Microfluidics for Polymer Microparticles: Opinion on Sustainability and Scalability
Abstract
1. Introduction
2. Microfluidics
2.1. Description of the Process
2.2. Advantages over Conventional Processes
3. Sustainability Assessment
3.1. Waste Generation
3.2. Nature of Solvents
3.3. Raw Materials
4. Scalability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekanem, E.E.; Zhang, Z.; Vladisavljević, G.T. Facile Microfluidic Production of Composite Polymer Core-Shell Microcapsules and Crescent-Shaped Microparticles. J. Colloid Interface Sci. 2017, 498, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Santos, L. Encapsulation of Cosmetic Active Ingredients for Topical Application—A Review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, B.F.; Kermasha, S.; Alli, I.; Mulligan, C.N. Encapsulation in the Food Industry: A Review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [CrossRef]
- Arshady, R. Suspension, Emulsion, and Dispersion Polymerization: A Methodological Survey. Colloid Polym. Sci. 1992, 270, 717–732. [Google Scholar] [CrossRef]
- Gu, X.L.; Zhu, X.; Kong, X.Z.; Tan, Y. Comparisons of Simple and Complex Coacervations for Preparation of Sprayable Insect Sex Pheromone Microcapsules and Release Control of the Encapsulated Pheromone Molecule. J. Microencapsul. 2010, 27, 355–364. [Google Scholar] [CrossRef]
- Oxley, J. Overview of Microencapsulation Process Technologies; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780124045682. [Google Scholar]
- Márquez-Gómez, M.; Galicia-García, T.; Márquez-Meléndez, R.; Ruiz-Gutiérrez, M.; Quintero-Ramos, A. Spray-Dried Microencapsulation of Orange Essential Oil Using Modified Rice Starch as Wall Material. J. Food Process. Preserv. 2018, 42, e13428. [Google Scholar] [CrossRef]
- Oxley, J.D. Spray Cooling and Spray Chilling for Food Ingredient and Nutraceutical Encapsulation; Elsevier Masson SAS: San Antonio, TX, USA, 2012. [Google Scholar]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of Bioactive Compounds by “Extrusion” Technologies: A Review. Crit. Rev. Food Sci. Nutr. 2020, 61, 3100–3118. [Google Scholar] [CrossRef]
- Xu, Q.; Hashimoto, M.; Dang, T.T.; Hoare, T.; Kohane, D.S.; Whitesides, G.M.; Langer, R.; Anderson, D.G.; David, H. Preparation of Monodisperse Biodegradable Polymer Microparticles Using a Microfluidic Flow-Focusing Device for Controlled Drug Delivery. Small 2009, 5, 1575–1581. [Google Scholar] [CrossRef]
- Zhang, Y.; Cattrall, R.W.; Kolev, S.D. Fast and Environmentally Friendly Microfluidic Technique for the Fabrication of Polymer Microspheres. Langmuir 2017, 33, 14691–14698. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Nisisako, T. Polymer Capsules with Tunable Shell Thickness Synthesized via Janus-to-Core Shell Transition of Biphasic Droplets Produced in a Microfluidic Flow-Focusing Device. Sci. Rep. 2020, 10, 4549. [Google Scholar] [CrossRef] [PubMed]
- Frey, C. Fluid Bed Coating-Based Microencapsulation; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780124045682. [Google Scholar]
- Pandey, P.; Turton, R.; Joshi, N.; Hammerman, E.; Ergun, J. Scale-up of Pan-Coating Process. AAPS PharmSciTech 2006, 7, E125–E132. [Google Scholar] [CrossRef] [PubMed]
- El Itawi, H.; Fadlallah, S.; Allais, F.; Perré, P. Green Assessment of Polymer Microparticles Production Processes: A Critical Review. Green Chem. 2022, 24, 4237–4269. [Google Scholar] [CrossRef]
- Fadlallah, S.; Roy, P.S.; Garnier, G.; Saito, K.; Allais, F. Are Lignin-Derived Monomers and Polymers Truly Sustainable? An in-Depth Green Metrics Calculations Approach. Green Chem. 2021, 23, 1495–1535. [Google Scholar] [CrossRef]
- Fadlallah, S.; Mouterde, L.M.M.; Garnier, G.; Saito, K.; Allais, F. Cellulose-Derived Levoglucosenone, a Great Versatile Chemical Platform for the Production of Renewable Monomers and Polymers. ACS Symp. Ser. 2020, 1373, 77–97. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor 25 Years on: The Rise of Green Chemistry and Sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor: Fifteen Years On. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Boskovic, D.; Loebbecke, S. Synthesis of Polymer Particles and Capsules Employing Microfluidic Techniques. Nanotechnol. Rev. 2013, 3, 27–38. [Google Scholar] [CrossRef]
- Chen, P.W.; Erb, R.M.; Studart, A.R. Designer Polymer-Based Microcapsules Made Using Microfluidics. Langmuir 2012, 28, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, Y.; Hong, X.; Liu, Z.; Yuan, W. Porous Microsphere and Its Applications. Int. J. Nanomed. 2013, 8, 1111–1120. [Google Scholar] [CrossRef]
- Elveflow.com Microfluidics: A General Overview of Microfluidics. Available online: https://www.elveflow.com/microfluidic-reviews/general-microfluidics/microfluidics-definitions/ (accessed on 7 February 2023).
