Removal of Myclobutanil and Detoxification in Modified Biomixtures: Fungal Bioaugmentation and Biochar Amendment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Biomixture Materials, Experimental Setup, and Sampling
2.3. Extraction and Quantification of Myclobutanil
2.4. Estimation of the Dissipation Rate of Myclobutanil
2.5. Ecotoxicological Assays
2.5.1. Seed Germination Tests in L. sativa
2.5.2. Algal Growth Inhibition Test
3. Results and Discussion
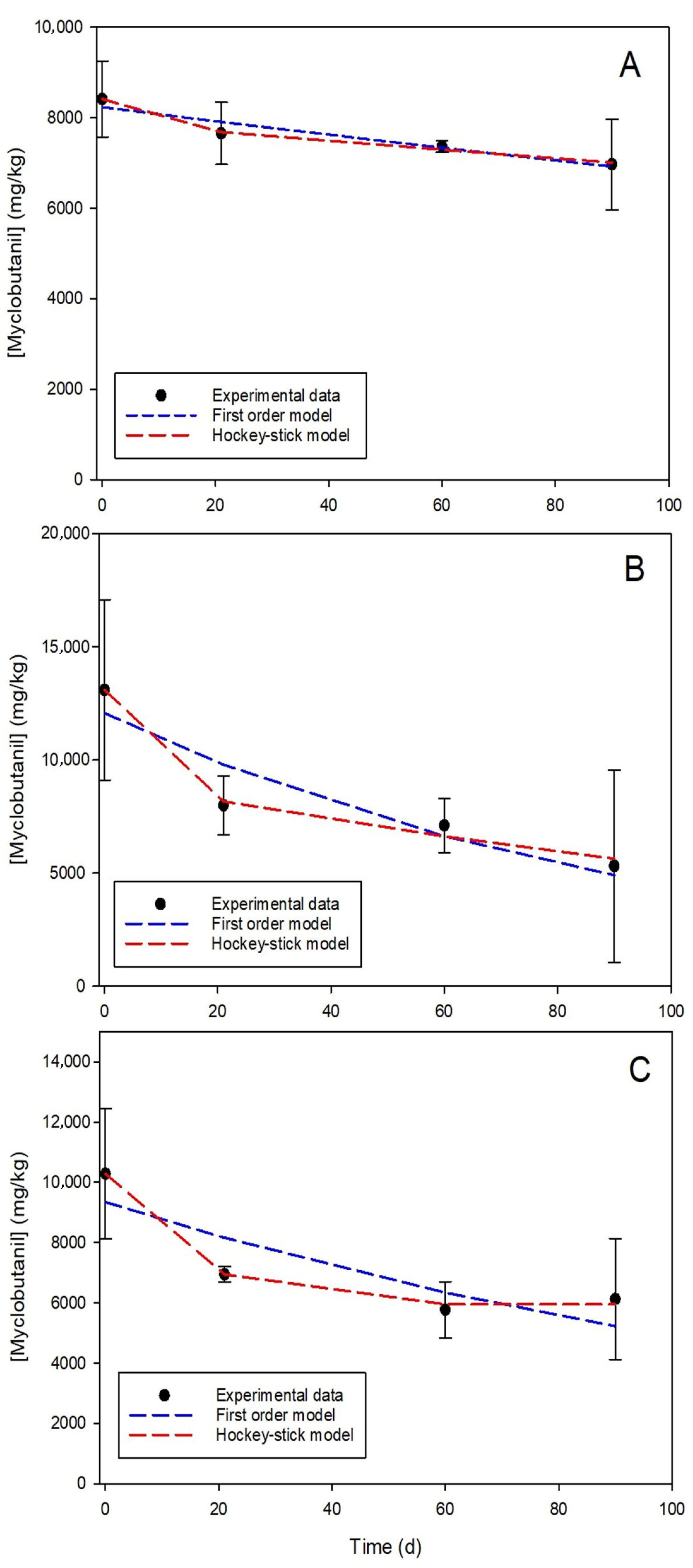
3.1. Dissipation Rate of Myclobutanil
3.2. Impact of Biochar on Myclobutanil Dissipation
3.3. Impact of T. versicolor on Myclobutanil Dissipation
3.4. Ecotoxicological Changes During the Treatment of Myclobutanil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/water-and-sanitation/ (accessed on 13 May 2024).
