Cyrene™, a Sustainable Solution for Graffiti Paint Removal
Abstract
1. Introduction
2. Materials and Methods
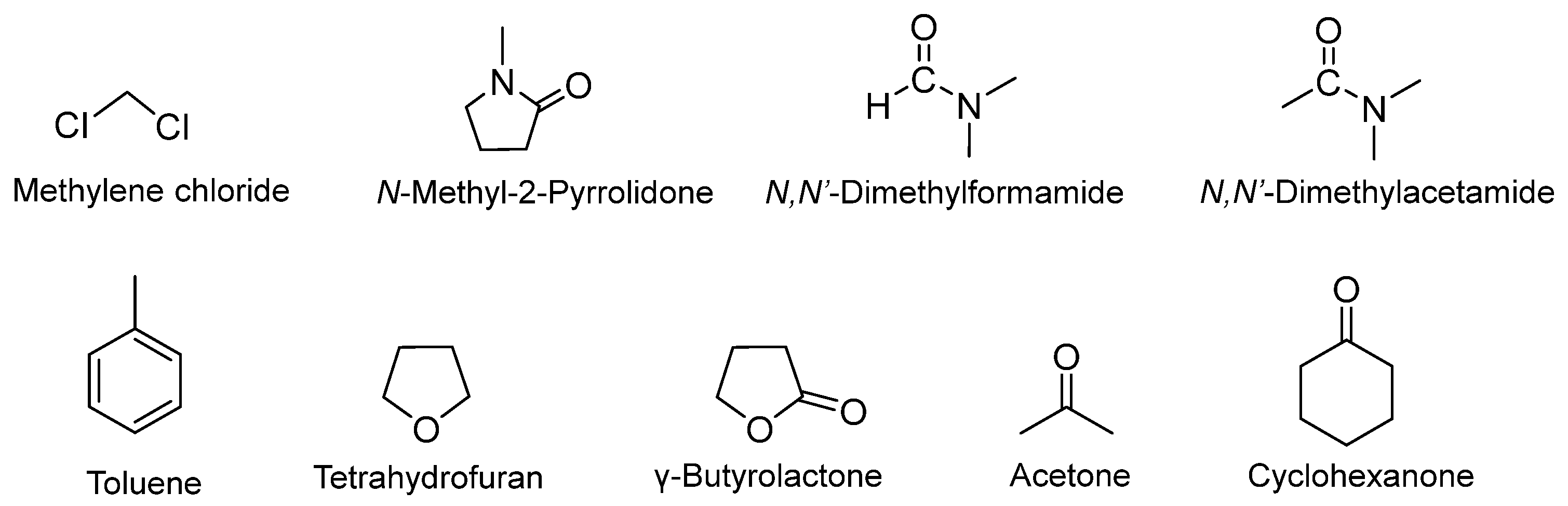
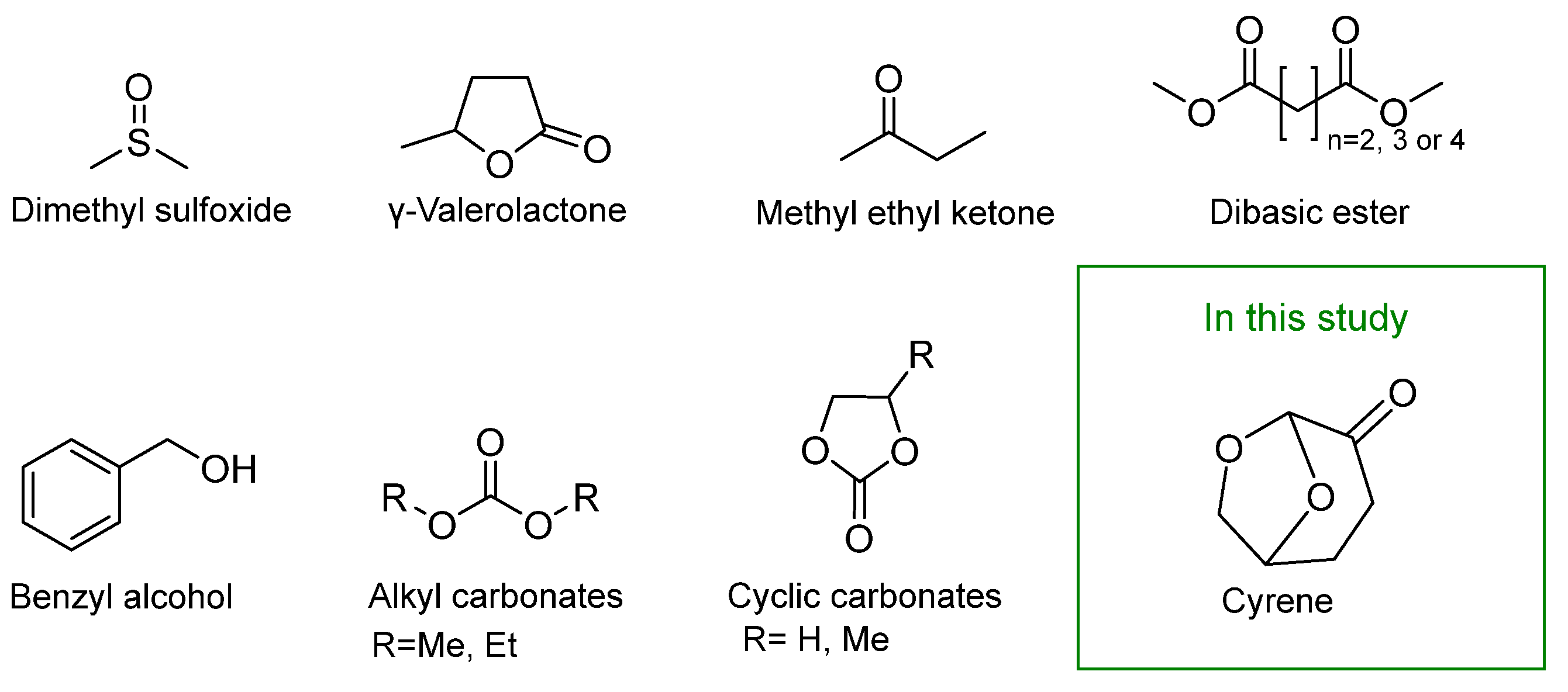
2.1. Solvents
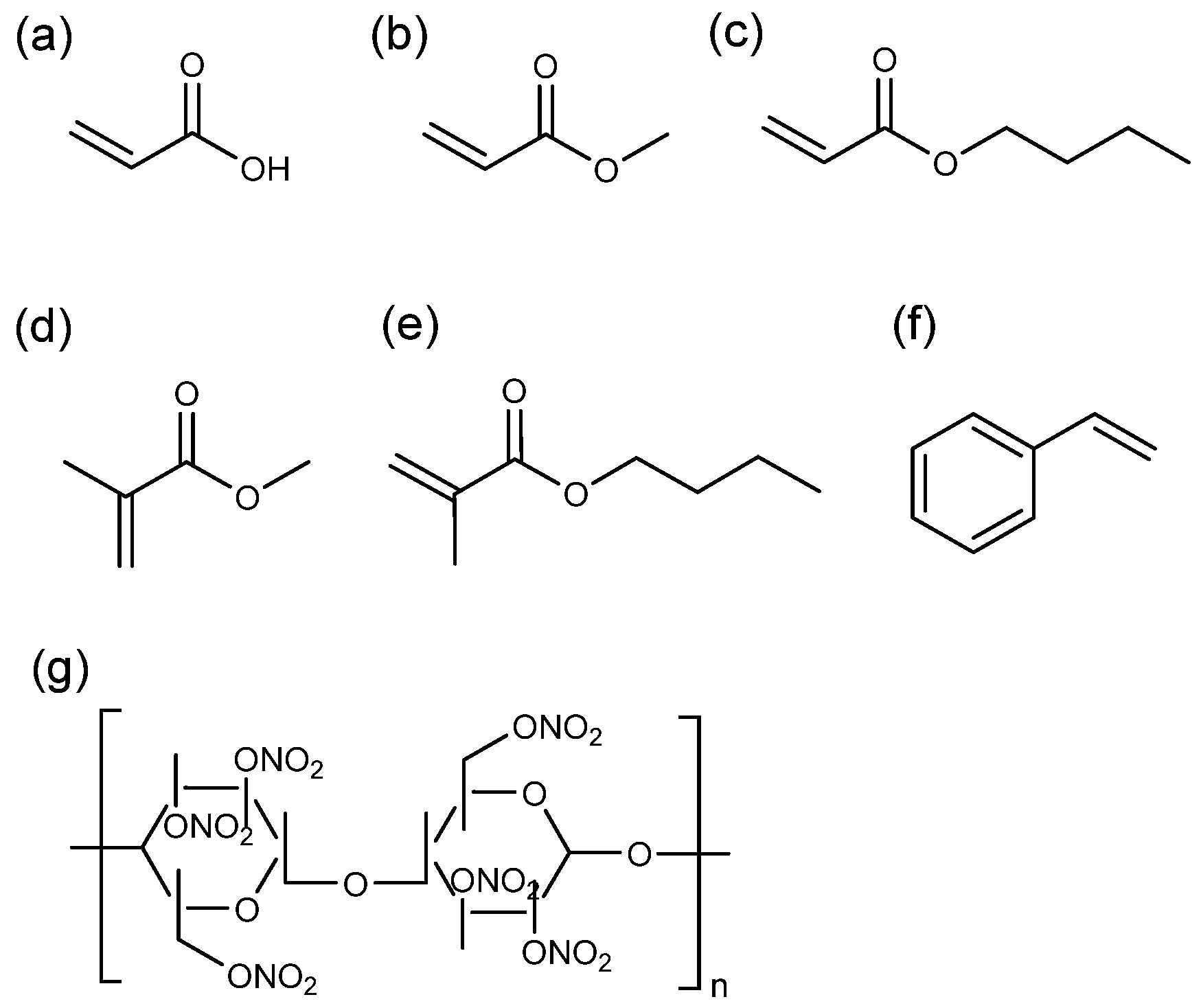
2.2. Substrates and Spray Paints
2.3. Samples Preparation
2.4. Immersion Tests
2.5. Poultice Test
2.6. Paint Characterisation
2.7. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) Analysis
2.8. Hansen Solubility Parameters and Laboratory Cleaning Tests
2.9. Water Content
2.10. Viscosity Measurements
3. Results
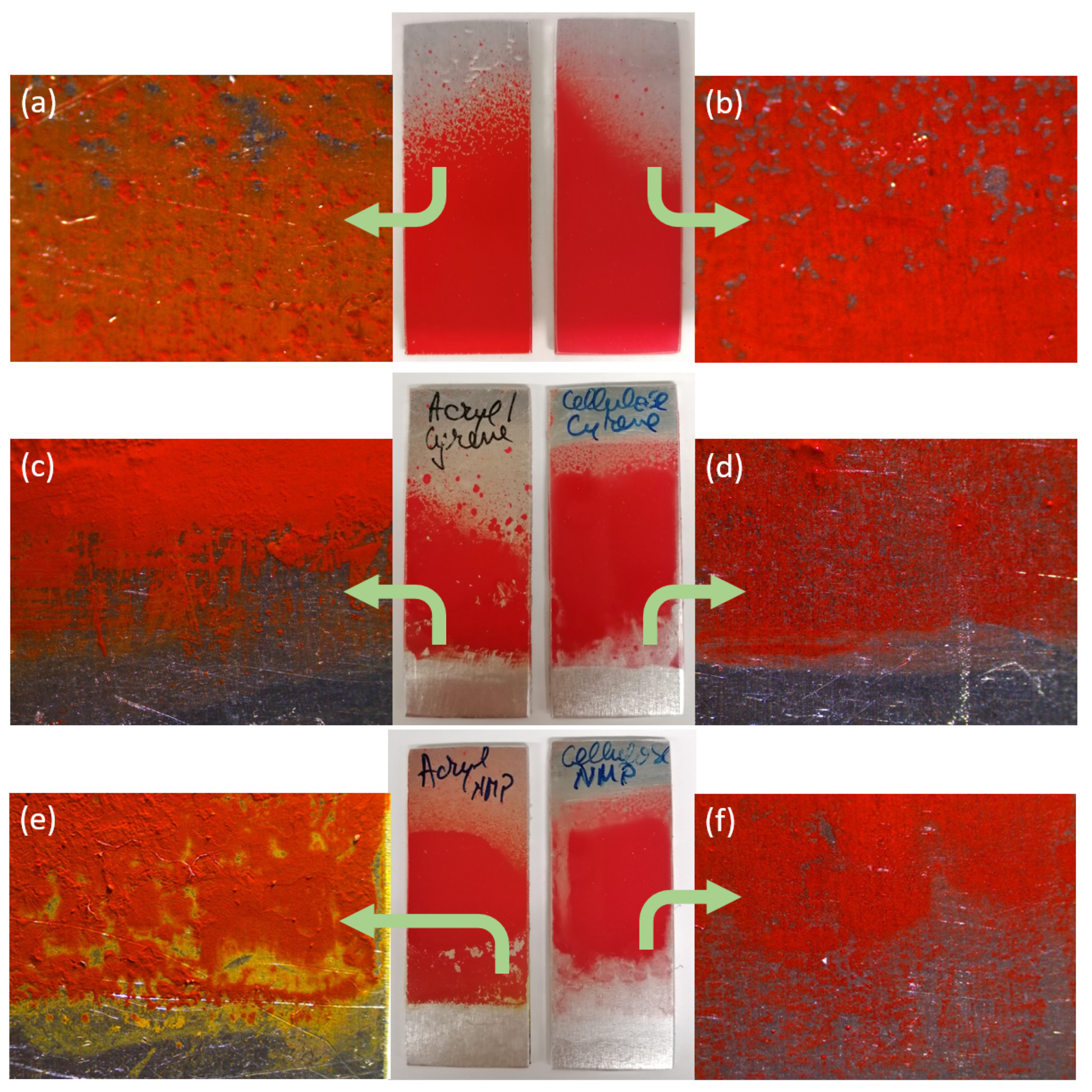
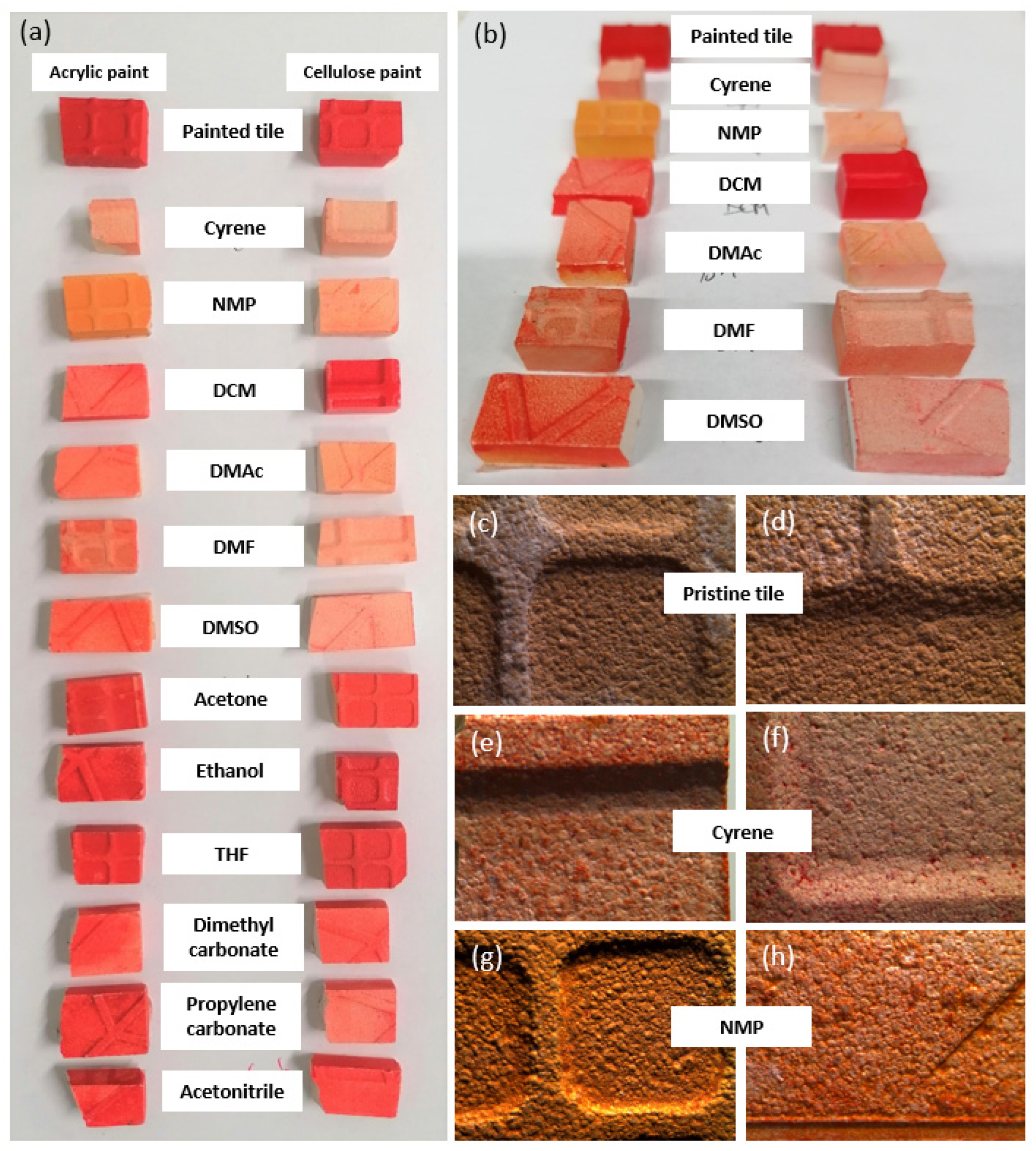
3.1. Paint Removal by Immersion
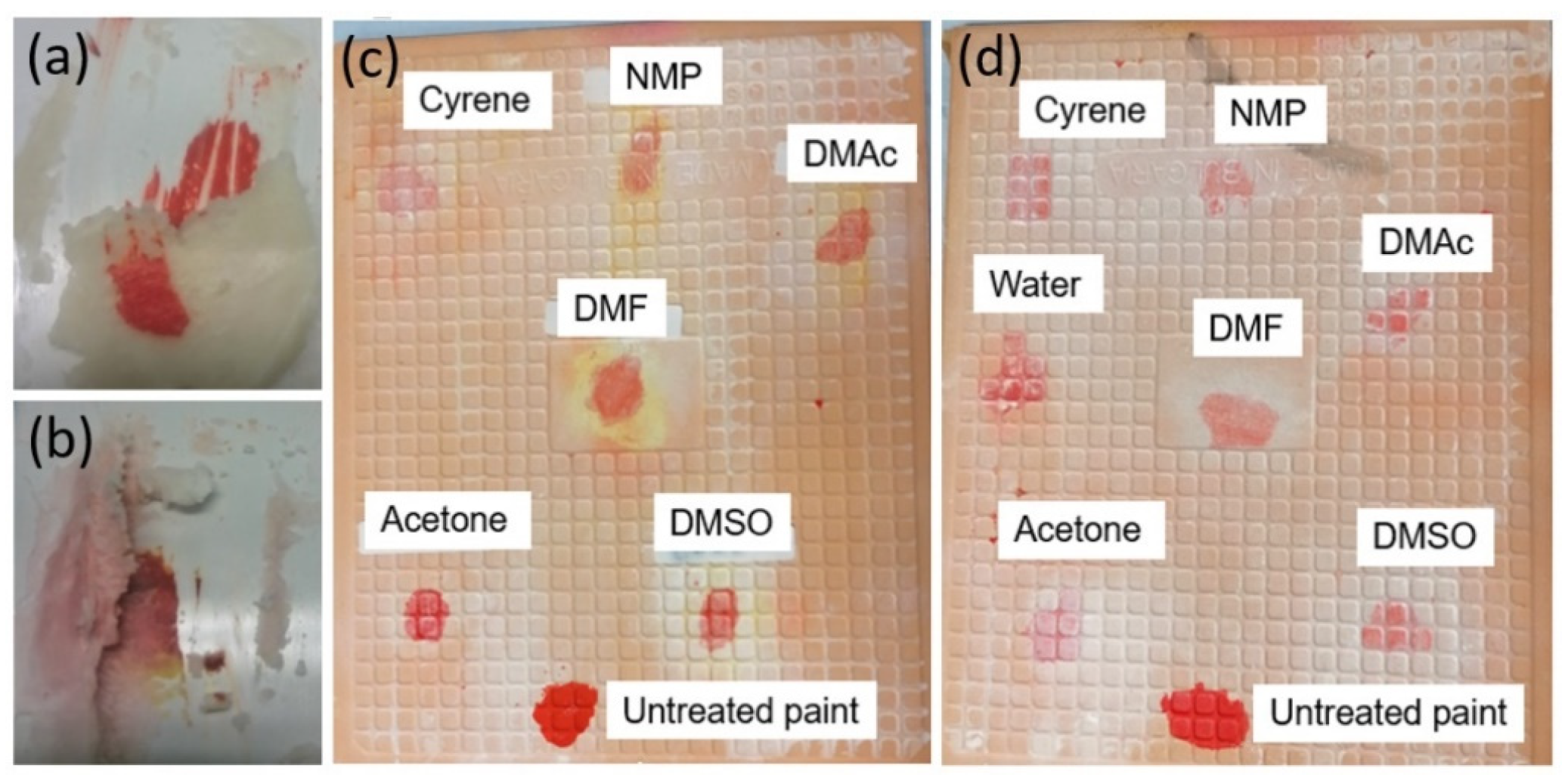
3.2. Paint Removal by Poultices
3.3. The Effect of the Solvent on the Paint
3.4. Infrared Spectroscopy
3.5. ICP-MS Analysis
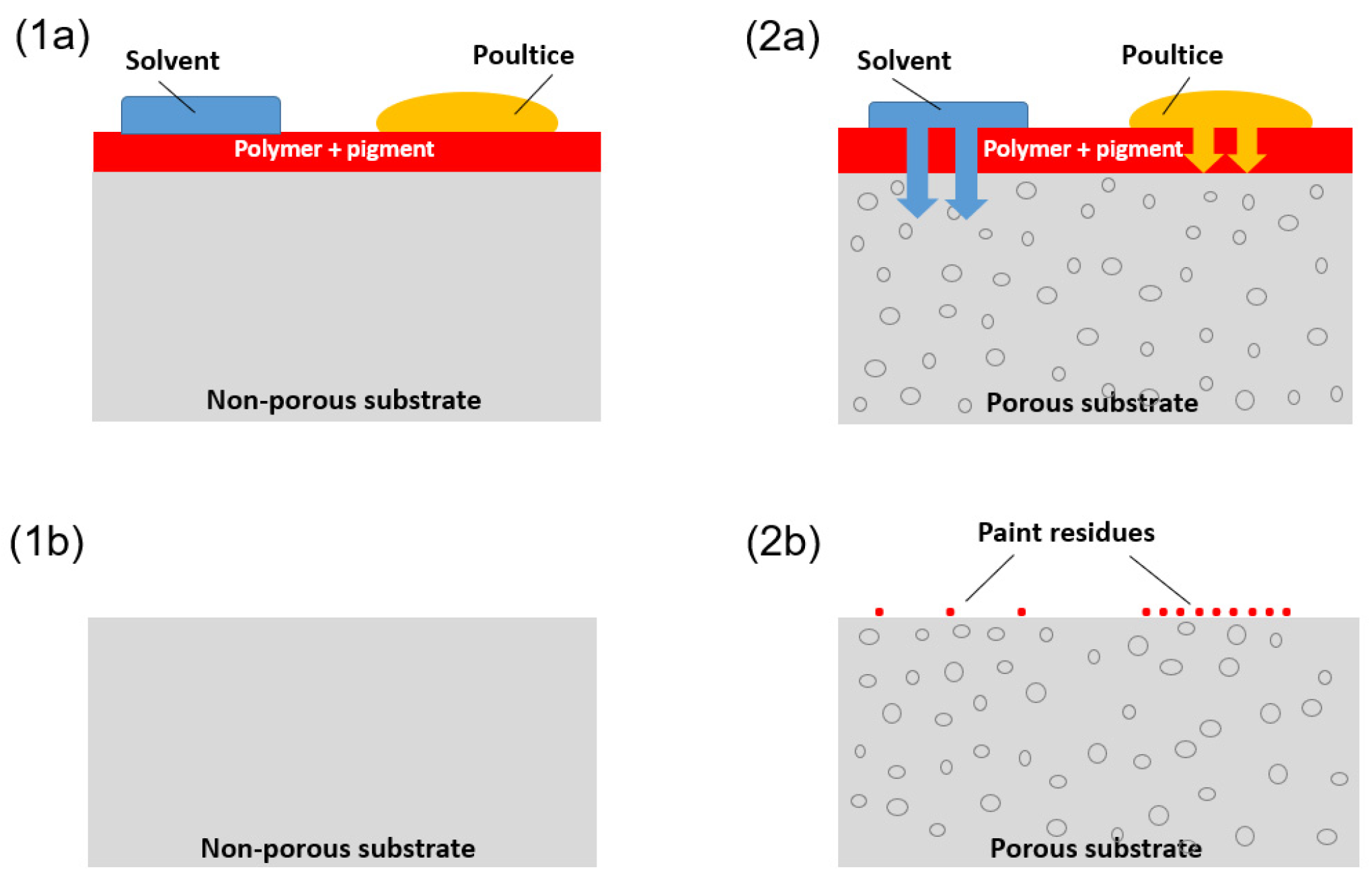
3.6. Paint Removal Mechanism
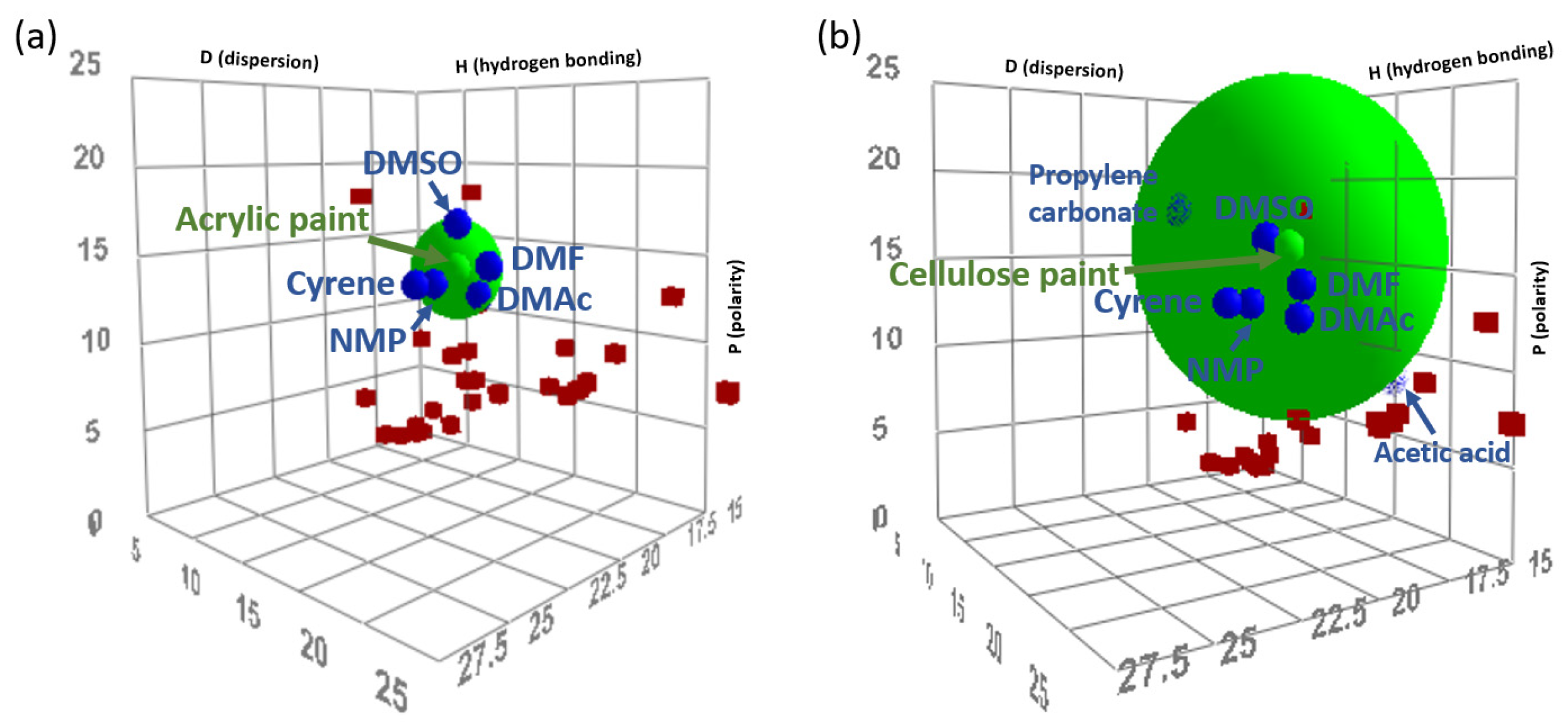
3.7. Solvent–Polymer Interactions Predicted by Solubility Parameters
