Studies on Biobased Non-Isocyanate Polyurethane Coatings with Potential Corrosion Resistance
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
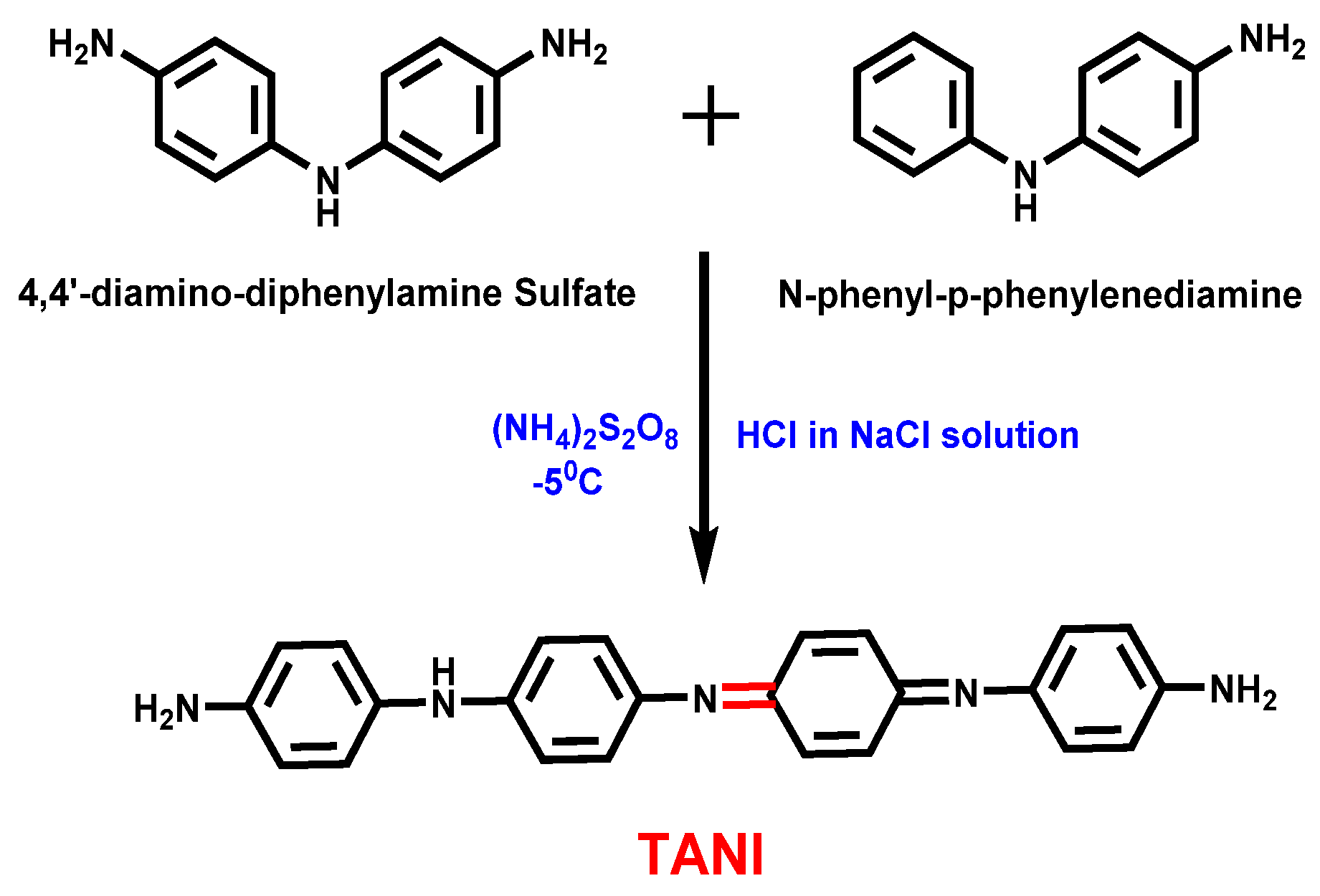
2.2.1. Synthesis of Tetraniline
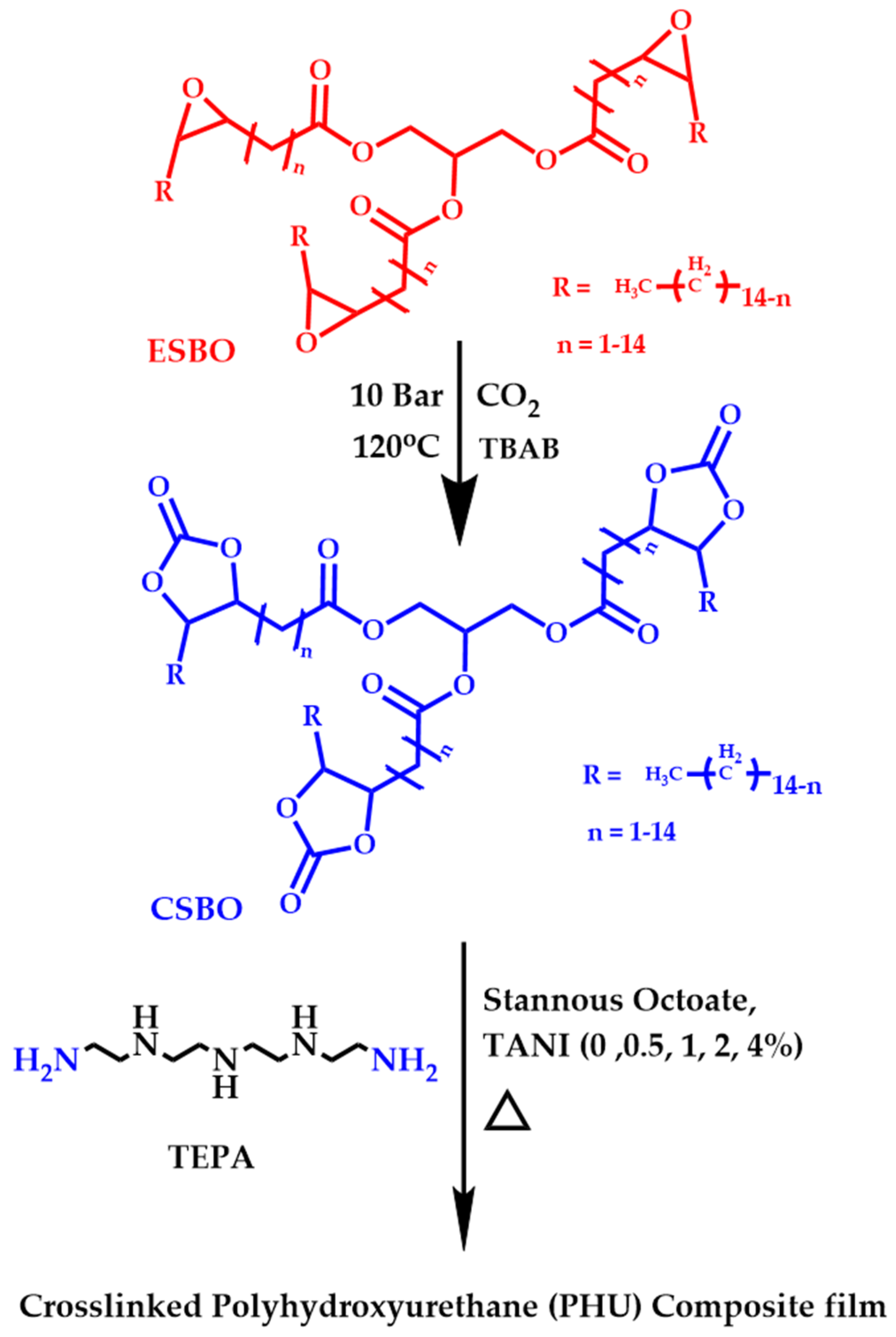
2.2.2. Synthesis of Carbonated Soyabean Oil
2.2.3. Synthesis of NIPU and Its Composite
2.3. Characterization of ESBO, CSBO and Cured Film
2.3.1. Viscosity
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.3.4. Electrospray Ionization Mass Spectroscopy (ESI-MS)
2.3.5. Water Contact Angle Analysis
2.3.6. Thermogravimetric Analysis (TGA)
2.3.7. Dynamic Mechanical Thermal Analyzer (DMA)
2.3.8. The Universal Testing Machine (UTM)
2.3.9. Scanning Electron Microscope
2.3.10. Tafel Polarization
2.3.11. The Salt Spray Fog Test
3. Results and Discussion
3.1. Characterization of TANI
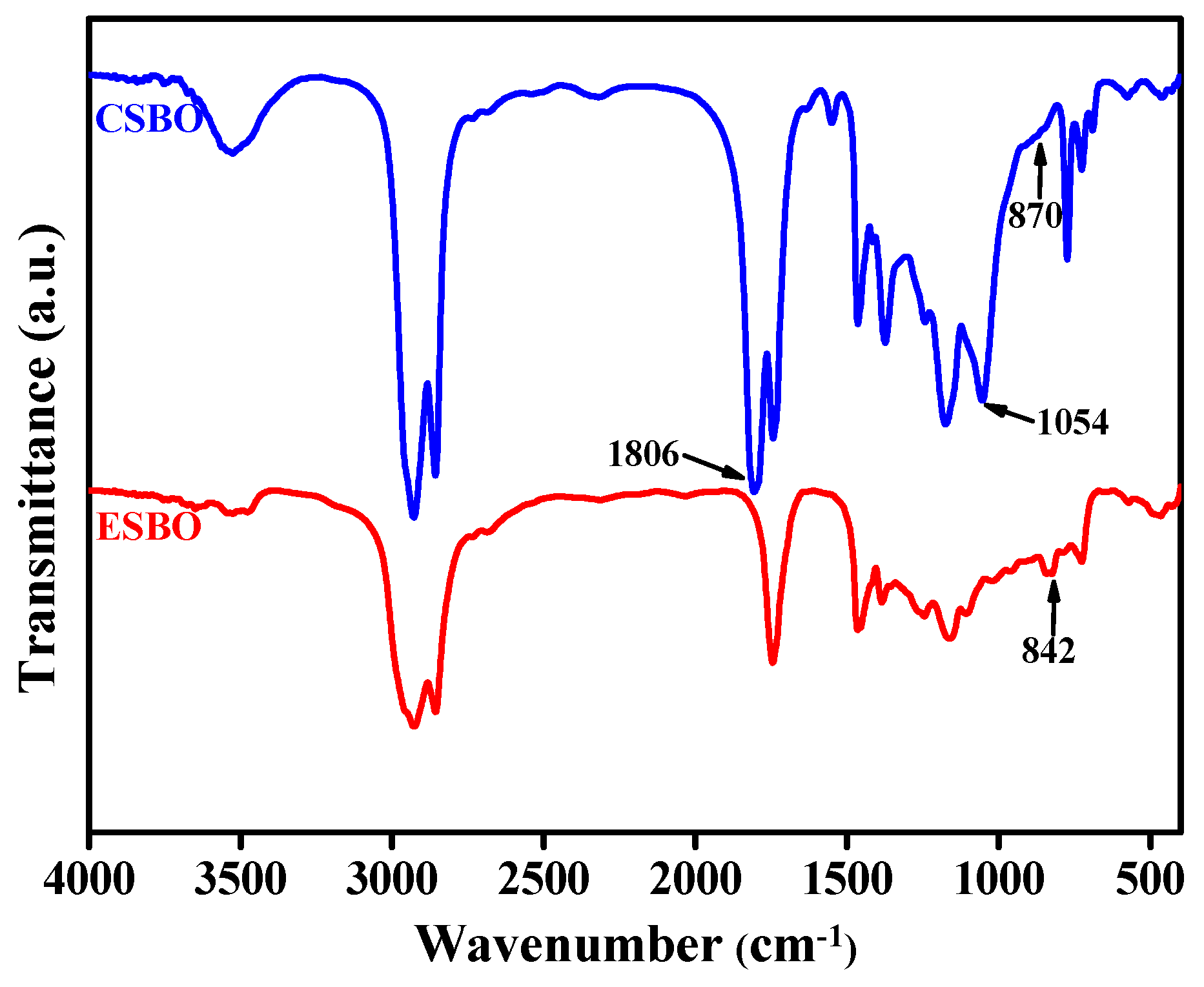
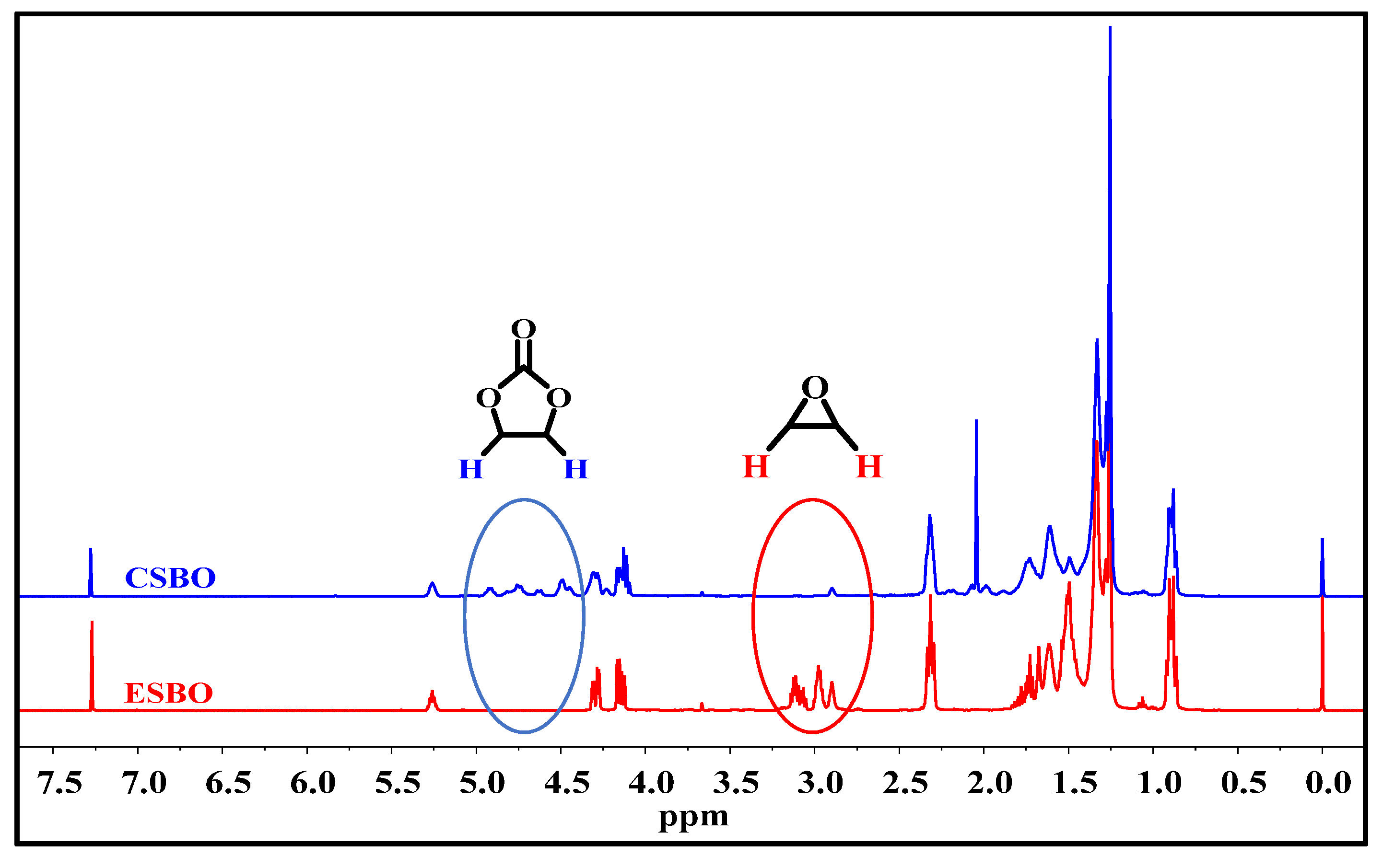
3.2. Characterization of CSBO
3.3. Characterization of NIPU Composites
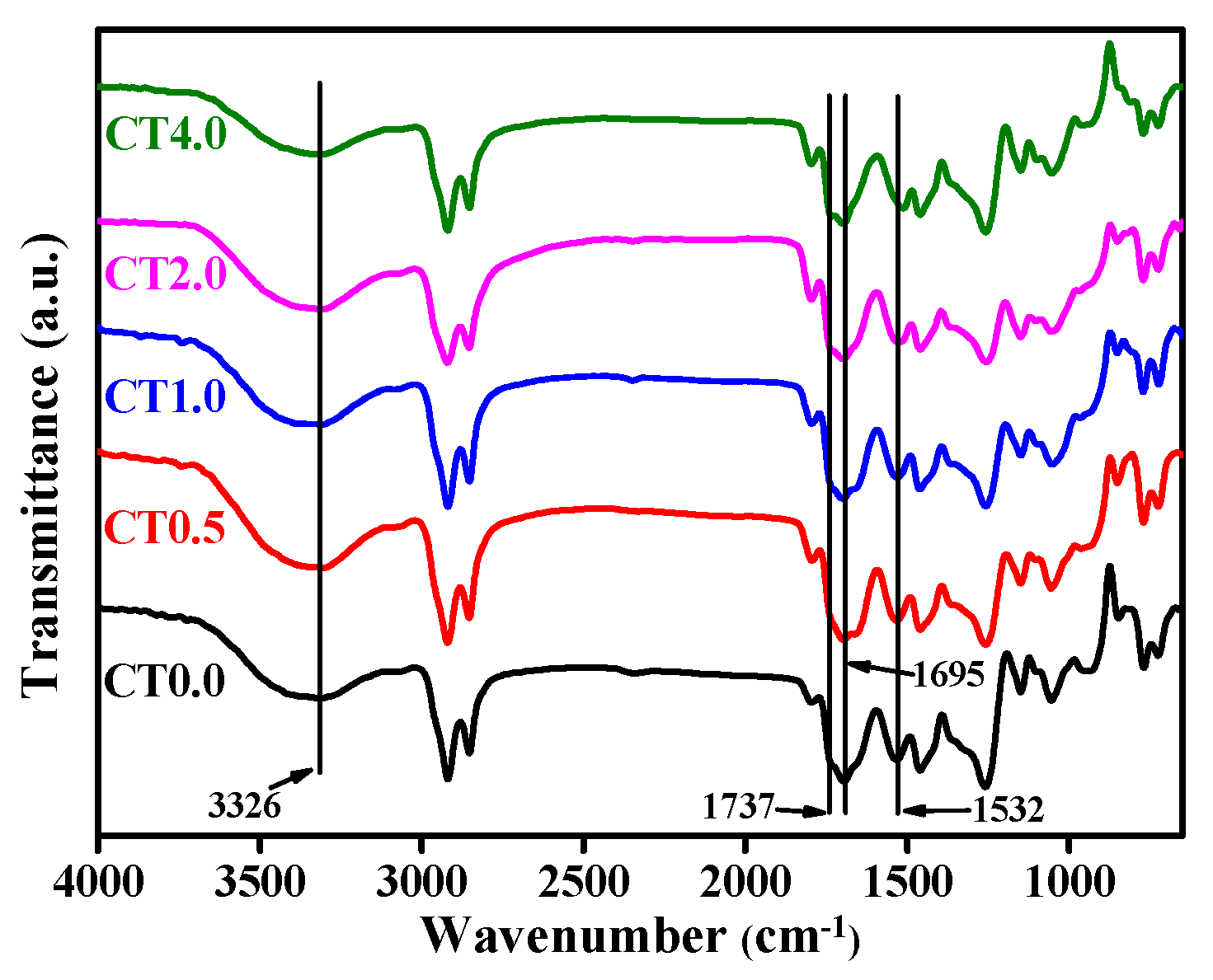
3.3.1. Fourier Transform Infrared Spectroscopy of NIPU–TANI Composites (FTIR)
3.3.2. Thermal Properties
3.3.3. Thermomechanical Properties
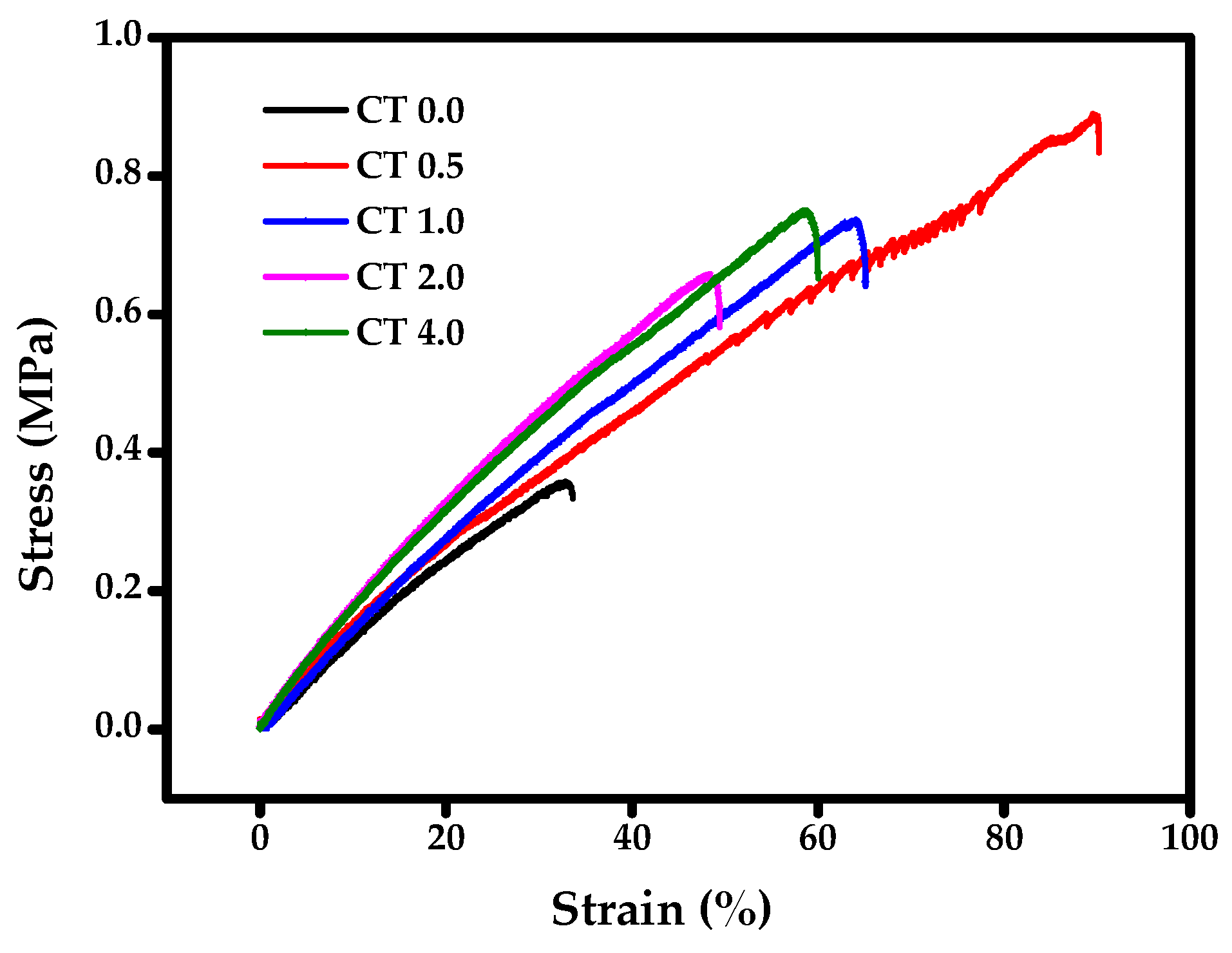
3.3.4. Static mechanical Properties
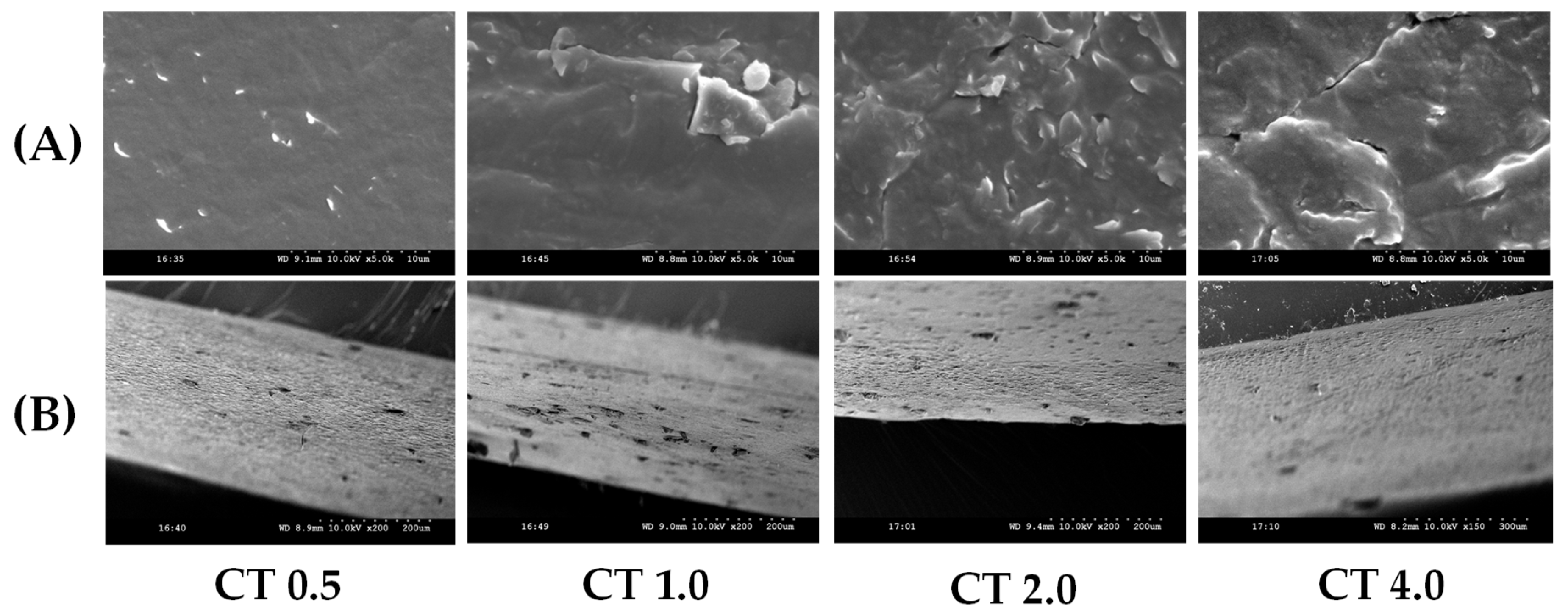
3.3.5. SEM Analysis
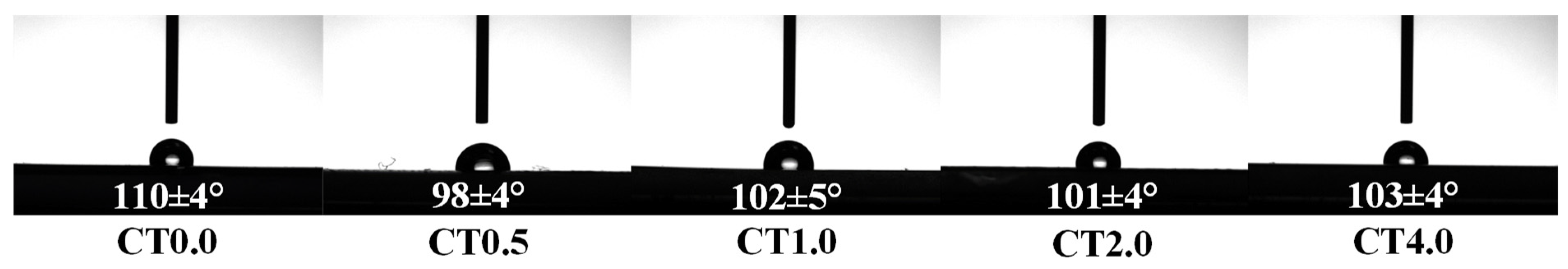
3.3.6. The Water Contact Angle
3.3.7. Polarization Studies
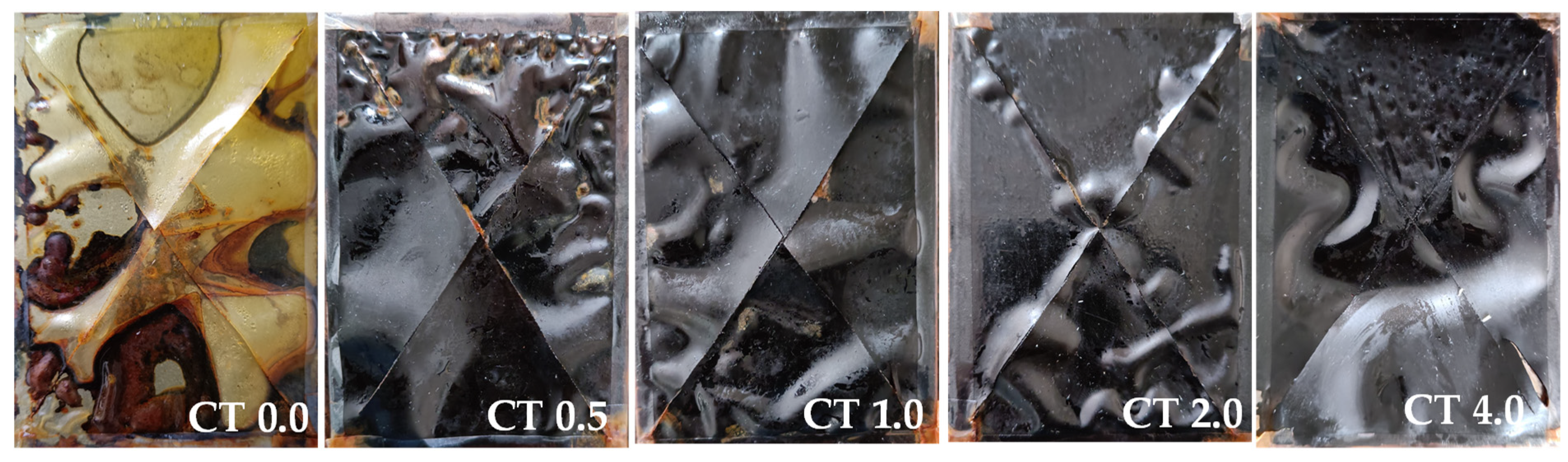
3.3.8. The Salt Spray Fog Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Li, X.; Wang, Y.; Huang, J.; Li, K.; Nie, X.; Jiang, J. Synthesis and application of environmental soybean oil-based epoxidized glycidyl ester plasticizer for poly(vinyl chloride). Eur. J. Lipid Sci. Technol. 2017, 119, 1600216. [Google Scholar] [CrossRef]
