Sustainable Production of Chitin Nanowhiskers from Crustacean Biomass Using Cost-Effective Ionic Liquids: Strategies to Avoid Byproduct Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Chitin Content in Biomass
2.3. Preparation of Chitin Nanowhiskers
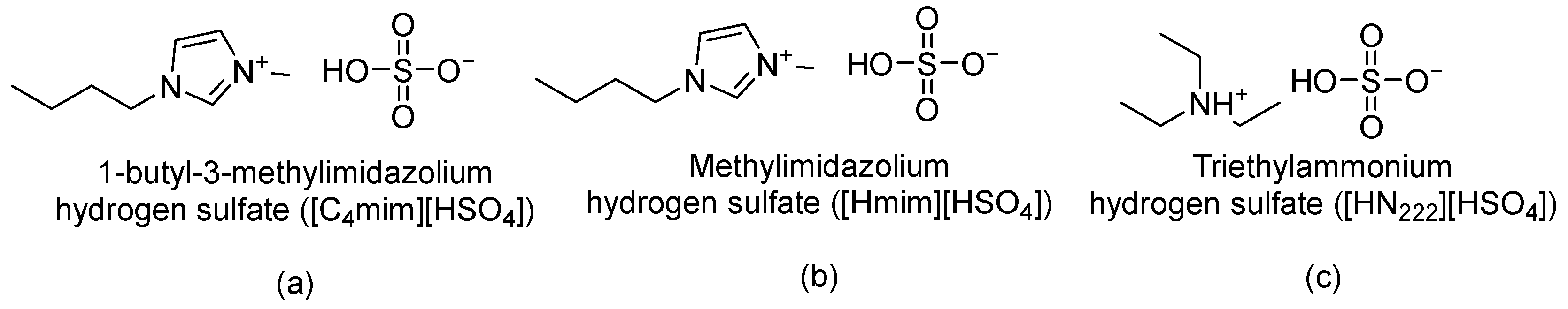
- Pretreatment with ILs—For chitin pretreatment, a suspension of chitin in IL (1:10 w/w) was prepared by thoroughly mixing chitin in the IL (for example, 0.76 g chitin was added and mixed in 7.64 g [HSO4]−-based IL), resulting in a well-blended paste. The prepared paste was then properly sealed, encased with a parafilm, and kept in the oven (45–65 °C) for 24 h.For biomass pretreatment, a 3 wt% crushed crustacean biomass was prepared by thoroughly mixing the biomass in the IL (for example, 1.5 g crushed crustacean biomass was added and mixed in 48.5 g of the [HSO4]−-based IL), resulting in a paste. The properly blended paste was subsequently sealed, covered with a parafilm, and kept in the oven (45–65 °C) for 24 h.
- Isolation of Chitin Nanowhiskers—After letting the mixture in the containers settle for 24 h, 30% DI water with respect to IL (i.e., 16.7 g of water per 48.5 g of the IL) was added to each flask. Each flask was equipped with a stirring bar and a condenser, and then heated to 110 °C in an oil bath, for 6–48 h. In the case of biomass, this resulted in a significant production of foam. After heating, 50 mL DI water was added to each flask. Following that, the mixtures were moved to 15 mL centrifuge tubes, and centrifuged (Eppendorf 5430 R, Enfield, CT; rotor CE 11017, 7830 rpm). The liquid was decanted, fresh water was added, the precipitate was stirred with a spatula, and the suspension was centrifuged again. This process was repeated 10 more times until a neutral pH was obtained. After washing, the suspensions were ultrasonicated (VibraCell Ultrasonicator, model CV 33, Newtown, CT, USA) for 10 min with 30 s cycles for the uniform distribution of particles. The suspension was then split into 2 parts. One part was diluted and analyzed using Transmission Electron Microscopy (TEM, Hitachi H-9500, Tokyo, Japan). The second part was frozen at −20 °C, and then subjected to a lyophilization (Labconco FreeZone Plus Cascade Benchtop Freeze Dryer System, Kansas City, MO, USA) process for approximately 72 h, resulting in the production of nanocrystals with precise dimensions.
2.4. Characterization
2.4.1. Estimation of Crystallinity
2.4.2. Crystallite Size Determination
2.4.3. Transmission Electron Microscopy
2.4.4. Field Emission Scanning Electron Microscopy
2.4.5. Thermal Gravimetric Analysis
2.4.6. Attenuated Total Reflectance (ATR) Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.7. Degree of Acetylation
3. Results and Discussion
3.1. Preparation and Characterization of Chitin Nanowhiskers from Pure Chitin
3.2. Preparation and Characterization of Chitin Nanowhiskers from Crustaceous Biomass
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, L.; Liu, L.; Esquivel, M.; Tardy, B.L.; Huan, S.; Niu, X.; Liu, S.; Yang, G.; Fan, Y.; Rojas, O.J. Nanochitin: Chemistry, Structure, Assembly, and Applications. Chem. Rev. 2022, 122, 11604–11674. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Revol, J.F.; Marchessault, R.H. Effect of degree of deacetylation of chitin on the properties of chitin crystallites. J. Appl. Polym. Sci. 1997, 65, 370–373. [Google Scholar] [CrossRef]
- Foster, A.B.; Webber, J.M. Chitin. In Advances in Carbohydrate Chemistry; Melville, L., Wolfrom, R., Tipson, S., Eds.; Academic Press: Oxford, UK, 1961; pp. 371–393. [Google Scholar]
- Visakh, P.M.; Thomas, S. Preparation of Bionanomaterials and Their Polymer Nanocomposites from Waste and Biomass. Waste Biomass Valor. 2010, 1, 121–134. [Google Scholar] [CrossRef]
- Lee, S.; Hao, L.T.; Park, J.; Oh, D.X.; Hwang, D.S. Nanochitin and nanochitosan: Chitin nanostructure engineering with multiscale properties for biomedical and environmental applications. Adv. Mater. 2023, 35, e2203325. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Boissiere, C.; Cassaignon, S.; Chaneac, C.; Durupthy, O.; Faustini, M.; Grosso, D.; Laberty-Robert, C.; Nicole, L.; Portehault, D.; et al. Molecular engineering of functional inorganic and hybrid materials. Chem. Mater. 2014, 26, 221–238. [Google Scholar] [CrossRef]
- Dominic, C.D.M.; Joseph, R.; Sabura Begum, P.M.; Raghunandanan, A.; Vackkachan, N.T.; Padmanabhan, D.; Formela, K. Chitin nanowhiskers from shrimp shell waste as green filler in acrylonitrile-butadiene rubber: Processing and performance properties. Carbohydr. Polym. 2020, 245, 116505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, M.; Yang, S.; Luo, B.; Zhou, C. Liquid crystalline behaviors of chitin nanocrystals and their reinforcing effect on natural rubber. ACS Sustain. Chem. Eng. 2018, 6, 325–336. [Google Scholar] [CrossRef]
- Chi-Yan Li, S.; Sun, Y.C.; Guan, Q.; Naguib, H. Effects of chitin nanowhiskers on the thermal, barrier, mechanical, and rheological properties of polypropylene nanocomposites. RSC Adv. 2016, 6, 72086–72095. [Google Scholar] [CrossRef]
- Wang, M.; Xue, H.; Feng, Z.; Cheng, B.; Yang, H. Increase of Tensile Strength and Toughness of Bio-Based Diglycidyl Ether of Bisphenol A with Chitin Nanowhiskers. PLoS ONE 2017, 12, e0177673. [Google Scholar] [CrossRef]
- Rajak, D.K.; Pagar, D.D.; Kumar, R.; Pruncu, C.I. Recent progress of reinforcement materials: A comprehensive overview of composite materials. J. Mater. Res. Technol. 2019, 8, 6354–6374. [Google Scholar] [CrossRef]
- Serventi, L.; He, Q.; Huang, J.; Mani, A.; Subhash, A.J. Advances in the preparations and applications of nanochitins. Food Hydrocoll. Health 2021, 1, 100036. [Google Scholar] [CrossRef]
- Mushi, N.E.; Utsel, S.; Berglund, L.A. Nanostructured biocomposite films of high toughness based on native chitin nanofibers and chitosan. Front. Chem. 2014, 2, 99. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, T.; Yamamoto, K.; Kadokawa, J.-I. Fabrication of highly flexible nanochitin film and its composite film with anionic polysaccharide. Carbohydr. Polym. 2021, 270, 118369. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Jian, G.; Chen, Z.; Wolcott, M.; Nassiri, S.; Fernandez, C.A. Interfacial interactions and reinforcing mechanisms of cellulose and chitin nanomaterials and starch derivatives for cement and concrete strength and durability enhancement: A review. Nanotechnol. Rev. 2022, 11, 2673–2713. [Google Scholar] [CrossRef]
- Haider, M.M.; Jian, G.; Zhong, T.; Li, H.; Fernandez, C.A.; Fifield, L.S.; Wolcott, M.; Nassiri, S. Insights into setting time, rheological and mechanical properties of chitin nanocrystals- and chitin nanofibers-cement paste. Cem. Concr. Compos. 2022, 132, 104623. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Aguirre, G. Biopolymer micro/nanogel particles as smart drug delivery and theranostic systems. Pharmaceutics 2023, 15, 2060. [Google Scholar] [CrossRef] [PubMed]
