Abstract
Heavy metal pollution in water resources, particularly cadmium and lead, poses a significant environmental and public health challenge, requiring the development of sustainable, efficient, and cost-effective water treatment methods. Therefore, this study investigates the biosorption capabilities of natural (SN) and surfactant-modified (SM) guava seed biosorbents to remove Cd and Pb from aqueous solutions. Guava seeds, an agricultural waste material, were treated with hexadecyltrimethylammonium bromide (HDTMA-Br) to enhance their adsorption efficiency. The biosorbents were characterized by FTIR, SEM-EDS, and zeta potential analysis to explain the surface modifications and their influence on the adsorption mechanisms. Batch experiments were performed to evaluate the effects of pH, contact time, temperature, biosorbent dosage, and concentration on Cd and Pb removal efficiencies. Adsorption isotherm and kinetic data were analyzed using mathematical models to obtain the basic parameters of the systems under study. The results showed that SM exhibited superior adsorption capacities of 328 mg/g for Cd and 594 mg/g for Pb at 25 °C, significantly outperforming SN. The study analyzed the thermodynamic parameters of adsorption systems, revealing endothermic and exothermic properties for SN and SM. Functional groups like hydroxyl and carbonyl were crucial for metal ion binding. HDTMA-Br introduced active sites and enhanced surface charge interactions. Regeneration tests showed reusability, maintaining over 85% efficiency after four cycles. Guava seeds could be cost-effective and sustainable biosorbents for heavy metal removal.
1. Introduction
Water pollution by heavy metals is a worldwide problem, mainly due to increased industrial, agricultural, metal finishing, landfill, energy production, and leather processing activities. These activities have generated high concentrations of heavy metals in surface and groundwater. The World Health Organization (WHO) has even established maximum permissible limits ranging from 0.01 to 1 mg/L of these metal ions in water for health protection [1,2]. It is also worrying that heavy metal concentrations have been reported in rivers, lakes, soils, and air in urban areas and marine environments [3], and even metals such as lead and cadmium have been detected in food, raising safety and health concerns. Regularly monitoring these metal levels is essential to mitigate health risks associated with food consumption [4,5]. Heavy metals tend to accumulate at different levels of the food chain, causing serious problems for human health and ecosystems [2].
Heavy metal contamination in water resources is a significant concern in developing countries, where inadequate sanitation and wastewater treatment facilities exacerbate the issue, threatening the achievement of Sustainable Development Goals (SDGs) related to clean water, sanitation, and life below water [6]. The surge of industrial activities contributes to the release of untreated wastewater containing heavy metals, which aggravates the problem of water pollution. Therefore, addressing the water pollution problem is crucial to protecting human health, preserving aquatic ecosystems, and achieving SDGs.
Heavy metals are introduced into the environment through natural and anthropogenic sources, including the weathering of bedrock and volcanic eruptions [5], industrial processes such as metal plating, battery manufacturing, petroleum refining, and pigment production, and fossil fuel combustion, coal burning in power plants, and leaded gasoline [4,5,7]. In agriculture, fertilizers and pesticides can introduce heavy metals into soil, potentially entering metals into the food chain and groundwater; also, urban runoff and wastewater discharge transport heavy metals from industrial and residential areas into water bodies [4,5]. Lead, a non-biodegradable metal found in dust and soil, disrupts cellular processes due to its bioaccumulative properties. However, effective management and reduction strategies can mitigate heavy metal pollution’s environmental and health impacts, highlighting the need for sustainable solutions [8,9].
The severity of heavy metal contamination is underscored by recent studies reporting the presence of toxic heavy metals, such as Hg, Pb, and Cd, in commonly consumed vegetables like lettuce, cabbage, squash, broccoli, and potatoes. These metals accumulate in the blood, particularly in fatty tissues, leading to toxicity in the human body [10]. For instance, prolonged exposure to small doses of lead, greater than 5 mg/dL, is associated with subclinical effects, such as hypertension, damage to kidney function, cognitive dysfunction, and reproductive disorders [10]. It is also considered the second largest toxin in the body, capable of causing neurological disorders, anemia, or kidney cancer. It adversely affects the nervous system and causes growth problems [11]. This metal is responsible for 600,000 new cases of intellectual disability per year worldwide, according to the WHO [12]. Lead is present in the environment and emissions derived from human activities such as batteries, pesticides, plumbing, paints, and oils [2,10]. The impact of these findings on human health is deeply concerning, and it underscores the urgent need for effective management and reduction strategies to mitigate its environmental and health consequences.
On the other hand, cadmium is found naturally in soils and rocks and synthetically in fertilizers, plastics, batteries, compounds associated with zinc, and paints. It is toxic for both humans and animals, causing acute short-term effects, as well as long-term chronic effects. It accumulates in plants and animals, entering the human food chain primarily through consuming contaminated food and water, cigarette smoke, and occupational exposure [13,14]. It mainly accumulates in organs such as the liver and kidneys; the half-life of this metal in the kidney is 18 to 33 years, and 200 μg/g is considered the critical cadmium concentration that causes damage to the renal cortex. A diet low in iron and, therefore, the deficiency of this element in the human body contributes to increased cadmium retention and increases its adsorption. This metal can cause bronchitis, infertility, neurological disorders, carcinogenesis, hypertension, and vascular diseases [10]. The metal induces oxidative stress by generating reactive oxygen species (ROS), which leads to DNA damage, apoptosis, and altered gene expression, further exacerbating its toxic effects. Cadmium’s ability to mimic essential metals like zinc disrupts metal homeostasis, a complex process contributing to its toxicity [8,14].
Several conventional methods are used to remove heavy metals from industrial wastewater. However, due to their high costs, high sludge production, and incomplete removal, they can be considered ineffective and unfavorable. These methods include precipitation, electrochemical treatment, membrane separation, evaporation, and coagulation. For this reason, sustainable technologies have been developed for removing metals using low-cost, efficient, easily acquired, and reusable biosorbents, among which agricultural wastes stand out for having these characteristics [15]. Among these technologies, biosorption stands out, as it is cost-effective and environmentally friendly for removing metals from water. It uses abundant and inexpensive natural or waste biomaterials, such as bacteria, fungi, algae, and agricultural waste, with high metal binding capacities [16]. The process is versatile and can be applied in fixed-bed columns for continuous treatment. The biosorption mechanism involves electrostatic interactions, ion exchange, and hydrogen bonding, which enhances its adsorption capacity. It can be tailored to specific metals, making it suitable for different types of wastewater [17,18]. Unlike other methods, biosorption offers cost-effectiveness, environmental sustainability, and operational efficiency.
Biosorption is a physicochemical process through which contaminants are adsorbed to living or inert organic materials, allowing for the rapid and reversible binding of specific ions to the biosorbent surface and also reusing waste from industrial or agricultural processes, like lignocellulosic biomass [1], whose main components are cellulose, hemicellulose, and lignin [19]. Among the agricultural wastes that have been used to produce a biosorbent for the uptake of heavy metals are mandarin peel [20], orange and mango peels [21], banana and potato peels [22], moringa seeds and leaves [23], coffee husks [24], and sugarcane bagasse [25]. Guava seeds (Psidium guajava L.) are agricultural waste with remarkable potential as efficient biosorbents for removing highly toxic pollutants [26,27,28,29,30]. However, our research focuses on using these natural seeds as biosorbents for metal cations such as cadmium and lead, a potential that has yet to be fully explored.
In addition, biosorbents can be chemically or physically modified to enhance their chemical and structural stability, mechanical strength, selectivity, and adsorption efficiency by introducing new functional groups on their surfaces [31,32]. Some adsorbents and biomasses have been modified with cationic surfactants, such as hexadecyltrimethylammonium bromide (HDTMA-Br) and octadecyltrimethylammonium bromide (ODTMA-Br), to increase the adsorption capacity of heavy metals. For example, the modification of clinoptilolite using these surfactants increased its anionic sorption capacity, allowing the removal of both chromates and iodide from aqueous solutions [33]. Also, sodium dodecyl sulfate (SDS) applied to Salacca zalacca skin improves its adsorption capacity for methylene blue and batik wastewater [34]. Bio-surfactant-modified ground grass using rhamnolipid has been explored for cadmium ion removal, offering high sorption ability, low cost, and biodegradability [7]. Moreover, cationic surfactants like cetyl trimethyl ammonium bromide (CTAB) have been shown to alter the surface charge of biomass, improving the uptake of metal anions like arsenic and chromium from wastewater [35]. This approach offers a cost-effective and efficient method for wastewater treatment, with potential for further optimization and application in various environmental remediation scenarios. Therefore, we hypothesized that surfactant-modified guava seeds would exhibit a significantly higher adsorption capacity for lead (Pb) and cadmium (Cd) than natural guava seeds due to the functional groups introduced via the modification. To the best of our knowledge, there are no previous reports on the use of natural or surfactant-modified guava seeds (Psidium guajava L.) as biosorbents for the removal of heavy metals such as cadmium (Cd) and lead (Pb). This study addresses this gap by exploring the potential of guava seeds, an abundant agricultural waste, as a low-cost and effective biosorbent for environmental remediation.
Guava (Psidium guajava L.) is among the world’s best-known and most appreciated fruits. It is a tropical and subtropical fruit widely cultivated across various countries, with India being the largest producer, contributing significantly to the global production of guava. Other major guava-producing countries include China, Thailand, Indonesia, Pakistan, Brazil, and Mexico, each contributing to the global supply of this fruit [36,37]. The United States and several South American countries, such as Colombia, also play a role in guava production, although to a lesser extent than the leading producers [38]. The adaptability of guava to various climatic conditions has allowed it to be cultivated in a wide range of countries, making it a significant fruit crop in both tropical and subtropical regions [36,39]. The global production of guava was estimated at 55 million tonnes in 2019, with India alone accounting for 45% of this total [37]. This widespread cultivation is supported by the fruit’s high nutritional value, pleasant aroma, and economic importance, which have led to its prominence in the agricultural sectors of these countries [36,38]. Guava pulp is industrialized, while seeds are agricultural waste. Research shows that guava seeds contain acid-rich lignocellulosic material, which can be broken down through thermal treatment [40]. These seeds can also be utilized as a precursor to activated carbon or biochar or directly as a biosorbent [26,30,41]. The above-mentioned implies that guava seeds possess the characteristics of effective biosorbents, such as wide availability, low cost, low toxicity, and surface chemical properties suitable for adsorbing various water pollutants.
Hence, this study aims to assess how effective natural and surfactant-modified guava seeds are in removing Pb and Cd from water via adsorption. This involved assessing the adsorption kinetics, isotherms, and thermodynamics and evaluating the potential for seed regeneration and reuse over multiple cycles. Additionally, the study examined the impact of different process parameters such as pH, biosorbent dose, and temperature.
2. Materials and Methods
2.1. Biosorbent Preparation
Guava seeds were obtained from the industrial processing of the fruit, as the seeds are considered waste. The leftover pulp was removed via washing, and the seeds were dried at 60 °C for 24 h. After that, the seeds were ground and sieved until they reached the desired particle size of 2 mm. This material, labeled SN, was used as a biosorbent and stored in a desiccator for further experiments.
The guava seeds (SN) were treated with the cationic surfactant hexadecyltrimethylammonium bromide (HDTMA-Br) by immersing the seeds in a 50 mmol solution of HDTMA-Br. The mixture was shaken at 30 °C and 100 rpm for 48 h. After this, the seeds were slightly rinsed with deionized water at 20 °C to remove excess surfactant. The treated material, now identified as SM, was then dried at 60 °C and stored in a desiccator, ready for later use.
2.2. Biosorbent Characterization
Fourier transform infrared spectroscopy (FTIR) analyzes the functional group bands of inorganic and organic substances in a material’s structure. It is an essential tool for studying adsorption processes by identifying and characterizing functional groups on adsorbent surfaces, such as hydroxyl, carbonyl, and carboxylic acids. These functional groups are essential for binding solid sites through surface complexation and cation exchange. FTIR analysis was conducted on SN and SM samples before and after removing Pb and Cd ions. The equipment used was a Mid-FTIR by Bruker, equipped with a fiber optic probe, allowing direct analysis of the object surface being studied. The spectral range was 4000 to 400 cm−1 with a resolution of 4 cm−1. The samples were pulverized and prepared with KBr to produce pellets for each biosorbent sample, enabling the identification of the main bands in the spectrogram.
Scanning electron microscopy (SEM), analysis was also performed for the observation and surface characterization of the biosorbent materials (SN and SM) to observe their texture, pore size, and morphology, as well as their semi-quantitative elemental composition using energy dispersive spectroscopy (EDS) analysis. Samples of SN and SM were collected before the removal process and after the adsorption of Cd and Pb. The samples were covered with copper and analyzed using a scanning electron microscope (JEOL JMS-6400, JEOL Ltd., Peabody, MA, USA) coupled with an EDS microanalysis detector (Bruker XFLASH 4010, Peabody, MA, USA).
Zeta potential analysis is a fundamental tool for studying adsorption processes. It provides information about the surface charge characteristics of adsorbents and their interaction with adsorbates. Zeta potential is also helpful in determining the isoelectric point or point of zero charge (PZC) of the adsorbent in an aqueous solution with zero or very close to zero charge. It is advantageous to monitor and characterize an adsorbent that behaves as a colloid in an aqueous solution since it indicates potential surface changes and repulsion forces between the adsorbent and the adsorbate. The PZC was determined by measuring the zeta potential of SN and SM using a Zeta-Meter System 3+. Different biosorbent solutions were prepared at various pH values, ranging from 3 to 12, and the pH was adjusted with 0.01 M HCl and 0.01 M KOH solutions. The experiments were carried out at 25 °C.
2.3. Biosorption Kinetics
Batch contact experiments were performed with solutions of these metals at different contact times to determine the biosorption kinetics of cadmium and lead. These experiments used 10 mL aliquots of 50 mg/L Cd(NO3)2 and Pb(NO3)2 solutions. They were separately contacted with 100 mg of SN and SM biomass in plastic flasks, i.e., a liquid-to-solid (L/s) ratio of 100 mL/g. They were then placed in a thermo-bath with reciprocal stirring for different periods, stirring at 100 rpm at 25 °C. Once the contact time was reached, the supernatants were separated through filtration, and the metal concentration was analyzed for Cd and Pb via atomic absorption (AA) spectroscopy in a PerkinElmer AAnalyst 200; this allowed the time at which equilibrium was reached to be determined. All biosorption experiments were conducted three times to ensure reproducibility. A control test was also performed without biosorbents to assess analyte (Cd or Pb) losses from processes other than biosorption.
