Recent Advances in Biogenic Silver Nanoparticles for Their Biomedical Applications
Abstract
1. Introduction
2. Biosynthesis of AgNPs
2.1. Methods of Biosynthesis: Physical, Chemical, and Biological
2.2. Biological Species for Nanoparticles Synthesis
2.2.1. Bacteria
2.2.2. Actinomycetes
2.2.3. Fungi
2.2.4. Yeast
2.2.5. Algae
2.2.6. Virus
2.2.7. Plants
| Plant | Morphological Characteristics | Application Studied | References |
|---|---|---|---|
| Whole plant | |||
| Swertia paniculata | Spherical, 31–44 nm (TEM) | Antimicrobial activity | [122] |
| Drosera ittatee Labill var. bakoensis | Spherical, 21 ± 4 nm (TEM) | Antimicrobial activity | [123] |
| Brassica oleracea var. botrytis and Raphanus sativus | Spherical, 4–18 nm (TEM) | Antibacterial activity against both Gram-negative (Escherichia coli, Myroides, Pseudomonas aeruginosa) and Gram-positive (Kocuria and Promicromonospora) bacteria | [124] |
| Ajuga bracteosa. | Spherical, 400 nm (SEM) | Antibacterial, antibiofilm, anticancer activity | [125] |
| Sida cordifolia | Spherical, 3–6 nm (TEM) | Antibacterial activity against Aeromonas hydrophila, Pseudomonas fluorescens, Flavobacterium branchiophilum, Edwardsiella tarda, and Yersinia ruckeri, Escherichia coli, Klebsiella pneumonia, Bacillus subtilis, Staphylococcus aureus | [126] |
| Aerial parts | |||
| Ephedra procera C. A. Mey. | Spherical, 20.4 nm (SEM) | Antimicrobial activity against Escherichia coli and Bacillus subtilis Antioxidant activity Antifungal activity against A. flavus, A. niger, and Mucor spp. Anticancer activity against HepG2 Cells | [127] |
| Perovskia abrotanoides | Spherical, 51 nm (SEM) | Antimicrobial activity against Staphylococcus aureus and Bacillus cereus and Gram-negative bacteria E. coli | [128] |
| Dorema ammoniacum D. | Spherical, 24.5 nm (TEM) | Antimicrobial activity against Gram-positive (Bacillus cereus, Staphylococcus aureus) and Gram-negative (Escherichia coli, Salmonella typhimurium) bacteria | [129] |
| Lythrum salicaria | Spherical, 45–65 nm (TEM) | Antimicrobial activity against E. coli and S. aureus Impregnation of AgNPs into organic nanofibers | [130] |
| Pistacia terebinthus (terebinth) | Spherical, 32 nm (SEM) | Antimicrobial, antioxidant, and anticancer effects | [131] |
| Glaucium corniculatum (L.) | Spherical, 45 nm (TEM) | Antibacterial activity | [132] |
| Calotropis procera | Spherical, 22.14 ± 0.42 nm (TEM) | Antibacterial activity against Pseudomonas aeruginosa, Klebsiella pneumonia, Staphylococcus aureus, and Bacillus subtilis bacteria Antibiofilm and photocatalytic degradation | [133] |
| Scurrula parasitica | Spherical, 295, 26.2 ± 0.7 nm (TEM) | Anticancer activity against human lung cancer cells (A549) | [134] |
| Anthemis atropatana | Spherical, 38.89 nm (TEM) | Anticancer activity against colon cancer cell lines (HT29) | [135] |
| Lampranthus coccineus | Spherical, 10.12–27.89 nm (TEM) | Antiviral activity against HAV-10 virus, HSV-1 virus, and CoxB4 virus | [136] |
| Leaves | |||
| Azadirachta indica | Spherical, 40 nm (TEM) | Antimicrobial activity | [137] |
| Barleria longiflora L. | Spherical, 2.4 nm (TEM) | Antimicrobial activity | [138] |
| Thymus kotschyanus | Spherical, 22 nm (XRD) | Antimicrobial activity | [139] |
| Green tea | Spherical, 11 nm (TEM) | Antimicrobial and antibiofilm activity | [140] |
| Cyanthillium cinereum | Spherical, 19.25 ± 0.44 nm | Antimicrobial activity against Staphylococcus aureus, Klebsiella pneumonia, biosensor in neurobiology, catalytic properties, antioxidant potential | [141] |
| Phyla dulcis | Bead-like, 63–114 nm (DLS) | Antimicrobial activity against Escherichia coli O157:H7 (ATCC 43888), Salmonella Typhimurium (novobiocin and nalidixic acid-resistant strain), Listeria monocytogenes (4b; ATCC 19115), and Staphylococcus aureus (ATCC 6538) strains | [142] |
| Passiflora edulis f. flavicarpa | Spherical, 3–7 nm (TEM) | Antimicrobial, antioxidant, photocatalytic activity | [143] |
| Pteris ittate | Spherical, 17.2 nm (XRD) | Antimicrobial and antivirulence activity against P. aeruginosa | [144] |
| Green tea | Quasi-spherical, ~8.3 ± 3.6 nm (TEM) | Antimicrobial and anticancer activity | [145] |
| Populus ciliata | Spherical, 4 nm (TEM) | Antimicrobial activity against Gram-positive (Staphylococcus epidermidis, Streptococcus pyogenes) and Gram-negative bacteria (Klebsiella pneumoniae, Serratia marcescens, Pseudomonas pseudoalcaligenes) | [146] |
| Aloe vera | Spherical, 20.9 nm (XRD) | Antimicrobial activity | [147] |
| Green tea | Spherical, 11 nm | Antimicrobial and antibiofilm activity | [140] |
| Aloe vera | Spherical, 20.9 nm (XRD) | Antimicrobial activity | [147] |
| Stevia rebaudiana | Spherical, 50–100 nm (TEM) | Antibacterial activity | [148] |
| Thymbra spicata L. (Zahter) | Triangles, hexagons, spheres, and irregular shapes, 70.2 nm (TEM) | Shape-dependent antibacterial and cytotoxic activity | [149] |
| Cinnamomum tamala | Spherical, 10 to 12 nm (TEM) | Antibacterial activity against multidrug-resistant bacterial strains (Escherichia coli (EC-1), Klebsiella pneumonia (KP-1), and Staphylococcus aureus (SA-1)). | [150] |
| Cichorium intybus L. (chicory) | Spherical, 50 nm (DLS) | Antibacterial activity against Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) bacteria | [151] |
| Barleria buxifolia | Spherical, 80 nm (DLS) | Antibacterial, antibiofilm, antioxidant, and cytotoxic agent. | [152] |
| Taxus | Circular, 15 nm (SEM) | Antibacterial and anticancer activity | [153] |
| Handroanthus serratifolius | Spherical, 76.02 ± 3.08 nm (DLS) | Antibacterial activity E. coli | [154] |
| Crescentia cujete L. | Spherical, 39.74 nm (TEM) | Antibacterial activity against Bacillus subtilis, Staphylococcus epidermidis, Rhodococcus rhodochrous, Salmonella typhi, Mycobacterium smegmatis, Shigella flexneri, and Vibrio cholerae | [155] |
| Aesculus hippocastanum (horse chestnut) | Spherical, 50 ± 5 nm (SEM) | Antibacterial and antioxidant activity | [156] |
| Litchi chinensis | Spherical, 5–15 nm (TEM) | Antibacterial and sporicidal activity against Bacillus subtilis | [157] |
| Purple heart | Spherical, 98 nm (TEM) | Antibacterial activity | [158] |
| Taxus | Circular, 15 nm (SEM) | Antibacterial and anticancer activity | [153] |
| Datura stramonium | Spherical, 20.43 nm (DLS) | Antibacterial, antioxidant activity, and DNA cleavage activities | [31] |
| Lindera strychnifolia | Spherical, 161, 15.7 ± 1.2 nm (TEM) | Anticancer activity against human lung cancer cells (A549) | [134] |
| Indigofera tinctoria | Spherical, 16.46 nm (TEM) | Anticancer activity against lung cancer cell line (A549) Antimicrobial activity against Gram-positive (Bacillus pumilus, Staphylococcus aureus), Gram-negative (Pseudomonas sp, Escherichia coli) Antifungal activity against Aspergillus fumigatus, and Aspergillus niger Antioxidant activity | [159] |
| Cratoxylum formosum | Spherical, 8.8 ± 0.3 nm (TEM) | Anticancer activity against human lung cancer cells (A549) | [134] |
| Phoebe lanceolata | Spherical, 412, 8.8 ± 0.3 nm (TEM) | Anticancer activity against human lung cancer cells (A549) | [134] |
| Mentha longifolia L. | Spherical, 20–100 nm (SEM) | Anticancer activity against HCT116 colon cancer cells and Leishmania | [160] |
| Rubia cordifolia L. | Spherical, 20.98 nm (TEM) | Anticancer activity, antifungal activity against aflatoxigenic Aspergillus flavus, DNA-binding properties, and DPPH and ABTS free-radical inhibition | [161] |
| Vernonia amygdalina | Spherical, 41.555 ± 2.488 nm (TEM) | Anticancer activities on the human breast cancer cell line MCF-7. | [162] |
| Cinnamomum verum | Spherical, 10 to 45 nm (TEM) | Treatment of Lung Adenocarcinoma | [163] |
| Berberis thunbergii | Spherical, 15 nm (TEM) | Anticancer activity against pancreatic cancer | [164] |
| Aloe arborescens | Spherical, 40–50 nm (TEM) | Wound healing activity | [165] |
| Mentha piperita | Spherical, 35 nm (TEM) | Effect on acetylcholinesterase (AchE) to predict its neurotoxicity. | [166] |
| Aloe vera | Spherical to oval, 10–50 nm (TEM) | chaperone-like activity in the aggregation inhibition of α-chymotrypsinogen A | [167] |
| Stems | |||
| Picea abies | Spherical, 78.48 nm (DLS) | Antibacterial, antifungal, and antimitotic effects | [168] |
| Cannabis sativa (industrial hemp) | Triangular, rods and hexagonal-shaped, 20–40 nm (TEM) | Antibiofilm activity | [169] |
| Ceratostigma minus | Spherical, 16.4 ± 0.3 nm (TEM) | Anticancer activity against human lung cancer cells (A549) | [134] |
| Mucuna birdwoodiana | Spherical, 35.4 ± 5.9 nm (TEM) | Anticancer activity against human lung cancer cells (A549) | [134] |
| Roots | |||
| Jurinea dolomiaea | Spherical, cubic, and triangular 24.58 nm | Antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa Antioxidant activity | [170] |
| Saussurea lappa | Spherical, 20.15 nm (XRD) | Antimicrobial activity | [171] |
| Beta vulgaris L. | Round, 20–50 nm (TEM) | Anticancer activity | [172] |
| Shikonin | Spherical, 20 nm (TEM) | Anticancer activity in human lung carcinoma cell line A549 cells | [173] |
| Myrsine africana | Spherical, 11.4 ± 0.1 nm (TEM) | Anticancer activity against human lung cancer cells (A549) | [134] |
| Tubers | |||
| Turmeric powder | Spherical, 18 ± 0.5 nm (TEM) | Antimicrobial activity | [174] |
| Zingiber zerumbet (L.) | Spherical, 0.2–1 μm (TEM) | Antipneumonial potential in mycoplasmal pneumonia in experimental rats. | [175] |
| Zingiber officinale | Spherical, 12 nm | Antifungal activity against Candida albicans | [176] |
| Pueraria tuberosa | Spherical, 162.72 ± 5.02 nm (DLS) | Anticancer and antioxidant activities | [177] |
| Alpinia officinarum | Spherical, 100 nm (TEM) | Effect against the cisplatin-induced nephrotoxicity | [178] |
