Reduction in Chemical Oxygen Demand of Effluents from the Confectionery Sector of Agroindustry Using the Fenton Process
Abstract
1. Introduction
2. Materials and Methods
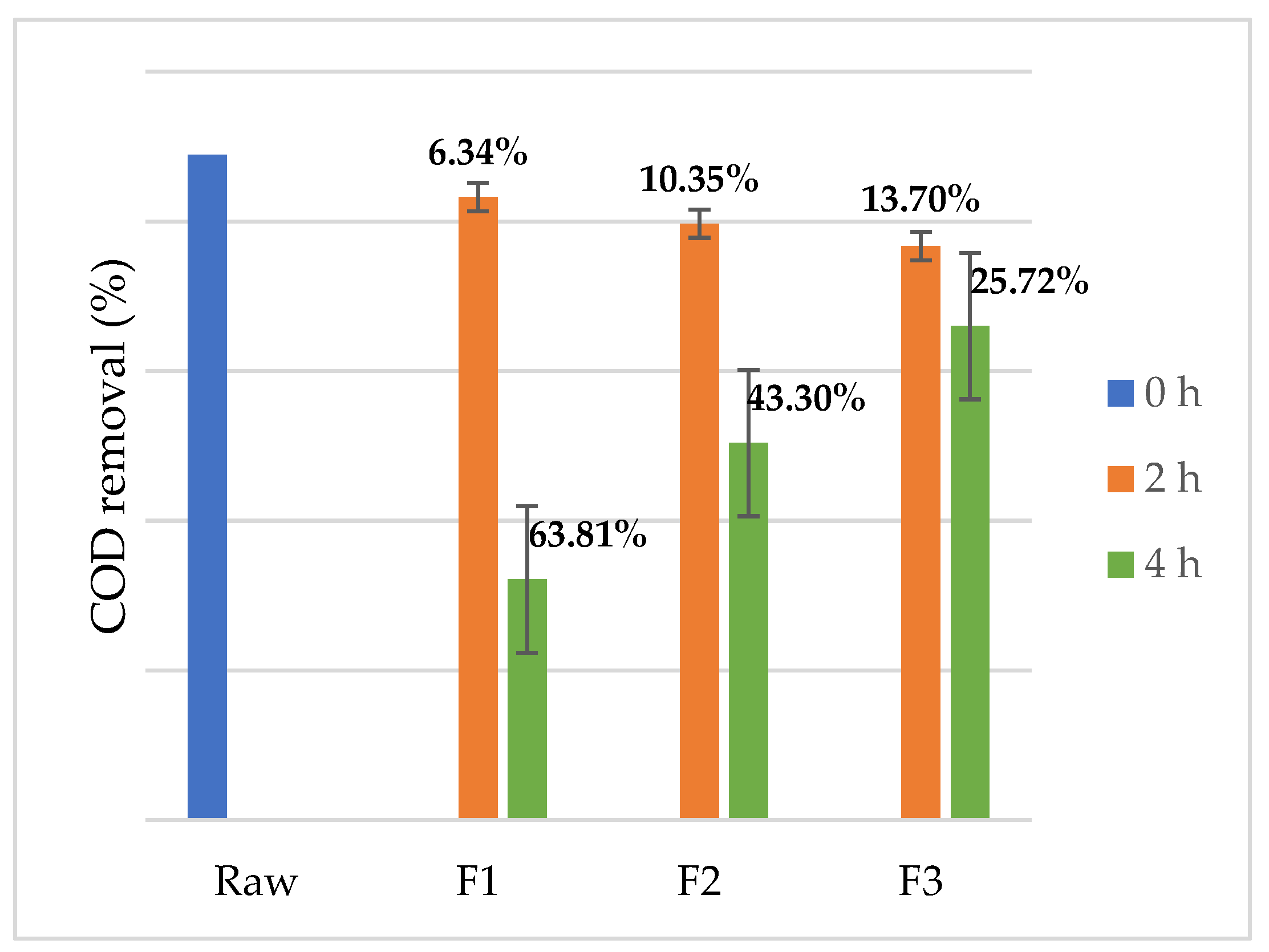
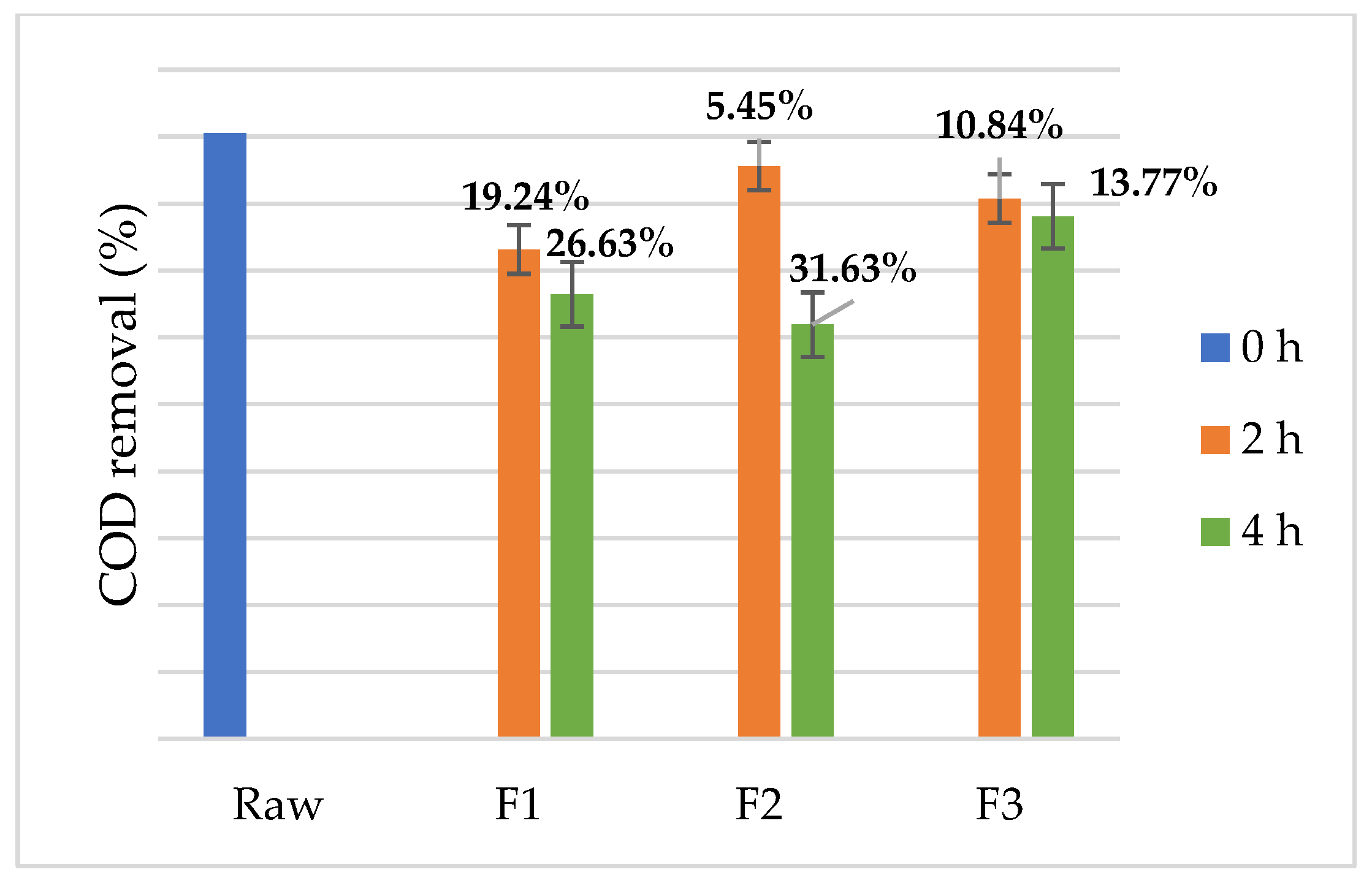
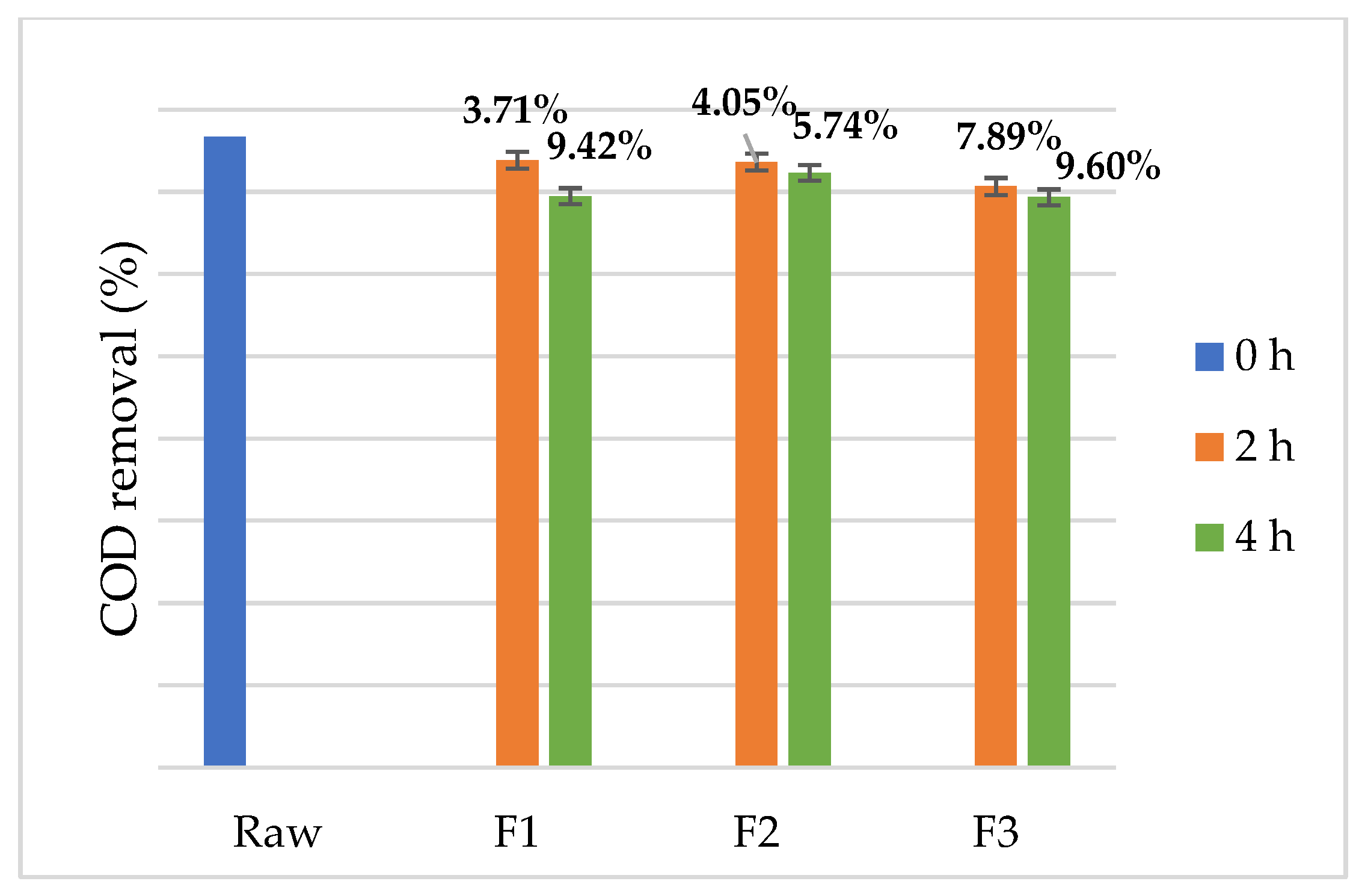
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| •OH | hydroxyl radicals |
| COD | chemical oxygen demand |
| TDS | total dissolved solids |
| DO | dissolved oxygen |
| EC | electrical conductivity |
References
- Kallel, M.; Belaid, C.; Mechichi, T.; Ksibi, M.; Elleuch, B. Removal of organic load and phenolic compounds from olive mill wastewater by Fenton oxidation with zero-valent iron. Chem. Eng. J. 2009, 150, 391–395. [Google Scholar] [CrossRef]
- de Almeida, F.S.C.; Cavalli, A.; Lenhard, D.C.; Genena, A.K. Determination of operational conditions for tertiary treatment of slaughterhouse wastewater by the integrated Fenton-Coagulation process. Ambiente E Agua-Interdiscip. J. Appl. Sci. 2015, 10, 565–573. [Google Scholar]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Bánkuti, S.M.; Bánkuti, F.I. Environmental management and business strategy: A study in a cosmetics company in Brazil. Manag. Prod. 2014, 21, 171–184. [Google Scholar] [CrossRef]
- Costa Filho, B.A.; Rosa, F. Maturity in environmental management: Revisiting best practices. Electron. J. Adm. 2017, 23, 110–134. [Google Scholar] [CrossRef][Green Version]