- Lescano, M.; Fussoni, N.; Vidal, E.; Zalazar, C. Biodegradation of pesticide-contaminated wastewaters from a formulation plant employing a pilot scale biobed. Sci. Total Environ. 2022, 807, 150758. [Google Scholar] [CrossRef]
- Singh, N.K.; Sanghvi, G.; Yadav, M.; Padhiyar, H.; Christian, J.; Singh, V. Fate of pesticides in agricultural runoff treatment systems: Occurrence, impacts and technological progress. Environ. Res. 2023, 237, 117100. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Union 2000, L327, 1–73. [Google Scholar]
- Papazlatani, C.V.; Karas, P.A.; Lampronikou, E.; Karpouzas, D.G. Using biobeds for the treatment of fungicide-contaminated effluents from various agro-food processing industries: Microbiome responses and mobile genetic element dynamics. Sci. Total Environ. 2022, 823, 153744. [Google Scholar] [CrossRef]
- Hao, W.; Zhang, Y.; Xie, Y.; Guo, B.; Chang, J.; Li, J.; Xu, P.; Wang, H. Myclobutanil accumulation, transcriptional alteration, and tissue injury in lizards (Eremias argus) treated with myclobutanil enantiomers. Ecotoxicol. Environ. Saf. 2019, 171, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Herrero-Hernández, E.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Effect of different organic amendments on the dissipation of linuron, diazinon and myclobutanil in an agricultural soil incubated for different time periods. Sci. Total Environ. 2014, 476–477, 611–621. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Wightwick, A.M.; Bui, A.D.; Zhang, P.; Rose, G.; Allinson, M.; Myers, J.H.; Reichman, S.M.; Menzies, N.W.; Pettigrove, V.; Allinson, G. Environmental fate of fungicides in surface waters of a horticultural-production catchment in southeastern Australia. Arch. Environ. Contam. Toxicol. 2012, 62, 380–390. [Google Scholar] [CrossRef]
- Smalling, K.L.; Kuivila, K.M.; Orlando, J.L.; Phillips, B.M.; Anderson, B.S.; Siegler, K.; Hunt, J.W.; Hamilton, M. Environmental fate of fungicides and other current-use pesticides in a central California estuary. Mar. Pollut. Bull. 2013, 73, 144–153. [Google Scholar] [CrossRef]
- Dias, L.A.; Gebler, L.; Niemeyer, J.C.; Itako, A.T. Destination of pesticide residues on biobeds: State of the art and future perspectives in Latin America. Chemosphere 2020, 248, 126038. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, V.I.; Baltierra-Trejo, E.; Gómez-Cruz, R.; Adams, R.H. Microbial growth in biobeds for treatment of residual pesticide in banana plantations. PeerJ 2021, 9, e12200. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Méndez, E.A.; Góngora-Echeverría, V.R.; González-Sánchez, A.; Quintal-Franco, C.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Pesticide treatment in biobed systems at microcosms level under critical moisture and temperature range using an Orthic Solonchaks soil from southeastern Mexico amended with corn husk as support. Sci. Total Environ. 2021, 772, 145038. [Google Scholar] [CrossRef]
- Parlakidis, P.; Tokamani, M.; Sandaltzopoulos, R.; Tokatlidis, I.; Sinapidou, E.; Vryzas, Z. Evaluation of the removal efficacy of three fungicides by biomixtures: Impact of bioaugmentation by plant growth promoting rhizobacteria and zeolite fortification. Int. J. Environ. Anal. Chem. 2024, 104, 6089–6108. [Google Scholar] [CrossRef]
- Chin-Pampillo, J.S.; Ruiz-Hidalgo, K.; Masís-Mora, M.; Carazo-Rojas, E.; Rodríguez-Rodríguez, C.E. Adaptation of biomixtures for carbofuran degradation in on-farm biopurification systems in tropical regions. Environ. Sci. Pollut. Res. 2015, 22, 9839–9848. [Google Scholar] [CrossRef]