| Curcuma longa | Spherical, 44.9 ± 2.2 nm (TEM) | Study human pterygium-derived keratinocytes | [179] |
| Flowers | |||
| Malva sylvestris | Spherical and hexagonal, 20–40 nm (TEM) | Antimicrobial activity against Escherichia coli, Staphylococcus aureus, Streptococcus pyogenes | [180] |
| Wedelia urticifolia (Blume) DC. | Spherical, <30 nm (TEM) | Antimicrobial activity | [181] |
| Abelmoschus esculentus (L.) | Spherical, 16.19 nm (TEM) | Antibacterial and anticancer activity | [182] |
| Madhuca longifolia | Spherical, oval, 30–50 nm (TEM) | Antibacterial activity | [183] |
| Fruits | |||
| Solanum viarum | Spherical, oval 2–40 nm (TEM) | Antimicrobial activity against Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus susp. Aureus, Aspergillus niger, and Candida albicans | [184] |
| Walnut | Spherical, 31.4 nm (DLS) | Antimicrobial, antioxidant, anticancer activity against the MCF-7 tumor cell line | [185] |
| Royal Jelly extract | Spherical, 30–100 nm (DLS) | Antibacterial activity | [186] |
| Brassica oleracea (curly kale) | Spherical | Antibacterial, antidiabetic, antioxidant, and anticancer activity | [187] |
| Benincasa hispida | Spherical, 27 ± 1 nm (TEM) | Antibacterial activity Antibiofilm activity Anticancer activities against the lung cancer cell line (A549) | [188] |
| Orange | Spherical and ovoid morphology | Antibacterial activity | [189] |
| Pomelo | 35 to 40 nm (XRD) | Antibacterial activity | [190] |
| Cocos nucifera (coconut) shell | Spherical, 14.2–22.96 nm (TEM) | Antibacterial activity against Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Salmonella typhimurium | [191] |
| Elm | Spherical, triangular, rod-shaped, 22.5–30.0 nm (TEM) | Antibacterial, anticancer, and catalytic activity | [192] |
| Grapes | Round-shaped, non-agglomerated 10–40 nm (TEM) | Antibacterial and antifungal activity against Gram-positive (Bacillus subtilis), Gram-negative (Escherichia coli), and Candida albicans wound pathogens. Photocatalytic | [193] |
| Pistachio | Spherical, polygonal 80–100 nm (TEM) | Antibiofilm activity against S. aureus, P. aeruginosa | [194] |
| Cornus sanguinea L. | Spherical, 18 nm (TEM) | Antioxidant and anti-inflammatory activities | [195] |
| Prunus serrulata | Spherical, 66 nm (DLS) | Anti-inflammatory | [196] |
| Red onion | Spherical, 12.5 nm (TEM) | antioxidant activity | [197] |
| Crataegus pentagyna | Spherical, 25–45 nm (TEM) | Photocatalytic degradation of organic pollutants and in the development of antibacterial materials. | [198] |
| Seeds | |||
| Artocarpus hirsutus | Spherical, 25–40 nm (SEM) | Antibacterial activity against Enterobacter aerogenes | [199] |
| Cassia tora | 60.78 nm (SEM) | Antibacterial activity | [200] |
| Catharanthus roseus | Spherical, 2–15 nm (TEM) | Antibacterial activity against Escherichia coli | [201] |
2.2.8. Human Cell Line
2.3. Characterization Techniques
3. Biomedical Applications
3.1. Antimicrobial Activity and Associated Applications
3.2. Antiviral Agents
3.3. Other Biological Applications of AgNPs
4. Toxicity Associated with AgNPs
5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderón-Jiménez, B.; Johnson, M.E.; Montoro Bustos, A.R.; Murphy, K.E.; Winchester, M.R.; Vega Baudrit, J.R. Silver Nanoparticles: Technological Advances, Societal Impacts, and Metrological Challenges. Front. Chem. 2017, 5, 6. [Google Scholar] [CrossRef]
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1689–1694. [Google Scholar] [CrossRef]
- Xu, R.; Wang, D.; Zhang, J.; Li, Y. Shape-dependent catalytic activity of silver nanoparticles for the oxidation of styrene. Chem. Asian J. 2006, 1, 888–893. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Jakubowska, M.; Młożniak, A.; Horaczek, M.; Rudka, D.; Free, C.; Carey, J.D. Enhanced Electrical Conductivity of Silver Nanoparticles for High Frequency Electronic Applications. ACS Appl. Mater. Interfaces 2012, 4, 7007–7010. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef]
- Ramezanpour, M.; Leung, S.S.W.; Delgado-Magnero, K.H.; Bashe, B.Y.M.; Thewalt, J.; Tieleman, D.P. Computational and experimental approaches for investigating nanoparticle-based drug delivery systems. Biochim. Biophys. Acta 2016, 1858, 1688–1709. [Google Scholar] [CrossRef]
- Muhammad, Z.; Raza, A.; Ghafoor, S.; Naeem, A.; Naz, S.S.; Riaz, S.; Ahmed, W.; Rana, N.F. PEG capped methotrexate silver nanoparticles for efficient anticancer activity and biocompatibility. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2016, 91, 251–255. [Google Scholar] [CrossRef]
- Petrov, P.D.; Yoncheva, K.; Gancheva, V.; Konstantinov, S.; Trzebicka, B. Multifunctional block copolymer nanocarriers for co-delivery of silver nanoparticles and curcumin: Synthesis and enhanced efficacy against tumor cells. Eur. Polym. J. 2016, 81, 24–33. [Google Scholar] [CrossRef]
- Al-Obaidi, H.; Kalgudi, R.; Zariwala, M.G. Fabrication of inhaled hybrid silver/ciprofloxacin nanoparticles with synergetic effect against Pseudomonas aeruginosa. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2018, 128, 27–35. [Google Scholar] [CrossRef]
- Kaur, A.; Goyal, D.; Kumar, R. Surfactant mediated interaction of vancomycin with silver nanoparticles. Appl. Surf. Sci. 2018, 449, 23. [Google Scholar] [CrossRef]
- Arumai Selvan, D.; Mahendiran, D.; Senthil Kumar, R.; Kalilur Rahiman, A. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: Phytochemical, antioxidant and in vitro cytotoxicity studies. J. Photochem. Photobiol. B 2018, 180, 243–252. [Google Scholar] [CrossRef]
- Jiang, Q.; Yu, S.; Li, X.; Ma, C.; Li, A. Evaluation of local anesthetic effects of Lidocaine-Ibuprofen ionic liquid stabilized silver nanoparticles in Male Swiss mice. J. Photochem. Photobiol. B 2018, 178, 367–370. [Google Scholar] [CrossRef]
- Karthik, C.S.; Manukumar, H.M.; Ananda, A.P.; Nagashree, S.; Rakesh, K.P.; Mallesha, L.; Qin, H.-L.; Umesha, S.; Mallu, P.; Krishnamurthy, N.B. Synthesis of novel benzodioxane midst piperazine moiety decorated chitosan silver nanoparticle against biohazard pathogens and as potential anti-inflammatory candidate: A molecular docking studies. Int. J. Biol. Macromol. 2018, 108, 489–502. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int. J. Pharm. 2015, 496, 159–172. [Google Scholar] [CrossRef]
- Stebounova, L.V.; Guio, E.; Grassian, V.H. Silver nanoparticles in simulated biological media: A study of aggregation, sedimentation, and dissolution. J. Nanoparticle Res. 2011, 13, 233–244. [Google Scholar] [CrossRef]
- Argentiere, S.; Cella, C.; Cesaria, M.; Milani, P.; Lenardi, C. Silver nanoparticles in complex biological media: Assessment of colloidal stability and protein corona formation. J. Nanoparticle Res. 2016, 8, 1–13. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Gorham, J.M.; Rohlfing, A.B.; Lippa, K.A.; MacCuspie, R.I.; Hemmati, A.; David Holbrook, R. Storage Wars: How citrate-capped silver nanoparticle suspensions are affected by not-so-trivial decisions. J. Nanoparticle Res. 2014, 16, 2339. [Google Scholar] [CrossRef]
- Kanti Das, T.; Ganguly, S.; Remanan, S.; Das, N.C. Temperature-Dependent Study of Catalytic Ag Nanoparticles Entrapped Resin Nanocomposite towards Reduction of 4-Nitrophenol. ChemistrySelect 2019, 4, 3665–3671. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Prasher, P.; Singh, M.; Mudila, H. Silver nanoparticles as antimicrobial therapeutics: Current perspectives and future challenges. 3 Biotech 2018, 8, 411. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public. Health 2022, 19, 674. [Google Scholar] [CrossRef]
- Ali, S.; Chen, X.; Ajmal Shah, M.; Ali, M.; Zareef, M.; Arslan, M.; Ahmad, S.; Jiao, T.; Li, H.; Chen, Q. The avenue of fruit wastes to worth for synthesis of silver and gold nanoparticles and their antimicrobial application against foodborne pathogens: A review. Food Chem. 2021, 359, 129912. [Google Scholar] [CrossRef]
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Lee, J.; Park, E.Y.; Lee, J. Non-toxic nanoparticles from phytochemicals: Preparation and biomedical application. Bioprocess Biosyst. Eng. 2014, 37, 983–989. [Google Scholar] [CrossRef]
- Saifuddin, N.; Wong, C.W.; Yasumira, A.A.N. Rapid Biosynthesis of Silver Nanoparticles Using Culture Supernatant of Bacteria with Microwave Irradiation. J. Chem. 2009, 6, 61–70. [Google Scholar] [CrossRef]
- Mousavi-Khattat, M.; Keyhanfar, M.; Razmjou, A. A comparative study of stability, antioxidant, DNA cleavage and antibacterial activities of green and chemically synthesized silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1022–S1031. [Google Scholar] [CrossRef]
- Kummara, S.; Patil, M.B.; Uriah, T. Synthesis, characterization, biocompatible and anticancer activity of green and chemically synthesized silver nanoparticles—A comparative study. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 84, 10–21. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Kronberg, F.; Spedalieri, C.; Munarriz, E.; Giacometti, R. Protein corona on biogenic silver nanoparticles provides higher stability and protects cells from toxicity in comparison to chemical nanoparticles. J. Environ. Manag. 2021, 297, 113434. [Google Scholar] [CrossRef] [PubMed]
- Sreelekha, E.; George, B.; Shyam, A.; Sajina, N.; Mathew, B. A Comparative Study on the Synthesis, Characterization, and Antioxidant Activity of Green and Chemically Synthesized Silver Nanoparticles. BioNanoScience 2021, 11, 489–496. [Google Scholar] [CrossRef]
