A Review of the Molecular Aggregation of Small-Molecule Anion Sensors for Environmental Contaminates in Aqueous Media
Abstract
1. Introduction
2. The Environmental Fate of Anions
2.1. Cyanide
2.2. Phosphorus Compounds
2.3. Disinfectant Byproducts (Chlorate, Chlorite, and Bromate)
2.4. Nitrite and Nitrate
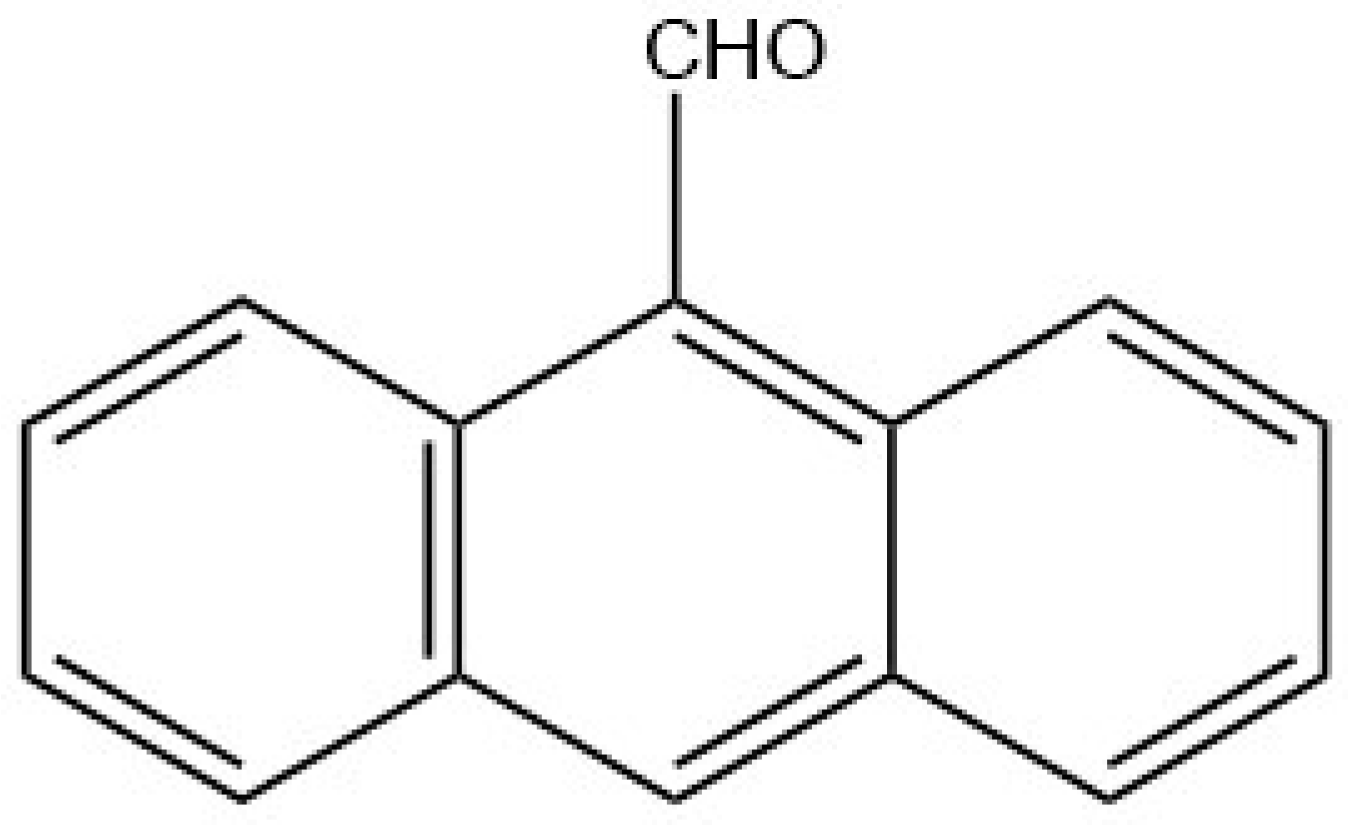
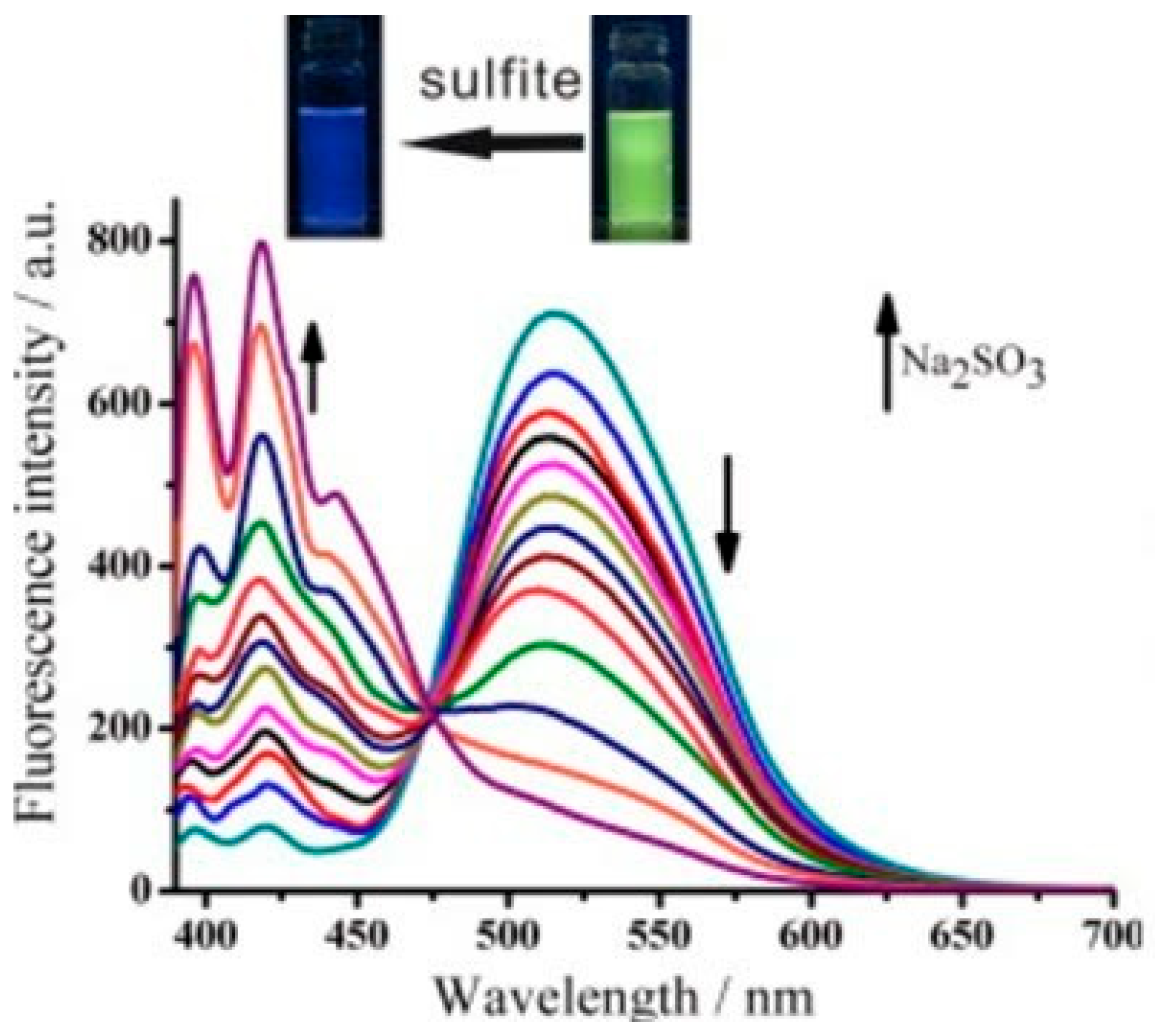
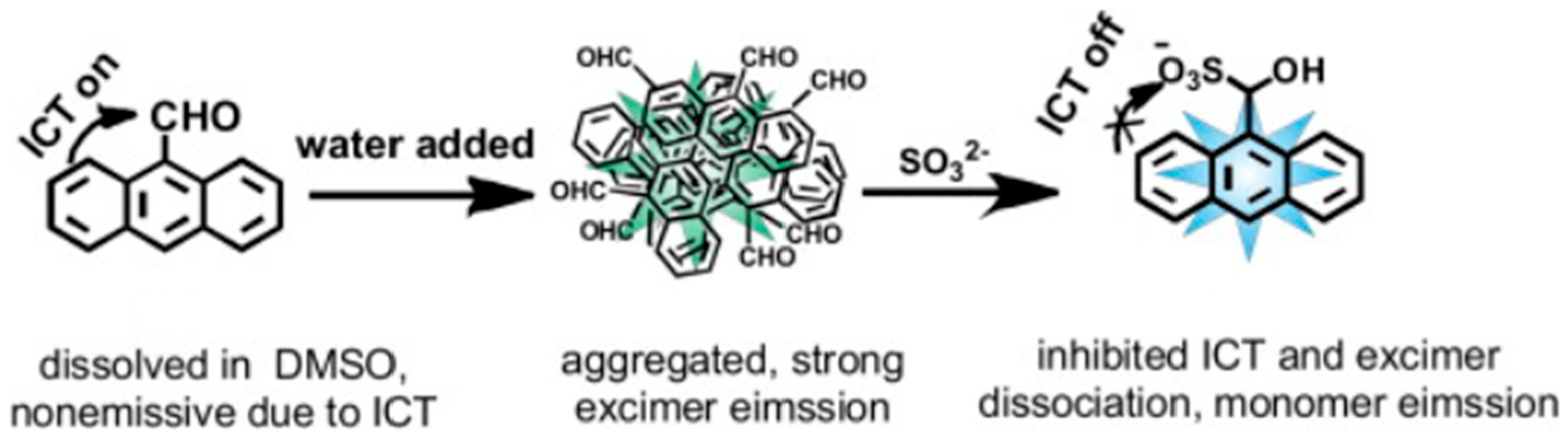
2.5. Sulfite and Sulfate
3. Methods of Sensing via Small Molecules
3.1. Hydrogen Bond Interactions
3.2. Electrostatic Interactions
3.3. Covalent Bond Formation or Cleavage
3.4. Supramolecular Interactions
4. Urea-Based Sensors
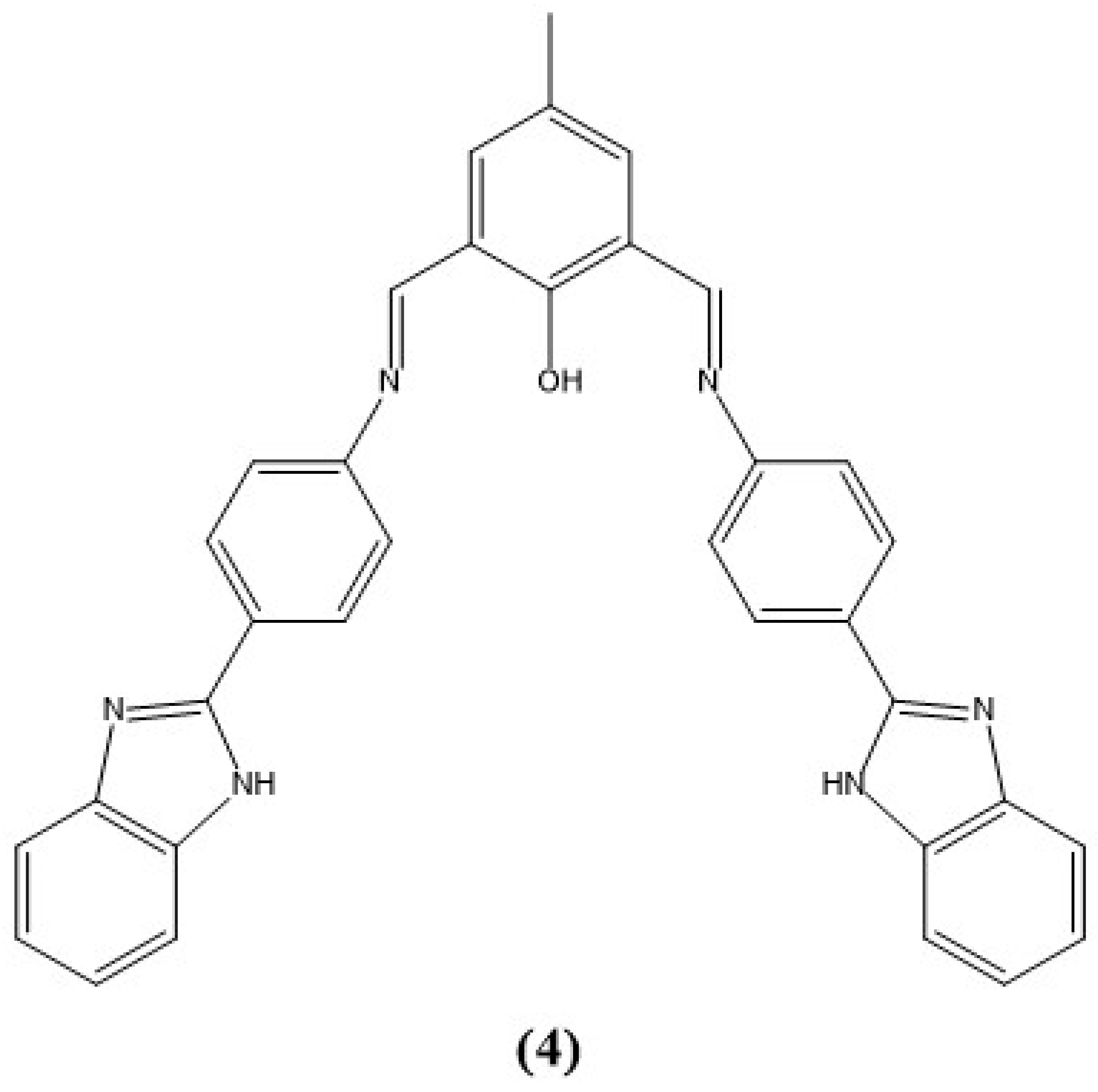
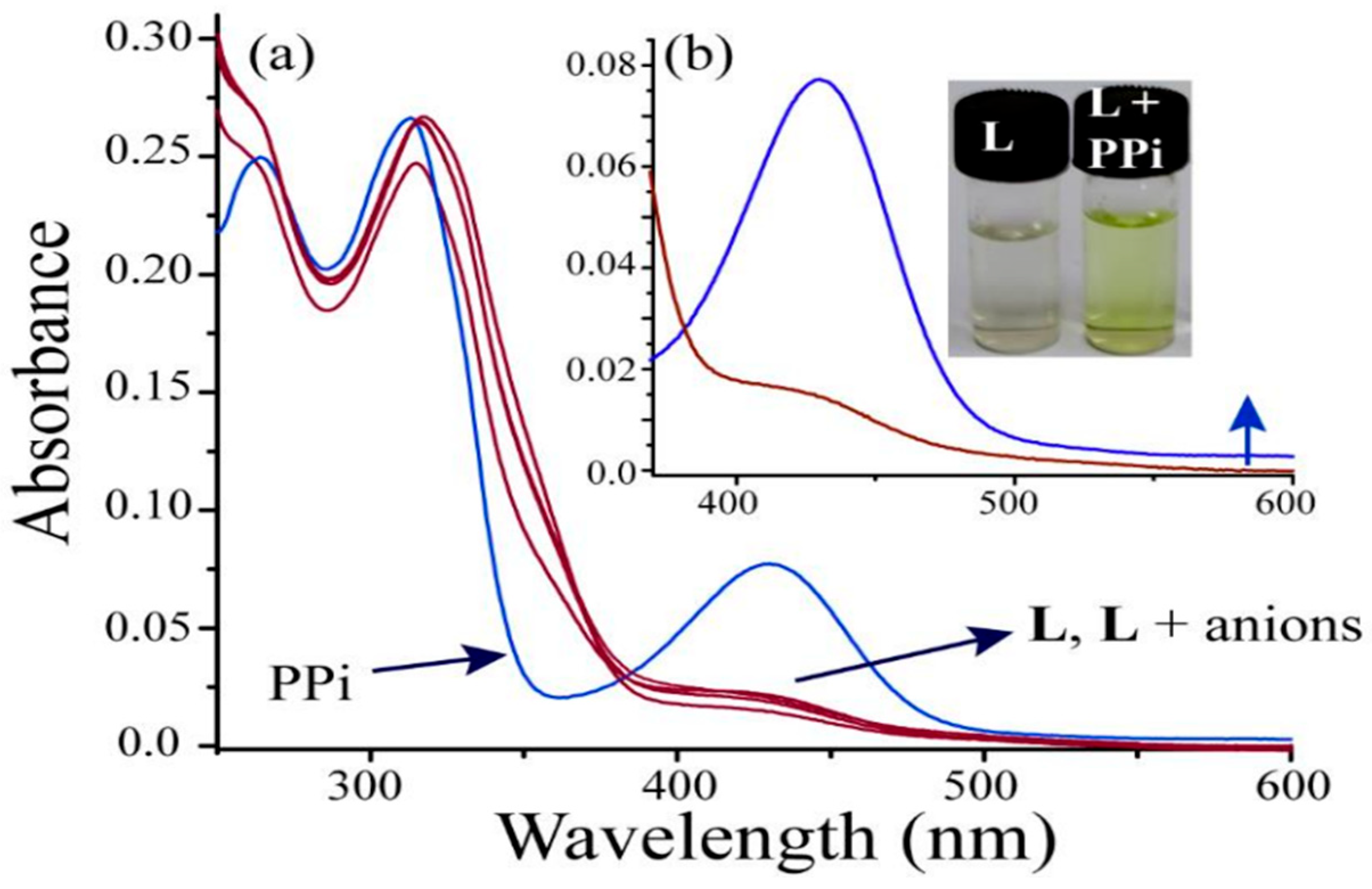
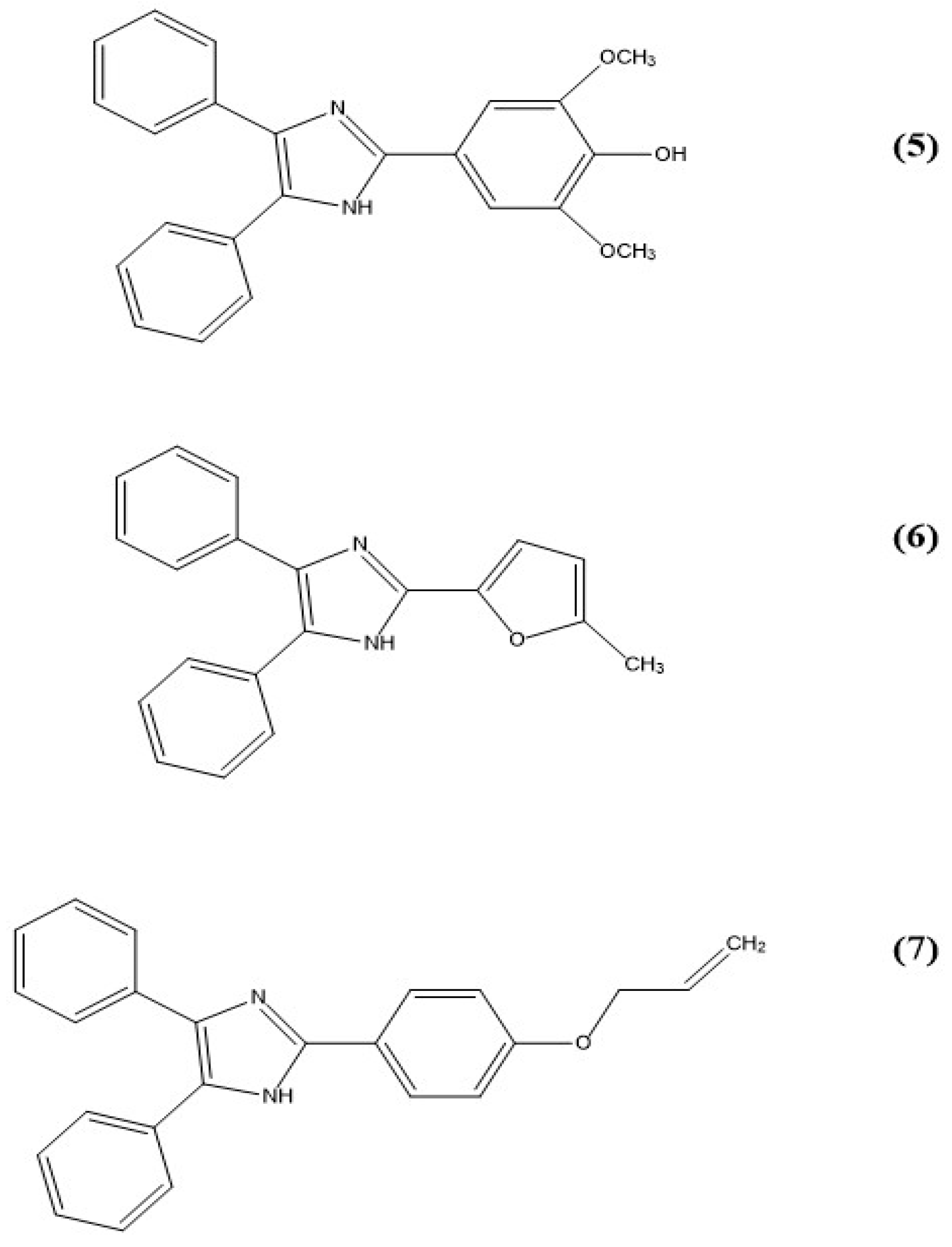
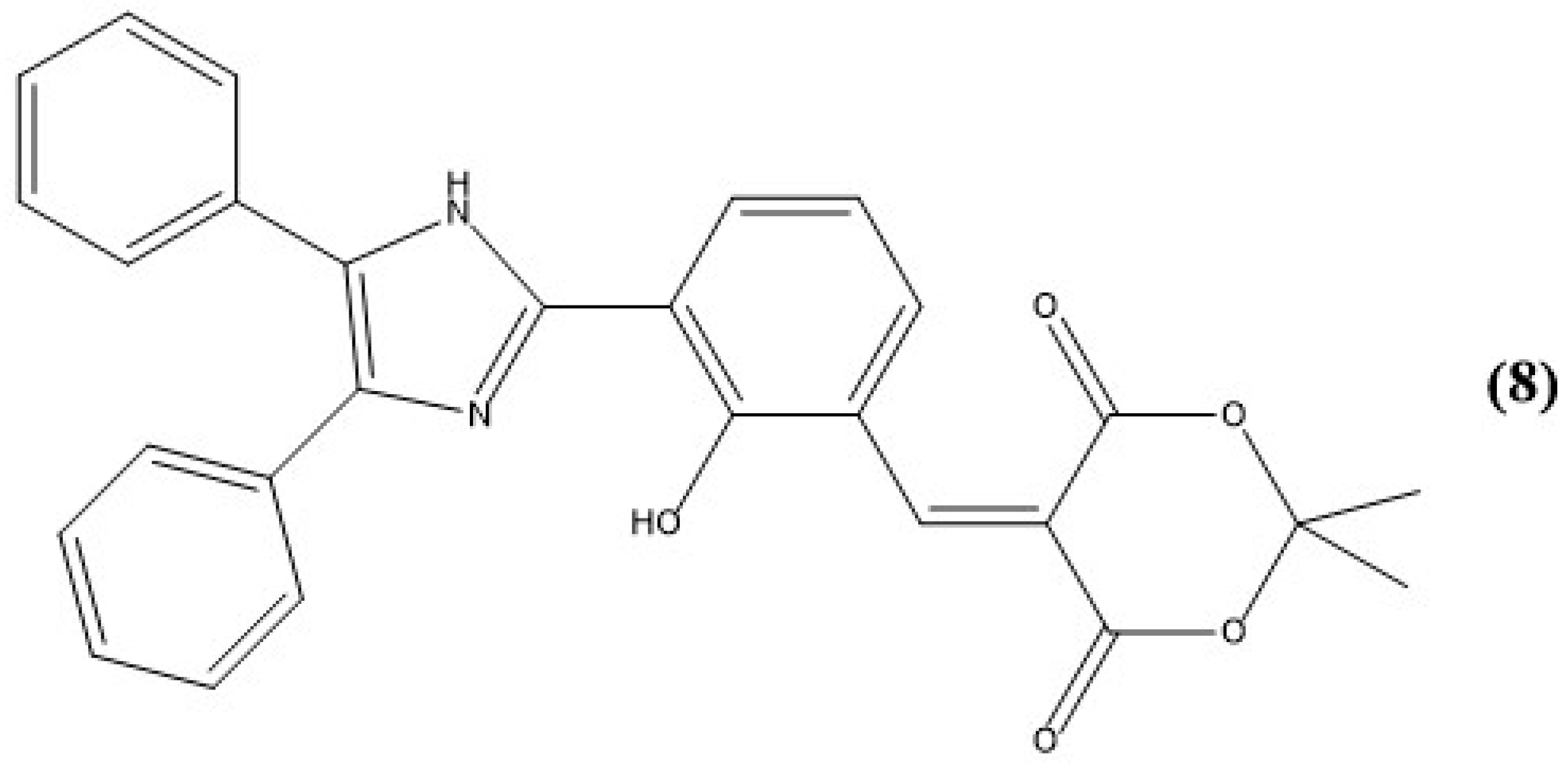
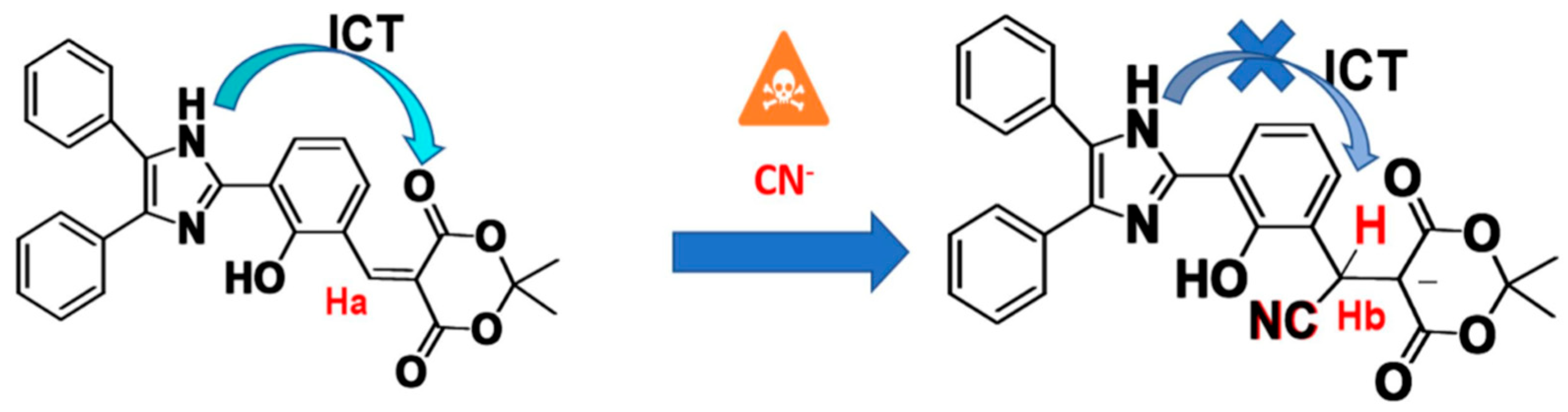
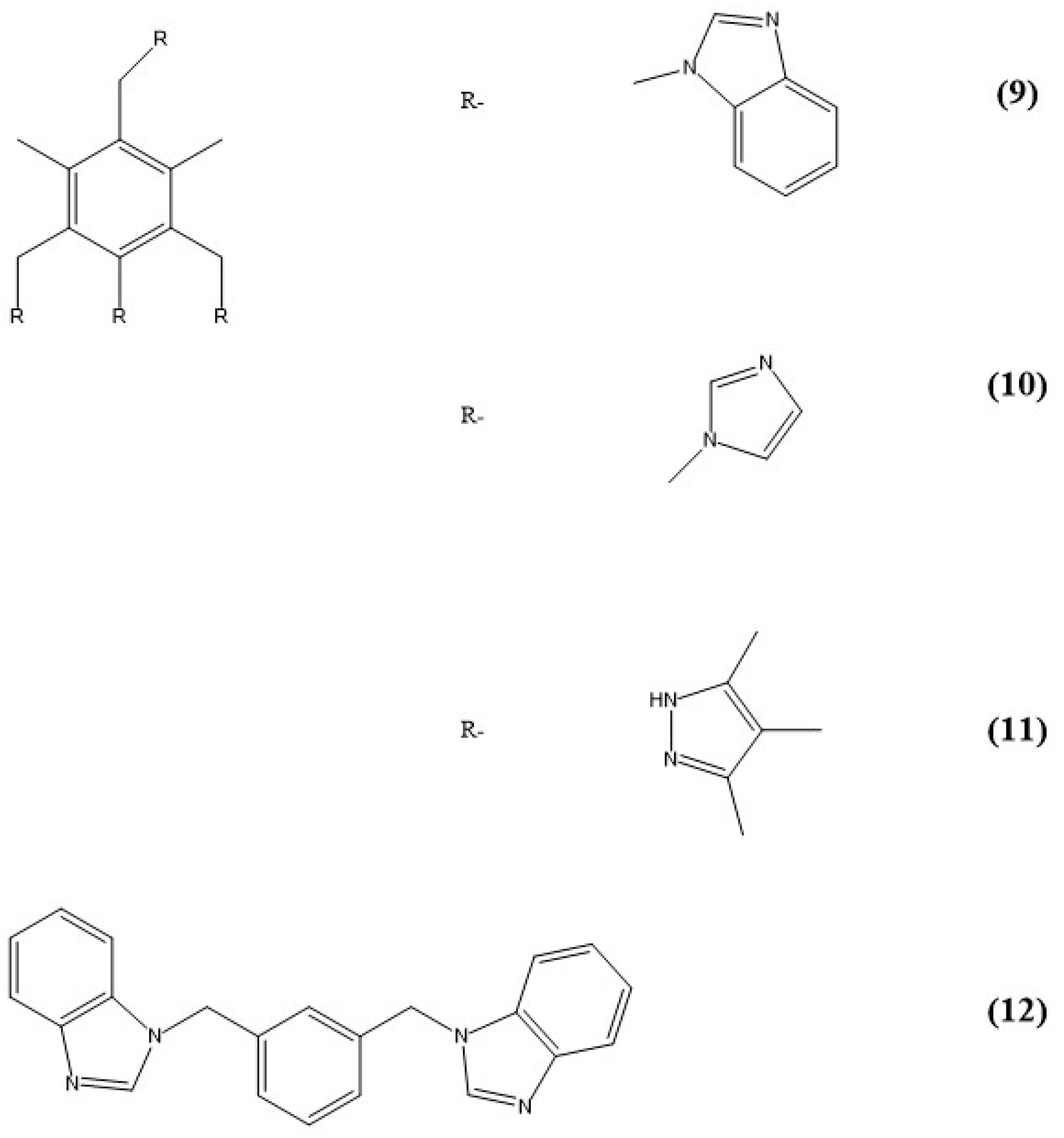
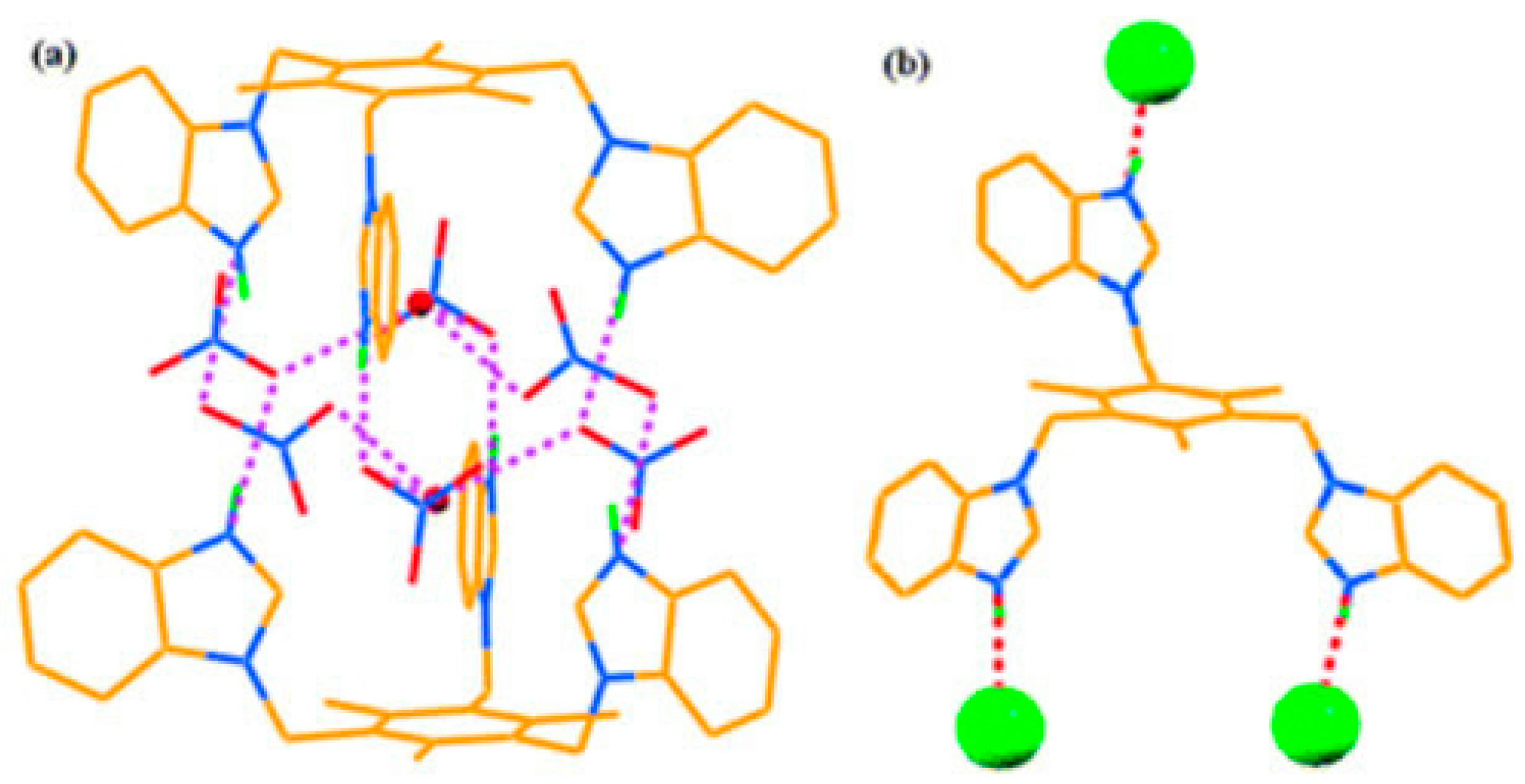
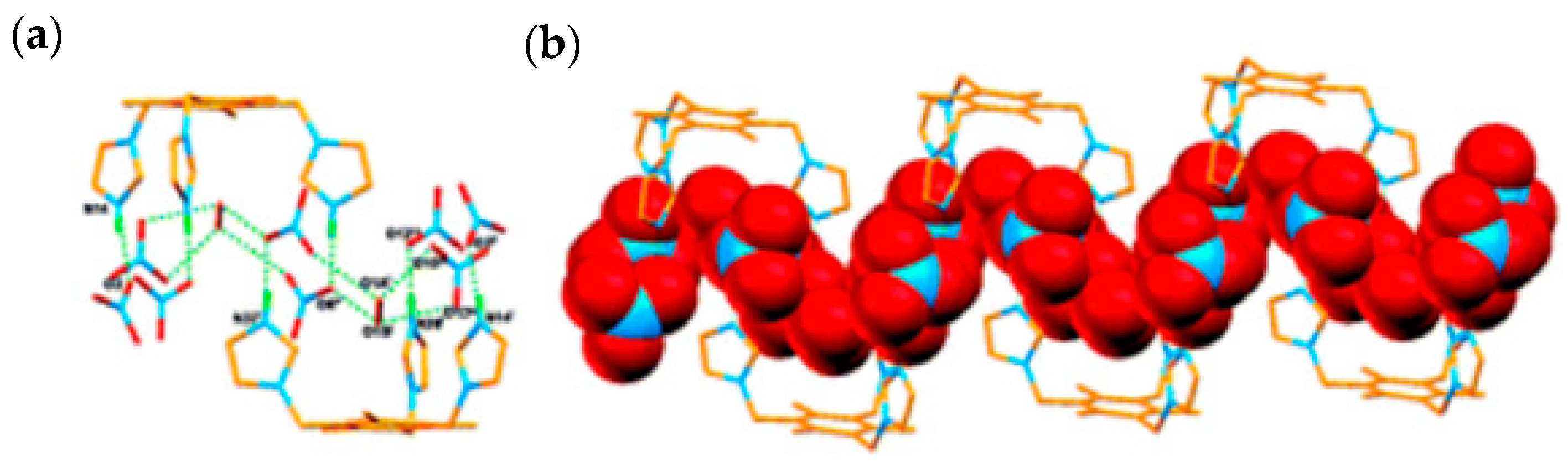
5. Imidazole-Based Sensors
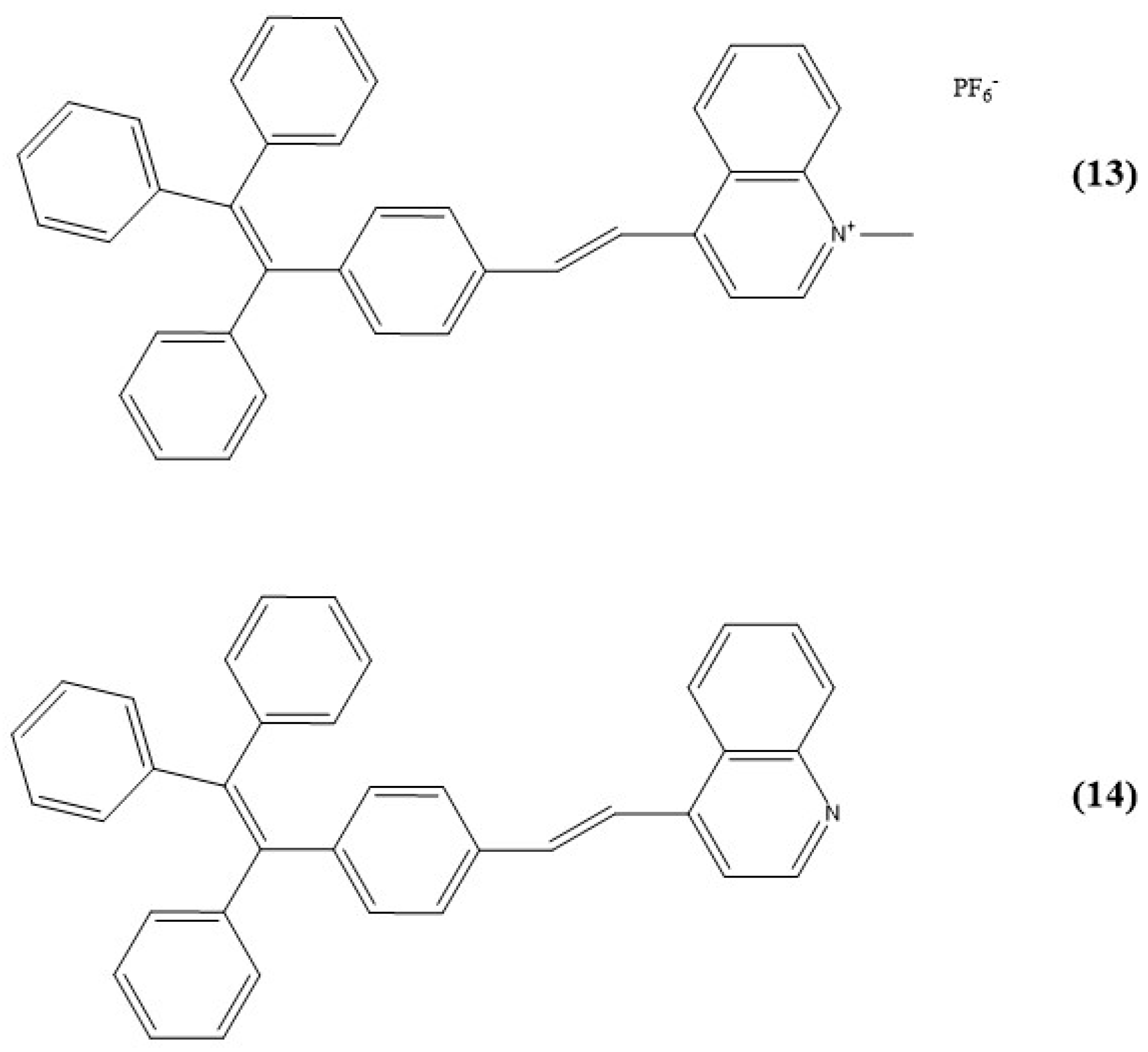
6. Quinolinium and Other Fused Ring-Based Sensors
7. Triazoles and Carbazole-Based Sensors
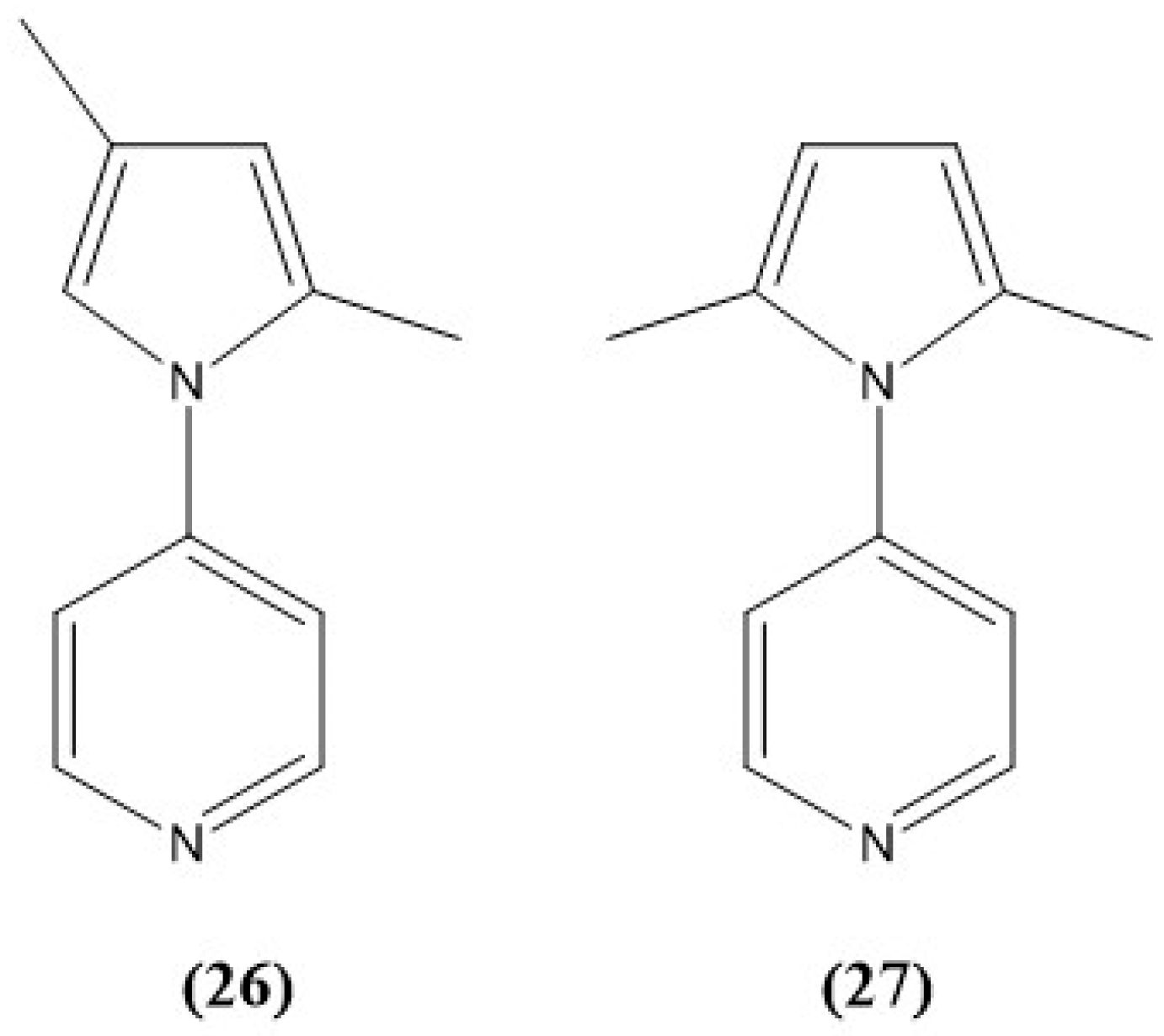
8. Pyridine and Pyrrole-Based Sensors
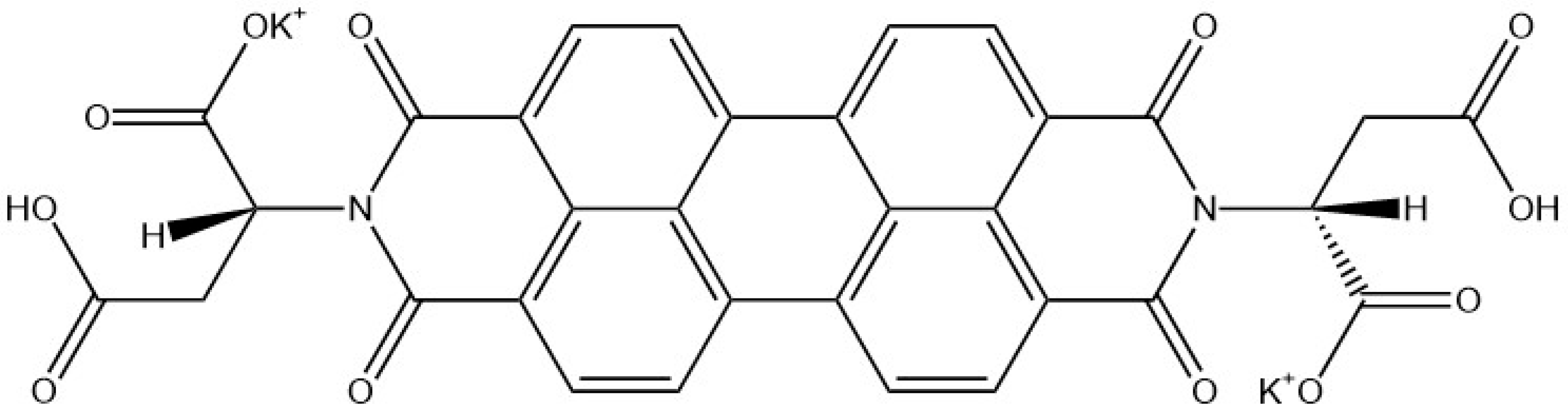
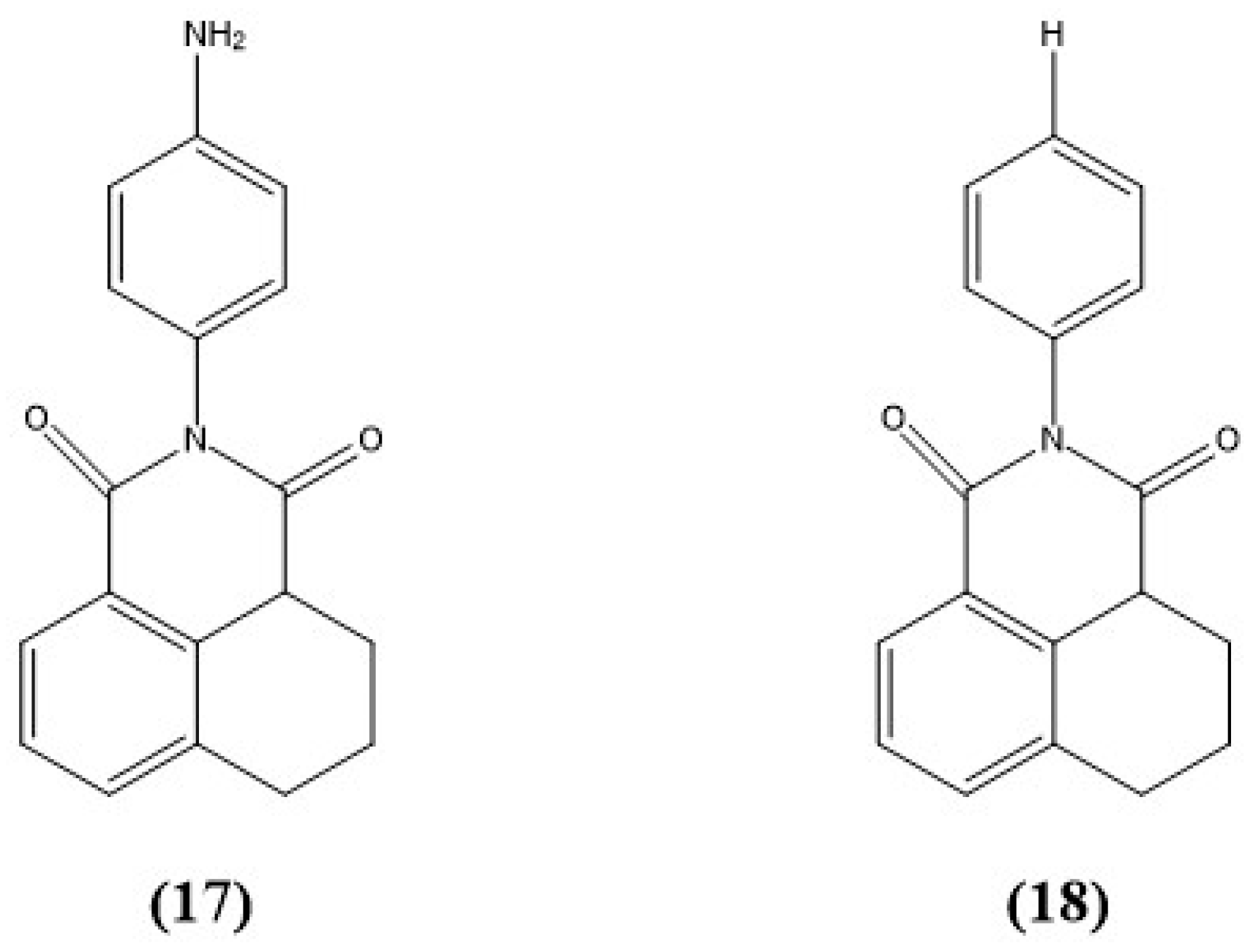
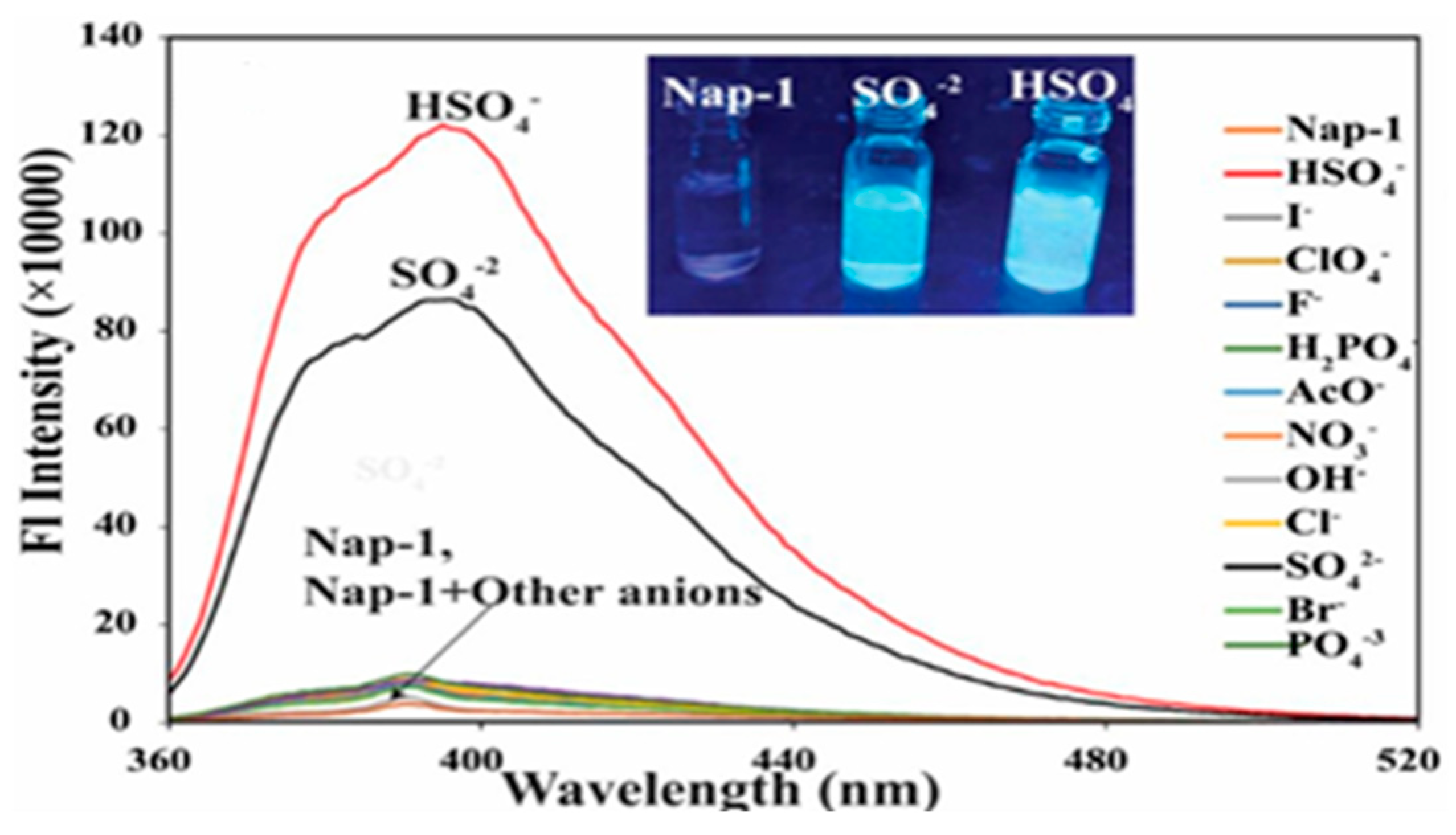
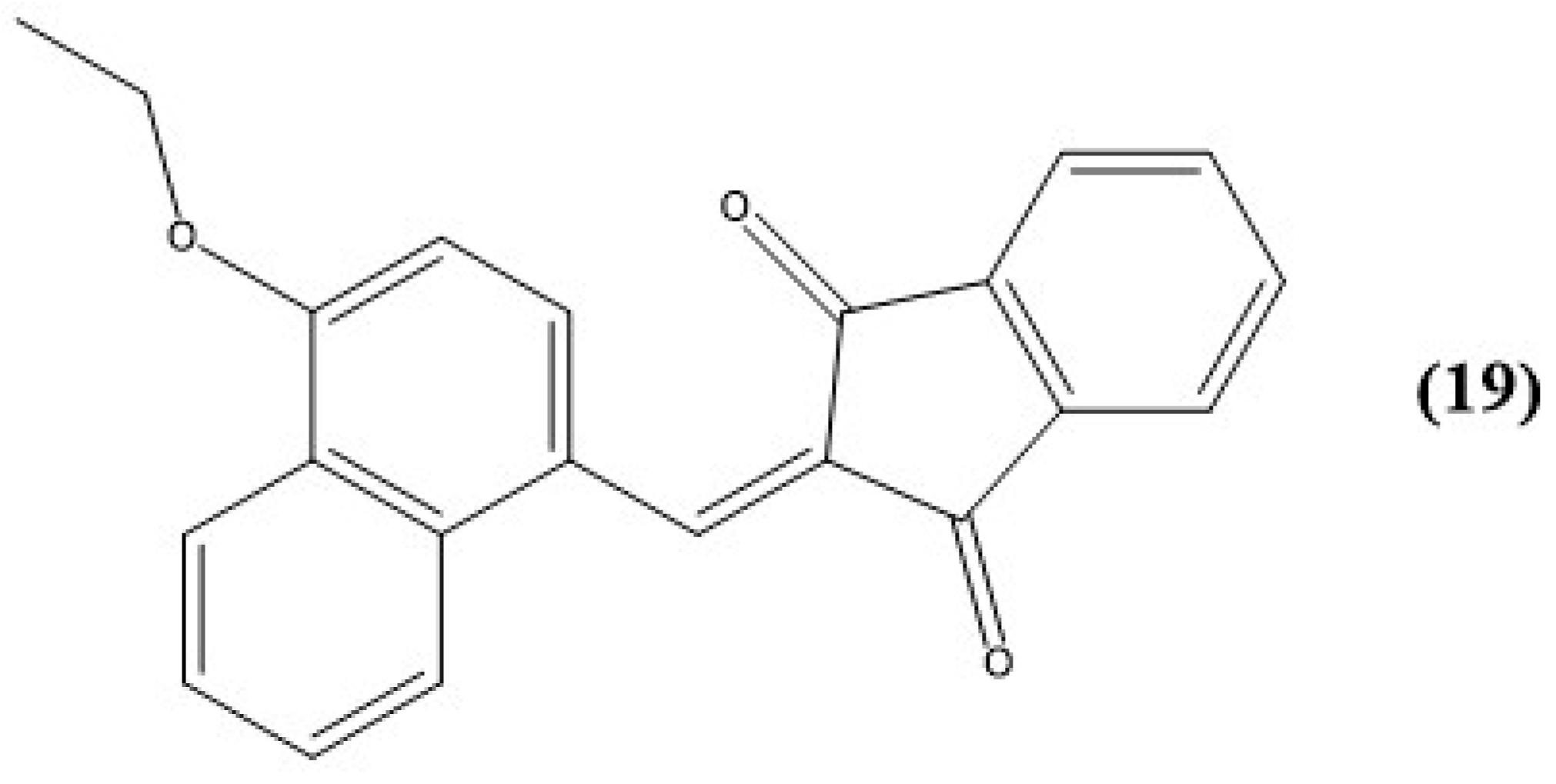
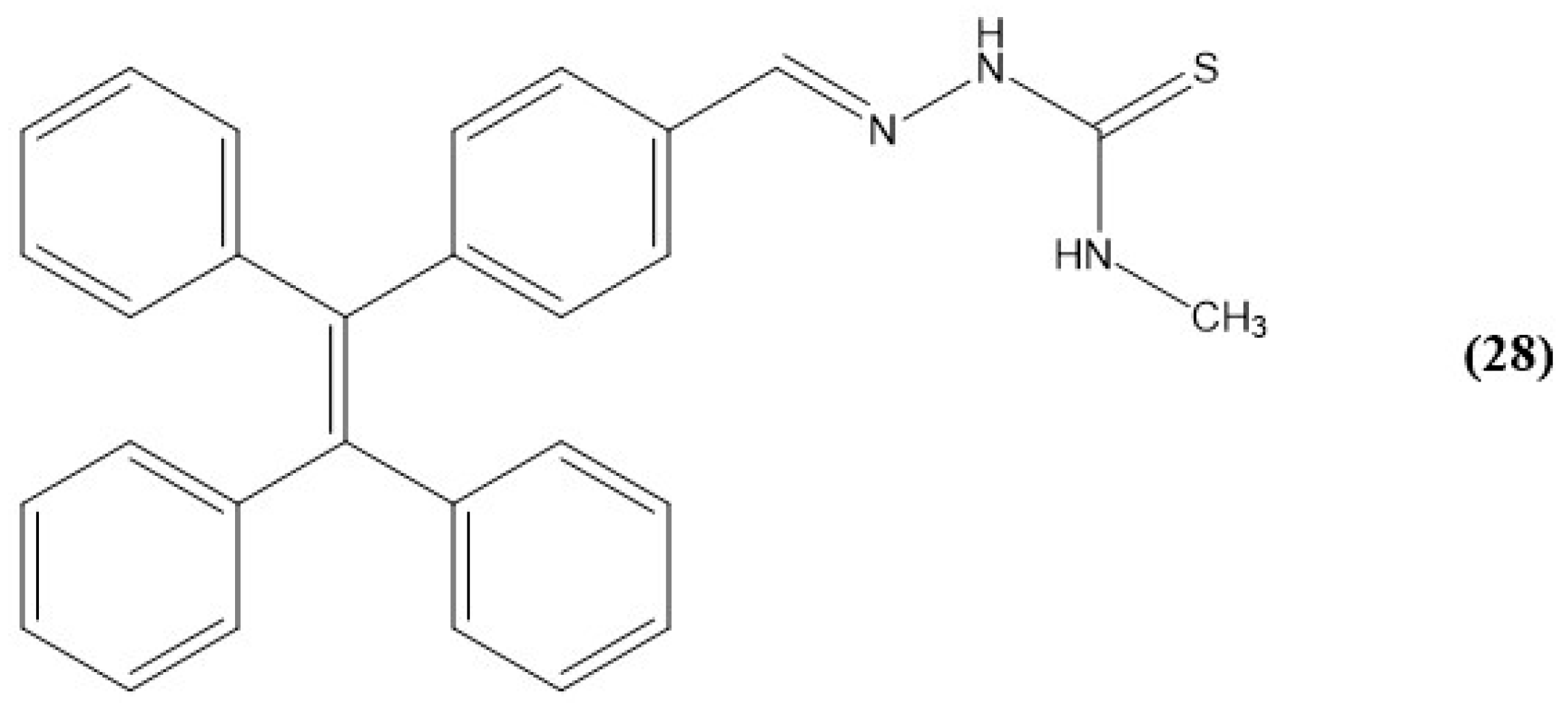
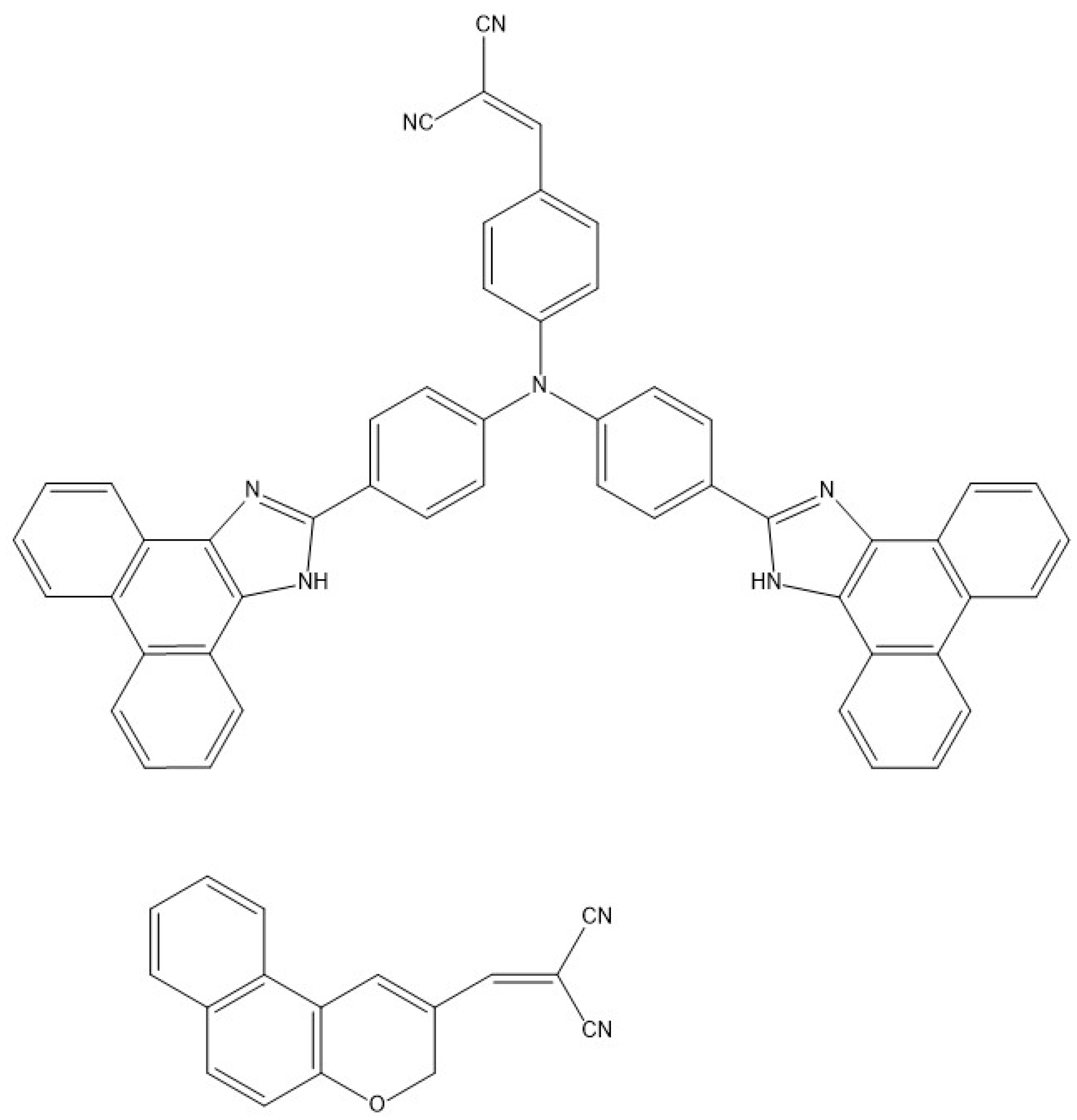
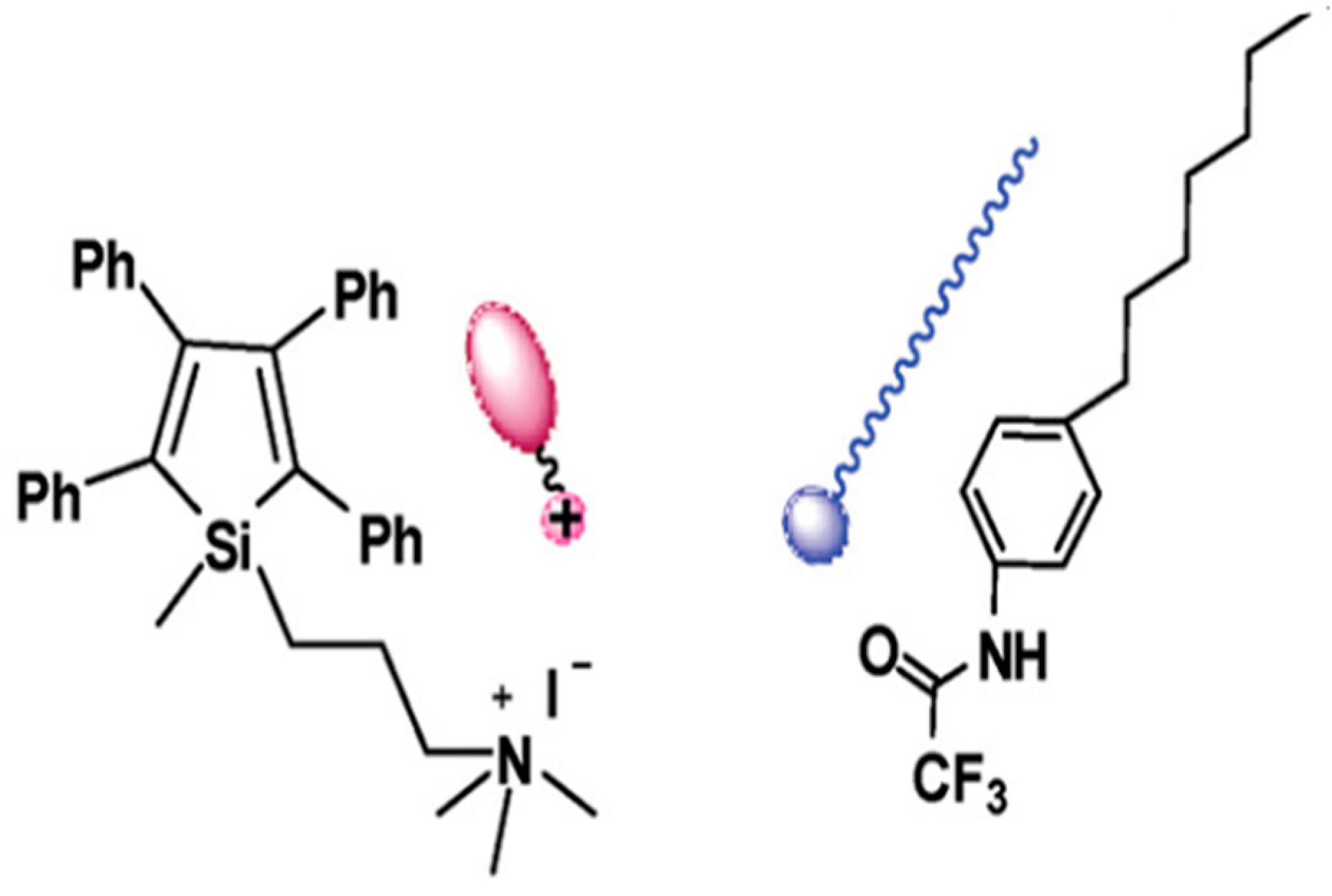
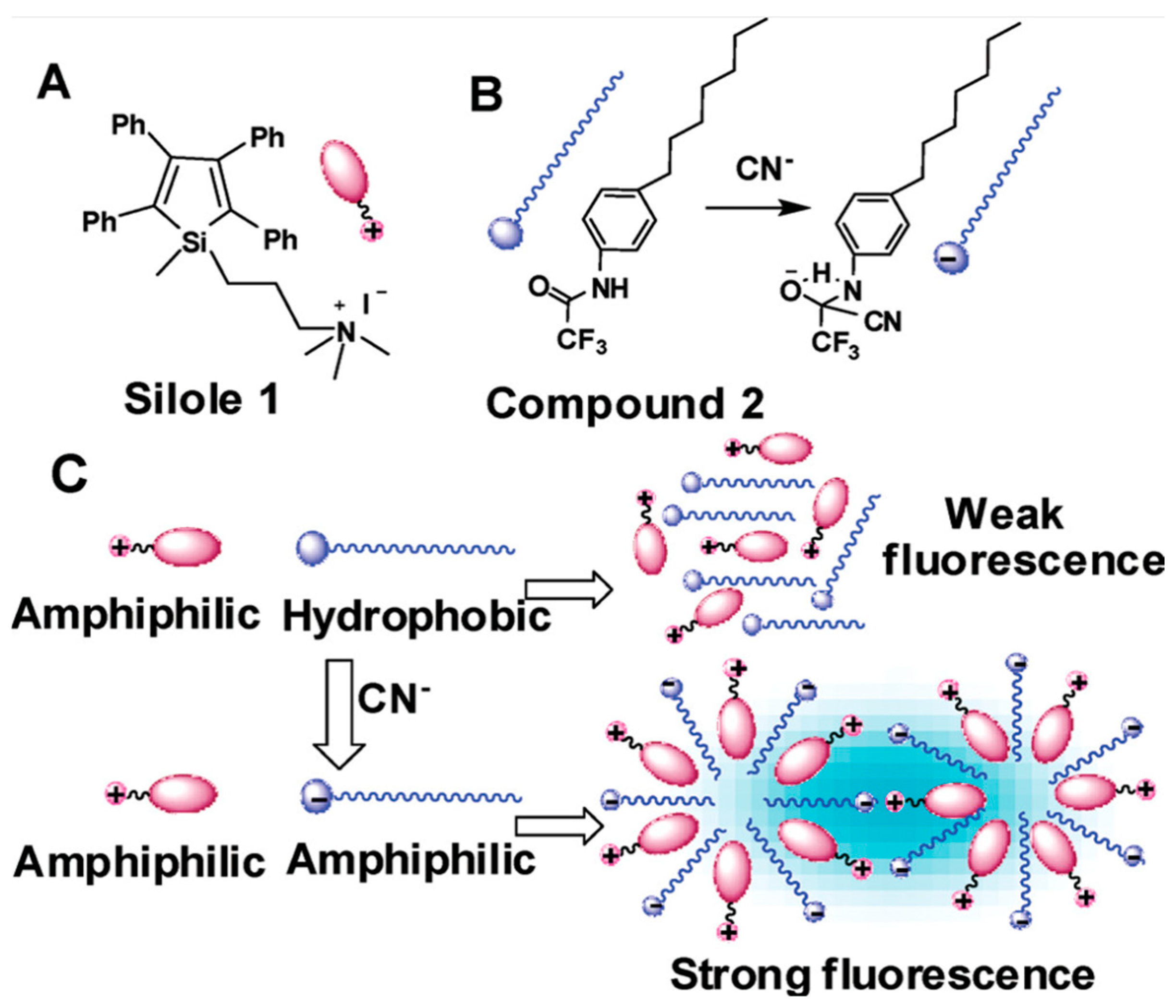
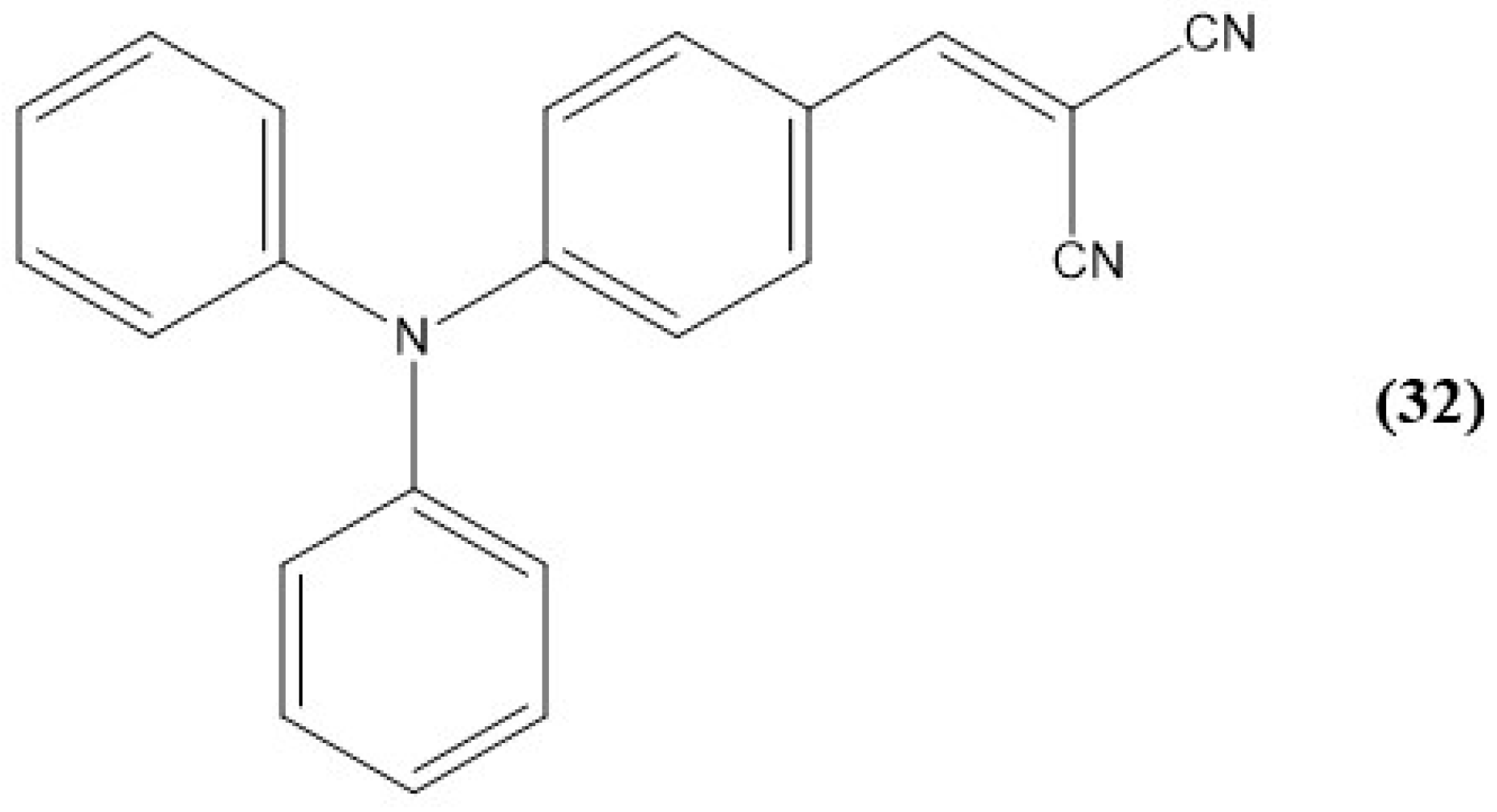
9. Other Examples
10. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, J.; Okunola, O.; Quesada, R. Recent advances in the transmembrane transport of anions. Chem. Soc. Rev. 2010, 39, 3843–3862. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tong, C. A Specific Turn-On Fluorescent Sensing for Ultrasensitive and Selective Detection of Phosphate in Environmental Samples based on Antenna Effect-Improved FRET by Surfactant. ACS Sens. 2018, 3, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bonizzoni, M. A Supramolecular Sensing Array for Qualitative and Quantitative Analysis of Organophosphates in Water. J. Am. Chem. Soc. 2018, 3, 1539–1545. [Google Scholar] [CrossRef]
- Dong, Z.-Z.; Yang, C.; Vellaisamy, K.; Li, G.; Leung, C.-H.; Ma, D.-L. Construction of a Nano Biosensor for Cyanide Anion Detection and Its Application in Environmental and Biological Systems. ACS Sens. 2017, 2, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Steed, J. Coordination and organometallic compounds as anion receptors and sensors. Chem. Soc. Rev. 2009, 38, 506–519. [Google Scholar] [CrossRef]
- Choudhury, N.; Saha, B.; Ruidas, B.; De, P. Dual-Action Polymeric Probe: Turn-On Sensing and Removal of Hg2+; Chemosensors for HSO4−. ACS Appl. Polym. Mater. 2019, 1, 461–471. [Google Scholar] [CrossRef]
- Chandra Rao, P.; Mandal, S. Europium-Based Metal-Organic Framework as a Dual Luminescence Sensor for the Selective Detection of the Phosphate Anion and Fe3+ Ion in Aqueous Media. Inorg. Chem. 2018, 57, 11855–11858. [Google Scholar] [CrossRef]
- Lin, B.; Xu, J.; Lin, K.; Li, M.; Lu, M. Low-Cost Automatic Sensor for in Situ Colorimetric Detection of Phosphate and Nitrite in Agricultural Water. ACS Sens. 2018, 3, 2541–2549. [Google Scholar] [CrossRef]
- Kaushik, R.; Ghosh, A.; Singh, A.; Cupta, P.; Mittal, A.; Jose, D.A. Selective Detection of Cyanide in Water and Biological Samples by an Off-the-Shelf Compound. ACS Sens. 2016, 1, 1265–1271. [Google Scholar] [CrossRef]
- Xiang, H.; Cai, Q.; Li, Y.; Zhang, Z.; Cao, L.; Li, K.; Yang, H. Sensors Applied for the Detection of Pesticides and Heavy Metals in Freshwaters. J. Sens. 2020, 2020, 8503491. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jabbari, J. Simple naked-eye colorimetric chemosensors based on Schiff-base for selective sensing of cyanide and fluoride ions. Can. J. Chem. 2016, 94, 631–636. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, C.; Gong, R.; Mu, H.; Fu, E. Ratiometric Fluorescent Chemodosimeter with Selective Recognition for Sulfite in Aqueous Solution. J. Org. Chem. 2009, 74, 7943–7946. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Li, K.; Li, C.-Y.; Hou, J.-T.; Yu, X.-Q. A water soluble near IR probe for colorimetric and ratiometric detection of sulfite in living cells. Chem. Commun. 2014, 50, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Su, S.-Y.; Cheng, X.-T.; Shen, L.-Y.; Zhang, Q.-L.; Ni, X.-L.; Xu, H.; Wang, Z.-Y.; Redshaw, C. A New Cationic Fluorescent Probe for HSO3− Based on Bisulfite Induced Aggregation Self-Assembly. Molecules 2022, 27, 2378. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, C.; Gale, P. Anion receptor chemistry: Highlights from 2007. Chem. Soc. Rev. 2009, 38, 520–563. [Google Scholar] [CrossRef]
- Gale, P.; Caltagirone, C. Anion sensing by small molecules and molecular assemblies. Chem. Soc. Rev. 2015, 44, 4212–4227. [Google Scholar] [CrossRef]
