef]
- Cheng, R.K.; MacDonald, C.J.; Meck, W.H. Differential effects of cocaine and ketamine on time estimation: Implications for neurobiological models of interval timing. Pharm. Biochem. Behav. 2006, 85, 114–122. [Google Scholar] [CrossRef]
- Lau, C.E.; Ma, F.; Foster, D.M.; Falk, J.L. Pharmacokinetic-pharmacodynamic modeling of the psychomotor stimulant effect of cocaine after intravenous administration: Timing performance deficits. J. Pharm. Exp. 1999, 288, 535–543. [Google Scholar]
- Matell, M.S.; King, G.R.; Meck, W.H. Differential modulation of clock speed by the administration of intermittent versus continuous cocaine. Behav. Neurosci. 2004, 118, 150–156. [Google Scholar] [CrossRef]
- Meck, W.H. Selective adjustment of the speed of internal clock and memory processes. J. Exp. Psychol. Anim. Behav. Process. 1983, 9, 171–201. [Google Scholar] [CrossRef] [PubMed]
- Meck, W.H. Neuropharmacology of timing and time perception. Brain Res. Cogn. Brain Res. 1996, 3, 227–242. [Google Scholar] [CrossRef]
- Matell, M.S.; Bateson, M.; Meck, W.H. Single-trials analyses demonstrate that increases in clock speed contribute to the methamphetamine-induced horizontal shifts in peak-interval timing functions. Psychopharmacology 2006, 188, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Oprisan, S.A.; Buhusi, C.V. Modeling pharmacological clock and memory patterns of interval timing in a striatal beat-frequency model with realistic, noisy neurons. Front Integr. Neurosci. 2011, 5, 52. [Google Scholar] [CrossRef]
- Katz, N.S.; Guiard, B.P.; El Mansari, M.; Blier, P. Effects of acute and sustained administration of the catecholamine reuptake inhibitor nomifensine on the firing activity of monoaminergic neurons. J. Psychopharmacol. 2010, 24, 1223–1235. [Google Scholar] [CrossRef]
- Basso, A.M.; Gallagher, K.B.; Bratcher, N.A.; Brioni, J.D.; Moreland, R.B.; Hsieh, G.C.; Drescher, K.; Fox, G.B.; Decker, M.W.; Rueter, L.E. Antidepressant-like effect of D(2/3) receptor-, but not D(4) receptor-activation in the rat forced swim test. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2005, 30, 1257–1268. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Blavet, N.; Deniel, M.; Jalfre, M. Immobility induced by forced swimming in rats: Effects of agents which modify central catecholamine and serotonin activity. Eur. J. Pharmacol. 1979, 57, 201–210. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Bayley, P.J.; Bentley, G.D.; Dawson, G.R. The effects of selected antidepressant drugs on timing behaviour in rats. Psychopharmacology 1998, 136, 114–122. [Google Scholar] [CrossRef]
- Ho, M.Y.; al-Zahrani, S.S.; Velazquez Martinez, D.N.; Lopez Cabrera, M.; Bradshaw, C.M.; Szabadi, E. Effects of desipramine and fluvoxamine on timing behavior investigated with the fixed-interval peak procedure and the interval bisection task. Psychopharmacology 1996, 125, 274–284. [Google Scholar] [CrossRef]
- Heilbronner, S.R.; Meck, W.H. Dissociations between interval timing and intertemporal choice following administration of fluoxetine, cocaine, or methamphetamine. Behav. Process. 2014, 101, 123–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [CrossRef]
- Buhusi, C.V.; Meck, W.H. Timing for the absence of a stimulus: The gap paradigm reversed. J. Exp. Psychol. Anim. Behav. Process. 2000, 26, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. (Eds.) The Rat Brain, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Roberts, S. Isolation of an internal clock. J. Exp. Psychol. Anim. Behav. Process. 1981, 7, 242–268. [Google Scholar] [CrossRef]