- Veeragoni, D.; Deshpande, S.; Rachamalla, H.K.; Ande, A.; Misra, S.; Mutheneni, S.R. In Vitro and In Vivo Anticancer and Genotoxicity Profiles of Green Synthesized and Chemically Synthesized Silver Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 2324–2339. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Durán, M.; Yadav, A.; Gade, A.; Rai, M. Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl. Microbiol. Biotechnol. 2011, 90, 1609–1624. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Long, D.; Wu, G.; Chen, S. Preparation of oligochitosan stabilized silver nanoparticles by gamma irradiation. Radiat. Phys. Chem. 2007, 76, 1126–1131. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Zhang, X.; Jin, Y. Microwave-assisted green synthesis of silver nanoparticles by carboxymethyl cellulose sodium and silver nitrate. Mater. Chem. Phys. 2008, 108, 421–424. [Google Scholar] [CrossRef]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Microwave assisted green synthesis of silver nanoparticles using leaf extract of elephantopus scaber and its environmental and biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 795–804. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green Synthesis of Silver Nanoparticles: A Review. Green Sustain. Chem. 2016, 06, 34–56. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Green and energy-efficient methods for the production of metallic nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2354–2376. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Nam, S.Y.; Oh, J. Marine microorganisms as potential biofactories for synthesis of metallic nanoparticles. Crit. Rev. Microbiol. 2016, 42, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Golinska, P.; Wypij, M.; Ingle, A.P.; Gupta, I.; Dahm, H.; Rai, M. Biogenic synthesis of metal nanoparticles from actinomycetes: Biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol. 2014, 98, 8083–8097. [Google Scholar] [CrossRef] [PubMed]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes- a review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef]
- Patil, M.P.; Kim, G.-D. Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 487–495. [Google Scholar] [CrossRef]
- Ammar, H.A.; El Aty, A.A.A.; El Awdan, S.A. Extracellular myco-synthesis of nano-silver using the fermentable yeasts Pichia kudriavzeviiHA-NY2 and Saccharomyces uvarumHA-NY3, and their effective biomedical applications. Bioprocess Biosyst. Eng. 2021, 44, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, J.K.; Zachara, J.M.; Balkwill, D.L.; Kennedy, D.; Li, S.W.; Kostandarithes, H.M.; Daly, M.J.; Romine, M.F.; Brockman, F.J. Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the hanford site, washington state. Appl. Environ. Microbiol. 2004, 70, 4230–4241. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, S.; Madhu, S.; Singh, S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem. 2011, 46, 1800–1807. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q.M. Magnetic nanocomposites with mesoporous structures: Synthesis and applications. Small Weinh. Bergstr. Ger. 2011, 7, 425–443. [Google Scholar] [CrossRef]
- Vaseghi, Z.; Nematollahzadeh, A.; Tavakoli, O. Green methods for the synthesis of metal nanoparticles using biogenic reducing agents: A review. Rev. Chem. Eng. 2018, 34, 529–559. [Google Scholar] [CrossRef]
- Chumpol, J.; Siri, S. Simple green production of silver nanoparticles facilitated by bacterial genomic DNA and their antibacterial activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 619–625. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yurtluk, T.; Akçay, F.A.; Avcı, A. Biosynthesis of silver nanoparticles using novel Bacillus sp. SBT8. Prep. Biochem. Biotechnol. 2018, 48, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Arzoo, S.; Naqvi, Z.; Hussain, M.; Shamim, S.; Zeb, T.F.; Ali, S. Production and antimicrobial activity of silver nanoparticles synthesized from indigenously isolated Pseudomonas aeruginosa from Rhizosphere. Pak. J. Pharm. Sci. 2020, 33, 2815–2822. [Google Scholar]
- Sable, S.V.; Kawade, S.; Ranade, S.; Joshi, S. Bioreduction mechanism of silver nanoparticles. Mater. Sci. Eng. C 2020, 107, 110299. [Google Scholar] [CrossRef]
- Saleem, S.; Iqbal, A.; Hasnain, S. Bacterial mediated silver nanoparticles and their efficacy against MRSA. Trop. Biomed. 2020, 37, 482–488. [Google Scholar] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garnæs, J.; Mijakovic, I. A Sustainable Approach for the Green Synthesis of Silver Nanoparticles from Solibacillus isronensis sp. and Their Application in Biofilm Inhibition. Molecules 2020, 25, 2783. [Google Scholar] [CrossRef]
- Li, J.; Tian, B.; Li, T.; Dai, S.; Weng, Y.; Lu, J.; Xu, X.; Jin, Y.; Pang, R.; Hua, Y. Biosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using protein extracts of Deinococcus radiodurans and evaluation of their cytotoxicity. Int. J. Nanomed. 2018, 13, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Elsharawy, K.; Abou-Dobara, M.; El-Gammal, H.; Hyder, A. Chitosan coating does not prevent the effect of the transfer of green silver nanoparticles biosynthesized by Streptomyces malachitus into fetuses via the placenta. Reprod. Biol. 2020, 20, 97–105. [Google Scholar] [CrossRef]
- Moein, M.; Imani Fooladi, A.A.; Mahmoodzadeh Hosseini, H. Determining the effects of green chemistry synthesized Ag-nisin nanoparticle on macrophage cells. Microb. Pathog. 2018, 114, 414–419. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, D.; Singh, S.K.; Singh, V.K.; Singh, A.V.; Kumar, A. Role of actinomycetes in bioactive and nanoparticle synthesis. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Woodhead Publishing: Amsterdam, The Netherlands; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–182. ISBN 978-0-12-817004-5. [Google Scholar]
- Das, S.; Lyla, P.S.; Khan, S.A. Distribution and generic composition of culturable marine actinomycetes from the sediments of Indian continental slope of Bay of Bengal. Chin. J. Oceanol. Limnol. 2008, 26, 166–177. [Google Scholar] [CrossRef]
- Abdeen, S.; Geo, S.; Praseetha, P.K.; Dhanya, R.P. Biosynthesis of silver nanoparticles from Actinomycetes for therapeutic applications. Int. J. Nano Dimens. 2014, 5, 155–162. [Google Scholar]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 2003, 14, 824–828. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Biosynthesis of Monodisperse Gold Nanoparticles by a Novel Extremophilic Actinomycete, Thermomonospora sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Actinobacteria mediated synthesis of nanoparticles and their biological properties: A review. Crit. Rev. Microbiol. 2016, 42, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Czarnecka, J.; Świecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J. Microbiol. Biotechnol. 2018, 34, 23. [Google Scholar] [CrossRef]
- Pallavi, S.S.; Rudayni, H.A.; Bepari, A.; Niazi, S.K.; Nayaka, S. Green synthesis of Silver nanoparticles using Streptomyces hirsutus strain SNPGA-8 and their characterization, antimicrobial activity, and anticancer activity against human lung carcinoma cell line A549. Saudi J. Biol. Sci. 2022, 29, 228–238. [Google Scholar] [CrossRef]
- Mabrouk, M.; Elkhooly, T.A.; Amer, S.K. Actinomycete strain type determines the monodispersity and antibacterial properties of biogenically synthesized silver nanoparticles. J. Genet. Eng. Biotechnol. 2021, 19, 57. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic Streptomyces spp. Biol. Trace Elem. Res. 2020, 195, 707–724. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R. de Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Korbekandi, H.; Asghari, G.; Chitsazi, M.R.; Bahri Najafi, R.; Badii, A.; Iravani, S. Green biosynthesis of silver nanoparticles using Althaea officinalis radix hydroalcoholic extract. Artif. Cells Nanomed. Biotechnol. 2016, 44, 209–215. [Google Scholar] [CrossRef]
- Fernández, J.G.; Fernández-Baldo, M.A.; Berni, E.; Camí, G.; Durán, N.; Raba, J.; Sanz, M.I. Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process Biochem. 2016, 51, 1306–1313. [Google Scholar] [CrossRef]
- Yadav, A.; Kon, K.; Kratosova, G.; Duran, N.; Ingle, A.P.; Rai, M. Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: Progress and key aspects of research. Biotechnol. Lett. 2015, 37, 2099–2120. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, P.; Mehrvar, A.; Michaud, J.P.; Vaez, N. Optimization of silver nanoparticle biosynthesis by entomopathogenic fungi and assays of their antimicrobial and antifungal properties. J. Invertebr. Pathol. 2022, 190, 107749. [Google Scholar] [CrossRef]
- Koli, S.H.; Mohite, B.V.; Suryawanshi, R.K.; Borase, H.P.; Patil, S.V. Extracellular red Monascus pigment-mediated rapid one-step synthesis of silver nanoparticles and its application in biomedical and environment. Bioprocess Biosyst. Eng. 2018, 41, 715–727. [Google Scholar] [CrossRef]
- Ansari, A.; Pervez, S.; Javed, U.; Abro, M.I.; Nawaz, M.A.; Qader, S.A.U.; Aman, A. Characterization and interplay of bacteriocin and exopolysaccharide-mediated silver nanoparticles as an antibacterial agent. Int. J. Biol. Macromol. 2018, 115, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-J.; Pan, J.-J.; Tao, L.-J.; Li, X.; Su, D.-L.; Shan, Y.; Li, H.-Y. Green Synthesis of Silver Nanoparticles by Extracellular Extracts from Aspergillus japonicus PJ01. Molecules 2021, 26, 4479. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci. Rep. 2021, 11, 10356. [Google Scholar] [CrossRef] [PubMed]
- Yeast—Industrial Applications; Morata, A., Loira, I., Eds.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3599-9. [Google Scholar]
- Jha, A.K.; Prasad, K.; Kulkarni, A.A.R. Yeast Mediated Synthesis of Silver Nanoparticles. Int. J. Nanosci. Nanotechnol. 2008, 4, 17–22. [Google Scholar]
- Boroumand Moghaddam, A.; Namvar, F.; Moniri, M.; Tahir, P.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef]