- Duffy, E.; Gibney, M.J. Use of a food-consumption database with packaging information to estimate exposure to food-packaging migrants: Epoxidized soybean oil and styrene monomer. Food Addit. Contam. 2007, 24, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hu, Y.; Tang, J.; Zhang, J.; Wang, C.; Shang, Q.; Feng, G.D.; Liu, C.; Zhou, Y.; Lei, W. High-Performance Soybean-Oil-Based Epoxy Acrylate Resins: “Green” Synthesis and Application in UV-Curable Coatings. ACS Sustain. Chem. Eng. 2018, 6, 8340–8349. [Google Scholar] [CrossRef]
- Alagi, P.; Ghorpade, R.; Jang, J.H.; Patil, C.; Jirimali, H.; Gite, V.; Hong, S.C. Functional soybean oil-based polyols as sustainable feedstocks for polyurethane coatings. Ind. Crops Prod. 2018, 113, 249–258. [Google Scholar] [CrossRef]
- Li, A.; Li, K. Pressure-Sensitive Adhesives Based on Epoxidized Soybean Oil and Dicarboxylic Acids. ACS Sustain. Chem. Eng. 2014, 2, 2090–2096. [Google Scholar] [CrossRef]
- Javni, I.; Hong, D.P.; Petrović, Z.S. Polyurethanes from soybean oil, aromatic, and cycloaliphatic diamines by nonisocyanate route. J. Appl. Polym. Sci. 2012, 128, 566–571. [Google Scholar] [CrossRef]
- Gholami, H.; Yeganeh, H. Soybean oil-derived non-isocyanate polyurethanes containing Azetidinium groups as antibacterial wound dressing membranes. Eur. Polym. J. 2020, 142, 110142. [Google Scholar] [CrossRef]
- Hu, S.; Chen, X.; Torkelson, J.M. Bio-based Reprocessable Polyhydroxyurethane Networks: Full Recovery of Cross-link Density with Three Concurrent Dynamic Chemistries. ACS Sustain. Chem. Eng. 2019, 7, 10025–10034. [Google Scholar] [CrossRef]
