Use of Domestic Polymeric Waste for Surfactant Removal from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Sample Preparation
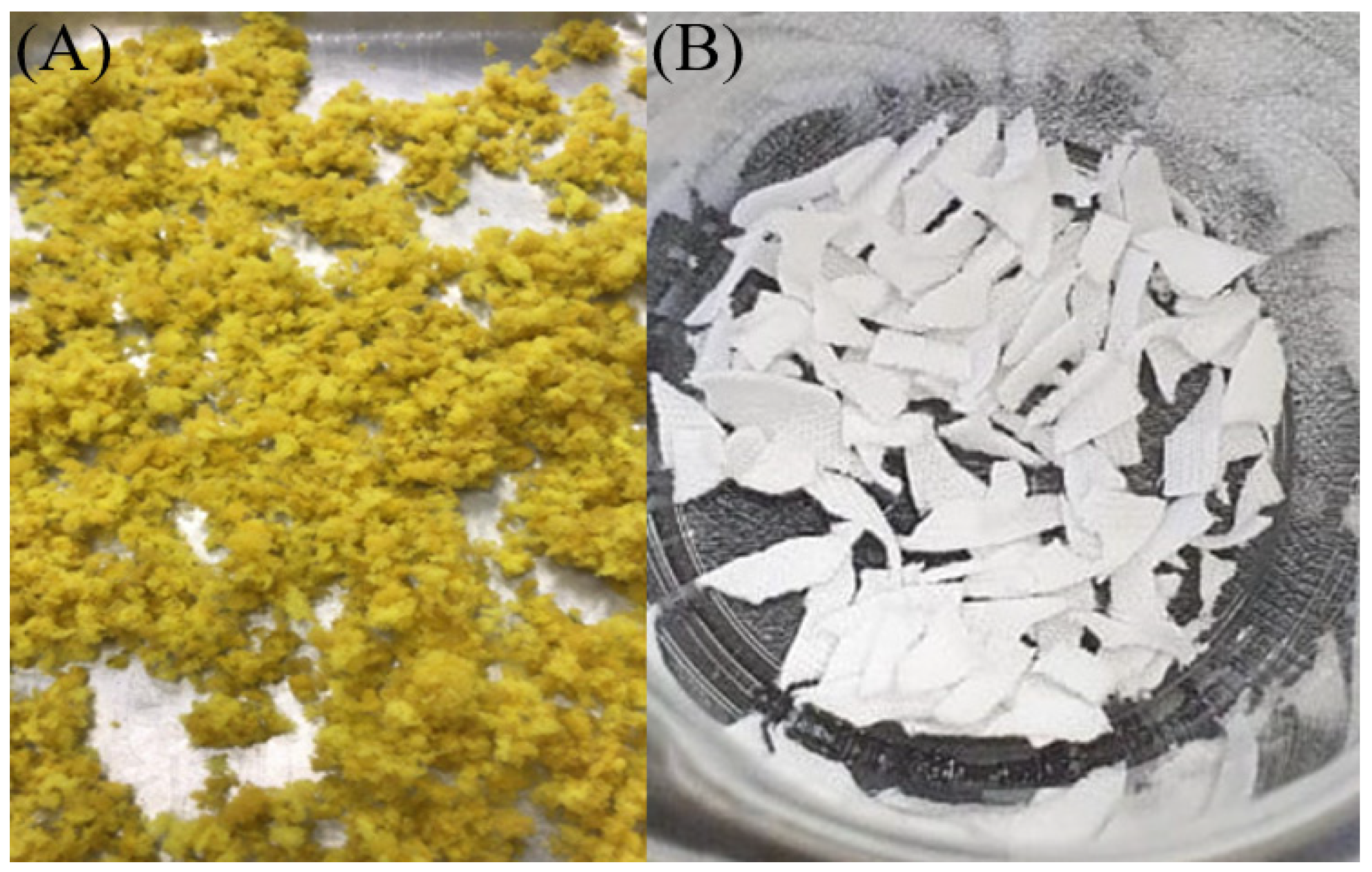
2.2. Characterizations
2.3. Factorial Design
3. Results and Discussion
3.1. LAS and DPC UV-Vis Characterization
3.2. Adsorbent Characterization
3.3. Factorial Design
3.4. Isotherm and Kinetic Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Guidelines on Hand Hygiene in Health Care (Advanced Draft): Global Safety Challenge 2005–2006: Clean Care Is Safer Care; World Health Organization: Geneva, Switzerland, 2006.
