Production of Bio-Oil from De-Oiled Karanja (Pongamia pinnata L.) Seed Press Cake via Pyrolysis: Kinetics and Evaluation of Anthill as the Catalyst
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Catalyst Characterisation
2.3. Pyrolysis Experiments
2.4. Kinetic Study
3. Results and Discussion
3.1. Characterisation of Catalyst
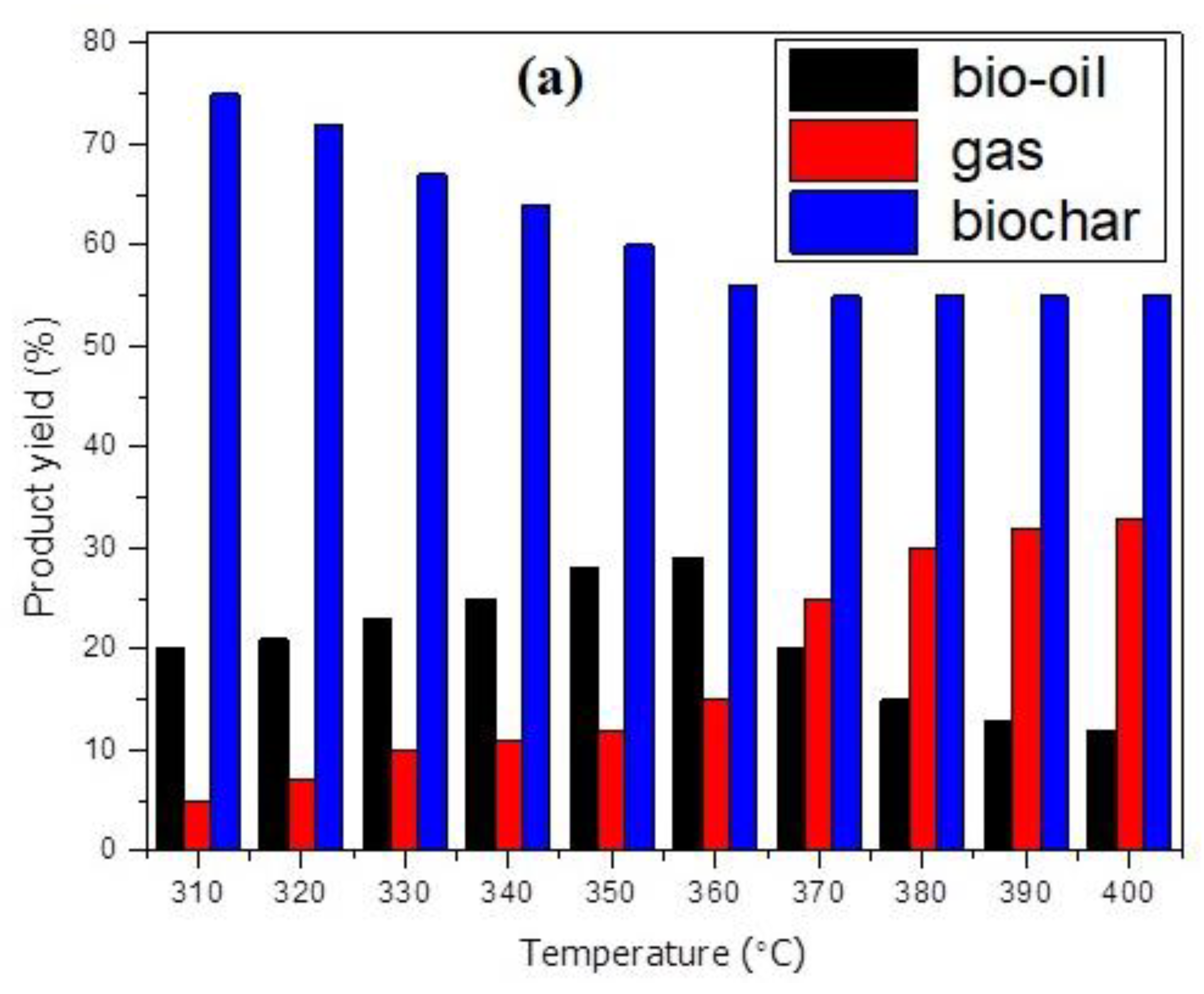
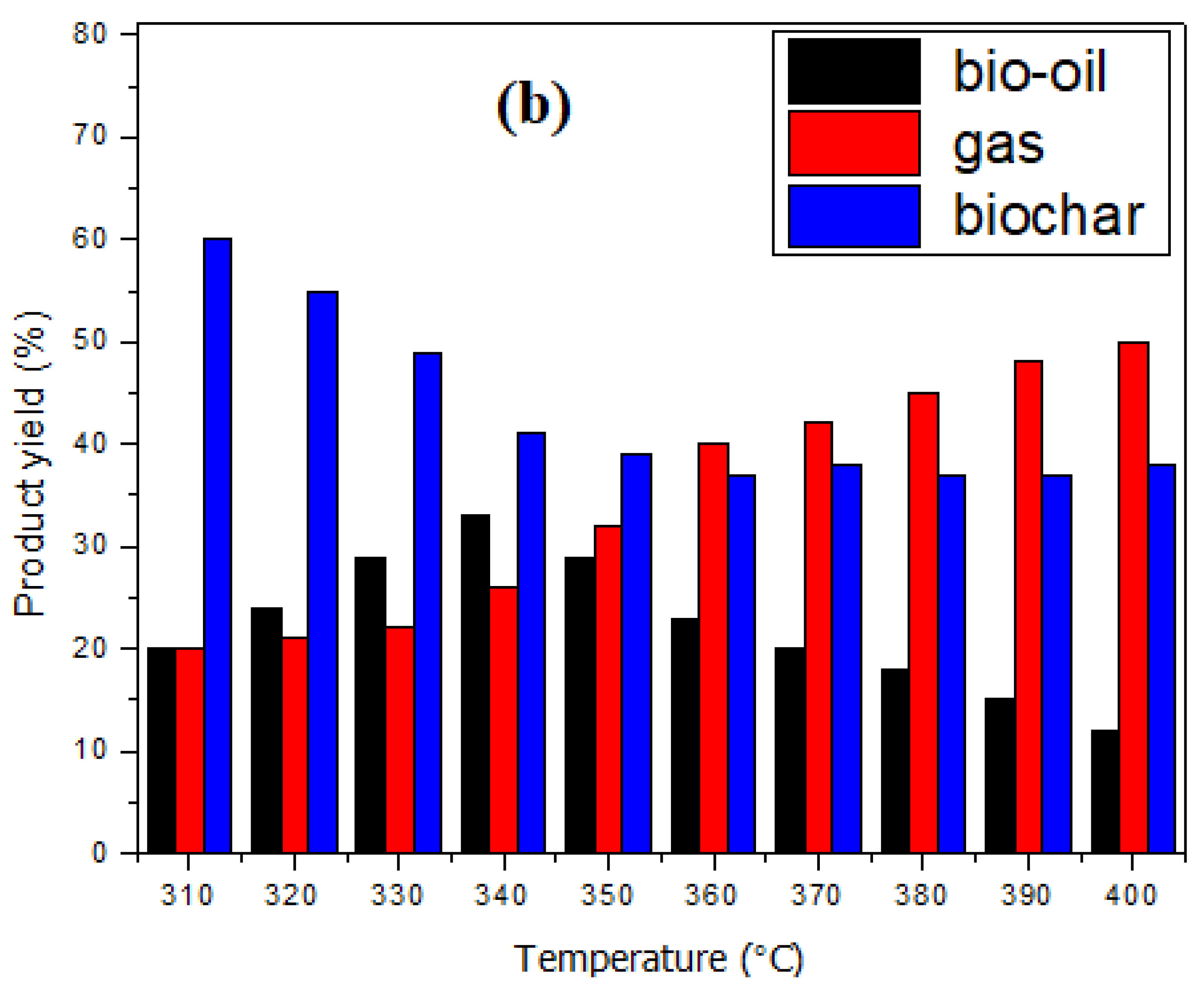
3.2. Pyrolysis and Characterisation of Products
3.3. Kinetic Study of Karanja Seed Press Cake Pyrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demirbas, A. Recent advances in biomass conversion technologies. Energy Educ. Sci. Technol. 2000, 6, 19–40. [Google Scholar]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Iqbal, J.; Howari, F.; Mohamed, A.-M.; Paleologos, E. Assessment of Radiation Pollution from Nuclear Power Plants. In Pollution Assessment for Sustainable Practices in Applied Sciences and Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1027–1053. [Google Scholar]
- Alhumade, H.; da Silva, J.C.G.; Ahmad, M.S.; Çakman, G.; Yıldız, A.; Ceylan, S.; Elkamel, A. Investigation of pyrolysis kinetics and thermal behavior of Invasive Reed Canary (Phalaris arundinacea) for bioenergy potential. J. Anal. Appl. Pyrolysis 2019, 140, 385–392. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis characteristics, fuel properties, and compositional study of Madhuca longifolia seeds over metal oxide catalysts. Biomass Convers. Biorefinery 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Das, B.; Mohanty, K. A review on advances in sustainable energy production through various catalytic processes by using catalysts derived from waste red mud. Renew. Energy 2019, 143, 1791–1811. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, G.K.; Mondal, M.K. Slow pyrolysis of chemically treated walnut shell for valuable products: Effect of process parameters and in-depth product analysis. Energy 2019, 181, 665–676. [Google Scholar] [CrossRef]
