1. Introduction
Grape is one of the world’s most widely developed fruit crops, with a reported annual production higher than 70 million tons. Around fifty per cent of grape production is transformed into wine and the main wine making by-product is represented by grape pomace (15–20% of the total grape weight). It is estimated that up to seven million tons grape pomace are generated annually worldwide [
1].
Winery by-products, such as grape skin, pomace, and vine leaves, are a promising source of phenolic compounds to be used as functional food ingredients in antioxidant dietary supplements. Its use would allow value to be created from wastes that are considered worthless nowadays and generated on a large scale by winemaking. Red grape skin is rich in anthocyanins and flavonols. The anthocyanins identified in V. Vinifera spp. Correspond to the 3-O-monoglucosides and the 3-O-acylated monoglucosides of the five main anthocyanidins: delphinidin, cyanidin, petunidin, peonidin, and malvidin. Acylation occurs at the C-6 position of the glucose molecule through esterification with acetic, p-coumaric, and caffeic acids [
2].
To extract anthocyanins from grape skins, polar solvents are used. For analysis, methanol–water acidified, or ethanol–water acidified (with HCl or acetic acid) is employed [
3,
4]. For food applications, ethanol–water is preferred due to its lower toxicity. In some cases, acetone–water or diluted hydrochloric acid is used to enhance extraction.
Recently, different deep eutectic solvents have been proposed as alternative extraction media. Eutectic solvents are mixtures that remain liquid at a desired temperature, even though at least one of their components would otherwise be a solid and unsuitable for use as a solvent. Deep eutectic solvents are composed of hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs), forming mixtures where the eutectic temperature is lower than that of an ideal system, exhibiting negative deviations from thermodynamic ideality [
5].
The use of DES has been proposed as a sustainable approach for the extraction of bioactive compounds from medicinal plants and agri-food industry wastes and as a safe alternative for nutritional, pharmaceutical, and cosmetic applications [
6,
7,
8]. However, numerous DESs have been reported to present limitations; for instance, those that are ethylene glycol-based may be toxic, or their use is restricted by regulations, such as choline chloride, whose use is banned in cosmetic products (Regulation (EC) No 1223/2009).
Brunetti et al. [
9] have reviewed the DES possibilities in food industry by-products utilization (artichoke bracts and leaves, grape pomace, and other agricultural by-products) as starting material for green feed formulations in poultry feeding. Recently, Kaoui et al. [
10] reviewed the applications of (natural) deep eutectic solvents as extraction solvents for bioactive compounds from plants and food samples. Moreover, the use of DES as a green and sustainable alternative to less safe solvents is already being incorporated for extracting bioactive phytochemicals on an industrial scale, for example, in the cosmetics industry [
11]. Despite that, in many of the studies included in these reviews, the use of choline chloride is proposed.
Polyphenols are the target compounds in many of the DES extraction applications. For instance, choline chloride-based DES with different hydrogen bond donors such as citric and lactic acid [
12], D-(+)-maltose [
13], malic acid and sugars [
14], propylene glycol [
15], oxalic acid [
16], and tartaric acid [
17] have been proposed as green solvents for the extraction of anthocyanins from grape skin and other fruit by-products. Additionally, choline chloride:citric acid and choline chloride:glucose DES have been reported for the extraction of other phenolic compounds from grape skins and seeds [
18,
19]. Choline chloride:levulinic acid:ethylene glycol (1:1:2) and choline chloride:proline:malic acid (1:1:1) have been reported as the DES providing the most optimal yield for the ultrasound-assisted extraction of phenolic acids and flavan-3-ols, respectively, from grape skins and seeds [
20]. Punzo et al. [
21] have investigated some properties, such as antioxidant and anti-inflammatory activities of red grape pomace extract obtained with betaine-based DES in combination with citric acid, urea, and ethylene glycol.
The recovery of anthocyanins from residues of blueberry processing using DES has also been reported by several authors [
22,
23,
24,
25]. For instance, Da Silva et al. [
24] propose a choline chloride:glycerol:citric acid-based DES for anthocyanin extraction from blueberry with a yield of 76% related to exhaustive extraction using a conventional organic solvent (50:48.5:1.5 (
v/
v) of CH
3OH/H
2O/HCOOH, 0.1:24 (
w/
v), sample:solvent ratio). On the other hand, Fu et al. [
25] obtained the highest yield of anthocyanins (9.32 ± 0.08 mg/g) from blueberry wine residues by ultrasound-assisted extraction with a water content of 29%, ultrasonic power of 380 W, extraction temperature of 55 °C, and extraction time of 40 min.
Given the vast number of possible hydrogen bond acceptors and hydrogen bond donor combinations that can form a deep eutectic solvent, silico methods have been employed to identify the most suitable DES for extraction processes. Among these methods, the COSMO-SAC (Conductor-like Screening Model for Segment Activity Coefficient) and COSMO-RS (Conductor-like Screening Model for Real Solvents) models are commonly used. A key selection parameter in these models is the activity coefficient at infinite dilution (γ∞), which provides an estimate of the solute’s solubility potential in the solvent. Typically, a low log γ∞ indicates a higher solubilization potential and, consequently, a greater likelihood of successful extraction.
For instance, Cui et al. [
26] evaluated the extractability of green tea polyphenols in various solvents using epigallocatechin gallate as a model solute. Their study revealed that DES formed by combining a strong HBA, such as choline chloride, with a relatively weaker HBD, like ethylene glycol, exhibited a strong affinity for this polyphenol. Similarly, Goltz et al. [
27] applied COSMO-RS to identify the optimal DES for extracting quercetin from chamomile. Among the evaluated DES, choline chloride:glycerol:water was determined to be the most effective in extracting quercetin and other phenolic compounds.