3.8. The Efficiency of Cyrene-Water Mixture in the Paint Removal
3.9. Other Greener Solvent Systems Proposed for Paint Removal
3.10. Cost and Renewability Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Whitford, M.J. Getting Rid of Graffiti: A Practical Guide to Graffiti Removal and Anti-Graffiti Protection, History and Trends in Graffiti, 1st ed.; Taylor & Francis: London, UK, 1992; Chapter 1; pp. 1–7. [Google Scholar]
- Giorgi, R.; Baglioni, M.; Baglioni, P. Nanofluids and chemical highly retentive hydrogels for controlled and selective removal of overpaintings and undesired graffiti from street art. Anal. Bioanal. Chem. 2017, 409, 3707–3712. [Google Scholar] [CrossRef] [PubMed]
- Garcia, O.; Malaga, K. Definition of the procedure to determine the suitability and durability of an anti-graffiti product for application on cultural heritage porous materials. J. Cult. Herit. 2012, 13, 77–82. [Google Scholar] [CrossRef]
- Sanmartin, P.; Cappitelli, F.; Mitchell, R. Current methods of graffiti removal: A review. Constr. Build. Mater. 2014, 71, 363–374. [Google Scholar] [CrossRef]
- Baglioni, M.; Alterini, M.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Removing Polymeric Coatings With Nanostructured Fluids: Influence of Substrate, Nature of the Film, and Application Methodology. Front. Mater. 2019, 6, 311. [Google Scholar] [CrossRef]
- Gomes, V.; Dionisio, A.; Pozo-Antonio, J.S. Conservation strategies against graffiti vandalism on Cultural Heritage stones: Protective coatings and cleaning methods. Prog. Org. Coat. 2017, 113, 90–109. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Rivas, T.; Lopez, A.J.; Fiorucci, M.P.; Ramil, A. Effectiveness of granite cleaning procedures in cultural heritage: A review. Sci. Total Environ. 2016, 571, 1017–1028. [Google Scholar] [CrossRef]
- Eck, R.W.; Martinelli, D.R. Assessment and mitigation measures for graffiti on highway structures. Maint. Manag. Bridge Struct. 1998, 1642, 35–42. [Google Scholar] [CrossRef]