- Olza, S.; Salaberria, A.M.; Alonso-Varona, A.; Samanta, A.; Fernandes, S.C.M. The role of nanochitin in biologically-active matrices for tissue engineering–Where do we stand? J. Mater. Chem. B 2023, 11, 5630–5649. [Google Scholar] [CrossRef] [PubMed]
- Zubillaga, V.; Salaberria, A.M.; Palomares, T.; Alonso-Varona, A.; Kootala, S.; Labidi, J.; Fernandes, S.C.M. Chitin nanoforms provide mechanical and topological cues to support growth of human adipose stem cells in chitosan matrices. Biomacromolecules 2018, 19, 3000–3012. [Google Scholar] [CrossRef]
- Suneetha, M.; Kim, H.; Han, S.S. Doxorubicin-loaded fungal-carboxymethyl chitosan functionalized polydopamine nanoparticles for photothermal cancer therapy. Pharmaceutics 2023, 15, 1281. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Bhat, A.H.; Khan, I. 1. Nanocellulose and Nanochitin for Water Remediation by Adsorption of Heavy Metals. In Nanomaterials for Water Remediation; Kumar Mishra, A., Hussain, M.C., Mishra, S.B., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2020; pp. 1–18. [Google Scholar] [CrossRef]
- CORDIS: Functional Membranes/Filters with Anti/Low-Fouling Surfaces for Water Purification through Selective Adsorption on Biobased Nanocrystals and Fibrils. Available online: https://cordis.europa.eu/project/id/280519 (accessed on 12 February 2024).
- Somasundaram, S.; Kumaravel, V. Application of Nanoparticles for Self-Cleaning Surfaces. In Home Emerging Nanostructured Materials for Energy and Environmental Science; Part of the Environmental Chemistry for a Sustainable World Book Series; Springer: Berlin/Heidelberg, Germany, 2019; Volume 23. [Google Scholar]
- He, Y.; Lin, X.; Feng, Y.; Luo, B.; Liu, M. Carbon nanotube ink dispersed by chitin nanocrystals for thermoelectric converter for self-powering multifunctional wearable electronics. Adv. Sci. 2022, 9, e2204675. [Google Scholar] [CrossRef] [PubMed]
- Schötz, S.; Reisbeck, F.; Schmitt, A.C.; Dimde, M.; Quaas, E.; Achazi, K.; Haag, R. Tunable polyglycerol-based redox-responsive nanogels for efficient cytochrome c delivery. Pharmaceutics 2021, 13, 1276. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.S.; dos Santos, D.M.; Mercante, L.A.; Facure, M.H.M.; Campana-Filho, S.P.; Mattoso, L.H.C.; Correa, D.S. Nanochitin-based composite films as a disposable ethanol sensor. J. Environ. Chem. Eng. 2020, 8, 104163. [Google Scholar] [CrossRef]
- Heidarian, P.; Gharaie, S.; Yousefi, H.; Paulino, M.; Kaynak, A.; Varley, R.; Kouzani, A.Z.A. 3D printable dynamic nanocellulose/nanochitin self-healing hydrogel and soft strain sensor. Carbohydr. Polym. 2022, 291, 119545. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Xi, C. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, M.; Sachindra, N.M.; Mahendrakar, N.S. Biotechnology for utilization of marine by-products. In Fish Processing Byproducts; Studium Press: Austin, TX, USA, 2015; pp. 43–62. [Google Scholar]
- Poeloengasih, C.D.; Hernawan, D.; Angwarndo, M. Isolation and characterization of chitin and chitosan prepared under various processing times. Indones. J. Chem. 2008, 8, 189–192. [Google Scholar] [CrossRef]
- Beaney, P.; Lizardi-Mendoza, J.; Healy, M. Comparison of chitins produced by chemical and bioprocessing methods. J. Chem. Tech. Biotech. 2005, 80, 145–150. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin Research Revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Joseph, B.; Sam, R.M.; Balakrishnan, P.; Maria, H.J.; Gopi, S.; Volova, T.; Fernandes, S.C.M.; Thomas, S. Extraction of nanochitin from marine resources and fabrication of polymer nanocomposites: Recent advances. Polymers 2020, 12, 1664. [Google Scholar] [CrossRef] [PubMed]
- Druzian, S.P.; Zanatta, N.P.; Côrtes, L.N.; Fátima, A.; Streit, M. Preparation of chitin nanowhiskers and its application for crystal violet dye removal from wastewaters. Environ. Sci. Pollut. Res. 2019, 26, 28548–28557. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yuan, Y.; Zhang, K.; Lian, H.; Liimatainen, H. Efficient Hydrolysis of Chitin in a Deep Eutectic Solvent Synergism for Production of Chitin Nanocrystals. Nanomaterials 2020, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nishimura, T.; Saito, T.; Kato, T. CaCO3/chitin-whisker hybrids: Formation of CaCO3 crystals in chitin-based liquid-crystalline suspension. Polym. J. 2010, 42, 583–586. [Google Scholar] [CrossRef]
- Revol, J.; Marchessaultf, R.H. In Vitro Chiral Nematic Ordering of Chitin Crystallites. Int. J. Biol. Macromol. 1993, 15, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Liu, T.; Lam, E.; Moores, A. Chitin and chitosan on the nanoscale. Nanoscale Horiz. 2021, 6, 505–542. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M.; Cheng, D.; Yang, R. Preparation of silver nano-particles immobilized onto chitin nano-crystals and their application to cellulose paper for imparting antimicrobial activity. Carbohydr. Polym. 2016, 151, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Saito, T.; Isogai, A. Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Different routes to turn chitin into stunning nano-objects. Eur. Polym. J. 2015, 68, 503–515. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, J.; Zhang, X.; Wang, Y.; Li, T.; Xia, B.; Jiang, J.; Dong, W. High strength chitin nanocrystal/alginate filament prepared by wet-spinning in “green” coagulating bath. Cellulose 2022, 29, 8611–8621. [Google Scholar] [CrossRef]
- Liu, P.; Liu, H.; Schäfer, T.; Gutmann, T.; Gibhardt, H.; Qi, H.; Tian, L.; Zhang, X.C.; Buntkowsky, G.; Zhang, K. Unexpected selective alkaline periodate oxidation of chitin for the isolation of chitin nanocrystals. Green Chem. 2021, 23, 745–751. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Effect of Oxidized Chitin Nanocrystals Isolated by Ammonium Persulfate Method on the Properties of Carboxymethyl Cellulose-Based Films. Carbohydr. Polym. 2017, 175, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, C.; Hong, S.; Lian, H.; Mei, C.; Lee, J.; Wu, Q.; Hubbe, M.A.; Li, M. Recent Advances in Extraction and Processing of Chitin Using Deep Eutectic Solvents. Chem. Eng. J. 2022, 446, 136953. [Google Scholar] [CrossRef]
- Berroci, M.; Vallejo, C.; Lizundia, E. Environmental impact assessment of chitin nanofibril and nanocrystal isolation from fungi, shrimp shells, and crab shells. ACS Sustain. Chem. Eng. 2022, 10, 14280–14293. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Abidi, N. Isolation of chitin nano-whiskers directly from crustacean biomass waste in a single step with acidic ionic liquid. ACS Sustain. Chem. Eng. 2022, 10, 11846–11855. [Google Scholar] [CrossRef]
- Abidi, N.; Shamshina, J.L. Preparation of Chitin Nanocrystals and Nanowhiskers from Crustacean Biomass Using Ionic Liquid. WO2023059499A1, 29 September 2022. Available online: https://worldwide.espacenet.com/patent/search?q=PCT%2FUS2022%2F045177 (accessed on 12 February 2024).