- Sperling, M.V. Introduction to Water Quality and Sewage Treatment, 3rd ed.; Department of Sanitary and Environmental Engineering, Federal University of Minas Gerais: Belo Horizonte, Brazil, 2005; Available online: https://www.google.com.br/books/edition/Introdu%C3%A7%C3%A3o_%C3%A0_qualidade_das_%C3%A1guas_e_a/1pxhLVxVFHoC?hl=pt-BR&gbpv=1 (accessed on 9 June 2025).[Green Version]
- Júnior, Ê.V. Sistema Integrado de Gestão Ambiental: Como Implementar um Sistema de Gestão que Atenda à Norma ISO 14001, a Partir de um Sistema Baseada na Norma ISO 9000; Editora Aquariana: São Paulo, Brazil, 1998; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=8nDS2Tcgn_4C&oi=fnd&pg=PA9&dq=viterbo+junior&ots=b7zFfxy0l8&sig=BkvMhxV7lgZGVDWNJOcaBsdObLQ&redir_esc=y#v=onepage&q=viterbo%20junior&f=false (accessed on 11 May 2025).[Green Version]
- Brillas, E. Recent development of electrochemical advanced oxidation of herbicides. A review on its application to wastewater treatment and soil remediation. J. Clean. Prod. 2021, 290, 125841. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Nunes, M.I. Recent trends and developments in Fenton processes for industrial wastewater treatment–A critical review. Environ. Res. 2021, 197, 110957. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Tekin, H.; Bilkay, O.; Ataberk, S.S.; Balta, T.H.; Ceribasi, I.H.; Sanin, F.D.; Dilek, F.B.; Yetis, U. Use of Fenton oxidation to improve the biodegradability of a pharmaceutical wastewater. J. Hazard. Mater. 2006, 136, 258–265. [Google Scholar] [CrossRef]