- Nisar, J.; Ali, G.; Ullah, N.; Awan, I.A.; Iqbal, M.; Shah, A.; Sirajuddin; Sayed, M.; Mahmood, T.; Khan, M.S. Pyrolysis of waste tire rubber: Influence of temperature on pyrolysates yield. J. Environ. Chem. Eng. 2018, 6, 3469–3473. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.; Spencer, N.; Jouhara, H. Potential of pyrolysis processes in the waste management sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Chowdhury, R.; Sarkar, A. Reaction Kinetics and Product Distribution of Slow Pyrolysis of Indian Textile Wastes. Int. J. Chem. React. Eng. 2012, 10, 456. [Google Scholar] [CrossRef]
- Shadangi, K.P.; Mohanty, K. Thermal and catalytic pyrolysis of Karanja seed to produce liquid fuel. Fuel 2014, 115, 434–442. [Google Scholar] [CrossRef]
- Patra, S.C.; Vijay, M.; Panda, A.K. Production and characterisation of bio-oil from Gold Mohar (Delonix regia) seed through pyrolysis process. Int. J. Ambient Energy 2016, 38, 788–793. [Google Scholar] [CrossRef]
- Sun, S.; Tian, H.; Zhao, Y.; Sun, R.; Zhou, H. Experimental and numerical study of biomass flash pyrolysis in an entrained flow reactor. Bioresour. Technol. 2010, 101, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Punsuwan, N.; Tangsathitkulchai, C. Product Characterization and Kinetics of Biomass Pyrolysis in a Three-Zone Free-Fall Reactor. Int. J. Chem. Eng. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Nisar, J.; Ahmad, A.; Ali, G.; Rehman, N.U.; Shah, A.; Shah, I. Enhanced Bio-Oil Yield from Thermal Decomposition of Peanut Shells Using Termite Hill as the Catalyst. Energies 2022, 15, 1891. [Google Scholar] [CrossRef]
- Olubunmi, B.E.; Karmakar, B.; Aderemi, O.M.; Auta, M.; Halder, G. Parametric optimization by Taguchi L9 approach towards biodiesel production from restaurant waste oil using Fe-supported anthill catalyst. J. Environ. Chem. Eng. 2020, 8, 104288. [Google Scholar] [CrossRef]
- Yusuff, A.S. Characterization of alkaline modified anthill and investigation of its catalytic behaviour in transesterification of Chrysophyllum albidium seed oil. South Afr. J. Chem. Eng. 2019, 29, 24–32. [Google Scholar] [CrossRef]