- Singh, V.; Kaur, S.; Sapehiyia, V.; Singh, J.; Kad, G.L. Microwave accelerated preparation of [bmim][HSO4] ionic liquid: An acid catalyst for improved synthesis of coumarins. Catal. Commun. 2005, 6, 57–60. [Google Scholar] [CrossRef]
- Tao, D.-J.; Li, Z.-M.; Cheng, Z.; Hu, N.; Chen, X.-S. Kinetics study of the ketalization reaction of cyclohexanone with glycol using Brønsted acidic ionic liquids as catalysts. Ind. Eng. Chem. Res. 2012, 51, 16263–16269. [Google Scholar] [CrossRef]
- Stocker, M.W.; Tsolaki, E.; Harding, M.J.; Healy, A.M.; Ferguson, S. Combining isolation-free and co-processing manufacturing approaches to access room temperature ionic liquid forms of APIs. J. Pharm. Sci. 2023, 112, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Alcalde, E.; Dinarès, I.; Ibáñez, A.; Mesquida, N. A Simple halide-to-anion exchange method for heteroaromatic salts and ionic liquids. Molecules 2012, 17, 4007–4027. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Simmons, B.A.; Blanch, H.W. Techno-economic analysis of a lignocellulosic ethanol biorefinery with ionic liquid pre-treatment. Biofuels Bioprod. Bioref. 2011, 5, 562–569. [Google Scholar] [CrossRef]
- Chen, L.; Sharifzadeh, M.; Dowell, N.M.; Welton, T.; Shah, N.; Hallett, J.P. Inexpensive ionic liquids: [HSO4]−-based solvent production at bulk scale. Green Chem. 2014, 16, 3098–3106. [Google Scholar] [CrossRef]
- George, A.; Brandt, A.; Tran, K.; Zahari, S.M.S.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015, 17, 1728–1734. [Google Scholar] [CrossRef]
- Zahari, S.M.S.N.S.; Azman, H.; Karim, L. Triethylammonium hydrogen sulfate ionic liquid as a low-cost solvent: A short review of synthesis, analysis and applications. In Proceedings of the MATEC Web conference, International Mechanical and Industrial Engineering Conference 2018 (IMIEC 2018), Malang, Indonesia, 30–31 August 2018; Volume 204. Available online: https://www.matec-conferences.org/articles/matecconf/abs/2018/63/matecconf_imiec2018_00006/matecconf_imiec2018_00006.html (accessed on 26 March 2024).
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Wealea, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078. [Google Scholar] [CrossRef]
- Karimi-Jaberi, Z.; Masoudi, B.; Rahmani, A.; Alborzi, K. Triethylammonium hydrogen sulfate [Et3NH][HSO4] as an efficient ionic liquid catalyst for the synthesis of coumarin derivatives. Polycycl. Aromat. Compd. 2020, 40, 99–107. [Google Scholar] [CrossRef]
- Black, M.M.; Schwartz, H.M. The estimation of chitin and chitin nitrogen in crawfish waste and derived products. Analyst 1950, 75, 185–189. [Google Scholar] [CrossRef]
- Scherrer, P. Determination of the size and internal structure of colloidal particles using X-rays. In Kolloidchemie Ein Lehrbuch; Springer: Berlin/Heidelberg, Germany, 1912; pp. 387–409. [Google Scholar]
- Fan, Y.; Fukuzumi, H.; Saito, T.; Isogai, A. Comparative characterization of aqueous dispersions and cast films of different chitin nanowhiskers/nanofibers. Int. J. Biol. Macromol. 2012, 50, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Beil, S.; Schamberger, A.; Naumann, W.; Machill, S.; van Pee, K.-H. Determination of the degree of N-acetylation (DA) of chitin and chitosan in the presence of water by first derivative ATR FTIR spectroscopy. Carbohydr. Polym. 2012, 87, 117–122. [Google Scholar] [CrossRef] [PubMed]
- PubChem. 1-Methylimidazolium (Compound). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1-Methylimidazolium#section=Synonyms (accessed on 6 April 2024).
