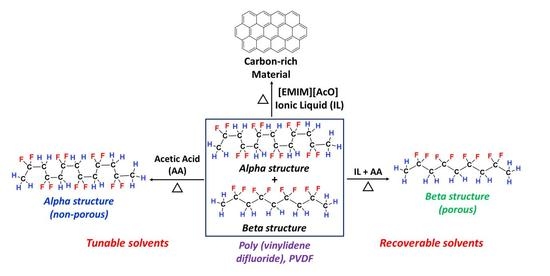
Poly (Vinylidene Difluoride) Polymer in 1-Ethyl-3-methylimidazolium Acetate and Acetic Acid Containing Solvents: Tunable and Recoverable Solvent Media to Induce Crystalline Phase Transition and Porosity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of [EMIM][AcO] IL and Acetic Acid Containing Mixtures
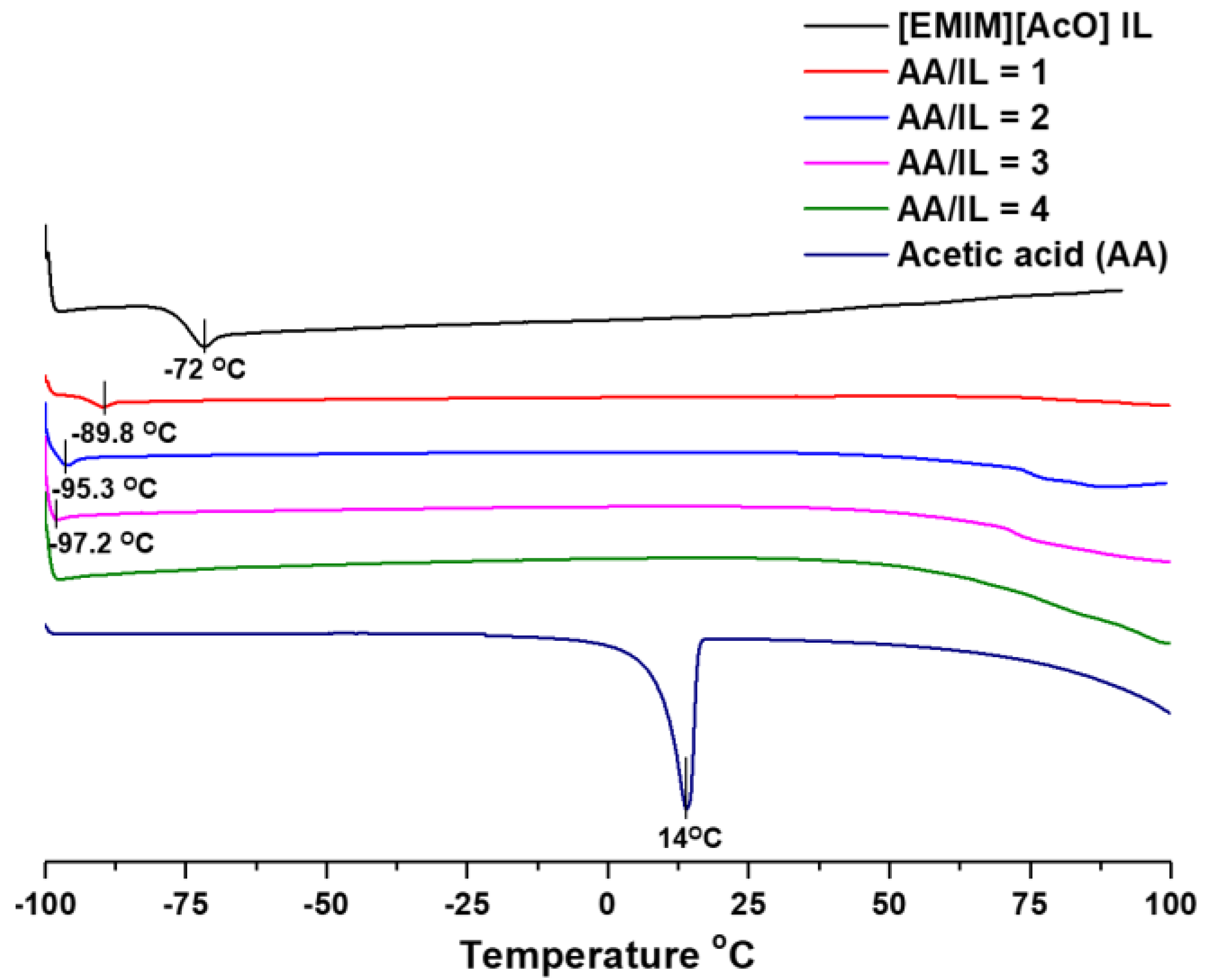
2.2.2. Differential Scanning Calorimetry (DSC) of [EMIM][AcO] IL and Acetic Acid Containing Mixtures
2.2.3. NMR Analysis (Liquid State) of [EMIM][AcO] IL and Acetic Acid Containing Mixtures
2.2.4. Kamlet-Taft Parameters Measurement of [EMIM][AcO] IL and Acetic Acid Containing Mixtures
2.2.5. Thermal Treatment of PVDF in [EMIM][AcO] IL and Acetic Acid Containing Mixtures
2.2.6. Analysis of PVDF after Treatment with Various Solvents
2.2.7. Recovery of [EMIM][AcO] IL and Acetic Acid Mixture (AA:IL Mole Ratio of 3)
3. Results
3.1. Differential Scanning Calorimetry (DSC) of [EMIM][AcO] IL and Acetic Acid Mixtures
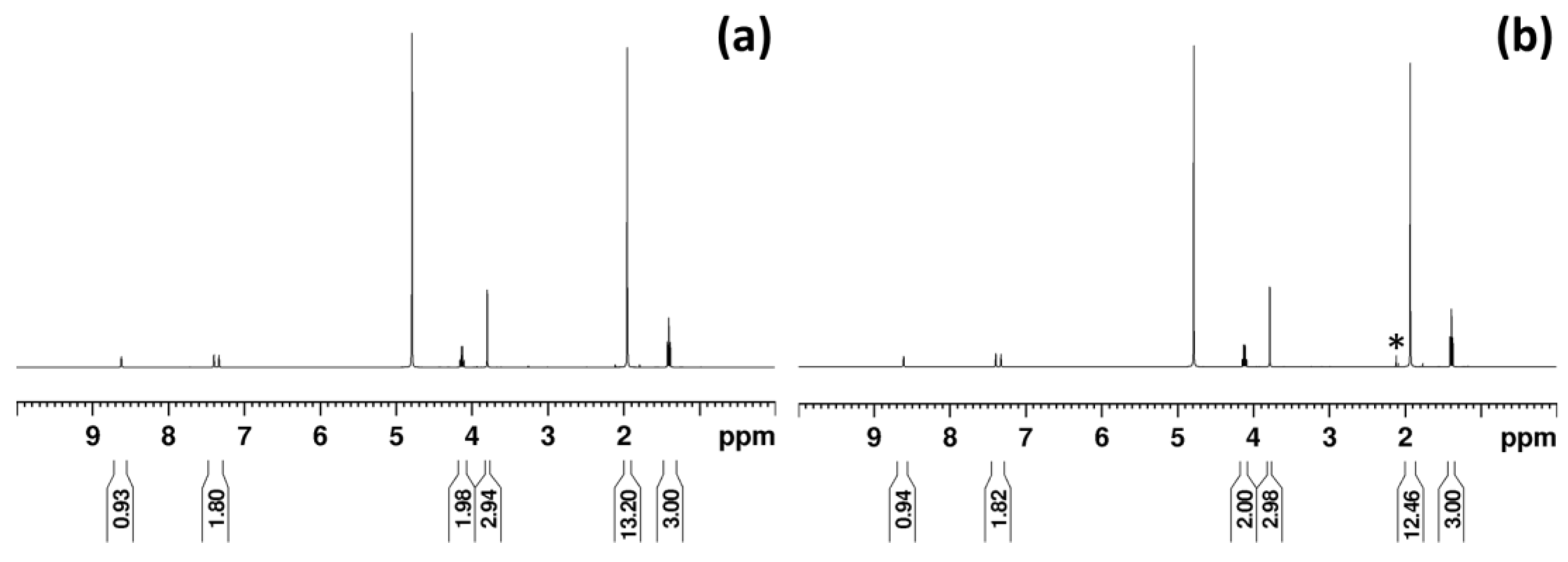
3.2. 1H NMR Analysis of [EMIM][AcO] IL and Acetic Acid Mixtures
3.3. Kamlet-Taft Measurements of [EMIM][AcO] IL and Acetic Acid Mixtures
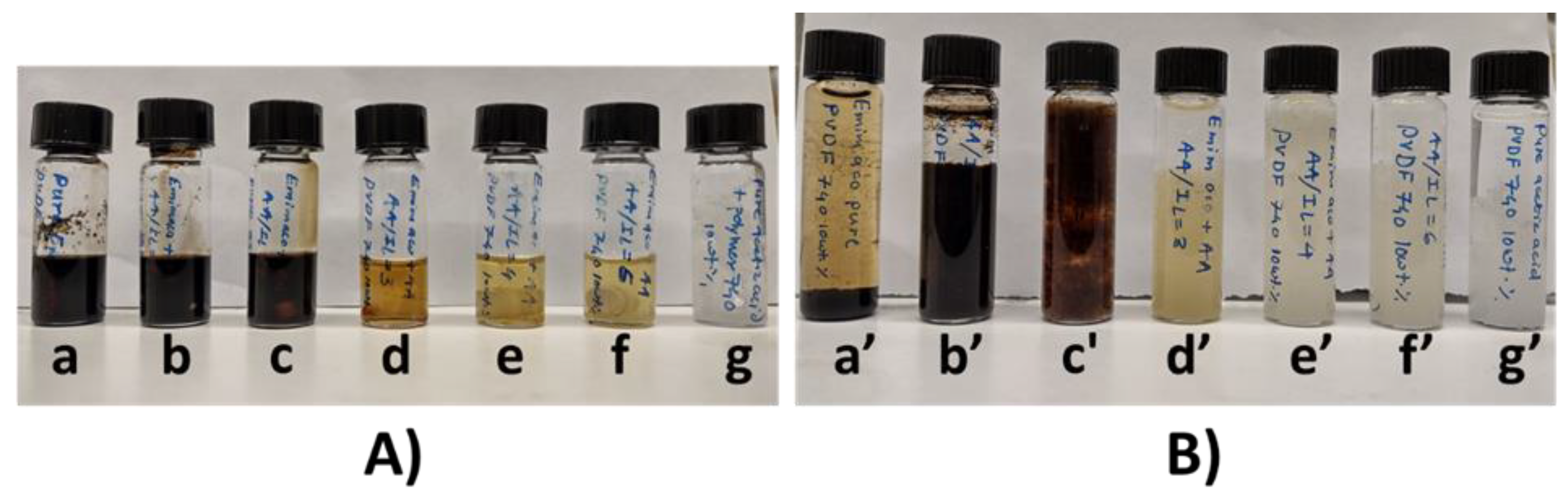
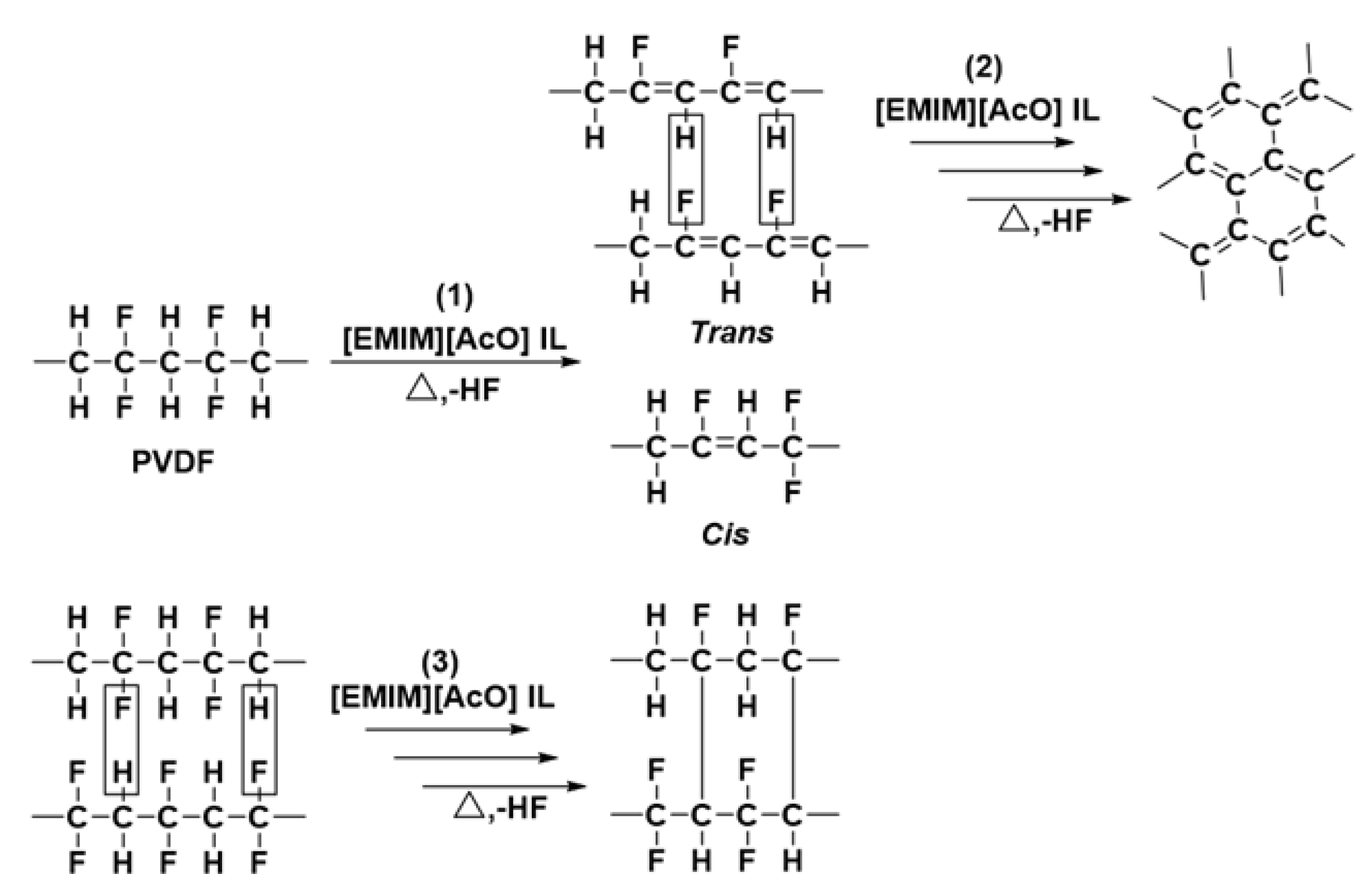
3.4. Thermal Processing and Recovery of PVDF in [EMIM][AcO] IL and Acetic acid Containing Mixtures
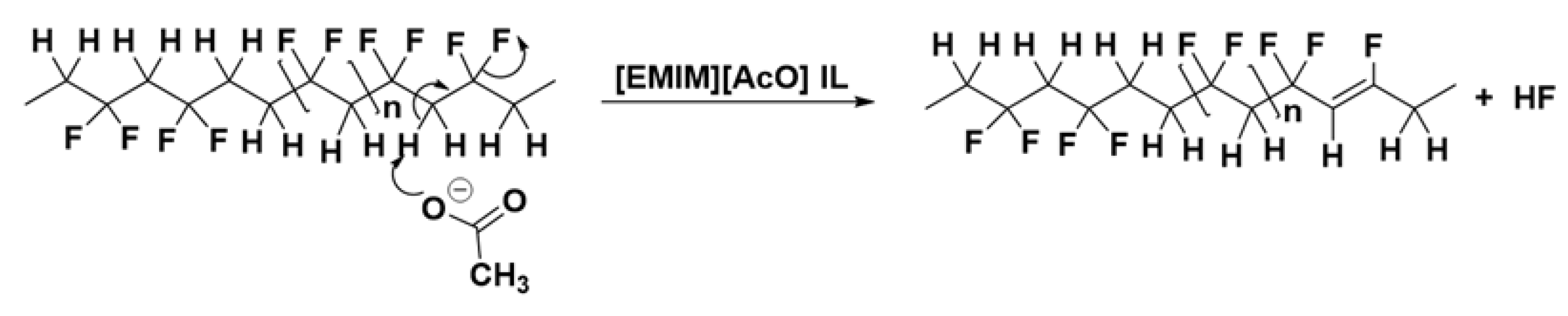
3.5. 19F NMR Analysis of Reaction Mixtures Obtained after Thermal Treatment of PVDF in Various Solvents
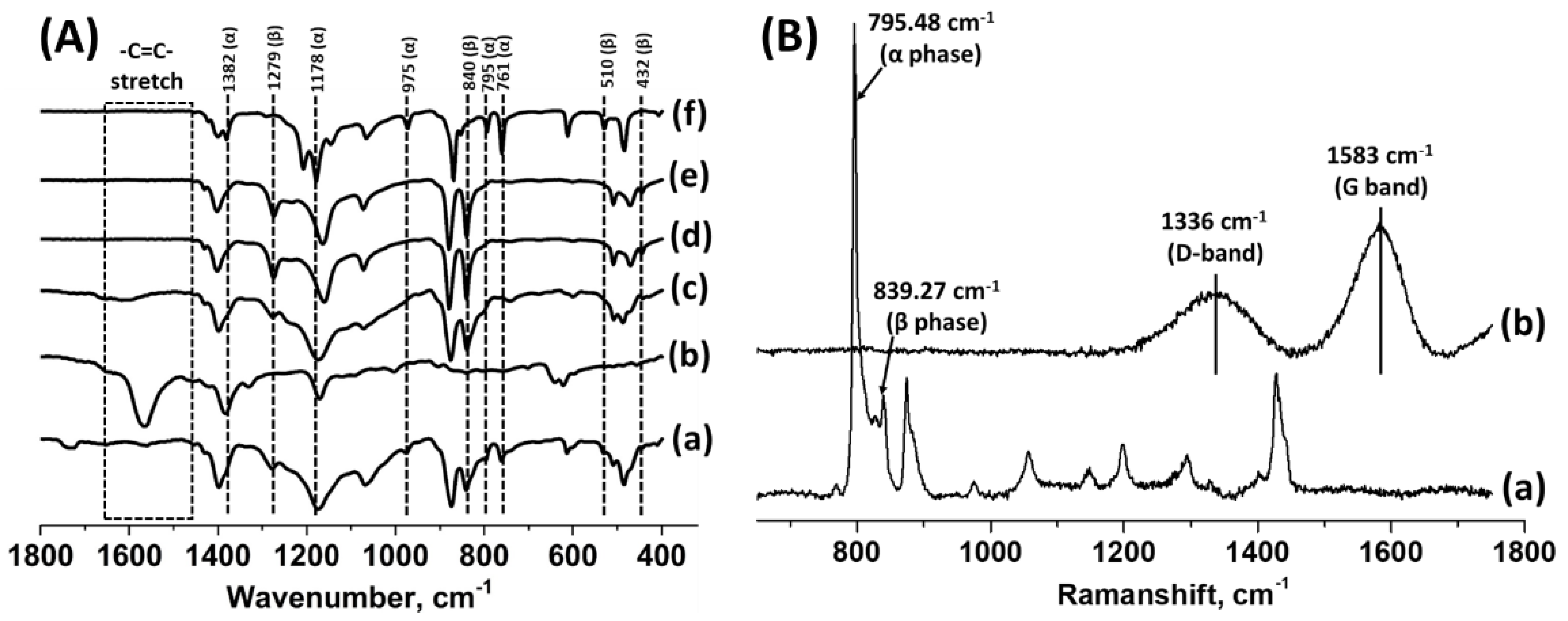
3.6. ATR-FTIR Analysis of Treated PVDF
3.7. Raman Analysis of Treated PVDF
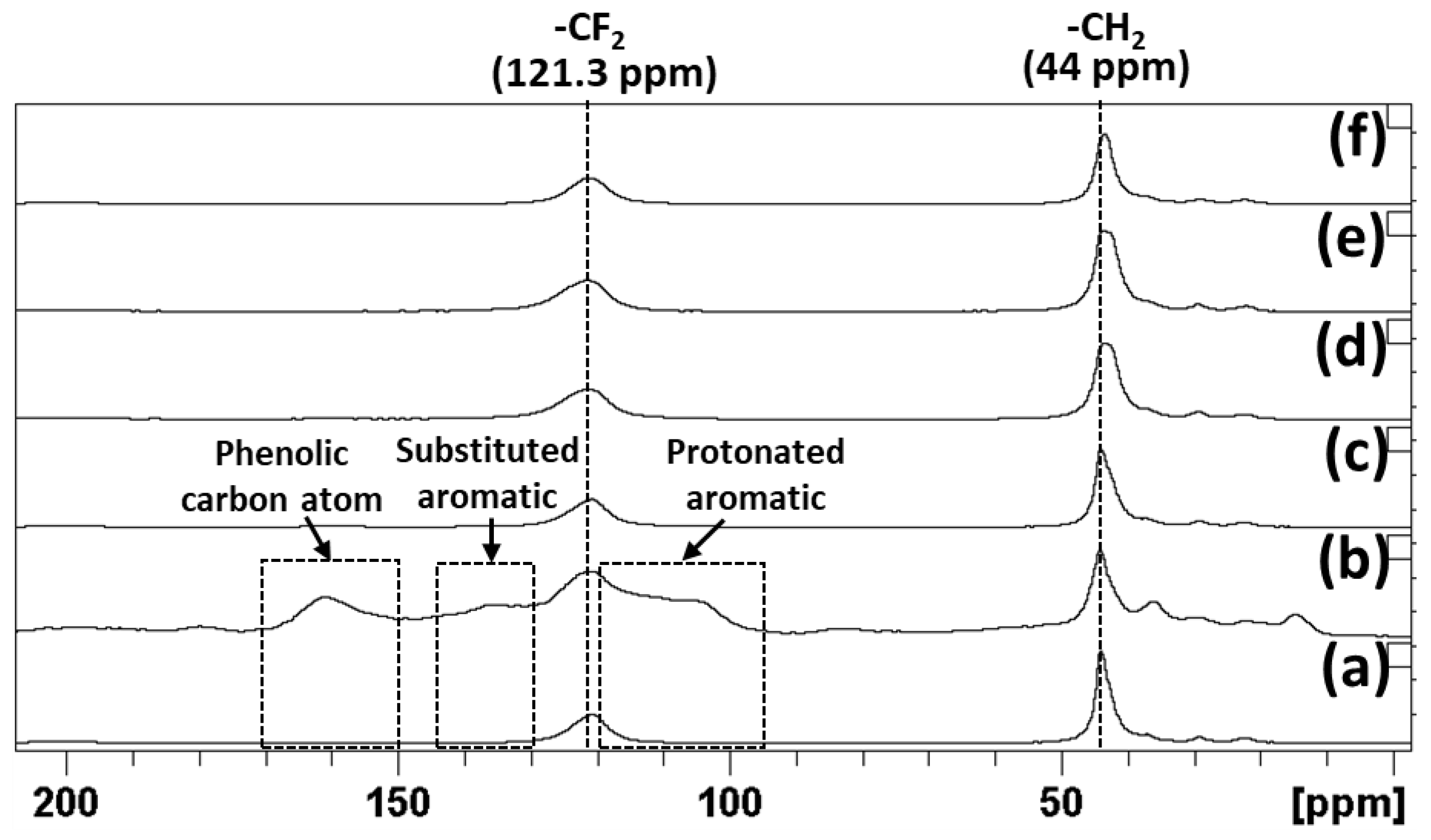
3.8. 13C MAS NMR Analysis of Treated PVDF