- Karanasios, E.; Papadi-Psyllou, A.; Karpouzas, D.G.; Tsiropoulos, N.G. Optimization of biomixture composition and water management for maximum pesticide dissipation in peat-free biobeds. J. Environ. Qual. 2012, 41, 1787–1795. [Google Scholar] [CrossRef]
- Kumari, U.; Singh, N. Ash and biochar mixed biomixtures for adsorption of atrazine and fipronil in the biopurification system. Int. J. Environ. Anal. Chem. 2022, 102, 7988–8003. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, C.E.; Madrigal-León, K.; Masís-Mora, M.; Pérez-Villanueva, M.; Chin-Pampillo, J.S. Removal of carbamates and detoxification potential in a biomixture: Fungal bioaugmentation versus traditional use. Ecotoxicol. Environ. Saf. 2017, 135, 252–258. [Google Scholar] [CrossRef]
- Elgueta, S.; Santos, C.; Lima, N.; Diez, M.C. Atrazine dissipation in a biobed system inoculated with immobilized white-rot fungi. Arch. Agron. Soil Sci. 2016, 62, 1451–1461. [Google Scholar] [CrossRef]
- Briceño, G.; Vergara, K.; Schalchli, H.; Palma, G.; Tortella, G.; Fuentes, M.S.; Diez, M.C. Organophosphorus pesticide mixture removal from environmental matrices by a soil Streptomyces mixed culture. Environ. Sci. Pollut. Res. 2018, 25, 21296–21307. [Google Scholar] [CrossRef]
- Saez, J.M.; Bigliardo, A.L.; Raimondo, E.E.; Briceño, G.E.; Polti, M.A.; Benimeli, C.S. Lindane dissipation in a biomixture: Effect of soil properties and bioaugmentation. Ecotoxicol. Environ. Saf. 2018, 156, 97–105. [Google Scholar] [CrossRef]
- Jatav, H.S.; Rajput, V.D.; Minkina, T.; Singh, S.K.; Chejara, S.; Gorovtsov, A.; Barakhov, A.; Bauer, T.; Sushkova, S.; Mandzhieva, S.; et al. Sustainable approach and safe use of biochar and its possible consequences. Sustainability 2021, 13, 10362. [Google Scholar] [CrossRef]
- Odukkathil, G.; Vasudevan, N. Bacteria amended clay biochar composite biobed system to treat agriculture runoff. J. Environ. Manag. 2020, 269, 110694. [Google Scholar] [CrossRef] [PubMed]
- Kumari, U.; Banerjee, T.; Singh, N. Evaluating ash and biochar mixed biomixtures for atrazine and fipronil degradation. Environ. Technol. Innov. 2021, 23, 101745. [Google Scholar] [CrossRef]
- Masin, C.E.; Lescano, M.R.; Rodríguez, A.R.; Godoy, J.L.; Zalazar, C.S. Earthworms to assess the innocuousness of spent biomixtures employed for glyphosate degradation. J. Environ. Sci. Health B 2018, 53, 519–525. [Google Scholar] [CrossRef]
- Chin-Pampillo, J.S.; Perez-Villanueva, M.; Masis-Mora, M.; Mora-Dittel, T.; Carazo-Rojas, E.; Alcañiz, J.M.; Chinchilla-Soto, C.; Domene, X. Amendments with pyrolyzed agrowastes change bromacil and diuron’s sorption and persistence in a tropical soil without modifying their environmental risk. Sci. Total Environ. 2021, 772, 145515. [Google Scholar] [CrossRef]
- Madrigal-Zúñiga, K.; Ruiz-Hidalgo, K.; Chin-Pampillo, J.S.; Masís-Mora, M.; Castro-Gutiérrez, V.; Rodríguez-Rodríguez, C.E. Fungal bioaugmentation of two rice husk-based biomixtures for the removal of carbofuran in on-farm biopurification systems. Biol. Fertil. Soils 2016, 52, 243–250. [Google Scholar] [CrossRef]
- Murillo-Zamora, S.; Castro-Gutiérrez, V.; Masís-Mora, M.; Lizano-Fallas, V.; Rodríguez-Rodríguez, C.E. Elimination of fungicides in biopurification systems: Effect of fungal bioaugmentation on removal performance and microbial community structure. Chemosphere 2017, 186, 625–634. [Google Scholar] [CrossRef]
