Abstract
Ammonia can be considered a relevant compound in the future energy sector, playing a significant role as an energy carrier, storage, or carbon-free fuel. However, the production of this molecule has a high energy demand, and the use of natural gas, which is not free of controversy due to the accidental leakage into the atmosphere produced during extraction and the fact that it is a nonrenewable source, contributes to increasing greenhouse gas emissions. Reducing the process’s energy demand and carbon footprint will be essential to making ammonia a clear alternative for a carbon-free economy. Given the vast research in ammonia production and handling, this gas seems to be the logical step forward in the evolution of the energy sector. However, the current uncertainty in the global market requires cautiousness in decision making. Several factors may impact economic growth and human welfare, thus needing a careful assessment before making any transcendental decisions that could affect worldwide energy prices and raw material availability.
1. Introduction
The aim of the European Commission of reaching an economy based on net-zero greenhouse emissions by 2050 [1] may probably be an overly optimistic objective understood from the perspective of great concerns regarding high CO2 concentration levels reached in the atmosphere and recent extreme climatic events experienced worldwide. However, the amount of energy needed to maintain industrial activity, employment, and healthcare is globally increasing despite efforts to attain higher process efficiency and reduce energy consumption. Two significant trends can be observed: one is the almost steady demand of European, and North and Latin American countries, and the other is the increasing energy demand of Asian, Pacific, African, and Middle Eastern countries [2], with oil, gas, and coal being the main energy sources.
Efforts in reducing CO2 emissions and increasing compliance with environmental measurements should keep pace with the country’s economic resources without affecting population wellbeing. Thus, Nigeria has experienced a great population increase and showed an energy demand of 167 Mtoe (millions of tons of oil equivalent) with a population of 213.4 million inhabitants by year 2021, which is in contrast to countries such as Saudi Arabia with a population of 35.6 million and an energy demand of 254 Mtoe [2].
The focus on reducing CO2 emissions is usually set on fossil sources and their replacement by renewable energy. Reducing the usage of fossil fuels is contradictory to social development since energy consumption is expected to increase along with overall economic development unless a change in energy structure is accomplished along with improvements in energy efficiency [3]. However, the solution is not simple. Renewable energies, such as wind and solar technologies, have an intermittent nature with low reliability under standalone configuration, thus requiring an excessive overdimension of installations [4,5]. To these disadvantages should be added the fact of the reduction in energy efficiency experienced by solar panels due to adverse climatic conditions, such as humidity, high temperature, and dust accumulation [6,7] and the effect of extreme climatic events, requiring special measures that allow grids with high penetration of wind power to withstand hurricane damage [8].
Great efforts are being made to increase energy efficiency and economic circularity, thus favoring the reuse of materials and waste valorization. However, several factors need to be considered when assuming a circular economic model, such as material dispersion, low quality of wastes, process feasible integration, and energy demand associated with treating recycled components [9]. The transition to decarbonizing the economy must cope with a global increasing energy demand and attain a gradual substitution of fossil fuels with alternative fuels. Alexander and Floyd [10] described an optimistic view of the economic transition, indicating that adapting to climate change via deep decarbonization may be challenging but manageable if negative emission technologies are implemented. Their approach uses fuel substitution, nuclear power, and carbon capture and storage. Ye and Tsang [11] proposed a similar starting point, reporting a feasible timeline for decarbonizing the ammonia synthesis process, which also involves capturing CO2 and subsequent process transformation by introducing hydrogen derived from electrolyzers and electricity production from renewable sources.
One important matter to carefully evaluate is the fact that decarbonizing the economy is costly, and although several technological alternatives are available, not all of them are mature enough to consider that a fast and smooth transition can be made in a relatively short period. Currently, fuel substitutes and electrification bring an unavoidable increase in production costs, which the producer cannot assume and, if translated to the consumer in full, may cause excessive market distortion. In a globalized market, producing goods at higher prices may cause consumers to decant their preferences for more affordable products from other regions despite being produced from higher-carbon-footprint manufacturing lines.
It should be taken into account that not all process or fuel alternatives may be directly linked to a decrease in energy intensity. An example of this feature is the production of hydrogen. This carbon-free gas has become the preferred green fuel due to the exclusive emission of water from combustion reactions and the high energy contained per unit of mass. However, the current production of hydrogen comes from fossil sources. Steam methane reforming (SMR) presents an energy demand of 10.84 kWh/kg H2, while the production of H2 from water electrolysis, which seems to be more environmentally friendly, requires even more energy (48 kWh/kg H2) [12]. A similar situation is found when implementing carbon capture and storage: CO2 capture, compression transportation, and further transformation will undoubtedly raise energy consumption [13].
The present document examines the feasibility of using ammonia as a fuel for energy production and as a complementary alternative fuel in road transport. The production process and combustion features of ammonia, when used with various fossil fuels and biofuels, are discussed. The emphasis is placed on the advantages of ammonia as an alternative fuel to be used in turbines and internal combustion engines (ICEs). This feature may help expedite the move towards a decarbonized economy.
2. Materials and Methods
The present review was carried out by using the following specific keywords for selecting proper references: for the section dedicated to alternative fuels, keywords were associated with the specific fuel analyzed, making preference for the newest published manuscripts. A survey of gray literature was also performed to obtain information regarding current commercial technologies. The subsequent sections regarding ammonia production and energetic valorization in engines used the following keywords: “ammonia production” AND “renewable energies”, “ammonia combustion” AND “alternative fuel” OR “gas turbines”, “spark ignition engines”, “compression ignition engines”. Preference was given to manuscripts published during the last ten years, except for relevant historical information.
3. Alternative Fuels
3.1. Hydrogen
Hydrogen may be considered the energy carrier of the future, and the concept of developing a hydrogen economy dates back to the 1970s by assuming hydrogen would replace fossil fuels, transforming transport and industrial sectors [14,15]. Since then, several authors have used this term and analyzed the feasibility of this approach [16,17]. Nevertheless, storage and delivery problems are still yet to be solved. Transporting and storing hydrogen requires a significant amount of energy to increase density, and there are no feasible solutions yet capable of attaining compact and lightweight storage [18].
Technological development attained in recent years has been astonishing. GE turbines are currently capable of operating with mixtures of hydrogen–methane at a low 5% content and up to 100% hydrogen capability [19] for stationary energy production. Airbus has announced a fuel cell engine and combustion engine development program to run these units using hydrogen as the main fuel [20,21]. Toyota introduced the Mirai model into the market in 2015, a pure-hydrogen-powered vehicle. However, the preferential institutional support for electric vehicles, high price (EUR 75,600 [22]), and difficulties of installing hydrogen gas stations set this technological pioneer in a standby position, waiting for a change in mobility trends.
Practical solutions regarding hydrogen handling, storage, safety measurements, and reliable supply seem to be a step behind. Kurien and Mittal [23] listed problems found when implementing hydrogen as an alternative fuel, including low volumetric density, embrittlement of engine components, safety concerns regarding onboard storage, and practical limitations associated with hydrogen production from water electrolyzers. The list of challenges is extensive, and even with an optimistic view, it is difficult to believe that hydrogen would become a viable option in the short term.
3.2. Biodiesel and Alcohols
Alternative fuels such as biodiesel and alcohols may also be considered suitable allies in decarbonizing the economy and the transport sector. Biodiesel can be obtained from the transesterification reaction of vegetable oils with alcohols or from hydrotreatment, with this later process resulting more interesting in terms of fuel quality (higher calorific value and greater chemical stability) [24]. Petroleum refining companies have succeeded in attaining biodiesel production from hydrotreatment of vegetable oils (HVOs) such as CEPSA and NESTE, among others [25,26], showing compatible characteristics with conventional diesel and better performance regarding pollutant emissions [27]. However, biofuel production must ensure that indirect land use change (ILUC) effects are carefully evaluated [25]. A guarantee should be given so that adverse impacts that may be expected by substituting land use for human and animal feed stocks into biofuel production are avoided.
A similar effect may also be associated with the ethanol production from sugars and cereals. Although ethanol derived from cellulosic substrates is considered a promising technology since it prevents these undesirable effects, various technical difficulties regarding high production costs and low yields have prevented the industrial deployment of this technology. As a result, several commercial plants remain idle or on hold until these issues are resolved [28]. The cultivation of cellulosic biomass for producing ethanol may add CO2 emissions to the balance, which may not always be easy to quantify due to the changes in land use (LUC) associated with the transformation of land use in the same region or a different region far from the local ethanol production activity [29]. Figure 1 shows a schematic representation of different alternative fuels currently available which can serve as allies in the decarbonization of the energy and transport sector.
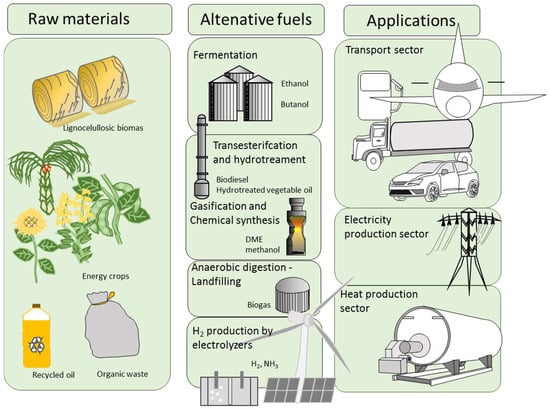
Figure 1.
Schematic representation of different process currently available at commercial and precommercial scale which can aid in decarbonizing the energy and transport sector.
Biodiesel and ethanol have the significant advantage of being compatible with current transport fleet and fuel distribution logistic. This feature enables a smooth transition to a greener transport sector, without requiring significant investments in technological infrastructure. Road transport constitutes the highest proportion of overall transport emissions (76% of all EU’s transport greenhouse gas (GHG) emissions) [30]. Therefore, any technical improvement in this sector regarding increasing fuel economy, fuel substitution, and electrification will significantly reduce CO2 emissions.
The European car fleet reduced average fuel economy by 36% from 1975 to 2015, simultaneously increasing average vehicle weight and producing more powerful engines [31]. Hybridization has allowed fuel consumption to be reduced even further. Mazda presented in 2021 commercialization of the e-skyactive X (2.0 L) engine with a fuel consumption of 5.6–4.9 L/100 km (NEDC cycle) capable of developing 186 HP at 6000 rpm (Mazda CX-30 model) [32]. Engine manufacturers’ efforts have significantly reduced CO2 emissions and pollutant levels (particles and NOx). Reducing the average age of the European vehicle fleet (which is 12 years currently [33]) would immediately impact global fuel consumption. This premise may be equally applied to any other country, with this measure having a much greater impact in developing countries (due to their older-vehicle-age fleet [34]), thus becoming a fast and effective solution, since CO2 emissions are a global problem.
3.3. Electrification of Transport Sector
Electrification of vehicles is currently considered the preferable choice in many developed countries for reducing dispersed emissions and improving air quality. However, a factor that should be carefully analyzed is the current energy mix of countries and the expected introduction of renewable energies in the short term. Electrification of the vehicle fleet may result in a costly and ineffective measure when the energy mix is mainly based on fossil fuels, but even in the case of an energy mix dominated by renewable sources, electrification implies significant changes in infrastructures, fleet vehicle production chains, and installation of a vast network of electric recharge points. The use of synthetic fuels should also be carefully analyzed. If the energy demand for producing these e-fuels is much higher than that needed for producing current fossil fuels, then, as reported by Sacchi et al. [35], the expected emission performance of a fleet based on fossil-fueled hybrid vehicles would be superior.
The introduction into the market of mild hybrid (MHV) and plug-in hybrid electric vehicles (PHEVs) opens an alternative scenario, reducing concerns regarding the extraction of minerals needed for batteries (because smaller batteries are used with these types of vehicles), and a reduced fuel consumption allows an increase in the percentage of biofuels with low cycle GHG emissions [36].
3.4. Biogas, Syngas and Syngas Derivates
Biogas is another attractive fuel that can be derived from landfilling and anaerobic digestion of wastes and energy crops. The process allows the stabilization of highly putrescible material, resulting in environmental benefits by avoiding uncontrolled degradation and the consequent release of methane into the atmosphere [37], as well as avoiding nutrient runoff. However, when energy crops are introduced into the equation, then similar concerns regarding LUC effects must be taken into account to avoid outweighing any potential benefit.
Biogas is composed mainly of methane and carbon dioxide. Upgrading technologies attain a quality similar to that of natural gas, making its introduction into the natural gas grid possible [9]. Thus, biomethane should be considered a natural gas substitute. Nevertheless, the amount of biogas produced in Europe is still small (191 TWh) compared to natural gas imports (3812 TWh) [38,39]. A slow development has characterized the biogas market, although it is expected to increase in the near future. However, the biogas potential extracted from wastes and residual biomass from agri-business may not be enough to provide the whole gas demand of European countries. Therefore, other technologies would need to come into play as it would be biomass gasification of forestry material and lignocellulosic wastes [40]. However, gasification technology has to overcome several challenges regarding syngas cleaning, tar production, and char quality along with inefficiencies caused by biomass transport logistics and energy densification [41,42].
Other alternative fuels that may be also considered are dimethyl ether (DME) and methanol. Both compounds can be either derived from the chemical conversion of syngas or from direct capture of CO2 and a subsequent hydrogenation process [43,44]. However, although technically feasible, the production of these fuels is costly and to this limitation should be added the challenge of obtaining a green hydrogen stream from renewable sources which further increases production costs.
3.5. Return of Ammonia as Alternative Fuel
Ammonia is again being considered as a suitable alternative fuel that is free of carbon. The early use of ammonia in internal combustion engines (ICEs) dates back to the 1940s due to oil scarcity imposed in World War II, with the first utilization of liquid anhydrous ammonia as fuel in motor buses reported in 1943 [45]. Dimitriou and Javaid [46] reported on the origins of ammonia as fuel, explaining that diesel engines’ high fuel efficiency and compatibility with ammonia combustion characteristics will make them play an important role in transforming the transport sector. The main benefits of using ammonia as a dual fuel come from the low degree of changes required in current combustion engines and the feasibility of pollutant abatement with already available catalytic technologies.
A technological transition should be favored. It would be irrational to consider an immediate switch from a fossil-fuel-based economy to a net-zero-emission economy. In contrast to hydrogen, ammonia is a commodity compound used in industrial processes and as fertilizer, with a global production of about 150 MMT (millions of metric tons) [47]. Ammonia is easier to handle, with several years of technical research in distribution and storage. The energy density of ammonia is 12.7 MJ/L at −33 °C [48], whereas liquefied hydrogen has an energy density of just 8.5 MJ/L [49].
The production process of ammonia is well established, with a large-scale capacity that could be easily extrapolated to increase global production based on demand in a much easier way than for other alternative fuels. Concerns regarding the use of fossil fuels in ammonia production may be mitigated with the implementation of carbon capture storage technologies until large-scale production from renewable sources overcomes efficiency and cost constraints.
The use of ammonia as a fuel in engines will endow flexibility to the power sector and is currently considered a net-zero CO2 emission fuel with great applicability in the long-haul shipping sector [50]. MAN Energy Solutions is developing a fuel-flexible ammonia engine and intends to attain the gradual rebuild of existing maritime engines to allow ammonia as complementary fuel [51]. Toyota presented the GT-89R Marangoni fitted with an ammonia engine system at the Geneva Motor Show of 2013 [52], and the company counts several patents regarding the ammonia engine and hydrogen generator associated with the engine [53,54]. Recently, the Toyota Corporation presented an ammonia engine suitable for passenger cars developed in collaboration with the Chinese car manufacturer GAC [55]. Although environmental concerns may arise regarding NOx levels during ammonia combustion, the technology is expected to meet regulation standards by performing suitable changes in combustion equivalence ratio, combustion chamber pressure, and applying emission control strategies [56].
4. Ammonia Production
Ammonia is one of the chemicals with the highest production worldwide due to its great application in the chemical industry and agriculture. Although it is a gas at room temperature conditions with a pungent smell and corrosive characteristics, there is wide research on its handling [57]. In fact, the strong smell may serve as an alarm, allowing a prompt awareness of leakages and facilitating safety precautions. The high hydrogen content of ammonia (17.7 %wt/wt) and low-pressure liquid state (8.58 bars) make this molecule a suitable hydrogen carrier or an energy storage molecule. Ammonia production can be thought of as a way to transform excess energy from renewables and equilibrate the balance between energy production and demand, a fact that is favored by the vast existing network of available pipelines, thus offering a lower transport cost than that estimated for hydrogen [5,58]. Therefore, the production of this molecule is expected to increase exponentially, with a forecast of 290 million metric tons by 2030 for the production capacity [59]. This value may experience a tremendous rise if expectations in the energy and transport sector are achieved.