- Doley, S.; Bora, A.; Saikia, P.; Ahmed, S.; Dolui, S.K. Blending of cyclic carbonate based on soybean oil and glycerol: A non-isocyanate approach towards the synthesis of polyurethane with high performance. J. Polym. Res. 2021, 28, 146. [Google Scholar] [CrossRef]
- Bähr, M.; Mülhaupt, R. Linseed and soybean oil-based polyurethanes prepared via the non-isocyanate route and catalytic carbon dioxide conversion. Green Chem. 2012, 14, 483–489. [Google Scholar] [CrossRef]
- Yu, A.Z.; Setien, R.A.; Sahouani, J.M.; Docken, J.; Webster, D.C. Catalyzed non-isocyanate polyurethane (NIPU) coatings from bio-based poly(cyclic carbonates). J. Coat. Technol. Res. 2018, 16, 41–57. [Google Scholar] [CrossRef]
- Mirzakhanzadeh, Z.; Kosari, A.; Moayed, M.H.; Naderi, R.; Taheri, P.; Mol, J.M.C. Enhanced corrosion protection of mild steel by the synergetic effect of zinc aluminum polyphosphate and 2-mercaptobenzimidazole inhibitors incorporated in epoxy-polyamide coatings. Corros. Sci. 2018, 138, 372–379. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, K.C.; Wang, H.; Zhou, Q. Anti-corrosion non-isocyanate polyurethane polysiloxane organic/inorganic hybrid coatings. Prog. Org. Coat. 2020, 148, 105855. [Google Scholar] [CrossRef]
- Wen, J.G.; Geng, W.; Geng, H.Z.; Zhao, H.; Jing, L.C.; Yuan, X.T.; Tian, Y.; Wang, T.; Ning, Y.J.; Wu, L. Improvement of Corrosion Resistance of Waterborne Polyurethane Coatings by Covalent and Noncovalent Grafted Graphene Oxide Nanosheets. ACS Omega 2019, 4, 20265–20274. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, L.; Dai, J.; Qu, J. Synthesis and properties of fluorinated non-isocyanate polyurethanes coatings with good hydrophobic and oleophobic properties. J. Coat. Technol. Res. 2019, 16, 1233–1241. [Google Scholar] [CrossRef]