- Torres-García, E.; Ramírez-Verduzco, L.; Aburto, J. Pyrolytic degradation of peanut shell: Activation energy dependence on the conversion. Waste Manag. 2020, 106, 203–212. [Google Scholar] [CrossRef]
- Nisar, J.; Nasir, U.; Ali, G.; Shah, A.; Farooqi, Z.H.; Iqbal, M.; Shah, M.R. Kinetics of pyrolysis of sugarcane bagasse: Effect of catalyst on activation energy and yield of pyrolysis products. Cellulose 2021, 28, 7593–7607. [Google Scholar] [CrossRef]
- Harreh, D.; Saleh, A.A.; Reddy, A.N.R.; Hamdan, S. An Experimental Investigation of Karanja Biodiesel Production in Sarawak, Malaysia. J. Eng. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Mukta, N.; Sreevalli, Y. Propagation techniques, evaluation and improvement of the biodiesel plant, Pongamia pinnata (L.) Pierre—A review. Ind. Crop. Prod. 2010, 31, 1–12. [Google Scholar] [CrossRef]
- Nisar, J.; Rahman, A.; Ali, G.; Shah, A.; Farooqi, Z.H.; Bhatti, I.A.; Iqbal, M.; Rehman, N.U. Pyrolysis of almond shells waste: Effect of zinc oxide on kinetics and product distribution. Biomass Convers. Biorefinery 2020, 12, 2583–2595. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Gbadamosi, A.O.; Popoola, L.T. Biodiesel production from transesterified waste cooking oil by zinc-modified anthill catalyst: Parametric optimization and biodiesel properties improvement. J. Environ. Chem. Eng. 2021, 9, 104955. [Google Scholar] [CrossRef]
- Taylor, J.; Miller, S.; Bibby, D. A Study of Decomposition Methods for Refinement of H+-ZSM5 Zeolite with Powder Diffraction Data. Zeitschrift für Kristallographie-Crystalline Mater. 1986, 176, 183–192. [Google Scholar] [CrossRef]
- Garcés, J.M.; Rocke, S.C.; Crowder, C.E.; Hasha, D.L. Hypothetical Structures of Magadiite and Sodium Octosilicate and Structural Relationships Between the Layered Alkali Metal Silicates and the Mordenite-and Pentasil-Group Zeolites. Clays Clay Miner. 1988, 36, 409–418. [Google Scholar] [CrossRef]
- Bosmans, H. Unit cell and crystal structure of nordstrandite, Al(OH)3. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1970, 26, 649–652. [Google Scholar] [CrossRef]