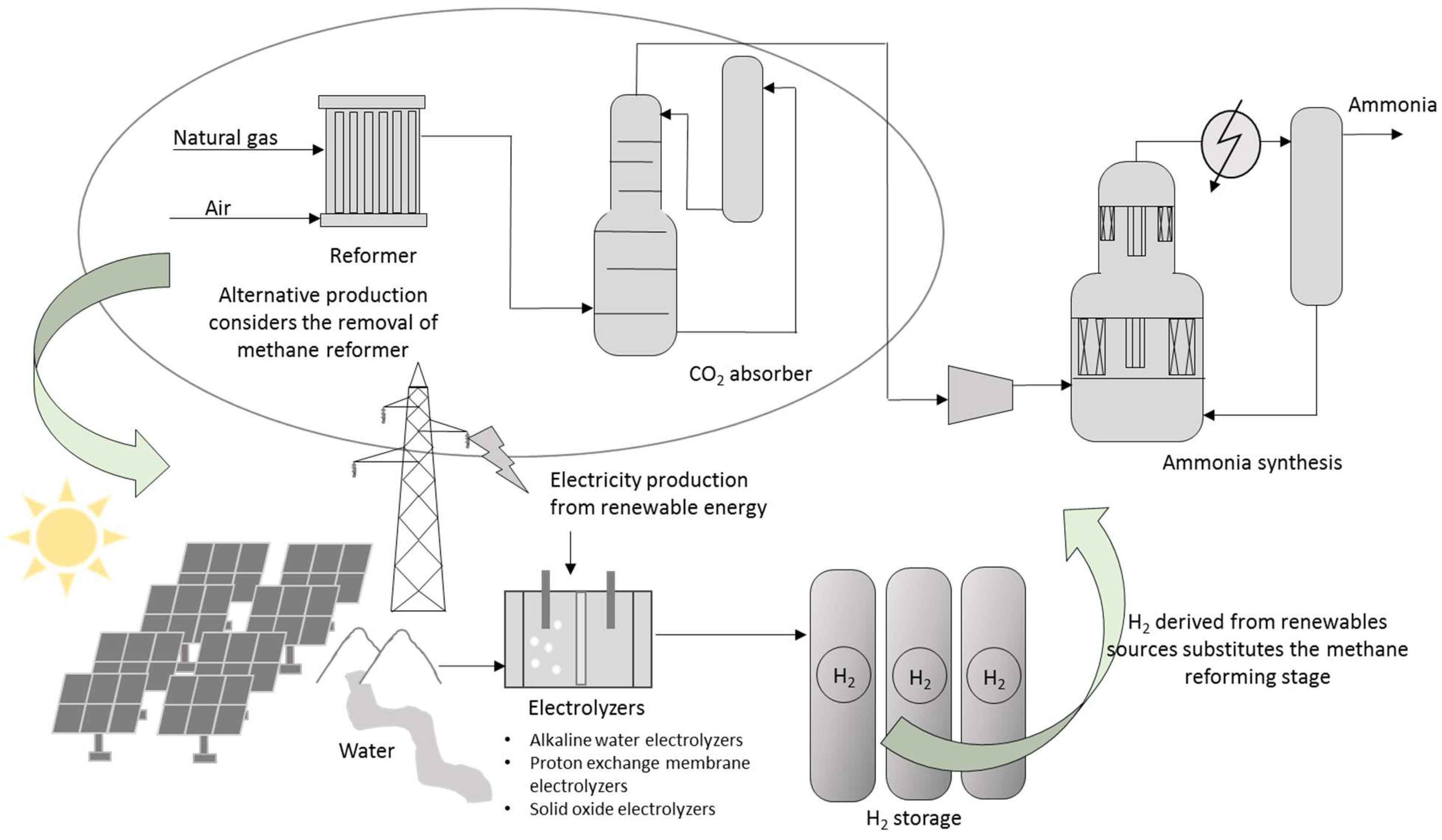
The industrial production of ammonia was dominated by the process developed by Fitz Haber in 1908 and industrialized by Carl Bosch thanks to solving material and pressure problems which allowed starting operation in 1913 of the first BASF industrial plant at Oppau site north of Ludwigshafen [60]. Today, Casale S.A. is the leading ammonia company in plant design and revamping (upgrading and retrofitting). Between 1984 and 2021, over 200 ammonia converters were retrofitted by Casale S.A. worldwide, increasing production yield by over 40% and the development of a revolutionary iron-based catalyst called AmoMax®-Casale [61]. Figure 2 shows a schematization of ammonia conventional production.
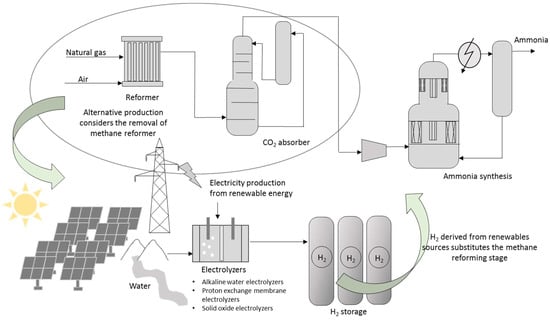
Figure 2.
Representation of the ammonia production process. Methane is used in a reformer unit to produce hydrogen. CO2 and other trace compounds are removed prior to reaching the catalytic converter. The methane reformer can be substituted by electrolysis units.
The catalytic conversion of ammonia from N2, previously separated from air, requires the production of H2, which is currently obtained from fossil fuels (natural gas or coal), thus making this process one of the primary candidates for proposing the use of water electrolyzers using renewable energy as power supply. This way, a significant reduction in the carbon footprint is attained. Smith et al. [62] estimated that a wind-powered ammonia process would have a carbon intensity of 0.12–0.53 t CO2-eq/t NH3 against a value of 1.5–1.6 estimated for a modern highly efficient methane-fed process [63]. However, the substitution is not so simple and estimation is not so straightforward.
The industrial market of water electrolyzers is dominated by alkaline water (AWE), proton exchange membrane (PEM) electrolyzers operating at low temperatures, and solid oxide electrolyzers currently under development, with high operating temperatures [64]. AWEs are preferred against PEM electrolyzers given their high installation costs and lower service life of the latter [65]. Companies offering commercial units, such as Nel hydrogen (Oslo, Norway) [66], offer atmospheric AWE with a production of 1.2 t H2/day and power demand of 2.2 MW (3.8 kWh/m3 (STP) H2), whereas McPhy Energy Italia Srl (San Miniato, Italy) [67] offers modules producing hydrogen at 30 bar with a consumption of 4.65 kWh/m3 (STP) H2. Additional energy is needed to increase H2 pressure to the Haber–Bosch process (200–400 atm) and the availability of enough power from renewable source is also needed to guarantee a reliable supply.
Electrolyzers present several drawbacks which need an urgent solution. Inefficiencies in electrolyzer performance are associated with low load (25–45% of rated load), favoring the diffusion of hydrogen into the oxygen container, creating safety concerns regarding the explosive nature of the mixture [68]. The flammability limits of the pure H2–O2 mixture are much wider than those expected for H2–air mixtures, and the problem is aggravated with pressure increase. Fluctuations in renewable energy production would affect performance, leading to start–stop cycles. After shutting down electrolyzers, a reverse current phenomenon associated with redox reactions of the electrodes is observed, causing severe degradation (irreversible oxidation of cathodes), and adversely affecting the service life of electrocatalysts [69,70].
Chau et al. [71] performed a study regarding different hydrogen production technologies evaluating safety, efficiency, and infrastructure, concluding that the SMR process was the most desirable technology. Even if becoming fully optimistic by considering that solutions will be available in the short term regarding safety measurements that allow for technology scale-up, the challenges are still enormous. As an example, the ammonia plants of Fertiberia are expected to reduce their carbon footprint by introducing green hydrogen derived from the use of renewable energy. The plant located at Puerto Llano (Spain) has a capacity of producing 200,000 t/year, and the company intends to install 200 MW electrolyzers which will obtain electricity from photovoltaic panels [72]. The intermittent nature of solar power and variations in irradiance and temperature deeply impact the reliability of electricity production [73]. Thus, the increase in renewables in the energy mix inevitably comes with a concomitant risk of uncertainty due to the intrinsic dependence on sun irradiance and daily climatic conditions. An equivalent amount of energy as backup is then necessary to cover for unavoidable low/null energy production periods. A similar feature can be ascribed to wind power, causing an overdimensioning of the power supply system. Therefore, interconnection with an electrical grid is needed as a warranty of steady energy supply to ensure electrolyzers’ energy requirements. The availability of other electricity sources, such as nuclear or large-scale energy storage systems, guarantees energy supply regardless of the intermittency of renewables.
An additional issue is water supply and quality. The water demand for electrolyzers is high, requiring pure water with low ion contents and low impurity levels. However, water scarcity and water distribution are among the greatest environmental concerns. During the past years, the global population faced changes in rain distribution patterns with extreme drought and flood episodes. This trend is expected to be maintained or even aggravated, requiring extraordinary measures to manage water availability for human consumption and ensure food supply due to the possible scarcity that will be probably faced in certain regions due to depletion of water reservoirs and incapacity of recharging [74,75]. Based on this scenario, it does not seem right to consider the production of H2 as a feasible option in regions characterized by water scarcity problems.
Using seawater or wastewater would offer a great advantage to the process since it avoids the treatment and transport of high-quality water to the hydrogen production site. Unfortunately, the presence of anions, such as chloride and bromide, and cations (Na, Ca, Mg) creates a severe decrease in performance [76]. El-Shafie [77] reviewed the technical barriers regarding the use of water sources other than pure water. These authors reported that the presence of organics increases cathode fouling rates, halide oxidation reactions occur under the presence of anionic impurities (chloride and bromide) instead of the oxygen evolution reaction [78], and cationic poisoning causes a complete electrolyzer failure. Therefore, finding a practical solution to all these problems seems far away, so the immediate alternative to overcome these difficulties may be the use of desalinated water.
The energy demand for water desalination is about 3.2–4.5 kWh/m3. However, water desalination for human consumption is considered a costly option and too intensively energy-demanding [79]. Based on the previous statement, it does not seem appropriate to think that the use of desalinated water would be an adequate solution for H2 production, particularly if 9 t of water are required to produce 1 t of H2 [80]; in contrast, it is considered an unadvisable choice for meeting water human consumption demand. Despite this controversy, this alternative is being categorized as a feasible option by several authors based on the currently high costs of hydrogen production systems [81,82,83].
It should be remembered that many current hydrogen projects are receiving public funds as an incentive for facilitating technological penetration. The objective should be to pursue economically viable activities along with climate protection. As a result, the costly installation and energy-intensive nature of the hydrogen production process should not be overlooked, and any additional equipment included in the process should not contribute to increasing the already high energy demand.
Saygin et al. [84] analyzed the energy demand of the ammonia-producing process. These authors indicated that the rise in oil and gas prices directly affected that of fertilizers since most of ammonia plants are based on the SMR process and are incapable of assuming such an increase in feedstock and energy prices, generating a curtailment in European production. The increase in ammonia prices also had a negative impact on food production consequently rising food prices. The interconnection between fuel prices and the economy is a factor that needs careful assessment. At its beginning, the Ukrainian war caused a great disruption in the cereal market; this, along with an increasing trend in inflation and oil prices, created food security risks [85].
The future increase in ammonia prices may be caused by a greater implementation of green ammonia production worldwide given the higher cost of this technology [86] or because of a higher demand if ammonia is used either as a hydrogen carrier or alternative fuel. Therefore, a careful deployment of green ammonia technology is necessary to avoid adverse perturbations in the food supply chain. An interesting review of the industrial ammonia production using water electrolysis was performed by Rouwenhorst et al. [87]. These authors described the expansion of the process in different countries, highlighting the relevance of the Lougi Casale process, indicating that the main factor for the installation of these plants was the availability of cheap hydropower electricity for producing hydrogen. The lower costs of fossil-fuel-based plants and better scale-up of electrolyzer-based ammonia plants led to a shut-down, with the only plant still operating located in Cuzco, Perú (Cachimayo plant, Enaex) [88].
Alternatives Routes for Producing Ammonia
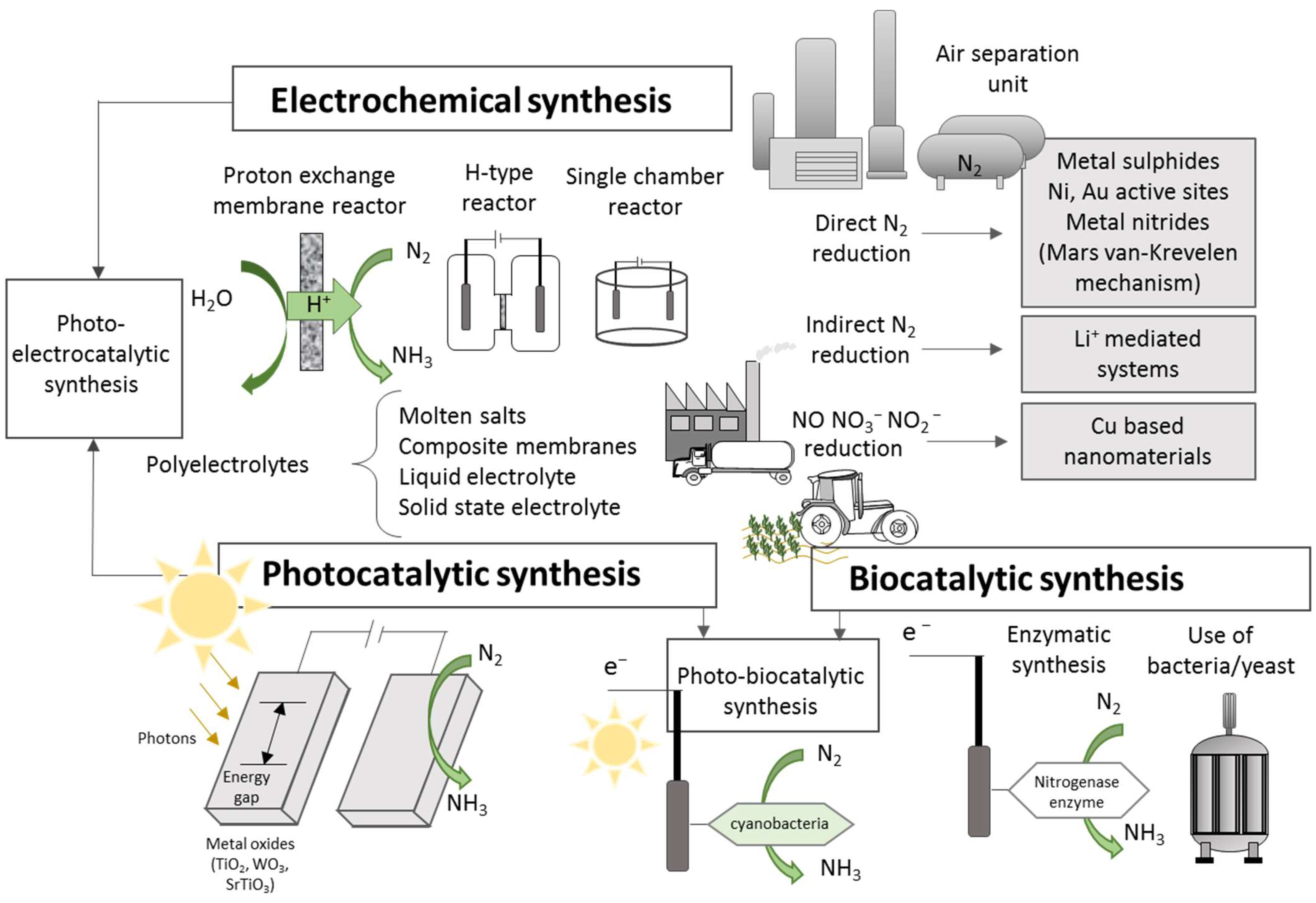
Alternatives to the Haber–Bosch process for ammonia production are electrocatalytic, photocatalytic, and biocatalytic ammonia synthesis. Other technologies include plasma catalysts and nonthermal plasma methods; in this latter case, an electric discharge generates the ionization stage [88,89,90]. However, commercial ammonia production is still dominated by the SMR due to the multiple difficulties associated with installation costs, energy demand, and process efficiency of the different alternatives.
In the case of electrochemical synthesis, the process can be carried out at different temperature ranges using liquid electrolytes, molten salts, composite membranes, and solid-state electrolytes [80]. These methods allow ammonia synthesis through nitrogen reduction (NRR), nitrogen oxide reduction (NORR), and nitrate/nitrite reduction (NtrRR). In some of these electrochemical processes, water is used as a source of protons and electrons as reducing agents, with the reaction taking place under ambient temperature and pressure conditions, directly using electricity and, thus, offering a sustainable strategy for ammonia production [91,92]. However, N2 is thermodynamically very stable under ambient conditions; thus, binding and subsequent activation to initiate conversion stages into ammonia becomes a great challenge [93]. A schematization of the different technological pathways for producing ammonia is presented in Figure 3, where the most commonly studied configurations are summarized.
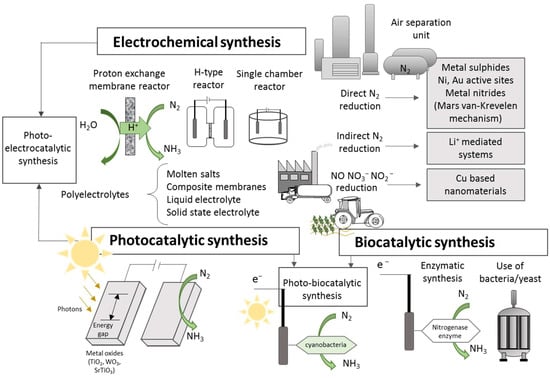
Figure 3.
Schematic representation of alternative ammonia production routes.
The direct reduction of nitrogen by electrochemical synthesis follows the equation:
N2(g) + 6H+ + 6e− → 2NH3(g)
The ammonia formation reaction has a higher potential than the hydrogen evolution reaction (2H+ + 2e− → H2(g)), thus compromising process efficiency. In addition to the low efficiency and high stability of the N2 molecule, its limited solubility in water is another factor that adversely affects the process [94], along with the high energetic costs due to the low faradic efficiencies of different catalysts [95,96], and the low stability and activity of some catalysts which degrade rapidly [97]. Even with theoretical faradic efficiencies of 100%, the energy consumption is 8.5 MWh/t, close to that of the Haber–Bosch process (9.5–10.5 MWh/t) [98]. Catalysts of different materials are being investigated to improve production, faradic efficiency, and inhibit the hydrogen evolution reaction [99,100].