- Hamidon, T.S.; Yun, T.P.; Zakaria, F.A.; Hussin, M.H. Potential of zinc based-graphene oxide composite coatings on mild steel in acidic solution. J. Indian Chem. Soc. 2021, 98, 100243. [Google Scholar] [CrossRef]
- Asemani, H.R.; Mannari, V.V. Dual-curable coatings obtained from multi-functional non-isocyanate polyurethane oligomers. J. Coat. Technol. Res. 2022, 5, 1393–1407. [Google Scholar] [CrossRef]
- Doley, S.; Dolui, S.K. Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties. Eur. Polym. J. 2018, 102, 161–168. [Google Scholar] [CrossRef]
- Kathalewar, M.; Sabnis, A.; Waghoo, G. Effect of incorporation of surface treated zinc oxide on non-isocyanate polyurethane based nano-composite coatings. Prog. Org. Coat. 2013, 76, 1215–1229. [Google Scholar] [CrossRef]
- Micić, D.; Šljukić, B.; Zujovic, Z.; Travas-Sejdic, J.; Ćirić-Marjanović, G. Electrocatalytic Activity of Carbonized Nanostructured Polyanilines for Oxidation Reactions: Sensing of Nitrite Ions and Ascorbic Acid. Electrochim. Acta 2014, 120, 147–158. [Google Scholar] [CrossRef]
- Pakapongpan, S.; Mensing, J.P.; Phokharatkul, D.; Lomas, T.; Tuantranont, A. Highly selective electrochemical sensor for ascorbic acid based on a novel hybrid graphene-copper phthalocyanine-polyaniline nanocomposites. Electrochim. Acta 2014, 133, 294–301. [Google Scholar] [CrossRef]
- Rana, U.; Paul, N.D.; Mondal, S.; Chakraborty, C.; Malik, S. Water soluble polyaniline coated electrode: A simple and nimble electrochemical approach for ascorbic acid detection. Synth. Met. 2014, 192, 43–49. [Google Scholar] [CrossRef]