The COSMO-RS model has also been extensively applied in solvent selection for flavonols and flavonol glycosides. The first study to be reviewed here should be Wojeicchowski et al. [
28]. Zurob et al. [
29] designed an extraction process for hydroxytyrosol from olive leaves using COSMO-RS to screen natural DES. Based on log γ values, mixtures of choline chloride (as HBA) with citric acid or lactic acid (as HBD) were identified as the most effective modifiers for extracting anthocyanins from black bean hulls using subcritical water [
12]. Lazarovic et al. [
30] assessed the extraction efficiency of DES for flavonols such as isoquercetin, rutin, quercitrin, astragalin, and quercetin from Agrimonia eupatoria by calculating their γ∞ values in nine different DES.
Additionally, Benvenutti et al. [
15] combined silico and experimental approaches to select DES compositions for the selective and sequential extraction of anthocyanins and pectin from Myrciaria cauliflora fruit, identifying choline chloride:propylene glycol as the most promising DES for anthocyanin extraction. More recently, Mesquita et al. [
31] successfully recovered anthocyanins from grape pomace using an aqueous solution of a eutectic solvent composed of nicotinamide and acetic acid. Their screening process, aided by COSMO-RS, evaluated nearly 2000 potential DES formulations. The optimized process achieved high anthocyanin yields (21 ± 1 mg anthocyanins per g of biomass).
In this study, the objective was to optimize anthocyanin extraction from red grape skin. Here, in silico studies were combined with design of experiments (DoE) to identify the optimal DES composition and extraction conditions for anthocyanins from red grape skin. Following the in silico screening, choline chloride, betaine, sodium acetate, potassium carbonate, and sodium propionate were evaluated as hydrogen bond acceptors and citric acid, lactic acid, 1,2-propanediol, glycerol, malic acid, oxalic acid, ethyleneglycol, and 1,6-hexanediol were evaluated as hydrogen bond donors with different molar ratios and percentages of water. As a novelty, ultrasound-assisted extraction (USAE) and microwave-assisted extraction (MAE) were compared for grape skin anthocyanin extraction, and the percentage of water in DES composition, temperature, and time of the extractions were optimized.
2. Materials and Methods
2.1. Samples
Red grape skins come from red berries collected during the September 2023 harvest in “Finca La Grajera” (Burgos Road, km 6, La Grajera, 26071, Logroño, La Rioja, Spain). La Grajera is an estate where ICVV (Institute of Grapevine and Wine Science
https://www.icvv.es/english/) is located. The skins were obtained by pressing the wine after fermentation, then they were dried and separated with sieves. The grape variety was Tempranillo. The material was crushed and homogenized with a kitchen mincer and frozen at −20 °C until use. Before proceeding with extraction, the skins were thawed. Moist skins (71 ± 4 wt% water) were extracted without prior drying. The water content in the skin was determined gravimetrically after removing the water by drying in an oven at 70 °C for 48 h.
2.2. Materials
Delphinidin-3-O-glucoside chloride (Del-3G), cyanidin-3-O-glucoside chloride (Cya-3G), petunidin-3-O-glucoside chloride (Pet-3G), peonidin-3-O-glucoside chloride (Peo-3G), and malvidin-3-O-glucoside chloride (Mal-3G) standards were obtained from Sigma-Aldrich (St. Louis, MO, USA). Individual standard solutions were prepared in methanol containing 100 µg/mL of each anthocyanin. Calibration solutions were prepared by diluting with a MilliQ water/acetonitrile/formic acid (85.5/10/4.5) mixture.
For the preparation of DES, choline chloride (>98%, Sigma Aldrich), betaine (>98%, Sigma Aldrich), potassium carbonate anhydrous (>99.5%, Fisher Scientific, Madrid, Spain), sodium acetate anhydrous (>99%, Carlo Ebra Reagents, Barcelona, Spain), and sodium propionate (>99%, Sigma Aldrich) were used as hydrogen bond acceptorsand the following compounds were selected as hydrogen bond donors: acid citric anhydrous (>99.5%, Carlo Ebra Reagents), L(+)-lactic acid (>99%, Carlo Erba Reagents), oxalic acid (>98%, Sigma Aldrich), DL-malic acid (>99%, Sigma Aldrich), 1,6-hexanediol (>97%, Sigma Aldrich), 1,2-propanediol (>99.5%, Sigma Aldrich), glycerol (>99.5%, Fisher Scientific), and ethylene glycol (>99.5%, Carlo Erba Reagents).
Supra-gradient acetonitrile, LC-MS methanol from Scharlab (Barcelona, Spain) and formic acid (>98%) from Fluka analytical were used.
2.3. COSMO-RS Simulations
Calculations using the Conductor-like Screening Model for Real Solvents (COSMO-RS) were carried out in a two-step process. Initially, the geometry of each molecule (HBA, HBD, and anthocyanin models) was optimized with the COSMO-BP-TZVP approach, utilizing the Turbomole software package (TmoleX19) (Dassault Systemes, Paris, France). Subsequently, the polarization charges (σ) and surface composition functions, p(σ), of the optimized molecules were determined using the COSMOtherm
® 21 software (Dassault Systemes, Paris, France), employing the BP_TZVP_21.ctd parameterization [
32]. The COSMO-BP-TZVP model integrates a def-TZVP basis set, DFT at the B-P83 functional level, and the COSMO solvation model. Following this, the σ-potentials of the DES components (HBA and HBD in a 1:1 molar ratio at 298.2 K) and the anthocyanin models (malvidin-3-O-glucoside and malvidin-3-O-(6-p-coumaroyl)glucoside) were used to evaluate the thermodynamic interactions among these molecules through the activity coefficient at infinite dilution (γ∞).