- Xu, S.; Nie, Z.; Seo, M.; Lewis, P.; Kumacheva, E.; Stone, H.A.; Garstecki, P.; Weibel, D.B.; Gitlin, I.; Whitesides, G.M. Generation of Monodisperse Particles by Using Microfluidics: Control over Size, Shape, and Composition. Angew. Chem. 2005, 117, 734–738. [Google Scholar] [CrossRef]
- Yang, B.; Lu, Y.; Luo, G. Controllable Preparation of Polyacrylamide Hydrogel Microspheres in a Coaxial Microfluidic Device. Ind. Eng. Chem. Res. 2012, 51, 9016–9022. [Google Scholar] [CrossRef]
- Dubey, R.; Shami, T.C.; Bhasker Rao, K.U. Microencapsulation Technology and Applications. Def. Sci. J. 2009, 59, 82–95. [Google Scholar]
- Dormer, N.H.; Berkland, C.J.; Singh, M. Monodispersed Microencapsulation Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780124045682. [Google Scholar]
- Etesami, N.; Nasr Esfahany, M.; Bagheri, R. Effect of the Phase Ratio on the Particle Properties of Poly(Vinyl Chloride) Resins Produced by Suspension Polymerization. J. Appl. Polym. Sci. 2010, 110, 2748–2755. [Google Scholar] [CrossRef]
- Teunou, E.; Poncelet, D. Rotary Disc Atomisation for Microencapsulation Applications-Prediction of the Particle Trajectories. J. Food Eng. 2005, 71, 345–353. [Google Scholar] [CrossRef]
- Abkarian, M.; Loiseau, E.; Massiera, G. Continuous Droplet Interface Crossing Encapsulation (CDICE) for High Throughput Monodisperse Vesicle Design. Soft Matter 2011, 7, 4610–4614. [Google Scholar] [CrossRef]
- El Itawi, H.; Lalanne, B.; Massiera, G.; Le Sauze, N.; Masbernat, O. Numerical Simulation of the Crossing of a Liquid-Liquid Interface by a Droplet. Phys. Rev. Fluids 2020, 5, 093601. [Google Scholar] [CrossRef]