- Muckerman, J.T.; Skone, J.H.; Ning, M.; Wasada-Tsutsui, Y. Toward the accurate calculation of pKa values in water and acetonitrile. Biochim. Biophys. Acta 2013, 1827, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Grishina, E.P.; Ramenskaya, L.M.; Gruzdev, M.S.; Kraeva, O.V. Water effect on physicochemical properties of 1-butyl-3-methylimidazolium based ionic liquids with inorganic anions. J. Mol. Liquids 2013, 177, 267–272. Available online: https://www.sciencedirect.com/science/article/pii/S0167732212003819?via%3Dihub (accessed on 10 April 2024). [CrossRef]
- Matuszek, K.; Chrobok, A.; Coleman, F.; Seddon, K.R.; Swadźba-Kwaśny, M. Tailoring ionic liquids: Structure, acidity and catalytic activity of a new class of protic ionic liquids based on oligomeric [(HSO4)xHx-1]-anions. Green Chem. 2014, 16, 3463–3471. [Google Scholar] [CrossRef]
- Belieres, J.P.; Angell, C.A. Protic ionic liquids: Preparation, characterization, and proton free energy level representation. J. Phys. Chem. B 2007, 111, 4926–4937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mao, Y.; Schwab, N.; Briber, R. An efficient method for chitin processing using phosphoric acid: Oligomers, nanocrystals, and high polymers. In Proceedings of the APS March Meeting 2022, Chicago, IL, USA, 14–18 March 2022. abstract id.N00.094. [Google Scholar]
- Muñoz-Núñez, C.; Fernández-García, M.; Muñoz-Bonilla, A. Chitin nanocrystals: Environmentally friendly materials for the development of bioactive films. Coatings 2022, 12, 144. [Google Scholar] [CrossRef]
- Mincea, M.; Negrulescu, A.; Ostafe, V. Preparation, modification, and applications of chitin nanowhiskers: A review. ons of chitin nanowhiskers: A review. Rev. Adv. Mater. Sci. 2012, 30, 225–242. [Google Scholar]
- Gómez Estaca, J.; Tovar, C.A.; Montero García, P.; Gómez Guillén, M.C. Structural, viscoelastic, and emulsifying properties of shrimp chitin nanowhisker dispersions as a function of acidic pHs. J. Food Eng. 2023, 351, 111519. [Google Scholar] [CrossRef]
- Morin, A.; Dufresne, A. Nanocomposites of chitin whiskers from Riftia tubes and poly(caprolactone). Macromolecules 2002, 35, 22190–22199. [Google Scholar] [CrossRef]
- Kumar, G.N.P.; Bhat, S.K. Preparation of chitin nano whiskers from mushrooms. Int. J. Sci. Res. Publ. 2018, 8, 130. [Google Scholar] [CrossRef]
- Goodrich, J.D.; Winter, W.T. Alpha-chitin nanocrystals prepared from shrimp shells and their specific surface area measurement. Biomacromolecules 2007, 8, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.D.; de Vries, R.; Stoyanov, S.D. Chitin nanowhiskers with improved properties obtained using natural deep eutectic solvent and mild mechanical processing. Green Chem. 2022, 24, 3834–3844. [Google Scholar] [CrossRef]
- Abdul Haleem, M.; Parker, K.D. X-ray Diffraction Studies on the Structure of a Chitin. Z. Naturforsch. C Biosci. 1976, 31, 383–388. [Google Scholar] [CrossRef]
- Focher, B.; Beltrame, P.L.; Naggi, A.; Torri, G. Alkaline N-deacetylation of chitin enhanced by flash treatments. Reaction kinetics and structure modifications. Carbohydr. Polym. 1990, 12, 405–418. [Google Scholar] [CrossRef]
- Ioelovich, M. Crystallinity and hydrophility of chitin and chitosan. Res. Rev. J. Chem. 2014, 3, 7–14. [Google Scholar]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Kinetics study of the solid-state acid hydrolysis of chitosan: Evolution of the crystallinity and macromolecular structure. Biomacromolecules 2010, 11, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Singhal, R.P. Carbon fibers prepared from tailored reversible-addition-fragmentation transfer copolymerization-derived poly(acrylonitrile)-co-poly(methylmethacrylate). J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 2243–2262. [Google Scholar] [CrossRef]
- Nansé, G.; Papirer, E.; Fioux, P.; Moguet, F.; Tressaud, A. Fluorination of carbon blacks: An X-ray photoelectron spectroscopy study: I. A literature review of XPS studies of fluorinated carbons. XPS investigation of some reference compounds. Carbon 1997, 35, 175–194. [Google Scholar] [CrossRef]
- Pereira, A.G.; Muniz, E.C.; Hsieh, Y.L. Chitosan-sheath and chitin-core nanowhiskers. Carbohydr. Polym. 2014, 107, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Rhim, J.W. Preparation of multifunctional chitin nanowhiskers/ZnO-Ag NPs and their effect on the properties of carboxymethyl cellulose-based nanocomposite film. Carbohydr. Polym. 2017, 169, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Böke, H.; Akkurt, S.; Özdemir, S.; Gökturk, E.H.; Saltik, E.N.C. Quantification of CaCO3–CaSO3·0.5H2O–CaSO4·2H2O mixtures by FTIR analysis and its ANN model. Mater. Lett. 2004, 58, 723–726. [Google Scholar] [CrossRef]
- Al Dabbas, M.; Eisa, M.Y.; Kadhim, W.H. Estimation of gypsum-calcite percentages using a fourier transform infrared spectrophotometer (FTIR), in Alexandria Gypsiferous soil-Iraq. Iraqi J. Sci. 2014, 55, 1916–1926. [Google Scholar]
- Morris, R.J. Infrared spectrophotometric analysis of calcium sulfate hydrates using internally standardized mineral oil mulls. Anal. Chem. 1963, 35, 1489–1492. [Google Scholar] [CrossRef]
- RRUFF Data Base. Gypsum. Available online: https://rruff.info/gypsum/display=default/R040029 (accessed on 6 April 2024).
- Kutschera, M.; Nicoleau, L.; Bräu, M. Nano-optimized Construction Materials by Nano-seeding and Crystallization Control. In Nanotechnology in Civil Infrastructure; Gopalakrishnan, K., Birgisson, B., Taylor, P., Attoh-Okine, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 195–205. [Google Scholar] [CrossRef]
- Shukla, J.; Mehta, M.J.; Kumar, A. Effect of ionic liquid additives on the solubility behavior and morphology of calcium sulfate dihydrate (gypsum) in the aqueous sodium chloride system and physicochemical solution properties at 30 °C. J. Chem. Eng. Data 2018, 63, 2743–2752. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Bourakhouadar, M. Kinetics and mechanism of the thermal degradation of biopolymers chitin and chitosan using thermogravimetric analysis. Polym. Degrad. Stab. 2016, 130, 1–9. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management, Life Cycle Assessment, Principles and Framework. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 10 April 2024).
- ISO 14044; Environmental Management, Life Cycle Assessment, Requirements and Guidelines. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/38498.html (accessed on 10 April 2024).
