Abstract
The facile and green synthesis of 1,1′-binaphthalene-2,2′-diamine (BINAM) derivatives was established via the anodic dehydrogenative homo-coupling of 2-naphthylamines. The sustainable protocol provided a series of BINAMs in excellent yields of up to 98% with good current efficiency (66%) and H2 as the sole coproduct without utilizing transition-metal reagents or stoichiometric oxidants.
1. Introduction
1,1′-Bi-2-naphthylamine (BINAM) and its derivatives are widely used as building blocks for transition-metal ligands and organocatalysts [1,2,3,4], as well as chiroptical materials for fluorescence sensing [5,6,7]. Among their syntheses, transition-metal mediated coupling of 2-naphthylamine derivatives has been well established [8,9,10,11,12]. In particular, the Ullmann [13] and metal-mediated C(sp2)–H oxidative [14,15,16,17,18,19,20] coupling reactions of 2-naphthylamines are the most popular synthetic approaches to obtaining BINAMs. Benzidine rearrangement [21,22,23] and Smiles rearrangement of phenolic compounds [24] have been reported to be alternative synthetic methods for BINAMs. However, the regio- and chemoselective C–C coupling of 2-naphthylamines remains challenging, probably because these strategies require the use of excess amounts of metals and oxidants, which leads to many side reactions of starting material and over-oxidation of coupling products.
Biaryls synthesis through anodic oxidation utilizing electrochemical synthesis has emerged as a promising green and sustainable approach over the last few decades. The electrochemical dehydrogenative coupling of aryls utilizes electricity as an alternative to oxidants and produces H2 as the sole coproduct without generating any toxic waste; thus, this approach exhibits great advantages in terms of high atom economy and environmentally benign synthesis protocols [25,26]. Although remarkable achievements have been made in the electrochemical dehydrogenative heterocoupling of anilines [27], few reports on the homocoupling of aniline derivatives [28,29], particularly 2-naphthylamines, are available. In the 1980s, Gossage [30] and Sereno [31] independently described the electrochemical dehydrogenative homocoupling of 2-naphthylamines, which afforded BINAMs, but in low yield. Thus, the development of efficient oxidative homocoupling protocols for 2-naphthylamines via electrochemical synthesis is of great importance.
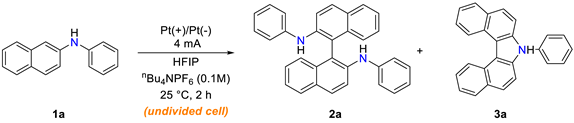
Because the discharge of the electrolyte or solvent leads to a low yield and poor current efficiency, we reexamined the anodic dehydrogenative homocoupling conditions of 2-naphthylamines to save energy and chemical loading in the pursuit of developing environmentally benign chemical reactions. To our delight, under the newly established conditions, the corresponding homocoupling products were obtained in 98% yield with good current efficiency (66%; Scheme 1).
Scheme 1.
Electrochemical dehydrogenative homocoupling of 2-naphthylamines.
2. Materials and Methods
2.1. Materials
N-Phenyl-2-napthylamine (1a) was purchased from Tokyo Chemical Industries (TCI). All commercially available organic and inorganic compounds were used directly without further purification.
2.2. Methods
2.2.1. Spectroscopy and Spectrometry
1H- and 13C-NMR spectra were recorded at 25 °C using a JEOL JMN ECS400 FT NMR instrument (1H-NMR 400 MHz; 13C-NMR 100 MHz). The 1H-NMR spectra are reported as follows: chemical shift in ppm downfield of tetramethylsilane and referenced to a residual solvent peak (CHCl3) at 7.26 ppm, integration, multiplicities (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), and coupling constants (Hz). The 13C-NMR spectra are reported in ppm relative to the central line of the triplet for CDCl3 at 77.16 ppm. ESI-MS spectra were obtained using a JMS-T100LC instrument (JEOL). FT-IR spectra were recorded using a JASCO FT-IR system (FT/IR4100). Thin-layer chromatography (TLC) analysis of the reaction mixture was performed on Merck silica gel 60 F254 TLC plates and visualized under UV light. Column chromatography on SiO2 was performed using Kanto Silica Gel 60 (63–210 μm).