- Dubinsky, S.; Zhang, H.; Nie, Z.; Gourevich, I.; Voicu, D.; Deetz, M.; Kumacheva, E. Microfluidic Synthesis of Macroporous Copolymer Particles. Macromolecules 2008, 41, 3555–3561. [Google Scholar] [CrossRef]
- El Itawi, H.; Fadlallah, S.; Leephakphumphanich, W.; Ruscassier, N.; Zoghlami, A.; Allais, F.; Perre, P. Online Microfluidic Production of Sustainable CyreneTM-Derived Porous Microparticles. Sustainability 2023, 15, 2023. [Google Scholar] [CrossRef]
- Kost, B.; Kunicka-Styczyńska, A.; Plucińska, A.; Rajkowska, K.; Basko, M.; Brzeziński, M. Microfluidic Preparation of Antimicrobial Microparticles Composed of L-Lactide/1,3-Dioxolane (Co)Polymers Loaded with Quercetin. Food Chem. 2022, 396, 133639. [Google Scholar] [CrossRef]
- Zhang, R.; Zhong, L.; Liu, X.; Liu, X.; Chen, Q.; Wu, P.; He, J.; Li, Y.; Zhao, Y.; Liu, Z.; et al. Microfluidic PLGA Microcapsules with PD-L1 Aptamers and Docetaxel Encapsulation for Enhancing Tumor Immunity. Appl. Mater. Today 2022, 27, 101484. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and Techniques for Solvent Selection: Green Solvent Selection Guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Baer, A. Not Enough Water to Go Round? Int. Soc. Sci. J. 1996, 48, 277–292. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and Biocompatibility of PLA and PLGA Microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H.; Picchioni, F. Material Encapsulation in Poly(Methyl Methacrylate) Shell: A Review. J. Appl. Polym. Sci. 2019, 136, 48039. [Google Scholar] [CrossRef]