3.9. XRD Analysis of Treated PVDF
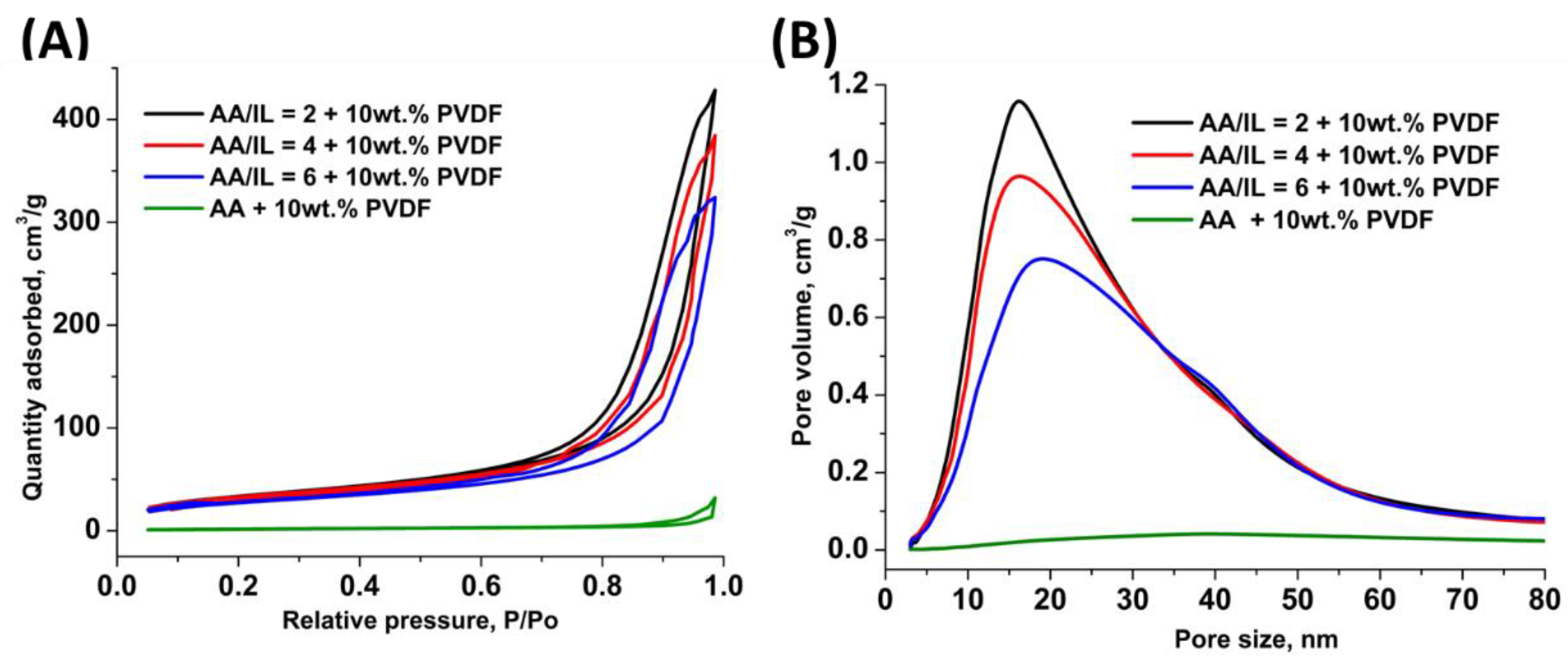
3.10. N2 Physisorption Analysis of Treated PVDF
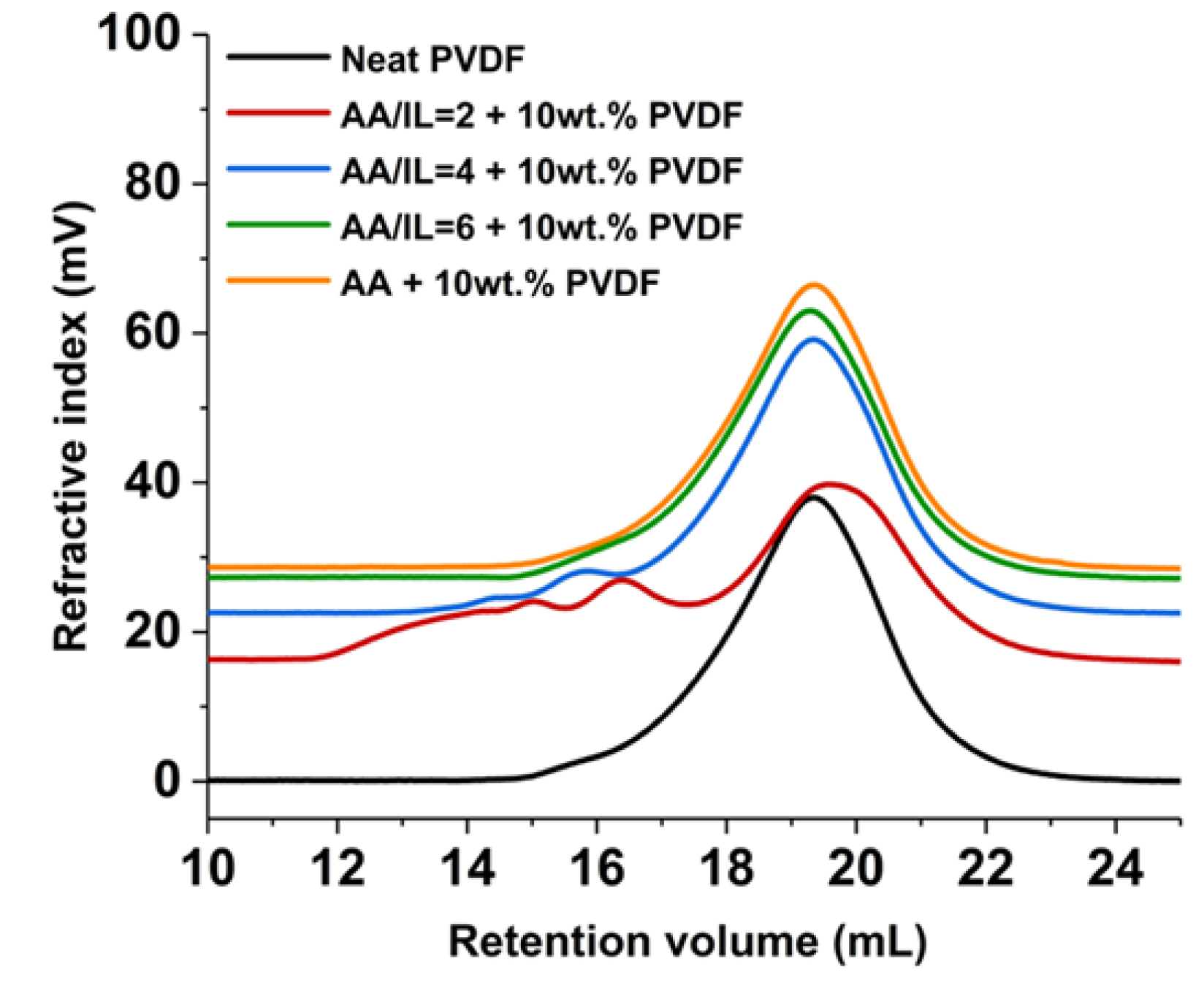
3.11. Size Exclusion Chromatography (SEC) Analysis of Treated PVDF
3.12. Recovery of [EMIM][AcO] IL and AA Containing Mixture (AA:IL Mole Ratio of 3:1)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and applications of the β phase poly (vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. A comprehensive review on fundamental properties and applications of poly(vinylidene fuoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. Chem. Eng. J. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Jain, A.; Prashanth, K.J.; Sharma, A.K.; Jain, A. Dielectric and piezoelectric properties of PVDF/PZT composites: A review. Polym. Eng. Sci. 2015, 55, 1589–1616. [Google Scholar] [CrossRef]
- Sukumaran, S.; Chatbouri, S.; Rouxel, D.; Tisserand, E.; Zineb, T.B. Recent advances in flexible PVDF based piezoelectric polymer devices for energy harvesting applications. J. Intell. Mater. Syst. Struct. 2021, 32, 746–780. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Application and modification of poly (vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Grasso, G.; Galiano, F.; Yoo, M.J.; Mancuso, R.; Park, H.B.; Gabriele, B.; Figoli, A.; Driol, E. Development of graphene-PVDF composite membranes for membrane distillation. J. Membr. Sci. 2020, 604, 118017–118028. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, J.; Xu, M.; Dou, H.; Zhang, H.; Yang, N.; Zhang, L. Multifunctional ternary deep eutectic solvent-based membranes for the cost-effective ethylene/ethane separation. J. Membr. Sci. 2020, 610, 118243–118252. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; He, T.; Hu, Q.; Yang, Y. Preparation of PVDF flexible piezoelectric film with high β-phase content by matching solvent dipole moment and crystallization temperature. J. Mater. Sci. Mater. Electron. 2019, 30, 20174–20180. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendeza, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Singh, N.; Madhav, H.; Yadav, S.; Jaiswar, G. Impact of vanadium-, sulfur-, and dysprosium-doped zinc oxide nanoparticles on various properties of PVDF/functionalized-PMMA blend nanocomposites: Structural, optical, and morphological studies. J. Appl. Polym. Sci. 2019, 136, 47116–47128. [Google Scholar] [CrossRef]
- Sultana, A.; Sadhukhan, P.; Alam, M.M.; Das, S.; Middya, T.R.; Mandal, D. Organo-Lead Halide Perovskite Induced Electroactive β-Phase in Porous PVDF films: An Excellent Material for Photoactive Piezoelectric energy Harvester and Photodetector. ACS Appl. Mater. Interfaces 2018, 10, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wang, H.; Li, H.; Yuan, C.; Wang, T.; Yang, H. Deep Eutectic Solvent—A Novel Additive to Induce Gamma Crystallization and Alpha-to-Gamma Phase Transition of PVDF. Macromol. Chem. Phys. 2022, 223, 2100416–2100425. [Google Scholar] [CrossRef]
- Xing, C.; Guan, J.; Li, Y.; Li, J. Effect of a room-temperature ionic liquid on the structure and properties of electrospun poly(vinylidene fluoride) nanofibers. ACS Appl. Mater. Interfaces 2014, 6, 4447–4457. [Google Scholar] [CrossRef]
- Vazquez, F.I.; Raghibi, M.; Bouzina, A.; Timperman, L.; Bigarréb, J.; Anouti, M. Protic ionic liquids/poly(vinylidene fluoride) composite membranes for fuel cell application. J. Energy Chem. 2021, 53, 197–207. [Google Scholar] [CrossRef]