- Wang, S.-L.; Johnston, C.T. Assignment of the structural OH stretching bands of gibbsite. Am. Miner. 2000, 85, 739–744. [Google Scholar] [CrossRef]
- Fernandez-Martinez, A.; Timon, V.; Roman-Ross, G.; Cuello, G.; Daniels, J.; Ayora, C. The structure of schwertmannite, a nanocrystalline iron oxyhydroxysulfate. Am. Miner. 2010, 95, 1312–1322. [Google Scholar] [CrossRef]
- Erdman, N.; Warschkow, O.; Asta, M.; Poeppelmeier, K.R.; Ellis, D.E.; Marks, L.D. Surface structures of SrTiO3 (001): A TiO2-rich reconstruction with ac (4 × 2) unit cell. J. Am. Chem. Soc. 2003, 125, 10050–10056. [Google Scholar] [CrossRef]
- Andersson, S.; Collén, B.; Kruuse, G.; Kuylenstierna, U.; Magnéli, A.; Pestmalis, H.; Asbrink, S. Identification of Titanium Oxides by X-Ray Powder Patterns. Acta Chem. Scand. 1957, 11, 1653–1657. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Olateju, I.I.; Adesina, O.A. TiO2/anthill clay as a heterogeneous catalyst for solar photocatalytic degradation of textile wastewater: Catalyst characterization and optimization studies. Materialia 2019, 8, 100484. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Adesina, O.S. Characterization and adsorption behaviour of anthill for the removal of anionic dye from aqueous solution. Int. J. Environ. Sci. Technol. 2019, 16, 3419–3428. [Google Scholar] [CrossRef]
- Westerhof, R.J.M.; Brilman, D.W.F.; Swaaij, W.P.M.; Kersten, S.R.A. Effect of Temperature in Fluidized Bed Fast Pyrolysis of Biomass: Oil Quality Assessment in Test Units. Ind. Eng. Chem. Res. 2009, 49, 1160–1168. [Google Scholar] [CrossRef]
- Nayan, N.K.; Kumar, S.; Singh, R. Characterization of the liquid product obtained by pyrolysis of karanja seed. Bioresour. Technol. 2012, 124, 186–189. [Google Scholar] [CrossRef]
- Soongprasit, K.; Sricharoenchaikul, V.; Atong, D. Pyrolysis of Millettia (Pongamia) pinnata waste for bio-oil production using a fly ash derived ZSM-5 catalyst. J. Anal. Appl. Pyrolysis 2019, 139, 239–249. [Google Scholar] [CrossRef]