- Souza, L.; Al-Tabbaa, A. Microfluidic Fabrication of Microcapsules Tailored for Self-Healing in Cementitious Materials. Constr. Build. Mater. 2018, 184, 713–722. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Roslan, M.R.; Nasir, N.F.M.; Cheng, E.M.; Amin, N.A.M. Tissue Engineering Scaffold Based on Starch: A Review. In Proceedings of the 2016 International Conference on Electrical, Electronics, and Optimization Techniques (ICEEOT), Chennai, India, 3–5 March 2016; pp. 1857–1860. [Google Scholar] [CrossRef]
- Mucha, M.; Ludwiczak, S.; Kawinska, M. Kinetics of Water Sorption by Chitosan and Its Blends with Poly(Vinyl Alcohol). Carbohydr. Polym. 2005, 62, 42–49. [Google Scholar] [CrossRef]
- No, H.K.; Prinyawiwatkul, W. Stability of Chitosan Powder during Long-Term Storage at Room Temperature M. J. Agric. Food Chem. 2009, 57, 8434–8438. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, T.; Sun, W.; Ren, X. The Depolymerization of Sodium Alginate by Oxidative Degradation. Pharm. Dev. Technol. 2012, 17, 763–769. [Google Scholar] [CrossRef]
- Sreenivasaya, M.; Pirie, N.W. The Disintegration of Tobacco Mosaic Virus Preparations with Sodium Dodecyl Sulphate. Biochem. J. 1938, 32, 1707–1710. [Google Scholar] [CrossRef]
- Llenado, R.A.; Neubecker, T.A. Surfactants. Anal. Chem. 1983, 55, 93–102. [Google Scholar] [CrossRef]