- Nowotny, T.K.; Velinsky, S.A.; Lasky, T.A.; Donohoe, S.P. Test Driven Design of a System for Removing Graffiti from Retroreflective Signs. Mech. Based Des. Struct. Mach. 2012, 40, 366–379. [Google Scholar] [CrossRef]
- Barreiro, P.; Andreotti, A.; Colombini, M.P.; Gonzalez, P.; Pozo-Antonio, J.S. Influence of the Laser Wavelength on Harmful Effects on Granite Due to Biofilm Removal. Coatings 2020, 10, 196. [Google Scholar] [CrossRef]
- Mueller, M.M.; Moore, J.W.; Doggett, R.A.; Tingstrom, D.H. The effectiveness of contingency-specific and contingency-nonspecific prompts in controlling bathroom graffiti. J. Appl. Behav. Anal. 2000, 33, 89–92. [Google Scholar] [CrossRef]
- Graffiti—Clean up Cost or WindfallG. Available online: https://www.penningtonslaw.com/news-publications/latest-news/graffiti-clean-up-cost-or-windfall (accessed on 18 January 2023).
- Costela, A.; Garcia-Moreno, I.; Gomez, C.; Caballero, O.; Sastre, R. Cleaning graffitis on urban buildings by use of second and third harmonic wavelength of a Nd: YAG laser: A comparative study. Appl. Surf. Sci. 2003, 207, 86–99. [Google Scholar] [CrossRef]