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 1876–1885. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Akpan, E.I.; Gbenebor, O.P.; Adeosun, S.O. Synthesis and characterisation of chitin from periwinkle (Tympanotonus fusatus (L.)) and snail (Lissachatina fulica (Bowdich)) shells. Int. J. Biol. Macromol. 2018, 106, 1080–1088. [Google Scholar] [CrossRef]
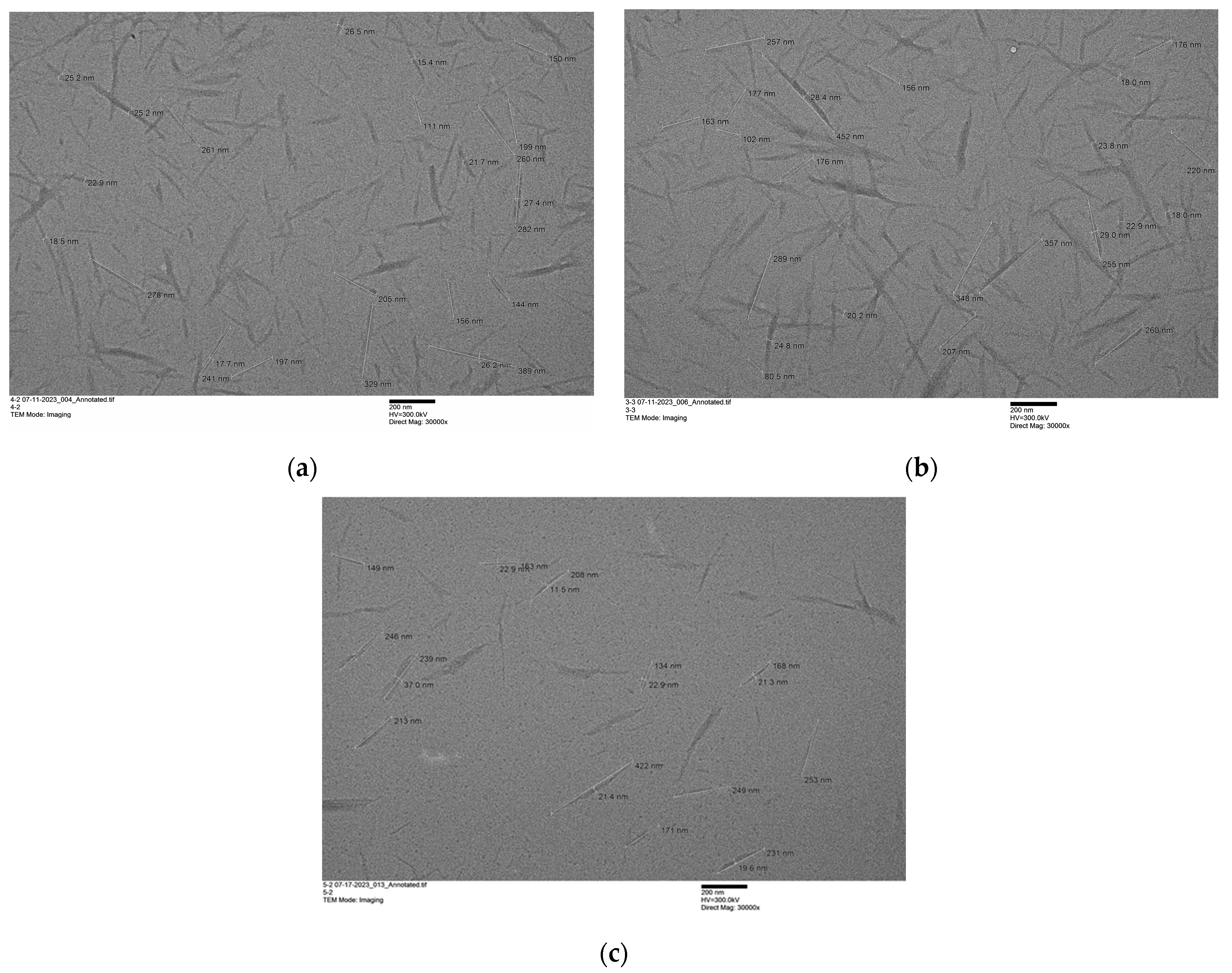
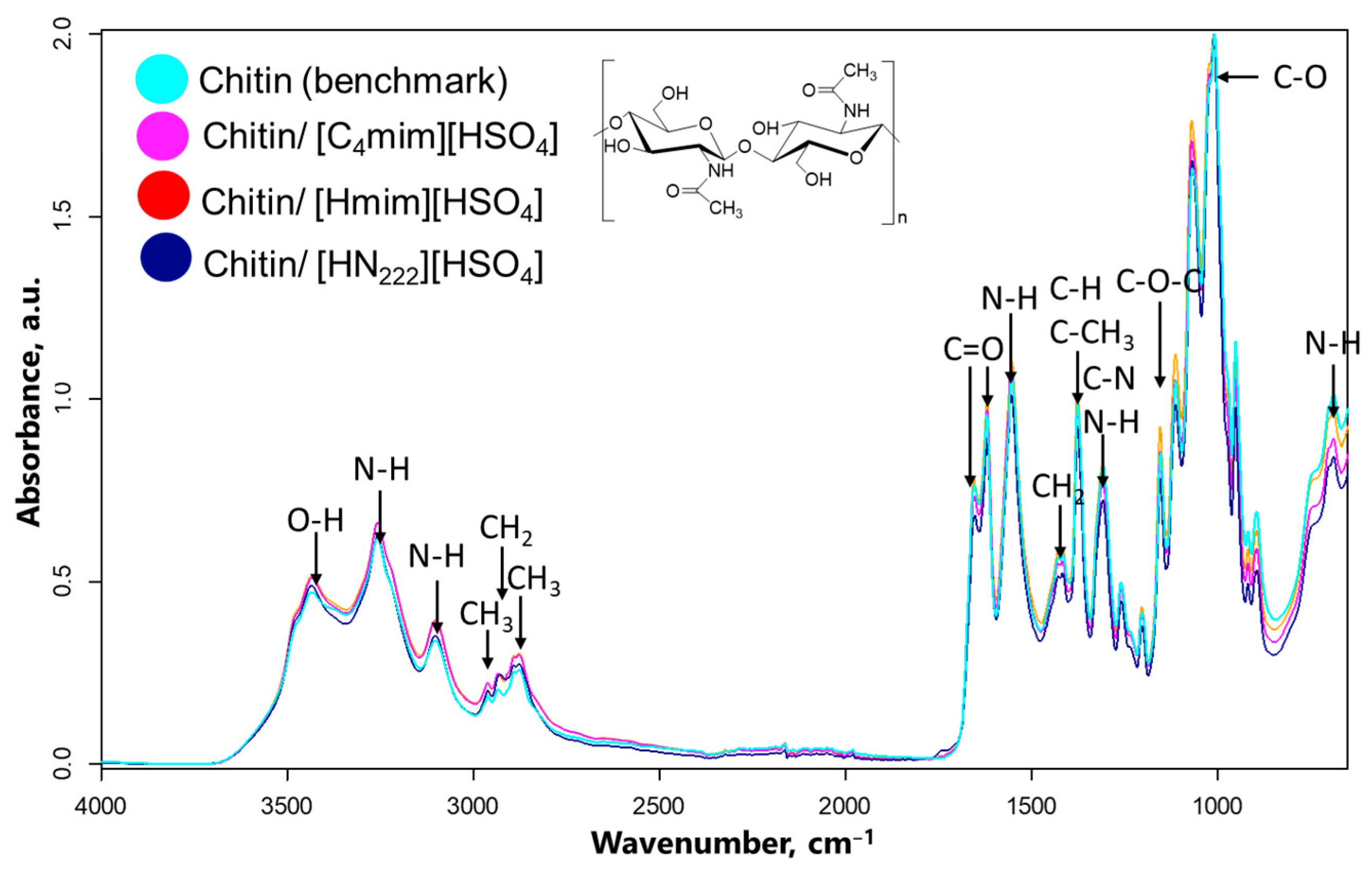
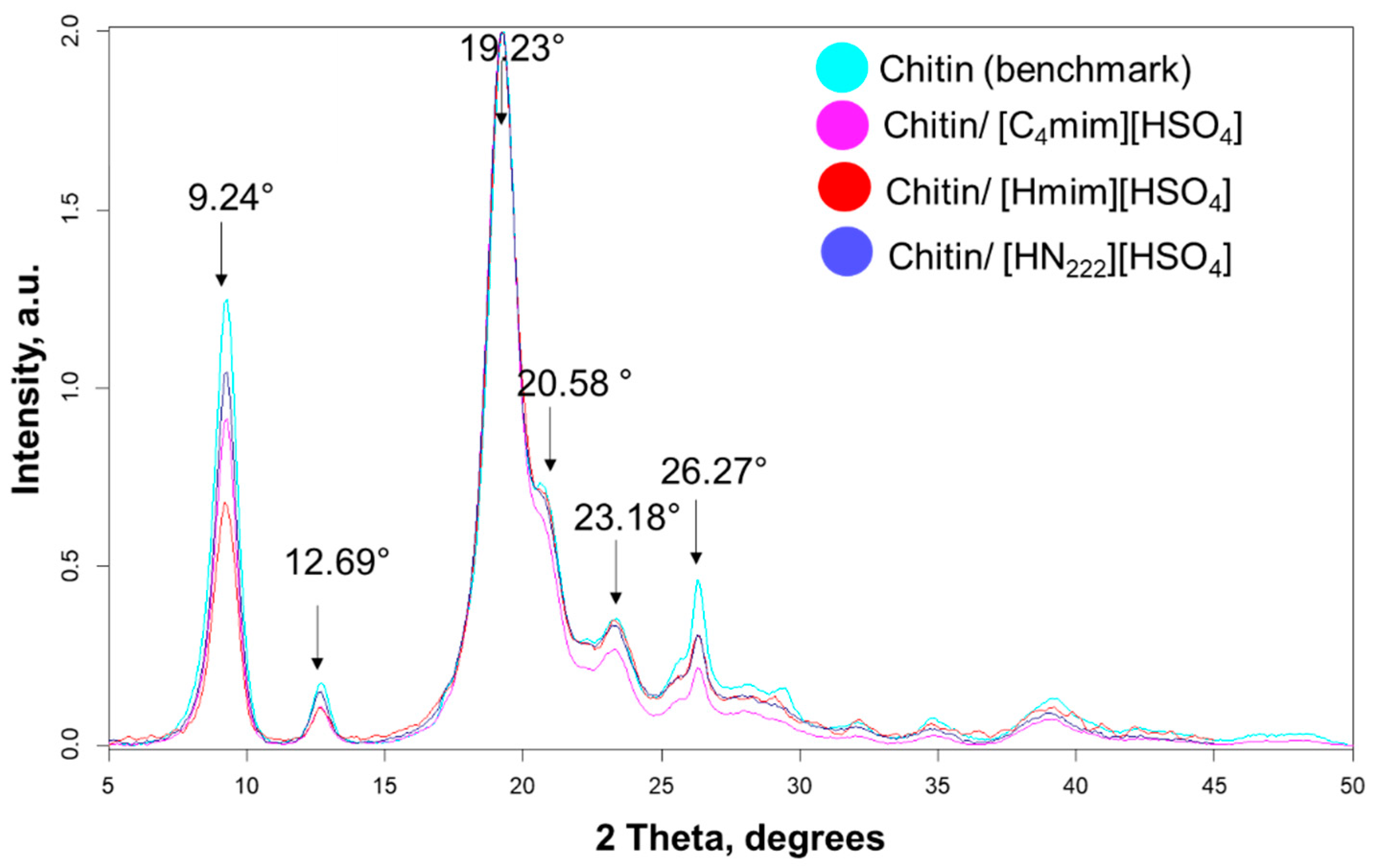
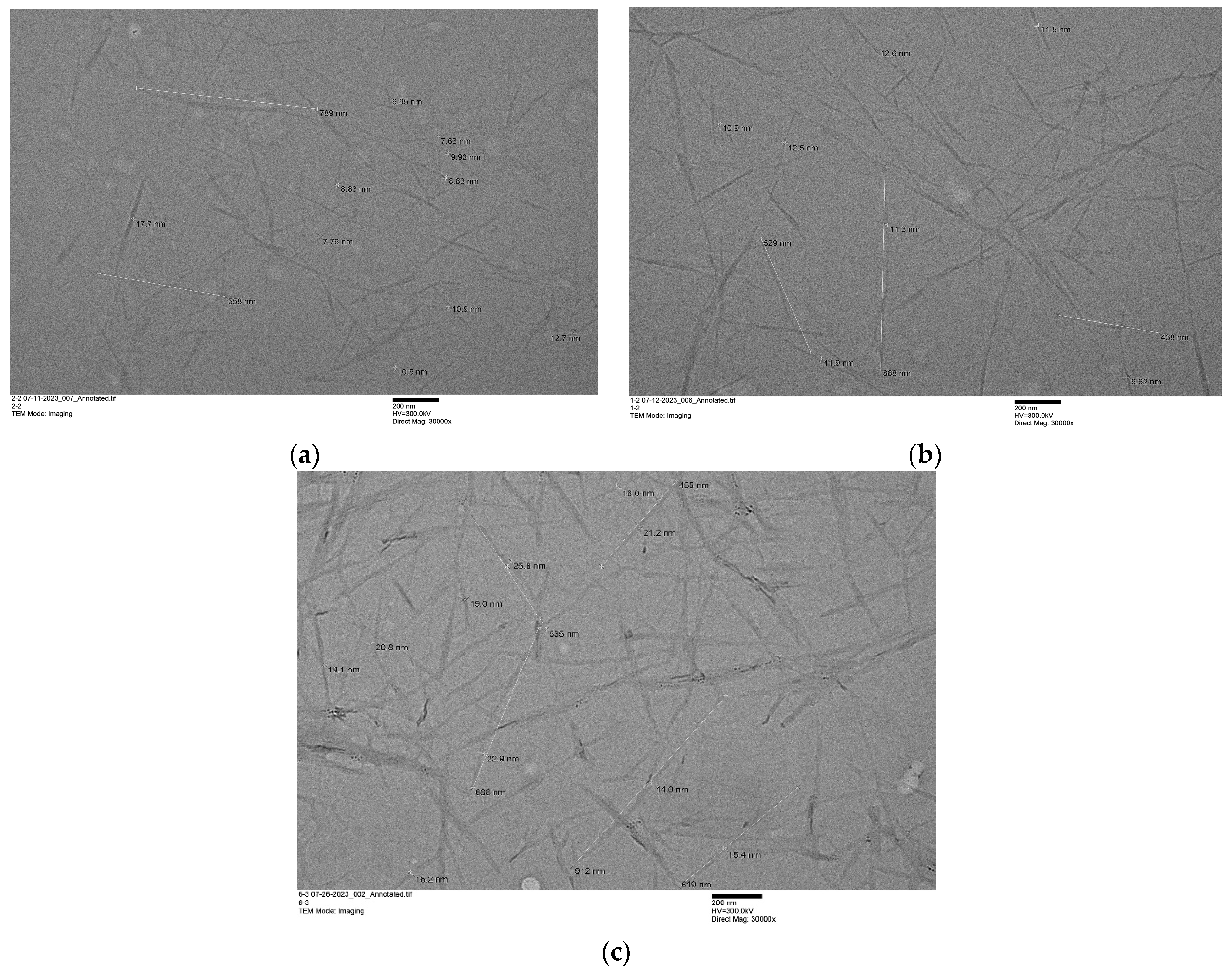
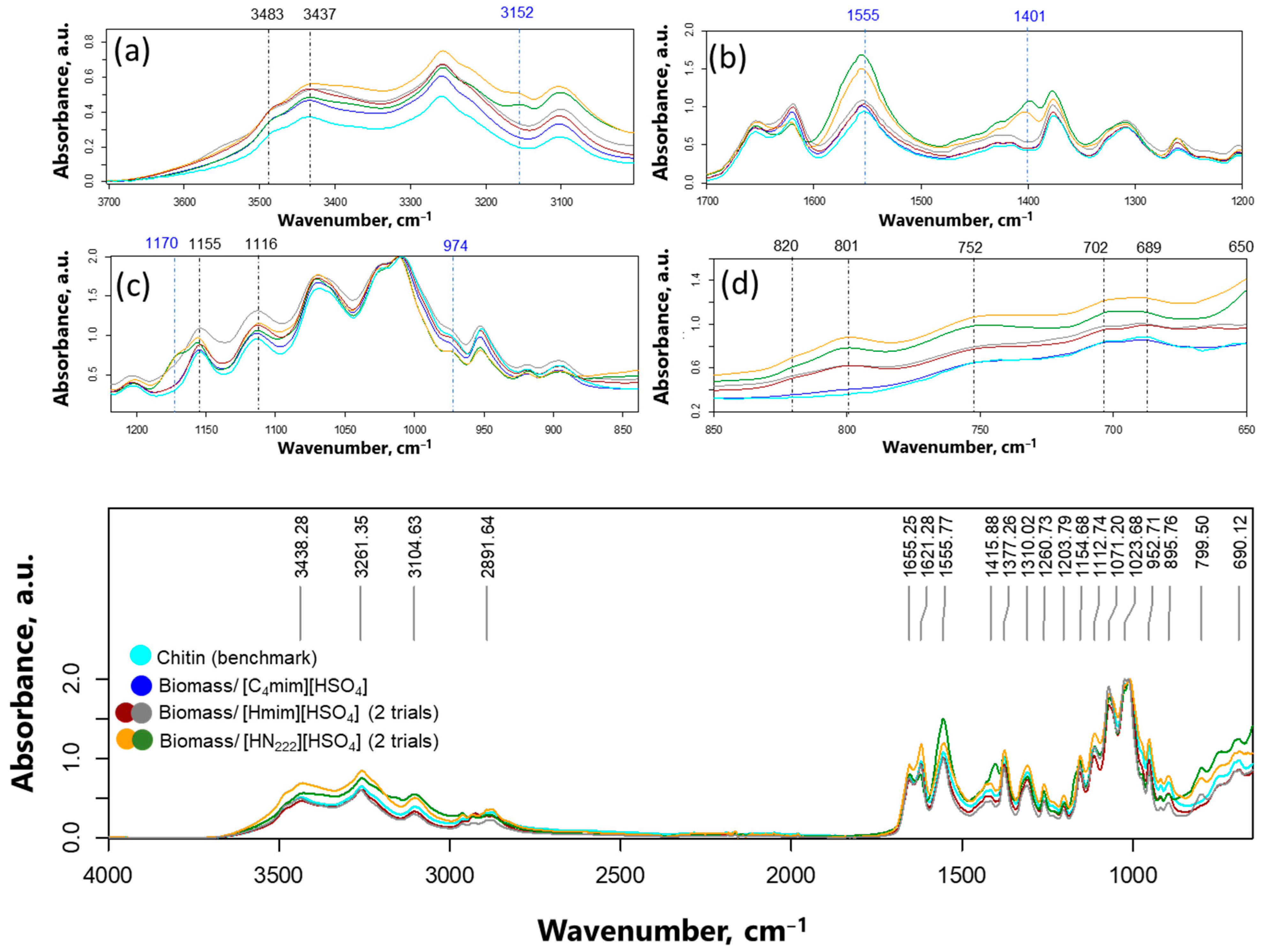
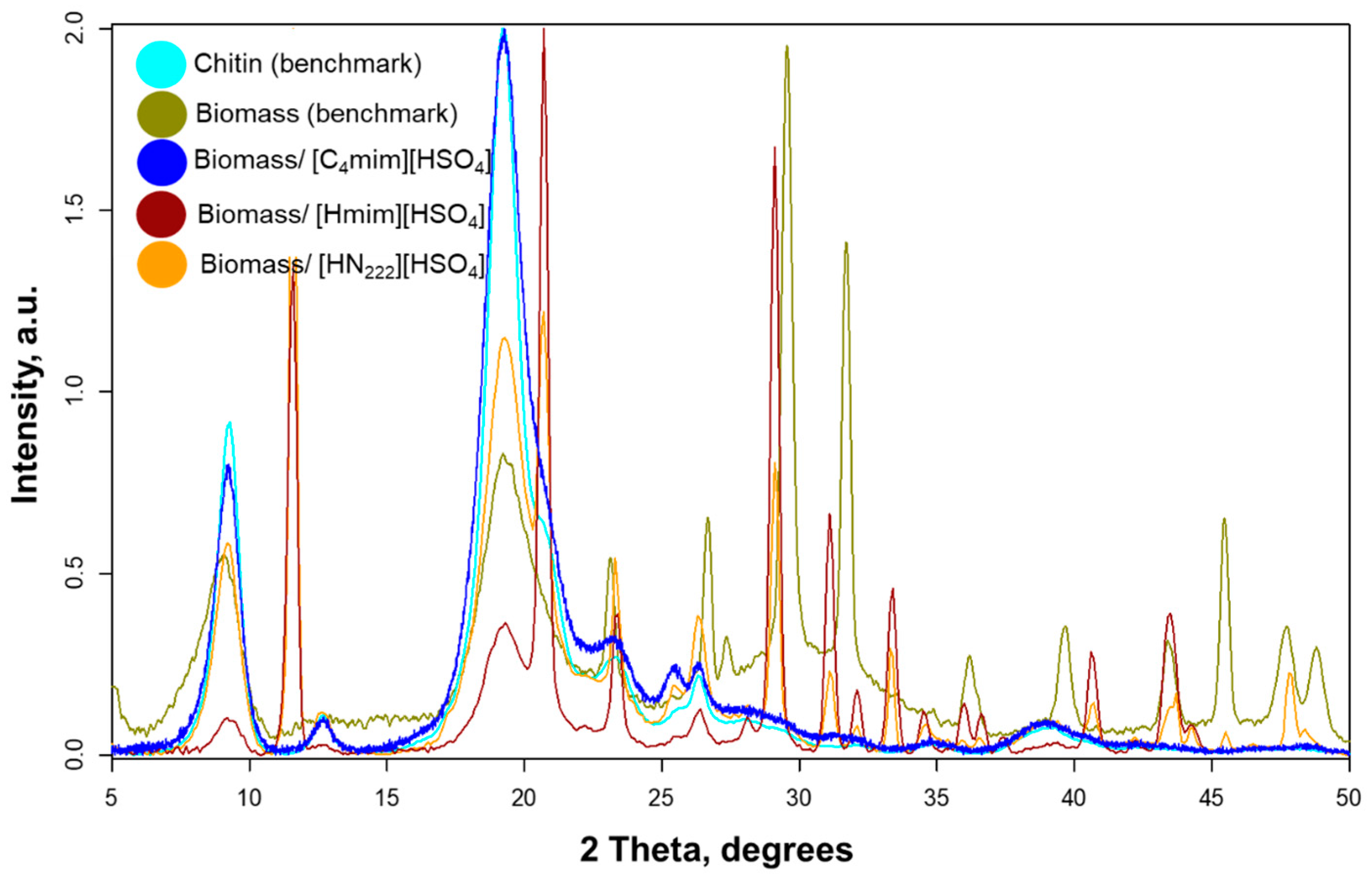
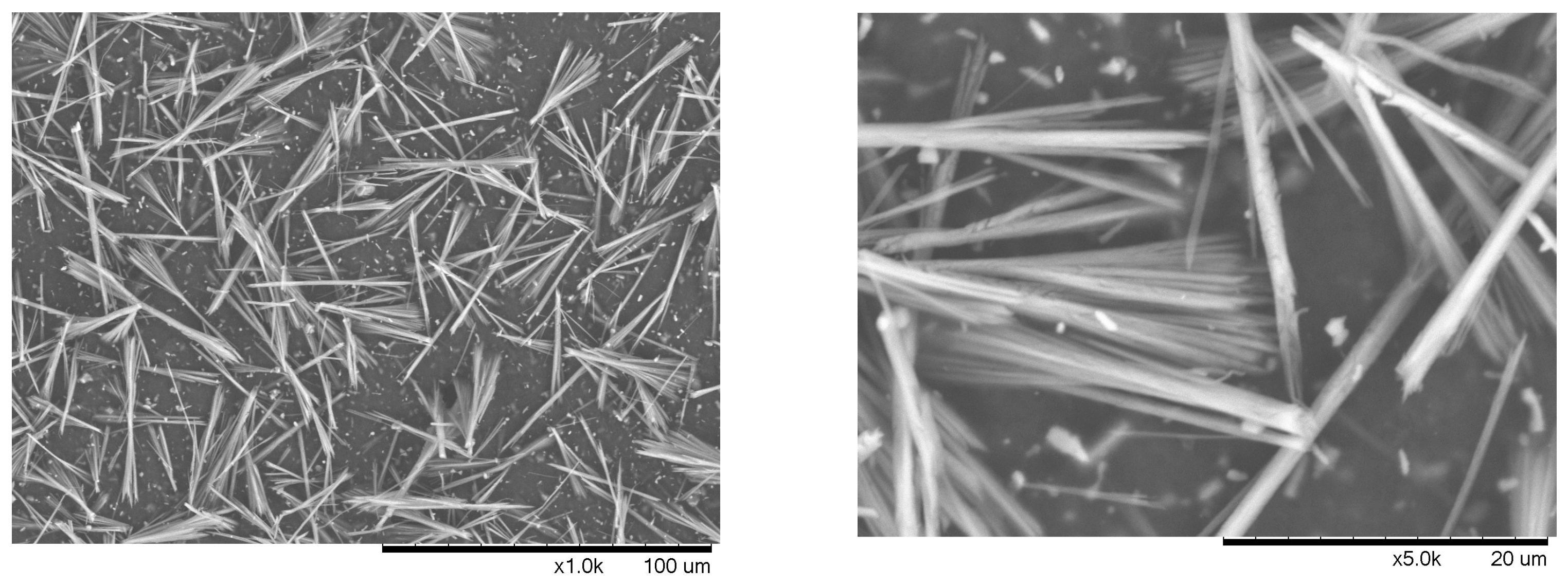
| ChNW Prepared from Chitin Hydrolyzed with | Yield % | Dimensions | Size (nm) | Sample Size | Aspect Ratio | Sample Size |
|---|---|---|---|---|---|---|
| Mean ± STD | Mean ± STD | |||||
| [C4mim][HSO4] * | 54 | Length | 216 ± 78 | 72 | 11.0 ± 4.9 | 38 |
| Diameter | 21.7 ± 5.5 | 38 | ||||