2.4. Effect of Biosorbent Dose
Batch-type contact experiments were performed to determine the effect of the biosorbent dose with Cd(NO3)2 and Pb(NO3)2 solutions at 50 mg/L and different masses of SN and SM ranging from 0.1 g to 1 g. Different doses of guava seeds were weighed and placed in plastic bottles separately; then, 10 mL of the cadmium and lead solution was added, and they were placed in a thermo-bath with shaking at a speed of 100 rpm and a fixed temperature (25 °C) until equilibrium was reached. The concentrations of the metals in the supernatants were determined as mentioned above. These experiments were performed in triplicate.
2.5. Influence of pH on Biosorption
Biosorption experiments were performed with cadmium and lead solutions at different pH values separately to determine which pH value caused the highest removal of contaminant and determine the influence of this parameter on the biosorbent material. This was carried out using the following procedure: SN and SM samples were weighed, and they were placed in plastic flasks to which 10 mL of the Cd(NO3)2 and Pb(NO3)2 solutions at 50 mg/L were added to yield an L/S ratio of 100 mL/g. The pH of each solution was adjusted with 0.1 M HCl or NaOH, as required, to different values ranging from 3 to 9. The flasks were shaken in a thermo-bath with reciprocal shaking at a speed of 100 rpm at 25 °C until adsorption equilibrium was reached. At the end of the contact time, the solution was filtered, and AA determined the total metal concentrations in the supernatant.
2.6. Biosorption Isotherms
Biosorption isotherms were obtained via batch contact experiments using SN and SM with Cd(NO3)2 and Pb(NO3)2 solutions at different concentrations ranging from 10 to 1000 mg/L. The tests were performed using plastic flasks in which different doses of SN and SM were put in contact, and 10 mL of the metal solutions were added. The plastic flasks were placed in a thermo-bath with reciprocal shaking and shaken at 100 rpm at 25 °C. The procedure was repeated to determine the isotherms at different temperatures (35 °C and 45 °C). At the end of the equilibrium time, the solution was filtered, and AA analyzed the supernatant for the total concentrations of each metal.
2.7. Desorption Tests
Specific biosorption experiments were initially conducted to perform desorption tests by removing Pb and Cd in order to saturate SN and SM with both metal ions. These previous tests were carried out using a dose of 1 g/L of each biosorbent and an initial concentration of 50 mg/L of each metal, separately, at a temperature of 25 °C and pH of 5. The contact times to reach adsorption equilibrium were 120 min for Cd and 280 min for Pb. The biomass was then separated from the metal solution. The recovered masses of SN and SM were rinsed lightly with deionized water to remove the excess metal solution remaining in the seeds, and they were dried in an oven at 60 °C. Subsequently, desorption tests were performed by adding 10 mL of 0.1 N HNO3 into plastic flasks containing the contaminated biosorbents (SN and SM) with Cd(II) and Pb(II) ions. The flasks were shaken at 100 rpm using a reciprocating stirring thermo-bath for 60 min at 25 °C to achieve desorption equilibrium. Subsequently, the recovered SN and SM biomasses were dried and used again in the adsorption and desorption cycle. The above procedure was repeated for four consecutive cycles. As mentioned above, AA analyzed the concentrations of the adsorbed and desorbed metals.
3. Results
3.1. FTIR Analyses
Infrared spectroscopy data identified the functional groups present in guava seeds. FTIR analysis was performed on natural and modified seed samples before and after the cadmium and lead sorption process. Figure 1 shows the FTIR spectra of natural seed (SN) and modified seed (SM). In SN (black line), a broad band at 3407.2 cm−1 is observed, corresponding to stretching vibrations of alcohols and phenols containing O-H groups and bands at 3007.7 and 732.4 cm−1 attributed to C-H groups. Carboxyl groups (COOH) can be found in bands near 2847.4 cm−1, as well as carbonyl groups of aldehydes (C=O) in peaks at 1736.5 cm−1, the latter related to the hemicellulose content in SN. Bands near 1656.8 cm−1 characterize the amino groups (N-H), while other types of OH groups corresponding to aliphatic alcohols can be observed in peaks near 1514.3 cm−1 and 1372.7 cm−1, which can be attributed to the presence of lignin in the structure of the biosorbent [42]. At wavenumbers 1168.3 cm−1 and 1097.1 cm−1, characteristic ether bands (COC) are present; this C-O stretching is due to the main cellulose chain. On the other hand, Figure 1 also shows the FTIR spectrum of SM (red line), in which a wide band is observed at wavenumber 3385.1 cm−1 corresponding to the hydroxyl group (-OH), as well as bands of different functional groups such as CH at 3011.1 cm−1, CH2 from 2862.7 cm−1 to 2925.1 cm−1, and CH3 at 1373.5 cm−1; these bands, in particular the one near 1400 cm−1, are directly related to the well-organized structure of the aliphatic chain of HDTMA [43], including also N-H bending and C-N stretching vibrations, which are characteristic of the ammonium groups in cationic surfactants [44]. Carbonyl groups (C=O) are also observed at 1748.4 cm−1 and 1646.6 cm−1, the nitro group (NO2) at 1553.3 cm−1, and (O=C-O) forms in peaks near 1241.2 cm−1 and 1155.6 cm−1. At wavenumbers near 1101.3 cm−1 and 1046.2 cm−1, characteristic bands of the C-N alkyl group are also related to cationic surfactant chains.
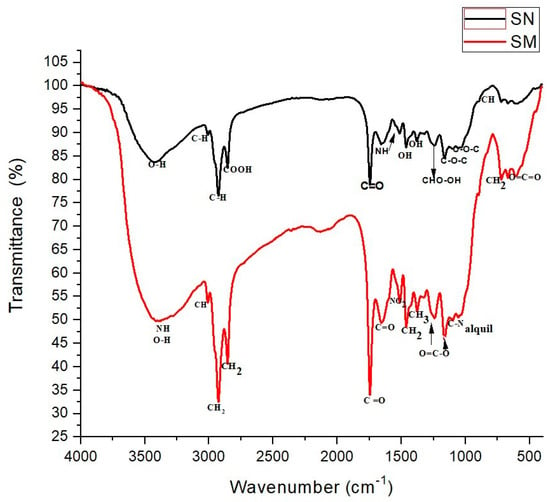
Figure 1.
FTIR spectrograms of SN and SM.
When comparing the SN and SM spectra, a typical broad peak separated within the 3600 to 3100 cm−1 range is observed. This range is associated with the stretching mode of the O-H groups, hydrogen-bonded O-H, and chemisorbed water [43]. However, variations can be observed in the absorption bands at 3407.2 cm−1, corresponding to the –OH groups, which can be attributed to the fact that intermolecular hydrogen bonds may exist due to the hydroxyl groups and the cationic surfactant, forming complexes between the ionic groups (NH+) of the surfactant and the surface structure of the biosorbent. The shifts in vibrational and stretching frequencies around 3385.1 and 1373.5 cm−1, corresponding to the amino and alkane groups, indicate the presence of surfactant on the SM surface through Van der Waals-type interactions. Furthermore, more intense transmittance bands are observed in SM after HDTMA-Br modification, particularly in the range from 1514.3 cm−1 to 1101.3 cm−1, indicative of a higher presence of surfactant-related groups, as mentioned above. Additionally, a shift in the absorption band at 1748.4 cm−1 corresponding to the C=O group was observed, which has been attributed to the formation of coordination bonds between this group (O atoms) and HDTMA-Br; this phenomenon is observed when the oxygen atoms of the C=O group interact with the bromine in HDTMA-Br, leading to a shift in the vibrational frequency of the C=O bond [45]. A shift in the band at 1646 cm−1 is also observed, indicating the presence of amide bonds (-NH-C=O) derived from the interaction of the surfactant’s amino groups with the biosorbent.
Figure 2 and Figure 3 show the FTIR spectra for SN and SM, respectively, after the adsorption of Cd and Pb ions. A comparison of the FTIR spectra for SN biosorbent (Figure 2) shows significant alterations in the functional groups upon exposure to these ions. Notable changes include the appearance of new C-N stretching bands at 1530 cm−1 and 1380 cm−1 in SN-Cd and SN-Pb, indicative of metal complex formation. The decrease in the intensity of the O-H and C=O bands supports the interaction between the biosorbent and heavy metal ions. These observations confirm that chemical interactions and complex formation play an important role in the biosorption mechanism of both cadmium and lead. Also, the medium peak at 2920 cm−1 reduced intensity after metal adsorption. The CH stretching band indicates the presence of methoxy groups in lignin, which can participate in metal complexation [46]. The weak shoulder observed at 1640 cm−1, corresponding to C=O stretching, has decreased in intensity, with a slight shift observed, particularly intensified for SN-Pb. This shift indicates the metal–lignin interactions [47], concluding that the carbonyl groups are involved in the binding process with the metal ions. The C-O and C-N stretching, around 1000 to 1500 cm⁻1, of SN-Cd and SN-Pb show changes in intensities and slight shifts, suggesting that both aromatic and aliphatic C-N and C-O bonds participate in metal ion binding, indicating complexation or interaction with cadmium and lead. Functional groups such as hydroxyl, carbonyl, carboxyl, and amine, found in the cell walls of biomaterials, play an essential role in biosorption processes. These groups, which contain C-O and C-N bonds, promote the sorption of metal ions and can change the molecular conformation of proteins, in this case, the proteins contained in SN, where peptide bonds (amide I and II bands) are involved. For example, it has been shown that the binding of metal ions such as Au(III) involves oxygenated and nitrogenous active groups, including C-O and C-N bonds in polysaccharides and proteins [48], which is consistent with what was observed in this case (SN-Cd and SN-Pb). The potential of SN for Cd and Pb adsorption is also highlighted by minor shifts in the C-H bending region, located below 1000 cm⁻1, indicating that these bonds are only partially involved in the biosorption process. Finally, it can be stated that the observed changes in the peak intensities and shifts, particularly in the OH, C=O, and C-N regions, suggest that these groups present in the SN biosorbent structure are directly involved in the biosorption mechanisms of Pb and Cd ions.

Figure 2.
FTIR spectrograms of SN, SNCd, and SNPb.
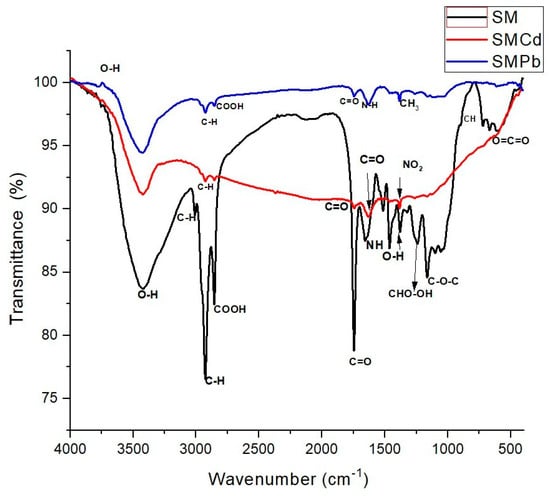
Figure 3.
FTIR spectrograms of SM, SMCd, and SMPb.
On the other hand, Figure 3 shows the FTIR spectra of the modified biosorbent before metal ion biosorption (SM) and after the biosorption process (SMCd and SMPb). When comparing these spectra, a broad O-H band can be observed in SM, which, as previously mentioned, is characteristic of free hydroxyl groups on the biosorbent surface interacting with NH+ ions from surfactant molecules and presents a significant intensity. However, its intensity decreases after Cd and Pb adsorption, particularly in SMCd, indicating an interaction between the surface hydroxyl groups and the adsorbed metals. Furthermore, if these spectra are compared with those obtained for SN, in the case of SMPb, the band’s intensity is higher, suggesting that the Pb biosorption mechanism is different in the modified seeds. Additionally, after the adsorption of Cd and Pb, this band shifts to lower wavenumbers, particularly in SMCd, indicating that a complexation mechanism through coordination bonds may exist between these groups and metal ions. The 2950–2850 cm⁻1 (C-H) band in the SM spectrum is visible for alkyl groups (Figure 3). After the adsorption of metal ions, the intensity of these bands decreases slightly in both SMCd and SMPb cases, possibly due to the rearrangement of the alkyl group on the surface. As observed in Figure 1, the presence of these groups increases after modification with HDTMA-Br since it introduces long-chain alkyl groups, which play an essential role in the adsorption process. From the above, it can be established that these functional groups are directly involved in the adsorption of Cd and Pb and are directly related to the modification of the biosorbent with the cationic surfactant.
Similarly, a strong band corresponding to carboxyl and carbonyl groups (between 1700 and 1600 cm−1) is shown in the SM spectrum (Figure 3). After the adsorption of Cd and Pb, this band shifts and decreases in intensity, particularly in SMCd, suggesting that carboxyl groups are involved in the binding of these metals. It is well known that HDTMA-Br modification increases the hydrophobicity and adsorption sites of the biosorbent, facilitating the binding of metal ions. Carbonyl and carboxyl groups contribute to this process by providing active sites for ion exchange and complexation with metal ions. This interaction is further enhanced by the structural changes induced via HDTMA-Br modification, which increases the surface area and availability of functional groups for metal ion binding. Particularly, the carbonyl groups, often in conjunction with carboxyl groups, participate in charge transfer complexes that facilitate metal ion binding. The inductive effect of bromine in HDTMA-Br enhances the dissociation of hydrogen ions from carboxyl groups, increasing the ion exchange capacity [49]. The NH2 groups can interact with metal ions through electrostatic forces, which are enhanced by the presence of HDTMA-Br [50]. Also, the NH2 groups can form chelate complexes with metal ions, which is a strong and stable form of binding [51]. Figures S1 and S2 show a schematic representation of the main sorption mechanisms for SN and SM, respectively (see Supplementary Information).