- Musolino, M.; Arico, F.; Tundo, P. An innovative and sustainable approach to spray paint graffiti removal from Istrian stone through the silica sol-gel chemistry: A preliminary assessment. J. Cult. Herit. 2019, 36, 268–274. [Google Scholar] [CrossRef]
- Samolik, S.; Walczak, M.; Plotek, M.; Sarzynski, A.; Pluska, I.; Marczak, J. Investigation into the removal of graffiti on mineral supports: Comparison of nanosecond Nd:YAG laser cleaning with traditional mechanical and chemical methods. Stud. Conserv. 2015, 60, S58–S64. [Google Scholar] [CrossRef]
- Weaver, M.E. Removing Graffiti from Historic Masonry. National Park Service, Technical Preservation Services, 1995, 38. Available online: https://www.nps.gov/tps/how-to-preserve/briefs/38-remove-graffiti.htm (accessed on 18 January 2023).
- Gomes, V.; Dionisio, A.; Pozo-Antonio, J.S. The influence of the SO2 ageing on the graffiti cleaning effectiveness with chemical procedures on a granite substrate. Sci. Total Environ. 2018, 625, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.; Wrbitzky, R.; Blaszkewicz, M.; Schaper, M.; van Thriel, C. Human volunteer study on the inhalational and dermal absorption of N-methyl-2-pyrrolidone (NMP) from the vapour phase. Arch. Toxicol. 2008, 82, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Langford, N.P.; Erismann, D.W. Paint Stripper Containing Benzyl Alcohol Oralkyl-Substituted Derivative and Methylene Chloride or Other Chlorinated Alkane. US5518661A, 21 May 1996. [Google Scholar]
- Lallier, J.P.; Marie, P. Aprotic Polar Solvent/Ether Paint Stripping Compositions. US5308527A, 3 May 1994. [Google Scholar]
- Withers, P.J.; Smart, E.J.; Boulineau, L.; Vlasblom, J.T. Graffiti Removal Composition and Method. WO2011041837A1, 14 April 2011. [Google Scholar]
- Matthews, P.A. Graffiti Removal Composition. GB2191501A, 16 December 1987. [Google Scholar]
- Cvengros, J.; Lengyel, J.; Rotheneder, H. Removal Agent of Graffiti. SK50982007A3, 2007.