- FOCUS. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU registration. In Report of the FOCUS Work Group on Degradation Kinetics, EC Document Reference Sanco/10058/2005 Version 2.0; European Commission: Brussels, Belgium, 2006; 434p. [Google Scholar]
- U.S. EPA. Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analyses: Technical Manual; EPA 823-B-01-002; Office of Water: Washington, DC, USA, 2001. [Google Scholar]
- Lizano-Fallas, V.; Masís-Mora, M.; Espinoza-Villalobos, D.; Lizano-Brenes, M.; Rodríguez-Rodríguez, C.E. Removal of pesticides and ecotoxicological changes during the simultaneous treatment of triazines and chlorpyrifos in biomixtures. Chemosphere 2017, 182, 106–113. [Google Scholar] [CrossRef]
- U.S. EPA—USACE. Great Lakes Dredged Material Testing and Evaluation Manual—Appendix G; Environmental Protection Agency & U.S. Army Corps of Engineers: Washington, DC, USA, 1998; 242p. [Google Scholar]
- OECD. OECD guidelines for the testing of chemicals—Algal growth inhibition test. In Test Guideline 201; Organisation for Economic Co-Operation and Development: Paris, France, 2006. [Google Scholar]
- Antunes, S.C.; Pereira, J.L.; Cachada, A.; Duarte, A.C.; Gonçalves, F.; Sousa, J.P.; Pereira, R. Structural effects of the bioavailable fraction of pesticides in soil: Suitability of elutriate testing. J. Hazard. Mater. 2010, 184, 215–225. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Bangar, V.R.; Kolase, S.V.; Sable, S.B.; Latake, S.B. Screening for fungicide degrading potential of isolated bacterial strains and identification of potent degrading strains. J. Pharmacogn. Phytochem. 2020, 9, 201–206. [Google Scholar]
- Hatzinger, P.B.; Alexander, M. Effect of aging of chemicals in soil on their biodegradability and extractability. Environ. Sci. Technol. 1995, 29, 537–545. [Google Scholar] [CrossRef]
- Castillo, M.P.; Torstensson, L.; Stenström, J. Biobeds for environmental protection from pesticide use—A review. J. Agric. Food Chem. 2008, 56, 6206–6219. [Google Scholar] [CrossRef]
- Rezende, S.; Cesio, M.V.; Archondo, L.; Russi, C.; Martínez, P.; Rivero, A.; Hladki, R.; Heinzen, H.; Besil, N. Pilot study of biobeds application for the remediation of citrus agro-industrial wastewaters. Int. J. Environ. Anal. Chem. 2023, 103, 9698–9714. [Google Scholar] [CrossRef]
- Ma, H.; Egamberdieva, D.; Wirth, S.; Li, Q.; Omari, R.A.; Hou, M.; Bellingrath-Kimura, S.D. Effect of biochar and irrigation on the interrelationships among soybean growth, root nodulation, plant P uptake, and soil nutrients in a sandy field. Sustainability 2019, 11, 6542. [Google Scholar] [CrossRef]
- Novak, J.M.; Ippolito, J.A.; Ducey, T.F.; Watts, D.W.; Spokas, K.A.; Trippe, K.M.; Sigua, G.C.; Johnson, M.G. Remediation of an acidic mine spoil: Miscanthus biochar and lime amendment affects metal availability, plant growth, and soil enzyme activity. Chemosphere 2018, 205, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Cara, I.G.; Țopa, D.; Puiu, I.; Jităreanu, G. Biochar a promising strategy for pesticide-contaminated soils. Agriculture 2022, 12, 1579. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar–microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, P.; Zhao, L.; Sun, H. Sorption and degradation of carbaryl in soils amended with biochars: Influence of biochar type and content. Environ. Sci. Pollut. Res. 2016, 23, 2724–2734. [Google Scholar] [CrossRef]