- Pradhan, D.; Biswasroy, P.; Kumar Naik, P.; Ghosh, G.; Rath, G. A review of current interventions for COVID-19 prevention. Arch. Med. Res. 2020, 51, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Chirani, M.R.; Kowsari, E.; Teymourian, T.; Ramakrishna, S. Environmental impact of increased soap consumption during COVID-19 pandemic: Biodegradable soap production and sustainable packaging. Sci. Total Environ. 2021, 796, 149013. [Google Scholar] [CrossRef] [PubMed]
- Voidarou, C.; Rozos, G.; Stavropoulou, E.; Giorgi, E.; Stefanis, C.; Vakadaris, G.; Vaou, N.; Tsigalou, C.; Kourkoutas, Y.; Bezirtzoglou, E. COVID-19 on the spectrum: A scoping review of hygienic standards. Front. Public Health 2023, 11, 1202216. [Google Scholar] [CrossRef] [PubMed]
- Săveanu, C.I.; Porsega, A.; Anistoroaei, D.; Iordache, C.; Bobu, L.; Săveanu, A.E. Cross-sectional study to evaluate knowledge on hand hygiene in a pandemic context with SARS-CoV-2. Medicina 2022, 58, 304. [Google Scholar] [CrossRef]
- Kalbusch, A.; Henning, E.; Brikalski, M.P.; de Luca, F.V.; Konrath, A.C. Impact of coronavirus (COVID-19) spread-prevention actions on urban water consumption. Resour. Conserv. Recycl. 2020, 163, 105098. [Google Scholar] [CrossRef] [PubMed]
- Arifin, M.Z.; Maizunati, N.A. Soap liquid waste due to COVID-19 pandemic in Magelang City: Challenges and recommendations. Sanit. Value Chain 2021, 5, 18–19. [Google Scholar] [CrossRef]
- Suárez, L.; Díez, M.A.; García, R.; Riera, F.A. Membrane technology for the recovery of detergent compounds: A review. J. Ind. Eng. Chem. 2012, 18, 1859–1873. [Google Scholar] [CrossRef]
- Ingrassia, E.B.; Luna, M.F.; Rubin, J.C. When the use of derived wastes and effluents treatment is part of a responsible industrial production: A review. Chem. Eng. Process. Process Intensif. 2024, 201, 109826. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Nunes, R.F.; Teixeira, A.C.S.C. An overview on surfactants as pollutants of concern: Occurrence, impacts, and persulfate-based remediation technologies. Chemosphere 2022, 300, 134507. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 19th ed.; APHA: Washington, DC, USA, 1995.
- Brasil. Sistema Integrado de Resolução Ambiental. Resolução CONAMA nº 357, de 17 de março de 2005. Available online: https://www.siam.mg.gov.br/sla/download.pdf?idNorma=2747 (accessed on 7 November 2022).
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; A Saleh, T. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Jena, G.; Dutta, K.; Daverey, A. Surfactants in water and wastewater (greywater): Environmental toxicity and treatment options. Chemosphere 2023, 341, 140082. [Google Scholar] [CrossRef]
- Rasheed, T.; Shafi, S.; Bilal, M.; Hussain, T.; Sher, F.; Rizwan, K. Surfactants-based remediation as an effective approach for removal of environmental pollutants—A review. J. Mol. Liq. 2020, 318, 113960. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Low, A.; Rabat, N.E. A review on recent developments on adsorption of surfactants from wastewater. J. Environ. Manag. 2020, 254, 109750. [Google Scholar] [CrossRef]
- Ankit, A.; Banerjee, A.; Bhardwaj, S. Environmental impact of COVID-19 pandemic: More negatives than positives. Environ. Sustain. 2021, 4, 447–454. [Google Scholar] [CrossRef]
- Svobodová, Z.; Lloyd, R.; Machala, M. Water quality and fish health. In EIFAC Technical Paper; FAO: Rome, Italy, 1993; Volume 54, p. 59. Available online: http://www.fao.org/3/t1623e/t1623e.pdf (accessed on 14 July 2020).
- Plastics Europe. The Facts 2019. An Analysis of European Plastics Production, Demand, and Waste Data. Available online: https://www.plasticseurope.org/en/resources/market-data (accessed on 15 June 2022).