- Breierová, E.; Vajcziková, I.; Sasinková, V.; Stratilová, E.; Fišera, M.; Gregor, T.; Šajbidor, J. Biosorption of Cadmium Ions by Different Yeast Species. Z. Für Nat. C 2002, 57, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Baron, M.; Sochor, J. Nanoparticles Biosynthesized by Yeast: A Review of their application. Kvas. Prum. 2017, 63, 290–292. [Google Scholar] [CrossRef]
- Cunha, F.A.; da, C.S.O. Cunha, M.; da Frota, S.M.; Mallmann, E.J.J.; Freire, T.M.; Costa, L.S.; Paula, A.J.; Menezes, E.A.; Fechine, P.B.A. Biogenic synthesis of multifunctional silver nanoparticles from Rhodotorula glutinis and Rhodotorula mucilaginosa: Antifungal, catalytic and cytotoxicity activities. World J. Microbiol. Biotechnol. 2018, 34, 127. [Google Scholar] [CrossRef]
- Vazquez-Rodriguez, A.; Vasto-Anzaldo, X.G.; Leon-Buitimea, A.; Zarate, X.; Morones-Ramirez, J.R. Antibacterial and Antibiofilm Activity of Biosynthesized Silver Nanoparticles Coated With Exopolysaccharides Obtained From Rhodotorula mucilaginosa. IEEE Trans. Nanobioscience 2020, 19, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; He, F.; Li, Z.; Zhu, X.; Ma, Y.; Zhou, Z.; Yang, Z.; Gao, F.; Zeng, M. Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Yeast Extract as Reducing and Capping Agents. Nanoscale Res. Lett. 2020, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Suganya, M.; Preethi, P.S.; Narenkumar, J.; Prakash, A.A.; Devanesan, S.; AlSalhi, M.S.; Rajasekar, A.; Nanthini, A.U.R. Synthesis of Silver Nanoparticles From Indian Red Yeast Rice and Its Inhibition of Biofilm in Copper Metal in Cooling Water Environment. Environ. Sci. Pollut. Res. 2022, 29, 77800–77808. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sharma, N. Industrial and Biotechnological Applications of Algae: A Review. J. Adv. Plant Biol. 2017, 1, 1–25. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Sharma, K.; Chetri, S.P.K.; Vashishtha, A.; Singh, P.; Kumar, R.; Rathi, B.; Agrawal, V. Algae as crucial organisms in advancing nanotechnology: A systematic review. J. Appl. Phycol. 2016, 28, 1759–1774. [Google Scholar] [CrossRef]
- Schröfel, A.; Kratošová, G.; Bohunická, M.; Dobrocka, E.; Vávra, I. Biosynthesis of gold nanoparticles using diatoms—Silica-gold and EPS-gold bionanocomposite formation. J. Nanoparticle Res. 2011, 13, 3207–3216. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.M.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology—A review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Sathishkumar, R.S.; Sundaramanickam, A.; Srinath, R.; Ramesh, T.; Saranya, K.; Meena, M.; Surya, P. Green synthesis of silver nanoparticles by bloom forming marine microalgae Trichodesmium erythraeum and its applications in antioxidant, drug-resistant bacteria, and cytotoxicity activity. J. Saudi Chem. Soc. 2019, 23, 1180–1191. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Wujcik, E.K.; Jeffryes, C. Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzym. Microb. Technol. 2016, 95, 13–27. [Google Scholar] [CrossRef]
- Rao, S.S.; Saptami, K.; Venkatesan, J.; Rekha, P.D. Microwave-assisted rapid synthesis of silver nanoparticles using fucoidan: Characterization with assessment of biocompatibility and antimicrobial activity. Int. J. Biol. Macromol. 2020, 163, 745–755. [Google Scholar] [CrossRef]
- Bao, Z.; Cao, J.; Kang, G.; Lan, C.Q. Effects of reaction conditions on light-dependent silver nanoparticle biosynthesis mediated by cell extract of green alga Neochloris oleoabundans. Environ. Sci. Pollut. Res. Int. 2019, 26, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Verma, S.K.; Yasin, D.; Hemlata, N.A.N.; Rizvi, M.M.; Fatma, T. Facile green bio-fabricated silver nanoparticles from Microchaete infer dose-dependent antioxidant and anti-proliferative activity to mediate cellular apoptosis. Bioorganic Chem. 2021, 107, 104535. [Google Scholar] [CrossRef] [PubMed]
- Dixit, D.; Gangadharan, D.; Popat, K.M.; Reddy, C.R.K.; Trivedi, M.; Gadhavi, D.K. Synthesis, characterization and application of green seaweed mediated silver nanoparticles (AgNPs) as antibacterial agents for water disinfection. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2018, 78, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Han, S.S. Helical plant viral nanoparticles-bioinspired synthesis of nanomaterials and nanostructures. Bioinspir. Biomim. 2017, 12, 031001. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tomita, S.; Sawada, K.; Shiba, K.; Yanagi, H.; Yamashita, I.; Uraoka, Y. Chiral meta-molecules consisting of gold nanoparticles and genetically engineered tobacco mosaic virus. Opt. Express 2012, 20, 24856–24863. [Google Scholar] [CrossRef]
- Young, M.; Debbie, W.; Uchida, M.; Douglas, T. Plant Viruses as Biotemplates for Materials and Their Use in Nanotechnology. Annu. Rev. Phytopathol. 2008, 46, 361–384. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Marchiol, L. Synthesis of metal nanoparticles in living plants. Ital. J. Agron. 2012, 7, 37. [Google Scholar] [CrossRef]
- Park, Y.; Hong, Y.N.; Weyers, A.; Kim, Y.S.; Linhardt, R.J. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The Relative Antioxidant Activities of Plant-Derived Polyphenolic Flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Tamuly, C.; Hazarika, M.; Bordoloi, M.; Bhattacharyya, P.K.; Kar, R. Biosynthesis of Ag nanoparticles using pedicellamide and its photocatalytic activity: An eco-friendly approach. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2014, 132, 687–691. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Y.; He, Z.; Stoffella, P.J. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2005, 18, 339–353. [Google Scholar] [CrossRef]
- Milner, M.J.; Kochian, L.V. Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann. Bot. 2008, 102, 3–13. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Mukunthan, K.S.; Balaji, S. Cashew Apple Juice ( Anacardium occidentale L.) Speeds Up the Synthesis of Silver Nanoparticles. Int. J. Green Nanotechnol. 2012, 4, 71–79. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Subramaniam, S.; Veerappan, G.; Hari, N.; Sivasubramanian, A.; Veerappan, A. β-Sitosterol-D-glucopyranoside isolated from Desmostachya bipinnata mediates photoinduced rapid green synthesis of silver nanoparticles. RSC Adv. 2014, 4, 59130–59136. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Elumalai, S.; Kumar, V.; Kumar, S.; Sangwan, R.S. Nano silver particle synthesis using Swertia paniculata herbal extract and its antimicrobial activity. Microb. Pathog. 2018, 114, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.A.; Kotakadi, V.S.; Subramanyam, G.K.; Penchalaneni, J.; Challagundla, V.N.; Dvr, S.G.; Pasupuleti, V.R. Multifaceted phytogenic silver nanoparticles by an insectivorous plant Drosera spatulata Labill var. bakoensis and its potential therapeutic applications. Sci. Rep. 2021, 11, 21969. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, B.; Deswal, R. Green silver nanoparticles from novel Brassicaceae cultivars with enhanced antimicrobial potential than earlier reported Brassicaceae members. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2018, 47, 1–11. [Google Scholar] [CrossRef]
- Nazer, S.; Andleeb, S.; Ali, S.; Gulzar, N.; Iqbal, T.; Khan, M.A.R.; Raza, A. Synergistic Antibacterial Efficacy of Biogenic Synthesized Silver Nanoparticles using Ajuga bractosa with Standard Antibiotics: A Study Against Bacterial Pathogens. Curr. Pharm. Biotechnol. 2020, 21, 206–218. [Google Scholar] [CrossRef]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Pammi, S.V.N.; Yoon, S.-G. Ultra Small, mono dispersed green synthesized silver nanoparticles using aqueous extract of Sida cordifolia plant and investigation of antibacterial activity. Microb. Pathog. 2018, 124, 63–69. [Google Scholar] [CrossRef]
- Nasar, M.Q.; Khalil, A.T.; Ali, M.; Shah, M.; Ayaz, M.; Shinwari, Z.K. Phytochemical Analysis, Ephedra Procera C. A. Mey. Mediated Green Synthesis of Silver Nanoparticles, Their Cytotoxic and Antimicrobial Potentials. Med. Kaunas Lith. 2019, 55, 369. [Google Scholar] [CrossRef] [PubMed]
- Mirsadeghi, S.; Koudehi, M.F.; Rajabi, H.R.; Pourmortazavi, S.M. Green and Simple Synthesis of Silver Nanoparticles by Aqueous Extract of Perovskia abrotanoides: Characterization, Optimization and Antimicrobial Activity. Curr. Pharm. Biotechnol. 2020, 21, 1129–1137. [Google Scholar] [CrossRef]
- Zandpour, F.; Allafchian, A.R.; Vahabi, M.R.; Jalali, S.A.H. Green synthesis of silver nanoparticles with the Arial part of Dorema ammoniacum D. extract by antimicrobial analysis. IET Nanobiotechnol. 2018, 12, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalinejhad, S.; Almasi, H.; Esmaiili, M. Simultaneous green synthesis and in-situ impregnation of silver nanoparticles into organic nanofibers by Lythrum salicaria extract: Morphological, thermal, antimicrobial and release properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110115. [Google Scholar] [CrossRef]
- Naghmachi, M.; Raissi, A.; Baziyar, P.; Homayoonfar, F.; Amirmahani, F.; Danaei, M. Green synthesis of silver nanoparticles (AgNPs) by Pistacia terebinthus extract: Comprehensive evaluation of antimicrobial, antioxidant and anticancer effects. Biochem. Biophys. Res. Commun. 2022, 608, 163–169. [Google Scholar] [CrossRef]
- Allafchian, A.R.; Jalali, S.A.H.; Aghaei, F.; Farhang, H.R. Green synthesis of silver nanoparticles using Glaucium corniculatum (L.) Curtis extract and evaluation of its antibacterial activity. IET Nanobiotechnol. 2018, 12, 574–578. [Google Scholar] [CrossRef]