Other methodologies, such as indirect N2 reduction using lithium as a mediator, are also being explored, which improves productivity but still presents high energy consumption to compete with the Haber–Bosch process [101]. NO, nitrate, and nitrite reduction have also been proposed as an ammonia production route. NO is a major air pollutant generated mainly from fossil fuel combustion in industrial activities and transport [102]. The NO reduction shows better performance than NRR, since NO is a less stable nitrogen source than N2. Copper is the most effective metal for catalyzing the reaction, achieving faradic efficiencies above 90% and ammonia production levels comparable to the Haber–Bosch process [91,103]. However, the low solubility of NO in water requires the use of concentrated or pure NO, limiting its practical application [104].
The electrochemical reduction of nitrates and nitrites to ammonia allows the removal of these pollutant ions, resulting from agricultural and industrial activities, becoming an alternative for wastewater treatment [105,106]. However, the hydrogen evolution reaction is the main secondary reaction at the cathode in this production route, thus also limiting process efficiency [104]. Numerous studies have been carried out with different catalysts and electrolytes [107,108,109], studying the phenomena at the atomic level to obtain more efficient catalysts. Yin et al. [110] reviewed many of these studies. Although this route presents good current densities and faradic efficiencies, it still presents low productivity [97]. As for its performance under real conditions, almost all tests used synthetic streams, and no studies have been conducted on large-scale treatment of real wastewater, where its long-term performance and techno-economic evaluation should also be considered [104].
Other processes currently under study involve the photocatalytic and biocatalytic processes. In the first case, energy is provided by a light source. Photons transport the energy needed for electrons to surpass the energy gap of the semiconductor. Electrons can migrate and participate in reactions (nitrogen reduction or hydrogen formation), leaving behind valence holes where water is oxidized to O2 and H+ [111,112]. In the second case, the process attempts to mimic the biological synthesis of ammonia using nitrogenase enzymes (commonly containing MoFe protein), focusing the interest on artificially supplying electrons to the catalytic protein [113,114]. However, the stabilization of the enzyme shows extreme difficulties, and production and purification costs seem too excessive to consider the process a feasible alternative to the traditional Haber–Bosch process [115]. Other biological alternatives consider the conversion of protein wastes using bacteria or yeast or new hybrid systems combining photocatalysis and cyanobacteria [116,117].
5. Use of Ammonia as Alternative Fuel: Ammonia Combustion
The search for alternative fuels complying with characteristics regarding carbon dioxide emissions and renewable sources has set the focus on carbon-free molecules. The immediate candidate to think of is hydrogen, but given the difficulties of attaining adequate volumetric densities at a reasonable cost, the attention has moved forward to hydrogen-rich molecules such as methane and ammonia. In the case of methane, this gas may be produced from the biological degradation of wastes under anaerobic conditions, or by the Sabatier process using syngas derived from biomass or CO2 absorbed directly from the atmosphere. The Sabatier reaction requires a special nickel-based catalyst and a source of hydrogen, transforming 1 mole unit of CO2 into methane by the use of 4 mole units of hydrogen [118,119], making the process complex, with only one plant built until now. The e-gas Audi plant was built in Werlte, Germany, and uses CO2 derived from an anaerobic digester, thus complying with the requirement of producing synthetic methane from renewable sources [120]. The plant was transferred to Kiwi AG and recently acquired by Hy2Gen [121].
Given the scarce research in producing synthetic methane at an industrial scale, synthetic ammonia production seems a better option if a fast transition to a carbon-free economy is to be achieved. Ammonia has a higher energy volumetric density than hydrogen, although combustion properties are poor compared to hydrogen and methane, with poor ignition and flame propagating behavior [122]. The range of flammability limits is narrower than that of methane or other hydrocarbons, and the flame speed is just 7 cm/s, whereas this value is in the range of 35 to 100 for conventional fuels and biofuels, reaching values as high as 291 for hydrogen [123]. Ammonia’s lower energy content per unit mass impacts combustion characteristics by releasing a lower amount of energy in the reaction zone and thereby reaching lower flame temperatures. This affects reactivity because a lower concentration of radicals is present in the flame front [124].
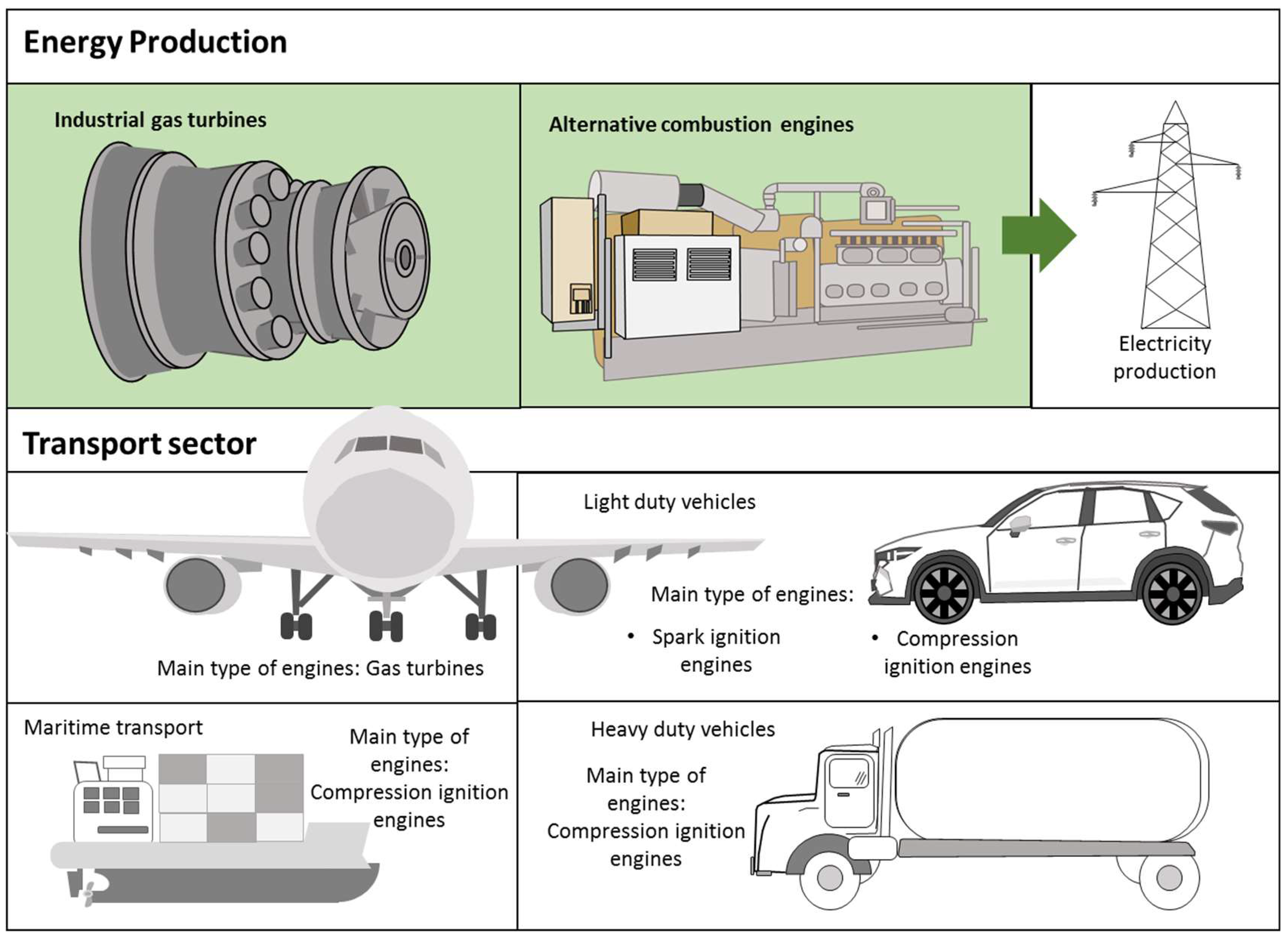
Despite these previous characteristics, ammonia has been proposed as a complementing fuel in ICEs to decrease the use of fossil fuels since the absence of carbon in its molecular structures avoids releasing greenhouse gas emissions. The poor combustion performance can be compensated by introducing modifications in engines to attain either neat ammonia combustion or its use as a dual fuel. The transition to a decarbonized economy may be faster if an important reduction in fossil fuel consumption is achieved by using suitable and available substitutes that have a proven track record of large-scale production and handling. ICEs commonly used in the power generation and transport sector can be classified as compression ignition, spark ignition, and gas turbines. The different operating characteristics of these engines translate into special features and combustion requirements. Thus, different techniques are necessary to adapt engines and attain a suitable performance when using mixtures of ammonia and conventional fuels. Figure 4 shows the main types of engines used in the electricity production and transport sectors.
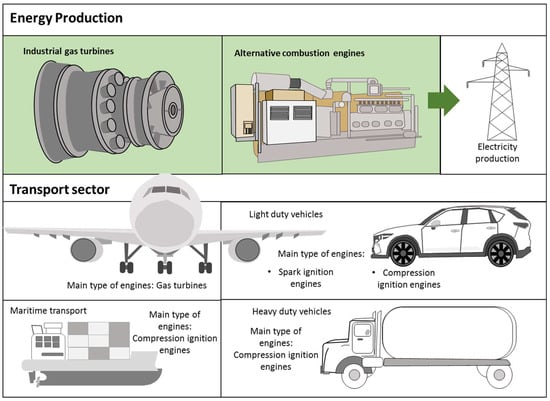
Figure 4.
Internal combustion engines used for electricity production and in the transport sector.
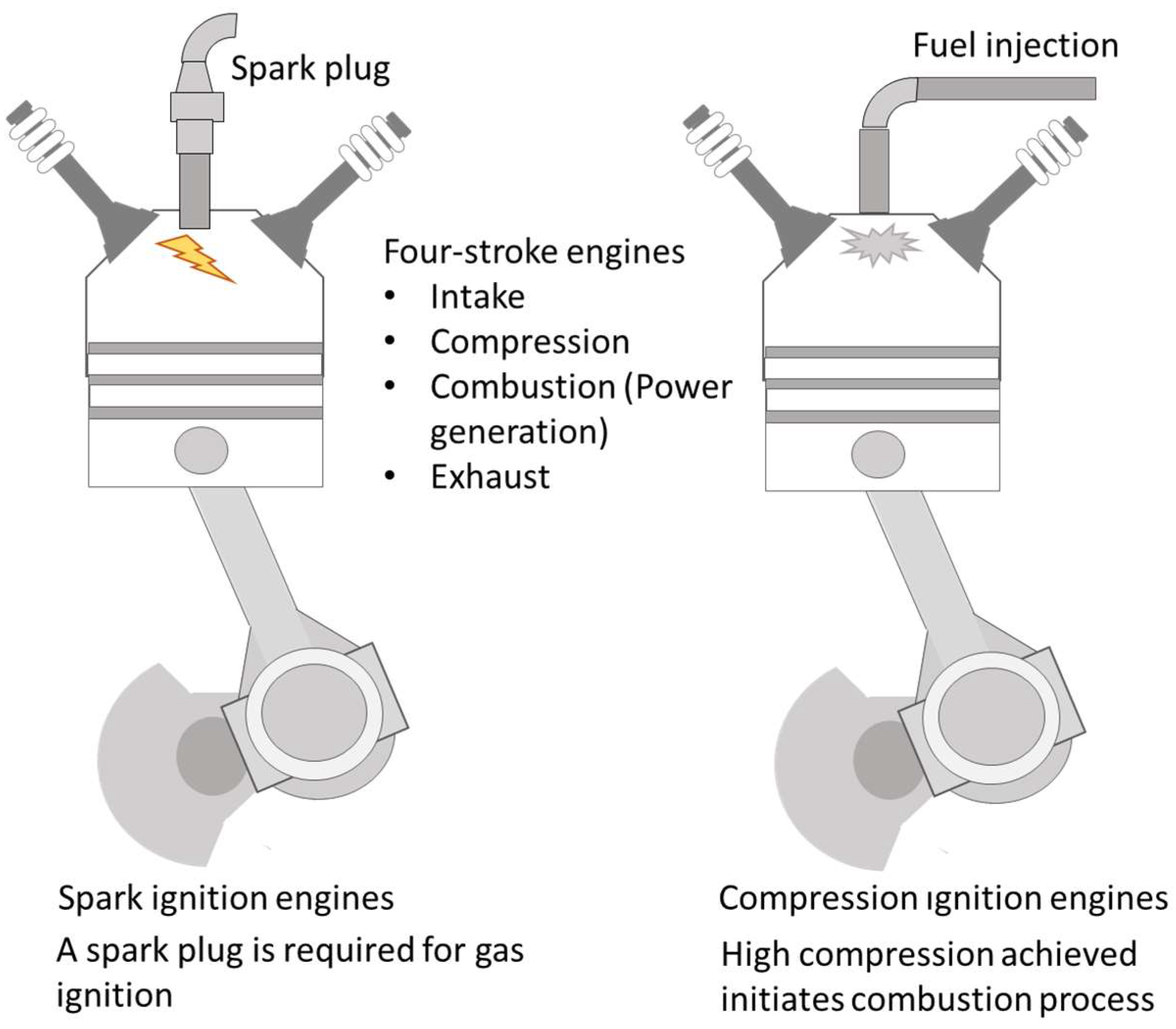
Compression ignition engines require a fuel with a high cetane number to allow the combustion chamber to reach a high compression stage, attaining the mixture’s autoignition by the associated temperature increase. In this system, direct injection of fuel is carried out once the air has been fully admitted into the cylinder and once the piston is closed to reach the top dead center position. On the contrary, spark ignition engines require a spark plug to initiate fuel–air mixture ignition (see Figure 5). Traditionally, in these later systems, the admission of fuel and air takes place in a premixed mode prior to the cylinder’s intake ports. Modifications in fuel injection have been implemented in current modern engines, with many counting with direct or stratified fuel injection. These modifications were introduced to improve fuel use efficiency and reduce combustion temperature to lower NOx emissions. Two examples are homogenous charge compression ignition (HCCI), in which the mixture enters the cylinder, achieving a full homogenous state prior to autoignition, and premixed charge combustion ignition (PCCI), where a partial premix is created, allowing for fuel stratification before combustion. Thus, this concept may be classified as being between conventional combustion and HCCI [125].
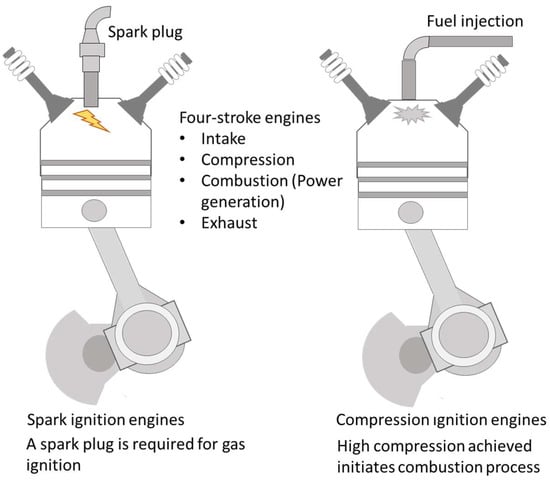
Figure 5.
Internal combustion engines: spark and compression ignition engines. Four-stroke engines comprise a cycle of four stages to produce power every two cycles of the piston.
The amount of fuel–air added to a combustion system can be expressed in terms of the equivalence ratio (φ). This parameter gives an idea of the combustion conditions, referencing the stoichiometric point (ratio between the stoichiometric air and the amount of air added). Thus, lean mixtures are defined by values of φ lower than unity due to the addition of excess air. Modifying the amount of air added to combustion allows for controlling temperature and emissions produced. The recirculation of combustion gases also reduces combustion temperature by returning to the reaction zone inert species, creating a quenching effect and acting as a NOx control strategy. Exhaust gas recirculation (EGR) is a common feature of diesel engines and, recently, of new spark ignition engines [126,127]. The different operating concepts in ICEs will need to be adapted based on the combustion characteristics of ammonia. This gas has a high ignition energy (8 mJ for ammonia, whereas propane only requires 0.5 mJ [128]) and low flame speed. Therefore, attaining stable performance under different engine operating regimes will be a challenging work. Several experimental research works deal with the use of ammonia–fuel mixtures of conventional (diesel, gasoline) and alternative fuels (ethanol, biodiesel) [129,130,131].
The release of pollutants is a characteristic of ammonia combustion, which is a cause of great concern; a higher release of nitrogen oxides and unburnt ammonia in the exhaust may be expected [132]. Combustion of conventional fuels has been extensively studied to reduce the production of NOx emissions. Changes in operating conditions in the combustion chamber have been introduced to lower temperatures and avoid thermal NOx emissions derived from the Zeldovich mechanism [133,134]. Prompt NOx emissions (Fenimore mechanism) are associated with the presence of CH radicals following a different route, where HCN and CN are formed [135]. Additional emissions may be expected from the nitrogen fuel content. Burning under lean fuel conditions, combustion staging, or including a recirculation stream of exhaust gases to create reducing atmospheres are just some examples of the alternatives available for avoiding NOx formation. However, these strategies need additional adjustment and operational modifications when considering the addition of ammonia as a dual fuel.