- Sullivan, C.J. Paint Stripper Compositions Containing N-methyl-2-pyrrolidone, Aliphatic Hydrocarbons, and Aromatic Hydrocarbons. US5015410A, 14 May 1991. [Google Scholar]
- DCM Restricted by REACH in 2010. Available online: https://echa.europa.eu/documents/10162/0ea58491-bb76-4a47-b1d2-36faa1e0f290 (accessed on 6 July 2020).
- Schumann, D.; Surkow, R. Formula for Removing Color Coats and Various Soil Layers from Surfaces, Method for Producing the Agent, and Method for Cleaning. US20140274855A1, 18 September 2014. [Google Scholar]
- Regulations.gov—Docket Folder Summary. Available online: https://www.regulations.gov/docket?D=EPA-HQ-OPPT-2016-0231 (accessed on 18 January 2023).
- Anundi, H.; Langworth, S.; Johanson, G.; Lind, M.L.; Akesson, B.; Friis, L.; Itkes, N.; Soderman, E.; Jonsson, B.A.G.; Edling, C. Air and biological monitoring of solvent exposure during graffiti removal. Int. Arch. Occup. Environ. Health 2000, 73, 561–569. [Google Scholar] [CrossRef]
- Akesson, B.; Jonsson, B.A.G. Major metabolic pathway for N-methyl-2-pyrrolidone in humans. Drug Metab. Dispos. 1997, 25, 267–269. [Google Scholar]
- Leira, H.L.; Tiltnes, A.; Svendsen, K.; Vetlesen, L. Irritant cutaneous reactions to N-methyl-2-pyrrolidone (NMP). Contact Dermat. 1992, 27, 148–150. [Google Scholar] [CrossRef]
- Saillenfait, A.M.; Gallissot, F.; Langonne, I.; Sabate, J.P. Developmental toxicity of N-methyl-2-pyrrolidone administered orally to rats. Food Chem. Toxicol. 2002, 40, 1705–1712. [Google Scholar] [CrossRef]
- Saillenfait, A.M.; Gallissot, F.; Morel, G. Developmental toxicity of N-methyl-2-pyrrolidone in rats following inhalation exposure. Food Chem. Toxicol. 2003, 41, 583–588. [Google Scholar] [CrossRef]
- Kenneth, C.J. Method of Removing Paint, Varnish, and Lacquer Films from Surfaces. US2438038A, 16 March 1948. [Google Scholar]
- Sullivan, C.J. Paint Stripper Compositions Containing Gamma-Butyrolactone. US5106525A, 21 April 1992. [Google Scholar]
- Bergemann, E.P.; Opre, J.E.; Henneberry, M. Environmentally Friendly Solvent. US6096699A, 1 August 2000. [Google Scholar]
- Leenen, A.; Richardt, P. Graffiti Removal Compositions and the Use Thereof. AU2015278250B2, 7 December 2017. [Google Scholar]
- Trivedi, S.; Fluck, D.; Sehgal, A.; Osborne, A.; Dahanayake, M.S.; Talingting-Pabalan, R.; Ruiz, J.; Aymes, C. Cleaning Compositions Incorporating Green Solvents and Methods for Use. US8222194B2, 17 July 2012. [Google Scholar]
- Durrani, T.; Clapp, R.; Harrison, R.; Shusterman, D. Solvent-based paint and varnish removers: A focused toxicologic review of existing and alternative constituents. J. Appl. Toxicol. 2020, 40, 1325–1341. [Google Scholar] [CrossRef]
- Sherwood, J.; De Bruyn, M.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunt, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.; Zhang, Y.Z.; Xu, G.L.; Christianson, L.; Luengo, F.; Halkoski, T.; Gao, P. Cyrene (TM) blends: A greener solvent system for organic syntheses. Green Chem. 2022, 24, 7184–7193. [Google Scholar] [CrossRef]
- Milescu, R.A.; Zhenova, A.; Vastano, M.; Gammons, R.; Lin, S.L.; Lau, C.H.; Clark, J.H.; McElroy, C.R.; Pellis, A. Polymer Chemistry Applications of Cyrene and its Derivative Cygnet 0.0 as Safer Replacements for Polar Aprotic Solvents. Chemsuschem 2021, 14, 3367–3381. [Google Scholar] [CrossRef]
- Citarella, A.; Amenta, A.; Passarella, D.; Micale, N. Cyrene: A Green Solvent for the Synthesis of Bioactive Molecules and Functional Biomaterials. Int. J. Mol. Sci. 2022, 23, 15960. [Google Scholar] [CrossRef] [PubMed]
- Warne, C.M.; Fadlallah, S.; Whitwood, A.C.; Sherwood, J.; Mouterde, L.M.M.; Allais, F.; Guebitz, G.M.; McElroy, C.R.; Pellis, A. Levoglucosenone-derived synthesis of bio-based solvents and polyesters. Green Chem. Lett. Rev. 2023, 16, 2154573. [Google Scholar] [CrossRef]
- Lin, S.L.; He, S.S.; Sarwar, S.; Milescu, R.A.; McElroy, C.R.; Dimartino, S.; Shao, L.; Lau, C.H. Spray coating polymer substrates from a green solvent to enhance desalination performances of thin film composites. J. Mater. Chem. A 2023, 11, 891–900. [Google Scholar] [CrossRef]
- Sanmartin, P.; Pozo-Antonio, J.S. Weathering of graffiti spray paint on building stones exposed to different types of UV radiation. Constr. Build. Mater. 2020, 236, 117736. [Google Scholar] [CrossRef]
- Govaert, F.; Bernard, M. Discriminating red spray paints by optical microscopy, Fourier transform infrared spectroscopy and X-ray fluorescence. Forensic Sci. Int. 2004, 140, 61–70. [Google Scholar] [CrossRef]
- Giacomucci, L.; Toja, F.; Sanmartin, P.; Toniolo, L.; Prieto, B.; Villa, F.; Cappitelli, F. Degradation of nitrocellulose-based paint by Desulfovibrio desulfuricans ATCC 13541. Biodegradation 2012, 23, 705–716. [Google Scholar] [CrossRef]
- Baglioni, P.; Berti, D.; Bonini, M.; Carretti, E.; Dei, L.; Fratini, E.; Giorgi, R. Micelle, microemulsions, and gels for the conservation of cultural heritage. Adv. Colloid Interface Sci. 2014, 205, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Antonio, J.S.; Rivas, T.; Fiorucci, M.P.; Lopez, A.J.; Ramil, A. Effectiveness and harmfulness evaluation of graffiti cleaning by mechanical, chemical and laser procedures on granite. Microchem. J. 2016, 125, 1–9. [Google Scholar] [CrossRef]
- Scholz, W. Surface Coatings; Paint Additives; Springer: Dordrecht, The Netherlands, 1993; Chapter 31; pp. 539–581. [Google Scholar] [CrossRef]
- Learner, T. A review of synthetic binding media in twentieth-century paints. Conservator 2010, 24, 96–103. [Google Scholar] [CrossRef]
- Pigments Sorted by Elements. Available online: https://colourlex.com/pigments/pigments-by-elements/ (accessed on 27 August 2020).