- Chandhru, M.; Logesh, R.; Kutti Rani, S.; Ahmed, N.; Vasimalai, N. Green synthesis of silver nanoparticles from plant latex and their antibacterial and photocatalytic studies. Environ. Technol. 2022, 43, 3064–3074. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.-Y.; Jin, H.; Park, Y. Assessing the antioxidant, cytotoxic, apoptotic and wound healing properties of silver nanoparticles green-synthesized by plant extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 204–216. [Google Scholar] [CrossRef]
- Dehghanizade, S.; Arasteh, J.; Mirzaie, A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: Characterization and in vitro biological activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Salem, M.; Youssif, K.A.; Samir, A.; Bin Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomed. 2019, 14, 6217–6229. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Mathew, G.M.; Pancrecious, J.K.; Nair, J.B.; Rajan, T.P.D.; Maiti, K.K.; Pai, B.C. Biogenic Ag Nanoparticles from Neem Extract: Their Structural Evaluation and Antimicrobial Effects against Pseudomonas nitroreducens and Aspergillus unguis (NII 08123). ACS Biomater. Sci. Eng. 2020, 6, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Cittrarasu, V.; Balasubramanian, B.; Kaliannan, D.; Park, S.; Maluventhan, V.; Kaul, T.; Liu, W.C.; Arumugam, M. Biological mediated Ag nanoparticles from Barleria longiflora for antimicrobial activity and photocatalytic degradation using methylene blue. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2424–2430. [Google Scholar] [CrossRef]
- Gholami, M.; Shahzamani, K.; Marzban, A.; Lashgarian, H.E. Evaluation of antimicrobial activity of synthesised silver nanoparticles using Thymus kotschyanus aqueous extract. IET Nanobiotechnol. 2018, 12, 1114–1117. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Rolim, W.R.; Viana, M.M.; Souza, T.R.; Gonçalves, F.; Tanaka, C.J.; Bueno-Silva, B.; Seabra, A.B. Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications. J. Dent. 2020, 96, 103327. [Google Scholar] [CrossRef]
- Anjana, V.N.; Joseph, M.; Francis, S.; Joseph, A.; Koshy, E.P.; Mathew, B. Microwave assisted green synthesis of silver nanoparticles for optical, catalytic, biological and electrochemical applications. Artif. Cells Nanomed. Biotechnol. 2021, 49, 438–449. [Google Scholar] [CrossRef]
- Carson, L.; Bandara, S.; Joseph, M.; Green, T.; Grady, T.; Osuji, G.; Weerasooriya, A.; Ampim, P.; Woldesenbet, S. Green Synthesis of Silver Nanoparticles with Antimicrobial Properties Using Phyla dulcis Plant Extract. Foodborne Pathog. Dis. 2020, 17, 504–511. [Google Scholar] [CrossRef]
- Thomas, B.; Vithiya, B.S.M.; Prasad, T.A.A.; Mohamed, S.B.; Magdalane, C.M.; Kaviyarasu, K.; Maaza, M. Antioxidant and Photocatalytic Activity of Aqueous Leaf Extract Mediated Green Synthesis of Silver Nanoparticles Using Passiflora edulis f. flavicarpa. J. Nanosci. Nanotechnol. 2019, 19, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Zamani, S.; Kumar, A. Green synthesis and characterization of silver nanoparticles using Pteris vittata extract and their therapeutic activities. Biotechnol. Appl. Biochem. 2022, 69, 1653–1662. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef]
- Hafeez, M.; Zeb, M.; Khan, A.; Akram, B.; Abdin, Z.-U.; Haq, S.; Zaheer, M.; Ali, S. Populus ciliata mediated synthesis of silver nanoparticles and their antibacterial activity. Microsc. Res. Tech. 2021, 84, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Muraro, P.C.L.; Pinheiro, L.D.S.M.; Chuy, G.; Vizzotto, B.S.; Pavoski, G.; Espinosa, D.C.R.; Rech, V.C.; da Silva, W.L. Silver nanoparticles from residual biomass: Biosynthesis, characterization and antimicrobial activity. J. Biotechnol. 2022, 343, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Timotina, M.; Aghajanyan, A.; Schubert, R.; Trchounian, K.; Gabrielyan, L. Biosynthesis of silver nanoparticles using extracts of Stevia rebaudiana and evaluation of antibacterial activity. World J. Microbiol. Biotechnol. 2022, 38, 196. [Google Scholar] [CrossRef]
- Erci, F.; Cakir-Koc, R.; Isildak, I. Green synthesis of silver nanoparticles using Thymbra spicata L. var. spicata (zahter) aqueous leaf extract and evaluation of their morphology-dependent antibacterial and cytotoxic activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 150–158. [Google Scholar] [CrossRef]
- Dash, S.S.; Samanta, S.; Dey, S.; Giri, B.; Dash, S.K. Rapid Green Synthesis of Biogenic Silver Nanoparticles Using Cinnamomum tamala Leaf Extract and its Potential Antimicrobial Application Against Clinically Isolated Multidrug-Resistant Bacterial Strains. Biol. Trace Elem. Res. 2020, 198, 681–696. [Google Scholar] [CrossRef]
- Ferreyra Maillard, A.P.V.; Gonçalves, S.; Santos, N.C.; López de Mishima, B.A.; Dalmasso, P.R.; Hollmann, A. Studies on interaction of green silver nanoparticles with whole bacteria by surface characterization techniques. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1086–1092. [Google Scholar] [CrossRef]
- Sekar, V.; Balakrishnan, C.; Kathirvel, P.; Swamiappan, S.; Alshehri, M.A.; Sayed, S.; Panneerselvam, C. Ultra-sonication-enhanced green synthesis of silver nanoparticles using Barleria buxifolia leaf extract and their possible application. Artif. Cells Nanomed. Biotechnol. 2022, 50, 177–187. [Google Scholar] [CrossRef]
- Sarli, S.; Kalani, M.R.; Moradi, A. A Potent and Safer Anticancer and Antibacterial Taxus-Based Green Synthesized Silver Nanoparticle. Int. J. Nanomed. 2020, 15, 3791–3801. [Google Scholar] [CrossRef]
- Araujo Neto, L.A.; Pereira, T.M.; Silva, L.P. Evaluation of behavior, growth, and swarming formation of Escherichia coli and Staphylococcus aureus in culture medium modified with silver nanoparticles. Microb. Pathog. 2020, 149, 104480. [Google Scholar] [CrossRef]
- Prabukumar, S.; Rajkuberan, C.; Sathishkumar, G.; Illaiyaraja, M.; Sivaramakrishnan, S. One pot green fabrication of metallic silver nanoscale materials using Crescentia cujete L. and assessment of their bactericidal activity. IET Nanobiotechnol. 2018, 12, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Küp, F.Ö.; Çoşkunçay, S.; Duman, F. Biosynthesis of silver nanoparticles using leaf extract of Aesculus hippocastanum (horse chestnut): Evaluation of their antibacterial, antioxidant and drug release system activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110207. [Google Scholar] [CrossRef] [PubMed]
- Tehri, N.; Kaur, R.; Maity, M.; Chauhan, A.; Hooda, V.; Vashishth, A.; Kumar, G. Biosynthesis, characterization, bactericidal and sporicidal activity of silver nanoparticles using the leaves extract of Litchi chinensis. Prep. Biochem. Biotechnol. 2020, 50, 865–873. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Javed, M.N.; Alam, M.S.; Rishishwar, P.; Rishishwar, S.; Ali, S.; Nayak, A.K.; Beg, S. Purple heart plant leaves extract-mediated silver nanoparticle synthesis: Optimization by Box-Behnken design. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, R.; Joseph, S.; Mathew, B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif. Cells Nanomed. Biotechnol. 2018, 46, 861–871. [Google Scholar] [CrossRef]
- Javed, B.; Mashwani, Z.-U.-R.; Sarwer, A.; Raja, N.I.; Nadhman, A. Synergistic response of physicochemical reaction parameters on biogenesis of silver nanoparticles and their action against colon cancer and leishmanial cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1340–1353. [Google Scholar] [CrossRef]
- Chandraker, S.K.; Lal, M.; Khanam, F.; Dhruve, P.; Singh, R.P.; Shukla, R. Therapeutic potential of biogenic and optimized silver nanoparticles using Rubia cordifolia L. leaf extract. Sci. Rep. 2022, 12, 8831. [Google Scholar] [CrossRef]
- Joseph, J.; Khor, K.Z.; Moses, E.J.; Lim, V.; Aziz, M.Y.; Abdul Samad, N. In vitro Anticancer Effects of Vernonia amygdalina Leaf Extract and Green-Synthesised Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 3599–3612. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, X.; Cai, Q.; Song, C. Introducing a Novel Chemotherapeutic Drug for the Treatment of Lung Adenocarcinoma: Silver Nanoparticles Green-formulated by Cinnamomum verum. J. Oleo Sci. 2022, 71, 371–378. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Yu, Z.; Chen, L.; Chinnathambi, A.; Almoallim, H.S.; Alharbi, S.A.; Liu, L. Novel green synthesis and characterization of a chemotherapeutic supplement by silver nanoparticles containing Berberis thunbergii leaf for the treatment of human pancreatic cancer. Biotechnol. Appl. Biochem. 2022, 69, 887–897. [Google Scholar] [CrossRef]
- Dhilip Kumar, S.S.; Houreld, N.N.; Abrahamse, H. Selective Laser Efficiency of Green-Synthesized Silver Nanoparticles by Aloe arborescens and Its Wound Healing Activities in Normal Wounded and Diabetic Wounded Fibroblast Cells: In vitro Studies. Int. J. Nanomed. 2020, 15, 6855–6870. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, A.; Khan, F.; Ahmad, N.; Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Al-Qahtani, M.H.; Abuzenadah, A.M.; Tabrez, S.; Ahmed, A.B.F.; et al. Silver nanoparticles from leaf extract of Mentha piperita: Eco-friendly synthesis and effect on acetylcholinesterase activity. Life Sci. 2018, 209, 430–434. [Google Scholar] [CrossRef]
- Alam, T.; Rauf, M.A.; Siddiqui, G.A.; Owais, M.; Naeem, A. Green synthesis of silver nanoparticles, its characterization, and chaperone-like activity in the aggregation inhibition of α-chymotrypsinogen A. Int. J. Biol. Macromol. 2018, 120, 2381–2389. [Google Scholar] [CrossRef]
- Macovei, I.; Luca, S.V.; Skalicka-Woźniak, K.; Sacarescu, L.; Pascariu, P.; Ghilan, A.; Doroftei, F.; Ursu, E.-L.; Rimbu, C.M.; Horhogea, C.E.; et al. Phyto-Functionalized Silver Nanoparticles Derived from Conifer Bark Extracts and Evaluation of Their Antimicrobial and Cytogenotoxic Effects. Molecules 2021, 27, 217. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Altaf, M.; Khan, M.Q.; Manzoor, S.; Shekheli, M.A.; Shah, M.A.; Ilyas, S.Z.; Hussain, Z. Green Synthesis of Silver Nanoparticles Using Jurinea dolomiaea and Biological Activities. J. Nanosci. Nanotechnol. 2018, 18, 8386–8391. [Google Scholar] [CrossRef]