3.2. SEM-EDS Analyses
Scanning electron microscopy (SEM) analysis was performed on two samples of SN and SM before and after the metal ion biosorption process in order to determine differences in their structure, morphology, and elemental microanalysis via EDS. Figure 4 shows micrographs of SN before and after Cd and Pb biosorption. The SEM image in Figure 4a reveals a complex porous structure in the SN sample. The visible surface is composed of interconnected layers and ridges, with an intricate network of voids and channels. The pores vary in size and are irregularly distributed along the surface, indicating a heterogeneous structure. The image also shows fibrous and rough surfaces, characteristic of plant-based materials with lignocellulosic composition [18]. The porous and fibrous nature of the SN structure makes it suitable for applications in adsorption processes, as these characteristics can enhance the surface area and provide sites for interaction with contaminants. The visible pores and channels facilitate fluid movement within the structure, improving adsorption efficiency in applications targeting contaminants such as fluoride or heavy metals. The natural texture and irregularity in the surface morphology suggest a great potential for surface modifications since it is quite likely that multiple reactive sites could be functionalized with surfactants or nanoparticles, as considered in fluoride and arsenic adsorption studies [30]. An elemental microanalysis of this sample indicates that the main elements present in SN are oxygen and carbon, also highlighting the presence of elements such as phosphorus, potassium, sulfur, and traces of sodium.
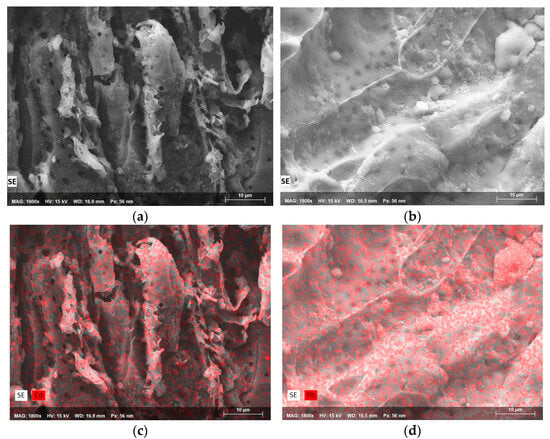
Figure 4.
SEM micrographs of (a) SN at 1800×, (b) SN at 1800×, (c) Cd mapping on SN at 1800×, and (d) Pb-mapping on SN at 1800×.
When both SEM micrographs of SN (Figure 4a,b) are compared, some differences in surface texture, pore distribution, and the presence of particles can be observed. The first image (Figure 4a) shows a fibrous and irregular structure with larger voids and interconnected channels, while the second image has a smoother and denser appearance with a compact surface layer. Figure 4b shows smaller and rounded particles, suggesting areas where the surface particles have not been removed and the seed components have not been altered through pretreatment. These differences indicate heterogeneity within the SN structure’s fibrous and porous regions. Optimizing pretreatment methods, such as grinding or surface modification with acids or alkalis, could expose more porous areas and improve adsorption performance.
The EDS analysis of SN prior to cadmium adsorption confirmed the predominance of oxygen (O) and carbon (C) as the main constituents of the biosorbent. Trace amounts of other elements, such as phosphorus (P) and potassium (K), were also identified, likely originating from the lignocellulosic composition of guava seeds. No cadmium peaks were detected in the initial analysis, as expected. However, the EDS spectrum after cadmium removal revealed a prominent Cd peak after cadmium sorption, confirming the successful adsorption of cadmium ions onto the SN surface. The increase in cadmium content was accompanied by a slight reduction in oxygen levels, indicating that functional groups containing oxygen, such as hydroxyl and carbonyl groups, were actively involved in the binding of cadmium ions. This supports the hypothesis concerning chemical interactions between Cd ions and the active sites of SN. EDS mapping of cadmium, depicted in Figure 4c, shows that cadmium is widely distributed along the surface of the SN, with red areas indicating cadmium ions adsorbed to its surface or filling the pores and channels of the biosorbent material. This suggests that SN’s porous and fibrous characteristics provide multiple active sites for the adsorption of this metal ion, allowing it to be adsorbed along the entire surface. The adsorption has notable homogeneity, with a slight concentration in porous regions. The widespread and uniform presence of Cd ions indicates strong interactions that likely involve multiple binding mechanisms on the biosorbent’s surface. EDS mapping confirms that cadmium adsorption has occurred throughout the SN structure, supporting its potential as an effective biosorbent. The broad but incomplete coverage suggests that SN could be further optimized for removing heavy metals from aqueous solutions.
After lead adsorption on SN, the EDS analysis indicated a significant presence of lead (Pb) on the biosorbent surface. The mapping highlighted a high lead concentration in specific areas (Figure 4d), particularly along compact ridges and spherical deposits observed in the SEM images. This localized distribution suggests that some regions of SN contained a higher density of functional groups capable of binding Pb ions. The increase in lead content (0.46% w/w) was more pronounced than that of cadmium (0.28% w/w), reflecting the biosorbent’s favorable interaction with lead ions. Moreover, as in the case of cadmium, a decrease in oxygen levels was observed, indicating the role of oxygen-containing functional groups in lead sorption. No lead peaks were observed in the EDS spectrum of SN before adsorption. The EDS mapping of SN after Pb biosorption (Figure 4d) reveals a widespread presence, with high concentrations in specific areas. The red overlay indicates that lead is distributed throughout the visible surface in this SN sample field, suggesting multiple active sites capable of binding lead ions. Higher concentrations are observed in certain regions, such as ridges and compact sections. The lead mapping appears more saturated and uniformly dense, suggesting a stronger or more extensive interaction with SN active sites. Differences in distribution patterns between cadmium and lead may provide information to optimize conditions, depending on the metal to be removed. In this case, it can be observed in the upper right (Figure 4b,d) that the SN surface shows accumulations of Pb in spherical shapes of the material, which could indicate metal adsorption and aggregation mechanisms. These accumulations may be the result of ions adsorbing at high-affinity sites, nucleating and forming aggregates, or the seed surface may have a higher density of functional groups in these protruding forms where they bind more easily to Pb ions, resulting in visible hemispherical deposits. These accumulations could indicate a high adsorption potential and reveal information about the adsorption mechanisms of SN. The localized formation of lead aggregates suggests that some areas of the seed structure reach saturation faster than others. Understanding these lead-rich “spheres” could inform further modifications to improve biosorbent performance.
Figure 5 shows the micrographs and EDS mappings of the surfactant-modified seeds (SM) before and after metal removal. The micrograph in Figure 5a shows the morphology of SM where the heterogeneity of its surface is also observed, as previously discussed (Figure 4), where the small protuberances, channels, pores, and smooth and rough surfaces, characteristic of the seeds, stand out again. In the micrograph in Figure 5b, the Cd mapping in SM is observed, where the distribution of the adsorbed metal ion is also generalized on the external surface of the material. In this case, it is observed that the highest concentration of Cd in SM coincides with the roughest and most protruding parts of the biosorbent rather than on the smooth surfaces, which again indicates the different types of active sites and suggests different sorption mechanisms. Before the natural biosorbent’s surface modification, the SN’s EDS spectrum showed no cadmium peaks. Following HDTMA-Br treatment, bromine (Br) peaks appeared in the SM spectrum, confirming the surfactant’s incorporation onto the biosorbent surface, with an approximate Br content of 0.2% w/w. This modification introduced additional functional groups and positively charged sites, enhancing cadmium adsorption. After cadmium sorption, the EDS spectrum of SM revealed a significantly stronger cadmium signal than SN, with an approximate content of 0.37% w/w, reflecting the modified biosorbent’s higher adsorption capacity. The elemental mapping displayed a uniform distribution of cadmium across the entire surface, suggesting that the HDTMA-Br treatment effectively enhanced the availability of active sites. The bromine peaks persisted after adsorption, suggesting that the surfactant coating remained intact, facilitating electrostatic interactions between the biosorbent and cadmium ions. A similar behavior is observed for the adsorption of Pb ions in SM (Figure 5c). However, a higher density of adsorbed Pb ions (indicated by a yellow arrow) can be observed on the material’s outer surface, which may indicate the interaction of these ions with the surfactant. A higher superficiality of the Cd and Pb ions is generally observed in SM (Figure 5a,b) compared to the mappings in SN (Figure 4c,d). Finally, Figure 5d shows an EDS mapping; the small blue dots represent the bromine adsorbed in SM, directly related to the surfactant HDTMA-Br. This fact indicates that guava seeds may also have the capacity to adsorb anionic species, thus broadening the spectrum of use of this biosorbent material and increasing its sustainable potential in water treatment processes. After lead sorption, the EDS spectrum showed distinct lead peaks, confirming its adsorption onto the SM surface. The intensity of the lead peaks was higher (~1.15% w/w) compared to lead removal via SN, indicating that Pb adsorption was more effective using SM as a biosorbent. Elemental mapping (Figure 5c) revealed a moderately uniform distribution of Pb across the surface, with localized areas of higher concentration. Interestingly, bromine peaks remained visible in the EDS spectrum after lead adsorption, indicating that the surfactant layer was not disrupted during adsorption. This stability may contribute to SM’s ability to support subsequent adsorption cycles.
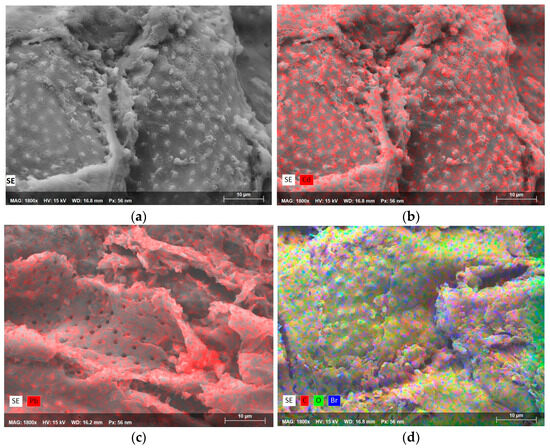
Figure 5.
SEM micrographs of (a) SM at 1800×, (b) Cd mapping on SM at 1800×, (c) Pb-mapping on SM at 1800×, and (d) EDS mapping on SM.
3.3. Zeta Potential (Ᵹ)
Figure 6 shows the results of the zeta potential determination for both SN and SM. The natural guava seed graph (Figure 6a) shows a descending trend in ᵹ that is observed as the pH increases. Initially, at low pH (approximately 2), the zeta potential is positive, indicating that the surface of the natural seed is positively charged, probably due to the protonation of surface functional groups such as carboxyl and amino groups. As the pH increases, ᵹ decreases and crosses the point of zero charge (pHPZC) around pH 3.5. After this point, the zeta potential becomes negative, suggesting the deprotonation of surface groups, which tend to be negatively charged in more basic solutions. This behavior is characteristic of materials with ionizable surface functional groups. The tendency of ᵹ to become more negative as pH increases suggests that surface sites are exposed to a greater negative charge due to the ionization of such groups. This phenomenon is important for anion adsorption since the surface charge determines the affinity of the biosorbent toward certain types of ions. This may represent an advantage for the natural seeds since it could help remove both metal ions and highly toxic anionic species in polluted waters.
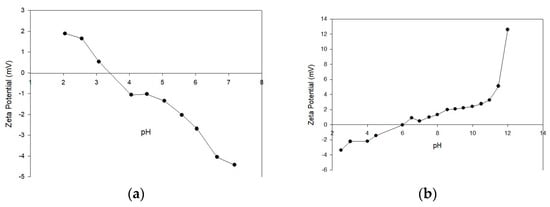
Figure 6.
Zeta potential as a function of pH for (a) SN and (b) SM.
On the other hand, concerning the SM, the graph (Figure 6b) shows a significant change in the behavior of ᵹ compared to the natural seed. At a low pH, the potential starts with values close to zero, indicating that the treatment with HDTMA-Br has altered the seed surface. The point of zero charge (pHPZC) for SM is around pH 6. As the pH increases, ᵹ becomes positive and increases progressively, reaching high positive values in the pH range of 12 to 13. This change can be clearly attributed to the surface modification with HDTMA-Br, a cationic surfactant, as well as several interconnected parameters, primarily pH, which influences the ionization of surface groups on the particles, subsequently affecting the surface charge density. The presence of this compound on the seed surface provides quaternary ammonium groups, which remain positively charged, regardless of the pH, which explains the positive behavior of the potential at high pH. This modification may also affect the adsorption of priority pollutant anions due to the electrostatic attraction between the positively charged surface of the modified seed and these chemical species. This suggests a significant advantage for this material in the sustainable decontamination of various wastewater or natural water pollutants. This modification may also affect the adsorption of priority pollutant anions due to the electrostatic attraction between the positively charged surface of the modified seed and these chemical species. This suggests a significant advantage for this material in the sustainable decontamination of various wastewater or natural water pollutants. Comparatively, the modified seeds (SM) are more versatile and could be more effective in alkaline pH conditions, while the natural seeds (SN) could be more efficient in acidic conditions or close to their isoelectric point. The choice of one or the other material will depend on the type of pollutant and the pH conditions of the solution to be treated.
3.4. Biosorption Kinetics
Figure 7 shows the biosorption kinetics of Cd on both biosorbents. Firstly, the biosorption of Cd on SN exhibits a constant increase at the beginning, with the removal percentage reaching around 80% within the first 50 min. After this point, the curve stabilizes, indicating it is approaching equilibrium. Over the whole time range (up to 500 min), the Cd removal percentage remains relatively stable, at around 85%, suggesting that SN reaches equilibrium relatively quickly. This plateau indicates that natural guava seeds occupy most of their available active sites within the first 50–100 min. Conversely, in the case of SM, the Cd removal percentage consistently exceeds that of SN throughout the entire time range. Within the first 50 min, SM reaches a removal percentage close to 90%, indicating rapid initial and overall adsorption kinetics. We also observe that the curve for SM quickly reaches a plateau, stabilizing near 95% removal. The fact that this plateau is higher and moves faster than SN suggests that the HDTMA-Br modification improves guava seeds’ biosorption rate and capacity.
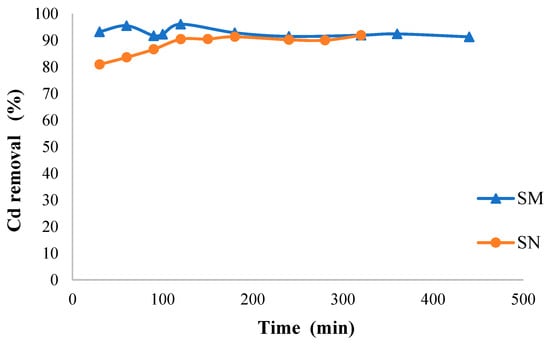
Figure 7.
Cadmium biosorption as a function of time by SN and SM (Cd concentration = 50 mg/L; adsorbent dose = 10 g/L; pH = 6; temperature = 25 °C).
It is important to mention that faster SM kinetics can provide economical and sustainable benefits by enabling shorter treatment times, reducing material requirements, and improving the practicality of these alternative water treatment methods. Modified guava seeds offer a cost-effective, sustainable solution to heavy metal contamination in water, reducing reliance on synthetic materials and supporting circular economy practices.