- Dhanavath, K.N.; Islam, M.S.; Bankupalli, S.; Bhargava, S.K.; Shah, K.; Parthasarathy, R. Experimental investigations on the effect of pyrolytic bio–oil during the liquefaction of Karanja Press Seed Cake. J. Environ. Chem. Eng. 2017, 5, 4986–4993. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Z.; Min, M.; Ding, K.; Xie, Q.; Ruan, R. Catalytic fast co-pyrolysis of biomass and food waste to produce aromatics: Analytical Py–GC/MS study. Bioresour. Technol. 2015, 189, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Pang, S.; Mercader, F.D.M.; Torr, K.M. The effect of biomass pretreatment on catalytic pyrolysis products of pine wood by Py-GC/MS and principal component analysis. J. Anal. Appl. Pyrolysis 2018, 138, 145–153. [Google Scholar] [CrossRef]
- Bhoi, P.; Ouedraogo, A.; Soloiu, V.; Quirino, R. Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew. Sustain. Energy Rev. 2020, 121, 109676. [Google Scholar] [CrossRef]
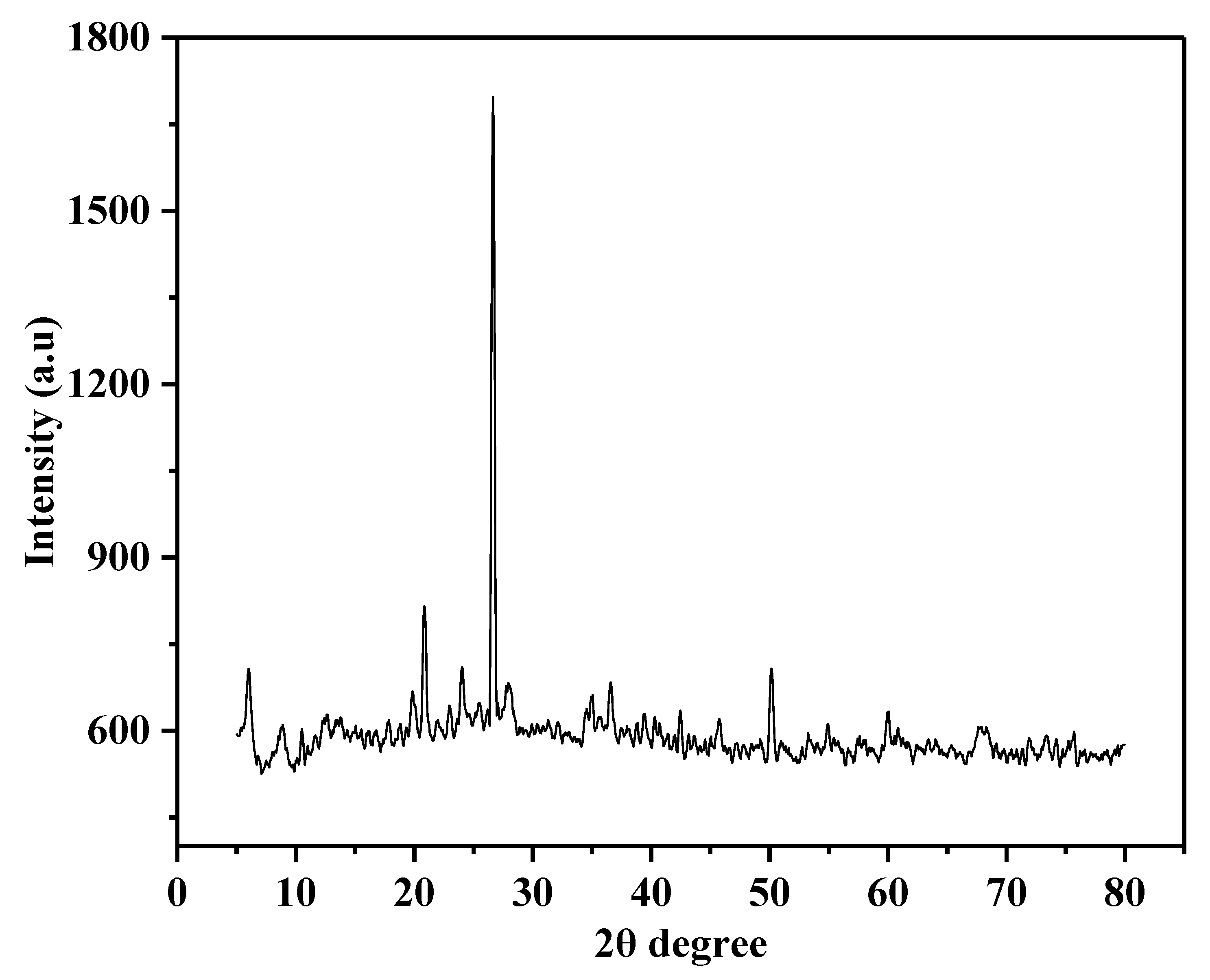

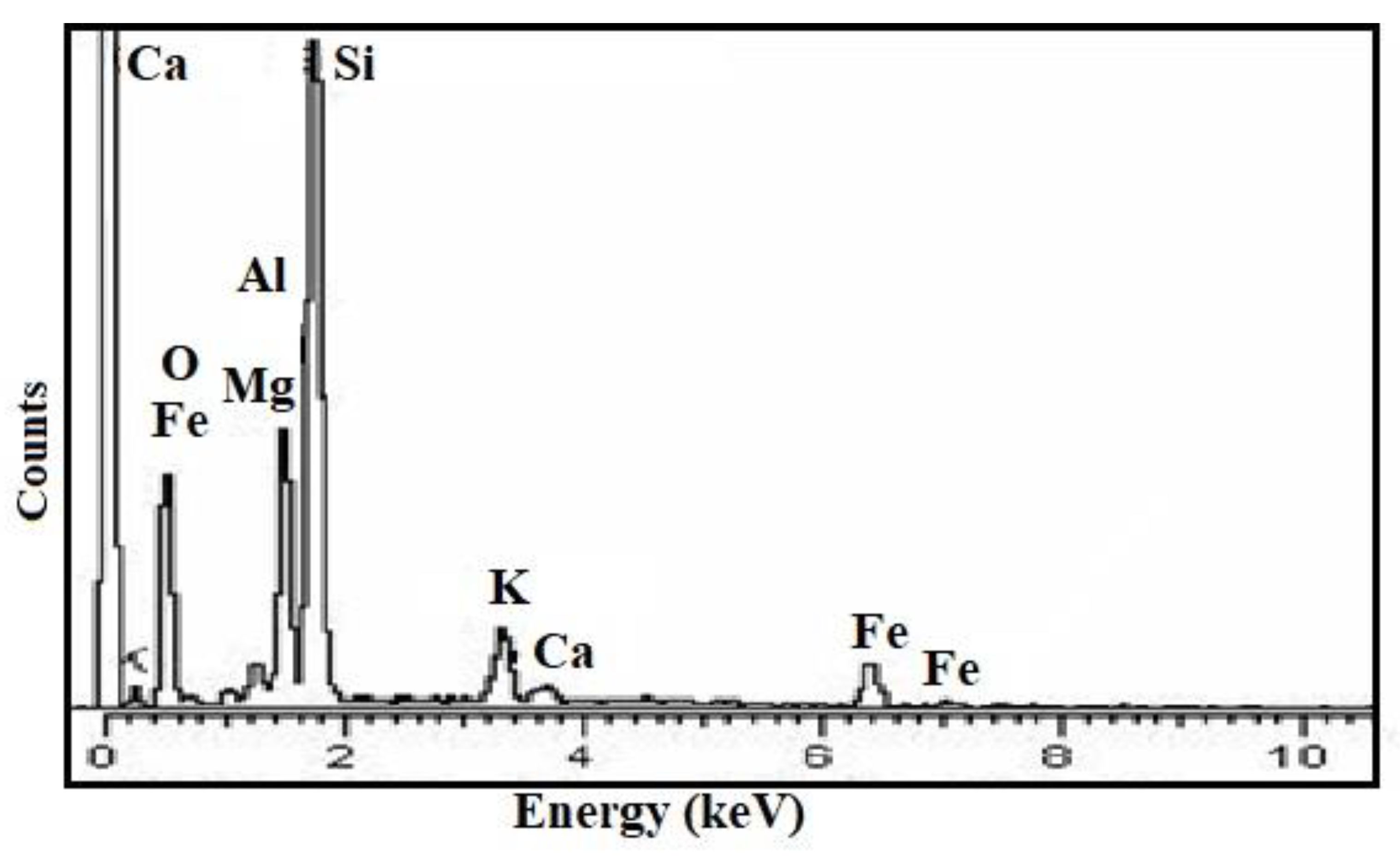

| S. No. | Compound | % Composition |
|---|---|---|
| 01 | SiO2 | 64.787 |
| 02 | Al2O3 | 20.399 |
| 03 | Fe2O3 | 8.306 |
| 04 | K2O | 3.510 |
| 05 | CaO | 1.615 |
| 06 | TiO2 | 1.039 |
| 07 | MnO | 0.166 |
| 07 | V2O5 | 0.041 |
| 08 | ZrO2 | 0.039 |
| 09 | Cr2O3 | 0.025 |
| 10 | CuO | 0.021 |
| 11 | SrO | 0.019 |
| 12 | ZnO | 0.016 |
| S. No. | R/T | Component | Chem. Formula | Mol. wt. | Area% |
|---|---|---|---|---|---|
| 1 | 2.40 | Furan, 2-methoxy | C5H6O2 | 98 | 46.79 |
| 2 | 3.20 | 2-Furanmethanol | C5H6O2 | 98 | 12.78 |
| 3 | 3.67 | 3-Octanamine | C8H19N | 129 | 3.28 |
| 4 | 4.67 | E-2-Tetradecen-1-ol | C14H28O | 212 | 0.19 |
| 5 | 5.34 | Phenol | C6H6O | 94 | 2.67 |
| 6 | 5.93 | Sorbic Acid | C6H8O2 | 112 | 0.94 |
| 7 | 6.42 | 1,2,3-Trimethylpiperidin-4-one | C8H15NO | 141 | 1.69 |
| 8 | 6.78 | Phenol, 4-methyl | C7H8O | 108 | 1.22 |
| 9 | 7.01 | 2,5-Heptadien-4-one,2,6-dimethyl | C9H14O | 138 | 1.55 |
| 10 | 7.68 | Glycyl-D-asparagine | C6H11N3O4 | 189 | 1.97 |
| 11 | 8.48 | 3,4-Dihydro-1-methylpyrrolo[1,2a]pyrazine | C8H10N2 | 134 | 2.81 |
| 12 | 9.15 | 4-Piperidinamine, N,1-dimethyl | C7H16N2 | 128 | 6.77 |