- Riaz, M.; Altaf, M.; Faisal, A.; Shekheli, M.A.; Miana, G.A.; Khan, M.Q.; Shah, M.A.; Ilyas, S.Z.; Khan, A.A. Biogenic Synthesis of AgNPs with Saussurea lappa C.B. Clarke and Studies on Their Biochemical Properties. J. Nanosci. Nanotechnol. 2018, 18, 8392–8398. [Google Scholar] [CrossRef] [PubMed]
- Bin-Jumah, M.; Al-Abdan, M.; Albasher, G.; Alarifi, S. Effects of Green Silver Nanoparticles on Apoptosis and Oxidative Stress in Normal and Cancerous Human Hepatic Cells in vitro. Int. J. Nanomed. 2020, 15, 1537–1548. [Google Scholar] [CrossRef]
- Fayez, H.; El-Motaleb, M.A.; Selim, A.A. Synergistic Cytotoxicity Of Shikonin-Silver Nanoparticles As An Opportunity For Lung Cancer. J. Label. Compd. Radiopharm. 2020, 63, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Alsammarraie, F.K.; Wang, W.; Zhou, P.; Mustapha, A.; Lin, M. Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids Surf. B Biointerfaces 2018, 171, 398–405. [Google Scholar] [CrossRef]
- Yang, Y.; Song, W.; Chen, Z.; Li, Q.; Liu, L. Ameliorative effect of synthesized silver nanoparticles by green route method from Zingiber zerumbet on mycoplasmal pneumonia in experimental mice. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2146–2154. [Google Scholar] [CrossRef]
- Mohammadi, M.; Shahisaraee, S.A.; Tavajjohi, A.; Pournoori, N.; Muhammadnejad, S.; Mohammadi, S.R.; Poursalehi, R.; Delavari H, H. Green synthesis of silver nanoparticles using Zingiber officinale and Thymus vulgaris extracts: Characterisation, cell cytotoxicity, and its antifungal activity against Candida albicans in comparison to fluconazole. IET Nanobiotechnol. 2019, 13, 114–119. [Google Scholar] [CrossRef]
- Satpathy, S.; Patra, A.; Ahirwar, B.; Delwar Hussain, M. Antioxidant and anticancer activities of green synthesized silver nanoparticles using aqueous extract of tubers of Pueraria tuberosa. Artif. Cells Nanomed. Biotechnol. 2018, 46, S71–S85. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, G.; Zhou, G.; Li, Q.; Veeraraghavan, V.P.; Krishna Mohan, S.; Wang, D.; Liu, F. Green synthesis of silver nanoparticles from Alpinia officinarum mitigates cisplatin-induced nephrotoxicity via down-regulating apoptotic pathway in rats. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3212–3221. [Google Scholar] [CrossRef]
- Stati, G.; Rossi, F.; Trakoolwilaiwan, T.; Tung, L.D.; Mourdikoudis, S.; Thanh, N.T.K.; Di Pietro, R. Development and Characterization of Curcumin-Silver Nanoparticles as a Promising Formulation to Test on Human Pterygium-Derived Keratinocytes. Molecules 2022, 27, 282. [Google Scholar] [CrossRef]
- Mahmoodi Esfanddarani, H.; Abbasi Kajani, A.; Bordbar, A. Green synthesis of silver nanoparticles using flower extract of Malva sylvestris and investigation of their antibacterial activity. IET Nanobiotechnol. 2018, 12, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.Y.; Shincy, M.; Sundarapandian, S. Silver nanoparticles synthesis using Wedelia urticifolia (Blume) DC. flower extract: Characterization and antibacterial activity evaluation. Microsc. Res. Tech. 2020, 83, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Devanesan, S.; AlSalhi, M.S. Green Synthesis of Silver Nanoparticles Using the Flower Extract of Abelmoschus esculentus for Cytotoxicity and Antimicrobial Studies. Int. J. Nanomed. 2021, 16, 3343–3356. [Google Scholar] [CrossRef]
- Patil, M.P.; Singh, R.D.; Koli, P.B.; Patil, K.T.; Jagdale, B.S.; Tipare, A.R.; Kim, G.-D. Antibacterial potential of silver nanoparticles synthesized using Madhuca longifolia flower extract as a green resource. Microb. Pathog. 2018, 121, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Vanlalveni, C.; Adhikari, P.P.; Lalfakzuala, R.; Rokhum, L. Green biosynthesis, characterisation and antimicrobial activities of silver nanoparticles using fruit extract of Solanum viarum. IET Nanobiotechnol. 2018, 12, 933–938. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef]
- Gevorgyan, S.; Schubert, R.; Falke, S.; Lorenzen, K.; Trchounian, K.; Betzel, C. Structural characterization and antibacterial activity of silver nanoparticles synthesized using a low-molecular-weight Royal Jelly extract. Sci. Rep. 2022, 12, 14077. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.-S.; Patra, J.K. Multitherapeutic Efficacy of Curly Kale Extract Fabricated Biogenic Silver Nanoparticles. Int. J. Nanomed. 2022, 17, 1125–1137. [Google Scholar] [CrossRef]
- Baker, A.; Iram, S.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Ahmad, K.; Sajid Khan, M.; Kim, J. Fruit Derived Potentially Bioactive Bioengineered Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 7711–7726. [Google Scholar] [CrossRef]
- Quiroz-Hernández, J.E.; Kharissova, O.V.; Aguirre-Arzola, V.E.; Martinez-Avila, G.C.G.; Castillo-Velazquez, U. Evaluation of the Conditions for the Synthesis of Silver Nanoparticles from Orange Peels and its Antibacterial Effect. Recent Pat. Nanotechnol. 2020, 14, 250–258. [Google Scholar] [CrossRef]
- Barbhuiya, R.I.; Singha, P.; Asaithambi, N.; Singh, S.K. Ultrasound-assisted rapid biological synthesis and characterization of silver nanoparticles using pomelo peel waste. Food Chem. 2022, 385, 132602. [Google Scholar] [CrossRef] [PubMed]
- Sinsinwar, S.; Sarkar, M.K.; Suriya, K.R.; Nithyanand, P.; Vadivel, V. Use of agricultural waste (coconut shell) for the synthesis of silver nanoparticles and evaluation of their antibacterial activity against selected human pathogens. Microb. Pathog. 2018, 124, 30–37. [Google Scholar] [CrossRef]
- He, M.; Han, Z.; Liang, Y.; Zhao, H.; Ji, X.; Ma, G.; Cui, Y.; Wang, L. Green synthesis of Ag nanoparticles using elm pod polysaccharide for catalysis and bacteriostasis. Int. J. Biol. Macromol. 2022, 213, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Vorobyova, V.; Vasyliev, G.; Uschapovskiy, D.; Lyudmyla, K.; Skiba, M. Green synthesis, characterization of silver nanoparticals for biomedical application and environmental remediation. J. Microbiol. Methods 2022, 193, 106384. [Google Scholar] [CrossRef]
- Gonca, S.; Özidemir, S.; Isik, Z.; M’barek, I.; Shaik, F.; Dizge, N.; Balakrishnan, D. Synthesis of silver nanoparticles from red and green parts of the pistachio hulls and their various in-vitro biological activities. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2022, 165, 113170. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B.; Baldea, I.; Olteanu, D.; Bolfa, P.; Clichici, S.; Filip, G.A. Modulatory effects of Cornus sanguinea L. mediated green synthesized silver nanoparticles on oxidative stress, COX-2/NOS2 and NFkB/pNFkB expressions in experimental inflammation in Wistar rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110709. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ahn, S.; Kang, J.-P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; El-Agamy Farh, M.; Aceituno, V.C.; Kim, Y.J.; et al. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: A green synthetic approach. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2022–2032. [Google Scholar] [CrossRef]
- Tan Sian Hui Abdullah, H.S.; Aqlili Riana Mohd Asseri, S.N.; Khursyiah Wan Mohamad, W.N.; Kan, S.-Y.; Azmi, A.A.; Yong Julius, F.S.; Chia, P.W. Green synthesis, characterization and applications of silver nanoparticle mediated by the aqueous extract of red onion peel. Environ. Pollut. Barking Essex 1987 2021, 271, 116295. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.A.; Naghizadeh, A.; Amiri, O.; Shirzadi-Ahodashti, M.; Mortazavi-Derazkola, S. Green and facile synthesis of Ag nanoparticles using Crataegus pentagyna fruit extract (CP-AgNPs) for organic pollution dyes degradation and antibacterial application. Bioorganic Chem. 2020, 94, 103425. [Google Scholar] [CrossRef]
- Shobana, S.; Veena, S.; Sameer, S.S.M.; Swarnalakshmi, K.; Vishal, L.A. Green Synthesis of Silver Nanoparticles Using Artocarpus hirsutus Seed Extract and its Antibacterial Activity. Curr. Pharm. Biotechnol. 2020, 21, 980–989. [Google Scholar] [CrossRef]
- Nawabjohn, M.S.; Sivaprakasam, P.; Anandasadagopan, S.K.; Begum, A.A.; Pandurangan, A.K. Green Synthesis and Characterisation of Silver Nanoparticles Using Cassia tora Seed Extract and Investigation of Antibacterial Potential. Appl. Biochem. Biotechnol. 2022, 194, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Osibe, D.A.; Chiejina, N.V.; Ogawa, K.; Aoyagi, H. Stable antibacterial silver nanoparticles produced with seed-derived callus extract of Catharanthus roseus. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Shanmugam, K. Extracellular and intracellular synthesis of silver nanoparticles. Asian J. Pharm. Clin. Res. 2016, 9, 133–139. [Google Scholar] [CrossRef]
- Rabia Kanwar, R.F.; Khalid, A. 2. Biological, physical and chemical synthesis of silver nanoparticles and their non-toxic bio-chemical application: A brief review. Pure Appl. Biol. PAB 2021, 11, 421–438. [Google Scholar]
- Sharma, N.K.; Vishwakarma, J.; Rai, S.; Alomar, T.S.; AlMasoud, N.; Bhattarai, A. Green Route Synthesis and Characterization Techniques of Silver Nanoparticles and Their Biological Adeptness. ACS Omega 2022, 7, 27004–27020. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Ranoszek-Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Optical Properties and Ultrafast Dynamics of Metallic Nanocrystals. Annu. Rev. Phys. Chem. 2003, 54, 331–366. [Google Scholar] [CrossRef] [PubMed]
- Noginov, M.A.; Zhu, G.; Bahoura, M.; Adegoke, J.; Small, C.; Ritzo, B.A.; Drachev, V.P.; Shalaev, V.M. The effect of gain and absorption on surface plasmons in metal nanoparticles. Appl. Phys. B 2007, 86, 455–460. [Google Scholar] [CrossRef]