| [Hmim][HSO4] | 55 | Length | 230 ± 84 | 65 | 12.5 ± 6.0 | 65 |
| Diameter | 20.0 ± 7.7 | 79 | ||||
| [HN222][HSO4] | 50 | Length | 246 ± 89 | 94 | 12.1 ± 5.9 | 72 |
| Diameter | 22.2 ± 5.9 | 72 |
| Crystallinity Index via Peak Height (CrI), % | Crystallinity Index via Deconvolution (CrI), % | Crystallite Size at the (0 2 0) Plane, nm | Crystallite Size at the (1 1 0) Plane, nm | |
|---|---|---|---|---|
| Chitin Standard | 98.0 | 84.0 | 6.9 | 5.2 |
| ChNWs/Ch/[HN222][HSO4] | 98.0 | 84.5 | 8.5 | 7.5 |
| ChNWs/Ch/[Hmim][HSO4] | 98.0 | 79.8 | 8.1 | 5.8 |
| ChNWs/Ch/[C4mim][HSO4] | 98.1 | 92.6 | 8.6 | 6.3 |
| ChNW Prepared from SS Hydrolyzed with | Yield % | Dimensions | Size, nm Mean ± STD | Sample Size | Aspect Ratio Mean ± STD | Sample Size |
|---|---|---|---|---|---|---|
| [C4mim][HSO4] * | 75 | Length | 561 ± 157 | 36 | 55.5 ± 22.1 | 36 |
| Diameter | 10.0 ± 3.6 | 90 | ||||
| [Hmim][HSO4] | 80 | Length | 576 ± 168 | 21 | 55.2 ± 28.1 | 21 |