| 13 | 9.60 | Tricyclo[4.3.1.1(3,8)]undecan-1amine | C11H19N | 165 | 0.09 |
| 14 | 10.27 | Indole | C8H7N | 117 | 1.45 |
| 15 | 11.16 | Isosorbide | C6H10O4 | 146 | 8.67 |
| 16 | 11.69 | 1H-Indole, 3-methyl | C9H17N | 131 | 0.70 |
| 17 | 12.06 | 1,3;2,4-Di-O-methylene-d-arabitol | C7H12O5 | 176 | 0.61 |
| 18 | 12.41 | Acetic acid,2-(1,2,3,4-tetrahydro6methyl-2,4dioxo-5pyrimidylmethylthio | C8H10N2O4S | 230 | 0.52 |
| 19 | 13.30 | à-[2[NAziridyl]ethylamino]iso butyronitrile | C8H15N3 | 153 | 0.89 |
| 20 | 14.83 | 2H-1-Benzopyran, 7-methoxy-2,2-dimethyl | C12H14O2 | 190 | 0.19 |
| 21 | 16.56 | 9,10a-dimethyl-6-methylene-3á-isopropyl | C20H32O2 | 304 | 0.05 |
| 22 | 17.05 | Benzeneethanamine, 3-hydroxy-4-methoxy | C9H13NO2 | 167 | 0.38 |
| 23 | 17.62 | á-Himachalenoxide | C15H24O | 220 | 0.19 |
| 24 | 17.85 | Dimethoxyamphetamine, 2,5- | C11H17NO2 | 195 | 0.14 |
| 25 | 18.33 | N-(O-Nitrophenylthio)-l-leucine | C12H16N2O4S | 284 | 0.14 |
| 26 | 18.66 | Pyrrolo[1,2]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | C11H18N2O2 | 210 | 0.09 |
| 27 | 19.35 | 5,10-Diethoxy-2,3,7,8-tetrahydro-H,6H-dipyrrolo[1,2,1,2]pyrazine | C14H22N2O2 | 250 | 1.22 |
| 28 | 20.35 | 8-Octadecenoic acid, methyl ester | C19H36O2 | 296 | 0.89 |
| 29 | 23.49 | 2-[5-(2-Methyl-benzooxazol-7-yl)1H-pyrazol-3-yl]-phenol | C17H13N3O2 | 291 | 0.89 |
| S. No. | R/T | Component | Chem. Formula | Mol. wt | Area % |
|---|---|---|---|---|---|
| 1 | 7.12 | Piperidine, 1-methyl- | C6H13N | 99 | 2.51 |
| 2 | 7.52 | Acetamide | C2H5NO | 59 | 4.73 |
| 4 | 7.84 | 2,3-Butanediol | C4H10O2 | 90 | 1.98 |
| 5 | 10.39 | 2-Furanmethanol | C5H6O2 | 98 | 32.15 |
| 6 | 10.92 | Acetamide, N,N-dimethyl- | C4H9NO | 87 | 1.39 |
| 7 | 12.05 | 2-n-Butyl furan | C8H12O | 124 | 16.84 |
| 8 | 12.62 | 2(5H)-Furanone | C4H4O2 | 84 | 5.01 |
| 9 | 13.05 | [1,3,4]Thiadiazol, 2-amino-5-(2-piperidin-1-ylethyl)- | C9H16N4S | 244 | 0.71 |
| 10 | 13.83 | 3(2H)-Furanone, 2-(1-hydroxy-1-methyl-2-oxopropyl)-2,5-dimethyl- | C10H14O4 | 198 | 0.96 |
| 11 | 14.15 | Mannosamine | C6H13NO5 | 179 | 0.66 |
| 12 | 15.26 | Phenol | C6H6O | 94 | 9.27 |