2.2.2. General Protocol for the Anodic Homocoupling of 2-Naphthylamines
ElectraSyn 2.0 and platinum were utilized as the reaction device and electrode, respectively. A suspension of 2-naphthylamines (0.1 mmol) and nBu4NPF6 (0.1 M) in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) (5 mL) was added to an undivided vessel and stirred under a constant current of 4 mA for 2 h. The electrolyte was removed using short silica gel column chromatography (nhexane/ethyl acetate = 1/1). The fraction was dried, and the crude product was purified by silica-gel column chromatography (nhexane/ethyl acetate = 20/1) to afford the pure homocoupling product.
3. Results
3.1. Optimization of the Reaction Conditions
Initially, we screened the electrodes, solvent systems, currents, and electrolytes to determine the optimal conditions (Table 1, also see supporting information). The electrodes were screened by employing 0.1 mmol N-phenyl-2-naphthylamine (1a, oxidative potential 1.05 eV; see Supporting Information) as the model substrate. The platinum electrode exhibited good reactivity, affording homocoupling product 2a in 98% yield (current efficiency, 66%) without the formation of any side product (entry 1). In contrast, the carbon–platinum [32] and fluorine-doped tin oxide(FTO) [33] electrodes gave 2a in 43% and 29% yields, respectively, with low current efficiencies (entries 2 and 3). Other alcoholic reaction solvents, such as methanol, ethanol, and trifluoroethanol, reduced the yield of 2a to 6–12% with current efficiencies of 4–8% (entries 4–6), along with a 5% yield of aza [5] helicene. HFIP, an appropriate solvent for the reaction, serves as an excellent hydrogen bond donor and provides highly persistent radical cations [34,35]. The effect of a constant current was also investigated. As shown in entry 7, when a current of 2 mA was employed for the electrosynthesis process, the current efficiency increased to 80%, but the yield of 2a decreased to 60%. In contrast, employing a current of 6 mA for the electrosynthesis process led to the formation of 2a in 49% yield, with a current efficiency of 22% (entry 8). Suppressing the discharge of the electrolyte or solvent resulted in higher yield and current efficiency. Among the electrolytes we screened, nBu4NPF6 proved to be superior to LiClO4 (2a, 57% yield due to low solubility in HFIP) and nBu4NClO4 (2a, 29% yield) (entries 9 and 10). No reaction occurred in the absence of electricity (entry 11).

Table 1.
Optimization of the conditions for the electrochemical homocoupling of 2-naphthylamines using 1a as the model substrate.
3.2. Scope of Substrates
With the optimized conditions in hand (reaction solvent: HFIP, electrolyte: nBu4NPF6 (0.1 M), electrode: platinum, constant current: 4 mA, and reaction temperature: 25 °C), we investigated the substrate scope of the 2-naphthylamines (Figure 1). The electrochemical homocoupling of 2-naphthylamines 1 proceeded smoothly at moderate current efficiencies (44–66%). N-Phenyl-2-naphthylamine (1a) produced homocoupling product 2a in 98% yield. N-4-Tolyl and N-2-tolyl-2-naphthylamines (1b and 1c), respectively, were also found to be appropriate coupling precursors, giving the corresponding homocoupling products 2b and 2c in 92% and 83% yields, respectively. The reaction of substrates 1d and 1e with electron-donating or electron-withdrawing groups, such as N-2,3-dimethoxyphenyl and N-4-bromophenyl, on the nitrogen atom, showed good functional group tolerance, giving binaphthylamines 2d and 2e in good yields. When N-(1,1′-biphenyl)-4-yl-2-naphthylamine (1f) was used as the substrate, a moderate yield of the coupling product 2f was obtained because of the poor solubility of 1f in HFIP. The reactions of N-alkyl-2-naphthylamines, such as N-methyle-2-naphthylamine (1g), N-ethyl-2-naphthylamine (1h), N-isopropyl-2-naphthylamine (1i), and N-t-butyle-2-naphthylamine (1j) afforded the corresponding homocoupling products 2g, 2h, 2i and 2j in 30%, 87%, 85% and 72% yields, respectively. The dehydrogenative coupling reactions of N-aryl-2-naphthylamines (1k–1n) with various substituents were also conducted. Products 2k and 2l with methoxy groups 2m and 2n with phenyl and cyano groups were readily obtained in good to excellent yields (65–95% yields). N-Naphthyl-2-naphthyl amine (1o) could not be tolerated. Finally, N-benzyl-naphthylamine (1p) was employed in the electrochemical homocoupling reaction. The corresponding product 2p was obtained an 85% yield and could be transformed into BINAM with Lewis acid (See Supporting Information).