- He, R.; Qian, X.; Yin, J.; Zhu, Z. Preparation of polychrome silver nanoparticles in different solvents. J. Mater. Chem. 2002, 12, 3783–3786. [Google Scholar] [CrossRef]
- Rani, P.; Kumar, V.; Singh, P.P.; Matharu, A.S.; Zhang, W.; Kim, K.-H.; Singh, J.; Rawat, M. Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants. Environ. Int. 2020, 143, 105924. [Google Scholar] [CrossRef] [PubMed]
- Majeed Khan, M.A.; Kumar, S.; Ahamed, M.; Alrokayan, S.A.; AlSalhi, M.S. Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanoscale Res. Lett. 2011, 6, 434. [Google Scholar] [CrossRef]
- Parit, S.B.; Karade, V.C.; Patil, R.B.; Pawar, N.V.; Dhavale, R.P.; Tawre, M.; Pardesi, K.; Jadhav, U.U.; Dawkar, V.V.; Tanpure, R.S.; et al. Bioinspired synthesis of multifunctional silver nanoparticles for enhanced antimicrobial and catalytic applications with tailored SPR properties. Mater. Today Chem. 2020, 17, 100285. [Google Scholar] [CrossRef]
- Mehta, B.K.; Chhajlani, M.; Shrivastava, B.D. Green synthesis of silver nanoparticles and their characterization by XRD. J. Phys. Conf. Ser. 2017, 836, 012050. [Google Scholar] [CrossRef]
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal. Methods 2014, 6, 7324–7334. [Google Scholar] [CrossRef]
- Hall, J.B.; Dobrovolskaia, M.A.; Patri, A.K.; McNeil, S.E. Characterization of nanoparticles for therapeutics. Nanomed. 2007, 2, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Reymond-Laruinaz, S.; Saviot, L.; Potin, V.; Marco de Lucas, M. del C. Protein–nanoparticle interaction in bioconjugated silver nanoparticles: A transmission electron microscopy and surface enhanced Raman spectroscopy study. Appl. Surf. Sci. 2016, 389, 17–24. [Google Scholar] [CrossRef]
- Qayyum, S.; Khan, A.U. Biofabrication of broad range antibacterial and antibiofilm silver nanoparticles. IET Nanobiotechnol. 2016, 10, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A. The microbiology of a pharmaceutical effluent and its public health implications. World J. Microbiol. Biotechnol. 2004, 20, 167–171. [Google Scholar] [CrossRef]
- Lateef, A.; Oloke, J.K.; Gueguim-Kana, E.B. Antimicrobial resistance of bacterial strains isolated from orange juice products. Afr. J. Biotechnol. 2004, 3, 334–338. [Google Scholar] [CrossRef]
- Adewoye, S.O.; Lateef, A. Assessment of the Microbiological Quality of Clarias gariepinus Exposed to an Industrial Effluent in Nigeria. Environmentalist 2004, 24, 249–254. [Google Scholar] [CrossRef]
- Eltarahony, M.; Zaki, S.; ElKady, M.; Abd-El-Haleem, D. Biosynthesis, Characterization of Some Combined Nanoparticles, and Its Biocide Potency against a Broad Spectrum of Pathogens. J. Nanomater. 2018, 2018, 5263814. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Seetharaman, P.; Krishnan, M.; Gnanasekar, S.; Sivaperumal, S. Carica papaya (Papaya) latex: A new paradigm to combat against dengue and filariasis vectors Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). 3 Biotech 2018, 8, 83. [Google Scholar] [CrossRef]
- Arunasri, K.; Mohan, S.V. Biofilms. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 295–313. ISBN 978-0-444-64052-9. [Google Scholar]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Malheiro, J.; Simões, M. Antimicrobial resistance of biofilms in medical devices. In Biofilms and Implantable Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 97–113. ISBN 978-0-08-100382-4. [Google Scholar]
- Almaguer-Flores, A. Biofilms in the oral environment. In Bio-Tribocorrosion in Biomaterials and Medical Implants; Woodhead Publishing Limited: Sawston, UK; Elsevier: Sawston, UK, 2013; pp. 169–186. ISBN 978-0-85709-540-4. [Google Scholar]
- Loza-Correa, M.; Ramírez-Arcos, S. Detection of bacterial adherence and biofilm formation on medical surfaces. In Biofilms and Implantable Medical Devices; Woodhead Publishing: Duxford, UK; Woodhead Publishing: Cambridge, MA, USA; Woodhead Publishing: Kidlington, UK; Elsevier: Duxford, UK; Elsevier: Cambridge, MA, USA; Elsevier: Kidlington, UK, 2017; pp. 181–193. ISBN 978-0-08-100382-4. [Google Scholar]
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef]
- van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Z.; Zara, J.N.; Hsu, C.; Soofer, D.E.; Lee, K.S.; Siu, R.K.; Miller, L.S.; Zhang, X.; Carpenter, D.; et al. The antimicrobial and osteoinductive properties of silver nanoparticle/poly (DL-lactic-co-glycolic acid)-coated stainless steel. Biomaterials 2012, 33, 8745–8756. [Google Scholar] [CrossRef]
- Zheng, Z.; Yin, W.; Zara, J.N.; Li, W.; Kwak, J.; Mamidi, R.; Lee, M.; Siu, R.K.; Ngo, R.; Wang, J.; et al. The use of BMP-2 coupled—Nanosilver-PLGA composite grafts to induce bone repair in grossly infected segmental defects. Biomaterials 2010, 31, 9293–9300. [Google Scholar] [CrossRef]
- Magalhães, A.P.R.; Santos, L.B.; Lopes, L.G.; Estrela, C.R.D.A.; Estrela, C.; Torres, M.; Bakuzis, A.F.; Cardoso, P.C.; Carrião, M.S. Nanosilver Application in Dental Cements. Int. Sch. Res. Not. 2012, 2012, e365438. [Google Scholar] [CrossRef]
- Akhavan, A.; Sodagar, A.; Mojtahedzadeh, F.; Sodagar, K. Investigating the effect of incorporating nanosilver/nanohydroxyapatite particles on the shear bond strength of orthodontic adhesives. Acta Odontol. Scand. 2013, 71, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Prospects of Nanobiomaterials for Biosensing. Int. J. Electrochem. 2011, 2011, 125487. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Grodzinski, P.; Cai, W.; Liu, C.H. Big Potential from Small Agents: Nanoparticles for Imaging-Based Companion Diagnostics. ACS Nano 2018, 12, 2106–2121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Franková, J.; Pivodová, V.; Vágnerová, H.; Juránová, J.; Ulrichová, J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J. Appl. Biomater. Funct. Mater. 2016, 14, 137–142. [Google Scholar] [CrossRef]
- Lee, C.G.; Link, H.; Baluk, P.; Homer, R.J.; Chapoval, S.; Bhandari, V.; Kang, M.J.; Cohn, L.; Kim, Y.K.; McDonald, D.M.; et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat. Med. 2004, 10, 1095–1103. [Google Scholar] [CrossRef]
- Barnes, P.J. Th2 cytokines and asthma: An introduction. Respir. Res. 2001, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Imtiyaz, H.Z.; Simon, M.C. Hypoxia-Inducible Factors as Essential Regulators of Inflammation. In Diverse Effects of Hypoxia on Tumor Progression; Simon, M.C., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 345, pp. 105–120. ISBN 978-3-642-13328-2. [Google Scholar]
- Lin, N.; Simon, M.C. Hypoxia-inducible factors: Key regulators of myeloid cells during inflammation. J. Clin. Investig. 2016, 126, 3661–3671. [Google Scholar] [CrossRef]
- Kanipandian, N.; Kannan, S.; Ramesh, R.; Subramanian, P.; Thirumurugan, R. Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater. Res. Bull. 2014, 49, 494–502. [Google Scholar] [CrossRef]
- Reddy, N.J.; Nagoor Vali, D.; Rani, M.; Rani, S.S. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 115–122. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Radhakrishnan, M.; Gopikrishnan, V.; Pazhanimurugan, R.; Balagurunathan, R. A study of the bactericidal, anti-biofouling, cytotoxic and antioxidant properties of actinobacterially synthesised silver nanoparticles. Colloids Surf. B Biointerfaces 2013, 111, 680–687. [Google Scholar] [CrossRef]
- Karami Mehrian, S.; Heidari, R.; Rahmani, F. Effect of silver nanoparticles on free amino acids content and antioxidant defense system of tomato plants. Indian J. Plant Physiol. 2015, 20, 257–263. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Valadbeigi, T. Antibacterial, Antibiofilm, Antiquorum Sensing, Antimotility, and Antioxidant Activities of Green Fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via Protoparmeliopsis muralis Lichen Aqueous Extract against Multi-Drug-Resistant Bacteria. ACS Biomater. Sci. Eng. 2019, 5, 4228–4243. [Google Scholar] [CrossRef]
- Jones, A.-A.D.; Mi, G.; Webster, T.J. A Status Report on FDA Approval of Medical Devices Containing Nanostructured Materials. Trends Biotechnol. 2019, 37, 117–120. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Jawhari, F.Z.; Almehdi, A.M.; Elmsellem, H.; Fikri Benbrahim, K.; Bousta, D.; Bari, A. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens L. Bioorganic Chem. 2019, 93, 103337. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mishra, P.K.; Talegaonkar, S.; Vaidya, B. Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov. Today 2015, 20, 1143–1151. [Google Scholar] [CrossRef]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 298–309. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; B, V.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef]
- Davalos, D.; Akassoglou, K. Fibrinogen as a key regulator of inflammation in disease. Semin. Immunopathol. 2012, 34, 43–62. [Google Scholar] [CrossRef]
- Levi, M.; Schultz, M.; van der Poll, T. Disseminated intravascular coagulation in infectious disease. Semin. Thromb. Hemost. 2010, 36, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, T.; Li, P.; Huang, W.; Tang, J.; Wang, P.; Liu, J.; Yuan, Q.; Bai, R.; Li, B.; et al. Use of Synchrotron Radiation-Analytical Techniques To Reveal Chemical Origin of Silver-Nanoparticle Cytotoxicity. ACS Nano 2015, 9, 6532–6547. [Google Scholar] [CrossRef] [PubMed]
- Tolaymat, T.M.; El Badawy, A.M.; Genaidy, A.; Scheckel, K.G.; Luxton, T.P.; Suidan, M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: A systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total Environ. 2010, 408, 999–1006. [Google Scholar] [CrossRef]