- Huston, M.; Akkaya, E.; Czarnik, A. Chelation Enhanced Fluorescence Detection of Non-Metal Ions. J. Am. Chem. Soc. 1989, 111, 8735–8737. [Google Scholar] [CrossRef]
- Suksai, C.; Tuntulani, T. Chromogenic anion sensors. Chem. Soc. Rev. 2003, 32, 192–202. [Google Scholar] [CrossRef]
- Sakai, R.; Barasa, E.; Sakai, N.; Sato, S.-I.; Satoh, T.; Kakuchi, T. Colorimetric Detection of Anions in Aqueous Solution by using Poly(phenylacetylene) with Sulfonamide Receptors Activated by Electron Withdrawing Group. Macromolecules 2012, 45, 8221–8227. [Google Scholar] [CrossRef]
- Sigalov, M.V.; Shainyan, B.A.; Chipanina, N.N.; Oznobikhina, L.P. Intra-and Intermolecular Hydrogen Bonds in Pyrrolylindandione Derivatives and Their Interaction with Fluoride and Acetate: Possible Anion Sensing Properties. J. Phys. Chem. 2013, 117, 11346–11356. [Google Scholar] [CrossRef]
- Kim, D.-S.; Chung, Y.-M.; Jun, M.; Ahn, K.-H. Selective Colorimetric Sensing of Anions in Aqueous Media through Reversible Covalent Bonding. J. Org. Chem. 2009, 74, 4849–4854. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ly, P.; Han, X.-W.; Zheng, M.; Fang, H.-T. A Highly Efficient Fluorescent Sensor Based on AIEgen for Detection of Nitrophenolic Explosives. Molecules 2022, 28, 181. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhong Tang, B. Fluorescent Sensors based on Aggregation-Induced Emission: Recent Advances and Perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Manez, R.; Sancenon, F. Fluorogenic and Chromogenic Chemosensors and Reagents for Anions. Chem. Rev. 2003, 103, 4419–4476. [Google Scholar] [CrossRef]
- Sessler, J.; Camiolo, S.; Gale, P. Pyrrolic and polypyrrolic anion binding agents. Coord. Chem. Rev. 2003, 240, 17–55. [Google Scholar] [CrossRef]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated Polymer-Based Chemical Sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef]
- Aldakov, D.; Palacios, M.; Anzenbacher, P. Benzothiadiazoles and Dipyrrolyl Quinoxalines with Extended Conjugated Chromophores-Fluorophores and Anion Sensors. Chem. Mater. 2005, 17, 5238–5241. [Google Scholar] [CrossRef]
- Sokkalingam, P.; Lees, C.-H. Highly Sensitive Fluorescence “Turn-On” Indicator for Fluoride Anion with Remarkable Selectivity in Organic and Aqueous Media. J. Org. Chem. 2011, 76, 3820–3828. [Google Scholar] [CrossRef]
- Oshchepkov, A.S.; Shumilova, T.A.; Namashivaya, S.R.; Fedorova, O.A.; Dorovatovskii, P.V.; Khrustalev, V.N.; Kataev, E.A. Hybrid Macrocycles for Selective Binding and Sensing of Fluoride in Aqueous Solution. J. Org. Chem. 2018, 83, 2145–2153. [Google Scholar] [CrossRef]
- Avinash, I.; Parveen, S.; Anantharaman, G. Backbone Boron-Functionalized Imidazoles/Imidazolium Salts: Synthesis, Structure, Metalation Studies, and Fluoride Sensing Properties. Inorg. Chem. 2020, 59, 5646–5661. [Google Scholar] [CrossRef]
- Yu, H.; Fan, M.; Su, Z.; Pan, Q.; Hu, X. Two Highly Water-Stable Imidazole-Based Ln-MOFs for Sensing Fe3+, Cr2O72−/CrO42− in a Water Environment. Inorg. Chem. 2020, 59, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.-S.; Ng, M.; Yeung, M.; Yam, V. Platinum(II)-Based Host-Guest Coordination-Driven Supramolecular Co-Assembly Assisted by Pt-Pt and π-π Stacking Interactions: A Dual- Selective Luminescence Sensor for Cations and Anions. JACS 2021, 143, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Moretto, L.M.; Ugo, P.; Zanata, M.; Guerriero, P.; Martin, C.R. Nitrate Biosensor Based on the Ultrathin-Film Composite Membrane Concept. Anal. Chem. 1998, 70, 2163–2166. [Google Scholar] [CrossRef]
- Hein, R.; Beer, P.D.; Davis, J.J. Electrochemical Anion Sensing: Supramolecular Approaches. Chem. Rev. 2020, 123, 1888–1935. [Google Scholar] [CrossRef]
- Choi, S.-J.; Yoon, B.; Lin, S.; Swager, T. Functional Single-Walled Carbon Nanotubes for Anion Sensing. ACS Appl. Mater. Inter. 2020, 12, 28375–28382. [Google Scholar] [CrossRef]
- Kwon, H.; Jiang, W.; Kool, E. Pattern-based detection of anion pollutants in water with DNA polyfluoropores. Chem. Sci. 2015, 6, 2575–2583. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P. Handbook of Water and Wastewater Treatment Techologies; Butterworth-Heinemann: Boston, MA, USA, 2022; pp. 446–495. [Google Scholar]
- Vennesland, B.; Comm, E.E.; Knownles, C.J.; Westly, J.; Wissing, F. Cyanide in Biology; Academic Press: London, UK, 1981. [Google Scholar]
- Noh, J.Y.; Hwang, I.H.; Kim, H.; Song, E.J.; Kim, K.B.; Kim, C. Salicylimine-Based Colorimetric and Fluorescent Chemosensor for Selective Detection of Cyanide in Aqueous Buffer. Bull. Korean Chem. Soc. 2013, 34, 1985–1989. [Google Scholar] [CrossRef][Green Version]
- Park, G.J.; Hwang, I.G.; Song, E.J.; Kim, H.; Kim, C. A colorimetric and fluorescent sensor for sequential detection of copper ion and cyanide. Tetrahedron 2014, 70, 2822–2828. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, P.; Zhong, K.; Hou, S. Fluorescence relay enhancement sequential recognition of Cu2+ and CN− by a new quinazoline derivative. Sens. Act. B 2013, 182, 439–445. [Google Scholar] [CrossRef]