The presence of nitrogen in the ammonia molecule would make anyone think of extremely high related fuel NOx emissions associated with direct ammonia oxidation. However, the reaction mechanism is complex, and the presence of different intermediary species allows for a window where the production of this undesirable contaminant is mitigated. The overall combustion reaction is the following:
4 NH3 + 3 O2 → 2 N2 + 6 H2O
The mechanism of ammonia combustion comprises the presence of HNO and NH as intermediary species participating in NO formation [136]. Nitrogen-related contaminants are high [137] but not as much as one may have inferred from its molecular composition. Shu et al. [138] studied the mechanism of ammonia combustion, reporting that the presence of NH2 intermediary species plays a fundamental role in autoignition, and its reaction with NO is crucial for the reaction outcome, either increasing or reducing the presence of this pollutant in exhaust gases. Other pathways responsible for NOx formation are those involving HNO and the Zeldovich pathway [139].
Cai et al. [137] reviewed different studies regarding ammonia combustion mechanisms and strategies to mitigate its formation. Operating under fuel-rich conditions, increasing pressure, staging combustion, and steam addition are some of the relevant strategies that have demonstrated success in controlling NOx presence [140,141,142,143].
Lean ammonia conditions favor the presence of O/H radicals, which are precursors to the formation of NO contaminants. Thus, lean ammonia flames show peaking NO levels at φ of 0.9, with higher values than those obtained from methane/air flames, but showing a minimum at slightly rich conditions (φ = 1) [144]. The presence of H, OH, and HNO radicals is crucial in the formation of NOx by the following reactions:
NO2 + H ↔ NO + OH
HNO + H ↔ NO + H2
HNO + OH ↔ NO + H2O
Therefore, adding H2 to ammonia combustion under lean conditions increases the availability of these radicals in the flame front. On the contrary, increasing the φ over unity values significantly reduces NOx formation because oxygen availability decreases as well as temperature [145].
NO2 and N2O are also part of NOx emissions. NO2 is responsible for smog formation, whereas N2O is a compound that causes great concern because of its high warming potential (about 300 times greater than that of CO2) [146]. Therefore, benefits associated with CO2 emission reduction may be in part mitigated by the release of N2O or by the adverse effects associated with acid rain. The proportion of N2O in the global NOx emission is believed to be closely related with incomplete combustion in ICEs, decreasing the yield of N2O formation with temperature in the 850–1350 K range [147,148].
5.1. Gas Turbines
Generating a flame and attaining stable conditions in a combustion chamber can be achieved by the use of different strategies. However, attaining stable performance under different operating conditions with low levels of contaminants was a great challenge, which was only made possible after several years of research and experience. In a very simplistic way, flames can be catalogued as premixed or diffusion types. In the first case, mixing of the fuel and oxidizer is performed prior to reaching the flame front, and the combustion temperature reached corresponds to the φ supplied. The second case refers to the separate supply of fuel and air. Thus, diffusion flames are said to always reach higher combustion temperatures since there is a preference for the flame front to be located at the stoichiometric mixing condition point. Since the diffusion of the fuel and oxidizer to the reaction zone is required, these flames are diffusion-limited, thus explaining their name.
The previous categorization is far from explaining all complexities taking place during the combustion. Burning fuels with excess air (lean conditions) reduces flame temperature and, thus, NOx emissions, but it also affects flame speed and may create acoustic interactions that adversely affect flame stability. In the case of burning fuels with air deficiency (rich conditions), flame temperature is also lowered, but unburnt hydrocarbons and CO emissions are a concern. Combustion science must deal with reaction chemistry, flow interactions, heat transport phenomena, and acoustic waves, thus explaining the difficulties in attaining stable performance under all possible engine operating regimes.
The use of swirling flow in combustion chambers increases flame stability by creating inner and outer recirculation zones that transport hot products and active radicals back to the reaction region, mixing with the fresh, unburnt mixture, increasing residence time and allowing for the fuel to be consumed [149]. The presence of these zones allows for operation with high inlet flow velocities, although the flame speed of the fuel used may be low. A swirl flow creates a central vortex zone and low-pressure gradient, giving rise to a reverse flow [150] that allows flame anchorage.
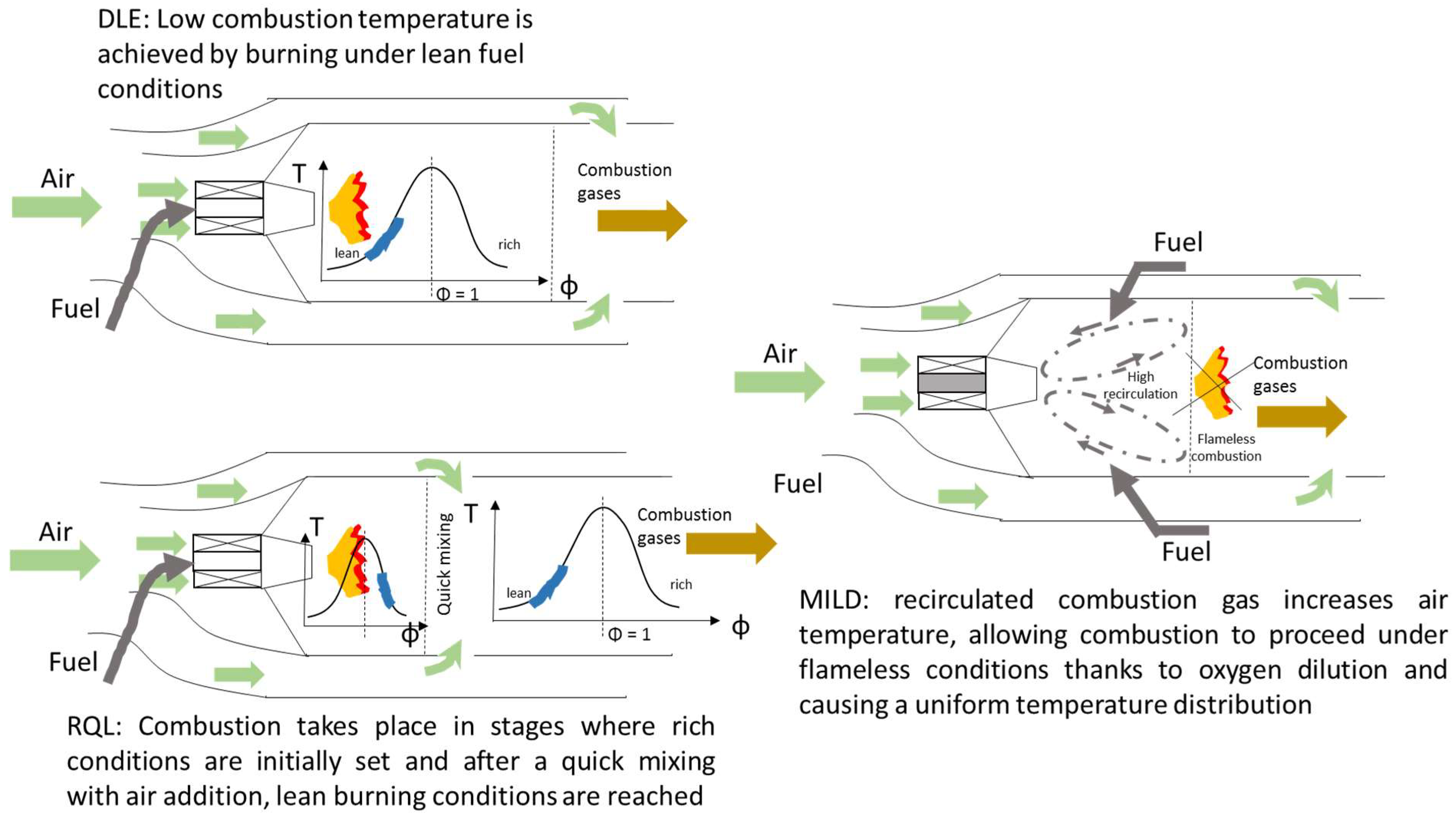
The strategies widely applied in gas turbines for attaining acceptable performance regarding combustion efficiency and emission control are dry low emission (DLE), rich-burn, quick-quench, and lean-burn (RQL), and moderate or intense low oxygen dilution (MILD). The first refers to lean burn conditions where low adiabatic flame temperatures avoid NO formation under the thermal mechanism. The reduction in NOx emission is achieved without using water or steam as a heat sink, hence the denomination of dry [151]. The RQL concept is based on the separation of combustion into two clearly distinct zones where rich conditions are initially favored, creating a pool of radicals that are subsequently oxidized in a secondary zone where excess air is sufficiently added, attaining a fast temperature drop and avoiding thermal NOx formation. MILD combustion refers to the recirculation of hot combustion gases, causing an oxygen dilution effect in the reaction zone and, thus, lowering peak temperatures [152]. Figure 6 shows the different operating concepts.
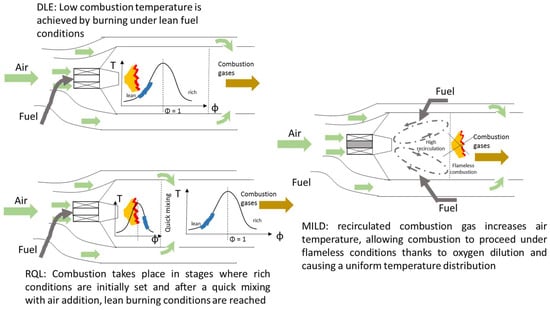
Figure 6.
Operating strategies in gas turbines to achieve low levels of pollutants. DLE: dry low emission, RQL: rich-burn, quick-quench, and lean-burn, and MILD: moderate or intense low oxygen dilution.
Ammonia/air flames are less stable than those of methane under similar conditions, with lower laminar burning velocities, heat release rate, and lower flame temperature [144]. However, combustion of this gas has been possible with success by attaining appropriate hydrodynamic conditions in the combustion chamber. Many industrial applications for energy production use methane in gas turbines. Therefore, there is a great interest in retrofitting current engines to allow for ammonia use, either as a mixture of methane/ammonia or as a single fuel, keeping always in mind that NOx regulation should be fully complied with. Iki et al. [153] studied the performance of a microturbine using methane and mixtures with ammonia and neat ammonia as fuel. Changes in air supply were needed to provide air at higher temperatures and lower flow rates. Staging combustion was also necessary to create two zones (rich–lean) and avoid an excessive production of NOx. Okafor et al. [154] tested single and two-stage (rich–lean) combustion using methane/ammonia mixtures, reporting high NO emissions in the first case but much lower ones in the latter due to the enhanced fuel consumption in the primary zone (where rich conditions are set). The methane/ammonia mixture was characterized by higher flame speed compared to that of neat ammonia flames, a feature that ensured lower NOx production in the secondary zone.
Gubbi et al. [56] performed a numerical study, evaluating the NO emission expected from ammonia combustion by changing combustor pressure and residence time. These authors indicated that current NOx regulation limits can be complied with by changing operating parameters and allowing higher residence time in the combustor. Increasing pressure lowers NOx emissions when using ammonia as fuel since it reduces the availability of O and H radicals [144], and the effect of pressure is just opposite to the behavior obtained when burning conventional fuels [155]. This study opens a door for opportunities regarding the use of ammonia for reducing CO2 emissions since it is expected that the problem of NO formation will be resolved. However, the increase in pressure also causes a decrease in the laminar flame speed, and the presence of ammonia causes a decrease in temperature and net heat release rate [156], thus requiring modifications intended to attain flame stabilization under such peculiar fuel characteristics.
Rocha et al. [157] also performed a numerical evaluation of ammonia combustion and its emission characteristics by considering the three typical gas turbine operating concepts for keeping low NOx emissions, that is, DLE, RQL, and MILD. Their results indicated that the DLE concept was inappropriate when dealing with ammonia as fuel, whereas RQL and MILD combustion achieved the lowest emissions. The presence of a post-combustion zone at high temperatures enhanced the consumption of NO previously formed by the reaction NH2 + NO → H2O + N2 [158].
The poor combustion characteristics of ammonia were the main reason for considering the use of more reactive fuels as additives either for starting up the system until steady combustion is attained or using ammonia in dual systems to warrant stable performance at all loading conditions. H2 has been added as an ignition enhancer to increase the pool of radicals, flame speed, and stability [159,160], but the supplementation of this gas brings along problems associated with storage and handling, along with the use of separate deposits for ammonia and hydrogen. The autothermal cracking of ammonia to release a fraction of hydrogen contained in the ammonia molecule, or the use of catalysts to aid in the thermal decomposition of ammonia, has been proposed as a solution for avoiding the presence of an additional high-pressure container and reducing safety risks.
Regardless of difficulties encountered when attempting to use ammonia as an alternative fuel, several studies have demonstrated that technical advances can overcome combustion inefficiencies observed and reduce the emission of pollutants. Therefore, there is a great interest in considering ammonia as a suitable substitute for conventional fuels and an enabler of the hydrogen economy thanks to its feasible use in power generation and the transport sector (aircraft propulsion and road and marine transport) [161].
Table 1 shows different experimental studies carried out to evaluate the combustion characteristics of ammonia and its performance in gas turbines as single fuel and as dual systems. In these studies, it can be observed that the addition of ammonia under certain operating conditions causes an increase in NO emissions, specifically when working under premixed conditions [162,163] or using a swirl burner [163,164]. Verkamp et al. [128] proposed partial dissociation of ammonia as a method for flame stabilization, achieving stable operation by working only with ammonia as fuel; it was also possible to keep NO emissions under control. Khateeb et al. [164] succeeded in keeping NO emissions under control in a swirl burner under rich operating conditions, thus reducing the risk of backfiring. These studies showed that using ammonia as a fuel in turbines may be possible in the future with certain improvements in the current state of the art.

Table 1.
Experimental studies evaluating ammonia combustion characteristics and performance in gas turbines in a single- and dual-fuel configuration.
5.2. Spark Ignition Engines
ICEs are widely used in the transport sector and power generation. Spark ignition engines are the preferred option in passenger cars, although there was higher efficiency of diesel engines and advancements performed in the last decade regarding particle emission control and NOx production. The diesel scandal damaged public perception towards diesel passenger cars, and political pressures set a regulation preference for gasoline and electric vehicles. Even though diesel vehicles have an equivalent environmental classification in Spain to gasoline and lower CO2 emission per kilometer, the market experienced a severe drop in sales of this type of vehicle [175].
Spark ignition (SI) engines can be easily adapted to use gaseous fuels such as natural gas and liquefied petroleum gas (LPG), currently becoming a practical solution for many consumers due to their lower fuel consumption and price. SI engines are also widely used for valorizing biogas since these engines produce electricity and heat recovery concomitantly, which is essential for keeping digestion temperature. When operating under lean burn conditions, NOx emissions are restricted to 400–500 ppm [176,177]. A further reduction in NOx levels, then, requires the use of selective catalytic reactors (SCRs). The main commercial combined heat and power (CHP) engines include Jenbacher®, MWM®, Caterpillar®, MTU®, Waukesha®, and MAN® [178,179,180,181,182,183], and many of these engines are currently adapted for also using hydrogen as fuel, just as in the case of gas turbines.
Ammonia has been considered a fuel worthy of research by industrial manufacturers regarding its use in gas turbines and compression ignition engines, but this is not the case for spark ignition engines, where the main research is found in academic sites. Early research performed by Cornelius et al. [184] and Starkman et al. [185] already provided a clear insight into the modifications required when using this gas in SI engines, such as the need for increasing compression ratio and using supercharges due to the lower energy content of this molecule, the lower air-to-fuel ratio to attain stoichiometric conditions, and the need for partial ammonia dissociation to release hydrogen (or direct hydrogen addition), which is critical to attain stable performance under different engine loading. Hydrogen enrichment or increased intake pressure may serve as suitable measurements to attain operation with satisfactory combustion efficiency [50,186].
Natural gas engines are more suitable for retrofitting to ammonia–hydrogen operation due to their higher compression ratio and the lower dependence on fuel laminar flame speed of their combustion chamber [187]. However, there is a limit to the addition of hydrogen in the mixture based on the risk of backfire and wall heat losses [188]. Low levels of hydrogen addition allow stability improvement, acting as an ignition promoter and increasing laminar flame speed, but this single factor does not fully explain the improvement in operation achieved [189]. The high octane number of ammonia (130) makes the engine operate under higher compression ratios than the one used for gasoline [190], and the different burning characteristics of hydrogen and ammonia allow for attaining a mixture proportion capable of reaching a similar performance regarding work output to that obtained with gasoline [191]. This approach’s main disadvantage is the need for separate fuel storing tanks, making the system too complex for onboard applications. However, in the case of liquid and gaseous supplementary fuels, the technological configuration resembles that of the already tested technology for LPG and petrol passenger cars.
Studies carried out by Grannell et al. [192] using a Cooperative Fuel Research (CFR) engine showed that ammonia could be used in up to 70–30% ammonia–gasoline mixtures, although under idle conditions, the engine needed to be operated with gasoline as single fuel. Ryu et al. [193] indicated that gaseous injection of ammonia is preferred because of the high value of the latent heat of vaporization (1370 kJ/kg) compared with that of gasoline (348.7 kJ/kg), causing a significant reduction in in-cylinder temperature. In addition, the low flame speed of ammonia translates into an increment in combustion duration, thus requiring a greater advancement in spark timing than that applied for typical SI engines [194].