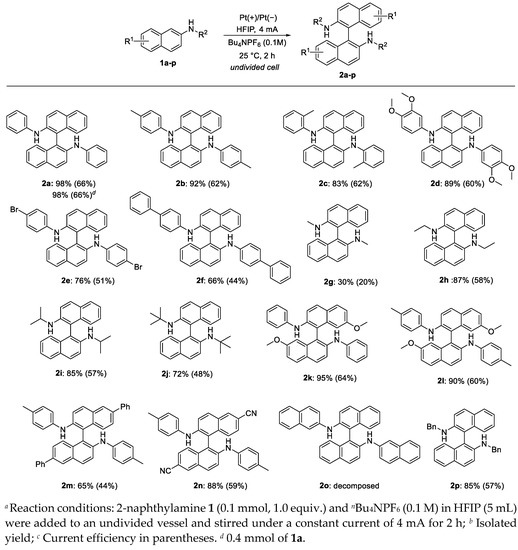
Figure 1.
Substrate scope of N-naphthylamines a–d.
4. Discussion
In our study, the homocoupling reaction of 2-naphthylamines proceeded smoothly with good current efficiency. To further understand the relevant mechanism, we conducted the heterocoupling reactions of 1b with N-4-tolyl (an electron-donating group) and 1e with N-4-bromo-phenyl (an electron-withdrawing group) under the optimal conditions (Scheme 2).
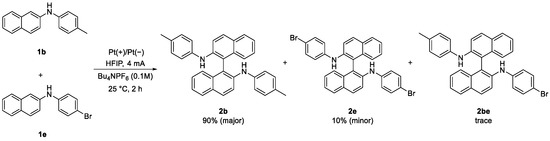
Scheme 2.
Electrochemical heterocoupling of substrate 1b and 1e.
Homocoupling product 2b was obtained in 90% yield as the major product, along with homocoupling product 2e in 10% yield and trace amounts of heterocoupling product 2be. The relative proportions of the products arising from the radical–radical coupling reactions are aligned with their relative reactivities [36,37,38]. In principle, 1e is less oxidizable than 1b, which should result in the formation of homocoupling 2b as the major product. The results support our hypothesis that the present coupling reaction of 1 proceeds through radical–radical coupling, as shown in Scheme 3. Triggering by single-electron transfer (SET) of 1a on the anode made the formation of intermediate I. The generated I species could be in equilibrium into Ia and Ib by electron transfer. Then a radical–radical coupling of Ib proceeded to afford the coupling product 2b through the oxidation of intermediate II. On the cathode, the generated H+ was reduced to give H2 as a sole coproduct.
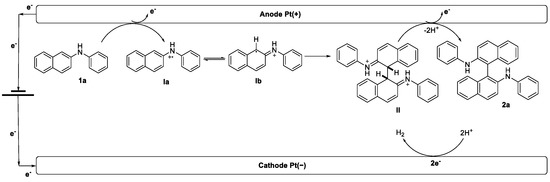
Scheme 3.
Proposed mechanism for the homocoupling of 2-naphthylamines.
5. Conclusions
We developed a facile and sustainable protocol for the homocoupling of various 2-naphthylamines with up to 98% yield and good current efficiency (66%). This new protocol not only saves energy and chemical loading but also significantly improves the product yield, thus representing a significant improvement to the previous electrosynthesis approach. Investigations of the further applications of homocoupling products are ongoing in our laboratory.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/suschem3040034/s1; Figure S1: IKA device ElectraSyn 2.0 standard setup; Table S1. Screening solvent of constant current for optimizing reaction conditions; Figure S2: CV experiments (MeCN) as a solvent with Bu4NPF6 (0.1 M) as an electrolyte.