- ABIPLAST—Associação Brasileira da Indústria do Plástico. Estudo Encomendado pelo Picplast Mapeia a Indústria de Reciclagem do Plástico no Brasil. 2020. Available online: http://www.abiplast.org.br/noticias/estudo-encomendado-pelo-picplast-mapeia-a-industria-de-reciclagem-do-plastico-no-brasil (accessed on 3 September 2021).
- Kemona, A.; Piotrowska, M. Polyurethane recycling and disposal: Methods and prospects. Polymers 2020, 12, 1752. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7464512/ (accessed on 28 June 2022). [CrossRef] [PubMed]
- Yu, T.M.; Meira, A.C.R.; Kreutz, J.C.; Effting, L.; Giona, R.M.; Gervasoni, R.; de Moura, A.A.; Bezerra, F.M.; Bail, A. Exploring the surface reactivity of the magnetic layered double hydroxide lithium-aluminum: An alternative material for sorption and catalytic purposes. Appl. Surf. Sci. 2019, 467–468, 1195–1203. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Swisher, L.; Hirsh, I.J. Brain damage and the ordering of two temporally successive stimuli. Neuropsychologia 1972, 10, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; Gerke, J.; Ziechmann, W. Photodegradation of the surfactants Na-dodecylbenzenesulfonate and dodecylpyridinium-chloride as affected by humic substances. Water Air Soil Pollut. 1997, 98, 43–55. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Tarbuk, A.; Pusic, T. Electrokinetic properties of textile fabrics. Color. Technol. 2005, 121, 221–227. [Google Scholar] [CrossRef]
- Bouraie, M.E.; Abdelghany, A. Sorption features of polyurethane foam functionalized with salicylate for chlorpyrifos: Equilibrium, kinetic models, and thermodynamic studies. Polymers 2020, 12, 2036. [Google Scholar] [CrossRef]
- Mindivan, F. Effect of crystalline form (γ) of polyamide 6/graphene nanoplatelets (pa6/gn) nanocomposites on its structural and thermal properties. Mach. Technol. Mater. 2016, 10, 56–59. [Google Scholar]
- Asefnejad, A.; Khorasani, M.T.; Behnamghader, A.; Farsadzadeh, B.; Bonakdar, S. Manufacturing of biodegradable polyurethane scaffolds based on polycaprolactone using a phase separation method: Physical properties and in vitro assay. Int. J. Nanomed. 2011, 6, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Nakhaei, M.; Elhami, S. Chapter 5—Adsorption processes for water treatment in emerging contaminants. In Membrane Technology for Water and Wastewater Treatment in Emerging Contaminants, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 139–192. [Google Scholar]
- Chapman, A.; Butler, C. Nanostructured Water Purification Systems: Recent Advances and Challenges; CRC Press: Boca Raton, FL, USA, 2020; pp. 19–34. [Google Scholar]
- Duman, O.; Ayranci, E. Adsorptive removal of cationic surfactants from aqueous solutions onto high-area activated carbon cloth monitored by in situ UV spectroscopy. J. Hazard. Mater. 2010, 174, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Bandyopadhyay, M.; Pal, A. Removal of anionic surfactant from wastewater by alumina: A case study. Colloids Surf. A Physicochem. Eng. Asp. 2005, 254, 165171. [Google Scholar] [CrossRef]