- Gebrekrstos, A.; Muzata, T.S.; Ray, S.S. Nanoparticle-Enhanced β-Phase Formation in Electroactive PVDF Composites: A Review of Systems for Applications in Energy Harvesting, EMI Shielding, and Membrane Technology. ACS Appl. Nano Mater. 2022, 5, 7632–7651. [Google Scholar] [CrossRef]
- Marshall, J.E.; Zhenova, A.; Roberts, S.; Petchey, T.; Zhu, P.; Dancer, C.E.J.; McElroy, C.R.; Kendrick, E.; Goodship, V. On the solubility and stability of polyvinylidene fluoride. Polymers 2021, 13, 1354. [Google Scholar] [CrossRef] [PubMed]
- Diorazio, L.J.; Hose, D.R.J.; Adlington, N.K. Toward a more holistic framework for solvent selection. Org. Process Res. Dev. 2016, 20, 760–773. [Google Scholar] [CrossRef]
- Low, Y.K.A.; Tan, L.Y.; Tan, L.P.; Boey, F.Y.C.; Ng, K.W. Increasing Solvent Polarity and Addition of Salts Promote β-Phase Poly(vinylidene fluoride) Formation. J. Appl. Polym. Sci. 2013, 128, 2902–2910. [Google Scholar] [CrossRef]
- Marino, T.; Russo, F.; Figoli, A. The Formation of Polyvinylidene Fluoride Membranes with Tailored Properties via Vapour/Non-Solvent Induced Phase Separation. Membranes 2018, 8, 71. [Google Scholar] [CrossRef]
- Nishiyama, T.; Sumihara, T.; Sato, E.; Horibe, H. Effect of solvents on the crystal formation of poly (vinylidene fluoride) film prepared by a spin-coating process. Polym. J. 2017, 49, 319–325. [Google Scholar] [CrossRef]
- Okabe, M.; Wada, R.; Tazaki, M.; Homma, T. The flory−huggins interaction parameter and thermoreversible gelation of poly(vinylidene fluoride) in organic solvents. Polym. J. 2003, 35, 798–803. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S. Green and sustainable membrane fabrication development. Sustain. Technol. Green Econ. 2021, 1, 14–23. [Google Scholar] [CrossRef]