- Kalyanaraman, C.; Kanchinadham, S.B.K.; Vidya Devi, L.; Porselvam, S.; Rao, J.R. Combined advanced oxidation processes and aerobic biological treatment for synthetic fatliquor used in tanneries. Ind. Eng. Chem. Res. 2012, 51, 16171–16181. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A. Removal of COD from olive mill wastewater by Fenton’s reagent: Kinetic study. J. Hazard. Mater. 2009, 168, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Catalkaya, E.C.; Kargi, F. Color, TOC and AOX removals from pulp mill effluent by advanced oxidation processes: A comparative study. J. Hazard. Mater. 2007, 139, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Jamil, T.S.; Ghaly, M.Y.; El-Seesy, I.E.; Souaya, E.R.; Nasr, R.A. A comparative study among different photochemical oxidation processes to enhance the biodegradability of paper mill wastewater. J. Hazard. Mater. 2011, 185, 353–358. [Google Scholar] [CrossRef]
- Pala, A.; Erden, G. Decolorization of a baker’s yeast industry effluent by Fenton oxidation. J. Hazard. Mater. 2005, 127, 141–148. [Google Scholar] [CrossRef]
- De Julio, M.; Neves, E.F.A.; Trofino, J.C.; Di Bernardo, L. Fenton’s reagent as coagulant agent on removing humic substances from water through dissolved air flotation and filtration. Eng. Sanit. Ambient. 2006, 11, 260–268. [Google Scholar] [CrossRef]
- Jiang, W.X.; Zhang, W.; Li, B.J.; Duan, J.; Lv, Y.; Liu, W.D.; Ying, W.C. Combined fenton oxidation and biological activated carbon process for recycling of coking plant effluent. J. Hazard. Mater. 2011, 189, 308–314. [Google Scholar] [CrossRef]