- Adak, A.; Bandyopadhyay, M.; Pal, A. Adsorption of anionic surfactant on alumina and reuse of the surfactantmodified alumina for the removal of crystal violet from aquatic environment. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2005, 40, 167–182. [Google Scholar] [CrossRef]








| Experiments | A: pH | B: Temperature (°C) | C: Time (min) | D: Material | LAS Qe (mg g−1) | DPC Qe (mg g−1) |
|---|---|---|---|---|---|---|
| 1 | 4 | 20 | 30 | PA | 0.45 | 4.54 |
| 2 | 10 | 20 | 30 | PA | 0.42 | 0 |
| 3 | 4 | 40 | 30 | PA | 0.80 | 4.44 |
| 4 | 10 | 40 | 30 | PA | 0.78 | 0 |
| 5 | 4 | 20 | 120 | PA | 0.81 | 4.87 |
| 6 | 10 | 20 | 120 | PA | 0.76 | 0 |
| 7 | 4 | 40 | 120 | PA | 1.23 | 4.4 |
| 8 | 10 | 40 | 120 | PA | 1.47 | 0 |
| 9 | 4 | 30 | 75 | PA | 1.29 | 4.47 |
| 10 | 10 | 30 | 75 | PA | 1.21 | 0 |
| 11 | 7 | 20 | 75 | PA | 0.04 | 13.44 |
| 12 | 7 | 40 | 75 | PA | 0.96 | 13.34 |
| 13 | 7 | 30 | 30 | PA | 0.17 | 13.21 |
| 14 | 7 | 30 | 120 | PA | 0 | 12.68 |
| 15 | 7 | 30 | 75 | PA | 0 | 12.77 |
| 16 | 7 | 30 | 75 | PA | 0.06 | 13.29 |
| 17 | 4 | 20 | 30 | PU | 3.73 | 4.70 |
| 18 | 10 | 20 | 30 | PU | 4.03 | 1.55 |
| 19 | 4 | 40 | 30 | PU | 2.67 | 4.42 |
| 20 | 10 | 40 | 30 | PU | 2.74 | 2.1 |
| 21 | 4 | 20 | 120 | PU | 2.72 | 4.79 |
| 22 | 10 | 20 | 120 | PU | 2.80 | 0 |
| 23 | 4 | 40 | 120 | PU | 2.75 | 4.24 |
| 24 | 10 | 40 | 120 | PU | 2.66 | 1.16 |
| 25 | 4 | 30 | 75 | PU | 2.89 | 4.13 |
| 26 | 10 | 30 | 75 | PU | 2.99 | 1.73 |
| 27 | 7 | 20 | 75 | PU | 3.73 | 13.32 |
| 28 | 7 | 40 | 75 | PU | 3.42 | 13.34 |
| 29 | 7 | 30 | 30 | PU | 4.14 | 13.35 |
| 30 | 7 | 30 | 120 | PU | 3.70 | 13.12 |
| 31 | 7 | 30 | 75 | PU | 3.32 | 13.43 |
| 32 | 7 | 30 | 75 | PU | 3.67 | 13.54 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 56.85 | 13 | 4.37 | 15.93 | <0.0001 |
| A: pH | 0.014 | 1 | 0.014 | 0.049 | 0.8269 |
| B: Temperature | 5.00 × 10−6 | 1 | 5.00 × 10−6 | 1.82 × 10−5 | 0.9966 |
| C: Time | 0.053 | 1 | 0.053 | 0.19 | 0.6654 |
| D: Material | 53.85 | 1 | 53.85 | 196.20 | <0.0001 |
| AB | 6.25 × 10−4 | 1 | 6.25 × 10−4 | 2.27 × 10−3 | 0.9625 |
| AC | 1.22 × 10−3 | 1 | 1.22 × 10−3 | 4.46 × 10−3 | 0.9475 |
| AD | 8.00 × 10−3 | 1 | 8.00 × 10−3 | 0.029 | 0.8663 |
| BC | 0.44 | 1 | 0.44 | 1.61 | 0.2205 |
| BD | 1.53 | 1 | 1.53 | 5.57 | 0.0297 |
| CD | 0.94 | 1 | 0.94 | 3.42 | 0.0811 |
| A2 | 0.013 | 1 | 0.013 | 0.047 | 0.8304 |
| B2 | 3.31 × 10−4 | 1 | B2 | 1.20 × 10−3 | 0.9727 |
| C2 | 9.71 × 10−3 | 1 | C2 | 0.035 | 0.8528 |
| Residual | 4.94 | 18 | 0.27 | ||
| Lack of Fit | 4.88 | 16 | 0.30 | 9.67 | 0.0977 |
| Pure Error | 0.063 | 2 | 0.032 | ||
| Cor. Total | 61.79 | 31 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 932.34 | 13 | 71.72 | 711.75 | <0.0001 |
| A: pH | 73.96 | 1 | 73.96 | 733.98 | <0.0001 |
| B: Temperature | 0.0026 | 1 | 0.0026 | 0.0262 | 0.8731 |