| 13 | 16.1 | Butanamide, 3-methyl- | C5H11NO | 101 | 5.22 |
| 14 | 16.93 | 1,2-Cyclopentanedione, 3-methyl- | C6H8O2 | 112 | 9.77 |
| 15 | 17.21 | Formamide, N-[1-[(1-cyano-2-methylpropyl)hydroxyamino]ethyl]- | C8H15N3O2 | 185 | 2.57 |
| 16 | 18.07 | Ethanone, 1-(1H-pyrrol-2-yl)- | C6H7NO | 109 | 2.81 |
| 17 | 19.24 | 2-Vinyl-9-[3-deoxy-β-d-ribofuranosyl]hypoxanthine | C12H14N4O4 | 278 | 4.96 |
| 18 | 19.87 | Maltol | C6H6O3 | 126 | 12 |
| 19 | 23.39 | 1,2-Benzenediol | C6H6O2 | 110 | 3.38 |
| 20 | 25.04 | Acetic acid, 2-[2-methyl-4-(1-piperidylmethyl)-1,3-dioxolan-2-yl]-, ethyl ester | C14H25NO4 | 271 | 5.12 |
| 21 | 25.81 | Isosorbide | C6H10O4 | 146 | 1.00 |
| 22 | 28.11 | Dodecanoic acid, 3-hydroxy- | C12H24O3 | 216 | 8.11 |
| 23 | 33.36 | Cholestan-3-ol, 2-methylene-, (3β,5α)- | C28H48O | 400 | 3.99 |
| 24 | 34.14 | 2-Methylenecholestan-3- | C28H48O | 400 | 2.94 |
| 25 | 36.31 | Uric acid | C5H4N4O3 | 168 | 0.39 |
| 26 | 37.04 | Nitro-L-arginine | C6H13N5O4 | 219 | 6.8 |
| 27 | 37.44 | Propyl-3,6-diazahomoadamantan-9-ol | C12H22N2O | 210 | 1.3 |
| 28 | 40.32 | Didemnin B | C57H89N7O15 | 1112 | 15.14 |
| 29 | 42.85 | Actinomycin C2 | C63H88N12O16 | 1268 | 5.57 |
| 30 | 43.71 | 1,3-Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione | C11H18N2O2 | 210 | 9.17 |
| 31 | 44.01 | 5,10-Diethoxy-2,3,7,8-tetrahydro-1H,6H-dipyrrolo[1,2-a;1′,2′-d]pyrazine | C14H22N2O2 | 250 | 7.9 |
| 32 | 44.4 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | C11H18N2O2 | 210 | 7.58 |
| 33 | 56.24 | Oleic Acid | C18H34O2 | 282 | 7.09 |
| 34 | 61.72 | Ethyl iso-allocholate | C26H44O5 | 436 | 0.61 |
| 35 | 61.87 | Ergotaman-3,6,18-trione,9,10-dihydro-12-hydroxy-2-methyl-5′-(phenylmethyl)-(5′α,10α) | C33H37N5O5 | 583 | 2.71 |
| 36 | 62.07 | Didemnin B | C57H89N7O15 | 1112 | 0.33 |
| 37 | 62.16 | Oxiraneoctanoic acid, 3-octyl cis- | C18H34O3 | 298 | 0.7 |
| 38 | 62.58 | Pregn-4-ene-3,20-dione,17,21-dihydroxy,bis(O-methyloxime) | C23H36N2O4 | 404 | 1.77 |
| 39 | 63.11 | Phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-methyl- | C23H32O2 | 340 | 2.57 |