- Ghumra, D.P.; Agarkoti, C.; Gogate, P.R. Improvements in effluent treatment technologies in Common Effluent Treatment Plants (CETPs): Review and recent advances. Process Saf. Environ. Prot. 2021, 147, 1018–1051. [Google Scholar] [CrossRef]
- Moravia, W.G.; Lange, L.C.; Amaral, M.C.S. Evaluate of advanced oxidative process by Fenton reagent under optimized conditions for landfill leachate treatment of with emphasis on collective parameters and characterization of sludge generated. Química Nova 2011, 34, 1370–1377. [Google Scholar] [CrossRef]
- Forti, J.C.; Loretti, G.H.; Tadayozzi, Y.S.; de Andrade, A.R. A phytotoxicity assessment of the efficiency 2,4-D degradation by different oxidative processes. J. Environ. Manag. 2020, 266, 110588. [Google Scholar] [CrossRef]
- Tadayozzi, Y.S.; Santos, F.A.D.; Vicente, E.F.; Forti, J.C. Application of oxidative process to degrade paraquat present in the commercial herbicide. J. Environ. Sci. Health Part B 2021, 56, 670–674. [Google Scholar] [CrossRef]
- Silva, V.E.; Tadayozzi, Y.S.; Putti, F.F.; Santos, F.A.; Forti, J.C. Degradation of commercial glyphosate-based herbicide via advanced oxidative processes in aqueous media and phytotoxicity evaluation using maize seeds. Sci. Total Environ. 2022, 840, 156656. [Google Scholar] [CrossRef]
- United Nations Brazil. UN The Sustainable Development Goals in Brazil. 2024. Available online: https://brasil.un.org/pt-br/sdgs (accessed on 2 February 2025).