- Yang, R.; Wu, W.; Wang, W.; Li, Z.; Qin, J. Copolymer of Fluorene and Triphenylamine Moieties: Direct and Post-Functionalization Strategy, Structural Characterization, and Chemosensoring Behavior. Macromol. Chem. Phys. 2010, 211, 18–26. [Google Scholar] [CrossRef]
- Dong, S.; Ou, D.; Quin, J.; Li, Z.J. New Imidazole-Functionalized Polyfluorene Derivatives: Convenient Postfunctional Syntheses, Sensitive Probes for Metal Ions and Cyanide, and Adjustable Output Signals with Diversified Fluorescence Color. Polym. Sci. A1. 2011, 49, 3314–3327. [Google Scholar] [CrossRef]
- Chen, X.; Nam, S.; Kim, G.; Song, N.; Jeong, Y.; Shin, I.; Park, S.; Kim, K.J.; Park, S.; Yoon, J. A near-infrared fluorescent sensor for detection of cyanide in aqueous solution and its application for bioimaging. Chem. Commun. 2010, 46, 8953–8955. [Google Scholar] [CrossRef]
- Yong Jo, H.; Lee, S.A.; Na, Y.J.; Park, G.J.; Kim, C. A colorimetric Schiff base chemosensor for CN− by naked-eye in aqueous solution. Inorg. Chem. Comm. 2015, 54, 73–76. [Google Scholar]
- Kulig, K.W. Cyanide Toxicity; U.S. Department of Health and Human Services: Atlanta, GA, USA, 1991. [Google Scholar]
- Sumiya, S.; Doi, T.; Shiraishi, Y.; Hirai, T. Colorimetric sensing of cyanide anion in aqueous media with a fluorescein-spiropyran conjugate. Tetrahedron 2012, 68, 690–696. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Chem, X.; Yoon, J. Recent progress in the development of fluorometric and colorimetric chemosensros for detection of cyanide ions. Chem. Soc. Rev. 2014, 43, 4312–4324. [Google Scholar] [CrossRef]
- Kim, H.J.; Ko, K.C.; Lee, J.H.; Lee, J.Y.; Kim, S.S. KCN sensor: Unique chromogenic and ‘turn-on’ fluorescent chemodosimeter: Rapid response and high selectivity. Chem. Commun. 2011, 47, 2886–2888. [Google Scholar] [CrossRef]
- Odago, M.; Colabello, M.; Lees, A.J. A simple thiourea based colorimetric sensor for cyanide anion. Tetrahedron 2010, 66, 7465–7471. [Google Scholar] [CrossRef]
- Yuan, Y.-X.; Wang, J.-H.; Zheng, Y.-S. Selective Fluorescence Turn-On Sensing of Phosphate Anion in Water by Tetraphenylethylene Dimethylformamidine. Chem. Asian J. 2019, 14, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Swamy, K.M.K.; Ki, S.K.; Kwon, J.-Y.; Kim, Y.; Kim, S.-J.; Yoon, Y.J.; Yoon, J. Simple but Effective Way to Sense Pyrophosphate and Inorganic Phosphate by Fluorescence Changes. Org. Lett. 2007, 9, 243–246. [Google Scholar] [CrossRef]
- Aydin, I.; Atdin, F.; Saydut, A.; Bakirdere, E.G.; Hamamci, C. Hazardous metal geochemistry of sedimentary phosphate rock used for fertilizer. Microchem. J. 2010, 96, 247–251. [Google Scholar] [CrossRef]
- Lawal, A.T.; Adeloju, S.B. Polypyrrole Based amperometric and potentiometric phosphate biosensors: A comparative study. J. Appl. Sci. 2012, 12, 315–325. [Google Scholar] [CrossRef][Green Version]
- Warwick, C.; Guerreiro, A.; Soares, A. Sensing and analysis of soluble phosphates in environmental samples: A review. Biosens. Bioelect. 2013, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.T.; Adeloju, S.B. Progress and recent advances in phosphate sensors: A review. Talanta 2013, 114, 191–203. [Google Scholar] [CrossRef]
- Berchmans, S.T.; Issa, T.B.; Sing, P. Determination of inorganic phosphate by electroanalytical methods. Anal. Chimi. Acta 2012, 729, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Reza, M.N.; Jeong, J.-T.; Lee, K.-H. Sensing Technology for Rapid Detection of Phosphorus in Water: A review. J. Biosyst. Eng. 2016, 41, 139–144. [Google Scholar] [CrossRef]
- Parsons, S.; Smith, J. Phosphates and Global Sustainability. Phorphorus Removal and Recovery from Municipal Wastewaters. Elements 2008, 4, 109–112. [Google Scholar] [CrossRef]
- Worsfold, P.J.; Gimbert, L.J.; Mankasingh, U.; Omaka, O.N.; Hanrahan, G.; Gardolinski, P.C.; Haygart, P.M.; Turner, B.L.; Keith-Roach, M.J.; McKelvie, I.D. Sampling, sample treatment and quality assurance issues for the determination of phosphorus species in natural waters and soils. Talanta 2005, 66, 273–293. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. Development of a Sequential Extraction Method for Different Forms of Phosphorus in Marine Sediments. Limnol. Oceanogr. 1992, 37, 1460–1482. [Google Scholar] [CrossRef]
- Lu, C.; Wang, B.; He, J.; Vogt, R.D.; Zhou, B.; Guan, R.; Zuo, L.; Wang, W.; Xie, Z.; Wang, J.; et al. Responses of Organic Phosphorus Fractionation to Environmental Conditions and Lake Evolution. Environ. Sci. Technol. 2016, 50, 5007–5016. [Google Scholar] [CrossRef]
- Jin, X.; Jiang, X.; Yao, Y.; Li, L.; Wu, F. Effects of light and oxygen on the uptake and distribution of phosphorus at the sediment-water interface. Sci. Total Environ. 2006, 357, 231–236. [Google Scholar] [CrossRef]
- Tallberg, P.; Treguer, P.; Beucher, C.; Corvaisier, R. Potentially mobile pools of phosphorus and silicon in sediment from the Bay of Brest: Interactions and implications for phosphorus dynamics. Estuarine. Coastal Shelf. Sci. 2008, 76, 85–94. [Google Scholar] [CrossRef]