2.4. DES Preparation
Table 1 lists the compounds and mole ratios used to prepare the different DES used in this work. The mixtures were heated at 80 °C in a beaker for about 2–4 h until a homogeneous transparent liquid was obtained. After that, 30 wt% water was added to reduce the viscosity of the DES in order to be able to handle it.
Moreover, HBD liquids (glycerol and lactic acid) were mixed with 30 wt% water and stirred until a homogeneous transparent liquid was obtained.
2.5. Extractions of Grape Skins
Two extraction techniques were used in this study: ultrasound and microwave-assisted extraction.
Ultrasound-assisted extraction was used to assess the anthocyanin extraction yields. This technique is faster and easier than microwave-assisted extraction, which is why it was used to experimentally evaluate the extraction power of different DES previously selected according to in silico results. Experimentally, the best DES was the one that led to the highest extraction rate.
Moreover, the aqueous percentage and extraction time were optimized for DES 4 using a design of experiments based on the response surface methodology carried out with software Statgraphics Centurion XV.
An amount of 2.5 g of wet skin was weighed in a Falcon tube, and 10 g of DES were added. The mixture was sonicated at room temperature for 30 min, except in the case of DES 5, whose temperature was 40 °C, as this is the lowest temperature at which it could be kept stable, and then centrifuged at 9000 rpm for 15 min. The supernatant was separated, and 1 g of the extract obtained was diluted in a 10 mL volumetric flask with the mobile phase MilliQ water/acetonitrile/formic acid (85.5/10/4.5). The solution was filtered through a 0.22 µm Nylon syringe filter before analysis by HPLC. In the same way, the extraction was carried out with methanol/MilliQ water/formic acid (79:20:1,
v/
v/
v) to compare the results with this solvent traditionally used in polyphenols extractions [
3].
On the other hand, the extraction parameters of microwave-assisted extraction were also optimized based on the response surface methodology for DES 5. A sample of 2.5 g of wet skin was weighed and mixed with 10 g of DES in a sealed vial and processed in a Mars 5 microwave extractor (CEM corporation, Matthews, NC, USA). Extractions were performed at 800 W, 100% power, with temperature and time kept in the range: 40–80 °C and 10–45 min. Then, the mixtures were centrifuged at 9000 rpm for 15 min. The supernatant was separated, and 1 g of the extract obtained was diluted in a 10 mL volumetric flask with the mobile phase MilliQ water/acetonitrile/formic acid (85.5/10/4.5). The solution was filtered through a 0.22 µm nylon syringe filter before analysis by HPLC.
2.6. Design of Experiments
DES 4 and DES 5 were selected to optimize the extraction conditions: time and percentage of water for ultrasound-assisted extraction, and temperature, time, and percentage of water for microwave-assisted extraction.
To establish the most favorable extraction conditions, the sonication time and the water concentration in the DES 4 were studied with ranges of 5–60 min and 20–80%, respectively. This study was carried out using a design of experiments based on the response surface methodology.
Table S4 shows 12 experiments that correspond to a 2
2-factorial design of 2 factors at 2 levels (−1 and 1), 4-star points located at a distance α = 1.414 from the center, in order to obtain a rotatable design, and 4 central points.
In addition, for microwave-assisted extraction, the experimental domain studied was 10–45 min, 20–50% of water, and 30–80 °C.
Table S5 (in Supplementary Materials) shows the 18 experiments that correspond to a 2
3-factorial design of 3 factors at 2 levels (−1 and 1), 6-star points located at a distance α = 1.682 from the center, in order to achieve a rotatable design, and 4 central points.
2.7. Analysis of the Extracts by HPLC-DAD
The determination of anthocyanins in the extracts was performed using an Agilent 1100/1200 modular liquid chromatography system (Agilent Technologies, Palo Alto, CA, USA) composed of a G1379A degasser, a G1311A HPLC quaternary pump, a G1329A autosampler, and a G1315D diode array detector. The chromatographic separation was performed with a Phenomenex Luna
® 5 µm C18 100 Å column (150 mm × 4.6 mm i.d.) using a mobile phase formed by a mixture of water/formic acid (95:5,
v/
v) (solvent A) and acetonitrile (solvent B). The composition of the mobile phase varied according to the following gradient program: 10% B for 3 min, 10–20% B linear for 3–10 min, 20–50% linear for 10–15 min, 50–100% B linear for 15–20 min, 100% B for 20–25 min. The injection volume was 20 μL, separation was performed at room temperature, and detection was performed at 520 nm.
Figure S1 shows a chromatogram corresponding to the standard and a grape skin extract.
Calibration curves were prepared using standard solutions of delphinidin-3-O-glucoside chloride, cyanidin-3-O-glucoside chloride, petunidin-3-O-glucoside chloride, peonidin-3-O-glucoside chloride, and malvidin-3-O-glucoside chloride with concentrations ranging from 0.5 to 4.5 µg/mL of each anthocyanin. The malvidin derivative: malvidin-3-O-(6-O-p-coumaroyl)-glucoside (Mal-3G-cou), was quantified by a malvidin-3-O-glucoside chloride calibration curve considering the molecular weight adjustment. Regression parameters of the calibration curves are summarized in
Table S1.
2.8. Analysis of the Extracts by UPLC-MS/MS
A Shimadzu Nexera ultra high-performance liquid chromatograph (Shimadzu Corporation, Kyoto, Japan), coupled to a Sciex 3200QTRAP® mass spectrometer (Sciex, Framingham, MA, USA) was used for the identification of anthocyanins in grape skin extract. The analytical column was a Water Acquity BEH C18 (100 mm × 2.1 mm i.d., 1.7 µm) equipped with a VanGuardTM precolumn Acquity BEH C18 (5 mm × 2.1 mm, 1.7 µm), both from Waters. The mobile phase consisted of a gradient of 2% formic acid in water (A) and 2% formic acid in acetonitrile (B), ranging from 1% B to 90% B over 15 min, with several steps of isocratic and ramp segments. The flow rate was 0.45 mL/min, and the injection volume was 2.5 µL.