- D1252-06R20; Standard Test Methods for Chemical Oxygen Demand (Dichromate Oxygen Demand) of Water. American Society for Testing and Materials (ASTM): Philadelphia, PA, USA, 1995. Available online: https://www.astm.org/Standards/D1252.htm (accessed on 11 May 2025).
- Lipps, W.C.; Baxter, T.E.; Braun-Howland, E. (Eds.) Standard Methods For the Examination of Water and Wastewater; APHA Press: Washington, DC, USA, 1995. [Google Scholar]
- ISO 15705:2002; Water Quality—Determination of the Chemical Oxygen Demand Index (ST-COD)—Small-Scale Sealed-Tube Method. International Organization for Standardization: Geneva, Switzerland, 2002.
- O’Dell, J.W. (Ed.) Method 410.4: The Determination of Chemical Oxygen Demand by Semi-Automated Colorimetry; Revision 2.0; Environmental Monitoring Systems Laboratory, Office of Research and Development, US Environmental Protection Agency: Cincinnati, OH, USA, 1993. [Google Scholar]
- CONAMA, Resolution 357. Council National Environment Agency. Brazil 2005. Available online: https://conama.mma.gov.br/?option=com_sisconama&task=arquivo.download&id=450 (accessed on 23 May 2025).
- CONAMA, Resolution 430. Council National Environment Agency. Brazil 2011. Available online: https://www.saude.mg.gov.br/index.php?option=com_gmg&controller=document&id=13281-resolucao-no-430-de-13-de-maio-de-2011?layout=print#:~:text=Disp%C3%B5e%20sobre%20as%20condi%C3%A7%C3%B5es%20e,Nacional%20do%20Meio%20Ambiente%2DCONAMA (accessed on 11 May 2025).
- Environmental Company of the State of São Paulo (CETESB). Appendix D—Environmental and Sanitary Significance of Quality Variables; CETESB: São Paulo, Brazil, 2014. Available online: https://cetesb.sp.gov.br/aguas-interiores/publicacoes-e-relatorios/ (accessed on 12 May 2025).
- Davies, F.D.; Stulp, S. Determination of methane gas (CH4) generated in effluent treatment plant, with evaluation of calorific power for boiler burning. Rev. Destaques Acadêmicos 2016, 8, 4. [Google Scholar] [CrossRef]
- Ruiz, G.S.; Tannuri, S.T.; Mauad, C.R.; Vendramel, M.R.; Veneu, D.M. Evaluation of COD, nitrogen and phosphorus removal efficiency in the co-treatment of sewage with leachate in an activated sludge system. Holos Environ. 2022, 22, 1–17. [Google Scholar] [CrossRef]
- Companhia de Saneamento Básico do Estado de São Paulo (SABESP). Non-Domestic Sewers Reported 03/19; Government of the State of São Paulo: São Paulo, Brazil, 2019. Available online: https://www.sabesp.com.br/site/interna/Default.aspx?secaoId=586 (accessed on 12 May 2025).
- São Paulo (State), Decree no. 8468/76. It Approves the Regulation of Law No. 997 of May 31, 1976, Which Provides for the Prevention and Control of Environmental Pollution. Government of the State of São Paulo: São Paulo, Brazil, 1976. Available online: https://www.cetesb.sp.gov.br/Institucional/documentos/Dec8468.pdf (accessed on 12 May 2025).
- Derisio, J.C. Introdução ao Controle de Poluição Ambiental, 5th ed.; Oficina de Textos: São Paulo, Brazil, 2017; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=dw-PDAAAQBAJ&oi=fnd&pg=PP9&dq=Introdu%C3%A7%C3%A3o+ao+controle+de+polui%C3%A7%C3%A3o+ambiental.+&ots=sm65TkC2po&sig=4r4bNF-Os6l9d3VsPFX_nHYIZ3c&redir_esc=y#v=onepage&q&f=false (accessed on 5 June 2025).