| C: Time | 0.4651 | 1 | 0.4651 | 4.62 | 0.0455 |
| D: Material | 1.74 | 1 | 1.74 | 17.31 | 0.0006 |
| AB | 0.6045 | 1 | 0.6045 | 6.00 | 0.0248 |
| AC | 0.4523 | 1 | 0.4523 | 4.49 | 0.0483 |
| AD | 2.44 | 1 | 2.44 | 24.18 | 0.0001 |
| BC | 0.0001 | 1 | 0.0001 | 0.0006 | 0.9814 |
| BD | 0.1232 | 1 | 0.1232 | 1.22 | 0.2833 |
| CD | 0.3302 | 1 | 0.3302 | 3.28 | 0.0870 |
| A2 | 598.15 | 1 | 598.15 | 5936.18 | <0.0001 |
| B2 | 0.0844 | 1 | 0.0844 | 0.8380 | 0.3721 |
| C2 | 0.1085 | 1 | 0.1085 | 1.08 | 0.3132 |
| Residual | 1.81 | 18 | 0.1008 | ||
| Lack of Fit | 1.67 | 16 | 0.1045 | 1.48 | 0.4772 |
| Pure Error | 0.1412 | 2 | 0.0706 | ||
| Cor. Total | 934.16 | 31 |
| Assay | Conditions | Model | R2 | Parameters | |
|---|---|---|---|---|---|
| 1 | PU/LAS pH = 10 T = 20 °C | Langmuir | 0.8269 | KL (L g−1) | 8.32 × 10−2 |
| qmax (mg g−1) | 54.5 | ||||
| Freundlich | 0.9980 | KF (mg g−1) | 1.4 × 10−2 | ||
| n | 1.82 | ||||
| SIPS | 0.9955 | KL (L g−1) | 1.2 × 10−2 | ||
| qmax (mg g−1) | 91.6 | ||||
| n | 2.61 | ||||
| 2 | PU/DPC pH = 6.8 T = 25 °C | Langmuir | 0.8782 | KL (L g−1) | 2.77 × 10−2 |
| qmax (mg g−1) | 16.5 | ||||
| Freundlich | 0.8851 | KF (mg g−1) | 3.21 | ||
| n | 0.76 | ||||
| SIPS | 0.8848 | KL (L g−1) | 2.24 × 10−2 | ||
| qmax (mg g−1) | 36.2 | ||||
| n | 0.76 | ||||
| 3 | PA/LAS pH = 4 T = 40 °C | Langmuir | 0.9721 | KL (L g−1) | 4.93 × 10−3 |
| qmax (mg g−1) | 5.7 | ||||
| Freundlich | 0.9728 | KF (mg g−1) | 0.233 | ||
| n | 1.04 | ||||
| SIPs | 0.9794 | KL (L g−1) | 9.13 × 10−3 | ||
| qmax (mg g−1) | 16.2 | ||||
| n | 1.52 | ||||
| 4 | PA/DPC pH = 6.65 T = 20 °C | Langmuir | 0.9928 | KL (L g−1) | 1.2 × 10−3 |
| qmax (mg g−1) | 4.59 | ||||
| Freundlich | 0.9916 | KF (mg g−1) | 0.593 | ||
| n | 0.978 | ||||
| SIPS | 0.9980 | KL (L g−1) | 1.23 × 10−3 | ||
| qmax (mg g−1) | 9.54 | ||||
| n | 1.41 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, T.R.d.; Tolari, D.L.M.; da Silva, A.C.P.; Bonafé, E.G.; Samulewski, R.B.; Tessaro, A.L. Use of Domestic Polymeric Waste for Surfactant Removal from Wastewater. Sustain. Chem. 2025, 6, 6. https://doi.org/10.3390/suschem6010006
Reis TRd, Tolari DLM, da Silva ACP, Bonafé EG, Samulewski RB, Tessaro AL. Use of Domestic Polymeric Waste for Surfactant Removal from Wastewater. Sustainable Chemistry. 2025; 6(1):6. https://doi.org/10.3390/suschem6010006
Chicago/Turabian StyleReis, Thaiara Ramires dos, Donizeti Leonardo Mancini Tolari, Ana Claudia Pedrozo da Silva, Elton Guntendorfer Bonafé, Rafael Block Samulewski, and André Luiz Tessaro. 2025. "Use of Domestic Polymeric Waste for Surfactant Removal from Wastewater" Sustainable Chemistry 6, no. 1: 6. https://doi.org/10.3390/suschem6010006
APA StyleReis, T. R. d., Tolari, D. L. M., da Silva, A. C. P., Bonafé, E. G., Samulewski, R. B., & Tessaro, A. L. (2025). Use of Domestic Polymeric Waste for Surfactant Removal from Wastewater. Sustainable Chemistry, 6(1), 6. https://doi.org/10.3390/suschem6010006