- Xue, C.; Wang, X.; Zhu, W.; Han, Q.; Zhu, C.; Hong, J.; Zhou, X.; Jiang, H. Electrochemical serotonin sensing interface based on double-layered membrane of reduced graphene oxide/polyaniline nanocomposites and molecularly imprinted polymers embedded with gold nanoparticles. Sens. Actuators B Chem. 2014, 196, 57–63. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Y.; Luo, J.; Zhang, L.; Xiao, D. Diameter-controlled synthesis of polyaniline microtubes and their electrocatalytic oxidation of ascorbic acid. J. Mater. Chem. B 2014, 2, 4122–4129. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.Y.; Du, S.; Guo, W.J.; Pei, M.S. Micro/nanostructures of PANI obtained in the presence of water soluble polymers and their electrochemical sensing properties. RSC Adv. 2015, 5, 69067–69074. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.; Sai-Anand, G.; Gopalan, A.I.; Lee, H.G.; Yeo, H.K.; Kang, S.W.; Lee, K.P. Direct electrochemistry of cytochrome c with three-dimensional nanoarchitectured multicomponent composite electrode and nitrite biosensing. Sens. Actuators B Chem. 2016, 228, 737–747. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, C.; Ding, T. A one-step method to synthesize N,N′-bis(4′-aminophenyl)-1,4-quinonenediimine and its derivatives. Tetrahedron Lett. 1996, 37, 731–734. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Y.; Mao, H.; Lu, X.; Zhang, W.; Wei, Y. Synthesis of parent aniline tetramer and pentamer and redox properties. Mater. Lett. 2005, 59, 2446–2450. [Google Scholar] [CrossRef]
- Peng, C.W.; Hsu, C.; Lin, K.H.; Li, P.L.; Hsieh, M.F.; Wei, Y.; Yeha, J.M.; Yuc, Y.H. Electrochemical corrosion protection studies of aniline-capped aniline trimer-based electroactive polyurethane coatings. Electrochim. Acta 2011, 58, 614–620. [Google Scholar] [CrossRef]