Tandem MS analysis was carried out with a 3200QTRAP triple quadrupole mass spectrometer (AB Sciex, Framingham, MA, USA) equipped with an electrospray ionization source (ESI Turbo VTM source). Ionization was performed in positive mode. Ionization source parameters were 4.5 kV ion spray voltage and 700 °C source temperature. Nitrogen (>99.99% purity) was used as the source and collision gas.
Data were acquired through multiple reaction monitoring (MRM). The two most sensitive MRM transitions were used. MRM transitions and parameters are listed in
Table S2. In addition, a chromatogram was acquired in scan mode in order to identify degradation products. Data acquisition and quantification were carried out with the Analyst
® 1.7.3 and Sciex OS MQ 3.0 software (Sciex, Framingham, MA, USA).
3. Results and Discussion
3.1. COSMO-RS Simulations
Several studies in the literature explore DES composition through experimental screening [
33,
34,
35,
36,
37,
38,
39]. However, in this work, in silico studies were employed to reduce the experimental workload. The activity coefficient at infinite dilution (
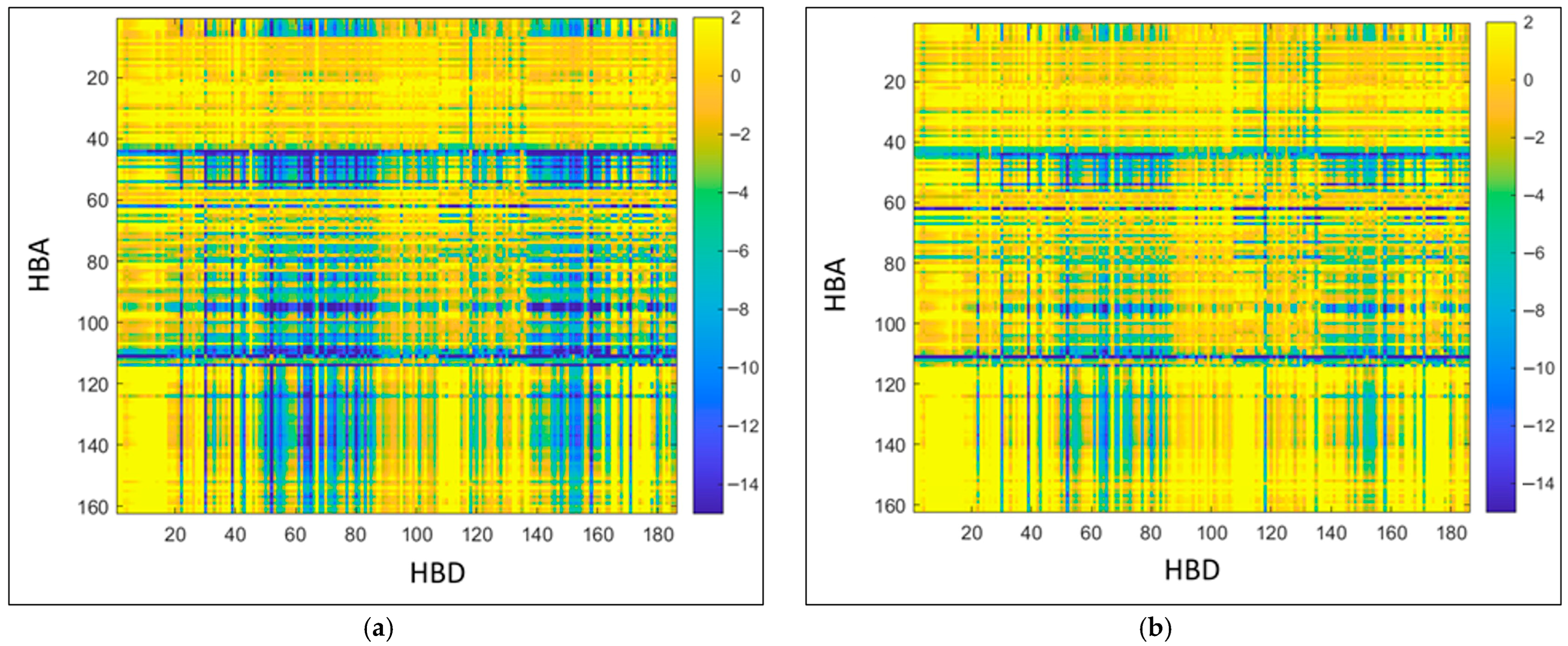
)) of two anthocyanin model compounds, malvidin-3-O-glucoside and malvidin-3-O-(6-p-coumaroyl)glucoside, was evaluated for a comprehensive set of DES, comprising 162 hydrogen bond acceptors (HBA) and 186 hydrogen bond donors (HBD), totaling 30,132 combinations.
Figure 1 and
Table S3A present the results, where the horizontal axis denotes the HBD, and the vertical axis represents the HBA. According to Huggins [
40], lower
) values indicate a higher solvation capacity of the solvent. The α-surface and the α-profile of the model molecules Mal-3G and Mal-3G-cou are shown in
Figure S2.
As illustrated in
Figure 1, DES exhibiting the highest dissolution potential for anthocyanins are identified in the blue regions, whereas those with the lowest dissolution capacity appear in the yellow areas. The solubility trends for both anthocyanin models follow a similar pattern. The most effective HBAs for anthocyanin dissolution include choline chloride (68), sodium acetate (113), sodium propionate (112), and potassium carbonate (111), particularly when paired with carboxylic acids (citric (63), malic (72), lactic (33), formic (65), oxalic (38)) or polyalcohols (glycerol (20), ethylene glycol (19), 1,2-propanol (3), 1,6-hexanediol (7)) as HBDs.