- Plagemann, P.; Weise, J.; Zockoll, A. Zinc-magnesium-pigment rich coatings for corrosion protection of aluminum alloys. Prog. Org. Coat. 2013, 76, 616–625. [Google Scholar] [CrossRef]
- Synthetic Inorganic Pigments. Available online: https://www.handprint.com/HP/WCL/pigmt1b.html#magnesium (accessed on 22 September 2020).
- Hansen, C.M. The three dimensional solubility parameter II Dyes, emulsifiers, mutual solubility and compatibility, and pigments. J. Paint. Technol. 1967, 511, 505–510. [Google Scholar]
- Hansen, C.M. Universality of Solubility Parameter. Ind. Eng. Chem. Prod. Res. Dev. 1969, 8, 2–11. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Sherwood, J.; De Bruyn, M.; Budarin, V.L.; Ellis, G.J.; Clark, J.H.; Shuttleworth, P.S. Identification of high performance solvents for the sustainable processing of graphene. Green Chem. 2017, 19, 2550–2560. [Google Scholar] [CrossRef]
- Cyrene-GVL and Cyrene-MeTHF (Both 50-50%) Are Commercially Available. Available online: https://www.sigmaaldrich.com/GB/en/search/cyrene-valerolactone%20blend?focus=products&page=1&perpage=30&sort=relevance&term=cyrene-valerolactone%2520blend&type=product (accessed on 18 January 2023).


| Solvent | Graffiti Paint | Substrate | Cleaning Method |
|---|---|---|---|
| Cyrene | acryl, cellulose nitrate | aluminium foil, ceramic tile (both sides) | immersion, poultice |
| N-Methyl-2-Pyrrolidone | acryl, cellulose nitrate | aluminium foil, ceramic tile (both sides) | immersion, poultice |
| N,N′-Dimethylformamide | acryl, cellulose nitrate | aluminium foil, ceramic tile (both sides) | immersion, poultice |
| N,N′-Dimethylacetamide | acryl, cellulose nitrate | ceramic tile (both sides) | immersion, poultice |
| Dimethyl Sulfoxide | acryl, cellulose nitrate | ceramic tile (both sides) | immersion, poultice |
| Methylene Chloride | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Acetone | acryl, cellulose nitrate | ceramic tile (both sides) | immersion, poultice |
| Acetonitrile | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Dimethyl Carbonate | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Propylene Carbonate | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Tetrahydrofuran | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| 2,2,5,5,-Tetramethyloxolane | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Oxymethylene Dimethyl Ethers | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Ethyl Acetate | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Acetic Acid | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Ethanol | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Methanol | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| 1-Butanol | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| 1-Propanol | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| 2-Propanol | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Water | acryl, cellulose nitrate | ceramic tile (both sides) | immersion, poultice |
| Lactic Acid | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Toluene | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Cyclohexene | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Hexane | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Heptane | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Chlorobenzene | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Diethyl Ether | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| 2-Methylfuran | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Propionic Acid | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| β-Pinene | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Benzyl Alcohol | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
| Dichloroethane | acryl, cellulose nitrate | ceramic tile (both sides) | immersion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milescu, R.A.; Farmer, T.J.; Sherwood, J.; McElroy, C.R.; Clark, J.H. Cyrene™, a Sustainable Solution for Graffiti Paint Removal. Sustain. Chem. 2023, 4, 154-170. https://doi.org/10.3390/suschem4020012
Milescu RA, Farmer TJ, Sherwood J, McElroy CR, Clark JH. Cyrene™, a Sustainable Solution for Graffiti Paint Removal. Sustainable Chemistry. 2023; 4(2):154-170. https://doi.org/10.3390/suschem4020012
Chicago/Turabian StyleMilescu, Roxana A., Thomas J. Farmer, James Sherwood, Con R. McElroy, and James H. Clark. 2023. "Cyrene™, a Sustainable Solution for Graffiti Paint Removal" Sustainable Chemistry 4, no. 2: 154-170. https://doi.org/10.3390/suschem4020012
APA StyleMilescu, R. A., Farmer, T. J., Sherwood, J., McElroy, C. R., & Clark, J. H. (2023). Cyrene™, a Sustainable Solution for Graffiti Paint Removal. Sustainable Chemistry, 4(2), 154-170. https://doi.org/10.3390/suschem4020012