- Park, J.; Lim, D.-H.; Lim, H.-J.; Kwon, T.; Choi, J.; Jeong, S.; Choi, I.-H.; Cheon, J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem. Commun. Camb. Engl. 2011, 47, 4382–4384. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Ji, J.H.; Song, K.S.; Lee, J.H.; Choi, K.H.; Lee, S.H.; Yu, I.J. Acute inhalation toxicity of silver nanoparticles. Toxicol. Ind. Health 2011, 27, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-M.; Mizuta, Y.; Akagi, J.; Toyoda, T.; Sone, M.; Ogawa, K. Size-dependent acute toxicity of silver nanoparticles in mice. J. Toxicol. Pathol. 2018, 31, 73–80. [Google Scholar] [CrossRef]
- Moradi-Sardareh, H.; Basir, H.R.G.; Hassan, Z.M.; Davoudi, M.; Amidi, F.; Paknejad, M. Toxicity of silver nanoparticles on different tissues of Balb/C mice. Life Sci. 2018, 211, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Roshan, R.; Ranjbar, S. Toxicological effects of silver nanoparticles in rats. Afr. J. Microbiol. Res. 2012, 6, 5587–5593. [Google Scholar] [CrossRef]
- Wen, H.; Dan, M.; Yang, Y.; Lyu, J.; Shao, A.; Cheng, X.; Chen, L.; Xu, L. Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS ONE 2017, 12, e0185554. [Google Scholar] [CrossRef]
- Mazen, N.F.; Saleh, E.Z.; Mahmoud, A.A.; Shaalan, A.A. Histological and immunohistochemical study on the potential toxicity of sliver nanoparticles on the structure of the spleen in adult male albino rats. Egypt. J. Histol. 2017, 40, 374–387. [Google Scholar] [CrossRef]
- Trickler, W.J.; Lantz, S.M.; Murdock, R.C.; Schrand, A.M.; Robinson, B.L.; Newport, G.D.; Schlager, J.J.; Oldenburg, S.J.; Paule, M.G.; Slikker, W., Jr.; et al. Silver Nanoparticle Induced Blood-Brain Barrier Inflammation and Increased Permeability in Primary Rat Brain Microvessel Endothelial Cells. Toxicol. Sci. 2010, 118, 160–170. [Google Scholar] [CrossRef]
- Khan, A.M.; Korzeniowska, B.; Gorshkov, V.; Tahir, M.; Schrøder, H.; Skytte, L.; Rasmussen, K.L.; Khandige, S.; Møller-Jensen, J.; Kjeldsen, F. Silver nanoparticle-induced expression of proteins related to oxidative stress and neurodegeneration in an in vitro human blood-brain barrier model. Nanotoxicology 2019, 13, 221–239. [Google Scholar] [CrossRef]
- Xu, F.; Piett, C.; Farkas, S.; Qazzaz, M.; Syed, N.I. Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol. Brain 2013, 6, 29. [Google Scholar] [CrossRef]
- Repar, N.; Li, H.; Aguilar, J.S.; Li, Q.Q.; Damjana, D.; Hong, Y. Silver nanoparticles induce neurotoxicity in a human embryonic stem cell-derived neuron and astrocyte network. Nanotoxicology 2018, 12, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Zanette, C.; Pelin, M.; Crosera, M.; Adami, G.; Bovenzi, M.; Larese, F.F.; Florio, C. Silver nanoparticles exert a long-lasting antiproliferative effect on human keratinocyte HaCaT cell line. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2011, 25, 1053–1060. [Google Scholar] [CrossRef]
- Huang, H.; Lai, W.; Cui, M.; Liang, L.; Lin, Y.; Fang, Q.; Liu, Y.; Xie, L. An Evaluation of Blood Compatibility of Silver Nanoparticles. Sci. Rep. 2016, 6, 25518. [Google Scholar] [CrossRef]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of silver nanoparticles to red blood cells: Size dependent adsorption, uptake, and hemolytic activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef]
- Shathviha, P.C.; Ezhilarasan, D.; Rajeshkumar, S.; Selvaraj, J. β-sitosterol Mediated Silver Nanoparticles Induce Cytotoxicity in Human Colon Cancer HT-29 Cells. Avicenna J. Med. Biotechnol. 2021, 13, 42–46. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef]
- Singh, S.; Bhargava, D.; Dubey, V.; Mishra, A.; Singh, Y. Silver nanoparticles: Biomedical applications, toxicity, and safety issues. Int. J. Res. Pharm. Pharm. Sci. 2017, 2, 1–10. [Google Scholar]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic Potential of Silver Nanoparticles. Yonsei Med. J. 2014, 55, 283. [Google Scholar] [CrossRef]
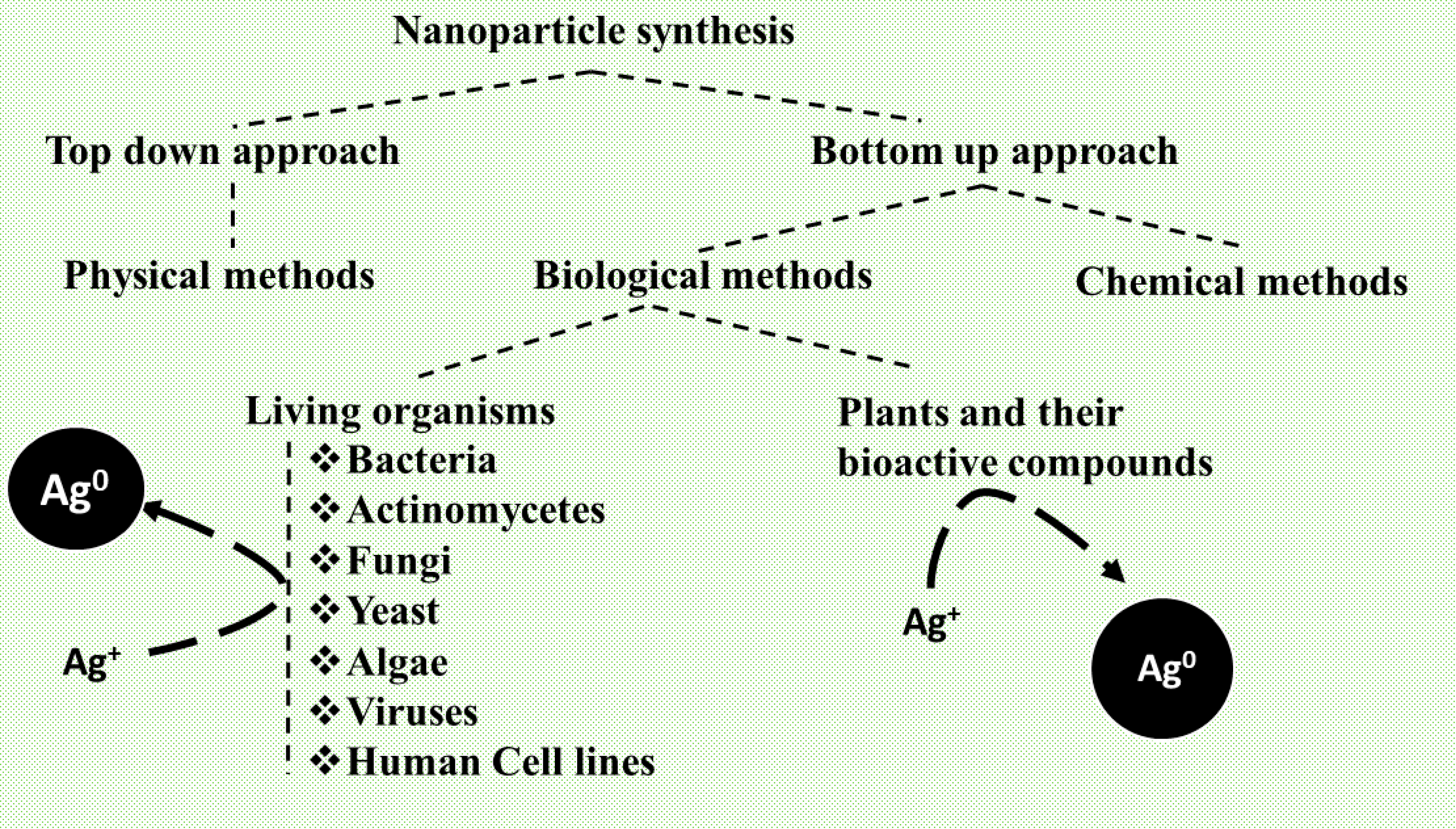
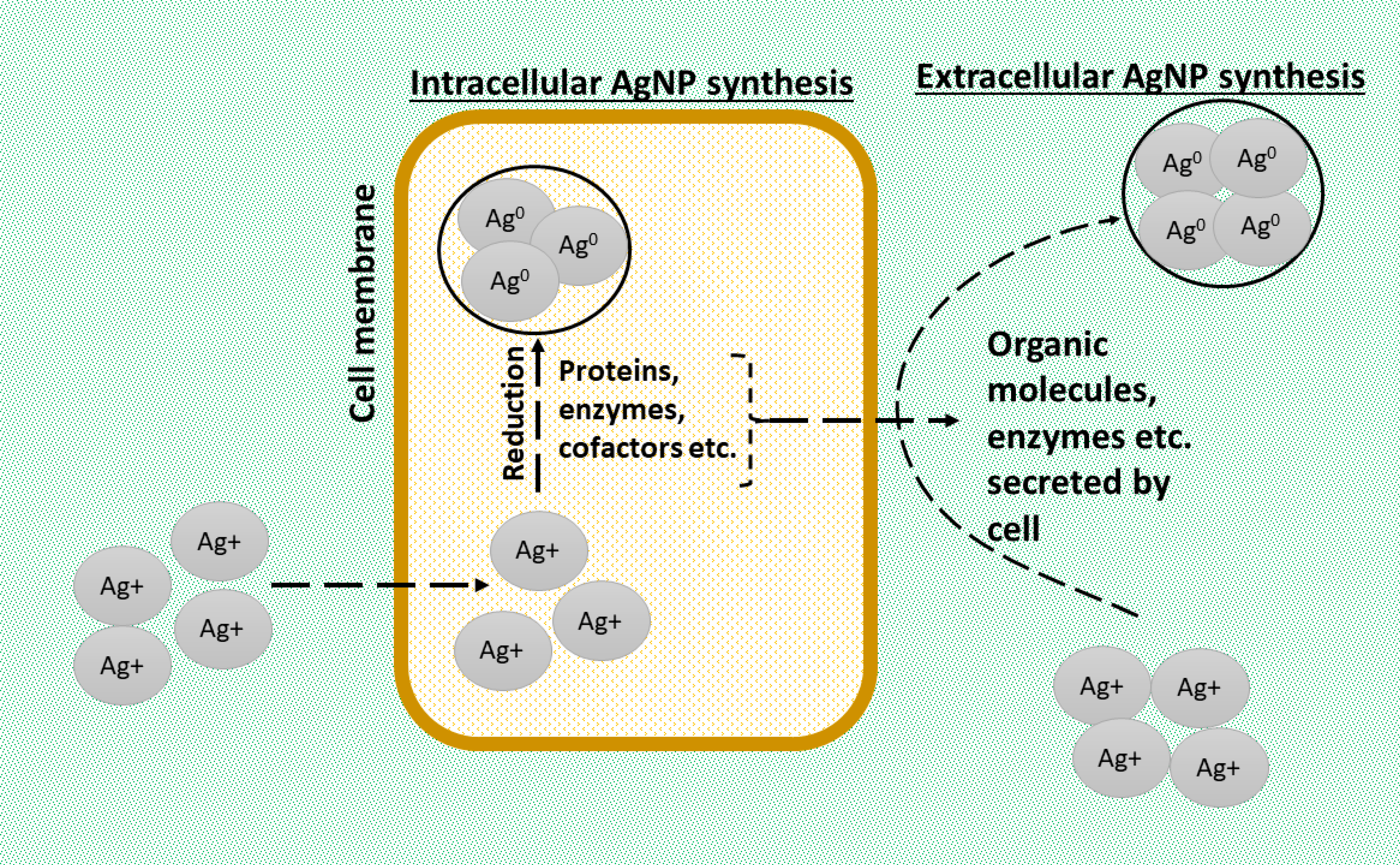

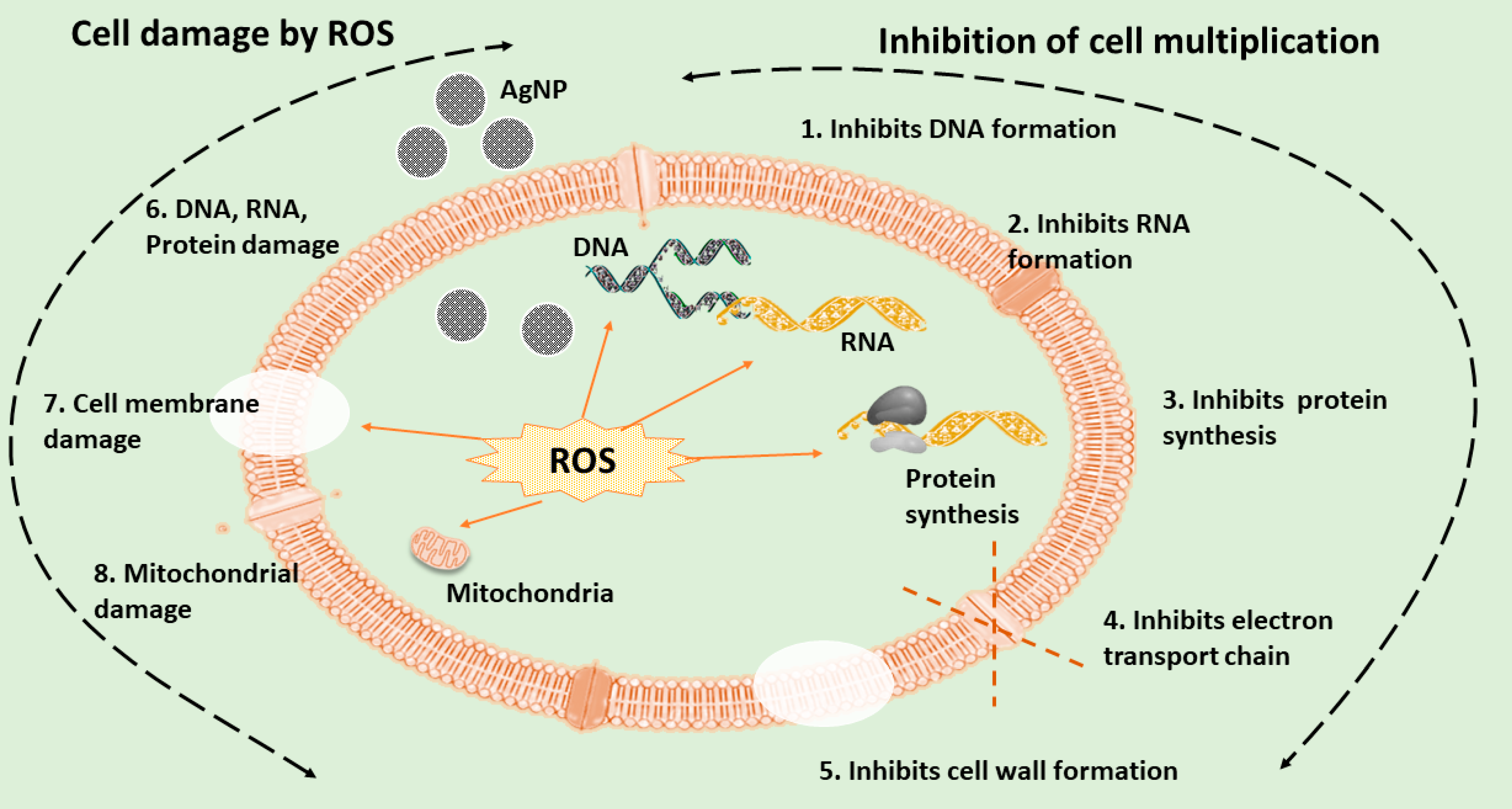

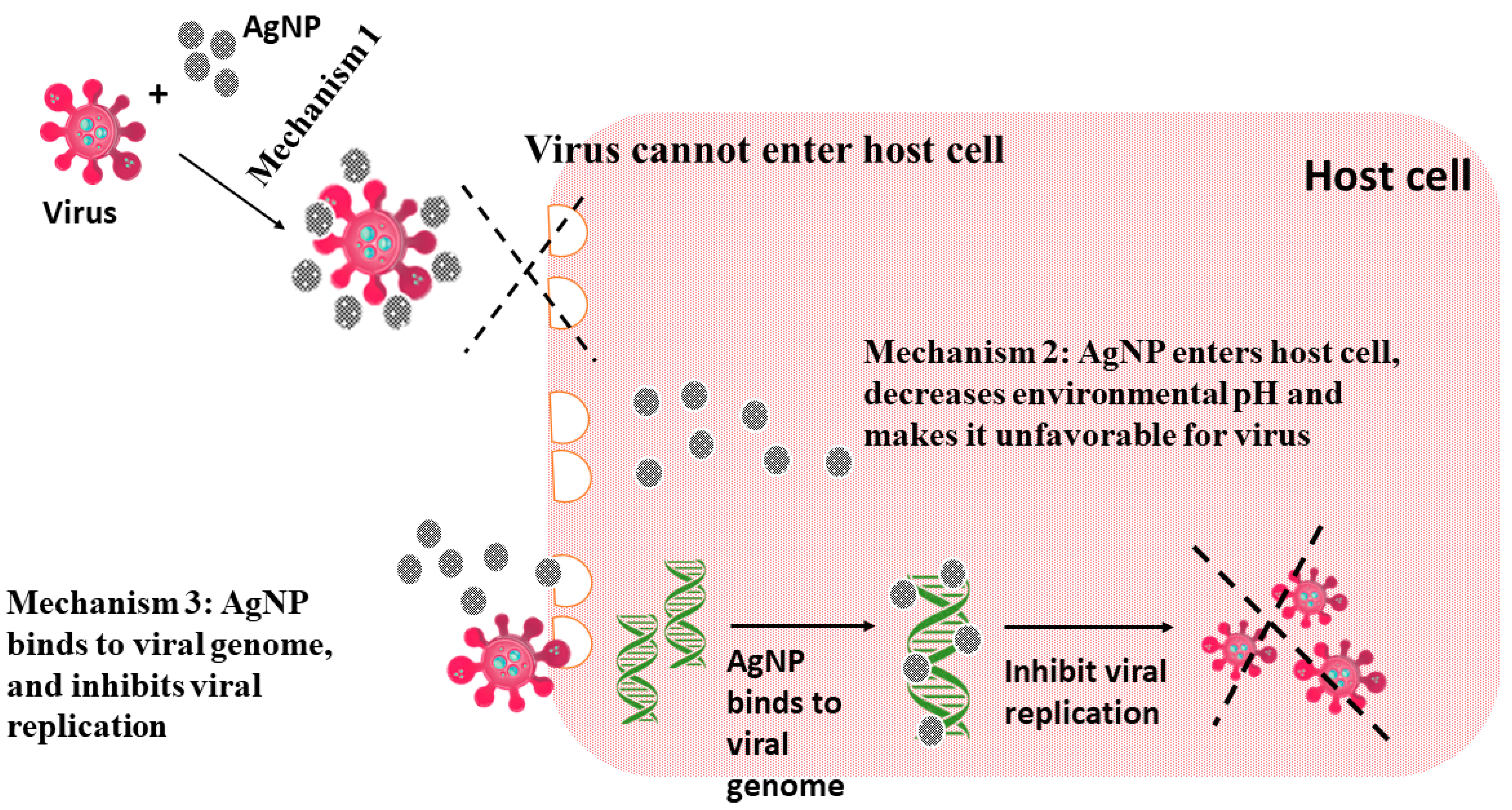

| Bacteria | Morphological Characteristics | Application Studied | References |
|---|---|---|---|
| Escherichia coli strain DH5α | Spherical, 15.0 ± 7.6 nm (TEM) | Antibacterial activity | [56] |
| Bacillus brevis NCIM 2533 | Spherical, 41–68 nm (SEM) | Antibacterial activity against multidrug-resistant pathogens such as Salmonella typhi and Staphylococcus aureus. | [57] |
| Bacillus sp. SBT8 | 20.7 ± 10.5 nm (SEM) | Antibacterial activity against Gram-positive and Gram-negative pathogens. | [58] |
| Pseudomonas aeruginosa | Spherical, 60 nm to 70 nm (SEM) | Antibacterial activity against Salmonella typhi, Shigella dysenteriae, Klebsiella pneumoniae, P. aeruginosa, Proteus mirabilis, and Streptococcus epidermidis | [59] |
| Bacillus subtilis spizizenii | Quasi-spherical, 10 ± 2 nm (TEM) | Antibacterial activity against Gram-negative (Escherichia coli, Pseudomonas aeruginosa, and Burkholderia cenocepacia) and Gram-positive (Staphylococcus aureus, Streptococcus faecalis, and Clostridium sporogenes) strains | [60] |
| Quasi-spherical, 23.8 ± 2 nm (TEM) | |||
| E. coli strain (wus1, wus2) Bacillus sp. (wus5) | Spherical, 10–60 nm (TEM) | Control the nosocomial infections triggered by Methicillin-resistant Staphylococcus aureus | [61] |
| Solibacillus isronensis | Quasi-spherical, 80–120 nm (TEM) | Antibiofilm activity against Escherichia coli and Pseudomonas aeruginosa | [62] |
| Deinococcus radiodurans | Spherical, 37.13 ± 5.97 nm (TEM) | Anticancer activity against MCF-10A cells | [63] |
| Streptomyces malachitus | Round, 19.50 ± 6.72 nm (XRD) | maternal-fetal transplacental transfer assay | [64] |
| Nisin | Spherical, 233 nm (TEM) | Evaluate inflammatory activity in macrophage cells | [65] |
| Actinomycetes | Morphological Characteristics | Application Studied | References |
|---|---|---|---|
| Streptomyces xinghaiensis OF1 | Spherical, 5–20 nm (TEM) | Antimicrobial and antibacterial activity | [73] |
| Streptomyces Hirsutus Strain SNPGA-8 | Spherical, 18.99 nm (TEM) | Antimicrobial and anticancer activity | [74] |
| Streptomyces spiralis | Spherical, 20–60 nm (TEM) | Antibacterial activity | [75] |
| Streptomyces rochei | Spherical, 5–40 nm (TEM) | ||
| Streptomyces capillispiralis Ca-1 | Spherical, 23.77–63.14 nm (TEM) | Antimicrobial, antioxidant, and larvicidal activities | [76] |
| Streptomyces zaomyceticus Oc-5 | Spherical, 11.32–36.72 nm (TEM) | ||
| Streptomyces pseudogriseolus Acv-11 | Spherical, 11.70–44.73 nm nm (TEM) |
| Fungi | Morphological Characteristics | Application Studied | References |
|---|---|---|---|
| Beauveria bassiana (JS1, JS2, and KA75) and Metarhizium anisopliae | Spherical, 23.30, 27.27, 76.61 nm, and 101.34 nm, respectively (SEM) | Antimicrobial and antifungal activity | [82] |
| Aspergillus niger | Spherical, 83.36 (DLS) | Antibacterial activity | [84] |
| Aspergillus fumigatus | Spherical, 88.8 (DLS) | ||
| Aspergillus flavus | Spherical, 208.2 (DLS) | ||
| Aspergillus terreus | Spherical, 113.8 (DLS) | ||
| Monascus pigment | Spherical, 10–40 nm (TEM) | Antibacterial activity against Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus Antibiofilm activity against antibiotic-resistant P. aeruginosa | [83] |
| Aspergillus japonicus PJ01 | Spherical or irregular, 3.8 nm (TEM) | Antibacterial and antifungal activities | [85] |
| Macrophomina phaseolina | Spherical, 40 nm (SEM) | Antibacterial activity, assessment of toxicity in Caenorhabditis elegans | [33] |
| Aspergillus sydowii | Spherical, 1–24 nm (TEM) | Antifungal activity anticancer activity to HeLa cells and MCF-7 cells | [86] |
| Yeast | Morphological Characteristics | Application Studied | References |
|---|---|---|---|
| Rhodotorula mucilaginosa UANL-001L | Spherical, 8.89 ± 6.95 nm (TEM) | Antibacterial and antibiofilm properties | [93] |
| Rhodotorula glutinis | Spherical, 15.45 ± 7.94 nm (TEM) | Antifungal, catalytic and cytotoxicity activities | [92] |
| Rhodotorula mucilaginosa | Spherical, 13.70 ± 8.21 nm (TEM) | ||
| Saccharomyces cerevisiae | Spherical, 10.3–18.9 nm (TEM) | Antimicrobial and anticancer activity | [94] |
| Indian Red Yeast Rice | Spherical, 6.81 nm to 30.93 nm (TEM) | Antibacterial and antibiofilm activity | [95] |
| Algae | Morphological Characteristics | Application Studied | References |
|---|---|---|---|
| Fucus vesiculosus | Spherical, 36.99 ± 12.39 nm (TEM) | Antimicrobial activity | [103] |
| Neochloris oleoabundans | Quasi-spherical, 16.63 nm (TEM) | Antibacterial activity | [104] |
| Ulva flexuosa | 4.93–6.70 nm (TEM) | Antibacterial activity against two Gram-positive (Bacillus subtilis, Staphylococcus aureus) and two Gram-negative (Escherichia coli, Pseudomonas aeruginosa) | [106] |
| Microchaete | Spherical, 7 nm (TEM) | Antioxidant, antiproliferative, and apoptotic activities | [105] |
| Padina tetrastromatica | spiracle or cubic structure, 166 nm (DLS) | Anticancer activity | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goel, M.; Sharma, A.; Sharma, B. Recent Advances in Biogenic Silver Nanoparticles for Their Biomedical Applications. Sustain. Chem. 2023, 4, 61-94. https://doi.org/10.3390/suschem4010007
Goel M, Sharma A, Sharma B. Recent Advances in Biogenic Silver Nanoparticles for Their Biomedical Applications. Sustainable Chemistry. 2023; 4(1):61-94. https://doi.org/10.3390/suschem4010007
Chicago/Turabian StyleGoel, Muskan, Anurag Sharma, and Bechan Sharma. 2023. "Recent Advances in Biogenic Silver Nanoparticles for Their Biomedical Applications" Sustainable Chemistry 4, no. 1: 61-94. https://doi.org/10.3390/suschem4010007
APA StyleGoel, M., Sharma, A., & Sharma, B. (2023). Recent Advances in Biogenic Silver Nanoparticles for Their Biomedical Applications. Sustainable Chemistry, 4(1), 61-94. https://doi.org/10.3390/suschem4010007