The kinetics data for Pb biosorption on SN and SM can be observed in Figure 8. Regarding SN, Pb biosorption shows a gradual increase in removal percentage with time. In the first 100 min, Pb removal reaches approximately 20%, which implies a relatively low initial rate. During the following minutes, the removal percentage increases steadily and reaches approximately 60% at 500 min, showing a slight plateau from 440 to 500 min, so the equilibrium time can be established in this range. This indicates that SN has a slower Pb adsorption rate than Cd (Figure 7). The removal efficiency is effective but requires longer to reach higher percentages, suggesting relatively slow kinetics for lead adsorption.

Figure 8.
Lead biosorption as a function of time by SN and SM (Pb concentration = 50 mg/L; adsorbent dose = 10 g/L; pH = 6; temperature = 25 °C).
On the other hand, Pb removal via SM is also gradual but initially slower compared to SN, with a lower removal percentage during the first 200 to 300 min. However, from 400 min onwards, the removal percentage starts to approach that of SN and reaches approximately 50% at 500 min, also presenting a small plateau after 480 min of contact. This behavior contrasts with the previous results for Cd (Figure 7), in which SM outperformed SN regarding the adsorption rate and capacity. Here, SM appears less effective for Pb biosorption than SN, taking longer to reach comparable removal levels. In this case, it can be experimentally established that SN demonstrates a faster adsorption rate for Pb in the initial stages, achieving higher removal percentages than SM within the same time intervals. This fact suggests that natural guava seeds may have surface characteristics that favor Pb adsorption, at least initially. At the end of the biosorption period, SN and SM reach similar removal efficiencies, around 50–60% (Figure 8). However, SN reaches this level more efficiently over time, while SM requires a more extended period to reach a comparable percentage. Unlike the case of Cd (Figure 7), modifying guava seeds with HDTMA-Br does not significantly improve the adsorption kinetics or the biosorption capacity of Pb ions. This behavior could be due to several factors, such as the interaction of Pb ions with specific sites on the SN surface that are altered or less accessible after modification with HDTMA-Br. It is possible that the modification mainly improves the removal of ions such as Cd through the increase in positive surface charges on SM, favoring complexation mechanisms with Cd, while Pb could interact with the surface through different mechanisms less affected by the modification with HDTMA-Br.
Finally, the modification has a less positive impact on Pb adsorption than Cd. This suggests that different adsorption mechanisms for Pb ions may be at play and that the natural surface properties of SN might be more suitable for Pb ion interaction. This finding highlights that HDTMA-Br modification might only sometimes improve biosorption performance for some heavy metals present in natural or wastewater, suggesting that further studies should be conducted regarding the selectivity of this biosorbent for other metals. Furthermore, the interaction of Pb with the biosorbent surface may be more dependent on specific binding sites or mechanisms present in natural guava seeds, making SN a better option for Pb removal, in this case, and under the study’s experimental conditions.
In order to quantify the effect of the modification on the biosorption kinetics of the metal ions, as well as to compare the removal rate in both materials, the biosorption kinetic data were fitted to well-known empirical models: the Lagergren model, pseudo-second-order model, and the Elovich equation [52,53,54], using non-linear regression, and thus obtain the global reaction kinetic constants for each model tested. The equations for these empirical models are detailed in the Supplementary Information (Equations (S1)–(S3)). The removal kinetic data (% removal) were previously converted to the concentration of each ion in the sorbent (qe) to facilitate fitting to these models using the following equation:
where Co is the initial concentration of metal in the supernatant; Ct is the concentration of metal in the supernatant at time t, V is the volume of solution (L), m is the mass of the biosorbent (g), and qe is the metal concentration in the biosorbent (mg/g). The results are shown in Table 1 and Table 2 for removing Cd and Pb, respectively, with both biosorbents.

Table 1.
Kinetic parameters and correlation coefficients (R) of Cd biosorption via SN and SM.

Table 2.
Kinetic parameters and correlation coefficients (R) of Pb biosorption via SN and SM.
From these results, it can be established that, for the case of Cd biosorption (Table 1), both biosorbents show high correlation coefficients (R values) in all cases, except for Elovich in SN, which decreases a little but is considered a good fit. It is also notable that SM shows remarkably higher kinetic constants and slightly higher adsorption capacities (qe) in all models than SN. Based on the above, it can be established that SM has a constant rate of enhanced adsorption due to the HDTMA-Br modification, and therefore, faster removal of Cd with SM is reflected. Furthermore, the initial adsorption rate of SM, established based on the constant α of the Elovich equation (α = 2.7142 mg/g min), is significantly higher than that of SN (α = 0.2040 mg/g min), which favors a faster initial biosorption of Cd once the seeds are modified with surfactant. Overall, SM modification significantly improves Cd biosorption kinetics, which is consistent with previous observations that SM rapidly reaches equilibrium with a higher removal percentage (Figure 7). This improvement is attributed to the HDTMA-Br creating a favorable surface for Cd binding.
In the case of Pb biosorption kinetics (Table 2), the results show some differences since SM does not surpass SN as observed with Cd, even though it can also be considered that all models describe Pb biosorption kinetics well due to their high R coefficient values, with a slight exception for Elovich in SM. Therefore, it can be established that SN presents a slightly faster Pb adsorption rate than SM, as well as a marginally higher adsorption capacity. Regarding their initial adsorption rate characterized by the Elovich constant α, in both biosorbents, it is significantly lower than the Cd biosorption. However, it can be observed that SN (α = 0.0371 mg/g min) has a slight advantage over SM (α = 0.0205 mg/g min), reflecting a slower Pb adsorption, particularly for SM. These results indicate that Pb ions interact more effectively with the natural surface of SN, while the HDTMA-Br modification of SM does not enhance the Pb removal rate as it does for Cd. This fact implies that Pb adsorption may depend on specific binding sites present in SN but are reduced or altered in SM, making SN more favorable for rapid Pb removal under the conditions studied. In summary, while SM shows a significant enhancement in Cd adsorption kinetics due to surface modification, this enhancement is not observed for Pb, highlighting the selective efficiency of guava seeds as a function of heavy metal type and surface modification.
3.5. Effect of Biosorbent Dose
Figure 9 presents the effect of biosorbent dosage on Cd and Pb removal using natural guava seeds (SN) and HDTMA-Br-modified guava seeds (SM). The experiments were conducted at different metal ion concentrations, pH 6, and a temperature of 25 °C. The results show metal removal percentages over a 10 to 100 g/L biosorbent dose range. Based on these results, it can be established that, for the case of SN removal (Figure 9a), the biosorbent dose increases, and the removal efficiency of both Cd and Pb generally improves. Specifically, Cd shows a consistently high removal efficiency, reaching almost 90% at low doses (10 g/L) and maintaining this level as the doses increase. The stability in Cd removal in the whole range suggests that the adsorption sites available on SN are effective and selective for binding Cd ions even at lower SN doses. This biosorption behavior of Cd on SN is notable due to its significant scientific and practical implications, indicating a robust adsorption mechanism and the applicability of this system in real-world scenarios such as wastewater and natural water treatment, where contaminant concentrations may vary [55]. Furthermore, maintaining high removal efficiency at lower doses can reduce the required biosorbent, leading to cost savings in large-scale applications [56]. Nevertheless, despite Cd removal at different SN doses being advantageous, potential limitations like biosorbent saturation, environmental impact, and regeneration feasibility must be considered for sustainable application.
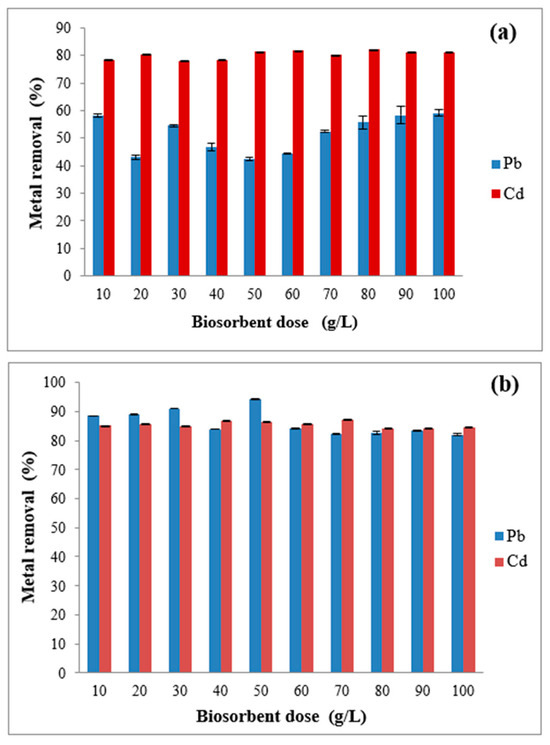
Figure 9.
Effect of biosorbent dose on Cd and Pb removal using (a) SN and (b) SM; ([Cd2+] = 16 mg/L; [Pb2+] = 8 mg/L; pH = 6; temperature = 25 °C).
Regarding Pb removal with SN (Figure 9a), the removal efficiency starts relatively low, around 58% at the lowest dose, which indicates that SN has a lower affinity for Pb than for Cd at these low concentration conditions. However, as the biosorbent dose increases, the Pb removal efficiency gradually decreases and begins to recover its effectiveness at 70 g/L, reaching its initial efficiency from the 80 g/L dose onwards. This dose-response relationship suggests that Pb removal by SN is highly dependent on the amount of biosorbent available, probably because Pb ions have a weaker interaction with the natural guava seed surface than Cd ions. This behavior can be attributed to the fact that, at low doses, fewer adsorption sites are available, limiting the amount of Pb that SN can capture. As the dosage increases, more binding sites become accessible, allowing more Pb ions to be adsorbed. For this reason, Pb removal efficiency improves with higher biosorbent doses. In summary, The implications of the observed dose-dependent behavior for Pb removal using SN indicate that achieving high removal efficiencies for Pb would require higher biosorbent doses than Cd. However, from a practical perspective, this may not be the most efficient approach as it requires more biosorbent material to achieve moderate Pb removal rates.
In Figure 9b, the effect of SM dosage on Cd and Pb removal shows a distinct behavior compared to SN. For example, modified guava seeds (SM) show high Cd removal in this case, consistently above 84% at all biosorbent doses. This suggests that HDTMA-Br modification improves the affinity of guava seeds for Cd, probably due to the higher availability of binding sites with complexation mechanisms, which facilitate the adsorption of Cd ions. In the case of Pb, removal in SM starts around 88% at a dose of 10 g/L and remains relatively stable, ranging between 80% and 90% as the dose increases. The HDTMA-Br modification enhances Pb removal efficiency compared to SN, enhancing Pb adsorption capacity at low concentrations. However, the effectiveness of SM for Pb slightly decreases at higher doses due to the initially increased surface area. As the dose increases, particle aggregation reduces the effective surface area for adsorption beyond a certain point [57,58]. Therefore, removing metal ions in the modified biosorbent (SM) has presented higher removal efficiencies for both metals than SN, particularly for Pb. The higher performance of SM highlights the role of HDTMA-Br in providing additional functional groups or charges that enhance metal ion binding. HDTMA-Br-modified guava seeds are more effective biosorbents for Pb than unmodified seeds, showing high removal efficiency at various doses. This behavior suggests that smaller doses of SM could reduce the cost and material requirements of water treatment processes. The modification process also enhances the surface’s ability to attract and retain Pb ions, making SM particularly useful in environments with prevalent Pb contamination. The increased availability of functional groups and favorable interactions introduced via HDTMA-Br make SM an effective and practical biosorbent for Pb removal, as high efficiency can be achieved without large quantities of biosorbent material. Based on these considerations, we have concluded that the lack of a clear trend in the effect of the biosorbent dose is attributable to the combination of saturation of the active sites, particle agglomeration, and competition between metal ions.
3.6. Influence of pH on Biosorption
Figure 10a shows the effect of pH on the equilibrium change adsorption capacity (qe) using SN and SM as adsorbents. The equilibrium diagram of change species in aqueous solution is also shown (Figure 10b). The results show that the pH value significantly affects qe when SN is used as a biosorbent. Generally, it can be established that the biosorption of cadmium is highly dependent on pH. In particular, it can be observed that, in SN, there is a gradual increase in the adsorption capacity with pH, reaching a maximum around pH 5 (Figure 10a); then, there is a notable decrease in qe starting from pH 6, but there is no significant increase or a slight stabilization beyond pH 8. The low adsorption capacity of cadmium in SN at low pH values can be attributed to the positive charge of the biosorbent (pHPZC = 3.5) and the predominance of the Cd2+ ion at these pH values in the solution (Figure 10b), quantitatively decreasing the adsorption mechanism via electrostatic attraction and also increasing the competition with H+ ions for the binding sites. The higher adsorption at pH 5 can be attributed to the deprotonation of the functional groups of SN, thus enhancing the binding of Cd2+ ions that continue to predominate in the aqueous system. Beyond pH 6 up to pH 9, the aqueous Cd(OH)+ species begins to form and predominate, suggesting that this change in the chemical speciation of Cd is responsible for the decrease in biosorption on SN. These species may be weaker concerning competition for binding sites with H+ ions. Similar behavior has been observed for other biosorbents, with removal decreasing from pH 5 to 6 [59,60].
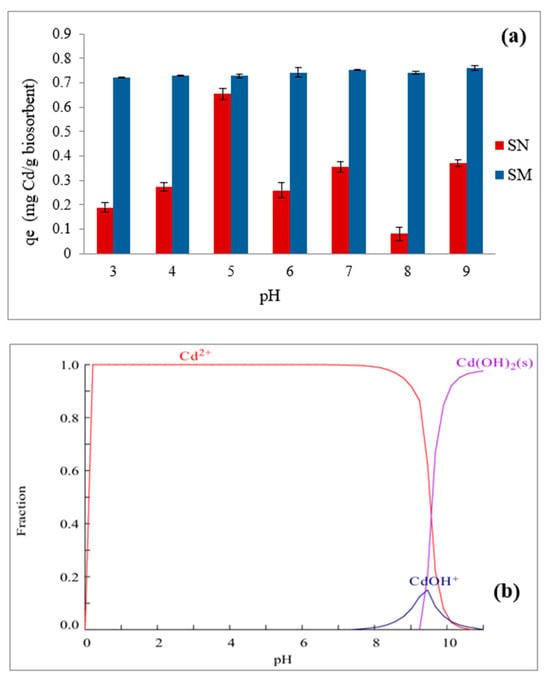
Figure 10.