Conversely, DESs composed of sugars (e.g., sorbitol (90), sucrose (105)) and fatty acids (e.g., myristic acid (36), oleic acid (38)) exhibit the highest ) values, indicating a lower dissolution capacity. Additionally, compounds such as choline fluoride (62), tetrabutylphosphonium chloride (43), and tetrabutylammonium chloride (44) theoretically possess good solvent properties. However, these were excluded from consideration due to their toxicity and/or high cost.
3.2. Identification of Anthocyanins in Extracts
During the analysis of the extracts, eight main peaks were detected in the chromatogram (
Figure S1). Four of them were identified with the corresponding analytical standards (Del-3G, Pet-3G, Peo-3G, and Mal-3G). Cya-3G was not detected in the extracts.
In order to identify the rest of the peaks detected in the chromatogram, the DES 5 extract was analyzed by Ultra-Performance Liquid Chromatography-Triple Quadrupole (UPLC-QQQ). The larger peak at 11.4 min (
Figure S1b) was identified as malvidin-3-O-(6-O-p-coumaroyl)-glucoside (Mal-3G-cou). The rest of the peaks detected between 10 min and 11 min were tentatively identified as: delphinidin-3-O-(6-O-p-coumaroyl)-glucoside cis and trans (Del-3G-cou), malvidin-3-O-(6-O-acetyl)-glucoside (Mal-3G-ace), malvidin-3-O-(6-O-caffeoyl)-glucoside (Mal-3G-caf), cyanidin-3-O-(6-O-p-coumaroyl)-glucoside (Cya-3G-cou), and petunidin-3-O-(6-O-p-coumaroyl)-glucoside (Pet-3G-cou).
Table S2 summarizes the m/z and transitions for the identifications.
3.3. Experimental Performance of Selected DES
Considering the COSMO-RS screening results, an experimental evaluation of the DES was conducted.
Table 1 summarizes the DES systems experimentally evaluated. According to the in silico study using COSMO-RS, the solubility of Mal-3G and Mal-3G-cou in potassium carbonate, sodium acetate, and sodium propionate is higher than those in choline chloride and betaine-based DES reported for extracting anthocyanins (
Figure 1,
Table S3A). Therefore, the mixture of these HBAs with glycerol and lactic acid as HBD was included in the study for DES evaluation. Previously reported molar ratios for these mixtures [
41,
42,
43,
44] were adopted for this study, as many of these molar ratios’ combinations do not form a liquid mixture at room temperature (
Table S3B).
Grape skins were extracted with methanolic extractant (methanol/MilliQ water/formic acid, 79:20:1,
v/
v/
v) and with the different DES prepared (all with 30% (
w/
w) water). The percentage of water for experimental screening was selected based on the literature. Fu et al. [
25] studied the extraction efficiency influenced by water content and its correlation with physicochemical properties, reporting 30% water as optimal in their studies.
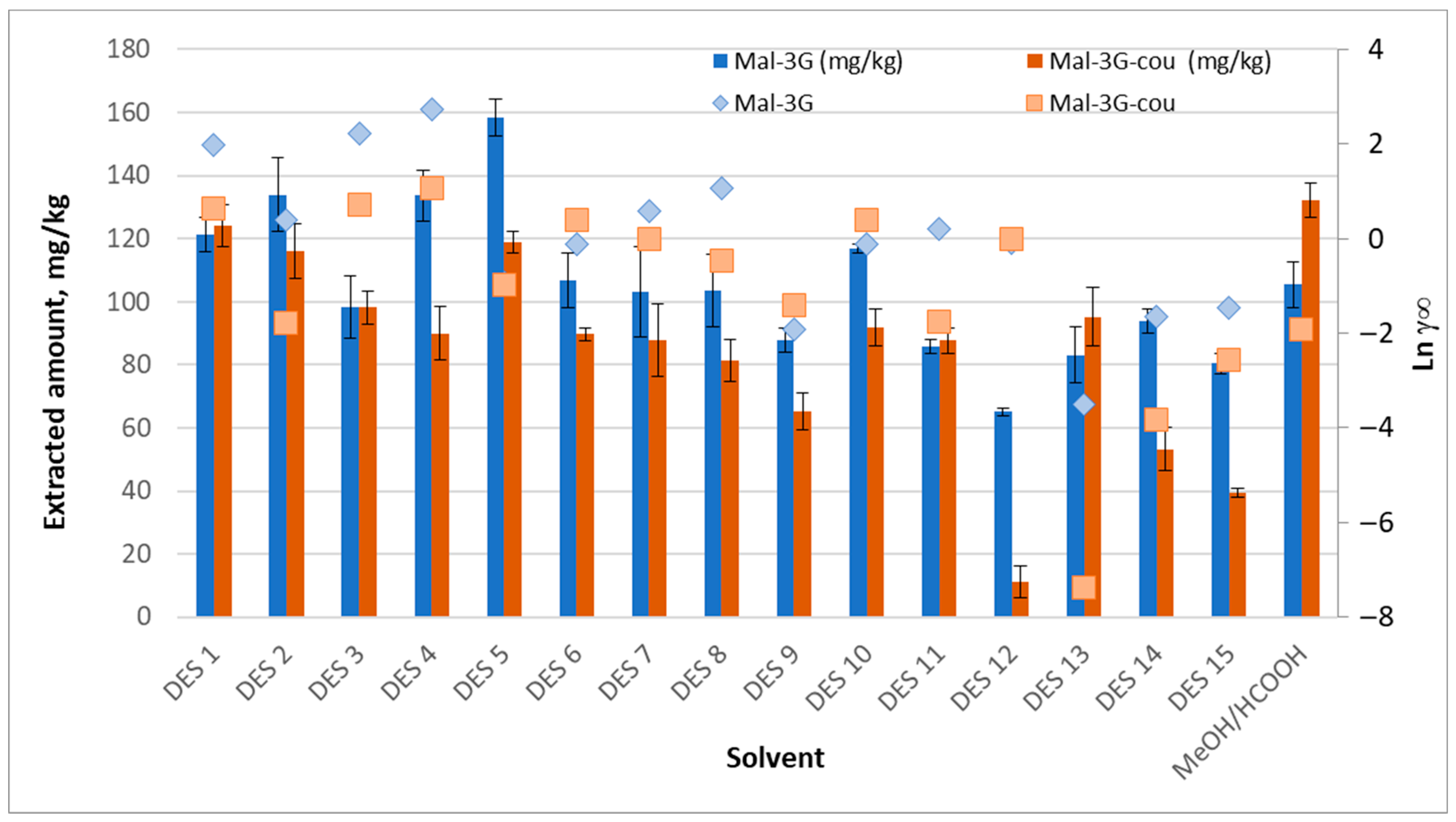
Figure 2 shows the extracted amounts of the two main anthocyanins presented in the grape skin analyzed, Mal-3G and Malv-3G-cou, with the different solvents studied and their respective
) values. The extracted amounts for Del-3G, Pet-3G, and Peo-3G are shown in
Figure S3. The extracts were red in all cases, except for those obtained with potassium carbonate-based DES, which were dark blue, indicating the presence of the flavylium ion at pH > 6. However, upon dilution in the acidic mobile phase, these extracts turned red.