- PROSAB Basic Sanitation Research Program. Reuse of Sewage, Including the Development of Treatment Technologies for this Purpose; Brazilian Association of Sanitary and Environmental Engineering: Rio de Janeiro, Brazil, 2006; p. 427. Available online: http://www.finep.gov.br/images/apoio-e-financiamento/historico-de-programas/prosab/Esgoto-Prosab_-_final.pdf (accessed on 5 June 2025).
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Villar, V.J.P. Degradation of trimethoprim antibiotic by UVA photoelectro-Fenton process mediated by Fe(III)–carboxylate complexes. Appl. Catal. B Environ. 2015, 162, 34–44. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, C.; Yoon, J. High temperature dependence of 2,4-dichlorophenoxyacetic acid degradation by Fe3+/H2O2 system. Chemosphere 2003, 51, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Ziembowicz, S.; Kida, M. Limitations and future directions of application of the Fenton-like process in micropollutants degradation in water and wastewater treatment: A critical review. Chemosphere 2022, 296, 134041. [Google Scholar] [CrossRef]
- Duesterberg, C.K.; Mylon, S.E.; Waite, T.D. pH Effects on Iron-Catalyzed Oxidation using Fenton’s reagent. Environ. Sci. Technol. 2008, 42, 8522–8527. [Google Scholar] [CrossRef]
- Pliego, G.; Zazo, J.A.; Garcia-Muñoz, P.; Munoz, M.; Casas, J.A.; Rodriguez, J.J. Trends in the Intensification of the Fenton Process for Wastewater Treatment: An Overview. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2611–2692. [Google Scholar] [CrossRef]