Effect of solution’s pH on Cd removal using (a) SN and SM as biosorbents; (b) Cd equilibrium diagram in aqueous solution at 25 °C.
Conversely, SM consistently has a higher adsorption capacity than SN over the entire pH range (Figure 10a). The qe values slightly increase to pH 6, after which they plateau, indicating maximum adsorption efficiency. The negative impact of pH on qe for SM is less pronounced than SN, suggesting a broader effective pH range. It is well known that surfactants modify the surface of the biosorbent, increasing its affinity for metal ions and making the adsorption process less sensitive to pH variations. This modification enhances the functional groups of the biosorbent, which are crucial for the binding of cadmium ions, stabilizing the adsorption efficiency over a range of pH levels. The presence of functional groups such as O-H, C-H, N-H, and P-O on the surface of the modified biosorbent facilitates the binding of cadmium ions, thereby reducing the impact of pH changes [7]. High qe values for SN and SM at pH 9 may be due to biosorption processes and microprecipitation of Cd(OH)2 species, causing a plateau or decline in qe. SM’s higher adsorption capacity over a wide pH range makes it suitable for environmental applications, especially in industrial wastewater treatment with varying pH conditions and particularly in aqueous systems. The significant difference in qe between SN and SM highlights the importance of biosorbent modification in improving cadmium biosorption performance. Future studies could focus on the influence of ionic strength or the presence of competing ions to complement the pH-dependent findings.
When examining the effect of pH on lead biosorption using SN and SM, it can be observed (Figure 11) that, at pH values lower than 6, SN exhibits slightly higher qe values than SM. However, the modification with surfactant gives SM a greater range of action with respect to the pH of the solution for the removal of Pb. The results also reveal that, at a low pH (between 3 and 4), both SN and SM have relatively low adsorption capacities (qe) for Pb2+ (Figure 11a). The low qe values at pH < 4 are probably due to the high concentration of H+ ions competing with Pb2+ ions for binding sites on both biosorbents; although, in the case of SM, the electrostatic charge may play an important role in biosorption because its charge is negative at these pH values (Figure 6), which slightly mitigates this effect when SM is used (Figure 11a). The adsorption capacity increases at pH values above 4, reaching a maximum at pH 6 in both biosorbents. This behavior can be attributed to the fact that the electrostatic attraction mechanism is favored in the case of SN (pHPZC = 3.5) since the carboxyl (-COO−) and amino (-NH3+) groups are negatively charged, favoring this mechanism between these groups and the positively charged lead ions. In contrast, other types of adsorption mechanisms, such as complexation with amino groups and the same electrostatic attraction, are predominant in this pH range for SM (pHPZC = 6). In addition, it should be considered that the ion exchange mechanism is more favorable at pH 6, where H+ ions are less competitive with lead ions for the binding sites; this allows more lead ions to be exchanged and adsorbed on the biosorbents [61,62]. From this pH value of 6, SM shows a significantly higher adsorption capacity compared to SN, which can be attributed to both its surface modification with HDMTA-Br and to microprecipitation processes of the Pb(OH)2 species that begin to form and predominate at values higher than 8 (Figure 11b). At pH values of 7 and higher, the adsorption capacity of SN drops sharply, while SM maintains high and stable qe values. As previously discussed, HDTMA-Br can increase the hydrophobicity and the number of active sites on the biosorbent surface, facilitating a better interaction with lead ions with SM. The decrease in SN biosorption can be attributed to the formation of PbOH+ species, which predominate at pH 7. However, the results suggest that these same species are highly amenable to biosorption on SM since the qe values remain stable at pH 7 for this biosorbent. Therefore, it can be established that modifying guava seeds with HDTMA-Br improves their surface functionality, providing stronger or more numerous active sites and making SM more effective in adsorbing Pb species under various pH conditions. Additionally, the interplay of electrostatic interactions (governed by zeta potential) and the distribution of Pb species (Figure 11b) explains the observed trends in adsorption capacity (qe) with pH.
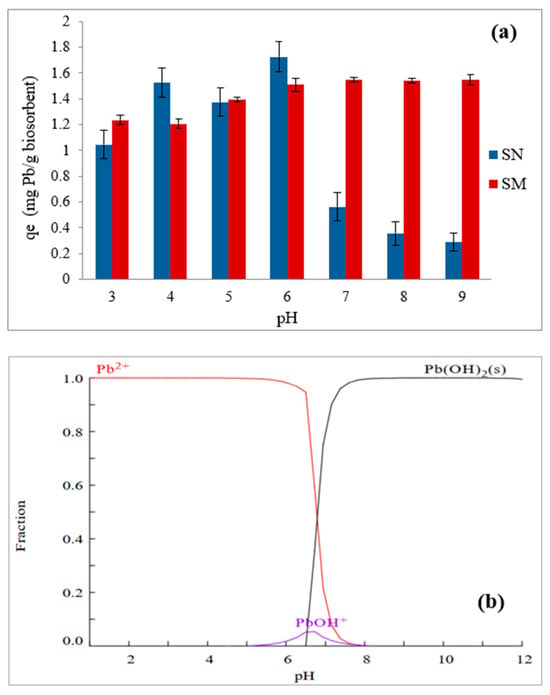
Figure 11.
Effect of solution’s pH on Pb removal using (a) SN and SM as biosorbents; (b) Pb equilibrium diagram in aqueous solution at 25 °C.
3.7. Biosorption Isotherms
Figure 10a shows the effect of pH on the equilibrium change adsorption capacity (qe) using SN and SM as adsorbents. The equilibrium diagram of change species in aqueous Figure 12a,b illustrates the biosorption isotherms of cadmium and lead using natural guava seeds (SN). They are presented as experimental data between the equilibrium concentration of cadmium in solution (Ce) and the amount adsorbed on SN (qe), obtained directly from batch processes at different temperatures (25, 35, and 45 °C).
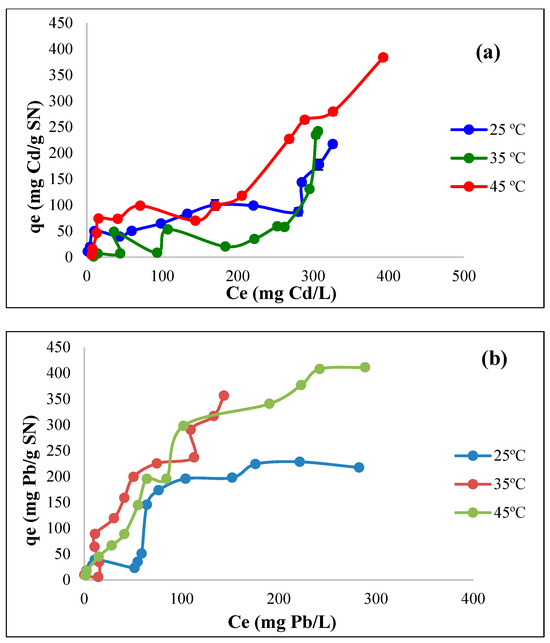
Figure 12.
Biosorption isotherms using SN at different temperatures for the removal of (a) cadmium (biosorbent dose = 10 g/L; pH = 5) and (b) lead (adsorbent dose = 10 g/L; pH = 6).
For the case of cadmium (Figure 12a), a similar trend can be observed in the adsorption of Cd at all temperatures since qe markedly increases as Ce increases, particularly at high solution concentrations. However, at 35 °C, the adsorption is initially similar but less consistent, with fluctuations suggesting competitive interactions, a less favorable adsorption process at this temperature, and experimental variations. At 45 °C, the adsorption capacity increases significantly with Ce, indicating potentially endothermic behavior and higher efficiency. The maximum experimental adsorption capacity (qe) is achieved at 45 °C, with a value of 383.8 mg Cd/g SN, indicating the positive influence of higher temperatures on biosorption. This fact can be attributed to several interrelated factors, including enhanced kinetic energy, the increased availability of active sites, and thermodynamic changes that favor the Cd2+ biosorption process on SN. For practical applications, it is also convenient to indicate the adsorption capacity at 25 °C for SN, which in this case was 217.4 mg Cd/g SN, in the concentration range studied.
It is also important to classify these isotherms according to the Giles et al. classification [63], as it provides a standardized framework to understand the adsorption mechanism, the behavior of the biosorbent, and the interaction between the adsorbate (cadmium) and the adsorbent (SN). In this case, the general trend of the curves resembles an L-type isotherm (Figure 12a), suggesting strong interactions between cadmium ions and the SN surface without significant competition from other ions in the solution, such as hydronium ions. It can also be established that, as Ce increases, the slope decreases slightly, indicating that, as the adsorption sites become saturated, the biosorption slows down, which is typical of L-type isotherms, which are indicative of a monolayer adsorption process in which the biosorbent surface has a finite number of active sites with a high affinity for cadmium ions. Additionally, the cadmium biosorption isotherms present different subtypes, depending on the temperature. At 25 °C and 35 °C, the adsorption capacity increases steadily with the equilibrium concentration, indicating an L4 subtype with moderate initial slopes. The L4 isotherm implies that the adsorbent–adsorbate interaction occurs at specific charge sites and reflects an adsorbent with a high initial affinity for the Cd ions [64]. The upward curve indicates that a second layer of adsorbate molecules forms at higher concentrations, possibly due to weak interactions on new adsorption sites. At 45 °C, the curve shows a steeper initial increase and a stabilization pattern, indicating stronger adsorption at the active sites and a transition toward saturation, aligning with the L3 subtype, which typically appears when the strength of adsorption increases due to temperature effects or enhanced interaction mechanisms. The L3 subtype indicates higher affinity at the initial adsorption stage, possibly due to enhanced adsorption mechanisms.
For the removal of lead via SN, the isotherms curves presented in Figure 12b show that the adsorption capacity of lead ions on SN increases with temperature, suggesting an endothermic process where higher temperatures favor biosorption. At 25 °C, a gradual increase is shown at lower equilibrium concentrations, but it plateaus at approximately 217 mg Pb/g SN, suggesting an early saturation of the biosorbent active sites at this temperature. At 35 °C, a more pronounced initial increase is observed, suggesting a stronger binding of lead to the active sites on SN. The isotherm reaches a higher adsorption capacity, indicating that increasing temperature enhances adsorption. On the other hand, at 45 °C, a sharp increase in the adsorption capacity is shown at low Pb concentrations in the solution, with the highest experimental maximum adsorption capacity of around 411 mg Pb/g SN. The upward trend persists over a wider concentration range, indicating a higher interaction strength and availability of active sites in SN at this temperature, and not only a higher capacity but also a smoother transition to saturation is obtained, suggesting a more effective utilization of the biosorbent surface. Giles’s classification based on curve shapes (Figure 12b) reveals L-type isotherms, which steeply rise in all curves, representing a high affinity between lead ions and the biosorbent’s surface at low concentrations. The plateau indicates the progressive saturation of adsorption sites, a characteristic of monolayer adsorption. At 25 °C, the L2 subtype exhibits a moderate rise, followed by a distinct plateau, indicating limited active site availability or reduced adsorption energy. At 35 °C and 45 °C, the L3 subtype has a steep slope, suggesting stronger binding and enhanced adsorption at higher temperatures. This transition highlights the impact of temperature on the adsorption mechanisms.
Figure 13a,b depict cadmium and lead biosorption isotherms, respectively, utilizing modified guava seeds (SM) as biosorbents. Similarly to SN, it can be observed that cadmium ions are adsorbed more efficiently on SM at higher temperatures (Figure 13a), resulting in an increased adsorption capacity. Notably, at 25 °C, the adsorption capacity in SM (qe = 328 mg Cd/g) surpasses that of SN at the same temperature. Furthermore, this behavior also suggests an endothermic biosorption process. The SM isotherms exhibit steeper initial slopes at 45 °C, suggesting stronger interactions between cadmium and the biosorbent. As previously observed, the stabilization at 25 °C and 35 °C as the Cd concentration increases suggests an early saturation of the binding sites. SN is significantly enhanced with surfactant modification, probably due to increased hydrophobicity, ionic interactions, and complexation mechanisms introduced via HDMTA-Br, as well as an improvement in surface area and the availability of binding sites. Additionally, the isotherms align with the S-type curves in the Giles et al. [63] classification, characterized by a sigmoidal shape (“S”-shaped curve). This type of isotherms indicates that adsorption is favored at the beginning. As the concentration of the liquid phase increases, it presents an inflection point due to two opposing mechanisms. The S-type isotherm at 25 °C has a sigmoidal shape, indicating a moderate initial interaction between the adsorbate (Cd) and the adsorbent (SM). Cd is effectively adsorbed at low equilibrium concentrations, but the slope decreases as the adsorption capacity is saturated. This behavior suggests that the surfactant on the seed initially enhances Cd adsorption, but as more Cd molecules are adsorbed, the available adsorption sites become limited. At 35 °C, the isotherm follows a sigmoid pattern, suggesting that an increasing temperature favors the adsorption process. An S-type suggests that competition between solvent and adsorbate for adsorption sites may decrease with increasing temperature, allowing more Cd to be adsorbed on the SM. At 45 °C, the sigmoid shape is even more pronounced, indicating that initial adsorption is very effective and continues until the adsorption sites are almost saturated. This behavior is typical in systems with a favorable initial interaction but is limited with increasing adsorbate concentration. S-type isotherms can be categorized into subgroups based on their adsorption patterns. Giles et al. [63] classified these subgroups into S1, S2, S3, etc., depending on the complexity of the curve. For example, at 25 °C, Subgroup S1 indicates moderate initial adsorption with a stable inflection point, indicating limited further adsorption due to saturation of available adsorption sites. Subgroup S2 shows a higher adsorption efficiency at 35 °C, indicating higher initial competition with the solvent but a reduction as the temperature increases, facilitating the adsorption of Cd. Subgroup S3 at 45 °C shows higher adsorption in all equilibrium concentrations, with a more pronounced inflection and higher maximum adsorption capacity, indicating an efficient interaction between the adsorbate and the adsorbent. This classification helps understand how different conditions affect the adsorption of Cd via SM.