- Milescu, R.A. Applications of the novel bioderived solvent Cyrene™ in polymer chemistry. Ph.D. Thesis, University of York, Heslington, UK, 2021. Available online: https://etheses.whiterose.ac.uk/29846/1/Milescu_201057084.pdf (accessed on 16 December 2021).
- Ravikumar, V.R.; Schröder, A.; Köhler, S.; Çetinel, F.A.; Schmitt, M.; Kondrakov, A.; Eberle, F.; Eichler-Haeske, J.O.; Klein, D.; Schmidt, H.B. γ-Valerolactone: An Alternative Solvent for Manufacturing of Lithium-Ion Battery Electrodes. ACS Appl. Energy Mater. 2021, 4, 696–703. [Google Scholar] [CrossRef]
- Zhenova, A.S. Green Solvents for Polymer Applications. Ph.D. Thesis, University of York, Heslington, UK, 2019. Available online: https://etheses.whiterose.ac.uk/25264/1/Anna_Zhenova_Thesis_2019.pdf (accessed on 28 January 2020).
- Lora, M.; Lim, J.S.; McHugh, M.A. Comparison of the Solubility of PVF and PVDF in Supercritical CH2F2 and CO2 and in CO2 with Acetone, Dimethyl Ether, and Ethanol. J. Phys. Chem. B 1999, 103, 2818–2822. [Google Scholar] [CrossRef]
- Dinoia, T.P.; Conway, S.E.; Lim, J.S.; McHugh, M.A. Solubility of Vinylidene Fluoride Polymers in Supercritical CO2 and Halogenated Solvents. J. Polym. Sci. B Polym. Phys. 2000, 38, 2832–2840. [Google Scholar] [CrossRef]
- Ueki, T.; Watanabe, M. Polymers in ionic liquids: Dawn of neoteric solvents and innovative materials. Bull. Chem. Soc. Jpn. 2012, 85, 33–50. [Google Scholar] [CrossRef]
- Ptak, S.; Zarski, A.; Kapusniak, J. The importance of ionic liquids in the modification of starch and processing of starch-based materials. Materials 2020, 13, 4479. [Google Scholar] [CrossRef]