- Swearingen, J.E.; Buhusi, C.V. The pattern of responding in the peak-interval procedure with gaps: An individual-trials analysis. J. Exp. Psychol. Anim. Behav. Process. 2010, 36, 443–455. [Google Scholar] [CrossRef]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Matthews, A.R. Effect of distracter preexposure on the reset of an internal clock. Behav. Process. 2014, 101, 72–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carboni, E.; Imperato, A.; Perezzani, L.; Di Chiara, G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience 1989, 28, 653–661. [Google Scholar] [CrossRef]
- Cass, W.A.; Gerhardt, G.A. In vivo assessment of dopamine uptake in rat medial prefrontal cortex: Comparison with dorsal striatum and nucleus accumbens. J. Neurochem. 1995, 65, 201–207. [Google Scholar] [CrossRef]
- Hunt, P.; Raynaud, J.P.; Leven, M.; Schacht, U. Dopamine uptake inhibitors and releasing agents differentiated by the use of synaptosomes and field-stimulated brain slices in vitro. Biochem. Pharm. 1979, 28, 2011–2016. [Google Scholar] [CrossRef]
- Hoffmann, I. Pharmacology of nomifensine. Int. Pharm. 1982, 17, 4–20. [Google Scholar] [CrossRef]
- Schacht, U.; Heptner, W. Effect of nomifensine (HOE 984), a new antidepressant, on uptake of noradrenaline and serotonin and on release of noradrenaline in rat brain synaptosomes. Biochem. Pharm. 1974, 23, 3413–3422. [Google Scholar] [CrossRef]
- Maricq, A.V.; Church, R.M. The differential effects of haloperidol and methamphetamine on time estimation in the rat. Psychopharmacology 1983, 79, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Wittenborn, J.R.; Flaherty, C.F., Jr.; McGough, W.E.; Bossange, K.A.; Nash, R.J. A comparison of the effect of imipramine, nomifensine, and placebo on the psychomotor performance of normal males. Psychopharmacology 1976, 51, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Blasi, G.; Taurisano, P.; Papazacharias, A.; Caforio, G.; Romano, R.; Lobianco, L.; Fazio, L.; Di Giorgio, A.; Latorre, V.; Sambataro, F.; et al. Nonlinear response of the anterior cingulate and prefrontal cortex in schizophrenia as a function of variable attentional control. Cereb. Cortex 2010, 20, 837–845. [Google Scholar] [CrossRef][Green Version]
- Vijayraghavan, S.; Wang, M.; Birnbaum, S.G.; Williams, G.V.; Arnsten, A.F. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007, 10, 376–384. [Google Scholar] [CrossRef]
- Zahrt, J.; Taylor, J.R.; Mathew, R.G.; Arnsten, A.F. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J. Neurosci. Off. J. Soc. Neurosci. 1997, 17, 8528–8535. [Google Scholar] [CrossRef]
- Tatsumi, M.; Groshan, K.; Blakely, R.D.; Richelson, E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 1997, 340, 249–258. [Google Scholar] [CrossRef]
- Blakely, R.D.; Bauman, A.L. Biogenic amine transporters: Regulation in flux. Curr. Opin. Neurobiol. 2000, 10, 328–336. [Google Scholar] [CrossRef]
- Richelson, E. The newer antidepressants: Structures, pharmacokinetics, pharmacodynamics, and proposed mechanisms of action. Psychopharmacol. Bull. 1984, 20, 213–223. [Google Scholar]
- Richelson, E.; Nelson, A. Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J. Pharm. Exp. 1984, 230, 94–102. [Google Scholar]
- Borsini, F.; Bendotti, C.; Velkov, V.; Rech, R.; Samanin, R. Immobility test: Effects of 5-hydroxytryptaminergic drugs and role of catecholamines in the activity of some antidepressants. J. Pharm. Pharm. 1981, 33, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Racke, K.; Sommer, M.; Burns, F.; Hering, B. Differential effects of electrical stimulation, blockade of neuronal amine uptake and activation of alpha 2-adrenoceptors on the release of endogenous noradrenaline and 5-hydroxytryptamine from the isolated rat pineal gland. Naunyn. Schmiedebergs. Arch. Pharm. 1991, 343, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Spencer, R.C. Differential cognitive actions of norepinephrine a2 and a1 receptor signaling in the prefrontal cortex. Brain Res. 2016, 1641, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, E.N. Higher nervous functions; the orienting reflex. Annu. Rev. Physiol. 1963, 25, 545–580. [Google Scholar] [CrossRef]