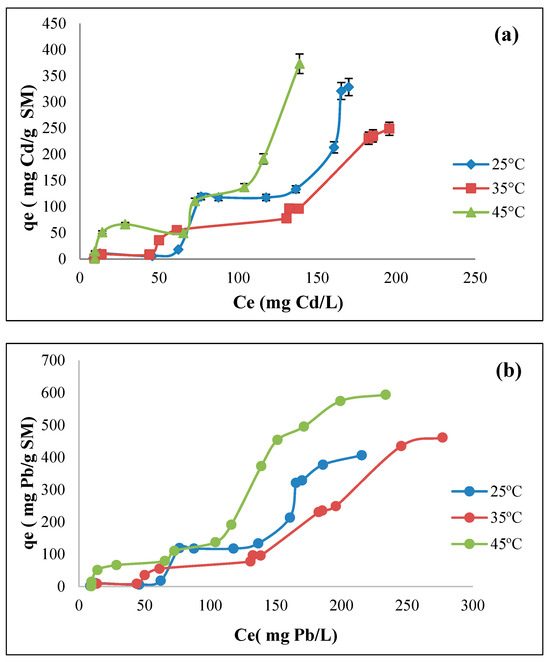
Figure 13.
Biosorption isotherms of SM at different temperatures for (a) cadmium (biosorbent dose = 10 g/L; pH = 5) and (b) lead (adsorbent dose = 10 g/L; pH = 6).
The SM lead biosorption isotherms (Figure 13b) show a behavior similar to that described for SN. In this case, a slower initial increase is observed at 25 °C, reaching a moderate plateau around 406 mg Pb/g SM. At 35 °C, a more pronounced increase is observed, reaching a biosorption capacity of 461 mg Pb/g SM. The increase is more pronounced for the 45 °C isotherm, and the highest adsorption capacity is presented compared to other systems for Pb, approaching 594 mg Pb/g SM. The adsorption of Pb on SM differs from the other systems analyzed in this study in the shape of its isotherms (Figure 13b), which can be classified as the S2 type [63]. This subtype of isotherms is characterized by an initial slow increase in adsorption at low concentrations, followed by a sharp rise and a gradual plateau. This pattern reflects cooperative adsorption, where the initial binding of molecules increases the likelihood of additional adsorption occurring nearby. S2 curves imply positive cooperativity, where adsorbed molecules already bound to the surface enhance the adsorption of additional molecules. It suggests a heterogeneous surface where initial adsorption occurs at less favorable sites. As coverage increases, the energy at neighboring sites improves, enhancing adsorption. The initial slow adsorption phase indicates a weak interaction with the adsorbent at low concentrations, while the sharp increase at intermediate concentrations is due to cooperative interactions or structural rearrangements at the surface. S2 isotherms are common in systems involving large molecules, such as surfactants or biomolecules, where cooperative or clustering effects are significant [65]. Finally, it can be clearly established that SM significantly improves the adsorption capacity of lead compared to natural guava seeds (SN) due to the increase in surface functional groups and better accessibility of the active sites. SN has a lower adsorption capacity and a less pronounced temperature effect. In this case, it is remarkable that the maximum adsorption capacity of SM at 45 °C showed an increase of almost 50% with respect to that of SN. Table 3 compares adsorption capacities for Cd and Pb using various adsorbents. Guava seed-based biosorbents (SN and SM) were more efficient for Cd removal than activated carbon from peanut shells, mango peel, and dodecyl dimethyl betaine-modified montmorillonite; the later adsorbent shows better Pb adsorption than most other adsorbents but is still below SM’s 406.4 mg/g. Organoclay with sodium cocoiminodipropionate has limited efficiency in heavy metal removal, while sophorolipid-functionalized rice husk-activated carbon reaches 361 mg/g. SN and SM outperformed most other listed adsorbents in terms of the maximum adsorption capacity for Cd and Pb, indicating their high potential for effective heavy metal removal. The impact of surface modification was also significant, with surfactant modification enhancing adsorption efficiency. SN and SM are natural and cost-effective, as they are derived from waste materials, making them more sustainable and economically viable for large-scale applications. The performance of SM is superior to that of most conventional and modified adsorbents, highlighting its potential for environmental remediation.

Table 3.
Adsorption capacities at 25 °C for Cd and Pb ions of various adsorbents.
The higher adsorption capacity observed for lead (Pb) compared to cadmium (Cd) in both SN and SM biosorbents can be attributed to several factors. The Pb2⁺ ion has a larger ionic radius (1.19 Å) than Cd2⁺ (0.97 Å), enhancing its interaction with functional groups in the biosorbent. This increased size allows for forming more stable coordinate bonds with these groups. Additionally, lead has an electronegativity of 2.33 on the Pauling scale, indicating that it can form stronger bonds with electron-donating groups in the biosorbent, thereby increasing its adsorption capacity. Electrostatic interactions between the Pb2⁺ ions and the negatively charged functional groups on the biosorbent surface further enhance this adsorption capacity. Certain lignocellulosic biosorbents demonstrate a higher selectivity for Pb due to their porous structure and active functional groups. Furthermore, the formation of surface complexes between the biosorbent and Pb2⁺ ions is likely more thermodynamically favorable than that with Cd2⁺, which explains the higher adsorption capacity observed for lead.
3.8. Desorption Tests
Figure 14 and Figure 15 show the cadmium and lead desorption efficiency, respectively. Percentage desorption was calculated using the following equation [58,72]:
Desorption efficiency (%) = (Cdes/Cads) × 100
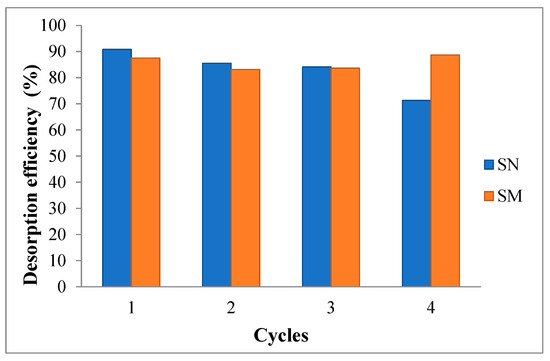
Figure 14.
Cadmium adsorption–desorption cycles for SN and SM with HNO3 used as regenerant.
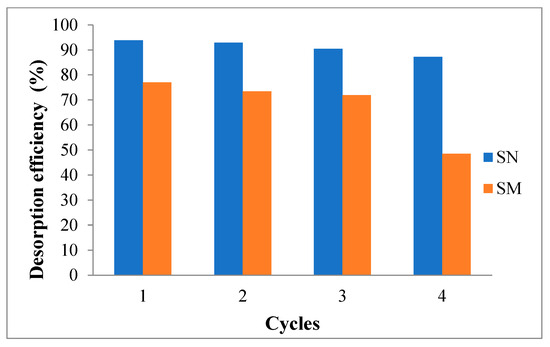
Figure 15.
Lead adsorption–desorption cycles for SN and SM with HNO3 used as regenerant.
Cad represents the concentration of metal ions adsorbed, and Cdes denotes the concentration of desorbed metal ions.
Figure 14 shows the adsorption–desorption cycles of cadmium for SN and SM. For SN, Cycle 1 shows a high desorption efficiency of around 90%; this indicates that cadmium was effectively desorbed during the initial cycle, suggesting good reusability at the early stages of the biosorbent’s lifetime. Cycle 2 results in a slight decrease in desorption efficiency, dropping to approximately 85%. This behavior could be attributed to the gradual saturation of the active sites or a loss of adsorption–desorption capacity due to structural changes in the biosorbent; after that, the efficiency progressively decreases until a more significant reduction in efficiency is observed in cycle 4, dropping below 72%. This decrease is likely a result of the cumulative degradation of the natural guava seed structure and the partial irreversible binding of cadmium ions to its active sites. The trend observed for SN suggests that, while it has good reuse potential for cadmium removal, its performance declines after several cycles due to a lack of chemical modification to stabilize or improve its surface properties. This may be attributed to the complex structure of lignocellulosic materials, including cellulose, hemicellulose, and lignin, which can hinder the diffusivity of cadmium ions for desorption, as well as the strong interaction with their functional groups, leading to incomplete desorption [73]. The effectiveness of desorption agents, in this case, HNO3, also influences the efficiency of cadmium removal [74]. On the other hand, SM shows high desorption efficiency, similar to SN, in cycles 1 and 2. The modified biosorbent maintains stability above 85%, indicating its durability. Cycle 3 shows consistent desorption performance, indicating HDTMA-Br’s enhanced structural integrity and chemical functionality. In cycle 4, the desorption efficiency slightly increases to nearly 90%, suggesting that the surfactant-modified structure enhances reusability and maintains surface activity over repeated cycles, possibly due to improved cadmium ion desorption facilitated via HDTMA-Br functional groups. SN and SM show varying desorption efficiencies, with SN decreasing over cycles due to lower structural robustness and potentially irreversible cadmium adsorption. On the other hand, SM maintains high desorption efficiencies, indicating the positive impact of HDTMA-Br modification, which facilitates cadmium desorption due to its hydrophobic surface. This behavior highlights surface modification’s importance in improving biosorbent reusability, which agrees with previous studies indicating higher stability and efficiency [7]. Moreover, HDMTA-Br protects the adsorbent structure, while HNO3 is a potent regenerating agent, protonating functional groups on biosorbent surfaces. It promotes metal ion release, achieving desorption efficiencies of over 80% and minimizing structural degradation.
Conversely, SN’s desorption is approximately 90% in the case of lead (Figure 15), indicating their significant capacity to adsorb and release lead ions. The efficiency remains consistent at 90% in the first three cycles, indicating good stability and reusability. The efficiency slightly decreases to 87% in the last cycle, indicating that the seeds maintain high performance even after four cycles, albeit with some reduction in efficiency. However, the Pb desorption efficiency of surfactant-modified guava seeds is around 77% in the first cycle (Figure 15), which is lower than that of SN. The efficiency of guava seeds remains constant throughout the cycle but does not significantly improve over cycles. With repeated use, efficiency drops to around 70%, indicating a decrease in effectiveness. The efficiency further decreases to around 50% at the last cycle, possibly reducing performance compared to natural guava seeds. This is due to distinct adsorption mechanisms of Pb ions on SM, which are stronger due to chelation. Therefore, it can be established that SN shows higher Pb desorption efficiency and stability over cycles compared to modified seeds (SM). Also, SN maintains high efficiency and reusability, while SM shows a decline in efficiency. SN could be more effective for long-term lead adsorption–desorption applications, while SM may offer a different level of reusability and stability.
Guava seeds have moderate biodegradability, meaning they can decompose in the environment over time, but the process can be slower than other organic materials. Factors influencing biodegradability include temperature, humidity, and the presence of specific microorganisms that can decompose organic matter. Temperature increases the metabolic activity of microorganisms responsible for decomposition, while it requires a certain amount of moisture for efficient decomposition. However, too much moisture can lead to the proliferation of fungi and bacteria, negatively affecting the seeds’ quality. Specific microorganisms, such as bacteria and fungi, are essential for the biodegradability of guava seeds [75,76]. This moderate biodegradability can be considered an advantage over other biosorbents concerning their shelf-life in water treatment applications. It can also benefit subsequent processes of properly disposing of the biosorbent once it is saturated and cannot be reused in further adsorption–desorption cycles.
3.9. Thermodynamic Parameters
Determining thermodynamic properties is crucial for optimizing and understanding adsorption processes, enhancing efficiency, selecting optimal materials, and designing more effective and sustainable systems. The thermodynamic parameters were calculated based on Cd and Pb biosorption data for SN and SM at various temperatures. The changes in standard Gibbs free energy (∆G°), standard enthalpy (∆H°), and standard entropy (∆S°) were determined using the Van’t Hoff equation. This technique entails graphing the natural logarithm of the equilibrium constant (ln Kc) vs. the inverse of temperature (1/T) to ascertain the enthalpy change (ΔH°) and entropy change (ΔS°) from the slope and intercept of the resulting graph, respectively [77]. The equilibrium constants (Kc) were calculated at different temperatures for the metal ions adsorbed by both biosorbent materials. These constants are crucial for applying the Van’t Hoff equation to determine thermodynamic parameters. One of our earlier works previously documented the equations related to this method [77].
Figures S3–S6 show the plots of ln Kc vs. 1/T for the cadmium and lead systems adsorption for both biosorbents. The figures exhibit excellent determination coefficients (ranging from 0.98 to 1) for the data across all systems in linear regression, demonstrating a highly predictable pattern in the adsorption process relative to temperature variations. This predictability supports the method’s reliability and the precise calculation of thermodynamic parameters from the slope and intercept of the linear regression equations.
Table 4 shows the thermodynamic parameters of Cd and Pb biosorption by SN. For cadmium (Cd), positive ΔG° values are observed, indicating that biosorption on SN is not spontaneous at all temperatures studied. The slight decrease in ΔG° with an increasing temperature suggests that the process becomes slightly more favorable at higher temperatures [78]. Generally, a positive ΔG° means that the process requires an input of energy. The positive ΔH° value indicates that Cd biosorption is an endothermic process requiring heat absorption from the surroundings. This fact implies that higher temperatures may enhance the biosorption capacity as the process gains more energy to drive the adsorption. Furthermore, the negative value of ΔS° indicates a decrease in randomness at the solid-liquid interface during the biosorption process; this suggests that Cd biosorption leads to a more ordered system, as Cd ions are likely to become more structured on the surface of guava seeds [78]. For Pb biosorption, negative ΔG° values (Table 4) indicate that Pb biosorption via SN is spontaneous at all temperatures studied. The increase in the magnitude of ΔG° with increasing temperature suggests that the process becomes more favorable at higher temperatures [78]. A negative ΔG° means that the process can occur without additional energy input. The negative ΔH° value indicates that Pb biosorption is an exothermic process that releases heat to the surroundings. This fact suggests that lower temperatures may enhance the biosorption capacity as the process does not require additional energy input. Furthermore, the positive ΔS° value indicates an increase in randomness at the solid–liquid interface during biosorption, suggesting that Pb biosorption leads to a more disordered system, as Pb ions are less structured on the surface of guava seeds than Cd ions. The thermodynamic behavior in Pb removal can be related to the mechanism of electrostatic attractions between the metal ion and the SN [79], which can also influence its reversibility, as previously demonstrated.

Table 4.
Thermodynamic parameters for Cd and Pb biosorption by SN.