- Huang, K.Y.; Shiu, C.L.; Wu, P.S.; Wei, Y.; Yeh, J.M.; Li, W.T. Effect of amino-capped aniline trimer on corrosion protection and physical properties for electroactive epoxy thermosets. Electrochim. Acta 2009, 54, 5400–5407. [Google Scholar] [CrossRef]
- Weng, C.J.; Huang, J.Y.; Huang, K.Y.; Jhuo, Y.S.; Tsai, M.H.; Yeh, J.M. Advanced anticorrosive coatings prepared from electroactive polyimide–TiO2 hybrid nanocomposite materials. Electrochim. Acta 2010, 55, 8430–8438. [Google Scholar] [CrossRef]
- Huang, T.C.; Yeh, T.C.; Huang, H.Y.; Ji, W.F.; Lin, T.C.; Chen, C.A.; Yang, T.I.; Yeh, J.M. Electrochemical investigations of the anticorrosive and electrochromic properties of electroactive polyamide. Electrochim. Acta 2012, 63, 185–191. [Google Scholar] [CrossRef]
- Yeh, L.C.; Huang, T.C.; Huang, Y.P.; Huang, H.Y.; Chen, H.H.; Yang, T.I.; Yeh, J.M. Synthesis electroactive polyurea with aniline-pentamer-based in the main chain and its application in electrochemical sensor. Electrochim. Acta 2013, 94, 300–306. [Google Scholar] [CrossRef]
- Ji, W.F.; Chu, C.M.; Hsu, S.C.; Lu, Y.D.; Yu, Y.C.; Santiago, K.S.; Yeh, J.M. Synthesis and characterization of organo-soluble aniline oligomer-based electroactive doped with gold nanoparticles, and application to electrochemical sensing of ascorbic acid. Polymer 2017, 128, 218–228. [Google Scholar] [CrossRef]
- Heng, Z.G.; Zhang, X.; Chen, Y.; Zou, H.; Liang, M. In-situ construction of “octopus”-like nanostructure to achieve high performance epoxy thermosets. Chem. Eng. J. 2019, 360, 542–552. [Google Scholar] [CrossRef]