Author Contributions
S.T. and H.S.: conceptualization, resources, writing—reviewing, and editing. D.F.: investigation, resources, visualization, validation, writing—original draft. M.I.K., and G.T.K.: investigation, resources, and validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Numbers 22K06502 in Grant-in-Aid for Scientific Research (C), Transformative Research Areas (A) 21A204 Digitalization-driven Transformative Organic Synthesis (DigiTOS), 22KK0073 in Fund for the Promotion of Joint International Research (Fostering Joint International Research (B)) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), and the Japan Society for the Promotion of Science (JSPS), JST CREST (No. JPMJCR20R1), and Hoansha Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Acknowledgments
We acknowledge the technical staff of the Comprehensive Analysis Center of SANKEN, Osaka University (Japan).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Telfer, S.; Kuroda, R. 1,1′-Binaphthyl-2,2′-diol and 2,2′-diamino-1,1′-binaphthyl: Versatile frameworks for chiral ligands in coordination and metallosupramolecular chemistry. Coord. Chem. Rev. 2003, 242, 33–46. [Google Scholar] [CrossRef]
- Hatano, M.; Ikeno, T.; Matsumura, T.; Torii, S.; Ishihara, K. Chiral lithium salts of phosphoric acids as Lewis acid-base conjugate catalysts for the enantioselective cyanosilylation of ketones. Adv. Synth. Catal. 2008, 350, 1776–1780. [Google Scholar] [CrossRef]
- Prasad, D.J.C.; Naidu, A.B.; Sekar, G. An efficient intermolecular C(aryl)–S bond forming reaction catalyzed by BINAM–copper(II) complex. Tetrahedron Lett. 2009, 50, 1411–1415. [Google Scholar] [CrossRef]
- Hannedouche, J.; Collin, J.; Trifonov, A.; Schulz, E. Intramolecular enantioselective hydroamination catalyzed by rare earth binaphthylamides. J. Organomet. Chem. 2011, 696, 255–262. [Google Scholar] [CrossRef]
- Averin, A.D.; Grigorova, O.K.; Malysheva, A.S.; Shaferov, A.V.; Beletskaya, I.P. Pd(0)-Catalyzed amination in the synthesis of chiral derivatives of BINAM and their evaluation as fluorescent enantioselective detectors. Pure Appl. Chem. 2020, 92, 1367–1386. [Google Scholar] [CrossRef]
- Yan, Z.-P.; Liu, T.-T.; Wu, R.; Liang, X.; Li, Z.-Q.; Zhou, L.; Zheng, Y.-X.; Zuo, J.-L. Chiral thermally activated delayed fluorescence materials based on R/S-N2,N2′-diphenyl-[1,1′-binaphthalene]-2,2′-diamine donor with narrow emission spectra for highly efficient circularly polarized electroluminescence. Adv. Funct. Mater. 2021, 31, 2103875. [Google Scholar] [CrossRef]
- Meng, Q.; Feng, Q.; Cui, L.; Li, F.; Cheng, Y.; Li, Y.; Wang, Y. Chiral binaphthylamine based emitters with donor-acceptor structures: Facile synthesis and circularly polarized luminescence. Dye. Pigment. 2022, 199, 110085. [Google Scholar] [CrossRef]
- Smrčina, M.; Vyskočil, S.; Máca, B.; Polášek, M.; Claxton, T.A.; Abbott, A.P.; Kočovský, P. Selective cross-coupling of 2-naphthol and 2-naphthylamine derivatives. A facile synthesis of 2,2′,3-trisubstituted and 2,2′,3,3′-tetrasubstituted 1,1′-binaphthyls. J. Org. Chem. 1994, 59, 2156–2163. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, J.; Gao, M.; Tang, S.; Li, W.; Shi, R.; Lei, A. Oxidative coupling between two hydrocarbons: An update of recent C–H functionalizations. Chem. Rev. 2015, 115, 12138–12204. [Google Scholar] [CrossRef]