Table 5 also shows the thermodynamic parameters for Cd and Pb removal for SM. The negative ΔG° values (−24.995 to −25.015 kJ/mol) for cadmium biosorption over the entire temperature range indicate that the process is spontaneous. The slight variation in ΔG° as the temperature increases suggests that the spontaneity of biosorption within the studied range is relatively independent of temperature. This behavior is characteristic of a stable and efficient adsorption process, which is attributable to the HDTMa-Br modification. The ΔH° value of −24.69 kJ/mol means that Cd biosorption is exothermic. An exothermic process implies that the biosorbent releases heat during adsorption, which may favor the process at lower temperatures, implying an advantage in water treatment applications since the range of effectiveness with respect to water temperature is increased. Additionally, the relatively low magnitude of ΔH° suggests weak interactions, possibly physisorption or combined physisorption and chemisorption [80], which directly positively influences the reversibility of biosorption in this system, as previously discussed. The positive value of ΔS° indicates high randomness at the solid–liquid interface during Cd adsorption, which can be attributed to the displacement of water molecules and the rearrangement of adsorbed ions, suggesting favorable interactions between the biosorbent and Cd ions [81]. Likewise, concerning Pb removal via SM, the ΔG° values for Pb biosorption are also negative (Table 5), confirming spontaneity. However, these values are less negative than Cd, indicating slightly lower spontaneity for Pb biosorption under the same conditions. Furthermore, the ΔH° value of −25.015 kJ/mol is comparable to Cd biosorption and confirms that Pb adsorption is exothermic. This indicates that Pb biosorption also favors lower temperatures and probably involves similar adsorption mechanisms. The positive ΔS° value (42.34 J/mol-K) for Pb biosorption is lower than that of Cd, suggesting a smaller increase in randomness at the solid–liquid interface compared to Cd. This behavior could be due to the differences in Pb versus Cd’s ionic radii or hydration characteristics.

Table 5.
Thermodynamic parameters for Cd and Pb biosorption by SM.
Finally, it can be established that both metals exhibit spontaneous biosorption; however, Cd shows higher spontaneity (more negative ΔG° values) than Pb, suggesting that SM has a higher affinity for Cd ions under the given conditions. Furthermore, the similar ΔH° values for both metals indicate that their biosorption involves comparable energy shifts, probably due to weak Van der Waals interactions or outer-sphere complexation mechanisms. Thermodynamic analysis suggests that SM is effective for the biosorption of both Cd and Pb, with higher affinity and increased spontaneity for Cd. The exothermic nature of the process indicates suitability at ambient or lower temperatures, and the positive entropy change highlights favorable interactions between the adsorbent and the metal. These insights are crucial for designing efficient biosorption systems for heavy metal removal using surfactant-modified guava seeds.
4. Conclusions
Surface modification with HDTMA-Br significantly improved the adsorption capacity of Cd and Pb due to introducing new functional groups and enhancing surface charge. The results showed that SM consistently outperformed SN in removing Cd and Pb, showing higher affinity for both metals and faster kinetics in the case of Cd. Furthermore, functional groups such as hydroxyls and carbonyls, together with cationic sites introduced by the surfactant, played a significant role in the adsorption mechanisms. Isotherm analyses suggest that the adsorption process follows a monolayer model, with endothermic behaviors favored at higher temperatures. Optimal adsorption conditions for Cd were identified at an optimal pH of 5, while for Pb, it was 6. SM showed lower sensitivity to pH variations, making them more applicable in industrial settings with fluctuating conditions. Regeneration tests demonstrated that both SN and SM can be efficiently reused for up to four cycles without significant loss of adsorption capacity (>85%). Furthermore, while both SN and SM showed favorable thermodynamic characteristics, SM presented better ΔG and ΔS values, reflecting that HDTMA-Br modification not only increases the adsorption capacity but also improves the thermodynamics of the process. This work highlights the potential of guava seeds as effective and low-cost biosorbents, contributing to the circular economy and reducing solid waste generation. The HDTMA-Br modification enhances biodegradability without compromising functionality. This technology can be scaled up, applied to real waste effluents, optimized, and cost-evaluated. When functionalized with surfactants, guava seeds are promising biosorbents for heavy metal removal, establishing a solid foundation for future research and industrial applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/suschem6010005/s1: Figure S1. Schematic representation of metal sorption mechanisms on SN (Me2+ = divalent metal). Figure S2. Schematic representation of metal sorption mechanisms on SM (Me2+ = divalent metal; A- = anionic counter-ions). Figure S3: Plot of ln Kc vs.1/T for cadmium biosorption on SN.; Figure S4: Plot of ln Kc vs.1/T for lead biosorption on SN.; Figure S5: Plot of ln Kc vs.1/T for cadmium biosorption on SM.; Figure S6. Plot of ln Kc vs.1/T for lead biosorption on SM.
Author Contributions
Conceptualization, G.E.T.-Q., S.A.V.-L. and R.C.-M.; methodology, G.E.T.-Q., S.A.V.-L. and A.V.-G.; software, S.A.V.-L., R.E.-G. and A.V.-G.; validation, S.A.V.-L., R.A.-C.-V. and R.C.-M.; formal analysis, R.A.-C.-V. and R.C.-M.; investigation, G.E.T.-Q., S.A.V.-L. and R.A.-C.-V.; resources, R.E.-G. and R.A.-C.-V.; data curation, G.E.T.-Q., S.A.V.-L. and A.V.-G.; writing—original draft preparation, G.E.T.-Q. and R.C.-M.; writing—review and editing, R.E.-G., R.A.-C.-V. and R.C.-M.; visualization, R.E.-G.; supervision, A.V.-G. and R.C.-M.; project administration, R.E.-G. and R.A.-C.-V.; funding acquisition, R.E.-G. and R.A.-C.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordinación de la Investigación Científica—UMSNH, grant number CIC-UMSNH-2024.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to acknowledge the technical support from D.M Bocanegra-González and N.Y. Mejía-Ramírez in language and style corrections.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sanchez-Silva, J.M.; González-Estrada, R.R.; Blancas-Benitez, F.J.; Fonseca-Cantabrana, Á.; Sanchez-Silva, J.M.; González-Estrada, R.R.; Blancas-Benitez, F.J.; Fonseca-Cantabrana, Á. Utilización de subproductos agroindustriales para la bioadsorción de metales pesados. TIP Rev. Espec. En Cienc. Quím.-Biológicas 2020, 23, 1–18. [Google Scholar] [CrossRef]
- Vallejo Aguilar, M.d.l.L.A.; Marín Castro, M.A.; Ramos Cassellis, M.E.; Silva Gómez, S.E.; Ibarra Cantún, D.; Tamariz Flores, J.V. Biosorción y tolerancia de Pb, Cr y Cd por la biomasa de Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. Rev. Mex. Cienc. Agríc. 2021, 12, 275–289. [Google Scholar] [CrossRef]
- Moreno, L.G.N.; Velasco, L.V.; Cordero, A.R.; González, J.M. Contaminación y hongos: Resistencia a metales pesados. LATAM Rev. Latinoam. Cienc. Soc. Humanidades 2022, 3, 215–232. [Google Scholar] [CrossRef]
- Hamid, N.H.A.; Rushdan, A.I.; Nordin, A.H.; Husna, S.M.N.; Faiz Norrrahim, M.N.; Knight, V.F.; Tahir, M.I.H.M.; Li, G.X.; Quan, T.L.; Abdullah, A.M.; et al. A State-of-Art Review on the Sustainable Technologies for Cadmium Removal from Wastewater. Water Reuse 2024, 14, 312–341. [Google Scholar] [CrossRef]
- Kumar, K.; Singh, D. Toxicity and Bioremediation of the Lead: A Critical Review. Int. J. Environ. Health Res. 2024, 34, 1879–1909. [Google Scholar] [CrossRef]
- Chong, Z.T.; Soh, L.S.; Yong, W.F. Valorization of Agriculture Wastes as Biosorbents for Adsorption of Emerging Pollutants: Modification, Remediation and Industry Application. Results Eng. 2023, 17, 100960. [Google Scholar] [CrossRef]
- Ghaith, E.-S.I.; Rizvi, S.; Namasivayam, C.; Rahman, P.K.S.M. Removal of Cd++ from Contaminated Water Using Bio-Surfactant Modified Ground Grass as a Bio-Sorbent. In Proceedings of the 2019 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 26 March–10 April 2019; pp. 1–7. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Ghorbani, M.; Seyedin, O.; Aghamohammadhassan, M. Adsorptive Removal of Lead (II) Ion from Water and Wastewater Media Using Carbon-Based Nanomaterials as Unique Sorbents: A Review. J. Environ. Manag. 2020, 254, 109814. [Google Scholar] [CrossRef]
- Bravo, C.; Quispe, L. Metales Pesados: Fuentes y su Toxicidad sobre la Salud Humana. Ciencias 2019, 2, 20–36. [Google Scholar] [CrossRef]
- Londoño Franco, L.F.; Londoño Muñoz, P.T.; Muñoz Garcia, F.G. Los Riesgos de los Metales Pesados en la Salud Humana y Animal. Biotecnoloía En El Sect. Agropecu. Agroindustrial 2016, 14, 145. [Google Scholar] [CrossRef]
- Figueroa, R.; Caicedo, D.; Echeverry, G.; Peña, M.; Méndez, F.; Figueroa, R.; Caicedo, D.; Echeverry, G.; Peña, M.; Méndez, F. Condición socioeconómica, patrones de alimentación y exposición a metales pesados en mujeres en edad fértil de Cali, Colombia. Biomédica 2017, 37, 341–352. [Google Scholar] [PubMed]
- Irfan, M.; Liu, X.; Hussain, K.; Mushtaq, S.; Cabrera, J.; Zhang, P. The Global Research Trend on Cadmium in Freshwater: A Bibliometric Review. Environ. Sci. Pollut. Res. 2023, 30, 71585–71598. [Google Scholar] [CrossRef]
- Khan, Z.; Elahi, A.; Bukhari, D.A.; Rehman, A. Cadmium Sources, Toxicity, Resistance and Removal by Microorganisms-A Potential Strategy for Cadmium Eradication. J. Saudi Chem. Soc. 2022, 26, 101569. [Google Scholar] [CrossRef]
- Domínguez, E.G.; Noriega, R.R.; Villanueva, J.L.; Francisco, R.B. Modelo de Thomas Para la Cinética de Adsorción del Azul de Metileno Mediante el Residuo eel Fruto de Encino: Thomas Model for Methylene Blue Adsorption Kinetics Using Oak Fruit Residue. South Fla. J. Dev. 2022, 3, 5250–5259. [Google Scholar] [CrossRef]
- Soliman, N.K.; Moustafa, A.F. Industrial Solid Waste for Heavy Metals Adsorption Features and Challenges; a Review. J. Mater. Res. Technol. 2020, 9, 10235–10253. [Google Scholar] [CrossRef]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Heavy Metal Removal by Fixed-Bed Column—A Review. ChemBioEng Rev. 2018, 5, 173–179. [Google Scholar] [CrossRef]
- Vázquez-Guerrero, A.; Alfaro-Cuevas-Villanueva, R.; Rutiaga-Quiñones, J.G.; Cortés-Martínez, R. Fluoride Removal by Aluminum-Modified Pine Sawdust: Effect of Competitive Ions. Ecol. Eng. 2016, 94, 365–379. [Google Scholar] [CrossRef]
- Sharma, A.; Anjana; Rana, H.; Goswami, S. A Comprehensive Review on the Heavy Metal Removal for Water Remediation by the Application of Lignocellulosic Biomass-Derived Nanocellulose. J. Polym. Environ. 2022, 30, 1–18. [Google Scholar] [CrossRef]
- Abdić, Š.; Memić, M.; Šabanović, E.; Sulejmanović, J.; Begić, S. Adsorptive Removal of Eight Heavy Metals from Aqueous Solution by Unmodified and Modified Agricultural Waste: Tangerine Peel. Int. J. Environ. Sci. Technol. 2018, 15, 2511–2518. [Google Scholar] [CrossRef]
- Ganesan, S. Waste Fruit Cortexes for the Removal of Heavy Metals from Water. In Green Adsorbents to Remove Metals, Dyes and Boron from Polluted Water; Inamuddin, Ahamed, M.I., Lichtfouse, E., Asiri, A.M., Eds.; Química ambiental para un mundo sostenible; Springer International Publishing: Cham, Switzerland, 2021; pp. 323–350. ISBN 978-3-030-47400-3. [Google Scholar]
- Nathan, R.J.; Martin, C.E.; Barr, D.; Rosengren, R.J. Simultaneous Removal of Heavy Metals from Drinking Water by Banana, Orange and Potato Peel Beads: A Study of Biosorption Kinetics. Appl. Water Sci. 2021, 11, 116. [Google Scholar] [CrossRef]
- Abatal, M.; Olguin, M.T.; Anastopoulos, I.; Giannakoudakis, D.A.; Lima, E.C.; Vargas, J.; Aguilar, C. Comparison of Heavy Metals Removal from Aqueous Solution by Moringa Oleifera Leaves and Seeds. Coatings 2021, 11, 508. [Google Scholar] [CrossRef]
- thi Quyen, V.; Pham, T.-H.; Kim, J.; Thanh, D.M.; Thang, P.Q.; Van Le, Q.; Jung, S.H.; Kim, T. Biosorbent Derived from Coffee Husk for Efficient Removal of Toxic Heavy Metals from Wastewater. Chemosphere 2021, 284, 131312. [Google Scholar] [CrossRef] [PubMed]
- Ezeonuegbu, B.A.; Machido, D.A.; Whong, C.M.Z.; Japhet, W.S.; Alexiou, A.; Elazab, S.T.; Qusty, N.; Yaro, C.A.; Batiha, G.E.-S. Agricultural Waste of Sugarcane Bagasse as Efficient Adsorbent for Lead and Nickel Removal from Untreated Wastewater: Biosorption, Equilibrium Isotherms, Kinetics and Desorption Studies. Biotechnol. Rep. 2021, 30, e00614. [Google Scholar] [CrossRef]