- Qutachi, O.; Vetsch, J.R.; Gill, D.; Cox, H.; Scurr, D.J.; Hofmann, S.; Müller, R.; Quirk, R.A.; Shakesheff, K.M.; Rahman, C.V. Injectable and Porous PLGA Microspheres That Form Highly Porous Scaffolds at Body Temperature. Acta Biomater. 2014, 10, 5090–5098. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Hughes, T.; Smith, C.; Hibbert, M.; Saito, K.; Simon, G.P. Development of Bio-Acrylic Polymers from CyreneTM: Transforming a Green Solvent to a Green Polymer. Polym. Chem. 2019, 10, 3334–3341. [Google Scholar] [CrossRef]
- Cheng, F.; Zhou, X.; Liu, Y. Methods for Improvement of the Thermal Efficiency during Spray Drying. In Proceedings of the E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 53, pp. 4–6. [Google Scholar]
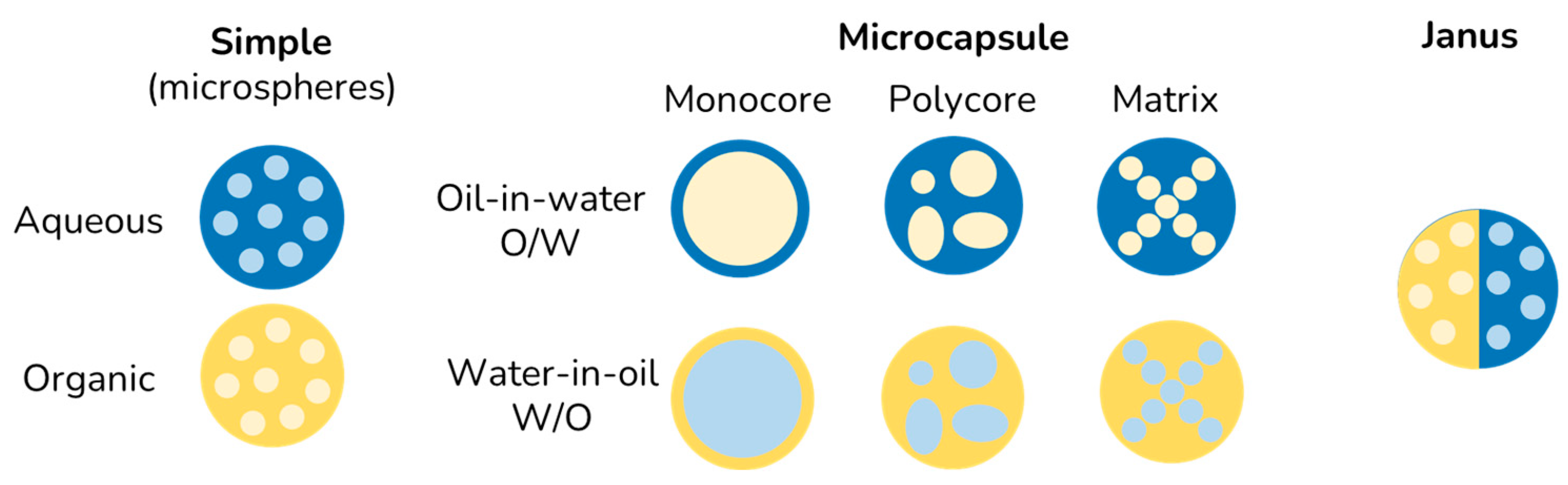
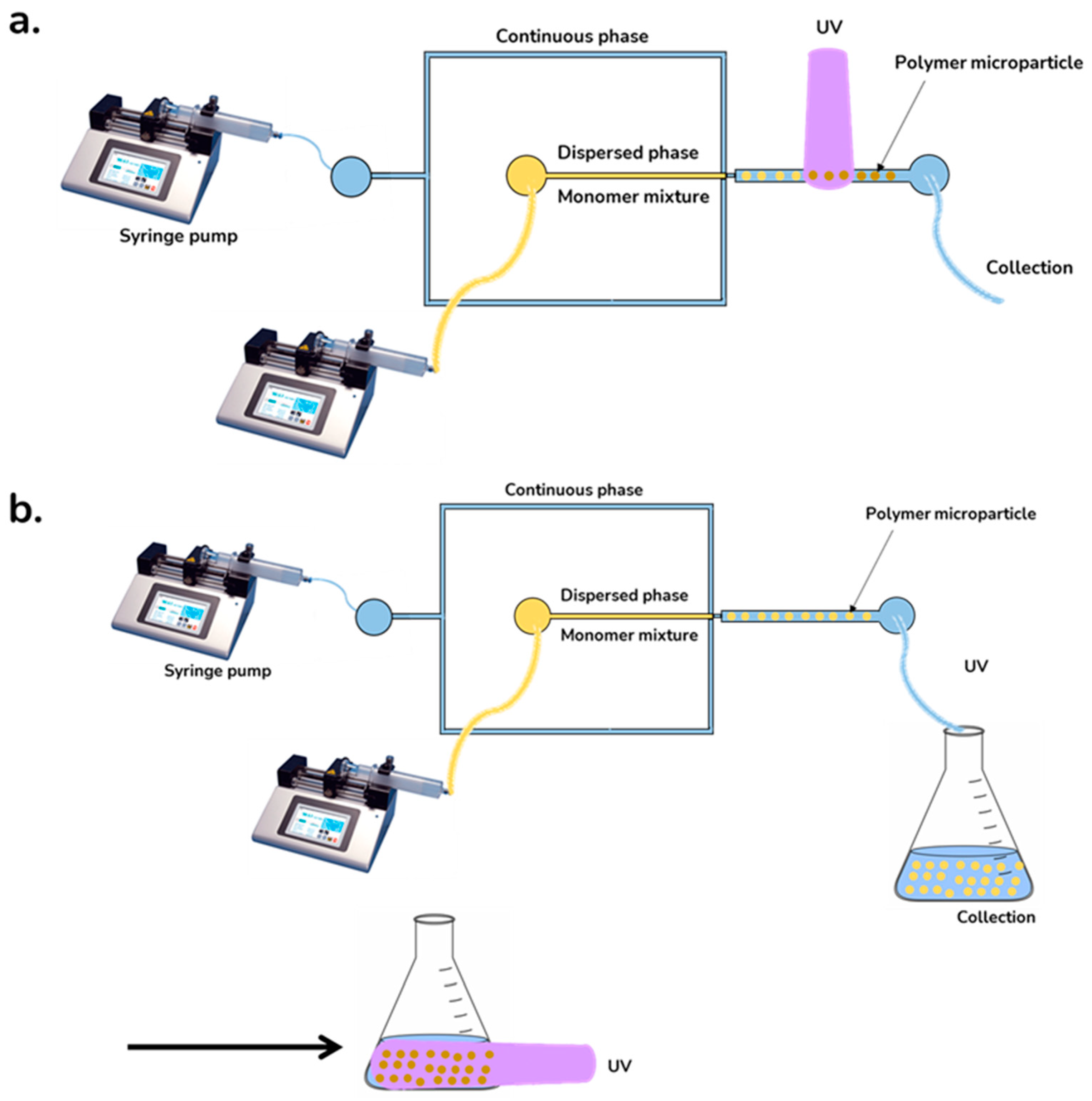
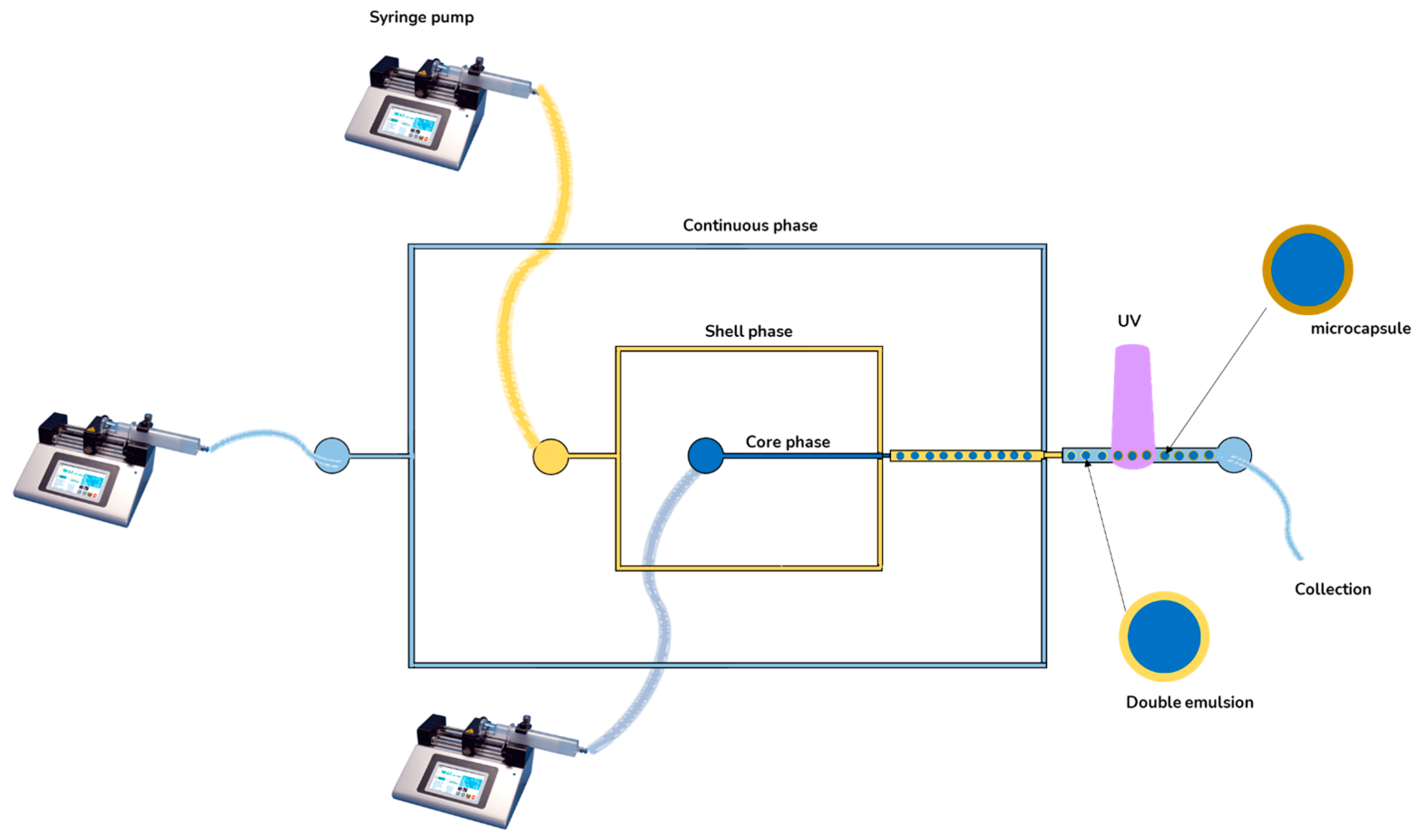
| Morphology | Encapsulated Ingredient | Shell | Solidification | (µm) | Yield (%) | cEF | Ref. | |
|---|---|---|---|---|---|---|---|---|
| P1 | Microspheres | - | GMA and EGDMA comonomer and DEP porogen | In situ UV polymerization with post-off-chip–UV polymerization | 110 ± 3.3 | 100 | 12.2 | [32] |
| P2 | Microspheres | - | GMA and EGDMA comonomer and DOP porogen | In situ UV polymerization with post-off-chip–UV polymerization | 75 ± 2.25 | 100 | 32.7 | [32] |
| P3 | Microcapsules | Silicone oil | HDDA monomer and photoinitiator | Off-chip–UV polymerization | 135 ± 2.7 | 100 | 16.3 | [12] |
| P4 | Microcapsules | Silicone oil | HDDA monomer and thermal initiator | Off-chip thermal polymerization | 135 ± 2.7 | 100 | 16.8 | [12] |
| P5 | Microspheres | - | m-Cyrene and MAN | In situ UV polymerization | 160 ± 4 | N/A | - | [33] |