| Diameter | 11.7 ± 2.9 | 62 | ||||
| [HN222][HSO4] | 79 | Length | 612 ± 198 | 41 | 34.8 ± 18.8 | 41 |
| Diameter | 17.3 ± 3.9 | 127 |
| ChNW Prepared from Biomass Hydrolyzed with | Yield % | Dimensions | Size Mean ± STD | DA % | CrI % | Crystallite Size, nm | |
|---|---|---|---|---|---|---|---|
| (0 2 0) | (1 1 0) | ||||||
| [C4mim][HSO4] * | 75 | Length | 561 ± 157 | ~100 a | 80.6 | 8.4 | 5.6 |
| Diameter | 10.0 ± 3.6 | ||||||
| [HN222][HSO4] | 72 | Length | 588 ± 163 | ~100 a | 79.4 | 7.1 | 6.1 |
| Diameter | 17.1 ± 4.1 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkuratov, A.S.; Panackal Shibu, R.; Therasme, O.; Berton, P.; Shamshina, J.L. Sustainable Production of Chitin Nanowhiskers from Crustacean Biomass Using Cost-Effective Ionic Liquids: Strategies to Avoid Byproduct Formation. Sustain. Chem. 2024, 5, 130-148. https://doi.org/10.3390/suschem5020010
Shkuratov AS, Panackal Shibu R, Therasme O, Berton P, Shamshina JL. Sustainable Production of Chitin Nanowhiskers from Crustacean Biomass Using Cost-Effective Ionic Liquids: Strategies to Avoid Byproduct Formation. Sustainable Chemistry. 2024; 5(2):130-148. https://doi.org/10.3390/suschem5020010
Chicago/Turabian StyleShkuratov, Alexander S., Reshma Panackal Shibu, Obste Therasme, Paula Berton, and Julia L. Shamshina. 2024. "Sustainable Production of Chitin Nanowhiskers from Crustacean Biomass Using Cost-Effective Ionic Liquids: Strategies to Avoid Byproduct Formation" Sustainable Chemistry 5, no. 2: 130-148. https://doi.org/10.3390/suschem5020010
APA StyleShkuratov, A. S., Panackal Shibu, R., Therasme, O., Berton, P., & Shamshina, J. L. (2024). Sustainable Production of Chitin Nanowhiskers from Crustacean Biomass Using Cost-Effective Ionic Liquids: Strategies to Avoid Byproduct Formation. Sustainable Chemistry, 5(2), 130-148. https://doi.org/10.3390/suschem5020010