An alternative to avoid using two separate storage fuel systems is to generate hydrogen from the ammonia decomposition. The cracking of ammonia has been studied by several authors [195,196,197], proposing the use of thermal catalytic reactors that allow reduction in reaction temperature. Catalysts commonly employed contain noble metals (Rh, Ru, Pd, and Pt, among others), but Fe and Ni have also been tested in order to reduce costs and dope the catalyst with rare-earth metals to increase activity, in addition to the use of bimetallic configurations [198,199]. The industrial application of this technology at a large scale was reviewed by Spatolisano et al. [200], analyzing the implications of technology deployment. Many technologies currently developed are still in the experimental stage; thus, information regarding long-term performance, economics, and specific environmental impacts are scarce [201]. Testing at a precommercial stage is necessary for technical maturity before large-scale implementation.
The ammonia cracking reaction is endothermic. Therefore, onboard applications may benefit from using exhaust combustion gases, which can provide the heat needed to release hydrogen, but at the cold start of the engine, external heaters are necessary. Comotti and Frigo [202] proposed using a Ru-based catalyst with electric heaters in the internal section of the reactor, and the outer section used the exhaust gases as heat supply. Their system allowed the use of ammonia as a single fuel in SI engines operating successfully under different loading conditions. One crucial aspect to consider is that when hydrogen is produced from ammonia cracking, the extra amount of N2 introduced into the cylinder should be taken into account to optimize operating conditions.
Table 2 shows some of the studies carried out using ammonia as fuel in spark ignition engines. The use of ammonia as the only fuel in a spark ignition engine is practically unfeasible due to the difficulty of ignition, low flame stability, combustion efficiency, and power delivery, so dual-fuel configuration seems to be the best option. Using hydrogen as a dual fuel in proportions between 10 and 15% allows for solving these problems [188,189]. The required hydrogen may be obtained from an additional storage tank or the partial dissociation of ammonia; in any case, it is necessary to use systems such as EGR to reduce ammonia and NO emissions [203]. Other fuels have also been evaluated in dual configuration with ammonia. Tutak et al. [204] used DME as a complement to ammonia, allowing stable operation to be achieved. Oh et al. [205] reported that using DME along with methane slightly decreased braking power.

Table 2.
Studies evaluating ammonia combustion in spark ignition engines using different ignition promoters.
5.3. Compression Ignition Engines
Compression ignition engines apply a higher compression ratio and, thus, achieve fuel ignition. Since the fuel is directly injected into the combustion chamber after compression, knock limitations are eliminated, fuel efficiency is high, and fuel consumption can be optimized to provide power. However, the direct injection of the fuel causes diffusion combustion, which translates into high localized temperatures, increasing the emission of NOx and particles. For this reason, it is said that CI engines, although highly efficient, have an emission problem [210]. Fortunately, advances in engine design and emission abatement technologies have guaranteed that post-combustion gases are adequately treated. Exhaust gas recirculation decreases combustion temperature, thus reducing thermal NOx production. Lean NOx trap technology has been developed to trap and reduce NOx compounds under lean operating conditions. Selective catalytic reactors use a nitrogen compound to transform NOx into N2, and particle filters are incorporated to remove soot [211,212]. Different technological developments have allowed diesel engines to keep playing a fundamental role in energy production and transport. The high energy density of diesel fuel (35.86 MJ/L [213]) makes it the ideal choice for heavy road transport, but it also makes difficult the task of finding a suitable alternative due to the low storage volume needed for onboard applications and the high efficiency reached by these type of engines.
After the initial work of Cornelius et al. [184], Gray et al. [214], and Starkman et al. [215], the studies of Pearsall and Garabedian [216] focused on using ammonia in diesel engines. However, in this case, dual-fuel engines were the best alternative for attaining an acceptable output by controlling ignition thanks to the injection of diesel fuel. Reiter and Kong [217] tested a dual-fuel (ammonia–diesel) engine and reported that NOx emissions did not increase, despite the presence of ammonia, as long as energy substitution attained by ammonia did not reach 60%. However, in a later study, the same authors [218] lowered the ammonia energy supply in the mixture below 40%. Otherwise, NOx emissions would increase significantly due to fuel-bound emissions. These authors also reported high ammonia slip (1000–3000 ppmv) in exhaust gases, which may be regarded as disappointing news for this technology.
The experimental and demonstration works have been extensive in the last decade due to the impact of greenhouse gas emissions and the feasibility of using a noncarbon gas for which industrial technology is already available. Reviews about the use of ammonia as fuel in compression ignition engines have been carried out by Dimitriou and Javaid, [46], Kurien and Mittal [23], and Voniati et al. [219], where it was reported the need to install several after-treatment devices for emission control, and this requirement also limits marine, power generation, and heavy-duty applications. In addition, the formation of N2O, which has a much greater global warming impact, is one of the main obstacles to surpass when considering the use of ammonia in ICEs [220].
The variety of options currently being considered to reduce CO2 emissions may add optimism and give hope to a future free of carbon fossil fuels, but it requires significant efforts from researchers and industry. It also comes along with an adverse effect by creating uncertainty regarding the future of the dominant technology and the limited space available in fuel stations and cargo ports since this variety of options also implies having installations for electrification, LPG, hydrogen, ammonia, and methane storage, which has been recently incorporated to the maritime transport sector, thus resulting in an excessive complexity of infrastructure. Space limitations can make it unfeasible for different technologies to coexist. As an example, Imhoff et al. [221] studied the feasibility of implementing ammonia power trains in current vessels, indicating that redesign and reconfiguration efforts are needed but achievable; thus, ammonia-fuel ships may represent a feasible alternative. However, the maritime transport sector needs the certainty that an investment, which may be extremely high, will stay long enough to be worth it. Balci et al. [222] studied critical factors for successfully implementing ammonia as an alternative fuel in shipping. They indicated that adopting green ammonia involves several complexities regarding safety regulation, storage capacity, and reliable renewable energy production, in addition to the evident claim of engine improvements. Resources for increasing research and development may be derived from stakeholders and carbon taxing. The intrinsic concatenation of events will aid in further developing infrastructures; that is, the development of efficient ammonia propulsion engines would aid in reducing operating costs, thus increasing ammonia demand and aiding in the development of port infrastructure.
Table 3 lists different studies dealing with the use of ammonia as fuel in ICEs. The risks of using anhydrous ammonia and pressurization tanks may be avoided if other forms of ammonia are considered. Schönborn [223] proposed the use of water ammonia solution in diesel engines. This way, ammonia can be stored in liquid form under ambient conditions, reducing the risk of vapor ammonia release. The author analyzed the behavior of a diesel engine when injecting a 25% ammonia solution as fuel using numerical simulation. The compression ratio needed to be increased to 27 to attain ignition of the ammonia solution since the high water content creates a dilution effect, making ignition possible only if higher energy is added. However, the dilution effect also allowed a reduction in NOx emissions. Therefore, in addition to facilitating storage, other benefits are associated with this approach, whereas improving ignition may be achievable by adding specific promoters such as hydrogen.

Table 3.
Studies evaluating ammonia combustion in compression ignition engines using dual-fuel configuration and ignition promoters.
Hydrogen has also been widely studied as an ignition promoter when testing compression ignition engines, but also methanol, ammonia nitrite, and diesel fuel (n-heptane in simulation runs) [228,229,230,231]. Although modifications in fuel injection strategies are needed, ammonia–diesel dual-fuel engines may reduce CO2 emissions by 90% and NOx emissions by 70% compared with original diesel engines [232]. Other organic solvents may also be proposed as a liquid phase for storing ammonia without causing detriments in combustion characteristics, such as for the case of water, with methanol being considered a better candidate [233]. Other alternatives proposed the use of spark-assisted ICEs to overcome poor ignition characteristics [234] or changing the injection strategy by allowing the entrance of a small quantity of ammonia that will aid in main fuel ignition, thanks to the precombustion stage during the compression cycle, thus increasing temperature and pressure and facilitating ammonia ignition [235]. Scharl and Sattelmayer [236] studied the presence of diesel-piloted injections and concluded that reliable ignition of ammonia was possible when adding a small fraction of energy as piloted injected fuel (3.2% of LHV based on loading conditions).
6. Conclusions
The aim of a net-zero carbon emission by 2050 is a desirable goal, but this target needs to be carefully evaluated since implications associated with the decarbonization of the economy bring along an increase in costs, which may create market distortions. Several alternatives are currently under development for reducing the use of fossil fuels. However, many of these options result in an increase in process energy demand. Electrification and electricity production from renewable sources aid in achieving decarbonization goals. Impacts regarding mineral extraction, battery recycling, and uncertainty in renewable energy production need careful evaluation. Given the immediacy of the targets, it seems more reasonable to concentrate efforts on well-known and worldwide applied technologies.
Ammonia as an alternative fuel has the main advantage of great research in large-scale production, storage, and handling. Although several aspects regarding emissions need to be addressed, vast research exists in technologies capable of mitigating combustion pollutants. Another matter that may be a cause of concern is the effect of massive ammonia use for other applications besides agriculture. The expected increase in price resulting from the higher costs in production chains caused by the adoption of green hydrogen in the manufacturing process, as well as the pressure induced by growing ammonia demand, are factors that should not be overlooked. These factors can have a significant impact on human wellbeing and the food supply chain.
There is still a long way to go before attaining a feasible solution to decarbonizing the economy. The use of ammonia may be one of them, but in the short term, it seems more reasonable to focus on reducing CO2 emissions by reducing fuel consumption and optimizing current technologies rather than forcing a complete shift towards electrification of the transport sector, which requires adaptation to a completely different technology, raising costs without the certainty of how production cycles and critical materials will be managed. Electrification can help reduce pollution in some urban regions. However, other alternatives, such as hybridization and current highly efficient combustion engines, can be more effective in achieving a global reduction in emissions by already having global technological deployment.
Author Contributions
Conceptualization, X.G. and R.G.; methodology, R.G.; validation, X.G.; formal analysis, R.G.; investigation, X.G.; resources, X.G.; data curation, R.G.; writing—original draft preparation, X.G.; writing—review and editing, R.G.; visualization, R.G.; supervision, X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 2050 Long-Term Strategy. Available online: https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2050-long-term-strategy_en (accessed on 12 December 2023).
- Total Energy Consumption. Available online: https://yearbook.enerdata.net/total-energy/world-consumption-statistics.html (accessed on 12 December 2023).
- Huang, Y.; Kuldasheva, Z.; Bobojanov, S.; Djalilov, B.; Salahodjaev, R.; Abbas, S. Exploring the links between fossil fuel energy consumption, industrial value-added, and carbon emissions in G20 countries. Environ. Sci. Pollut. Res. 2023, 30, 10854–10866. [Google Scholar] [CrossRef]
- Bakhtiari, H.; Naghizadeh, R.A. Multi-criteria optimal sizing of hybrid renewable energy systems including wind, photovoltaic, battery, and hydrogen storage with ɛ-constraint method. IET Renew. Power Gener. 2018, 12, 883–892. [Google Scholar] [CrossRef]
- González, R.; Cabeza, I.O.; Casallas-Ojeda, M.; Gómez, X. Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy. Environments 2023, 10, 82. [Google Scholar] [CrossRef]
- Touati, F.A.; Al-Hitmi, M.A.; Bouchech, H.J. Study of the effects of dust, relative humidity, and temperature on solar PV performance in Doha: Comparison between monocrystalline and amorphous PVS. Int. J. Green Energy 2013, 10, 680–689. [Google Scholar] [CrossRef]
- Salimi, H.; Mirabdolah Lavasani, A.; Ahmadi-Danesh-Ashtiani, H.; Fazaeli, R. Effect of dust concentration, wind speed, and relative humidity on the performance of photovoltaic panels in Tehran. Energy Sources Part A 2023, 45, 7867–7877. [Google Scholar] [CrossRef]
- Satkauskas, I.; Maack, J.; Reynolds, M.; Sigler, D.; Panda, K.; Jones, W. Simulating Impacts of Extreme Events on Grids with High Penetrations of Wind Power Resources. In Proceedings of the IEEE/PES Transmission and Distribution Conference and Exposition (T&D), New Orleans, LA, USA, 25–28 April 2022; pp. 1–5. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- Alexander, S.; Floyd, J. The Political Economy of Deep Decarbonization: Tradable Energy Quotas for Energy Descent Futures. Energies 2020, 13, 4304. [Google Scholar] [CrossRef]
- Ye, D.; Tsang, S.C.E. Prospects and challenges of green ammonia synthesis. Nat. Synth. 2023, 2, 612–623. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Abánades, A. Comparative Analysis of Energy and Exergy Performance of Hydrogen Production Methods. Entropy 2020, 22, 1286. [Google Scholar] [CrossRef]
- Huo, J.; Wang, Z.; Oberschelp, C.; Guillén-Gosálbez, G.; Hellweg, S. Net-zero transition of the global chemical industry with CO2-feedstock by 2050: Feasible yet challenging. Green Chem. 2023, 25, 415–430. [Google Scholar] [CrossRef]
- Bockris, J.O.; Nagy, Z. The Hydrogen Economy. In Electrochemistry for Ecologists; Springer: Boston, MA, USA, 1974. [Google Scholar] [CrossRef]
- Yap, J.; McLellan, B. A Historical Analysis of Hydrogen Economy Research, Development, and Expectations, 1972 to 2020. Environments 2023, 10, 11. [Google Scholar] [CrossRef]
- Ogden, J.M. Hydrogen: The Fuel of the Future? Phys. Today 2002, 55, 69–75. [Google Scholar] [CrossRef]
- Crabtree, G.W.; Dresselhaus, M.S.; Buchanan, M.V. The Hydrogen Economy. Phys. Today 2004, 57, 39–44. [Google Scholar] [CrossRef]
- Chanchetti, L.F.; Leiva, D.R.; Lopes de Faria, L.I.; Ishikawa, T.T. A scientometric review of research in hydrogen storage materials. Int. J. Hydrogen Energy 2020, 45, 5356–5366. [Google Scholar] [CrossRef]
- Hydrogen Fueled Gas Turbines. Available online: https://www.gevernova.com/gas-power/future-of-energy/hydrogen-fueled-gas-turbines?utm_campaign=h2&utm_medium=cpc&utm_source=google&utm_content=rsa&utm_term=Ge%20gas%20turbine%20hydrogen&gad_source=1&gclid=CjwKCAiApuCrBhAuEiwA8VJ6JkR3-lQPrF-s1tt3rgZ46itzqPULaD2Sr6OxvQAyNnk3cZkRnRca4RoCoKUQAvD_BwE (accessed on 12 December 2023).
- Airbus Reveals Hydrogen-Powered Zero-Emission Engine. Available online: https://www.airbus.com/en/newsroom/press-releases/2022-11-airbus-reveals-hydrogen-powered-zero-emission-engine (accessed on 17 December 2023).
- Airbus and CFM International to Pioneer Hydrogen Combustion Technology. Available online: https://www.airbus.com/en/newsroom/press-releases/2022-02-airbus-and-cfm-international-to-pioneer-hydrogen-combustion (accessed on 17 December 2023).
- Toyota. Available online: https://www.toyota.es/?gad_source=1&gclid=CjwKCAiApuCrBhAuEiwA8VJ6JvdD0b3ceOoBNrMc-iiOjzAn4NlXqK0WQOF8_CsaJ_IdrW6NkvsXdxoCWvQQAvD_BwE&gclsrc=aw.ds (accessed on 17 December 2023).
- Kurien, C.; Mittal, M. Review on the production and utilization of green ammonia as an alternate fuel in dual-fuel compression ignition engines. Energy Convers. Manag. 2022, 251, 114990. [Google Scholar] [CrossRef]
- Ershov, M.A.; Savelenko, V.D.; Makhova, U.A.; Makhmudova, A.E.; Zuikov, A.V.; Kapustin, V.M.; Abdellatief, T.M.; Burov, N.O.; Geng, T.; Abdelkareem, M.A.; et al. Current challenge and innovative progress for producing HVO and fame biodiesel fuels and their applications. Waste Biomass Valorization 2023, 14, 505–521. [Google Scholar] [CrossRef]
- Elía, M.F.; de la Torre, O.; Larraz, R.; Frontela, J. Cepsa: Towards The Integration of Vegetable Oils and Lignocellulosic Biomass into Conventional Petroleum Refinery Processing Units. In Industrial Biorenewables; Domínguez de María, P., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 141–174. [Google Scholar] [CrossRef]
- Power Your Future with Neste MY Renewable Diesel™ (HVO100). Available online: https://www.neste.com/products/all-products/renewable-road-transport/neste-my-renewable-diesel#1ff8d5dd (accessed on 19 December 2023).
- Dimitriadis, A.; Natsios, I.; Dimaratos, A.; Katsaounis, D.; Samaras, Z.; Bezergianni, S.; Lehto, K. Evaluation of a hydrotreated vegetable oil (HVO) and effects on emissions of a passenger car diesel engine. Front. Mech. Eng. 2018, 4, 7. [Google Scholar] [CrossRef]
- Padella, M.; O’Connell, A.; Prussi, M. What is still Limiting the Deployment of Cellulosic Ethanol? Analysis of the Current Status of the Sector. Appl. Sci. 2019, 9, 4523. [Google Scholar] [CrossRef]
- Tamburini, E.; Gaglio, M.; Castaldelli, G.; Fano, E.A. Is Bioenergy Truly Sustainable When Land-Use-Change (LUC) Emissions Are Accounted for? The Case-Study of Biogas from Agricultural Biomass in Emilia-Romagna Region, Italy. Sustainability 2020, 12, 3260. [Google Scholar] [CrossRef]
- Greenhouse Gas Emissions from Transport in Europe. Available online: https://www.eea.europa.eu/en/analysis/indicators/greenhouse-gas-emissions-from-transport (accessed on 19 December 2023).