- Gama, M.R. Processos fenton como alternativa na remoção de interferentes endócrinos e outros micropoluentes ambientais. Rev. Virtual Química 2012, 4, 777–787. [Google Scholar] [CrossRef]
- Santos, M.S.F.; Alves, A.; Madeira, L.M. Paraquat removal from water by oxidation with Fenton’s reagent. Chem. Eng. J. 2011, 175, 279–290. [Google Scholar] [CrossRef]
- Lucena, L.G.; Rocha, E.M. Solar photo-Fenton process for the treatment of landfill leachate. Rev. DAE 2015, 49–63. Available online: https://www.revistadae.com.br/artigos/artigo_edicao_200_n_1614.pdf (accessed on 12 June 2025). [CrossRef]
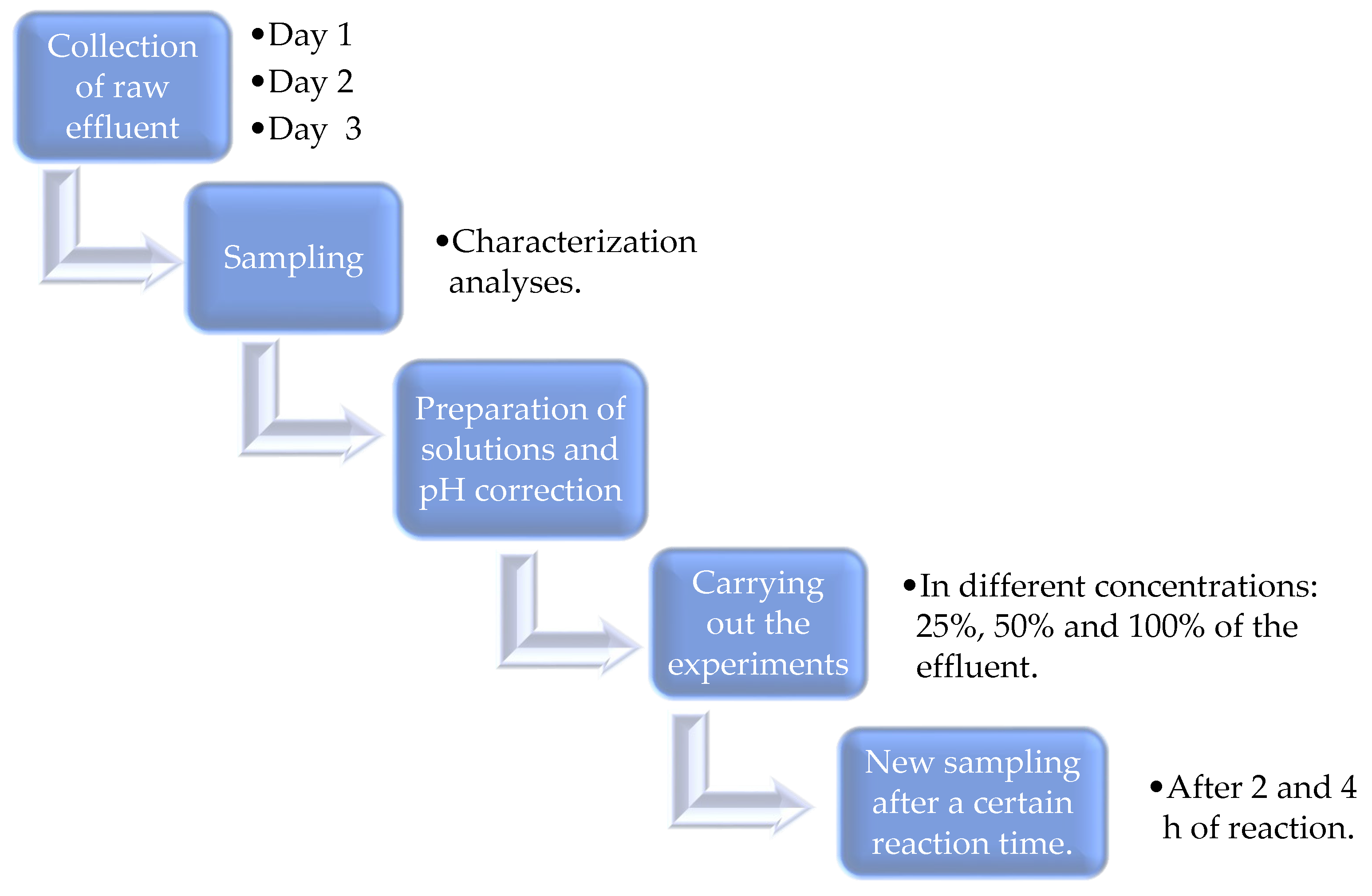

| Identification | Description | Concentration of Raw Effluent |
|---|---|---|
| F1 | 60 mL of distilled water | 25% |
| 20 mL of raw effluent | ||
| 600 µL of iron sulfate solution at 8.3 g L−1 | ||
| 31 µL of 29% hydrogen peroxide | ||
| F2 | 40 mL distilled water | 50% |
| 40 mL of raw effluent | ||
| 600 µL of iron sulfate solution at 8.3 g L−1 | ||
| 31 µL of 29% hydrogen peroxide | ||
| F3 | 80 mL of raw effluent | 100% |
| 600 µL of iron sulfate solution at 8.3 g L−1 | ||
| 31 µL of 29% hydrogen peroxide |
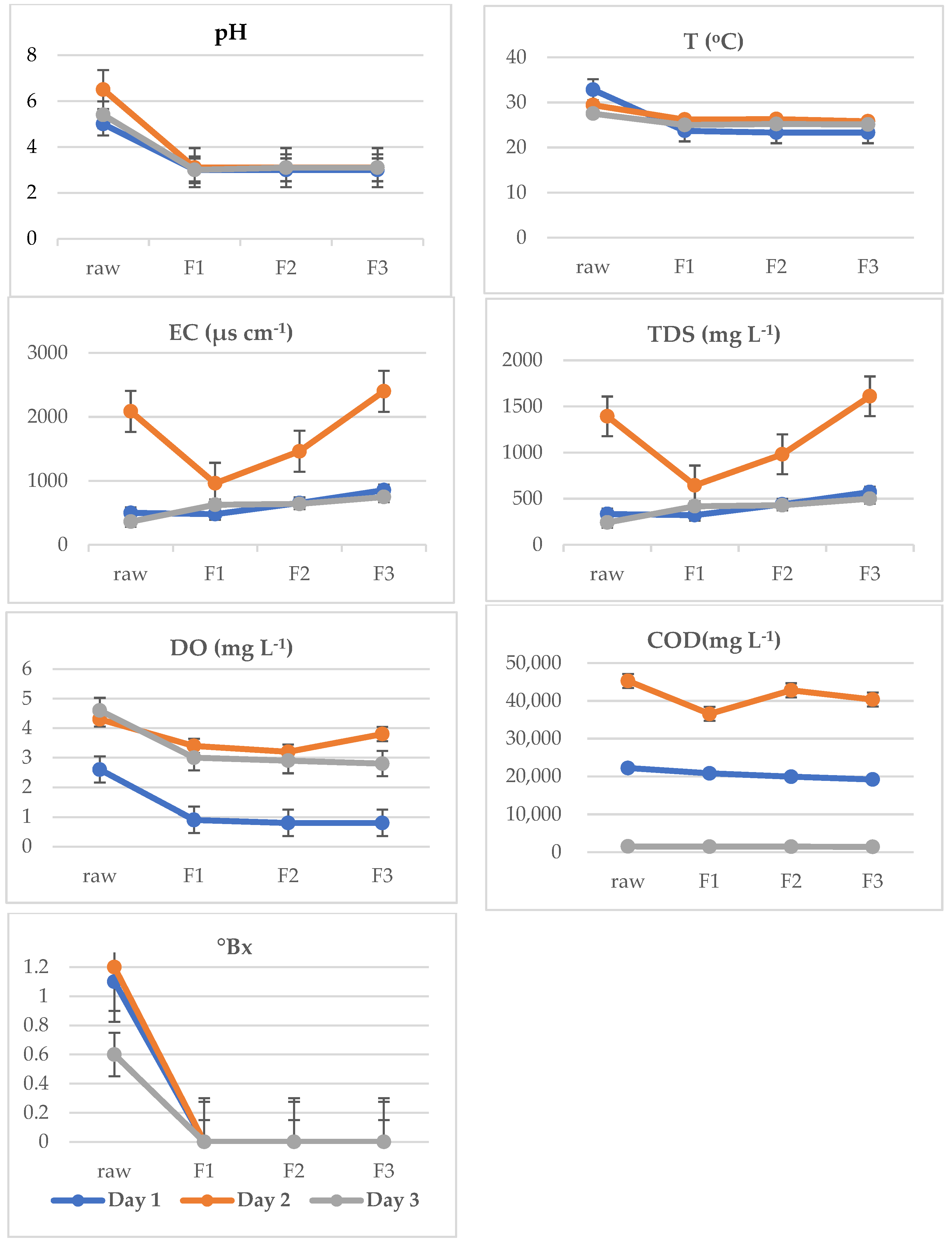
| Parameter | DAY 1 | DAY 2 | DAY 3 |
|---|---|---|---|
| pH | 50 ± 0.0 | 6.5 ± 0.0 | 5.4 ± 0.0 |