- Van der Zee, C.; Roevros, N.; Chou, L. Phosphorus speciation, transformation and retention in the Scheldt estuary (Belgium/The Netherlands) from the freshwater tidal limits to the North Sea. Mar Chem. 2007, 106, 76–91. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Zhao, H.; Zhou, X.; Wu, F. Effects of organic matter on phosphorus release kinetics in different trophic lake sediments and application of transition state theory. J. Environ. Manag. 2008, 88, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Huang, C.M.; Zeng, H.A.; Schleser, G.H.; Battarbee, R. Sedimentary evidence for recent eutrophication in the northern basin of Lake Taihu, China: Human impacts on a large shallow lake. J Paleolimnol. 2007, 38, 13–23. [Google Scholar]
- Turner, B.L.; Frossard, E.; Baldwin, D.S. Organic Phosphorus in the Environment; CABI Publishing: Cambridge, UK, 2005. [Google Scholar]
- Zhang, W.; Zhang, Y.; Fan, R.; Lewis, R. A facile TiO2/PVDF composite membrane synthesis and their application in water purification. J. Nanopart. Res. 2016, 18, 31. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Yang, Y.; Gaskin, P.; Seng Teng, L. Recent Advances on Electrochemical Sensors for the Detection of Organic Disinfection Byproducts in Water. ACS Sens. 2019, 4, 1138–1150. [Google Scholar] [CrossRef]
- Jose Farre, M.; Reungoat, J.; Argaud, F.X.; Ratter, M.; Keller, J.; Gernjak, W. Fate of N-nitrosodimethylamine, trihalomethane and haloacetic acid precursors in tertiary treatment including biofiltration. Water Res. 2011, 45, 5695–5704. [Google Scholar] [CrossRef]
- Ghernaout, D.; Ghernaout, B. From chemical disinfection to electrodisinfection: The obligatory itinerary? Desalin. Water Treat. 2010, 16, 156–175. [Google Scholar] [CrossRef]
- USEPA (US Environmental Protection Agency). National Primary Drinking Water Regulations: Stage 2 Disinfectants and Disinfection Byproducts Rule: Final Rule. Fed. Reg. 2006, 71, 387–493. [Google Scholar]
- Lee, Y.-G.; Jang, A. Application of sensitive electrochemical sensing system for detecting bromate from disinfection process in desalination plant. Desalination 2017, 423, 135–140. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking Water Quality; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- European Communities. European Communities (Drinking Water) (No. 2) Regulations; European Union: Brussels, Belgium, 2007. [Google Scholar]
- Wu, T.; Karimi-Maleg, H.; Dragoi, E.C.; Puri, P.; Zhang, D.; Zhang, Z. Traditional methods and biosensors for detecting disinfection by-products in water: A review. Environ. Res. 2023, 237, 116935. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, X.; Zhu, Y.; Huang, Y.; Chen, B. Ecotoxicological effects of DBPs on freshwater phytoplankton communities in co-culture systems. J. Haz. Mater. 2022, 421, 126679. [Google Scholar] [CrossRef]
- Xue, B.; Guo, X.; Cao, J.; Yang, S.; Qiu, Z.; Wang, J.; Shen, Z. The occurrence, ecological risk, and control of disinfection by-products from intensified wastewater disinfection during the COVID-19 pandemic. Sci. Tot. Environ. 2023, 900, 165602. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, S.; Qian, X.; Shimizu, T.; Dente, S.M.R.; Hasimoto, S.; Nakajima, J. Assessment of the municipal water cycle in China. Sci. Total Environ. 2017, 607, 761–770. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Xue, B.; Yang, X.; Wang, S.; Li, C.; Zhang, X.; Zhao, C.; Wang, X.; Qiu, Z.; Shen, Z.; et al. Low-concentration of trichloromethane and dichlororacetonitrile promote the plasmid-mediated horizontal transfer of antibiotic resistance genes. J. Haz. Mater. 2022, 425, 128030. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, V.; Siciliano, A.; Guida, M.; Libralato, G.; Saviano, L.; Luongo, G.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Disinfection by-products and ecotoxic risk associated with hypochlorite treatment of irbesartan. Sci. Total Environ. 2020, 712, 135625. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Yang, W.; Wang, L. Aquatic toxicity and aquatic ecological risk assessment of wastewater-derived halogenated phenolic disinfection byproducts. Sci. Total Environ. 2020, 809, 151089. [Google Scholar] [CrossRef]
- Krasner, S.W.; Westerhoff, P.; Chen, B.; Rittmann, B.E.; Amy, G. Occurrence of disinfection byproducts in United States wastewater treatment plant effluents. Environ. Sci. Technol. 2009, 43, 8320–8325. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Huang, Z.; Hu, S.; Wang, J.; Qian, Z.; Feng, J.; Xian, Q.; Gong, T. Occurrence and ecological risk assessment of disinfection byproducts from chlorination of wastewater effluents in East China. Water Res. 2019, 157, 247–257. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. National Primary Drinking Water Regulations: Contaminant Specific Fact Sheets, Inorganic Chemicals; Consumer Version: Washington, DC, USA, 1995.