- Hu, K.; Chen, Y. Technological growth of fuel efficiency in European automobile market 1975–2015. Energy Policy 2016, 98, 142–148. [Google Scholar] [CrossRef]
- Dosier de Prensa—Mazda Motor Europe, Mazda e-SKYACTIV X. Available online: https://es.mazda-press.com/api/assets/download/167c8a2c-bb33-4dbc-ac38-4144d1de6d89_Pdf?isDownload=false (accessed on 19 December 2023).
- Average Age of the EU Vehicle Fleet, by Country. Available online: https://www.acea.auto/figure/average-age-of-eu-vehicle-fleet-by-country/#:~:text=Passenger%20cars%20are%20now%20on,of%20the%20EU%20member%20states (accessed on 19 December 2023).
- Global Fleet the Executive: Wikifleet. Available online: https://www.globalfleet.com/en/wikifleet (accessed on 19 December 2023).
- Sacchi, R.; Bauer, C.; Cox, B.; Mutel, C. When, where and how can the electrification of passenger cars reduce greenhouse gas emissions? Renew. Sustain. Energy Rev. 2022, 162, 112475. [Google Scholar] [CrossRef]
- Andersson, Ö.; Börjesson, P. The greenhouse gas emissions of an electrified vehicle combined with renewable fuels: Life cycle assessment and policy implications. Appl. Energy 2021, 289, 116621. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- European Biogas Association. EBA Statistical Report 2021; European Biogas Association: Brussels, Belgium, 2021; Available online: https://www.europeanbiogas.eu/wp-content/uploads/2021/11/EBA-STATISTICAL-REPORT-2021-SHORT-VERSION.pdf (accessed on 20 December 2023).
- Eurostats: Natural Gas Supply Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Natural_gas_supply_statistics#Consumption_trends (accessed on 19 December 2023).
- Sulewski, P.; Ignaciuk, W.; Szymańska, M.; Wąs, A. Development of the Biomethane Market in Europe. Energies 2023, 16, 2001. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; Gil, M.V.; Gómez, X. Syngas Fermentation: Cleaning of Syngas as a Critical Stage in Fermentation Performance. Fermentation 2023, 9, 898. [Google Scholar] [CrossRef]
- Maitlo, G.; Ali, I.; Mangi, K.H.; Ali, S.; Maitlo, H.A.; Unar, I.N.; Pirzada, A.M. Thermochemical Conversion of Biomass for Syngas Production: Current Status and Future Trends. Sustainability 2022, 14, 2596. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 Recycling to Dimethyl Ether: State-of-the-Art and Perspectives. Molecules 2018, 23, 31. [Google Scholar] [CrossRef]
- Giuliano, A.; Freda, C.; Catizzone, E. Techno-Economic Assessment of Bio-Syngas Production for Methanol Synthesis: A Focus on the Water–Gas Shift and Carbon Capture Sections. Bioengineering 2020, 7, 70. [Google Scholar] [CrossRef]
- Kroch, E. Ammonia—A fuel for motor buses. J. Inst. Pet. 1945, 31, 213–223. Available online: https://claverton-energy.com/cms4/wp-content/files/NH3_bus_1945_JInstPetrol31_Pg213.pdf (accessed on 19 December 2023).
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Statista: Production of Ammonia Worldwide from 2010 to 2023. Available online: https://www.statista.com/statistics/1266378/global-ammonia-production/#:~:text=Ammonia production has remained fairly,approximately 64.6 million metric tons (accessed on 19 December 2023).
- Brown, A.E.; Hammerton, J.M.; Camargo-Valero, M.A.; Ross, A.B. Integration of Hydrothermal Carbonisation and Anaerobic Digestion for the Energy Valorisation of Grass. Energies 2022, 15, 3495. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Rousselle, C. Combustion Characteristics of Ammonia in a Modern Spark-Ignition Engine; No. 2019-24-0237; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2019. [Google Scholar] [CrossRef]
- Unlocking Ammonia’s Potential for Shipping. Available online: https://www.man-es.com/discover/two-stroke-ammonia-engine?gad_source=1&gclid=CjwKCAiA-P-rBhBEEiwAQEXhHwmTLNnS5Oc5d-BzX3NU_aCBfD0ieFAmPFtUdECGAzLdvRQvM2qdGBoCpZQQAvD_BwE (accessed on 19 December 2023).
- Fumanelli, F. Toyota GT86-R Marangoni Eco Explorer: Sport and Technology in the New 2013 Show Car, Fitted with Marangoni “M-Power EvoRed” Tyres. 2013. Available online: https://marangonipress.com/wp-content/uploads/2019/07/Toyota_Marangoni_GT86-R_Eco_Explorer_EN.pdf (accessed on 19 December 2023).
- Michikawauchi, R.; Ito, Y.; Iwatani, K.; Tanno, S. Ammonia Burning Internal Combustion Engine. U.S. Patent 8,904,994 B2, 9 December 2014. Available online: https://ppubs.uspto.gov/dirsearch-public/print/downloadPdf/8904994 (accessed on 20 December 2023).
- Tange, K.; Nakamura, N.; Nakanishi, H.; Arikawa, H. Hydrogen Generator, Ammonia-Burning Internal Combustion Engine, and Fuel Cell. U.S. Patent 9,506,400, 29 November 2016. Available online: https://ppubs.uspto.gov/dirsearch-public/print/downloadPdf/9506400 (accessed on 21 December 2023).
- Kable, G. GAC and Toyota Develop Ammonia Engine for 90% CO2 Reduction. Available online: https://www.autocar.co.uk/car-news/new-cars/gac-and-toyota-develop-ammonia-engine-90-co2-reduction (accessed on 20 December 2023).
- Gubbi, S.; Cole, R.; Emerson, B.; Noble, D.; Steele, R.; Sun, W.; Lieuwen, T. Air Quality Implications of Using Ammonia as a Renewable Fuel: How Low Can NOx Emissions Go? ACS Energy Lett. 2023, 8, 4421–4426. [Google Scholar] [CrossRef]
- Erdemir, D.; Dincer, I. A perspective on the use of ammonia as a clean fuel: Challenges and solutions. Int. J. Energy Res. 2021, 45, 4827–4834. [Google Scholar] [CrossRef]
- Hasan, M.H.; Mahlia, T.M.; Mofijur, M.; Rizwanul Fattah, I.M.; Handayani, F.; Ong, H.C.; Silitonga, A.S. A Comprehensive Review on the Recent Development of Ammonia as a Renewable Energy Carrier. Energies 2021, 14, 3732. [Google Scholar] [CrossRef]
- Statista Research Department: Production Capacity of Ammonia Worldwide from 2018 to 2022, with a Forecast for 2026 and 2030. Available online: https://www.statista.com/statistics/1065865/ammonia-production-capacity-globally/#:~:text=The global production capacity of,million metric tons by 2030 (accessed on 22 December 2023).
- Basf: BASF Hystory We Create Chemistry 1865–2015. Available online: https://www.basf.com/global/images/about-us/history/BASF_Chronik_Gesamt_en.pdf.assetdownload.pdf (accessed on 22 December 2023).
- Travis, A.S. Luigi Casale’s enterprise: Pioneer of global catalytic high-pressure industrial chemistry. Catal. Today 2022, 387, 4–8. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I.; Zamfirescu, C.; Vezina, G.; Raso, F. Comparative life cycle assessment of various ammonia production methods. J. Clean. Prod. 2016, 135, 1379–1395. [Google Scholar] [CrossRef]
- López-Fernández, E.; Sacedón, C.G.; Gil-Rostra, J.; Yubero, F.; González-Elipe, A.R.; de Lucas-Consuegra, A. Recent Advances in Alkaline Exchange Membrane Water Electrolysis and Electrode Manufacturing. Molecules 2021, 26, 6326. [Google Scholar] [CrossRef]
- Partidário, P.; Aguiar, R.; Martins, P.; Rangel, C.M.; Cabrita, I. The hydrogen roadmap in the Portuguese energy system—Developing the P2G case. Int. J. Hydrogen Energy 2020, 45, 25646–25657. [Google Scholar] [CrossRef]
- NEL Hydrogen Electrolizers. Available online: https://nelhydrogen.com/water-electrolysers-hydrogen-generators/?ppc_keyword=water electrolyzer&gclid=Cj0KCQiAm4WsBhCiARIsAEJIEzV6AsnlJMZVeXF022ipll_r21bThveR82DVWTJf4v2YeETcE9vQNwgaAtInEALw_wcB (accessed on 10 January 2024).
- Mcphy Electrolyzers. Available online: https://mcphy.com/en/ (accessed on 10 January 2024).
- Xia, Y.; Cheng, H.; He, H.; Wei, W. Efficiency and consistency enhancement for alkaline electrolyzers driven by renewable energy sources. Commun. Eng. 2023, 2, 22. [Google Scholar] [CrossRef]
- Haleem, A.A.; Huyan, J.; Nagasawa, K.; Kuroda, Y.; Nishiki, Y.; Kato, A.; Nakai, T.; Araki, T.; Mitsushima, S. Effects of operation and shutdown parameters and electrode materials on the reverse current phenomenon in alkaline water analyzers. J. Power Sources 2022, 535, 231454. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, S.M.; Kim, K.S.; Kim, H.Y.; Kwon, J.; Lee, J.; Cho, H.-S.; Kim, Y.T. Cathodic protection system against a reverse-current after shut-down in zero-gap alkaline water electrolysis. JACS Au 2022, 2, 2491–2500. [Google Scholar] [CrossRef]
- Chau, K.; Djire, A.; Khan, F. Review and analysis of the hydrogen production technologies from a safety perspective. Int. J. Hydrogen Energy 2022, 47, 13990–14007. [Google Scholar] [CrossRef]
- Fertiberia: Planta de Hidrógeno, Amoniaco y Fertilizantes Verdes. Available online: https://www.fertiberia.com/amoniacoverde/puertollano-proyecto-h2f-planta-de-hidrogeno-amoniaco-y-fertilizantes-verdes/ (accessed on 10 January 2024).
- Harrou, F.; Sun, Y.; Taghezouit, B.; Dairi, A. Artificial Intelligence Techniques for Solar Irradiance and PV Modeling and Forecasting. Energies 2023, 16, 6731. [Google Scholar] [CrossRef]
- Lehner, B.; Döll, P.; Alcamo, J.; Henrichs, T.; Kaspar, F. Estimating the impact of global change on flood and drought risks in Europe: A continental, integrated analysis. Clim. Chang. 2006, 75, 273–299. [Google Scholar] [CrossRef]
- Barbeta, A.; Mejía-Chang, M.; Ogaya, R.; Voltas, J.; Dawson, T.E.; Peñuelas, J. The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Glob. Chang. Biol. 2015, 21, 1213–1225. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Dresp, S.; Thanh, T.N.; Klingenhof, M.; Brückner, S.; Hauke, P.; Strasser, P. Efficient direct seawater electrolysers using selective alkaline NiFe-LDH as OER catalyst in asymmetric electrolyte feeds. Energy Environ. Sci. 2020, 13, 1725–1729. [Google Scholar] [CrossRef]
- Rao, P.; Morrow III, W.R.; Aghajanzadeh, A.; Sheaffer, P.; Dollinger, C.; Brueske, S.; Cresko, J. Energy considerations associated with increased adoption of seawater desalination in the United States. Desalination 2018, 445, 213–224. [Google Scholar] [CrossRef]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.; Styring, P. Sustainable ammonia production processes. Front. Energy Res. 2021, 9, 34. [Google Scholar] [CrossRef]
- Rezaei, M.; Mostafaeipour, A.; Qolipour, M.; Arabnia, H.R. Hydrogen production using wind energy from sea water: A case study on Southern and Northern coasts of Iran. Energy Environ. 2018, 29, 333–357. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Attas, T.; Roy, S.; Rahman, M.M.; Ghaffour, N.; Thangadurai, V.; Larter, S.; Hu, J.; Ajayan, P.M.; Kibria, M.G. Seawater electrolysis for hydrogen production: A solution looking for a problem? Energy Environ. Sci. 2021, 14, 4831–4839. [Google Scholar] [CrossRef]
- Calado, G.; Castro, R. Hydrogen Production from Offshore Wind Parks: Current Situation and Future Perspectives. Appl. Sci. 2021, 11, 5561. [Google Scholar] [CrossRef]
- Saygin, D.; Blanco, H.; Boshell, F.; Cordonnier, J.; Rouwenhorst, K.; Lathwal, P.; Gielen, D. Ammonia Production from Clean Hydrogen and the Implications for Global Natural Gas Demand. Sustainability 2023, 15, 1623. [Google Scholar] [CrossRef]
- Hamulczuk, M.; Pawlak, K.; Stefańczyk, J.; Gołębiewski, J. Agri-Food Supply and Retail Food Prices during the Russia–Ukraine Conflict’s Early Stage: Implications for Food Security. Agriculture 2023, 13, 2154. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Maréchal, F.; Desideri, U. Techno-economic comparison of green ammonia production processes. Appl. Energy 2020, 259, 114135. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Travis, A.S.; Lefferts, L. 1921–2021: A Century of Renewable Ammonia Synthesis. Sustain. Chem. 2022, 3, 149–171. [Google Scholar] [CrossRef]
- Enaex. Available online: https://www.enaex.com/pe/us/enaex-peru-history/ (accessed on 10 January 2024).
- Hong, J.; Prawer, S.; Murphy, A.B. Plasma catalysis as an alternative route for ammonia production: Status, mechanisms, and prospects for progress. ACS Sustain. Chem. Eng. 2018, 6, 15–31. [Google Scholar] [CrossRef]
- Meloni, E.; Cafiero, L.; Martino, M.; Palma, V. Structured Catalysts for Non-Thermal Plasma-Assisted Ammonia Synthesis. Energies 2023, 16, 3218. [Google Scholar] [CrossRef]
- Long, J.; Chen, S.; Zhang, Y.; Guo, C.; Fu, X.; Deng, D.; Xiao, J. Direct Electrochemical Ammonia Synthesis from Nitric Oxide. Angew. Chem. Int. Ed. 2020, 59, 9711–9718. [Google Scholar] [CrossRef]
- Ahmed, H.S.; Yahya, Z.; Ali Khan, W.; Faraz, A. Sustainable Pathways to Ammonia: A Comprehensive Review of Green Production Approaches. Clean Energy 2024, 8, 60–72. [Google Scholar] [CrossRef]
- Shen, H.; Choi, C.; Masa, J.; Li, X.; Qiu, J.; Jung, Y.; Sun, Z. Electrochemical ammonia synthesis: Mechanistic understanding and catalyst design. Chem 2021, 7, 1708–1754. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Zhou, J.; Liu, F.; Hao, F.; Fan, Z. Electrochemical Nitrate Reduction: Ammonia Synthesis and the Beyond. Adv. Mater. 2024, 36, 2304021. [Google Scholar] [CrossRef]
- Yang, D.; Chen, T.; Wang, Z. Electrochemical Reduction of Aqueous Nitrogen (N2) at a Low Overpotential on (110)-Oriented Mo Nanofilm. J. Mater. Chem. A 2017, 5, 18967–18971. [Google Scholar] [CrossRef]
- Sekhar, S.J.; Al-Shahri, A.S.A.; Glivin, G.; Le, T.H.T.; Mathimani, T. A Critical Review of the State-of-the-Art Green Ammonia Production Technologies—Mechanism, Advancement, Challenges, and Future Potential. Fuel 2024, 358, 130307. [Google Scholar] [CrossRef]
- Kong, J.; Choi, J.; Park, H.S. Advantages and Limitations of Different Electrochemical NH3 Production Methods under Ambient Conditions: A Review. Curr. Opin. Electrochem. 2023, 39, 101292. [Google Scholar] [CrossRef]
- Final Report for a Study on Composition and Drivers of Energy Prices and Costs in Energy Intensive Industries: The Case of the Flat Glass Industry; Publications Office of the European Union: Luxembourg, 2014; Available online: https://op.europa.eu/en/publication-detail/-/publication/b43ca37c-ae26-49f3-9341-7558a75d52da (accessed on 13 June 2024).