- Forkosh, H.; Vershinin, V.; Reiss, H.; Pappo, D. Stereoselective synthesis of optically pure 2-amino-2′-hydroxy-1,1′-binaphthyls. Org. Lett. 2018, 20, 2459–2463. [Google Scholar] [CrossRef]
- Hayashi, H.; Ueno, T.; Kim, C.; Uchida, T. Ruthenium-catalyzed cross-selective asymmetric oxidative coupling of arenols. Org. Lett. 2020, 22, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Shabade, A.B.; Punji, B. Advances in C(sp2)–H/C(sp2)–H oxidative coupling of (hetero)arenes using 3d transition metal catalysts. Adv. Synth. Catal. 2021, 363, 1998–2022. [Google Scholar] [CrossRef]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl–aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1469. [Google Scholar] [CrossRef] [PubMed]
- Smrčina, M.; Lorenc, M.; Hanuš, V.; Sedmera, P.; Kočovský, P. Synthesis of enantiomerically pure 2,2′-dihydroxy-1,1′-binaphthyl, 2,2′-diamino-1,1′-binaphthyl, and 2-amino-2′-hydroxy-1,1′-binaphthyl. Comparison of processes operating as diastereoselective crystallization and as second order asymmetric transformation. J. Org. Chem. 1992, 57, 1917–1920. [Google Scholar] [CrossRef]
- Vyskočil, Š.; Smrčina, M.; Lorenc, M.; Tišlerová, I.; Brooks, R.D.; Kulagowski, J.J.; Langer, V.; Farrugia, L.J.; Kočovský, P. Copper(II)-mediated oxidative coupling of 2-aminonaphthalene homologues. Competition between the straight dimerization and the formation of carbazoles. J. Org. Chem. 2001, 66, 1359–1365. [Google Scholar] [CrossRef]
- Zi, G.; Xiang, L.; Zhang, Y.; Wang, Q.; Zhang, Z. Synthesis, structure, and activity of (PhCH2NH2)2CuCl2 for oxidative coupling of 2-naphthylamine. Appl. Organomet. Chem. 2007, 21, 177–182. [Google Scholar] [CrossRef]
- Yusa, Y.; Kaito, I.; Akiyama, K.; Mikami, K. Asymmetric catalysis of homo-coupling of 3-substituted naphthylamine and hetero-coupling with 3-substituted naphthol leading to 3,3′-dimethyl-2,2′-diaminobinaphthyl and -2-amino-2′-hydroxybinaphthyl. Chirality 2010, 22, 224–228. [Google Scholar] [CrossRef]
- Li, X.-L.; Huang, J.-H.; Yang, L.-M. Iron(III)-promoted oxidative coupling of naphthylamines: Synthetic and mechanistic investigations. Org. Lett. 2011, 13, 4950–4953. [Google Scholar] [CrossRef]
- Matsumoto, K.; Dougomori, K.; Tachikawa, S.; Ishii, T.; Shindo, M. Aerobic oxidative homocoupling of aryl amines using heterogeneous rhodium catalysts. Org. Lett. 2014, 16, 4754–4757. [Google Scholar] [CrossRef]
- Fujimoto, S.; Matsumoto, K.; Iwata, T.; Shindo, M. Aerobic oxidative homocoupling of anilides using heterogeneous metal catalysts. Tetrahedron Lett. 2017, 58, 973–976. [Google Scholar] [CrossRef]
- Lim, B.; Choi, M.; Cho, C. Acid-catalyzed condensation of 2,2′-diamino-1,1′-biaryls for the synthesis of benzo[c]carbazoles. Tetrahedron Lett. 2011, 52, 6015–6017. [Google Scholar] [CrossRef]
- Li, G.-Q.; Gao, H.; Keene, C.; Devonas, M.; Ess, D.H.; Kürti, L. Organocatalytic aryl-aryl bond formation: An atroposelective [3,3]-rearrangement approach to BINAM derivatives. J. Am. Chem. Soc. 2013, 135, 7414–7417. [Google Scholar] [CrossRef] [PubMed]