- Moorcroft, M.J.; Davis, J.; Compton, R.G. Detection and determination of nitrate and nitrite: A review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- Fanning, J.C. The chemical reduction of nitrite in aqueous solution. Coord. Chem. Rev. 2000, 199, 159–179. [Google Scholar] [CrossRef]
- Nollet, L.M.L. Handbook of Water Analysis, Marcel Dekker: New York, NY, USA, 2000.
- World Health Organization. Health criteria and other supporting information. In Guidelines for Drinking Water Quality, 2nd ed.; World Health Organization: Geneva, Switzerland, 1998; Volume 2. [Google Scholar]
- Rajasulochana, P.; Ganesan, Y.; Kumar, P.S.; Mahalazmi, S.; Tasneem, F.; Ponnuchamy, M.; Kapoor, A. Paper-Based Microfluidic Colorimetric Sensor on a 3D Printed Support for Quantitative Detection of Nitrite in Aquatic Environments. Environ. Res. 2022, 208, 112745. [Google Scholar] [CrossRef]
- Burt, T.P.; Howden, N.J.K.; Worral, F.; Whelan, M.J. Long-term monitoring of river water nitrate: How much data do we need? J. Environ. Monit. 2010, 12, 71–79. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smit, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Zia, H.; Harris, N.R.; Merrett, G.V.; Rivers, M.; Coles, N. The impact of agricultural activities on water quality: A case for collaborative catchment-scale management using integrated wireless sensor networks. Comput. Electron. Agr. 2013, 96, 126–138. [Google Scholar] [CrossRef]
- Ye, D.; Luo, L.; Chen, Q.; Liu, X. A novel nitrite sensor based on graphene/polypyrrole/chitosan nanocomposite modified glassy carbon electrode. Analyst 2011, 136, 4563–4569. [Google Scholar] [CrossRef]
- Zuane, J.D. Handbook of Drinking Water Quality, 2nd ed.; Wiley: Hoboken, New Jersey, USA, 1996. [Google Scholar]
- Bryan, N.S.; Fernandez, B.O.; Bauer, S.M.; Garcia-Saura, M.F.; Milson, A.B.; Rassaf, T.; Maloney, R.E.; Bharti, A.; Rodriguez, J.; Feelisch, M. Nitrite is a Signalling Molecule and Regulator of Gene Expression in Mammalian Tissues. Nat. Chem. Biol. 2005, 1, 290–297. [Google Scholar] [CrossRef]
- Song, P.; Wu, L.; Guan, W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: A meta-analysis. Nutrients 2015, 7, 9872–9895. [Google Scholar] [CrossRef]
- Balimandawa, M.; de Meester, C.; Leonard, A. The mutagenicity of nitrite in the Salmonella/microsome test system. Mutat. Res. Genet. Toxicol. 1994, 321, 7–11. [Google Scholar] [CrossRef]
- Ma, L.; Hu, L.; Feng, X.; Wang, S. Nitrate and Nitrite in Health and Disease. Aging Dis. 2018, 9, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Shepard, S.E. Endogenous formation of N-nitroso compounds in relation to the intake of nitrate or nitrite. In Health Aspects of Nitrate and Its Metabolites; Council of Europe: Strasbourg, France, 1995; pp. 137–150. [Google Scholar]
- Cheng, X.H.; Jia, H.Z.; Long, T.; Feng, J.; Qin, J.; Li, Z. A “turn-on” fluorescent probe for hypochlorous acid: Convenient synthesis, good sensing performance, and a new design strategy by the removal of C = N isomerization. Chem. Commun. 2011, 47, 11978–11980. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhang, G.; Zhou, M.; Xin, X.; Chen, S.; Duan, X. Two Reaction-Based Fluorescent Sensors with Cationic Group Enable High-Selective Detection of HSO3− in the Environment. Ind. Eng. Chem. Res. 2019, 58, 9231–9238. [Google Scholar] [CrossRef]
- Chen, L.; Deborba, B.; Rohrer, J. Determination of Total and Free Sulfite in Foods and Beverages. Thermo Fish. Sci. 2016, 1–8. [Google Scholar]
- Duan, C.; Won, M.; Verwilst, P.; Xu, J.C.; Kim, H.S.; Zeng, L.T.; Kim, J.S. In vivo imaging of endogenously produced HClO in zebrafish and mice using a bright, photostable ratiometric fluorescent probe. Anal. Chem. 2019, 91, 4172–4178. [Google Scholar] [CrossRef]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef]
- Common Anion Analysis by U.S. EPA 300.0 & 300.1. Available online: https://www.thermofisher.com/us/en/home/industrial/environmental/environmental-learning-center/contaminant-analysis-information/anion-analysis/common-anions-analysis-epa-300-0-300-1.html (accessed on 10 February 2025).
- Scheiner, S. Hydrogen Bonding: A Theoretical Perspective; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Molina, P.; Zapata, F.; Caballero, A. Anion Recognition Strategies Based on Combined Noncovalent Interactions. Chem. Rev. 2017, 117, 9907–9972. [Google Scholar] [CrossRef]
- Maiti, N.C.; Maxumdar, S.; Periasamy, N. J-and H-aggregates of porphyrin-surfactant complexes: Time-resolved fluorescence and other spectroscopic studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, W.-H.; Xie, Y. Development of Ion Chemosensors Based on Porphyrin Analogues. Chem. Rev. 2017, 117, 2203–2256. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Amendola, V.; Fabbrizzi, L.; Mosca, L. Anion recognition by hydrogen bonding: Urea-based receptors. Chem. Soc. Rev. 2010, 39, 3889–3915. [Google Scholar] [CrossRef] [PubMed]
- Busschaert, N.; Caltagirone, C.; Van Rossom, W.; Gale, P.A. Applications of supramolecular anion recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef]
- Phipps, R.J.; Hamilton, G.L.; Toste, D. The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem. 2012, 4, 603–614. [Google Scholar] [CrossRef]
- Bell, K.J.; Westra, A.N.; Warr, R.J.; Chartres, J.; Ellis, R.; Tong, C.C.; Blake, A.J.; Tasker, P.A.; Schroder, M. Outer-Sphere Coordination Chemistry: Selective Extraction and Transport of the [PtCl6]2− Anion. Angew. Chem. Int Ed. 2008, 47, 1745–1748. [Google Scholar] [CrossRef] [PubMed]
- Pike, S.J.; Hutchinson, J.J.; Hunter, C.A. H-Bond Acceptor Parameters for Anions. J. Am. Chem. Soc. 2017, 139, 6700–6706. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Simmons, H.E. Macrobicyclic Amines. III. Encapsulation of Halide Ions by in,in-1,(k + 2)-Diazabicyclo [k.l.m.] alkane Ammonium Ions. J. Am. Chem. Soc. 1968, 90, 2431–2432. [Google Scholar] [CrossRef]
- Llinares, J.M.; Powell, D.; Bowman-James, K. Ammonium Based Anion Receptors. Coord. Chem. Rev. 2003, 240, 57–75. [Google Scholar] [CrossRef]
- Garcia-Espana, E.; Diaz, P.; Llinares, J.M.; Bianchi, A. Anion Coordination Chemistry in Aqueous Solution of Polyammonium Receptors. Coord. Chem. Rev. 2006, 250, 2952–2986. [Google Scholar] [CrossRef]
- Hosseini, M.W.; Lehn, J.-M. Anion Receptor Molecules. Chain Length Dependent Selective Binding of Organic and Biological Dicarboxylate Anions by Ditopic Polyammonium Macrocycles. J. Am. Chem. Soc. 1982, 104, 3525–3527. [Google Scholar] [CrossRef]
- Schmidtchen, F.P. Macrocyclic Quaternary Ammonium Salts. II. Formation of Inclusion Complexes with Anions in Solution. Chem. Ber. 1981, 114, 597–607. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Bencini, A.; Giorgi, C.; Valtancoli, B.; Lippolis, V.; Perra, A. Exploring the Binding Ability of Polyammonium Hosts for Anion Substrates: Selective Size-Dependent Recognition of Different Phosphate Anions by Bis-macrocyclic Receptors. Inorg. Chem. 2011, 50, 7202–7216. [Google Scholar] [CrossRef] [PubMed]
- Bencini, A.; Coluccini, C.; Garau, A.; Giorgi, C.; Lippolis, V.; Messori, L.; Pasini, D.; Puccioni, S. A BINOL-Based Chiral Polyammonium Receptor for Highly Enantioselective Recognition and Fluorescence Sensing of (S, S)-Tartaric Acid in Aqueous Solution. Chem. Commun. 2012, 48, 10428–10430. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Kang, S.A.; Kut, J.A.; Day, V.W.; Bowman-James, K. Influence of Charge on Anion Receptivity in Amide-Based Marocycles. Inorg. Chem. 2012, 51, 4833–4840. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.; Tarraga, A.; Oton, F. Imidazole Derivatives: A Comprehensive Survey of their Recognition Properties. Org. Biomol. Chem. 2012, 10, 1711–1724. [Google Scholar] [CrossRef]
- Cai, J.; Sessler, J.L. Neutral CH and Cationic CH Donor Groups as Anion Receptors. Chem. Soc. Rev. 2014, 43, 6198–6213. [Google Scholar] [CrossRef]
- Steiner, T. Cooperative C≡C-H···C≡C-H Interactions: Crystal Structure of DL-Prop-2-ynylglycine and Database Study of Terminal Alkynes. J. Chem. Soc. Chem. Commun. 1995, 1, 95–96. [Google Scholar] [CrossRef]
- Nishio, M.; Umezawa, Y.; Fantini, J.; Weiss, M.S.; Chakrabarti, P. CH-π Hydrogen Bonds in Biological Macromolecules. Phys. Chem. Phys. 2014, 16, 12648–12683. [Google Scholar] [CrossRef]
- Nishio, M.; Umezawa, Y.; Honda, K.; Tsuboyama, S.; Suezawae, H. CH/π Hydrogen Bonds in Organic and Organometallic Chemistry. Cryst. Eng. Commun. 2009, 11, 1757–1788. [Google Scholar] [CrossRef]
- Nishio, M.; Hirota, M.; Umezawa, Y. The CH/π Interaction: Evidence, Nature, Consequences; Wiley-VCH Varlag GmbH & Co. KGaA.: New York, NY, USA, 1998. [Google Scholar]
- Amendola, V.; Boiocchi, M.; Fabbrizzi, L. Anion Receptors Containing -NH Binding Sites: Hydrogen-Bond Formation or Neat Proton Transfer? Chem. Eur. J. 2004, 11, 120–127. [Google Scholar] [CrossRef]