- An, L.; Zhang, Z.; Liu, G.; Liu, W.; Fu, Y.; Qu, D.; Liu, Y.; Hu, P.; Sun, Z. Recent Advances in Transition Metal Electrocatalysts for Effective Nitrogen Reduction Reaction under Ambient Conditions. EcoEnergy 2024, 1–29. [Google Scholar] [CrossRef]
- Chen, J.; Guan, B.; Zhuang, Z.; Zheng, C.; Zhou, J.; Su, T.; Chen, Y.; Zhu, C.; Hu, X.; Zhao, S.; et al. Recent Advances of Structure-Performance Relationship and Improvement Methods of Catalysts for Photochemical and Electrochemical Reduction of Nitrogen to Green Ammonia. Fuel 2024, 371 Pt B, 131928. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.J.; Kim, D.Y.; Yoo, C.Y.; Choi, J.W.; Kim, J.N.; Woo, Y.; Yoon, H.C.; Han, J.I. Electrochemical synthesis of ammonia from water and nitrogen: A lithium-mediated approach using lithium-ion conducting glass ceramics. ChemSusChem 2018, 11, 120–124. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Z.-W.; Gu, K.; Chen, C.; Liu, Y.; Wei, X.; Singh, C.V.; Wang, S. Hexagonal Cobalt Nanosheets for High-Performance Electrocatalytic NO Reduction to NH3. J. Am. Chem. Soc. 2023, 145, 6899–6904. [Google Scholar] [CrossRef]
- Shao, J.; Wei, P.; Wang, S.; Song, Y.; Fu, Y.; Li, R.; Zhang, X.; Wang, G.; Bao, X. Copper Oxide Nanosheets for Efficient Electrochemical Reduction of Nitric Oxide. Sci. China Mater. 2024, 67, 1876–1881. [Google Scholar] [CrossRef]
- Ouyang, L.; Liang, J.; Luo, Y.; Zheng, D.; Sun, S.; Liu, Q.; Hamdy, M.S.; Sun, X.; Ying, B. Recent Advances in Electrocatalytic Ammonia Synthesis. Chin. J. Catal. 2023, 50, 6–44. [Google Scholar] [CrossRef]
- Zou, X.; Chen, C.; Wang, C.; Zhang, Q.; Yu, Z.; Wu, H.; Zhuo, C.; Zhang, T.C. Combining Electrochemical Nitrate Reduction and Anammox for Treatment of Nitrate-Rich Wastewater: A Short Review. Sci. Total Environ. 2021, 800, 149645. [Google Scholar] [CrossRef]
- Shafiq, F.; Yang, L.; Zhu, W. Recent progress in the advanced strategies, rational designs, and engineering of electrocatalysts for nitrate reduction toward ammonia. Phys. Chem. Chem. Phys. 2024, 26, 11208–112016. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, S.; Li, X.; Chang, C.; Xie, M.; Xu, J.; Yang, Z. A robust metal-free electrocatalyst for nitrate reduction reaction to synthesize ammonia. Mater. Today Phys. 2021, 19, 100431. [Google Scholar] [CrossRef]
- Liu, H.; Park, J.; Chen, Y.; Qiu, Y.; Cheng, Y.; Srivastava, K.; Shanks, B.H.; Roling, L.T.; Li, W. Electrocatalytic nitrate reduction on oxide-derived silver with tunable selectivity to nitrite and ammonia. ACS Catal. 2021, 11, 8431–8442. [Google Scholar] [CrossRef]
- Zhao, R.; Yan, Q.; Yu, L.; Yan, T.; Zhu, X.; Zhao, Z.; Liu, L.; Xi, J. A Bi-Co Corridor Construction Effectively Improving the Selectivity of Electrocatalytic Nitrate Reduction toward Ammonia by Nearly 100%. Adv. Mater. 2023, 35, 2306633. [Google Scholar] [CrossRef]
- Yin, S.; Cao, R.; Han, Y.; Shang, J.; Zhang, J.; Jiang, W.; Liu, G. Electrocatalysts with Atomic-Level Site for Nitrate Reduction to Ammonia. J. Energy Chem. 2024, 96, 642–668. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Shi, R.; Waterhouse, G.I.; Zhang, T. Photocatalytic ammonia synthesis: Recent progress and future. EnergyChem 2019, 1, 100013. [Google Scholar] [CrossRef]
- Han, Q.; Jiao, H.; Xiong, L.; Tang, J. Progress and challenges in photocatalytic ammonia synthesis. Mater. Adv. 2021, 2, 564–581. [Google Scholar] [CrossRef]
- Milton, R.D.; Minteer, S.D. Nitrogenase bioelectrochemistry for synthesis applications. Acc. Chem. Res. 2019, 52, 3351–3360. [Google Scholar] [CrossRef]
- Rapson, T.D.; Gregg, C.M.; Allen, R.S.; Ju, H.; Doherty, C.M.; Mulet, X.; Giddey, S.; Wood, C.C. Insights into nitrogenase bioelectrocatalysis for green ammonia production. ChemSusChem 2020, 13, 4856–4865. [Google Scholar] [CrossRef]
- Rapson, T.D.; Wood, C.C. Analysis of the Ammonia Production Rates by Nitrogenase. Catalysts 2022, 12, 844. [Google Scholar] [CrossRef]
- Watanabe, Y.; Aoki, W.; Ueda, M. Ammonia Production Using Bacteria and Yeast toward a Sustainable Society. Bioengineering 2023, 10, 82. [Google Scholar] [CrossRef]
- Kosem, N.; Shen, X.F.; Ohsaki, Y.; Watanabe, M.; Song, J.T.; Ishihara, T. Photobiocatalytic conversion of solar energy to NH3 from N2 and H2O under ambient condition. Appl. Catal. B 2024, 342, 123431. [Google Scholar] [CrossRef]
- Veselovskaya, J.V.; Parunin, P.D.; Okunev, A.G. Catalytic process for methane production from atmospheric carbon dioxide utilizing renewable energy. Catal. Today 2017, 298, 117–123. [Google Scholar] [CrossRef]
- Navarro, J.C.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Policies and Motivations for the CO2 Valorization through the Sabatier Reaction Using Structured Catalysts. A Review of the Most Recent Advances. Catalysts 2018, 8, 578. [Google Scholar] [CrossRef]
- Korosec, K. Audi E-Gas Plant Uses CO<sub>2</sub>, Renewables to Make Fuel. Environment + Energy Leader 2012. Available online: https://www.environmentenergyleader.com/2012/12/audi-e-gas-plant-uses-co2-renewables-to-make-fuel/ (accessed on 12 January 2024).
- Hy2Gen Acquires e-Gas Plant and Project Pipeline of Kiwi AG in Germany; Formerly Audi e-Gas Plant. Available online: https://www.greencarcongress.com/2023/12/20231212-hy2gen.html#:~:text=In%202013%2C%20the%20plant%20in,%2Dto%2DeMethane%20plant%20today (accessed on 12 January 2024).
- Cai, T.; Zhao, D. Overview of Autoignition and Flame Propagation Properties for Ammonia Combustion. AIAA J. 2023, 61, 2754–2778. [Google Scholar] [CrossRef]
- Chiong, M.; Chong, C.T.; Ng, J.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of combustion technologies in the ammonia-fuelled engines. Energy Convers. Manag. 2021, 244, 114460. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Wang, S.; Guiberti, T.F.; Roberts, W.L. Review on the recent advances on ammonia combustion from the fundamentals to the applications. Fuel Commun. 2022, 10, 100053. [Google Scholar] [CrossRef]
- Liang, X.; Zheng, Z.; Zhang, H.; Wang, Y.; Yu, H. A Review of Early Injection Strategy in Premixed Combustion Engines. Appl. Sci. 2018, 9, 3737. [Google Scholar] [CrossRef]
- Alger, T.; Gingrich, J.; Roberts, C.; Mangold, B. Cooled exhaust-gas recirculation for fuel economy and emissions improvement in gasoline engines. Int. J. Engine Res. 2011, 12, 252–264. [Google Scholar] [CrossRef]
- Piqueras, P.; Morena, J.D.; Sanchis, E.J.; Pitarch, R. Impact of Exhaust Gas Recirculation on Gaseous Emissions of Turbocharged Spark-Ignition Engines. Appl. Sci. 2020, 10, 7634. [Google Scholar] [CrossRef]
- Verkamp, F.; Hardin, M.; Williams, J. Ammonia combustion properties and performance in gas-turbine burners. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1967; Volume 11, pp. 985–992. [Google Scholar] [CrossRef]
- Haputhanthri, S.O.; Maxwell, T.T.; Fleming, J.; Austin, C. Ammonia and gasoline fuel blends for spark ignited internal combustion engines. J. Energy Resour. Technol. 2015, 137, 062201. [Google Scholar] [CrossRef]
- Rodríguez, C.G.; Lamas, M.I.; Rodríguez, J.D.D.; Abbas, A. Multi-Criteria Analysis to Determine the Most Appropriate Fuel Composition in an Ammonia/Diesel Oil Dual Fuel Engine. J. Mar. Sci. Eng. 2023, 11, 689. [Google Scholar] [CrossRef]
- Elumalai, R.; Ravi, K. Optimization of ideal engine parameters to adopt ammonia and algae biodiesel for reactivity controlled compression ignition engine using response surface methodology. Proc. Inst. Mech. Eng. Part C 2024, 238, 2456–2473. [Google Scholar] [CrossRef]
- Ma, F.; Guo, L.; Li, Z.; Zeng, X.; Zheng, Z.; Li, W.; Zhao, F.; Yu, W. A Review of Current Advances in Ammonia Combustion from the Fundamentals to Applications in Internal Combustion Engines. Energies 2023, 16, 6304. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Hayakawa, A.; Kobayashi, H. Success of ammonia-fired, regenerator-heated, diffusion combustion gas turbine power generation and prospect of low NOx combustion with high combustion efficiency. In Proceedings of the ASME Power Conference, Charlotte, NC, USA, 26–30 June 2017; American Society of Mechanical Engineers: New York, NY, USA, 2017; Volume 57601, p. V001T04A026. [Google Scholar] [CrossRef]
- Gubbi, S.; Cole, R.; Emerson, B.; Noble, D.; Steele, R.; Sun, W.; Lieuwen, T. Evaluation of Minimum NOx Emission from Ammonia Combustion. J. Eng. Gas Turbines Power 2024, 146, 031023. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kim, H.; Kim, H.; Kang, S.; Ryu, J.; Shim, S. Reduction of NOx Emission from the Cement Industry in South Korea: A Review. Atmosphere 2021, 13, 121. [Google Scholar] [CrossRef]
- Miller, J.A.; Smooke, M.D.; Green, R.M.; Kee, R.J. Kinetic modeling of the oxidation of ammonia in flames. Combust. Sci. Technol. 1983, 34, 149–176. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Gutmark, E. Overview of fundamental kinetic mechanisms and emission mitigation in ammonia combustion. Chem. Eng. J. 2023, 458, 141391. [Google Scholar] [CrossRef]
- Shu, B.; Vallabhuni, S.; He, X.; Issayev, G.; Moshammer, K.; Farooq, A.; Fernandes, R. A shock tube and modeling study on the autoignition properties of ammonia at intermediate temperatures. Proc. Combust. Inst. 2018, 37, 205–211. [Google Scholar] [CrossRef]
- Karimkashi, S.; Tamadonfar, P.; Kaario, O.; Vuorinen, V. A Numerical Investigation on Effects of Hydrogen Enrichment and Turbulence on NO Formation Pathways in Premixed Ammonia/Air Flames. Combust. Sci. Technol. 2023, 1–30. [Google Scholar] [CrossRef]
- Rocha, R.C.; Zhong, S.; Xu, L.; Bai, X.S.; Costa, M.; Cai, X.; Kim, H.; Brackmann, C.; Li, Z.; Alden, M. Structure and laminar flame speed of an ammonia/methane/air premixed flame under varying pressure and equivalence ratio. Energy Fuels 2021, 35, 7179–7192. [Google Scholar] [CrossRef]
- Khalil, A.T.; Manias, D.M.; Kyritsis, D.C.; Goussis, D.A. NO formation and autoignition dynamics during combustion of H2O-diluted NH3/H2O2 mixtures with air. Energies 2021, 14, 84. [Google Scholar] [CrossRef]
- Kim, N.; Lee, M.; Park, J.; Park, J.; Lee, T. A Comparative Study of NOx Emission Characteristics in a Fuel Staging and Air Staging Combustor Fueled with Partially Cracked Ammonia. Energies 2022, 15, 9617. [Google Scholar] [CrossRef]
- Woo, M.; Choi, B.C. Numerical study on fuel-NO formation characteristics of ammonia-added methane fuel in laminar non-premixed flames with oxygen/carbon dioxide oxidizer. Energy 2021, 226, 120365. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Nozari, H.; Karabeyoğlu, A. Numerical study of combustion characteristics of ammonia as a renewable fuel and establishment of reduced reaction mechanisms. Fuel 2015, 159, 223–233. [Google Scholar] [CrossRef]
- Westlye, F.R.; Ivarsson, A.; Schramm, J. Experimental investigation of nitrogen based emissions from an ammonia fueled SI-engine. Fuel 2013, 111, 239–247. [Google Scholar] [CrossRef]
- Pedersen, K.A.; Lewandowski, M.T.; Schulze-Netzer, C.; Pasternak, M.; Løvås, T. Ammonia in Dual-Fueled Internal Combustion Engines: Impact on NOx, N2O, and Soot Formation. Energy Fuels 2023, 37, 17585–17604. [Google Scholar] [CrossRef]
- Glarborg, P. The NH3/NO2/O2 system: Constraining key steps in ammonia ignition and N2O formation. Combust. Flame 2023, 257, 112311. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Syred, N.; Griffiths, A. Visualisation of isothermal large coherent structures in a swirl burner. Combust. Flame 2009, 156, 1723–1734. [Google Scholar] [CrossRef]
- Reddy, V.M.; Katoch, A.; Roberts, W.L.; Kumar, S. Experimental and numerical analysis for high intensity swirl based ultra-low emission flameless combustor operating with liquid fuels. Proc. Combust. Inst. 2015, 35, 3581–3589. [Google Scholar] [CrossRef]
- Faqih, M.; Omar, M.B.; Ibrahim, R.; Omar, B.A. Dry-Low Emission Gas Turbine Technology: Recent Trends and Challenges. Appl. Sci. 2021, 12, 10922. [Google Scholar] [CrossRef]
- Albin, T.; da Franca, A.A.; Varea, E.; Kruse, S.; Pitsch, H.; Abel, D. Potential and challenges of MILD combustion control for gas turbine applications. In Active Flow and Combustion Control 2014; King, R., Ed.; Notes on Numerical Fluid Mechanics and Multidisciplinary Design; Springer International Publishing: Manhattan, NY, USA; Cham, Switzerland, 2015; Volume 127, pp. 181–195. [Google Scholar] [CrossRef]
- Iki, N.; Kurata, O.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Koboyashi, H.; Hayakawa, A.; Okafor, E. NOx reduction in a swirl combustor firing ammonia for a micro gas turbine. In Proceedings of the Turbo Expo: Power for Land, Sea, and Air, Oslo, Norway, 11–15 June 2018; American Society of Mechanical Engineers: New York, NY, USA, 2018; Volume 51173, p. V008T26A009. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.K.A.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Kobayashi, H. Towards the development of an efficient low-NOx ammonia combustor for a micro gas turbine. Proc. Combust. Inst. 2019, 37, 4597–4606. [Google Scholar] [CrossRef]
- Tumanovskii, A.G.; Bulysova, L.A.; Vasil’ev, V.D.; Gutnik, M.N.; Gutnik, M.M. Development of Low-Emission Combustors for Power-Generating GTUs. Therm. Eng. 2021, 68, 473–480. [Google Scholar] [CrossRef]
- Jin, T.; Dong, W.; Qiu, B.; Xu, C.; Liu, Y.; Chu, H. Effect of Ammonia on Laminar Combustion Characteristics of Methane–Air Flames at Elevated Pressures. ACS Omega 2022, 7, 15326–15337. [Google Scholar] [CrossRef]
- Rocha, R.C.; Costa, M.; Bai, X.S. Combustion and emission characteristics of ammonia under conditions relevant to modern gas turbines. Combust. Sci. Technol. 2021, 193, 2514–2533. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Gutesa, M.; Xiao, H.; Pugh, D.; Giles, A.; Goktepe, B.; Marsh, R.; Bowen, P. Premixed ammonia/hydrogen swirl combustion under rich fuel conditions for gas turbines operation. Int. J. Hydrogen Energy 2019, 44, 8615–8626. [Google Scholar] [CrossRef]
- Mashruk, S.; Xiao, H.; Pugh, D.; Chiong, M.C.; Runyon, J.; Goktepe, B.; Giles, A.; Valera-Medina, A. Numerical analysis on the evolution of NH2 in ammonia/hydrogen swirling flames and detailed sensitivity analysis under elevated conditions. Combust. Sci. Technol. 2023, 195, 1251–1278. [Google Scholar] [CrossRef]
- Zhu, D.; Ruwe, L.; Schmitt, S.; Shu, B.; Kohse-Höinghaus, K.; Lucassen, A. Interactions in Ammonia and Hydrogen Oxidation Examined in a Flow Reactor and a Shock Tube. J. Phys. Chem. A 2023, 127, 2351–2366. [Google Scholar] [CrossRef]
- Stefanizzi, M.; Capurso, T.; Filomeno, G.; Torresi, M.; Pascazio, G. Recent Combustion Strategies in Gas Turbines for Propulsion and Power Generation toward a Zero-Emissions Future: Fuels, Burners, and Combustion Techniques. Energies 2021, 14, 6694. [Google Scholar] [CrossRef]
- Colson, S.; Hirano, Y.; Hayakawa, A.; Kudo, T.; Kobayashi, H.; Galizzi, C.; Escudie, D. Experimental and numerical study of NH3/CH4 counterflow premixed and non-premixed flames for various NH3 mixing ratios. Combust. Sci. Technol. 2021, 193, 2872–2889. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia–methane combustion in tangential swirl burners for gas turbine power generation. Appl. Energy 2017, 185, 1362–1371. [Google Scholar] [CrossRef]
- Khateeb, A.A.; Guiberti, T.F.; Zhu, X.; Younes, M.; Jamal, A.; Roberts, W.L. Stability limits and exhaust NO performances of ammonia-methane-air swirl flames. Exp. Therm. Fluid Sci. 2020, 114, 110058. [Google Scholar] [CrossRef]
- Henshaw, P.F.; D’andrea, T.I.N.A.; Mann, K.R.; Ting, D.S.K. Premixed ammonia-methane-air combustion. Combust. Sci. Technol. 2005, 177, 2151–2170. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Wang, P.; Mao, C.; Cheng, K.; Sun, Y.; Yin, Z. Combustion Performance of the Premixed Ammonia-Hydrogen-Air Flame in Porous Burner. Combust. Sci. Technol. 2023, 1–18. [Google Scholar] [CrossRef]
- Iki, N.; Kurata, O.; Matsunuma, T.; Inoue, T.; Suzuki, M.; Tsujimura, T.; Furutani, H. Micro gas turbine firing kerosene and ammonia. In Turbo Expo: Power for Land, Sea, and Air, Proceedings of the ASME Turbo Expo 2015: Turbine Technical Conference and Exposition, Montreal, QC, Canada, 15–19 June 2015; American Society of Mechanical Engineers: New York, NY, USA, 2015; Volume 56796, p. V008T23A023. [Google Scholar] [CrossRef]
- Iki, N.; Kurata, O.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Operation and flame observation of micro gas turbine firing ammonia. In Turbo Expo: Power for Land, Sea, and Air, Proceedings of the ASME Turbo Expo 2017: Turbomachinery Technical Conference and Exposition, Charlotte, NC, USA, 26–30 June 2017; American Society of Mechanical Engineers: New York, NY, USA, 2017; Volume 50954, p. V008T26A018. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3–air and NH3CH4–air combustion gas-turbine power generations. Proc. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Ávila, C.D.; Cardona, S.; Abdullah, M.; Younes, M.; Jamal, A.; Guiberti, T.F.; Roberts, W.L. Experimental assessment of the performance of a commercial micro gas turbine fueled by ammonia-methane blends. Appl. Energy Combust. Sci. 2023, 13, 100104. [Google Scholar] [CrossRef]
- Somarathne, K.D.K.A.; Colson, S.; Hayakawa, A.; Kobayashi, H. Modelling of ammonia/air non-premixed turbulent swirling flames in a gas turbine-like combustor at various pressures. Combust. Theory Model. 2018, 22, 973–997. [Google Scholar] [CrossRef]
- Bonasio, V.; Ravelli, S. Performance Analysis of an Ammonia-Fueled Micro Gas Turbine. Energies 2022, 15, 3874. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and other emissions in micro gas turbine combustors fuelled with mixtures of methane and ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]
- Ditaranto, M.; Saanum, I.; Larfeldt, J. Experimental study on high pressure combustion of decomposed ammonia: How can ammonia be best used in a gas turbine? In Turbo Expo: Power for Land, Sea, and Air, Proceedings of the ASME Turbo Expo 2021: Turbomachinery Technical Conference and Exposition, Virtual, Online, 7–11 June 2021; American Society of Mechanical Engineers: New York, NY, USA, 2021; Volume 84959, p. V03BT04A027. [Google Scholar] [CrossRef]
- Matriculaciones de Turismos en España. Available online: https://www.epdata.es/datos/matriculaciones-turimos-espana-carburante/206/espana/106 (accessed on 17 January 2024).