| COD (mg L−1) | 22,223.3 ± 1377.4 | 45,280.0 ± 2343.3 | 1534.0± 17.7 |
| T (°C) | 32.8 ± 0.0 | 29.4 ± 0.0 | 27.5 ± 0.0 |
| DO (mg L−1) | 2.6 ± 0.0 | 4.3 ± 0.0 | 4.6 ± 0.0 |
| TDS (mg L−1) | 333.3 ± 1.5 | 1393.0 ± 5.7 | 241.6 ± 0.5 |
| EC (µs cm−1) | 499.3 ± 3.5 | 2086.6 ± 11.5 | 362.3 ± 0.5 |
| °Bx | 1.1 ± 0.0 | 1.2 ± 0.0 | 0.0 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frigulio, M.A.P.; Morales, A.G.; Santos, F.A.; Forti, J.C. Reduction in Chemical Oxygen Demand of Effluents from the Confectionery Sector of Agroindustry Using the Fenton Process. Sustain. Chem. 2025, 6, 32. https://doi.org/10.3390/suschem6040032
Frigulio MAP, Morales AG, Santos FA, Forti JC. Reduction in Chemical Oxygen Demand of Effluents from the Confectionery Sector of Agroindustry Using the Fenton Process. Sustainable Chemistry. 2025; 6(4):32. https://doi.org/10.3390/suschem6040032
Chicago/Turabian StyleFrigulio, Maiara A. P., Angélica G. Morales, Felipe A. Santos, and Juliane C. Forti. 2025. "Reduction in Chemical Oxygen Demand of Effluents from the Confectionery Sector of Agroindustry Using the Fenton Process" Sustainable Chemistry 6, no. 4: 32. https://doi.org/10.3390/suschem6040032
APA StyleFrigulio, M. A. P., Morales, A. G., Santos, F. A., & Forti, J. C. (2025). Reduction in Chemical Oxygen Demand of Effluents from the Confectionery Sector of Agroindustry Using the Fenton Process. Sustainable Chemistry, 6(4), 32. https://doi.org/10.3390/suschem6040032