- Cametti, M.; Rissanen, K. Recognition and sensing of fluoride anion. Chem. Commun. 2009, 20, 2809–2829. [Google Scholar] [CrossRef]
- Fabbrizzi, L.; Licchelli, M.; Rabaioli, G.; Taglietti, A. The design of luminescent sensors for anions and ionizable analytes. Coord. Chem. Rev. 2000, 205, 85–108. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry, Concepts and Perspectives; VCH: Weinheim, Germany, 1995; pp. 31–35. [Google Scholar]
- De Silva, A.P.; Guanaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Fabbrizzi, L.; Poggi, A. Sensors and switches from supramolecular chemistry. Chem. Soc. Rev. 1995, 24, 197–202. [Google Scholar] [CrossRef]
- Isiklan, M.; Saeed, M.A.; Pramanki, A.; Wong, B.M.; Fronzcek, F.R.; Hossain, M.A. A C3 Symmetric Nitrate Complex with a Thiophene-Based Tripodal Receptor. Cryst. Growth Des. 2010, 10, 1478–1481. [Google Scholar]
- Li, A.-F.; Wang, J.-H.; Wang, F.; Jiang, Y.-B. Anion complexation and sensing using modified urea and thiourea-based receptors. Chem. Soc. Rev. 2010, 39, 3729–3745. [Google Scholar] [CrossRef]
- Cametti, M.; Rissanen, K. Highlights on contemporary recognition and sensing of fluoride anion in solution and in the solid state. Chem. Soc. Rev. 2013, 42, 2016–2038. [Google Scholar] [CrossRef]
- Bissel, R.A.; de Silva, A.P.; Guanaratne, H.Q.N.; Lynch, P.L.M.; Maguire, G.E.M.; Sandanayake, K.R.A.S. Molecular fluorescent signalling with ‘flour-spacer-receptor’ systems: Approaches to sensing and switching devices via supramolecular photophysics. Chem. Soc. Rev. 1992, 21, 187–195. [Google Scholar] [CrossRef]
- Azenbacher, P., Jr.; Jursikova, K.; Aldakov, D.; Marquex, M.; Pohl, R. Materials chemistry approach to anion-sensor design. Tetrahedron 2004, 60, 11163–11168. [Google Scholar] [CrossRef]
- Niikura, K.; Metzger, A.; Anslyn, E.V. Chemosensor ensemble with selectivity for inositol-triphosphate. J. Am. Chem. Soc. 1998, 120, 8533–8534. [Google Scholar] [CrossRef]
- Gale, P.A.; Sessler, J.L.; Kral, V.; Lynch, V. Calix [4] pyrroles: Old yet new anion-binding agents. J. Am. Chem. Soc. 1996, 118, 5140–5141. [Google Scholar] [CrossRef]
- Reetz, M.T.; Niemeyer, C.M.; Harms, K. Crown ethers with a Lewis acidic center: A new class of heterotropic host molecules. Angew. Chem. Int. Ed. Engl. 1991, 30, 1472–1474. [Google Scholar] [CrossRef]
- Morzherin, Y.; Rudkevich, D.M.; Verboom, W.; Reinhoudt, D.N. Chlorosulfonated calix [4] arenes: Precursors for neutral anion receptors with selectivity for hydrogen sulphate. J. Org. Chem. 1993, 58, 7602–7605. [Google Scholar] [CrossRef][Green Version]
- Casnati, A.; Fochi, M.; Minari, P.; Pochini, A.; Reggiani, M.; Ungaro, R.; Reinhoudt, D.N. Upper-rim urea-derivatised calix [4] arenes as neutral receptors for monocarboxylate anions. Gass. Chim. Ital. 1996, 126, 99–106. [Google Scholar]
- Cameron, B.R.; Love, S.J. Bis(amido)calix [4] arene in the pinched cone conformation as tuneable hydrogen-bonding anion receptors. J. Chem. Soc. Chem. Commun. 1997, 6, 573–574. [Google Scholar] [CrossRef]
- Beer, P.D.; Hazlewood, C.; Hesek, D.; Hodacova, J.; Stokes, S.E. Anion recognition by acyclic redox-responsive amide-linked cobaltocenium receptor. J. Chem. Soc. Dalton. Trans. 1993, 8, 1327–1332. [Google Scholar] [CrossRef]
- Raposo, C.; Perez, N.; Almaraz, M.; Luisa Mussons, M.; Cruz Cabarello, M.; Moran, J.R. A cyclohexane spacer for phosphate receptors. Tetrahedron Lett. 1995, 36, 3255–3258. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nuriman, N.A.; Verboom, W.; Reinhoudt, D.N. Tripodal Receptors for Cation and Anion Sensors. Sensors 2006, 6, 978–1017. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Fang, W.; Li, F. Luminescent Chemodosimeters for Bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Du, J.; Hu, M.; Fan, J.; Peng, X. Fluorescent chemodosimeters using “mild” chemical events for the detection of small anions and cations in biological and environmental media. Chem. Soc. Rev. 2012, 41, 4511–4535. [Google Scholar] [CrossRef]
- Abouderbala, L.O.; Belcher, W.J.; Boutelle, M.G.; Wallace, K.J. Cooperative anion binding and electrochemical sensing by modular podands. Proc. Natl. Acad. Sci. USA 2002, 99, 5001–5006. [Google Scholar] [CrossRef]
- Young Chae, M.; Czarnik, A.W. Fluorometric chemodosimetry. Mercury (II) and silver (I) indication in water via enhanced fluorescence signaling. J. Am. Chem. Soc. 1992, 114, 9704–9705. [Google Scholar] [CrossRef]
- Kaur, K.; Saini, R.; Kumar, A.; Luxami, V.; Kaur, N.; Singh, P.; Kumar, S. Chemodosimeters: An approach for detection and estimation of biologically and medically relevant metal ions, anions and thiols. Coord. Chem. Rev. 2012, 256, 1992–2028. [Google Scholar] [CrossRef]
- Jun, M.E.; Roy, B.; Han Ahn, K. “Turn-on” fluorescent sensing with “reactive” probes. Chem. Comm. 2011, 47, 7583–7601. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.; Manna, S.K.; Banik, D.; Mahapatra, A.K. Name reactions: Strategies in the design of chemodosimeters for analyte detection. New J. Chem. 2021, 45, 20046–20074. [Google Scholar] [CrossRef]
- AbhijinaKrishna, R.; Velmathi, S. A review on fluorimetric and colorimetric detection of metal ions by chemodosimetric approach 2013–2021. Coord. Chem. Rev. 2022, 459, 214401. [Google Scholar] [CrossRef]
- Chinna Ayya Swamy, P.; Mukherjee, S.; Thilagar, P. Dual Binding Site Assisted Chormogenic and Fluorogenic Recognition and Discrimination of Fluoride and Cyanide by a Peripherally Borylated Metalloporphyrin: Overcoming Anion Interference in Organoboron Based Sensors. Anal. Chem. 2014, 86, 3616–3624. [Google Scholar] [CrossRef]
- Mohr, G.J.; Wenzel, M.; Lehmann, F.; Czerney, P. A chromoreactand for optical sensing of amphetamines. Anal. Bioanal. Chem. 2002, 374, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Mohr, G.J. Covalent bond formation as an analytical tool to optically detect neutral and anionic analytes. Sens. Act. B Chem. 2005, 107, 2–13. [Google Scholar] [CrossRef]
- Jagessar, R.C. Molecular Recognition of Anions by Novel Functionalized Porphyrins. Nat. Sci. 2008, 6, 22–40. [Google Scholar]
- Zhang, Z.; Kim, A.D.; Lin, C.-Y.; Zhang, H.; Lammer, A.; Lynch, V.; Popov, I.; Anslyn, E.; Sessler, J. Explanded Porphyrin-Anion Supramolecular Assemblies: Environmentally Responsive Sensors for Organic Solvents and Anions. J. Am. Chem. Soc. 2015, 1337, 7769–7774. [Google Scholar] [CrossRef]
- Kim, B.F.; Bohandy, J. Spectroscopy of Porphyrins. Johns Hopkins APL Tech. Dig. 1981, 2, 153–163. [Google Scholar]
- Liu, W.; Wang, Y.; Bai, Z.; Li, Y.; Wang, Y.; Chen, L.; Xu, L.; Dimu, J.; Chai, Z.; Wang, S. Hydrolytically stable luminescent cationic metal organic framework for highly sensitive and selective sensing of chromate ions in natural water systems. ACS Appl. Mater. Inter. 2017, 9, 16448–16457. [Google Scholar] [CrossRef] [PubMed]
- Beyene, B.B.; Yibeltal, A.W.; Ayana, M.T. Colorimetric and Fluorescent on-off Detection of Cu2+, Sn2+ and Zn2+ by a Water-Soluble Porphyrin: Electronic Absorption and Emission Study. Res. Chem. 2020, 2, 100058. [Google Scholar] [CrossRef]
- Kalaiselvan, A.; Krishna, I.S.V.; Nambiar, A.P.; Edwin, A.; Reddy, V.S.; Gokulnath, S. Carbazole-Based Porphyrins: Synthesis, Structure-Photophysical Property Correlations, and Mercury Ion Sensing. Org. Lett. 2020, 22, 4494–4499. [Google Scholar] [CrossRef] [PubMed]
- Hestand, N.; Spano, C. Expanded Theory of H- and J-Molecular Aggregates: The Effects of Vibronic Coupling and Intermolecular Charge Transfer. Chem. Rev. 2018, 118, 7069–7163. [Google Scholar] [CrossRef]
- Wang, F.-M.; Zhou, L.; Lustig, W.P.; Hu, Z.; Li, J.-F.; Hu, B.-X.; Chen, L.-Z.; Li, J. Highly Luminescent Metal-Organic Frameworks Based on an Aggregation-Induced Emission Ligand as Chemical Sensors for Nitroaromatic Compounds. Cryst. Growth. Des. 2018, 18, 5166–5173. [Google Scholar] [CrossRef]
- Graham, D. Information Content in Organic Molecules: Aggregation States and Solvent Effects. J. Chem. Inf. Model. 2005, 45, 1223–1236. [Google Scholar] [CrossRef]
- Hestand, N.; Spano, F. Interference between Coulombic and CT-mediated couplings in molecular aggregates: H- to J-aggregate transformation in perylene-based π-stacks. J. Chem. Phys. 2015, 143, 244707. [Google Scholar] [CrossRef]
- Ma, S.; Du, S.; Pan, G.; Dai, S.; Xu, B.; Tian, W. Organic molecular aggregates: From aggregation structure to emission property. Aggregate 2021, 2, e96. [Google Scholar] [CrossRef]
- Magna, G.; Monti, D.; Di Natale, C.; Paolesse, R.; Stefanelli, M. The Assembly of Porphyrin Systems in Well-Defined Nanostructures: An Update. Molecules 2019, 24, 4307. [Google Scholar] [CrossRef]
- Lu, Y.; Shen, Z.; Lian, C.; Wu, J.; Liu, M.; Guo, Z. Tunable molecular packing modes via H- or J-aggregates in the supramolecular helical nanostructures from an achiral C3 symmetric molecule. Soft Matter 2023, 19, 4909–4915. [Google Scholar] [CrossRef] [PubMed]