- Dobslaw, D.; Engesser, K.; Störk, H.; Gerl, T. Low-cost process for emission abatement of biogas internal combustion engines. J. Clean. Prod. 2019, 227, 1079–1092. [Google Scholar] [CrossRef]
- Benato, A.; Macor, A.; Rossetti, A. Biogas Engine Emissions: Standards and On-Site Measurements. Energy Procedia 2017, 126, 398–405. [Google Scholar] [CrossRef]
- INNIO; Jenbacher. Available online: https://www.jenbacher.com/en/energy-solutions/applications/cogeneration-combined-heat-power (accessed on 17 January 2024).
- MWM Gas Engines and Gensets—Output. Reliability. Economy. For Your Success. Available online: https://www.mwm.net/en/gas-engines-gensets/ (accessed on 19 January 2024).
- Discover The Latest Gas-Powered Generator Sets. Available online: https://www.cat.com/en_GB/campaigns/npi/cg170b-gas-generator-sets.html (accessed on 19 January 2024).
- GAS GENERATOR SETS. Available online: https://www.mtu-solutions.com/eu/en/applications/power-generation/power-generation-products/gas-generator-sets.html (accessed on 20 January 2024).
- Powering Your Reliable, Responsible Energy Future. Available online: https://www.waukeshaengine.com/en (accessed on 19 January 2024).
- MAN Energy Solutions. A Cleaner Future with Gas Engines. Available online: https://www.man-es.com/energy-storage/products/gas-fuel-engines?utm_medium=sea&utm_source=google&utm_campaign=always_ao_sp_brand-energy-and-storage-products-all_230101&utm_term=sp&gad_source=1&gclid=CjwKCAiArfauBhApEiwAeoB7qJYQnAMEpJYX67n-whaDwvggV52Y3MwULQW-FY9PamNRH3MDEXz-cRoCtugQAvD_BwE&gclsrc=aw.ds (accessed on 19 January 2024).
- Cornelius, W.; Huellmantel, L.W.; Mitchell, H.R. Ammonia as an engine fuel. SAE Trans. 1966, 74, 300–326. [Google Scholar]
- Starkman, E.S.; Newhall, H.K.; Sutton, R.; Maguire, T.; Farbar, L. Ammonia as a spark ignition engine fuel: Theory and application. SAE Trans. 1967, 75, 765–784. [Google Scholar]
- Frigo, S.; Gentili, R.; Doveri, N. Ammonia Plus Hydrogen as Fuel in a S.I. Engine: Experimental Results; No. 2012-32-0019; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2012. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Z.; Liu, J. An evaluation of the conversion of gasoline and natural gas spark ignition engines to ammonia/hydrogen operation from the perspective of laminar flame speed. J. Energy Resour. Technol. 2022, 145, 012302. [Google Scholar] [CrossRef]
- Park, C.; Jang, Y.; Kim, S.; Kim, Y.; Choi, Y. Influence of Hydrogen on the Performance and Emissions Characteristics of a Spark Ignition Ammonia Direct Injection Engine. Gases 2023, 3, 144–157. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Experimental study on ammonia/hydrogen/air combustion in spark ignition engine conditions. Fuel 2020, 269, 117448. [Google Scholar] [CrossRef]
- Pyrc, M.; Gruca, M.; Tutak, W.; Jamrozik, A. Assessment of the co-combustion process of ammonia with hydrogen in a research VCR piston engine. Int. J. Hydrogen Energy 2023, 48, 2821–2834. [Google Scholar] [CrossRef]
- Ge, H.; Bakir, A.H.; Zhao, P. Knock Mitigation and Power Enhancement of Hydrogen Spark-Ignition Engine through Ammonia Blending. Machines 2023, 11, 651. [Google Scholar] [CrossRef]
- Grannell, S.M.; Assanis, D.N.; Bohac, S.V.; Gillespie, D.E. The fuel mix limits and efficiency of a stoichiometric, ammonia, and gasoline dual fueled spark ignition engine. J. Eng. Gas Turbines Power 2008, 130, 042802. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S. Effects of gaseous ammonia direct injection on performance characteristics of a spark-ignition engine. Appl. Energy 2014, 116, 206–215. [Google Scholar] [CrossRef]
- Lanni, D.; Galloni, E.; Fontana, G. Assessment of the Operation of an SI Engine Fueled with Ammonia. Energies 2022, 15, 8583. [Google Scholar] [CrossRef]
- Polanski, J.; Bartczak, P.; Ambrozkiewicz, W.; Sitko, R.; Siudyga, T.; Mianowski, A.; Szade, J.; Balin, K.; Lelątko, J. Ni-Supported Pd Nanoparticles with Ca Promoter: A New Catalyst for Low-Temperature Ammonia Cracking. PLoS ONE 2015, 10, e0136805. [Google Scholar] [CrossRef]
- Lee, Y.J.; Cha, J.; Kwak, Y.; Park, Y.; Jo, Y.S.; Jeong, H.; Sohn, H.; Chang, W.Y.; Kim, Y.; Kim, K.-B.; et al. Top-down syntheses of nickel-based structured catalysts for hydrogen production from ammonia. ACS Appl. Mater. Interfaces 2021, 13, 597–607. [Google Scholar] [CrossRef]
- Cechetto, V.; Agnolin, S.; Di Felice, L.; Pacheco Tanaka, A.; Llosa Tanco, M.; Gallucci, F. Metallic Supported Pd-Ag Membranes for Simultaneous Ammonia Decomposition and H2 Separation in a Membrane Reactor: Experimental Proof of Concept. Catalysts 2023, 13, 920. [Google Scholar] [CrossRef]
- Khan, W.U.; Alasiri, H.S.; Ali, S.A.; Hossain, M.M. Recent Advances in Bimetallic Catalysts for Hydrogen Production from Ammonia. Chem. Rec. 2022, 22, e202200030. [Google Scholar] [CrossRef]
- Huang, X.; Lei, K.; Mi, Y.; Fang, W.; Li, X. Recent Progress on Hydrogen Production from Ammonia Decomposition: Technical Roadmap and Catalytic Mechanism. Molecules 2023, 28, 5245. [Google Scholar] [CrossRef]
- Spatolisano, E.; Pellegrini, L.A.; de Angelis, A.R.; Cattaneo, S.; Roccaro, E. Ammonia as a carbon-free energy carrier: NH3 cracking to H2. Ind. Eng. Chem. Res. 2023, 62, 10813–10827. [Google Scholar] [CrossRef]
- Mashhadimoslem, H.; Safarzadeh Khosrowshahi, M.; Delpisheh, M.; Convery, C.; Rezakazemi, M.; Aminabhavi, T.M.; Kamkar, M.; Elkamel, A. Green ammonia to Hydrogen: Reduction and oxidation catalytic processes. Chem. Eng. J. 2023, 474, 145661. [Google Scholar] [CrossRef]
- Comotti, M.; Frigo, S. Hydrogen generation system for ammonia–hydrogen fuelled internal combustion engines. Int. J. Hydrogen Energy 2015, 40, 10673–10686. [Google Scholar] [CrossRef]
- Bréquigny, P.; Dumand, C.; Houillé, S. Operating Limits for Ammonia Fuel Spark-Ignition Engine. Energies 2021, 14, 4141. [Google Scholar] [CrossRef]
- Tutak, W.; Pyrc, M.; Gruca, M.; Jamrozik, A. Ammonia Combustion in a Spark-Ignition Engine Supported with Dimethyl Ether. Energies 2023, 16, 7283. [Google Scholar] [CrossRef]
- Oh, S.; Park, C.; Kim, S.; Kim, Y.; Choi, Y.; Kim, C. Natural gas–ammonia dual-fuel combustion in spark-ignited engine with various air–fuel ratios and split ratios of ammonia under part load condition. Fuel 2021, 290, 120095. [Google Scholar] [CrossRef]
- Mørch, C.; Bjerre, A.; Gøttrup, M.; Sorenson, S.; Schramm, J. Ammonia/hydrogen mixtures in an SI-engine: Engine performance and analysis of a proposed fuel system. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S. Performance characteristics of compression-ignition engine using high concentration of ammonia mixed with dimethyl ether. Appl. Energy 2014, 113, 488–499. [Google Scholar] [CrossRef]
- Ambalakatte, A.; Geng, S.; Cairns, A.; Harrington, A.; Hall, J.; Bassett, M. Evaluation of ammonia-gasoline co-combustion in a modern spark ignition research engine. Carbon Neutrality 2023, 2, 35. [Google Scholar] [CrossRef]
- Abd El Fattah, S.F.; Ezzat, M.F.; Mourad, M.A.; Elkelawy, M.; Youssef, I.M. Experimental Investigation of the Performance and Exhaust Emissions of a Spark-Ignition Engine Operating with Different Proportional Blends of Gasoline and Water Ammonia Solution. J. Eng. Res. 2022, 5, 38–45. [Google Scholar] [CrossRef]
- Ciatti, S.A. Compression Ignition Engines—Revolutionary Technology That has Civilized Frontiers all over the Globe from the Industrial Revolution into the Twenty-First Century. Front. Mech. Eng. 2015, 1, 145182. [Google Scholar] [CrossRef]
- Reşitoğlu, İ.A.; Altinişik, K.; Keskin, A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef]
- Ayodhya, A.S.; Narayanappa, K.G. An overview of after-treatment systems for diesel engines. Environ. Sci. Pollut. Res. 2018, 25, 35034–35047. [Google Scholar] [CrossRef]
- Fuels-Higher and Lower Calorific Values. Available online: https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html (accessed on 18 June 2024).
- Gray, J.T., Jr.; Dimitroff, E.; Meckel, N.T.; Quillian, R.D., Jr. Ammonia fuel—Engine compatibility and combustion. SAE Trans. 1967, 75, 785–807. [Google Scholar]
- Starkman, E.S.; James, G.E.; Newhall, H.K. Ammonia as a diesel engine fuel: Theory and application. SAE Trans. 1968, 76, 3193–3212. [Google Scholar]
- Pearsall, T.J.; Garabedian, C.G. Combustion of anhydrous ammonia in diesel engines. SAE Trans. 1968, 76, 3213–3221. [Google Scholar]
- Reiter, A.J.; Kong, S.C. Demonstration of compression-ignition engine combustion using ammonia in reducing greenhouse gas emissions. Energy Fuels 2008, 22, 2963–2971. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef]
- Voniati, G.; Dimaratos, A.; Koltsakis, G.; Ntziachristos, L. Ammonia as a Marine Fuel towards Decarbonization: Emission Control Challenges. Sustainability 2023, 15, 15565. [Google Scholar] [CrossRef]
- Yousefi, A.; Guo, H.; Dev, S.; Lafrance, S.; Liko, B. A study on split diesel injection on thermal efficiency and emissions of an ammonia/diesel dual-fuel engine. Fuel 2022, 316, 123412. [Google Scholar] [CrossRef]
- Imhoff, T.B.; Gkantonas, S.; Mastorakos, E. Analysing the Performance of Ammonia Powertrains in the Marine Environment. Energies 2021, 14, 7447. [Google Scholar] [CrossRef]
- Balci, G.; Phan, T.T.N.; Surucu-Balci, E.; Iris, Ç. A roadmap to alternative fuels for decarbonising shipping: The case of green ammonia. Res. Transp. Bus. Manag. 2024, 53, 101100. [Google Scholar] [CrossRef]
- Schönborn, A. Aqueous solution of ammonia as marine fuel. Proc. Inst. Mech. Eng. M 2020, 235, 142–151. [Google Scholar] [CrossRef]
- Nadimi, E.; Przybyła, G.; Lewandowski, M.T.; Adamczyk, W. Effects of ammonia on combustion, emissions, and performance of the ammonia/diesel dual-fuel compression ignition engine. J. Energy Inst. 2023, 107, 101158. [Google Scholar] [CrossRef]
- Nadimi, E.; Przybyła, G.; Emberson, D.; Løvås, T.; Ziółkowski, Ł.; Adamczyk, W. Effects of using ammonia as a primary fuel on engine performance and emissions in an ammonia/biodiesel dual-fuel CI engine. Int. J. Energy Res. 2022, 46, 15347–15361. [Google Scholar] [CrossRef]
- Gross, C.W.; Kong, S. Performance characteristics of a compression-ignition engine using direct-injection ammonia–DME mixtures. Fuel 2013, 103, 1069–1079. [Google Scholar] [CrossRef]
- Frost, J.; Tall, A.; Sheriff, A.M.; Schönborn, A.; Hellier, P. An experimental and modelling study of dual fuel aqueous ammonia and diesel combustion in a single cylinder compression ignition engine. Int. J. Hydrogen Energy 2021, 46, 35495–35510. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Wang, Y.; Wu, J.; Wang, X. Comparison of the Effect of Diesel and Hydrogen Addition on Ammonia Combustion Characteristics in a Marine Engine; No. 2023-32-0065; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2023. [Google Scholar] [CrossRef]
- Huang, Z.; Lyu, Z.; Luo, P.; Zhang, G.; Ying, W.; Chen, A.; Xiao, H. Effects of Methanol–Ammonia Blending Ratio on Performance and Emission Characteristics of a Compression Ignition Engine. J. Mar. Sci. Eng. 2023, 11, 2388. [Google Scholar] [CrossRef]
- Liu, L.; Tan, F.; Wu, Z.; Wang, Y.; Liu, H. Comparison of the combustion and emission characteristics of NH3/NH4NO2 and NH3/H2 in a two-stroke low speed marine engine. Int. J. Hydrogen Energy 2022, 47, 17778–17787. [Google Scholar] [CrossRef]
- Xu, L.; Bai, X.S. Numerical investigation of engine performance and emission characteristics of an ammonia/hydrogen/n-heptane engine under RCCI operating conditions. Flow Turbul. Combust. 2024, 112, 957–974. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, X.; Cui, L.; Wang, K.; Wang, X.; Zhu, S. Simulation Research on the Injection Strategy of a Diesel-Ammonia Dual-Fuel Marine Engine. Energy Fuels 2023, 37, 9736–9745. [Google Scholar] [CrossRef]
- Rehbein, M.C.; Meier, C.; Eilts, P.; Scholl, S. Mixtures of ammonia and organic solvents as alternative fuel for internal combustion engines. Energy Fuels 2019, 33, 10331–10342. [Google Scholar] [CrossRef]
- Mounaïm-Rousselle, C.; Mercier, A.; Brequigny, P.; Dumand, C.; Bouriot, J.; Houillé, S. Performance of ammonia fuel in a spark assisted compression Ignition engine. Int. J. Eng. Res. 2021, 23, 781–792. [Google Scholar] [CrossRef]
- Lee, D.; Song, H.H. Development of combustion strategy for the internal combustion engine fueled by ammonia and its operating characteristics. J. Mech. Sci. Technol. 2018, 32, 1905–1925. [Google Scholar] [CrossRef]
- Scharl, V.; Sattelmayer, T. Ignition and combustion characteristics of diesel piloted ammonia injections. Fuel Commun. 2022, 11, 100068. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).