The DES that showed larger amounts of anthocyanins extracted were DES 1, 2, 4, and 5. DES 1 was discarded for food industry applications because of the toxicity of ethyleneglycol. In view of the results, polyalcohols (ethyleneglycol, glycerol, and 1,2-propanediol) seem to function better as HBD than carboxylic acids (citric, lactic, and malic acid). Worthy of noting is the comparison of 1,6-hexanediol (DES 3) and ethyleneglycol (DES 1), which shows that the alkyl chain of the solvents influences the extractions. Based on the results obtained, the longer the alkyl chain, the worse the anthocyanins are extracted. Moreover, the number of hydroxyl groups in the alkyl chain might also affect the extraction yields. Glycerol, as HBD in DES 4, extracted more Mal-3G than ethyleneglycol (DES 1), while DES 1 was a better extractant of Mal-3G-cou than DES 4. This can be explained based on the polarity of the solvent and the anthocyanin. Glycerol is more polar than ethyleneglycol and 1,6-hexanediol. Additionally, Mal-3G is more polar than Mal-3G-cou. In most cases, choline chloride DESs are more effective than the traditional methanolic extractant for anthocyanins; however, this changes in the case of Mal-3G-cou. In addition, comparing DES 10 (ChCl:CA) and DES 11 (Bet:CA), it can be concluded that choline chloride is more effective as HBA for extracting anthocyanins than betaine.
On the other hand, according to in silico studies, potassium carbonate with glycerol (1:1) presents one of the most negative
) values (
Table S3A). The molar ratios selected in this work were based on the best results reported in the literature according to the stability of DES and the extraction yields [
33,
34,
35,
36,
37,
38,
41,
42,
43,
44,
45]. To compare the experimental yield values with the theoretical predictions for the mixtures used, the
) values corresponding to the actual HBA:HBD ratios in the experiments were also calculated (
Table S3B) and are presented in
Figure 2 (right
y-axis). As can be seen, the
) values at the actual HBA:HBD ratios fit better with the experimentally observed trend than those reported in
Table S3A. However, DES 13 and 14, with molar ratios 1:5 and 1:10, respectively, did not show significantly higher extraction yields compared with other solvents. A possible explanation could be that anthocyanin extraction from grape skins requires a low pH, which is not achieved with potassium carbonate, and that the process depends not only on solubility but also on mass transfer from the biomass. In fact, it has been reported [
46] that the extraction yield of anthocyanins from pomegranate waste is higher at acidic pH, around pH 2.
It is important to note that not all DES formulations improved anthocyanin extraction compared to their corresponding liquid HBD with a 30 wt% water (see
Figure S4 in Supplementary Materials). For instance, sodium acetate:lactic acid (1:3) proved to be a better extractant than lactic acid alone, but only for Peo-3G. For the other anthocyanins, DES either provided similar or somewhat worse results, as in the case of Mal-3G-cou, where the extracted amount was reduced by approximately 40%.
Regarding glycerol-based DES, significant improvements over glycerol alone were observed only with DES 13 (potassium carbonate:glycerol, 1:5), particularly for Mal-3G, where the yield doubled. For the other DES formulations, the presence of the additional DES component (HBA) had little to no effect, or was even detrimental, as, for instance, in the case of DES 12, where both Mal-3G and Mal-3G-cou showed a decrease in extraction efficiency.
3.4. Optimization of Extraction Parameters
DES are characterized by high viscosities because of their extensive hydrogen bonding, which is an issue in extraction processes as it makes the mixing processes difficult and slows down the transport properties, hindering the dissolution of target compounds from biomass. A usual approach to overcome this problem is the addition of water to decrease the viscosity of the DES and enhance its extraction efficiency. While the addition of water increases the polarity of the solvent, which could have a detrimental effect on the extraction yields of non-polar compounds, the hydrotropy and the solubility maxima induced by many DES or DES precursors may, in fact, lead to better solubilities in these mixed solvents [
47,
48]. If the water content in the DES system is further increased, the solubility will be decreased, and the extraction yields will be greatly influenced by the percentage of water. The purpose of this study was to evaluate two extraction techniques using different DES to broaden the scope of research for future studies. Consequently, two DES systems (DES 4 and DES 5) out of the 15 evaluated (
Table 1) were selected to optimize two different extraction techniques based on the highest extraction yields reported (in terms of maximum extracted amount, mg/kg) (
Figure 2), ease of preparation, superior stability, and ease of handling.