- Spano, F.; Silva, C. H- and J-Aggregate Behavior in Polymeric Semiconductors. Annu. Rev. Phys. Chem. 2014, 65, 477–500. [Google Scholar] [CrossRef] [PubMed]
- Eisfeld, A.; Briggs, J.S. The J- and H-bands of organic dye aggregates. Chem. Phys. 2006, 324, 376–384. [Google Scholar] [CrossRef]
- Jelly, E. Spectral Absorption and Fluorescence of Dyes in the Molecular State. Nature 1936, 138, 1009–1010. [Google Scholar] [CrossRef]
- You, L.; Zha, D.; Anslyn, E.V. Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, S.K.; Sing, N.J.; Kim, K.S. Imidazolium receptors for the recognition of anions. Chem. Soc. Rev. 2006, 35, 355–360. [Google Scholar] [CrossRef]
- Kilah, N.L.; Beer, P.D. Pyridine and Pyridinium Based Anion Receptors. In Anion Recognition in Supramolecular Chemistry; Gale, P.A., Dehaen, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 301–340. [Google Scholar]
- Evans, N.H.; Beer, P.D. Advances in Anion Supramolecular Chemistry: From Recognition to Chemical Applications. Angew. Chem. 2014, 53, 11716–11754. [Google Scholar] [CrossRef]
- Takkeuchi, M.; Shioya, T.; Swager, T. Allosteric Fluoride Anion Recognition by a Doubly Strapped Porphyrin. Angew. Chem. Int. Ed. 2001, 40, 3372–3376. [Google Scholar] [CrossRef]
- Panja, S.; Ghosh, S.; Ghosh, K. Pyridine/Pyridinium symmetrical bisamides as functional materials: Aggregation, selective sensing, and drug release. New J. Chem. 2018, 42, 6488–6497. [Google Scholar] [CrossRef]
- Feng, A.; Jiang, F.; Huang, G.; Liu, P. Synthesis of the cationic fluorescent probes for the detection of anionic surfactants by electrostatic self-assembly. Spectrochim. Acta. A Mol. Biomol. Spectr. 2020, 224, 117446. [Google Scholar] [CrossRef]
- Palacios, M.A.; Nishiyabu, R.; Marquez, M.; Anzenbacher, P. Supramolecular Chemistry Approach to the Design of a High-Resolution Sensor Array for Multianion Detection in Water. J. Am. Chem. Soc. 2007, 129, 7538–7544. [Google Scholar] [CrossRef] [PubMed]
- Gale, P.A.; Gunnlaugsson, T. Preface: Supramolecular chemistry o anionic species themed issue. Chem. Soc. Rev. 2010, 39, 3595–3596. [Google Scholar] [CrossRef]
- Frontera, A.; Gamez, P.; Mascal, M.; Mooibroek, T.; Reedijk, J. Putting Anion-π Interactions Into Perspective. Angew. Chem. Int. Ed. 2011, 50, 9564–9583. [Google Scholar] [CrossRef]
- Hossain, M.A.; Llinares, J.M.; Powell, D.; Bowman-James, K. Multiple hydrogen bond stabilization of a sandwich complex of sulfate between two macrocyclic tetraamides. Inorg. Chem. 2001, 40, 2936–2937. [Google Scholar] [CrossRef] [PubMed]
- Gale, P. Anion and ion-pair receptor chemistry: Highlights from 2000 and 2001. Coord. Chem. Rev. 2003, 240, 191–221. [Google Scholar] [CrossRef]
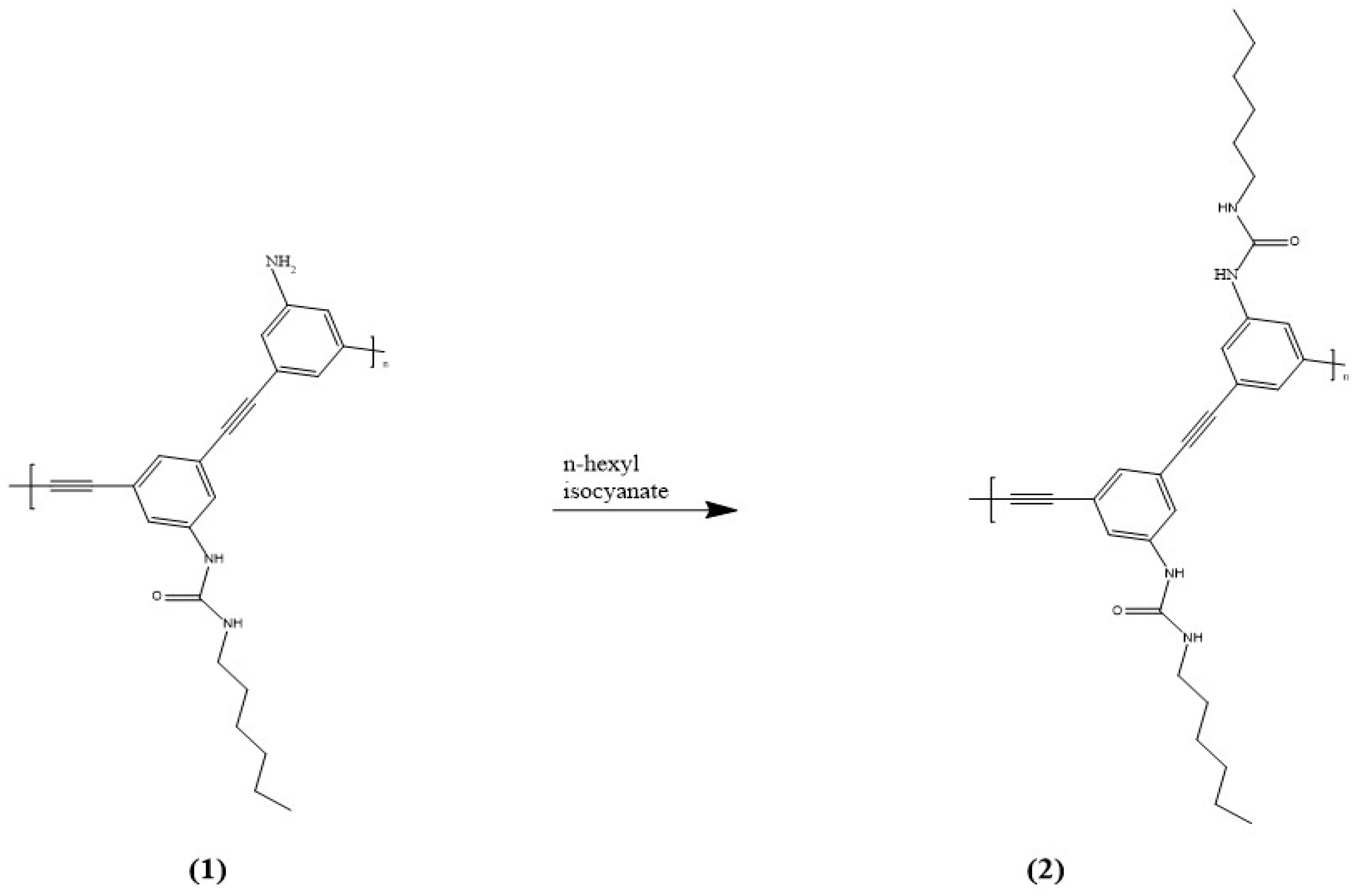
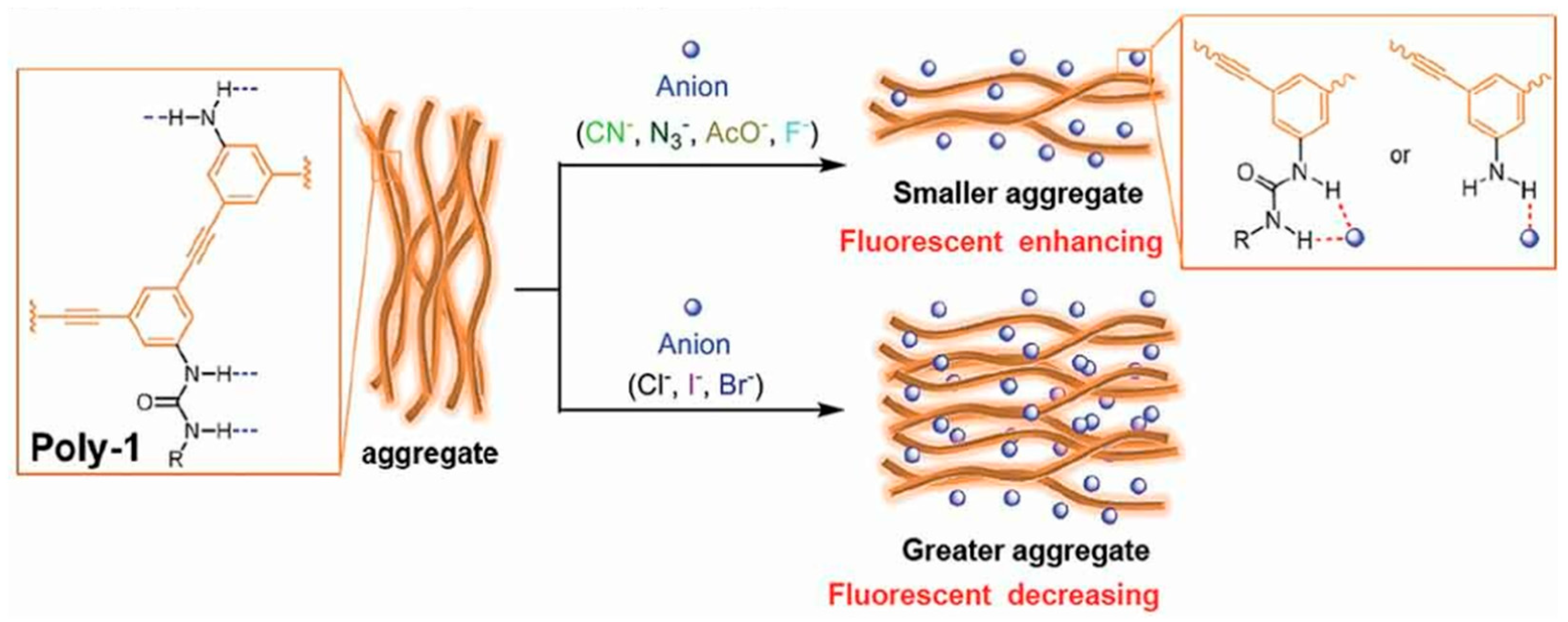
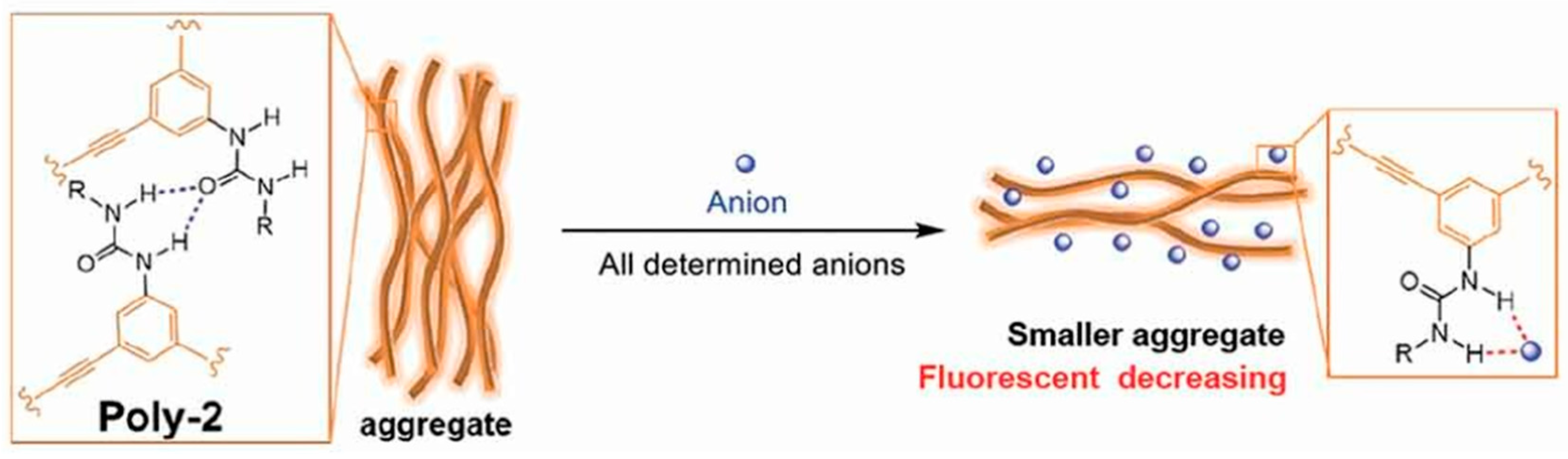
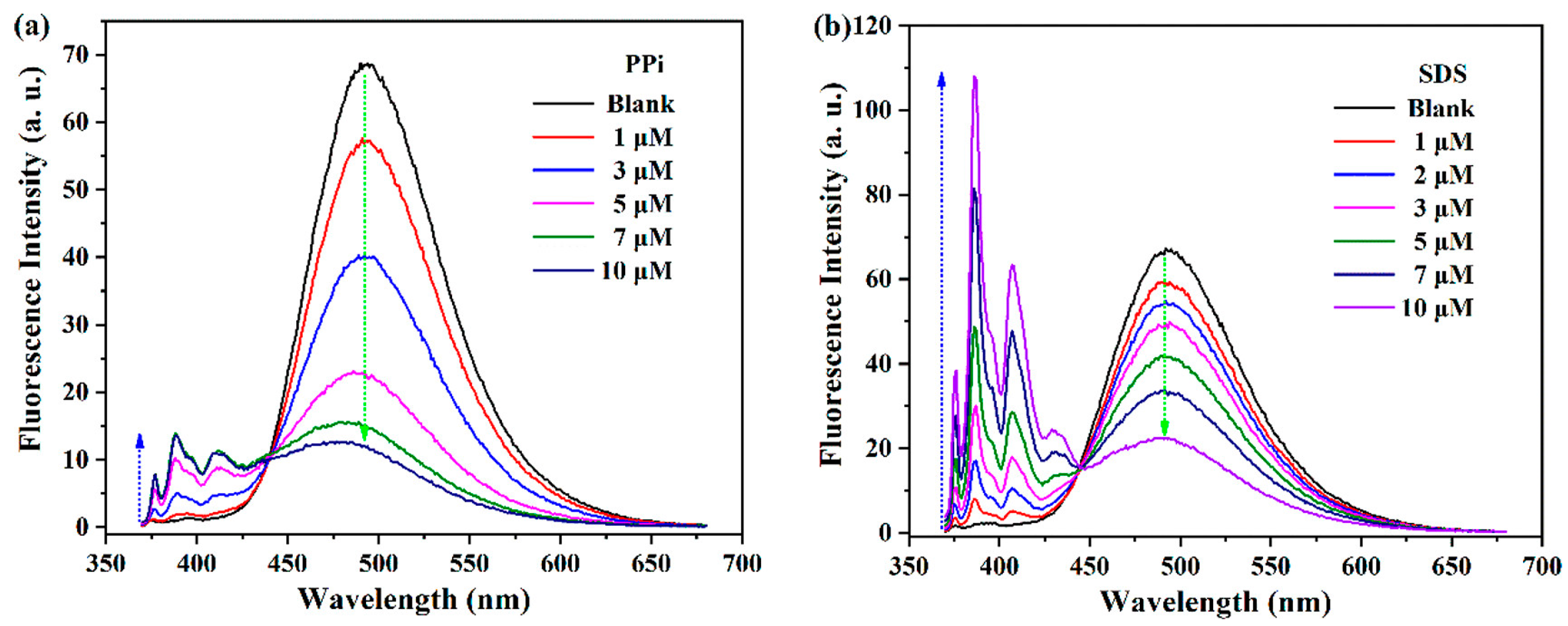
- Li, J.; Saleem, M.; Duan, Q.; Kakuchi, T.; Chen, Y. Aggregation-induced fluorescent response of urea-bearing polyphenyleneethynylenes toward anion sensing. Sci. Tech. Adv. Mater. 2021, 22, 597–606. [Google Scholar] [CrossRef]
- Nieuwenhuizen, M.M.L.; De Greef, T.F.A.; van der Bruggen, R.L.J.; Paulusse, J.M.J.; Appel, W.P.J.; Smulders, M.M.J.; Sijbesma, R.P.; Meijer, E.W. Self-assembly of ureido-pyrimidinone dimers into one-dimensional stacks by lateral hydrogen bonding. Chemistry 2010, 16, 1601–1612. [Google Scholar] [CrossRef]
- Qiao, M.; Zhang, R.; Liu, S.; Ding, L.; Fang, Y. Imidazolium-Modified Bispyrene-Based Fluorescent Aggregates for Discrimination of Multiple Anions in Aqueous Solution. ACS Appl. Mater. Inter. 2022, 14, 32706–32718. [Google Scholar] [CrossRef]
- Gogoi, A.; Mukherjee, S.; Ramesh, A.; Das, G. Aggregation-Induced Emission Active Metal-Free Chemosensing Platform for Highly Selective Turn-On Sensing and Bioimaging of Pyrophosphate Anion. Anal. Chem. 2015, 87, 6974–6979. [Google Scholar] [CrossRef]
- Halder, S.; Samanta, S.; Das, G. Exploring the potential of a urea derivative: An AIE-luminogen and its interaction with human serum albumin in aqueous medium. Analyst 2019, 144, 2696–2703. [Google Scholar] [CrossRef]
- Rajput, J.K. “On-Off’ novel fluorescent chemosensors based on nanoaggregates of triaryl imidazoles for superselective detection of nitro-explosive trinitrophenol in multiple solvent systems. Sens Act. B Chem. 2018, 259, 990–1005. [Google Scholar]
- Kumar, S.M.; Jothi, D.; Munusamy, S.; Enhanathan, S.; Iyer, S.K. Imidazole-derived new colorimetric/fluorometric chemosensor for the sensitive recognition of CN- ions: Real-time application in food samples and fluorescence bio-imaging. J Photochem. Photobiol. A Chem. 2023, 434, 114269. [Google Scholar]
- Arunachalam, M.; Ghosh, P. Nitrate directed organized assemblies of protonated arene based tripodal receptors. CrystEngComm. 2010, 12, 1621–1627. [Google Scholar] [CrossRef]
- Zhang, R.X.; Li, P.F.; Zhang, W.J.; Li, N.; Zhao, N. A highly sensitive fluorescent sensor with aggregation-induced emission characteristics for the detection of iodide and mercury ions in aqueous solution. J. Mater. Chem. C 2016, 4, 10479–10485. [Google Scholar] [CrossRef]
- Xie, H.; Jiang, X.; Zeng, F.; Yu, C.; Wu, S. A novel ratiometric fluorescent probe through aggregation-induced emission and analyte-induced excimer dissociation. Sens Act. B Chem. 2014, 203, 504–510. [Google Scholar] [CrossRef]
- Sun, B.J.; Yang, X.J.; Ma, L.; Niu, C.X.; Wang, F.F.; Na, N.; Wen, J.Y.; Ouyang, J. Design and application of anthracene derivative with aggregation-induced emission characteristics for visualization and monitoring of erythropoietin unfolding. Langmuir 2013, 29, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Chen, J.L.; Xu, B.; Wang, L.J.; Ma, S.Q.; Li, B.; Ye, L.; Tian, W.J. Remarkable fluorescence change based on the protonation-deprotonation control in organic crystals. Chem. Commun. 2013, 49, 3878–3880. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Sukul, P.K. Selective Detection of Pyrophosphate Anions in Aqueous Medium Using Aggregation of Perylene Diimide as a Fluorescent Probe. ACS Omega 2019, 4, 16191–16200. [Google Scholar] [CrossRef]
- Yan, Q.; Cai, K.; Zhao, D. Supramolecular Aggregates with Distinct Optical Properties from PDI Oligomers of Similar. Phys. Chem. Chem. Phys. 2016, 18, 1905–1910. [Google Scholar] [CrossRef]
- Gorl, D.; Zhang, X.; Wurthner, F. Molecular Aggregates of Perylene Bisimide Dyes in Water. Angew. Chem. Int. Ed. 2012, 51, 6328. [Google Scholar] [CrossRef]
- Pacheco-Linan, P.J.; Moral, M.; Nueda, M.L.; Cruz-sanches, R.; Fernandez-Sainz, J.; Garzon-ruiz, A.; Bravo, I.; Melguizo, M.; Laborda, J.; Albaladejo, J. Study on the pH-Dependence of the photophysical properties of a functionalized peryle ebisimide and its potential applications as a fluorescence lifetime-based pH probe. J. Phys. Chem. C 2017, 121, 24786–27497. [Google Scholar] [CrossRef]
- Sukul, P.K.; Sing, P.K.; Maji, S.K.; Malik, S. Aggregation Induced Chirality in a Self Assembled Perylene Based Hydrogel: Application of the Intracellular pH Measurement. J. Mater. Chem. B 2013, 1, 153–156. [Google Scholar] [CrossRef]
- Pandit, S.K.; Das, G. Naphthalimide-based AIE-active receptor: HSO4−/SO42− sensing and detection of Pb2+ by receptor-anion ensemble in aqueous medium. Spectrochim. Acta A, Mol. Biomol. Spectr. 2024, 310, 123879. [Google Scholar] [CrossRef]
- Padghan, S.D.; Wang, L.-C.; Lin, W.-C.; Hu, J.-W.; Liu, W.-C.; Chen, K.-Y. Rational Design of an ICT-Based Chemodosimeter with Aggregation-Induced Emission for Colorimetric and Ratiometric Fluorescent Detection of Cyanide in a Wide pH Range. ACS Omega 2021, 6, 5287–5296. [Google Scholar] [CrossRef]
- Zou, Q.; Tao, F.; Wu, H.; Yu, W.W.; Li, T.; Cui, Y. A new carbazole-based colorimetric and fluorescent sensor with aggregation induced emission for detection of cyanide anion. Dye. Pigment. 2019, 164, 165–173. [Google Scholar] [CrossRef]
- Hong, Y.N.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism, and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, Y.; Qiao, B.; Tao, F.; Li, T.; Ding, Y.; Raymo, F.M.; Cui, Y.Z. Facile fabrication of AIE/AIEE-active fluorescent nanoparticles based on barbituric for cell imaging applications. RSC Adv. 2017, 7, 30229–30241. [Google Scholar] [CrossRef]
- Li, K.; Yu, R.-H.; Shi, C.-M.; Tao, F.-R.; Li, T.-D.; Cui, Y.-Z. Electrospun nanofibrous membrane based on AIE-active compound for detecting picric acid in aqueous solution. Sens Act. B 2018, 262, 637–645. [Google Scholar] [CrossRef]
- Alizadeh, A.; Ghouzivand, S.; Khodaei, M.M. An interesting spectroscopic method for chromofluorogenic detection of cyanideion in aqueous solution: Disruption of intramolecular charge transfer (ICT). J. Chem. Sci. 2016, 128, 537–543. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision A.2; Gaussian Inc.: Wallingford, UK, 2009. [Google Scholar]