- Sargolini, F.; Roullet, P.; Oliverio, A.; Mele, A. Effects of lesions to the glutamatergic afferents to the nucleus accumbens in the modulation of reactivity to spatial and non-spatial novelty in mice. Neuroscience 1999, 93, 855–867. [Google Scholar] [CrossRef]
- Kety, S.S. The biogenic amines in the central nervous system: Their possible roles in arousal, emotion and learning. In The Neurosciences Second Study Program; Schmitt, F.O., Ed.; Rockefeller University Press: New York, NY, USA, 1970; pp. 324–335. [Google Scholar]
- Bodnoff, S.R.; Suranyi-Cadotte, B.; Quirion, R.; Meaney, M.J. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology 1989, 97, 277–279. [Google Scholar] [CrossRef]
- Lubow, R.E.; Gewirtz, J.C. Latent inhibition in humans: Data, theory, and implications for schizophrenia. Psychol. Bull. 1995, 117, 87–103. [Google Scholar] [CrossRef]
- Lubow, R.E. Latent inhibition: Effects of frequency of nonreinforced preexposure of the CS. J. Comp. Physiol. Psychol. 1965, 60, 454–457. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Paskalis, J.P.; Cerutti, D.T. Time-sharing in pigeons: Independent effects of gap duration, position and discriminability from the timed signal. Behav. Process. 2006, 71, 116–125. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Sasaki, A.; Meck, W.H. Temporal integration as a function of signal and gap intensity in rats (Rattus norvegicus) and pigeons (Columba livia). J. Comp. Psychol. 2002, 116, 381–390. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Perera, D.; Meck, W.H. Memory for timing visual and auditory signals in albino and pigmented rats. J. Exp. Psychol. Anim. Behav. Process. 2005, 31, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Lubow, R.E. Latent Inhibition and Conditioned Attention Theory; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Buhusi, C.V.; Gray, J.A.; Schmajuk, N.A. Perplexing effects of hippocampal lesions on latent inhibition: A neural network solution. Behav. Neurosci. 1998, 112, 316–351. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Honey, R.C. Involvement of the rat medial prefrontal cortex in novelty detection. Behav. Neurosci. 2002, 116, 498–503. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Chiang, C.; Alexinsky, T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog. Brain Res. 1991, 88, 501–520. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthews, A.R.; Buhusi, M.; Buhusi, C.V. Blockade of Catecholamine Reuptake in the Prelimbic Cortex Decreases Top-Down Attentional Control in Response to Novel, but not Familiar Appetitive Distracters, within a Timing Paradigm. NeuroSci 2020, 1, 99-114. https://doi.org/10.3390/neurosci1020010
Matthews AR, Buhusi M, Buhusi CV. Blockade of Catecholamine Reuptake in the Prelimbic Cortex Decreases Top-Down Attentional Control in Response to Novel, but not Familiar Appetitive Distracters, within a Timing Paradigm. NeuroSci. 2020; 1(2):99-114. https://doi.org/10.3390/neurosci1020010
Chicago/Turabian StyleMatthews, Alexander R., Mona Buhusi, and Catalin V. Buhusi. 2020. "Blockade of Catecholamine Reuptake in the Prelimbic Cortex Decreases Top-Down Attentional Control in Response to Novel, but not Familiar Appetitive Distracters, within a Timing Paradigm" NeuroSci 1, no. 2: 99-114. https://doi.org/10.3390/neurosci1020010
APA StyleMatthews, A. R., Buhusi, M., & Buhusi, C. V. (2020). Blockade of Catecholamine Reuptake in the Prelimbic Cortex Decreases Top-Down Attentional Control in Response to Novel, but not Familiar Appetitive Distracters, within a Timing Paradigm. NeuroSci, 1(2), 99-114. https://doi.org/10.3390/neurosci1020010