- Brandt, A.; Grasvik, J.; Hallett, J.P.; Welton, T. D deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Guyomard, L.A.; Buchtova, N.; Humbert, B.; Bideau, J.L. Ion segregation in an ionic liquid confined within chitosan based chemical ionogels. Phys. Chem. Chem. Phys. 2015, 17, 23947–23951. [Google Scholar] [CrossRef]
- Jablonski, P.; Nikjoo, D.; Warna, J.; Irgum, K.; Mikkola, J.P.; Khokarale, S.G. Sustainable, highly selective, and metal-free thermal depolymerization of poly-(3- hydroxybutyrate) to crotonic acid in recoverable ionic liquids. Green Chem. 2022, 24, 4130–4139. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; Bruinhorst, A.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. 2. The α-scale of solvent hydrogen-bond donor (HBD) acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. The solvatochromic comparison method. 6. The π* scale of solvent polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, X.; Ma, X.; Lu, L.; Deng, Y. Hydroxyl Ionic Liquids: The Differentiating Effect of Hydroxyl on Polarity due to Ionic Hydrogen Bonds between Hydroxyl and Anions. J. Phys. Chem. B 2010, 114, 3912–3920. [Google Scholar] [CrossRef]
- Lin, J.; Malakooti, M.H.; Sodano, H.A. Thermally Stable Poly(vinylidene fluoride) for High-Performance Printable Piezoelectric Devices. ACS Appl. Mater. Interfaces 2020, 12, 21871–21882. [Google Scholar] [CrossRef]
- Sierke, J.; Ellis, A.V. Cross-linking of dehydrofluorinated PVDF membranes with thiol modified polyhedral oligomeric silsesquioxane (POSS) and pure water flux analysis. J. Membr. Sci. 2019, 581, 362–372. [Google Scholar] [CrossRef]
- Chipara, D.; Kuncser, V.; Lozano, K.; Alcoutlabi, M.; Ibrahim, E.; Chipara, M. Spectroscopic investigations on PVDF-Fe2O3 nanocomposites. J. Appl. Polym. Sci. 2020, 137, 48907–48920. [Google Scholar] [CrossRef]