- Xie, Z.; Kong, X.; Feng, L.; Ma, J.; Li, Y.; Wang, X.; Bao, W.; Shi, W.; Hui, Y. A novel highly selective probe with both aggregation-induced emission enhancement and intramolecular charge transfer characteristics for CN-detection. Sens Act. B Chem. 2018, 257, 154–165. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, J.; Han, X.; Yin, J.; Yu, G.-A.; Liu, S.H. Fluorene-based novel highly emissive fluorescent molecules with aggregate fluorescence change or aggregation-induced emission enhancement characteristics. Dye. Pigment. 2015, 112, 59–66. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Al-Zahrani, F.A.M.; Al-amshany, Z.M.; Asiri, A.M. Synthesis of a new fluorescent cyanide chemosensor based on phenothiazine derivative. Sens. Act. B Chem. 2017, 240, 288–296. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Yang, J.; Qu, Y.; Hua, J. Colorimetric and ratiometric near-infrared fluorescent cyanide chemodosimeter based on phenazine derivatives. ACS Appl. Mater. Interfaces. 2013, 5, 1317–1326. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, W.; Hiu, Y.; Sun, X.; Xu, L.; Feng, L.; Xie, Z. A new highly selective fluorescent turn-on chemosensor for cyanide anion. Talanta 2015, 137, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Yan, S.; Xiong, M.; Nie, Y.; Tian, X.; Li, Y. Ultra-trace detection and efficient adsorption removal of multiple water-soluble volatile organic compounds by fluorescent sensor array. J. Haz. Mater. 2023, 443, 130182. [Google Scholar] [CrossRef]
- Lee, S.; Hua, Y.; Park, H.; Flood, A.H. Intramolecular Hydrogen Bonds Preorganize an Aryl-triazole Receptor into a Crescent for Chloride Binding. Org. Lett. 2010, 12, 2100–2102. [Google Scholar] [CrossRef]
- Li, Y.; Flood, A.H. Pure C-H hydrogen bonding to chloride ions: A preorganized and rigid macrocyclic receptor. Angew. Chem. Int. Ed. 2008, 47, 2649–2652. [Google Scholar] [CrossRef]
- Ramabhadra, R.O.; Hua, Y.; Li, Y.-J.; Flood, A.H.; Raghavachari, K. From Atomic to Molecular Anions: A Neutral Receptor Captures Cyanide Using Strong C-H Hydrogen bonds. Chem. Eur. J. 2011, 17, 9123–9129. [Google Scholar] [CrossRef]
- Chellappan, K.; Singh, N.J.; Hwang, I.-C.; Lee, J.W.; Kim, K.S. A Caliz [4] imidazolium [2] pyridine as an Anion Receptor. Angew. Chem. Int. Ed. 2005, 44, 2899–2905. [Google Scholar] [CrossRef]
- Ihm, H.; Yun, S.; Kim, H.G.; Kim, J.K.; Kim, K.S. Tripodal Nitro-Imidazolium Receptor for Anion Binding driven by (C-H)+—X hydrogen bonds. Org. Lett. 2002, 4, 2897–2900. [Google Scholar] [CrossRef] [PubMed]
- Schulze, B.; Friebe, C.; Hager, M.D.; Gunther, W.; Kohn, U.; Jahn, B.O.; Gorls, H.; Schubert, U.S. Anion Complexation by Triazolium “Ligands”: Mono- and Bis-tridentate Complexes of Sulfate. Org. Lett. 2010, 12, 2710–2713. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, P.S. Anion Recognition by 1,2,3-Triazolium Receptors: Application of Click Chemistry in Anion Recognition. Org. Lett. 2008, 10, 165–168. [Google Scholar] [CrossRef]
- Cao, Q.-Y.; Pradhan, T.; Hee Lee, M.; No, K.; Kim, J.S. Ferrocene-based anion receptor bearing amide and triazolium donor groups. Analyst 2012, 137, 4454–4457. [Google Scholar] [CrossRef]
- White, N.G.; Carvalho, S.; Feliz, V.; Beer, P.D. Anion binding in aqueous media by a tetra-triazolium macrocycle. Org. Biomol. Chem. 2012, 10, 6951–6959. [Google Scholar] [CrossRef]
- Sui, B.; Kim, B.; Zhang, Y.; Frazer, A.; Belfield, K.D. Highly Selective Fluorescence Turn-On Sensor for Fluoride Detection. ACS Appl. Mater. Interfaces 2013, 5, 2920–2923. [Google Scholar] [CrossRef]
- Peng, X.; Wu, Y.; Fan, J.; Tian, M.; Han, K. Colorimetric and Ratiometric Fluorescence Sensing of Fluoride: Tuning Selectivity in Proton Transfer. J. Org. Chem. 2005, 70, 10524–10531. [Google Scholar] [CrossRef]
- Descalzo, A.B.; Rurack, K.; Weisshoff, H.; Marinez-Manez, R.; Marcos, M.D.; Amoros, P.; Hoffman, K.; Soto, J. Rational Design of a Chromo- and Fluorogenic Hybrid Chemosensor Material for the Detection of Long-Chain Carboxylates. J. Am. Chem. Soc. 2005, 127, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Shenderovich, I.G.; Limbach, H.-H.; Smirnov, S.N.; Tolstoy, P.M.; Denisov, G.S.; Golubey, N.S. H/D isotope effects on the low-temperature NMR parameters and hydrogen bond geometries of (FH)2F− and (FH)3F− dissolved in CDF3/CDF2Cl. Phys. Chem. Chem. Phys. 2002, 4, 5488–5497. [Google Scholar] [CrossRef]
- Watt, M.M.; Engle, J.M.; Fairley, K.C.; Robitshek, T.E.; Haley, M.M.; Johnson, D.W. “Off-on” aggregation-based fluorescent sensor for the detection of chloride in water. Org. Biomol. Chem. 2015, 13, 4266–4270. [Google Scholar] [CrossRef]
- Alizadeh, N.; Akbarinejad, A.; Hosseinkhani, S.; Rabbani, F. Synthesis of highly fluorescent water-soluble polypyrrole for cell imaging and iodide ion sensing. Anal. Chim. Acta. 2019, 1084, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.E.; Schmitt, L.D.; Lees, A.J. A New, Rapid, Colorimetric Chemodosimeter, 4-(pyrrol-1-yl)pyridine, for Nitrite Detection in Aqueous Solution. ACS Omega 2024, 9, 37278–37287. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K. The Nature of Pi-Pi Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
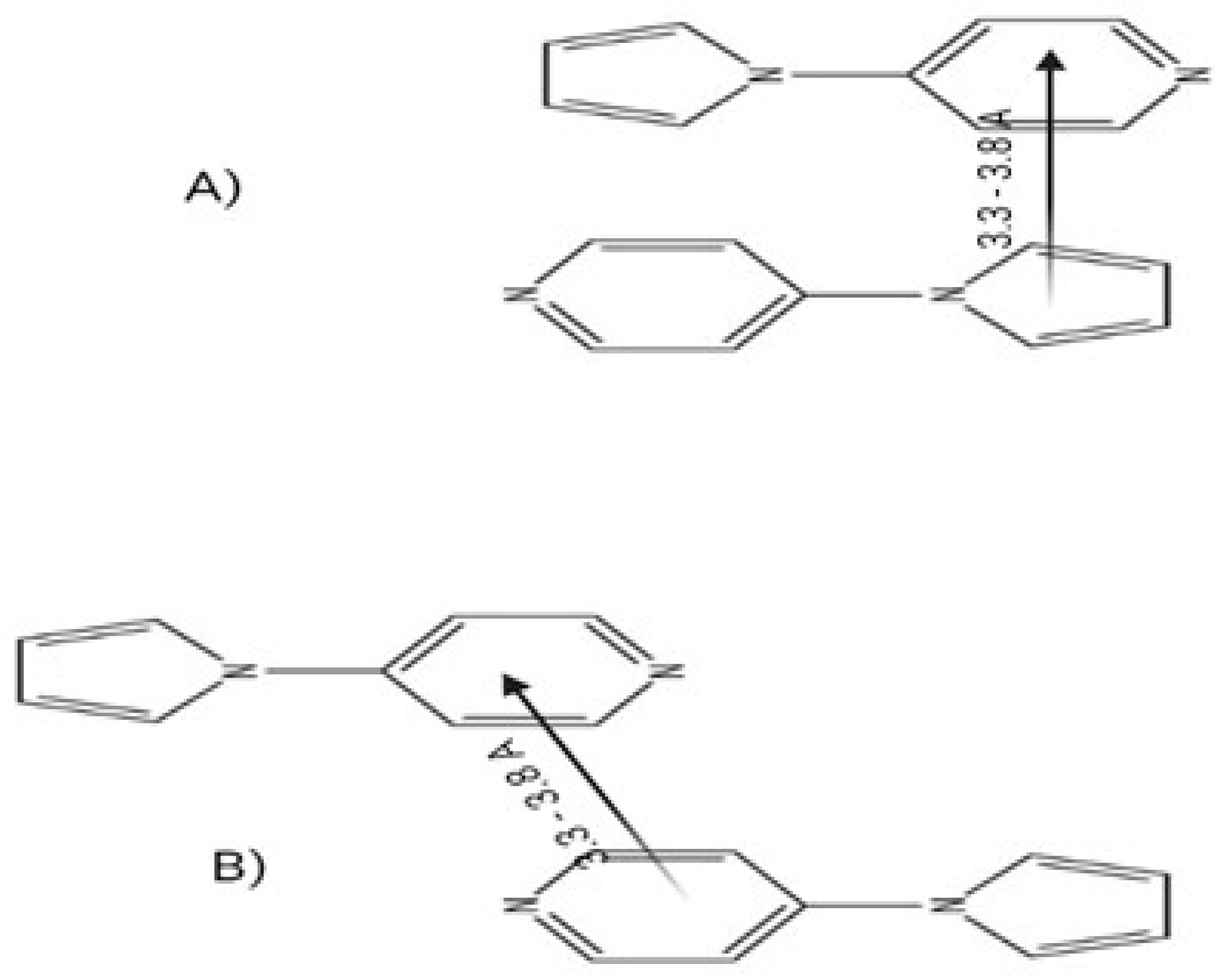
- Thomas, M.E.; Schmitt, L.D.; Lees, A.J. An Investigation into Anion Sensing of the Molecular Aggregate of 4-(pyrrol-1-yl)pyridine and its Derivatives. Molecules 2024, 29, 5692. [Google Scholar] [CrossRef]
- Rananaware, A.; Bhosale, R.S.; Ohkubo, K.; Patil, H.; Jones, L.A.; Jackson, S.L.; Fukuzumi, S.; Bhosale, S.V.; Bhosale, S.V. Tetraphenylethene-Based Star Shaped Porphyrins: Synthesis, Self-assembly, and Optical and Photophysical Study. J. Org. Chem. 2015, 80, 3832–3840. [Google Scholar] [CrossRef]
- Mahendran, V.; Pasumpon, K.; Thimmarayaperumal, S.; Thilagar, S.; Shanmugam, J. Tetraphenylethene-2-Pyrone Conjugate: Aggregation-Induced Emission Study and Explosives Sensor. J. Org. Chem. 2016, 81, 3597–3602. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, X.; Chen, Q.; Jiang, G.; Wang, H.; Wang, J. New switch on fluorescent probe with AIE characteristics for selective and reversible detection of mercury ion in aqueous solution. Anal. Biochem. 2019, 585, 113403. [Google Scholar] [CrossRef]
- Zhao, X.; Ji, C.; Ma, L.; Wu, Z.; Cheng, W.; Yin, M. An Aggregation-Induced Emission-Based “Turn-On” Fluorescent Probe for Facile Detection of Gaseous Formaldehyde. ACS Sens. 2018, 3, 2112–2117. [Google Scholar] [CrossRef] [PubMed]
- Jagadhane, K.S.; Bhosale, S.R.; Moyo, A.A.; Kolekar, G.B.; Sharma, K.K.; Yadav, H.M.; Anbhule, P.V. A Tetraphenylethene-Based Aggregation-Induced Emission Luminogen (AIEgen) with Mechanochromic Phenomena for Highly Selective Naked-Eye Detection of MnO4− Directly in Aqueous Media. Chem. Eur. 2022, 7, e202203185. [Google Scholar] [CrossRef]
- Dong, M.Z.; Ren, H.; Wang, J.N.; Wang, Y. A new naphthopyran-based chemodosimeter with aggregation-induced emission: Selective dual-channel detection of cyanide ion in aqueous medium and test strips. Microchem. J. 2020, 155, 104676. [Google Scholar] [CrossRef]
- Peng, L.; Wang, M.; Zhang, G.; Zhang, D.; Zhu, D. A Fluorescence Turn-on Detection of Cyanide in Aqueous Solution Based on the Aggregation-Induced Emission. Org. Lett. 2009, 11, 1943–1946. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, X.; Lu, X.; Yan, C.; Xu, Y.; Hang, X.; Qu, J.; Liu, R. A highly selective and sensitive fluorescent chemosensor for detection of CN−, SO32−, and Fe3+ based on aggregation-induced emission. J. Mater. Chem. C. 2016, 4, 383–390. [Google Scholar] [CrossRef]




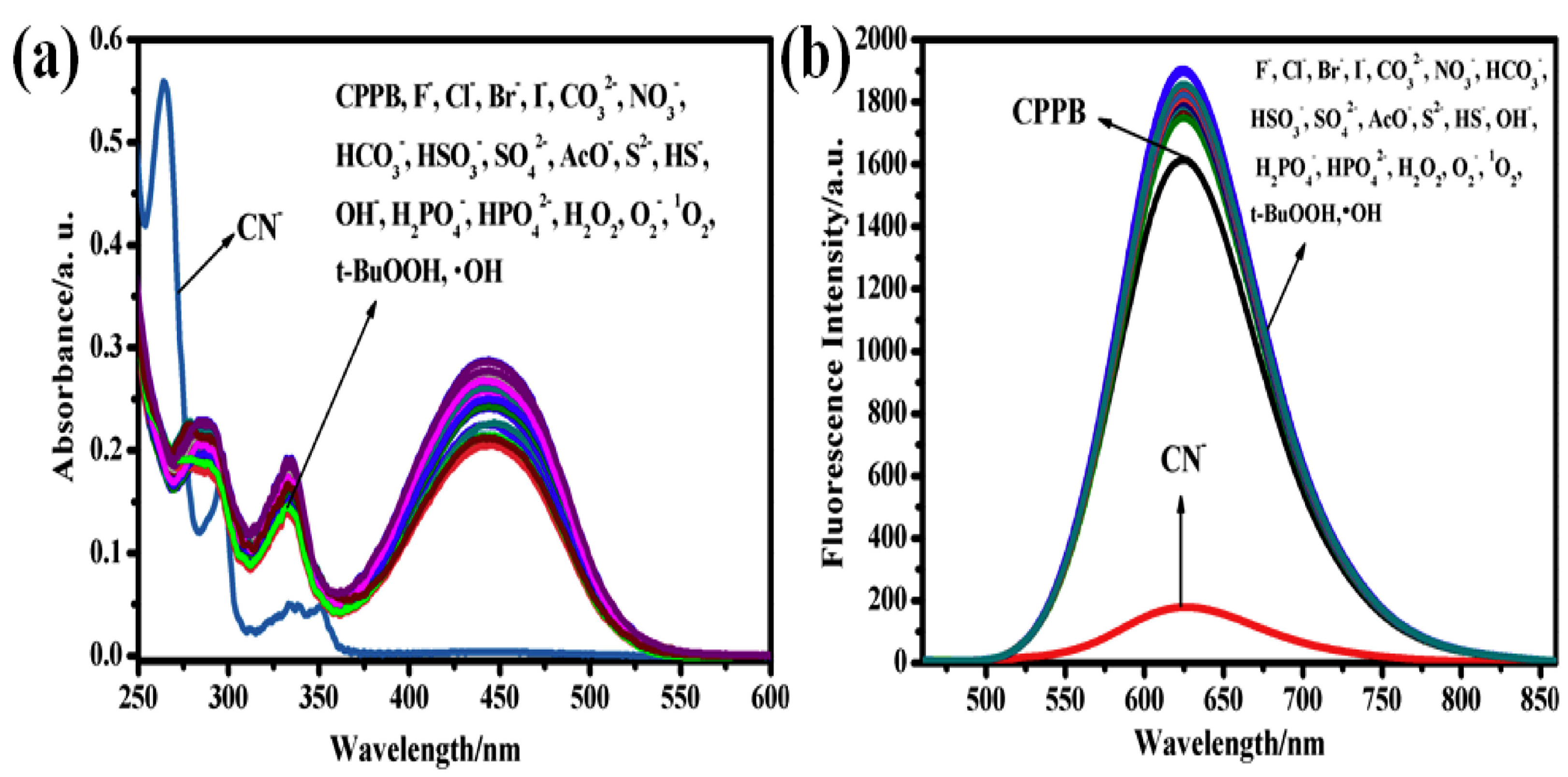
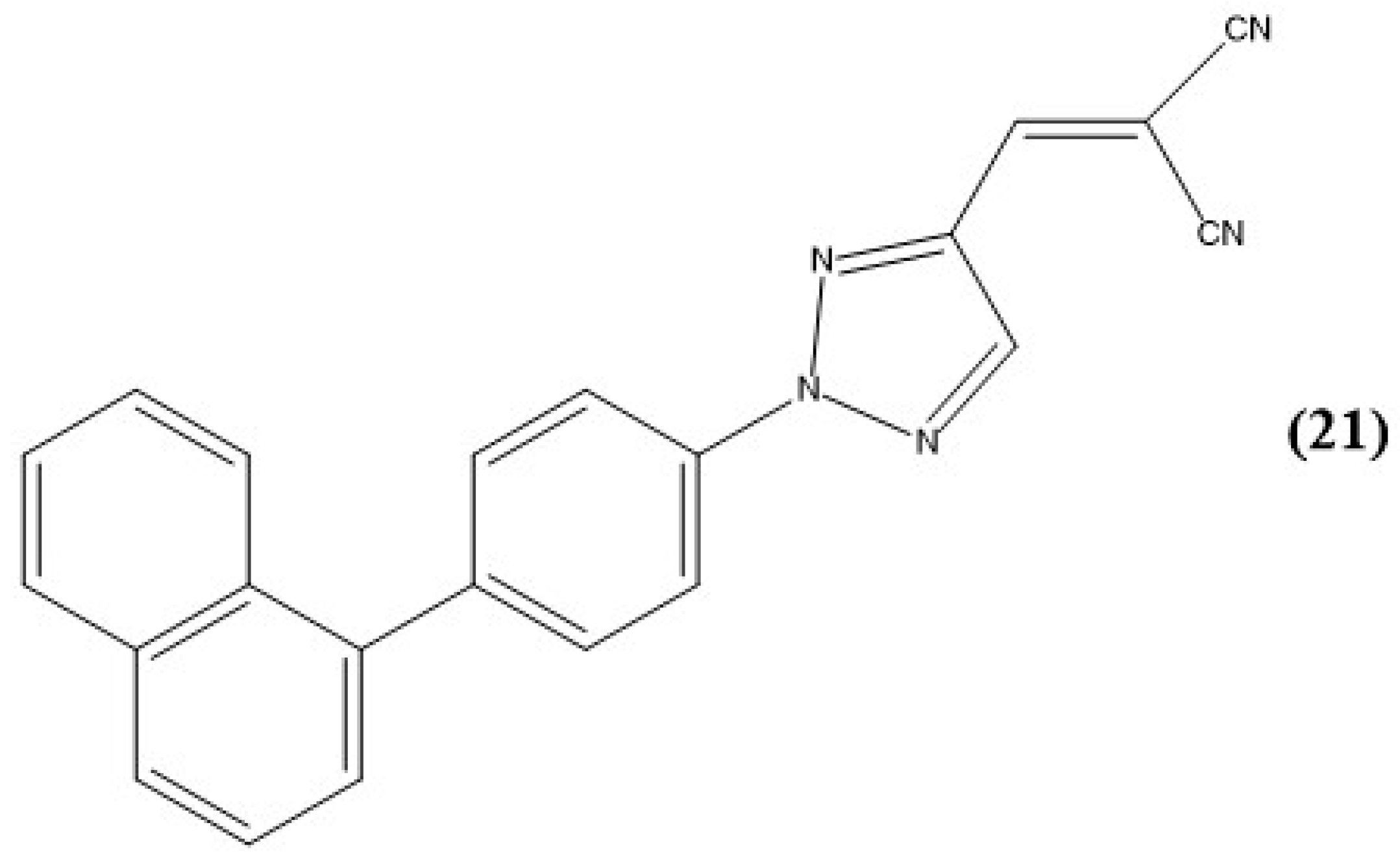
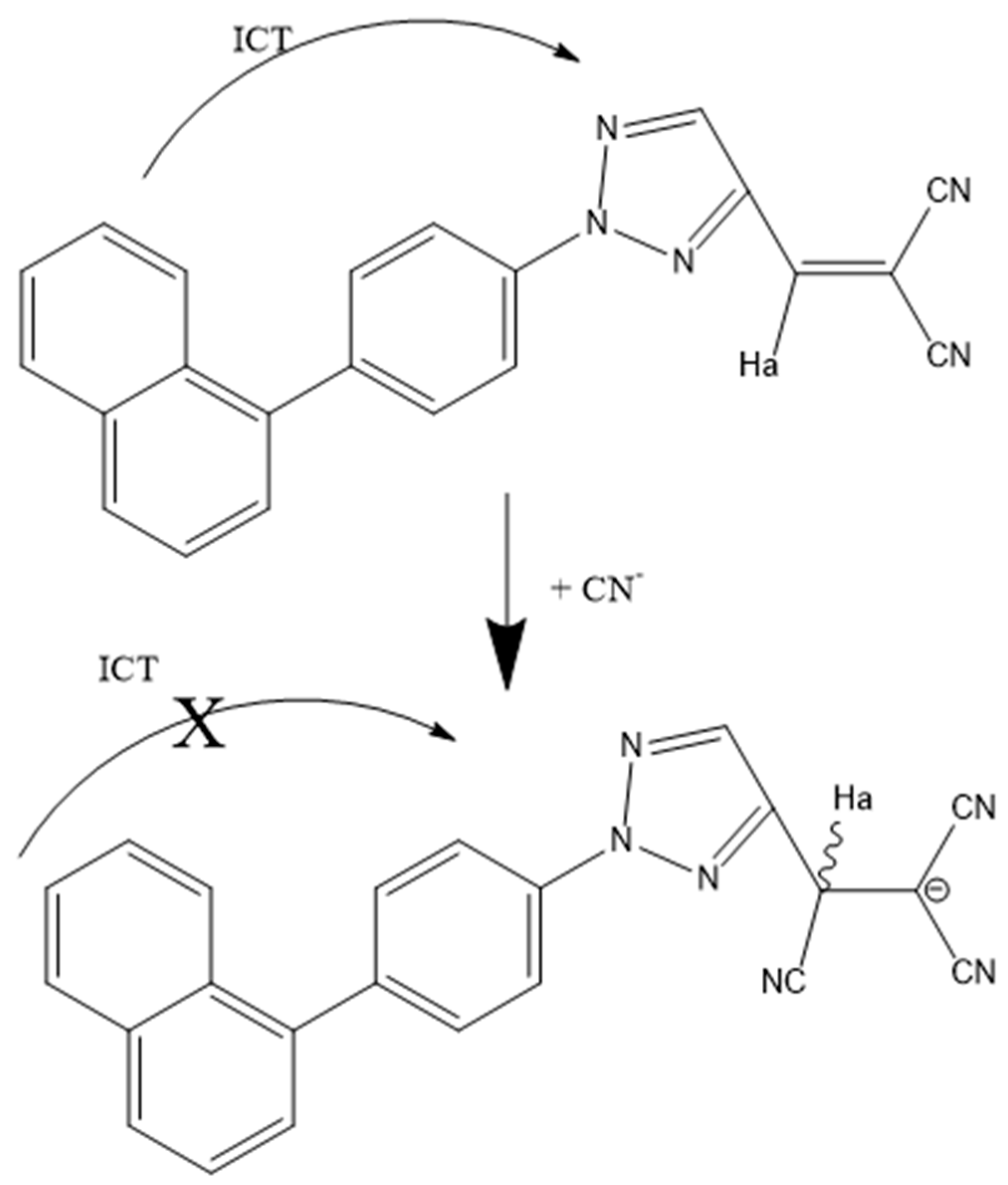
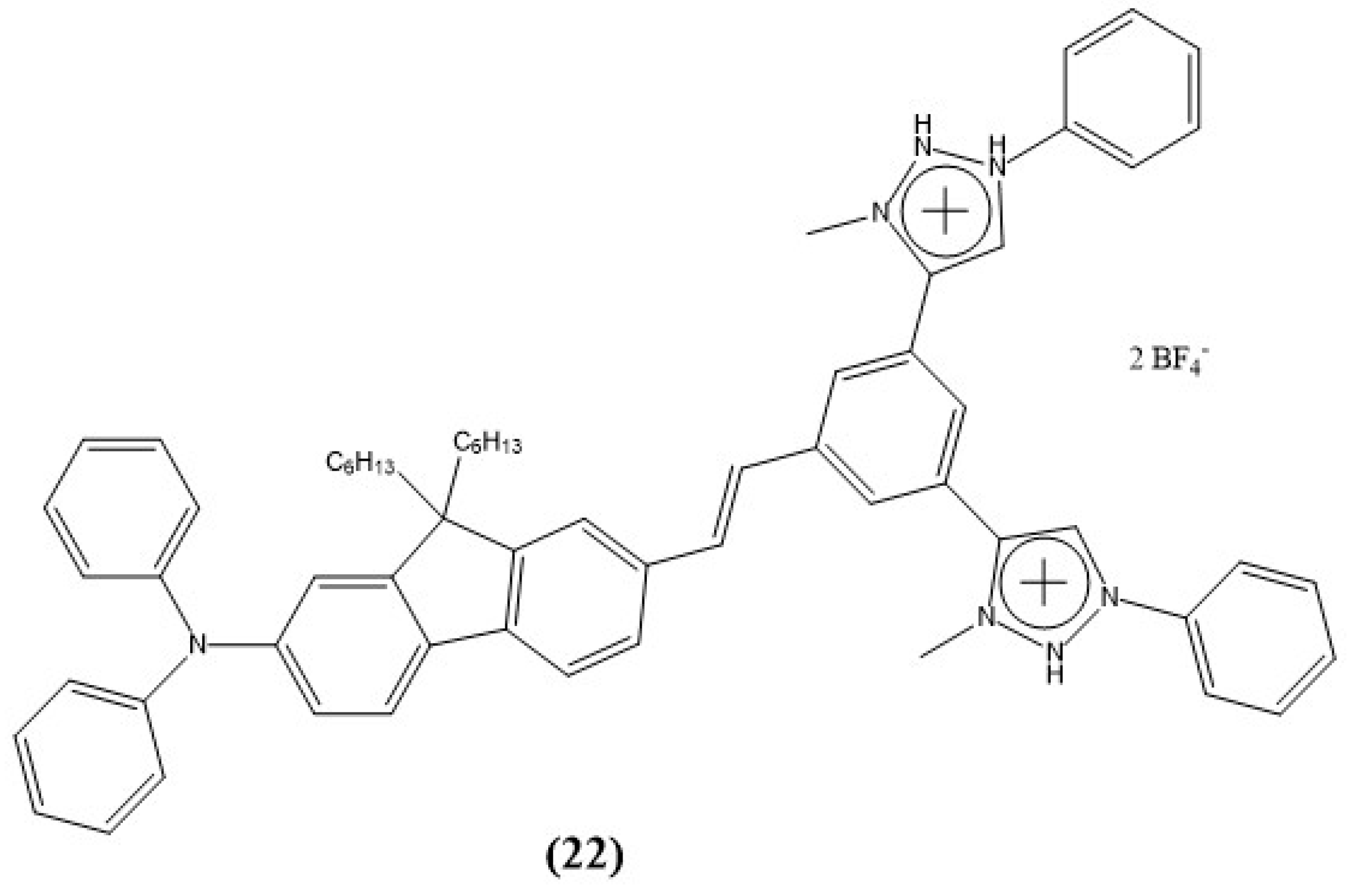
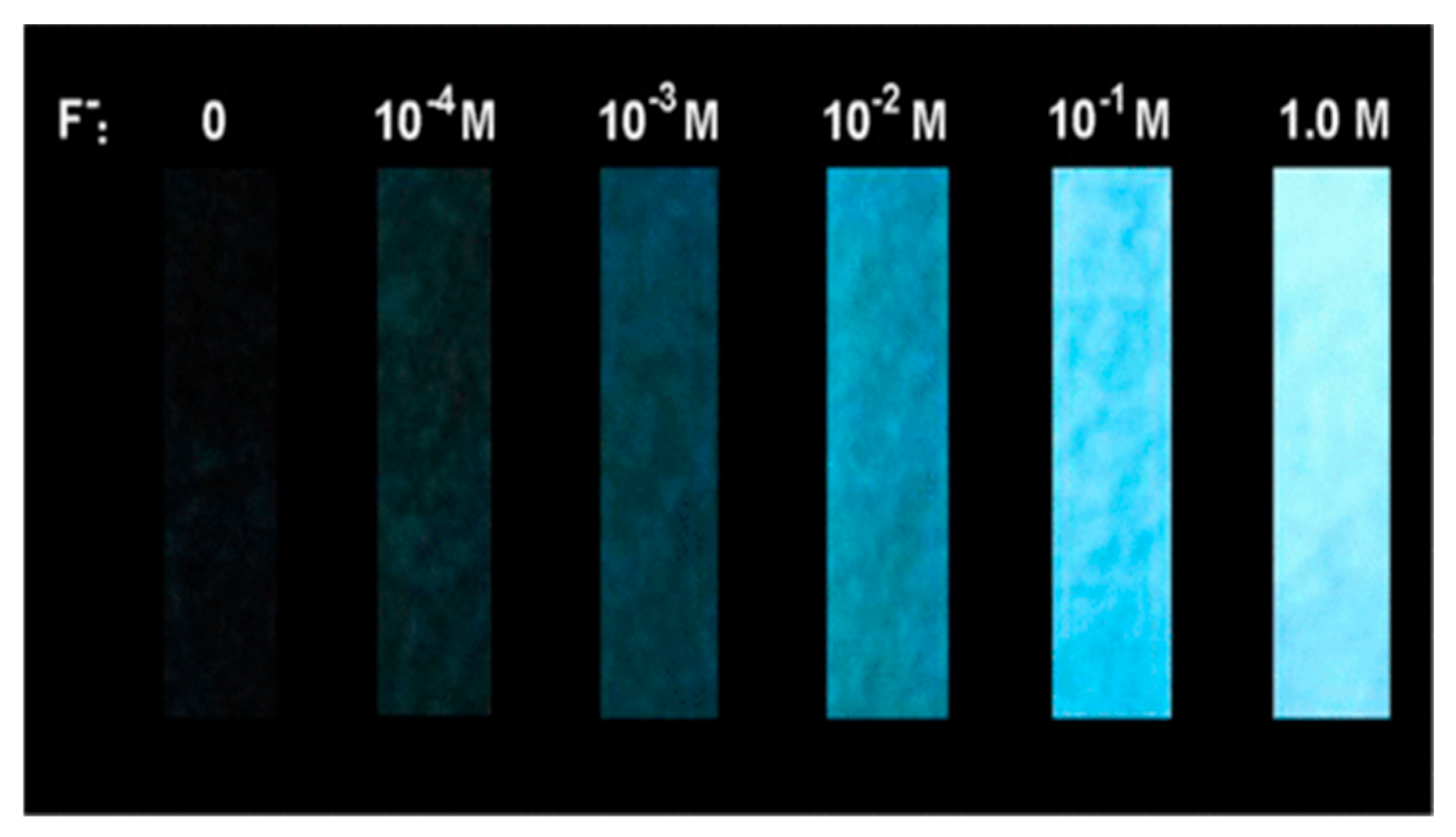
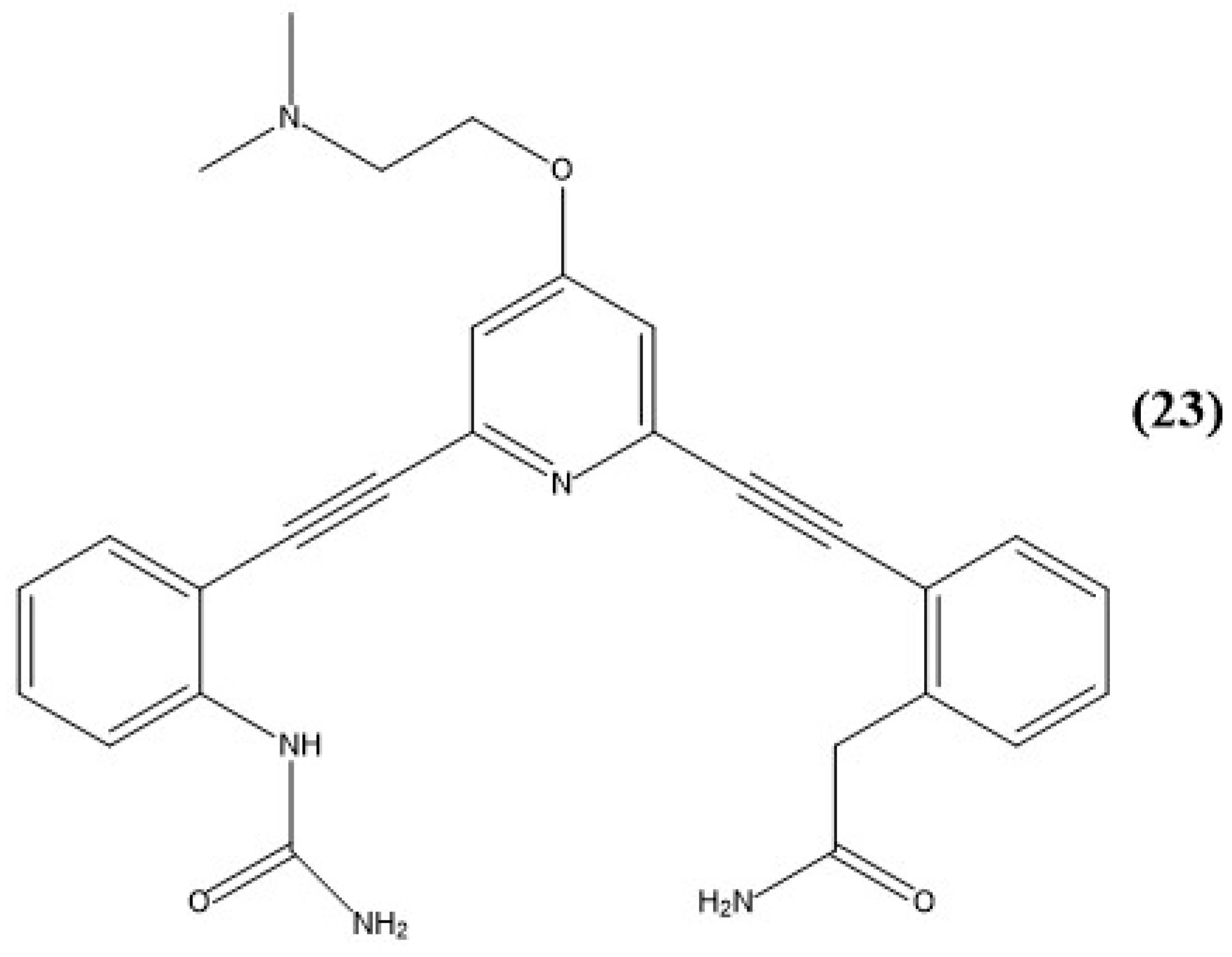
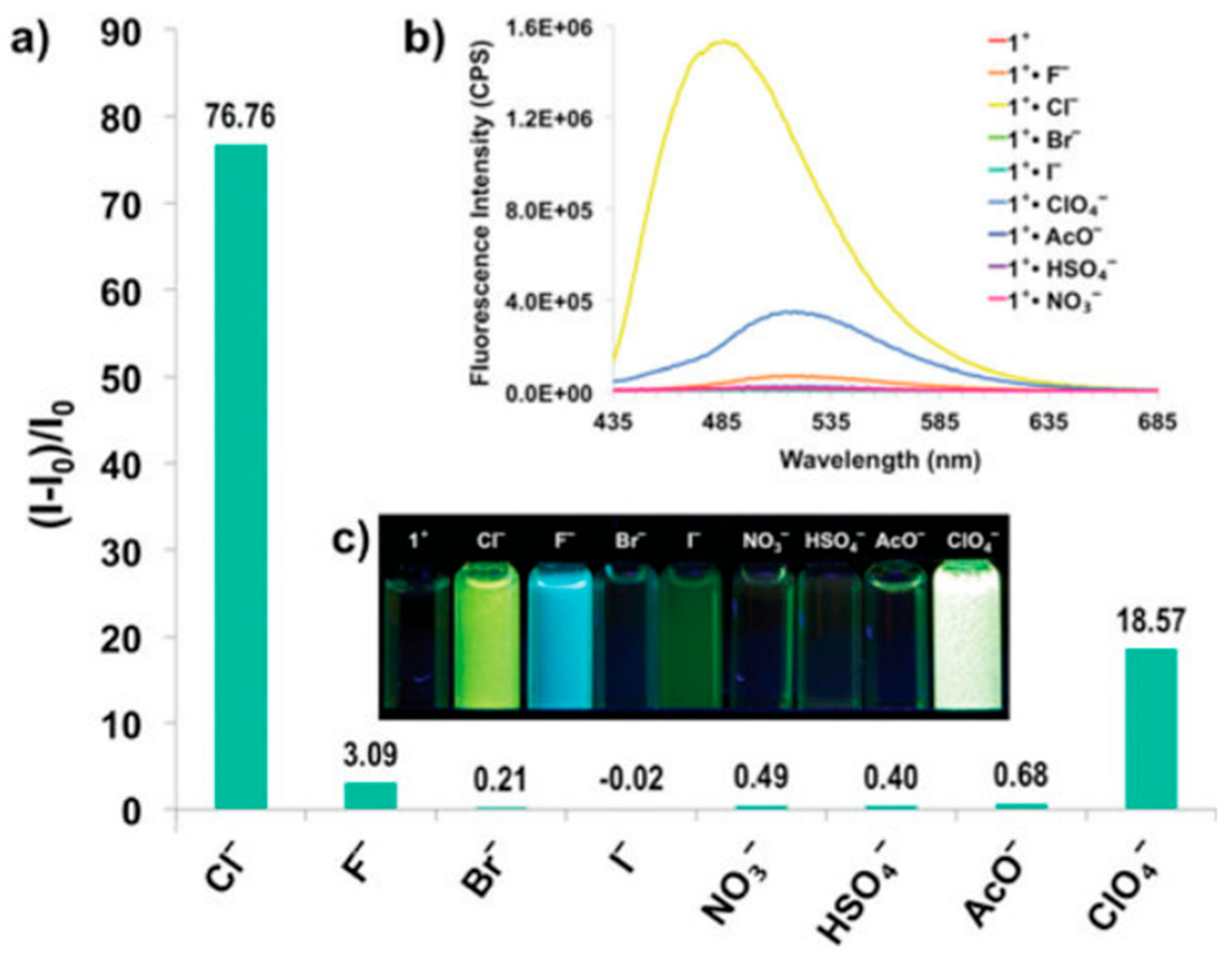
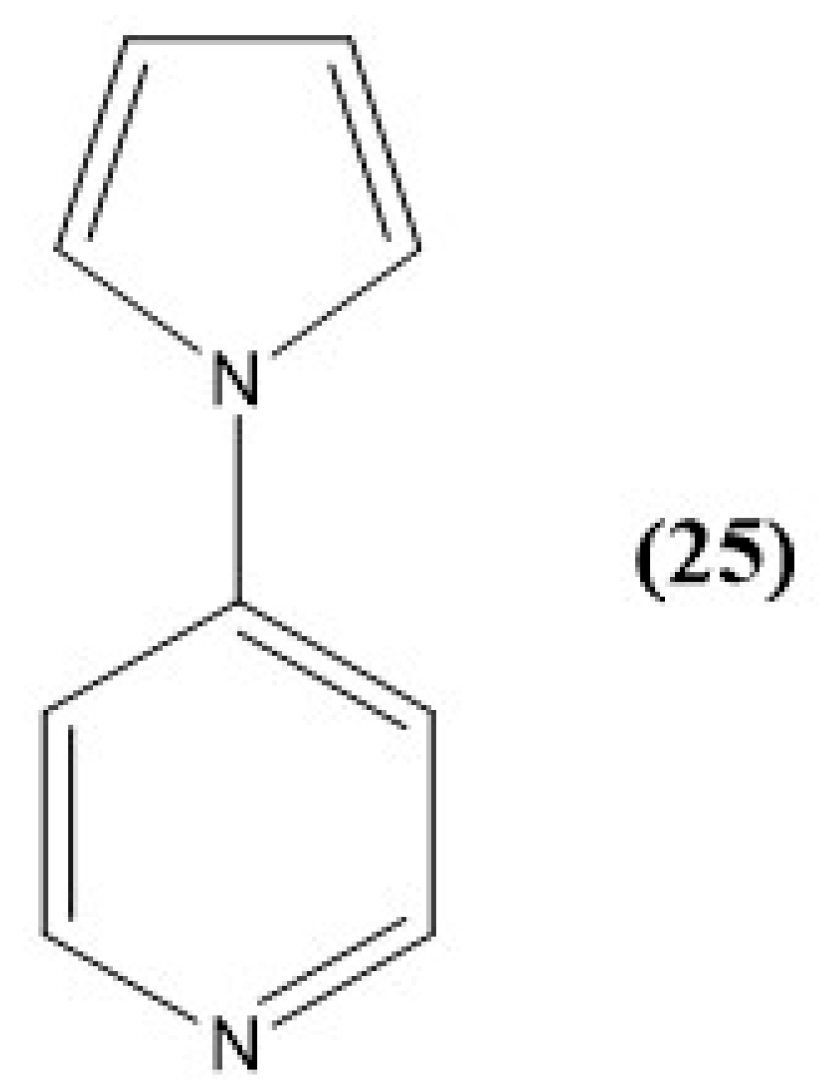


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, M.E.; Lees, A.J. A Review of the Molecular Aggregation of Small-Molecule Anion Sensors for Environmental Contaminates in Aqueous Media. Sustain. Chem. 2025, 6, 17. https://doi.org/10.3390/suschem6020017
Thomas ME, Lees AJ. A Review of the Molecular Aggregation of Small-Molecule Anion Sensors for Environmental Contaminates in Aqueous Media. Sustainable Chemistry. 2025; 6(2):17. https://doi.org/10.3390/suschem6020017
Chicago/Turabian StyleThomas, Mallory E., and Alistair J. Lees. 2025. "A Review of the Molecular Aggregation of Small-Molecule Anion Sensors for Environmental Contaminates in Aqueous Media" Sustainable Chemistry 6, no. 2: 17. https://doi.org/10.3390/suschem6020017
APA StyleThomas, M. E., & Lees, A. J. (2025). A Review of the Molecular Aggregation of Small-Molecule Anion Sensors for Environmental Contaminates in Aqueous Media. Sustainable Chemistry, 6(2), 17. https://doi.org/10.3390/suschem6020017