3.4.1. Ultrasound Assisted Extraction
Sonication time and the water concentration in the DES 4 were studied with ranges of 5–60 min and 20–80%, respectively. This study was carried out using a design of experiments based on the response surface methodology.
The statistical analysis of the responses, adjusted to a quadratic model, indicates that the effects of time and percentage of water in the mixture are significant for the five anthocyanins studied, and the sign of the effect is negative for water and positive for time. Significant interaction is observed between the two variables studied, with a negative sign, for Pet-3G and Mal-3G (
Table S6 and Figure S5). According to the sign of the effects it is expected that the best results will be obtained at low aqueous percentages and longer extraction times. This result agrees with a previous study of polyphenol extraction from the leaves of Apocynum venetum L., where 20% of water was also found to be optimal [
39]. On the contrary, Fu et al. [
25] report an ultrasonic time of 3.2 min as the optimal for anthocyanin extraction from blueberry pomace using choline chloride–oxalic acid at 76 °C. Higher temperature accelerates mass transfer in media as well as reduces viscosity, which may favor achieving the maximum extraction in less time.
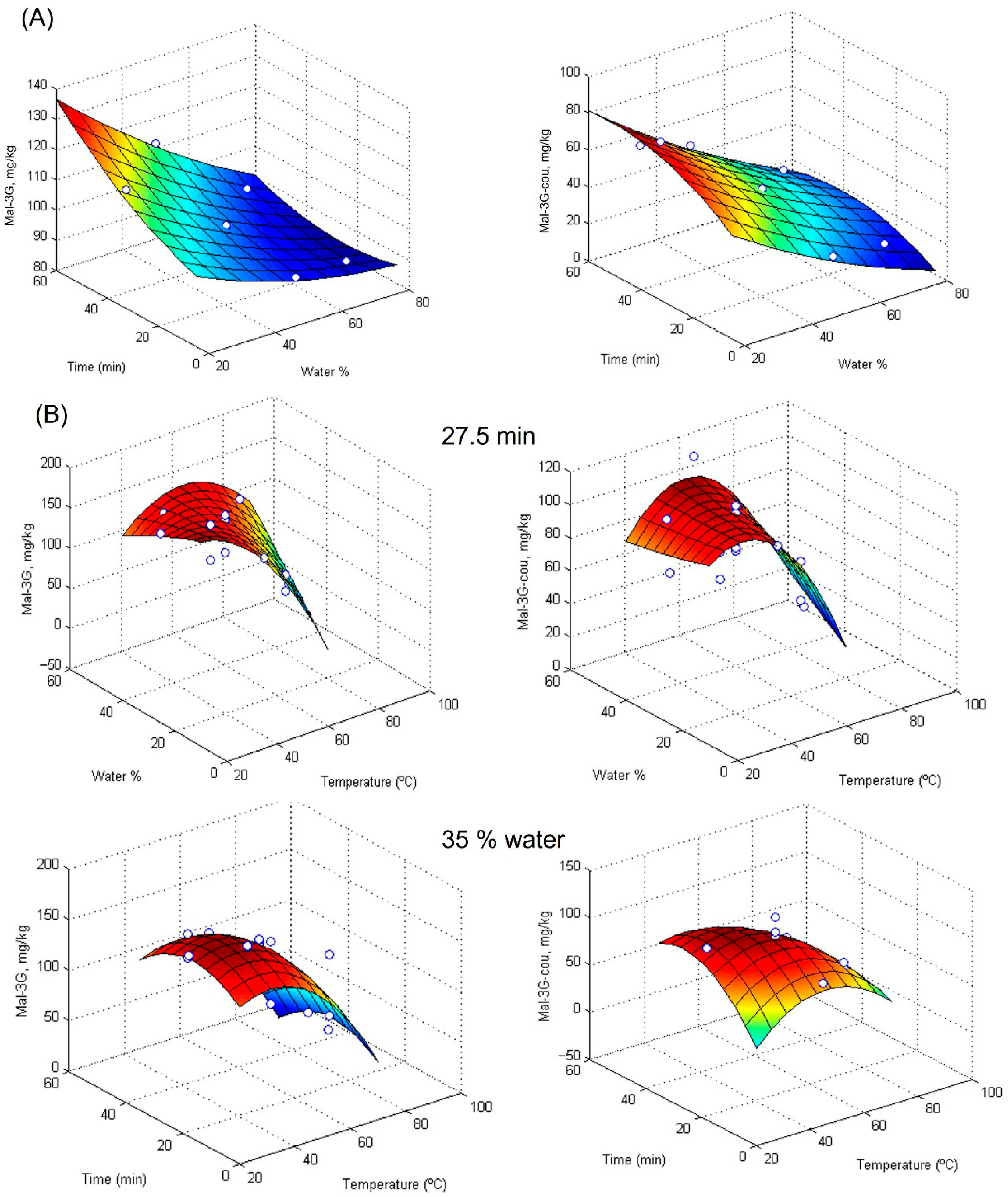
The experimental design results were fitted to a second-degree polynomial model, and the corresponding response surfaces for Mal-3G and Mal-3G-cou are shown in
Figure 3A, and those for the remaining anthocyanins are shown in
Figure S6. R
2 coefficients showed an excellent fit to the experimental data, between 88.9 and 97.5%. Moreover,
Figure S7 shows the desirability plot for the ultrasound-assisted extraction experimental design. Extraction time and aqueous percentages were significant, and according to their response surfaces, the optimal values for the extraction were 60 min of extraction time and 20% of aqueous percentage in DES 4. Under these conditions, the Mal-3G and Mal-3G-cou amounts extracted were around 135 and 80 mg/kg, respectively.