- Wei, Z.; Pan, R.; Hou, Y.; Yang, Y.; Liu, Y. Graphene-supported Pd catalyst for highly selective hydrogenation of resorcinol to 1, 3-cyclohexanedione through giant π-conjugate interactions. Sci. Rep. 2015, 5, 15664–15672. [Google Scholar] [CrossRef]
- Montina, T.; Wormald, P.; Hazendonk, P. 13C Solid-State NMR of the Mobile Phase of Poly(vinylidene fluoride). Macromolecules 2012, 45, 6002–6007. [Google Scholar] [CrossRef]
- Cheng, H.N.; Wartelle, L.H.; Klasson, K.T.; Edwards, J.C. Solid-state NMR and ESR studies of activated carbons produced from pecan shells. Carbon 2010, 48, 2455–2469. [Google Scholar] [CrossRef]
- Zhu, W.; Okada, K.; Li, Z.; Zhu, J.; Marina, E.; Pezzotti, G. Effect of component content variation on composition and structure of activated carbon in PVDF:K2CO3. Phys. Chem. Chem. Phys. 2019, 21, 2382–2388. [Google Scholar] [CrossRef] [PubMed]




| Solvent | α | β | π* | β-α |
|---|---|---|---|---|
| [EMIM][AcO] IL | 0.41 | 1.03 | 1.08 | 0.62 |
| AA/IL = 1 | 0.45 | 0.85 | 1.01 | 0.40 |
| AA/IL = 2 | 0.47 | 0.65 | 0.98 | 0.17 |
| AA/IL = 3 | 0.57 | 0.56 | 0.97 | −0.01 |
| AA/IL = 4 | 0.64 | 0.49 | 0.94 | −0.15 |
| AA/IL = 6 | 0.84 | 0.47 | 0.91 | −0.37 |
| Pure AA | 0.91 | 0.41 | 0.65 | −0.50 |
| Solvent | BET Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| AA/[EMIM][AcO] = 2:1 | 123 | 16.01 | 0.66 |
| AA/[EMIM][AcO] = 4:1 | 111 | 18.17 | 0.64 |
| AA/[EMIM][AcO] = 6:1 | 100 | 21.52 | 0.61 |
| Acetic acid (AA) | 6.68 | 27.40 | 0.05 |
| Solvent | Mn | Mw | Mw:Mn |
|---|---|---|---|
| Neat PVDF | 45,983 | 209,123 | 4.55 |
| AA/IL= 2:1 + PVDF | 327,821 | 856,936 | 2.61 |
| AA/IL= 4:1 + PVDF | 62,931 | 220,316 | 3.51 |