- Bouoidina, A.; Ech-chihbi, E.; El-Hajjaji, F.; El Ibrahimi, B.; Kaya, S.; Taleb, M. Anisole derivatives as sustainable-green inhibitors for mild steel corrosion in 1 M HCl: DFT and molecular dynamic simulations approach. J. Mol. Liq. 2021, 324, 115088. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, Y.; Wan, H.; Chen, L.; Zhou, H.; Chen, J. Chemical Modification ofGraphene Oxide to Reinforce the Corrosion Protection Performance of UV-Curable Polyurethane Acrylate Coating. Prog. Org. Coat. 2020, 141, 105547. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, Z.; Yu, L.; Zhang, L.; Bowmaker, G.A. Electrochemical Corrosion Behaviour of Carbon Steel Coated by Polyaniline Copolymers Micro/Nanostructures. RSC Adv. 2014, 4, 32718–32725. [Google Scholar] [CrossRef]
- Yongbo, D.; Liang, J.; Liu, G.; Ni, W.; Shen, L. Preparation and Anticorrosive Propertyof Soluble Aniline Tetramer. Coatings 2019, 9, 399. [Google Scholar]






| Characteristics | Specification |
|---|---|
| Appearance | Light yellow clear liquid |
| Specific gravity | 0.987 |
| Moisture content (%) | 0.1% |
| pH | 6.5 |
| Oxirane oxygen content (%) | 6.52 |
| Acid value (mg KOH/g) | 0.64 |
| Iodine value (g I2/100 g) | 1.62 |
| Properties | Value |
|---|---|
| Viscosity (cPs) @44 °C | 10,180 |
| Moisture Content (%) | 0.31 |
| Appearance | Brownish viscous liquid |
| Oxirane Oxygen Content (%) | 0.55 |
| Sample Code | Degradation Temperature (°C) | ||
|---|---|---|---|
| 5% Td | 50% Td | Residue @550 °C (%) | |
| CT 0.0 | 207 | 373 | 1.72 |
| CT 0.5 | 185 | 373 | 2.11 |
| CT 1.0 | 204 | 372 | 1.40 |
| CT 2.0 | 203 | 372 | 2.86 |
| CT 4.0 | 196 | 382 | 4.14 |
| Sample | Storage Modulus (MPa) | Tg (°C) | Tensile Strength (MPa) | Elongation (%) | |
|---|---|---|---|---|---|
| @ −50 °C | @ 25 °C | ||||
| CT 0.0 | 2543 | 30 | 21.44 | 0.45 ± 0.10 | 36.71 ± 3.1 |