- De, C.K.; Pesciaioli, F.; List, B. Catalytic asymmetric benzidine rearrangement. Angew. Chem. Int. Ed. 2013, 52, 9293–9295. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, Q.; Guo, C. Switchable Smiles rearrangement for enantioselective O-aryl amination. Org. Lett. 2019, 21, 4915–4918. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, H.J. Anodic and cathodic CC-bond formation. Angew. Chem. Int. Ed. Engl. 1981, 20, 911–934. [Google Scholar] [CrossRef]
- Kirste, A.; Elsler, B.; Schnakenburg, G.; Waldvogel, S.R. Efficient anodic and direct phenol-arene C,C cross-coupling: The benign role of water or methanol. J. Am. Chem. Soc. 2012, 134, 3571–3576. [Google Scholar] [CrossRef]
- Schulz, L.; Enders, M.; Elser, B.; Schollmeyer, D.; Dyballa, K.M.; Franke, R.; Waldvogel, S.R. Reagent- and metal-free anodic C-C cross-coupling of aniline derivatives. Angew. Chem. Int. Ed. 2017, 56, 4877–4881. [Google Scholar] [CrossRef]
- Schulz, L.; Franke, R.; Waldvogel, S.R. Direct anodic dehydrogenative cross- and homo-coupling of formanilides. ChemElectroChem 2018, 5, 2069–2072. [Google Scholar] [CrossRef]
- Schulz, L.; Husmann, J.-Å.; Waldvogel, S.R. Outstandingly robust anodic dehydrogenative aniline coupling reaction. Electrochim. Acta 2020, 337, 135786. [Google Scholar] [CrossRef]
- Hornback, J.M.; Gossage, H.E. Electrochemical oxidative dehydrodimerization of naphthylamines. An efficient synthesis of 8,8-dianilino-5,5′-binaphthalene-l,l′-disulfonate. J. Org. Chem. 1985, 50, 541–543. [Google Scholar] [CrossRef]
- Vettorazzi, N.; Silber, J.J.; Sereno, L. Anodic oxidation of 1-naphthylamine in acetonitrile. J. Electroanal. Chem. Interfacial Electrochem. 1981, 125, 459–475. [Google Scholar] [CrossRef]
- Luo, M.-J.; Li, Y.; Ouyang, X.-H.; Li, J.-H.; He, D.-L. Electrochemical dehydrogenative cross-coupling of two anilines: Facile synthesis of unsymmetrical biaryls. Chem. Commun. 2020, 56, 2707–2710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, X. Nickel catalysis enables convergent paired electrolysis for direct arylation of benzylic C–H bonds. Chem. Sci. 2020, 11, 10786–10791. [Google Scholar] [CrossRef] [PubMed]
- Eberson, L.; Hartshorn, M.P.; Persson, O. 1,1,1,3,3,3-Hexafluoropropan-2-ol as a solvent for the generation of highly persistent radical cations. J. Chem. Soc. Perkin Trans. 2 1995, 18, 1735–1744. [Google Scholar] [CrossRef]
- Eberson, L.; Persson, O.; Hartshorn, M.P. Detection and reactions of radical cations generated by photolysis of aromatic compounds with tetranitromethane in 1,1,1,3,3,3-hexafluoro-2-propanol at room temperature. Angew. Chem. Int. Ed. Engl. 1995, 34, 2268–2269. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Kozlowski, M.C. Enantioselective oxidative biaryl coupling reactions catalyzed by 1,5-diazadecalin metal complexes. Org. Lett. 2001, 3, 1137–1140. [Google Scholar] [CrossRef]
- Takizawa, S.; Katayama, T.; Somei, H.; Asano, Y.; Yoshida, T.; Kameyama, C.; Rajesh, D.; Onitsuka, K.; Suzuki, T.; Mikami, M.; et al. Dual activation in oxidative coupling of 2-naphthols catalyzed by chiral dinuclear vanadium complexes. Tetrahedron 2008, 64, 3361–3371. [Google Scholar] [CrossRef]
- Guo, Q.-X.; Wu, Z.-J.; Luo, Z.-B.; Liu, Q.-Z.; Ye, J.-L.; Luo, S.-W.; Cun, L.-F.; Gong, L.-Z. Highly enantioselective oxidative couplings of 2-naphthols catalyzed by chiral bimetallic oxovanadium complexes with either oxygen or air as oxidant. J. Am. Chem. Soc. 2007, 129, 13927–13938. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).