- Ahmad, R.; Kookana, R.S.; Alston, A.M.; Skjemstad, J.O. The nature of soil organic matter affects sorption of pesticides. 1. Relationships with carbon chemistry as determined by 13C CPMAS NMR spectroscopy. Environ. Sci. Technol. 2001, 35, 878–884. [Google Scholar] [CrossRef]
- Motoki, Y.; Iwafune, T.; Seike, N.; Otani, T.; Asano, M. Effects of organic carbon quality on the sorption behavior of pesticides in Japanese soils. J. Pestic. Sci. 2014, 39, 105–114. [Google Scholar] [CrossRef]
- Sun, K.; Gao, B.; Ro, K.S.; Novak, J.M.; Wang, Z.; Herbert, S.; Xing, B. Assessment of herbicide sorption by biochars and organic matter associated with soil and sediment. Environ. Pollut. 2012, 163, 167–173. [Google Scholar] [CrossRef]
- Yang, Y.; Sheng, G.; Huang, M. Bioavailability of diuron in soil containing wheat-straw-derived char. Sci. Total Environ. 2006, 354, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.; Velarde, P.; Cabrera, A.; Hermosín, M.C.; Cornejo, J. Dissolved organic carbon interactions with sorption and leaching of diuron in organic-amended soils. Eur. J. Soil Sci. 2007, 58, 714–721. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; Yang, Y.; Tao, S.; Xing, B. Sorption mechanisms of phenanthrene, lindane, and atrazine with various humic-acid fractions from a single soil sample. Environ. Sci. Technol. 2011, 45, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef]
- Petter, F.A.; Ferreira, T.S.; Sinhorin, A.P.; de Lima, L.B.; de Morais, L.A.; Pacheco, L.P. Sorption and desorption of diuron in oxisol under biochar application. Bragantia 2016, 75, 487–496. [Google Scholar] [CrossRef]
- Uchimiya, M.; Ohno, T.; He, Z. Pyrolysis-temperature-dependent release of dissolved organic carbon from plant, manure, and biorefinery wastes. J. Anal. Appl. Pyrolysis 2013, 104, 84–94. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chu, W.; Li, H.; Boyd, S.A.; Teppen, B.J.; Mao, J.; Lehmann, J.; Zhang, W. Quantification and characterization of dissolved organic carbon from biochars. Geoderma 2019, 335, 161–169. [Google Scholar] [CrossRef]
- Mukherjee, S.; Tappe, W.; Weihermueller, L.; Hofmann, D.; Köppchen, S.; Laabs, V.; Schroeder, T.; Vereecken, H.; Burauel, P. Dissipation of bentazone, pyrimethanil and boscalid in biochar and digestate based soil mixtures for biopurification systems. Sci. Total Environ. 2016, 544, 192–202. [Google Scholar] [CrossRef]
- Hu, K.; Peris, A.; Torán, J.; Eljarrat, E.; Sarrà, M.; Blánquez, P.; Caminal, G. Exploring the degradation capability of Trametes versicolor on selected hydrophobic pesticides through setting sights simultaneously on culture broth and biological matrix. Chemosphere 2020, 250, 126293. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.; Baldrian, P.; Rogers, H.J.; Boddy, L. Changes in oxidative enzyme activity during interspecific mycelial interactions involving the white-rot fungus Trametes versicolor. Fungal Genet. Biol. 2010, 47, 562–571. [Google Scholar] [CrossRef]
- McErlean, C.; Marchant, R.; Banat, I.M. An evaluation of soil colonisation potential of selected fungi and their production of ligninolytic enzymes for use in soil bioremediation applications. Antonie Van Leeuwenhoek 2006, 90, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Modin, O.; Yamamoto, K.; Fukushi, K.; Nakajima, F.; Nghiem, L.D. Pesticide removal by a mixed culture of bacteria and white-rot fungi. J. Taiwan Inst. Chem. Eng. 2012, 43, 459–462. [Google Scholar] [CrossRef]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef]