| P6 | Microspheres | Bupivacaine amphiphilic drug | 3 wt.% PLGA polymer in DCM | Off-chip solvent evaporation | 12 ± 0.46 | 100 | 165 | [10] |
| P7 | Microspheres | - | 1 wt.% PVC polymer in THF | In situ solvent dissolution | 80 ± 2.4 | 97 | 1024 | [11] |
| P8 | Microspheres | Quercetin drug | 5.25 wt.% L-lactide/1,3-dioxolane (co)polymers in DCM | Off-chip solvent evaporation | 60 ± 2 | N/A | - | [34] |
| P9 | Microcapsules | PD-L1 aptamers and chemotherapy drug docetaxel | 3 wt.% PLGA polymer in DCM | Off-chip solvent evaporation | 230 ± 8 | N/A | - | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Itawi, H.; Fadlallah, S.; Perré, P.; Allais, F. Microfluidics for Polymer Microparticles: Opinion on Sustainability and Scalability. Sustain. Chem. 2023, 4, 171-183. https://doi.org/10.3390/suschem4020013
El Itawi H, Fadlallah S, Perré P, Allais F. Microfluidics for Polymer Microparticles: Opinion on Sustainability and Scalability. Sustainable Chemistry. 2023; 4(2):171-183. https://doi.org/10.3390/suschem4020013
Chicago/Turabian StyleEl Itawi, Hassan, Sami Fadlallah, Patrick Perré, and Florent Allais. 2023. "Microfluidics for Polymer Microparticles: Opinion on Sustainability and Scalability" Sustainable Chemistry 4, no. 2: 171-183. https://doi.org/10.3390/suschem4020013
APA StyleEl Itawi, H., Fadlallah, S., Perré, P., & Allais, F. (2023). Microfluidics for Polymer Microparticles: Opinion on Sustainability and Scalability. Sustainable Chemistry, 4(2), 171-183. https://doi.org/10.3390/suschem4020013