- Hashem, A.; Aniagor, C.O.; Farag, S.; Aly, A.A. Adsorption of Acid Violet 90 Dye onto Activated Carbon and Guava Seed Powder Adsorbents. Biomass Convers. Biorefinery 2023, 1–15. [Google Scholar] [CrossRef]
- Zewail, T.M.; El-Garf, S.A.M. Preparation of Agriculture Residue Based Adsorbents for Heavy Metal Removal. Desalination Water Treat. 2010, 22, 363–370. [Google Scholar] [CrossRef]
- Parate, V.R.; Gulve, R.; Labhane, U.C.; Talib, M.I. Study of Metal Adsorbent Prepared from Guava Seeds. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 73–82. [Google Scholar] [CrossRef]
- Ortíz-Gutiérrez, M.; Alfaro-Cuevas-Villanueva, R.; Martínez-Miranda, V.; Hernández-Cristóbal, O.; Cortés-Martínez, R. Reduction and Biosorption of Cr(VI) from Aqueous Solutions by Acid-Modified Guava Seeds: Kinetic and Equilibrium Studies. Pol. J. Chem. Technol. 2020, 22, 36–47. [Google Scholar] [CrossRef]
- Ramos-Vargas, S.; Alfaro-Cuevas-Villanueva, R.; Huirache-Acuña, R.; Cortés-Martínez, R. Removal of Fluoride and Arsenate from Aqueous Solutions by Aluminum-Modified Guava Seeds. Appl. Sci. 2018, 8, 1807. [Google Scholar] [CrossRef]
- Garole, D.J.; Choudhary, B.C.; Paul, D.; Borse, A.U. Sorption and Recovery of Platinum from Simulated Spent Catalyst Solution and Refinery Wastewater Using Chemically Modified Biomass as a Novel Sorbent. Environ. Sci. Poll. Res. 2018, 25, 10911–10925. [Google Scholar] [CrossRef]
- Ngah, W.W.; Hanafiah, M.M. Removal of Heavy Metal Ions from Wastewater by Chemically Modified Plant Wastes as Adsorbents: A Review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar]
- Warchoł, J.; Misaelides, P.; Petrus, R.; Zamboulis, D. Preparation and Application of Organo-Modified Zeolitic Material in the Removal of Chromates and Iodides. J. Hazard. Mater. 2006, 137, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, I.; Sahroni, I.; Dahlyani, M.S.E.; Oktaviyani, A.M.N.; Nurillahi, R. Surfactant-Modified Salacca zalacca Skin as Adsorbent for Removal of Methylene Blue and Batik’s Wastewater. Mater. Today Proc. 2021, 44, 3211–3216. [Google Scholar] [CrossRef]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The Improved Methods of Heavy Metals Removal by Biosorbents: A Review. Environ. Pollut. 2020, 258, 113777. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Rani, R.; David, J. A Review on Utility of An Astonishing Fruit: Psidium Guajava (Guava). J. Sci. Technol. JST 2021, 6, 60–72. [Google Scholar]
- Kumar, K.; Ahmed, N.; Rizvi, Q.-U.-E.H.; Jan, S.; Thakur, P.; Chauhan, D.; Kaur, J. Guava Wastes and By-Products: Chemistry, Processing, and Utilization. In Handbook of Fruit Wastes and By-Products; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-00-316446-3. [Google Scholar]
- Gavhane, A.; Dighe, P.; Kour, A.; Author, C.; Chopade, S. The Nutritional and Bioactive Potential of Guava and Possibilities for Its Commercial Application in Value-Added Products. Pharma Innov. J. 2022, 11, 2643–2647. [Google Scholar]
- Omayio, D.G.; Abong’, G.O.; Okoth, M.W.; Gachuiri, C.K.; Mwang’ombe, A.W. Trends and Constraints in Guava (Psidium guajava L.) Production, Utilization, Processing and Preservation in Kenya. Int. J. Fruit Sci. 2020, 20, S1373–S1384. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, H.A.; Cortés-Martínez, R.; Alfaro-Cuevas, R. Fluoride Removal from Aqueous Solutions by Mechanically Modified Guava Seeds. Int. J. Sci. 2013, 11, 159–172. [Google Scholar]
- Masry, B.A.; Madbouly, H.A.; Daoud, J.A. Studies on the Potential Use of Activated Carbon from Guava Seeds (AC-GS) as a Prospective Sorbent for the Removal of Cr(VI) from Aqueous Acidic Medium. Int. J. Environ. Anal. Chem. 2023, 103, 378–395. [Google Scholar] [CrossRef]
- Shkliarenko, Y.; Halysh, V.; Nesterenko, A. Adsorptive Performance of Walnut Shells Modified with Urea and Surfactant for Cationic Dye Removal. Water 2023, 15, 1536. [Google Scholar] [CrossRef]
- Mohamed Pauzan, A.S.; Ahad, N. Biomass Modification Using Cationic Surfactant Cetyltrimethylammonium Bromide (CTAB) to Remove Palm-Based Cooking Oil. J. Chem. 2018, 2018, 5059791. [Google Scholar] [CrossRef]
- Barboza, J.A.T.; Penido, E.S.; Ferreira, G.M.D. Production of Surfactant-Modified Banana Peel Biosorbents Applied to Treatment and Decolorization of Effluents. Colloids Surf. Physicochem. Eng. Asp. 2024, 680, 132650. [Google Scholar] [CrossRef]
- Omeiza Aroke, U.; El-Nafaty, U. XRF, XRD and FTIR Properties and Characterization of HDTMA-Br Surface Modified Organokaolinite Clay. Int. J. Emerg. Technol. Adv. Eng. 2014, 4, 817–825. [Google Scholar]
- Kim, J.; Kim, Y.; Jung, S.; Yun, H.; Won, S.; Yeo, H.; Choi, I.-G.; Kwak, H.W. Green Lignocellulosic Superadsorbent for Superior Pd(II) Removal and Cascade Catalytic Conversion. Sep. Purif. Technol. 2024, 332, 125732. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.-C.; Sun, R.-C. Fractional and Structural Characterization of Lignin and Its Modification as Biosorbents for Efficient Removal of Chromium from Wastewater: A Review. J. Leather Sci. Eng. 2019, 1, 5. [Google Scholar] [CrossRef]
- Lin, Z.; Ye, Y.; Li, Q.; Xu, Z.; Wang, M. A Further Insight into the Biosorption Mechanism of Au(III) by Infrared Spectrometry. BMC Biotechnol. 2011, 11, 98. [Google Scholar] [CrossRef]
- Sura, N.; Okabe, H.; Omondi, B.A.; Mufundirwa, A.; Hidaka, Y.; Hara, K. Study on the Influence of Inductive Groups on the Performance of Carboxylate-Based Hydrogel Polymer Network. Polym. Test. 2019, 80, 106117. [Google Scholar] [CrossRef]
- Dinh, V.-P.; Nguyen, P.-T.; Tran, M.-C.; Luu, A.-T.; Hung, N.Q.; Luu, T.-T.; Kiet, H.A.T.; Mai, X.-T.; Luong, T.-B.; Nguyen, T.-L.; et al. HTDMA-Modified Bentonite Clay for Effective Removal of Pb(II) from Aqueous Solution. Chemosphere 2022, 286, 131766. [Google Scholar] [CrossRef]
- Chen, F.; Hong, M.; You, W.; Li, C.; Yu, Y. Simultaneous Efficient Adsorption of Pb2+ and MnO4− Ions by MCM-41 Functionalized with Amine and Nitrilotriacetic Acid Anhydride. Appl. Surf. Sci. 2015, 357, 856–865. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G.; Wase, D.A.J.; Forster, C.F. Study of the Sorption of Divalent Metal Ions on to Peat. Adsorpt. Sci. Technol. 2000, 18, 639–650. [Google Scholar] [CrossRef]
- Low, M.J.D. Kinetics of Chemisorption of Gases on Solids. Available online: https://pubs.acs.org/doi/pdf/10.1021/cr60205a003 (accessed on 28 August 2021).
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Abdallah, M.A.M.; Alprol, A.E. Utilization of Aquatic Biomass as Biosorbent for Sustainable Production of High Surface Area, Nano- Microporous, for Removing Two Dyes from Wastewater. Sci. Rep. 2024, 14, 4471. [Google Scholar] [CrossRef] [PubMed]
- Blaga, A.C.; Tanasă, A.M.; Cimpoesu, R.; Tataru-Farmus, R.-E.; Suteu, D. Biosorbents Based on Biopolymers from Natural Sources and Food Waste to Retain the Methylene Blue Dye from the Aqueous Medium. Polymers 2022, 14, 2728. [Google Scholar] [CrossRef] [PubMed]
- Dharsana, M.; Jose, J.P.A. Adsorption of Lead from Contaminated Water Using Biosorbent. Mater. Technol. 2022, 56, 171–177. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.; Abukari, M.A.; Pelig-Ba, K.B.; Mtei, K. Modeling and Optimization of Independent Factors Influencing Lead(II) Biosorption from Aqueous Systems: A Statistical Approach. Sci. Afr. 2022, 16, e01270. [Google Scholar] [CrossRef]
- Effect of pH on Cadmium Bio-Sorption by Myriophyllum spicatum. Available online: http://www.hjkxyj.org.cn/en/article/id/20091116 (accessed on 18 November 2024).
- Li, X.; Xiao, Q.; Shao, Q.; Li, X.; Kong, J.; Liu, L.; Zhao, Z.; Li, R. Adsorption of Cd (II) by a Novel Living and Non-Living Cupriavidus Necator GX_5: Optimization, Equilibrium and Kinetic Studies. BMC Chem. 2023, 17, 54. [Google Scholar] [CrossRef]
- Tunali Akar, S.; Arslan, D.; Alp, T. Ammonium Pyrrolidine Dithiocarbamate Anchored Symphoricarpus Albus Biomass for Lead(II) Removal: Batch and Column Biosorption Study. J. Hazard. Mater. 2012, 227–228, 107–117. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Naidoo, E.B.; Modise, S.J. Biosorption of Copper(II) and Lead(II) onto Potassium Hydroxide Treated Pine Cone Powder. J. Environ. Manag. 2010, 91, 1674–1685. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A General Treatment and Classification of the Solute Adsorption Isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Bruna, F.; Celis, R.; Pavlovic, I.; Barriga, C.; Cornejo, J.; Ulibarri, M.A. Layered Double Hydroxides as Adsorbents and Carriers of the Herbicide (4-Chloro-2-Methylphenoxy)Acetic Acid (MCPA): Systems Mg–Al, Mg–Fe and Mg–Al–Fe. J. Hazard. Mater. 2009, 168, 1476–1481. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, C. Influence of Nonionic Surfactants on the Adsorption and Elution of Atrazine in Agriculturally Modified Soils. Agriculture 2024, 14, 733. [Google Scholar] [CrossRef]
- Kumar, U.; Bandyopadhyay, M. Sorption of Cadmium from Aqueous Solution Using Pretreated Rice Husk. Bioresour. Technol. 2006, 97, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Yang, H.; Seo, C.W.; Marshall, W.E. Select Metal Adsorption by Activated Carbon Made from Peanut Shells. Bioresour. Technol. 2006, 97, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR Spectrophotometry, Kinetics and Adsorption Isotherms Modeling, Ion Exchange, and EDX Analysis for Understanding the Mechanism of Cd2+ and Pb2+ Removal by Mango Peel Waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Cheng, F.; Li, Z.; Wu, D. Regulating Interlayer and Surface Properties of Montmorillonite by Dodecyl Dimethyl Betaine for Enhanced Lead Ion Capture. Surf. Interfaces 2023, 42, 103348. [Google Scholar] [CrossRef]
- Gertsen, M.; Perelomov, L.; Kharkova, A.; Burachevskaya, M.; Hemalatha, S.; Atroshchenko, Y. Removal of Lead Cations by Novel Organoclays Derived from Bentonite and Amphoteric and Nonionic Surfactants. Toxics 2024, 12, 713. [Google Scholar] [CrossRef]
- Onuzulike, C.M.; Aniagor, C.O.; Modekwe, G.O.; Ejimofor, M.I.; Menkiti, M.C. Remediation of Lead Ion Contaminated Stream Using Biosurfactant-Functionalized Mesoporous Activated Carbon. Chem. Afr. 2023, 6, 711–718. [Google Scholar] [CrossRef]
- Katsou, E.; Malamis, S.; Tzanoudaki, M.; Haralambous, K.J.; Loizidou, M. Regeneration of Natural Zeolite Polluted by Lead and Zinc in Wastewater Treatment Systems. J. Hazard. Mater. 2011, 189, 773–786. [Google Scholar] [CrossRef]
- Tran, H.N.; Chao, H.-P. Adsorption and Desorption of Potentially Toxic Metals on Modified Biosorbents through New Green Grafting Process. Environ. Sci. Pollut. Res. 2018, 25, 12808–12820. [Google Scholar] [CrossRef]
- Stirk, W.A.; Staden, J. van Desorption of Cadmium and the Reuse of Brown Seaweed Derived Products as Biosorbents. Bot. Mar. 2002, 45, 9–16. [Google Scholar] [CrossRef]
- Guevara-Ohara, J.E.; Cardozo-Conde, C.I.; Santos-Meléndez, L.G.; Guevara-Ohara, J.E.; Cardozo-Conde, C.I.; Santos-Meléndez, L.G. Tolerancia a la desecación y almacenamiento de la semilla de guayaba (Psidium guajava). Agron. Costarric. 2019, 43, 107–121. [Google Scholar] [CrossRef]
- Suárez-Toledo, J.R.; Hernández-Aguilar, C.; Domínguez-Pacheco, F.A.; Aceves-Hernandez, F.J.; Suárez-Toledo, J.R.; Hernández-Aguilar, C.; Domínguez-Pacheco, F.A.; Aceves-Hernandez, F.J. Characterization of Guava Grown in Mexico. Rev. Mex. Cienc. Agríc. 2022, 13, 1233–1245. [Google Scholar] [CrossRef]
- Alfaro-Cuevas-Villanueva, R.; Hidalgo-Vázquez, A.R.; Cortés Penagos, C.D.J.; Cortés-Martínez, R. Thermodynamic, Kinetic, and Equilibrium Parameters for the Removal of Lead and Cadmium from Aqueous Solutions with Calcium Alginate Beads. Sci. World J. 2014, 2014, 647512. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of Heavy Metal Ions from Aqueous System by Ion-Exchange and Biosorption Methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic Parameters of Cadmium Adsorption onto Orange Peel Calculated from Various Methods: A Comparison Study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Costanzo, F.; Silvestrelli, P.L.; Ancilotto, F. Physisorption, Diffusion, and Chemisorption Pathways of H2 Molecule on Graphene and on (2,2) Carbon Nanotube by First Principles Calculations. J. Chem. Theory Comput. 2012, 8, 1288–1294. [Google Scholar] [CrossRef]
- Elanza, S.; Taouil, H.; Amine, A.; Doubi, M.; Lebkiri, A.; Rifi, E. Thermodynamic Study and Mathematical Modeling of Adsorption of Co2+ Ions on Biopolymers Based of Sugarcane Bagasse. Orient. J. Chem. 2017, 33, 3204–3210. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).