3.4.2. Microwave-Assisted Extraction
One of the DES advantages over conventional organic solvents is its low volatility. These features favor the development of extraction processes at higher temperatures. For this reason, the influence of temperature and extraction time was studied here. One aspect to consider is that higher temperature enhances the mass transfer in media, accelerating the extraction processes. Nevertheless, high temperatures may also lead to the degradation of the target compounds if they are thermolabile [
25]. Furthermore, Fu et al. [
25] report an intriguingly abrupt increase in viscosity at temperatures over 80 °C, which contributes to a lower anthocyanin yield. In a preliminary experiment, different flasks containing the mixture were heated on a hot plate, resulting in the almost complete degradation of anthocyanins at temperatures above 80 °C (see
Figures S8 and S9 in Supplementary Materials). For that reason, the range of temperatures in this study was limited to 80 °C. Subsequently, a study of the time and temperature in microwave-assisted extraction and the aqueous percentage in the DES 5 was carried out using a design of experiments based on response surface methodology. The experimental domain studied was 10–45 min, 20–50% of water, and 30–80 °C.
The statistical analysis of the responses indicates that the effect of temperature is significant in all cases, with a negative sign, except for Peo-3G with a positive effect. However, this Peo-3G result was misleading because at high temperatures, there was a degradation product coeluting with Peo-3G and which interfered with the Peo-3G quantification. For this reason, the Peo-3G results are discarded. The quadratic effect of temperature is significant in all cases. The effect of water was only significant for Mal-3G. The Pareto charts and values of the effects and signs are summarized in
Figure S10 and Table S7. The experimental design results were fit to a second-degree polynomial model, and the corresponding response surfaces for Mal-3G and Mal-3G-cou are shown in
Figure 3B, whereas response surfaces for Pet-3G and Del-3G are shown in
Figures S11 and S12.
The R
2 coefficients of the model were between 83.2 and 90.7, as presented in
Table S8. The desirability plot for the microwave-assisted extraction experimental design is shown in
Figure S13. According to these results, the optimized percentage of water in DES 5 for the extraction of the four anthocyanins included in the study is 20 wt%. It should be noted that little difference was observed between 20 wt% and 30 wt% water. In the aforementioned ultrasound-assisted extraction, the optimal percentage of water for DES4 was also 20 wt%. These results indicate that water is needed to reduce the viscosity of DES and be able to work with them. Nevertheless, the higher the percentage of water, the lower the anthocyanin amount extracted. As previously discussed, excessive water content in the DES system might lead to a decrease in the solubility of the anthocyanins by making the solvent too polar for effective dissolution. The time at which the maximum yield was achieved was 60 min and 27.5 min for ultrasound-assisted extraction with DES 4 and microwave-assisted extraction with DES 5, respectively. Finally, a temperature of 45 °C was selected as optimal for the microwave-assisted extraction of the anthocyanins studied.
Table 2 compares the maximum extracted amount of each anthocyanin by MAE and USAE with the yields obtained with the traditional solvent and ultrasound-assisted extraction during 30 min. Additionally, the methods USAE (DES 4) and MAE (DES 5) were statistically compared with the traditional solvent (methanol/H
2O/formic acid). The
p-values obtained in all cases were lower than 0.05 (
Table S9), illustrating a significant difference in the extracted amounts. It should be noted that the amount extracted with DES 5 (MAE) and DES 4 (USAE) is higher than the amount extracted with the traditional solvent, except for Peo-3G and Mal-3G-cou. Hence, not only are DES extraction systems, in most cases, more efficient than the conventional solvent, but also more environmentally friendly. On the other hand, microwave-assisted extraction with DES 5 extracted higher amounts of anthocyanins than ultrasound-assisted extraction with DES 4. It should be noted that the DES systems were different; DES 4 contains glycerol as HBD, meanwhile DES 5 contains oxalic acid. Moreover, ultrasound-assisted extraction experiments were performed at room temperature (20–25 °C approximately).
4. Conclusions
In this work, anthocyanin extraction yields from red grape skins were improved with the use of deep eutectic solvents. The combined computational and experimental approach enabled the selection of solvent systems, reducing experimental workload and improving process efficiency. The computational study revealed that the solubility of Mal-3G and Mal-3G-cou in potassium carbonate, sodium acetate, and sodium propionate are higher than that in choline chloride and betaine-based DES. However, potassium carbonate with glycerol (DES 13 and DES 14) did not exhibit significantly higher extraction yields compared to other solvents, as anthocyanin extraction from biomass appears to require acidic conditions.
Experimentally, choline chloride was found to be more effective as HBA for extracting anthocyanins than betaine. Furthermore, polyalcohols functioned better as HBD than carboxylic acids (citric, lactic, and malic acid). The alkyl chain of the HBD also influenced the extraction yields. A longer alkyl chain had a detrimental effect on the extraction yields of anthocyanins.
The best extraction yields in the experimental study were obtained for choline chloride:oxalic acid, and choline chloride:glycerol. For microwave-assisted extraction, the optimal parameters were 45 °C, 27.5 min, and 20 wt% water, while 60 min and 20 wt% water were found to be the best conditions for ultrasound-assisted extraction.
This study is a prospective analysis of the efficacy of DES as extractants, aiming to assess their current performance and explore potential future applications. To achieve this, it is essential to evaluate not only their extraction efficiency but also factors such as stability, toxicity, and environmental impact. These aspects are crucial for assessing their future applicability within the cosmetic, pharmaceutical, and food industries.