- Taskin, E.; Branà, M.T.; Altomare, C.; Loffredo, E. Biochar and hydrochar from waste biomass promote the growth and enzyme activity of soil-resident ligninolytic fungi. Heliyon 2019, 5, e02051. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, B.; Han, Y.; Guan, J.; Gao, H.; Guo, P. Role of reactive oxygen species (ROS) on biochar enhanced chromium phytoremediation in the soil-plant system: Exploration on detoxification mechanism. Environ. Int. 2025, 199, 109471. [Google Scholar] [CrossRef]
- Acosta-Sánchez, A.; Soto-Garita, C.; Masís-Mora, M.; Cambronero-Heinrichs, J.C.; Rodríguez-Rodríguez, C.E. Impaired pesticide removal and detoxification by biomixtures during the simulated pesticide application cycle of a tropical agricultural system. Ecotoxicol. Environ. Saf. 2020, 195, 110460. [Google Scholar] [CrossRef]
| Parameter | Value/Parameter |
|---|---|
| Molecular formula | C15H17ClN4 |
| Substance group | Triazoles |
| Structural formula | 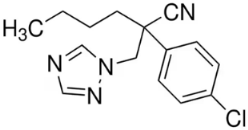 |
| Molecular weight (g/mol) | 288.78 |
| Water solubility at 20 °C (mg/L) | 132 |
| Octanol-water partition coefficient at 25 °C (LogKow) | 2.89 |
| Vapor pressure at 20 °C (mPa) | 0.198 |
| Adsorption coefficient Koc (L/Kg) | 278.9 |
| Henry’s Law constant at 25 °C (Pa m3/mol) | 4.33 × 10−4 |
| Parameters | Biomixture B | Biomixture BB | Biomixture BT |
|---|---|---|---|
| First-order model (FO) | |||
| r | 0.9549 | 0.9283 | 0.8529 |
| k (1/days) | 0.00193 | 0.01001 | 0.006455 |
| DT50 (days) | 360 | 69.3 | 107 |
| Hockey-stick model (HS) | |||
| r | 0.9967 | 0.9945 | 0.9975 |
| tb (days) | 20.98 | 20.09 | 29.28 |
| k1 (1/days) | 0.00431 | 0.0233 | 0.01868 |
| k2 (1/days) | 0.00133 | 0.005382 | 1.24 × 10−12 |
| DT50, k1 (days) | 161 | 29.8 | 37.1 |
| DT50, k2 (days) | 521 | 129 | ND |
| DT50, overall (days) | 474 | 61.9 | ND |
| Time (d) | Conventional Biomixture (B) | Biochar-Containing Biomixture (BB) | Fungal Bioaugmented Biomixture (BT) | |||
|---|---|---|---|---|---|---|
| GI (L. sativa) (%) | EC50 (Algae) (%) | GI (L. sativa) (%) | EC50 (Algae) (%) | GI (L. sativa) (%) | EC50 (Algae) (%) | |
| 0 | 37.6 | 0.00355 | 28.5 | 0.0124 | 20.6 | 0.0124 |
| 60 | 35.6 | 8.41 | 30.4 | 4.95 | 21.7 | 9.97 |
| 90 | 42.0 | 9.20 | 33.1 | 2.23 | 40.9 | 9.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parlakidis, P.; Castro-Gutiérrez, V.; Masís-Mora, M.; Vryzas, Z.; Rodríguez-Rodríguez, C.E. Removal of Myclobutanil and Detoxification in Modified Biomixtures: Fungal Bioaugmentation and Biochar Amendment. Sustain. Chem. 2025, 6, 40. https://doi.org/10.3390/suschem6040040
Parlakidis P, Castro-Gutiérrez V, Masís-Mora M, Vryzas Z, Rodríguez-Rodríguez CE. Removal of Myclobutanil and Detoxification in Modified Biomixtures: Fungal Bioaugmentation and Biochar Amendment. Sustainable Chemistry. 2025; 6(4):40. https://doi.org/10.3390/suschem6040040
Chicago/Turabian StyleParlakidis, Paraskevas, Víctor Castro-Gutiérrez, Mario Masís-Mora, Zisis Vryzas, and Carlos E. Rodríguez-Rodríguez. 2025. "Removal of Myclobutanil and Detoxification in Modified Biomixtures: Fungal Bioaugmentation and Biochar Amendment" Sustainable Chemistry 6, no. 4: 40. https://doi.org/10.3390/suschem6040040
APA StyleParlakidis, P., Castro-Gutiérrez, V., Masís-Mora, M., Vryzas, Z., & Rodríguez-Rodríguez, C. E. (2025). Removal of Myclobutanil and Detoxification in Modified Biomixtures: Fungal Bioaugmentation and Biochar Amendment. Sustainable Chemistry, 6(4), 40. https://doi.org/10.3390/suschem6040040