| CT 0.5 | 1910 | 29 | 29.78 | 0.85 ± 0.06 | 89.16 ± 0.7 |
| CT 1.0 | 2377 | 35 | 26.92 | 0.97 ± 0.07 | 92.00 ± 4.0 |
| CT 2.0 | 2071 | 18 | 24.54 | 0.57 ± 0.08 | 44.75 ± 4.3 |
| CT 4.0 | 2198 | 73 | 29.33 | 0.75 ± 0.08 | 61.6 ± 5.0 |
| Sample | ECorr (mV) | ICorr (nA) | Corrosion Rate (CR) (mm/year) | Polarization Resistance (Ω) |
|---|---|---|---|---|
| CT 0.0 | −377.3 | 23.19 | 2.69 × 10−4 | 5.59 × 105 |
| CT 0.5 | −213.66 | 11.99 | 1.39 × 10−4 | 9.09 × 105 |
| CT 1.0 | −203.06 | 7.97 | 9.26 × 10−5 | 15.39 × 105 |
| CT 2.0 | −331.08 | 5.8 | 6.81 × 10−5 | 25.22 × 105 |
| CT 4.0 | −276.09 | 1.43 | 1.67 × 10−5 | 73.80 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhore, N.; Prasad, E.; Narayan, R.; Rao, C.R.K.; Palanisamy, A. Studies on Biobased Non-Isocyanate Polyurethane Coatings with Potential Corrosion Resistance. Sustain. Chem. 2023, 4, 95-109. https://doi.org/10.3390/suschem4010008
Dhore N, Prasad E, Narayan R, Rao CRK, Palanisamy A. Studies on Biobased Non-Isocyanate Polyurethane Coatings with Potential Corrosion Resistance. Sustainable Chemistry. 2023; 4(1):95-109. https://doi.org/10.3390/suschem4010008
Chicago/Turabian StyleDhore, Nikhil, Ermiya Prasad, Ramanuj Narayan, Chepuri R. K. Rao, and Aruna Palanisamy. 2023. "Studies on Biobased Non-Isocyanate Polyurethane Coatings with Potential Corrosion Resistance" Sustainable Chemistry 4, no. 1: 95-109. https://doi.org/10.3390/suschem4010008
APA StyleDhore, N., Prasad, E., Narayan, R., Rao, C. R. K., & Palanisamy, A. (2023). Studies on Biobased Non-Isocyanate Polyurethane Coatings with Potential Corrosion Resistance. Sustainable Chemistry, 4(1), 95-109. https://doi.org/10.3390/suschem4010008