| 40 | 64.63 | 1,2-Benzenedicarboxylic acid, diisooctyl ester | C24H38O4 | 390 | 3.94 |
| 41 | 65.54 | 9,10-Anthracenedione, 1,2,3,4-tetrahydro-1,2,3,4,5-pentahydroxy-7-methoxy-2-methyl-, (1α,2β,3β,4α)- | C16H16O8 | 336 | 3.08 |
| 42 | 65.77 | 2-[5-(2-Methyl-benzooxazol-7-yl)-1H-pyrazol-3-yl]-phenol | C17H13N3O2 | 291 | 5.21 |
| Component | Catalytic Reaction | Non-Catalytic Reaction | ||||
|---|---|---|---|---|---|---|
| Ea (kJ·mol−1) | A (min−1) | R2 | Ea (kJ·mol−1) | A (min−1) | R2 | |
| Hemicellulose | 99.7 ± 0.4 | 4.8 × 108 | 0.859 | 74.8 ± 0.2 | 6.5 × 106 | 0.958 |
| Cellulose | 182.9 ± 0.5 | 8.4 × 1013 | 0.996 | 83.1 ± 0.4 | 2.3 × 108 | 0.962 |
| Lignin | 199.5 ± 0.7 | 9.7 × 1014 | 0.848 | 108.0 ± 0.5 | 8.8 × 109 | 0.872 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisar, J.; Waris, S.; Shah, A.; Anwar, F.; Ali, G.; Ahmad, A.; Muhammad, F. Production of Bio-Oil from De-Oiled Karanja (Pongamia pinnata L.) Seed Press Cake via Pyrolysis: Kinetics and Evaluation of Anthill as the Catalyst. Sustain. Chem. 2022, 3, 345-357. https://doi.org/10.3390/suschem3030022
Nisar J, Waris S, Shah A, Anwar F, Ali G, Ahmad A, Muhammad F. Production of Bio-Oil from De-Oiled Karanja (Pongamia pinnata L.) Seed Press Cake via Pyrolysis: Kinetics and Evaluation of Anthill as the Catalyst. Sustainable Chemistry. 2022; 3(3):345-357. https://doi.org/10.3390/suschem3030022
Chicago/Turabian StyleNisar, Jan, Salman Waris, Afzal Shah, Farooq Anwar, Ghulam Ali, Ali Ahmad, and Faisal Muhammad. 2022. "Production of Bio-Oil from De-Oiled Karanja (Pongamia pinnata L.) Seed Press Cake via Pyrolysis: Kinetics and Evaluation of Anthill as the Catalyst" Sustainable Chemistry 3, no. 3: 345-357. https://doi.org/10.3390/suschem3030022
APA StyleNisar, J., Waris, S., Shah, A., Anwar, F., Ali, G., Ahmad, A., & Muhammad, F. (2022). Production of Bio-Oil from De-Oiled Karanja (Pongamia pinnata L.) Seed Press Cake via Pyrolysis: Kinetics and Evaluation of Anthill as the Catalyst. Sustainable Chemistry, 3(3), 345-357. https://doi.org/10.3390/suschem3030022