| AA/IL= 6:1 + PVDF | 59,277 | 224,365 | 3.78 |
| Acetic acid + PVDF | 44,247 | 205,918 | 4.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khokarale, S.G.; Jablonski, P.; Nikjoo, D.; Dinh, V.M.; Sundman, O.; Irgum, K.; Mikkola, J.-P. Poly (Vinylidene Difluoride) Polymer in 1-Ethyl-3-methylimidazolium Acetate and Acetic Acid Containing Solvents: Tunable and Recoverable Solvent Media to Induce Crystalline Phase Transition and Porosity. Sustain. Chem. 2022, 3, 455-474. https://doi.org/10.3390/suschem3040028
Khokarale SG, Jablonski P, Nikjoo D, Dinh VM, Sundman O, Irgum K, Mikkola J-P. Poly (Vinylidene Difluoride) Polymer in 1-Ethyl-3-methylimidazolium Acetate and Acetic Acid Containing Solvents: Tunable and Recoverable Solvent Media to Induce Crystalline Phase Transition and Porosity. Sustainable Chemistry. 2022; 3(4):455-474. https://doi.org/10.3390/suschem3040028
Chicago/Turabian StyleKhokarale, Santosh Govind, Piotr Jablonski, Dariush Nikjoo, Van Minh Dinh, Ola Sundman, Knut Irgum, and Jyri-Pekka Mikkola. 2022. "Poly (Vinylidene Difluoride) Polymer in 1-Ethyl-3-methylimidazolium Acetate and Acetic Acid Containing Solvents: Tunable and Recoverable Solvent Media to Induce Crystalline Phase Transition and Porosity" Sustainable Chemistry 3, no. 4: 455-474. https://doi.org/10.3390/suschem3040028
APA StyleKhokarale, S. G., Jablonski, P., Nikjoo, D., Dinh, V. M., Sundman, O., Irgum, K., & Mikkola, J.-P. (2022). Poly (Vinylidene Difluoride) Polymer in 1-Ethyl-3-methylimidazolium Acetate and Acetic Acid Containing Solvents: Tunable and Recoverable Solvent Media to Induce Crystalline Phase Transition and Porosity. Sustainable Chemistry, 3(4), 455-474. https://doi.org/10.3390/suschem3040028