This section covers the discussion on the characterization of the CFA raw material and the extraction of critical REEs, particularly Dy, Nd, Er, Eu and Tb. There was no data obtained for Y because its presence was below the detection limit of the ICP-MS used in the analysis and hence it is not included in the discussion. The extraction of major elements Al, Fe, Ca and Fe are also presented in relation to the extraction of REEs.
3.1. Characterization of CFA
The REE composition of the CFA determined by ICP-MS analysis is summarized in
Table 3. The elements are presented according to the economic classification of Seredin and Dai (2010) which is based on the current market trend of the individual REE rather than on the traditional classification of light REE as not valuable and heavy REE as valuable. This economic classification is the basis for the grading of the REE ore through an index called outlook coefficient which measures the ratio of the total critical REE to the total excessive REE, and the relative amount of the critical REE. An ore is regarded as promising REE raw material for economic development if its outlook coefficient is within 0.7 to 1.9 and the relative amount of critical REE is between 30% and 51% [
15]. Based on this classification, the CFA used in this study qualifies as a promising REE raw material with an outlook coefficient of 1.03 and critical elements percentage of 33.3%.
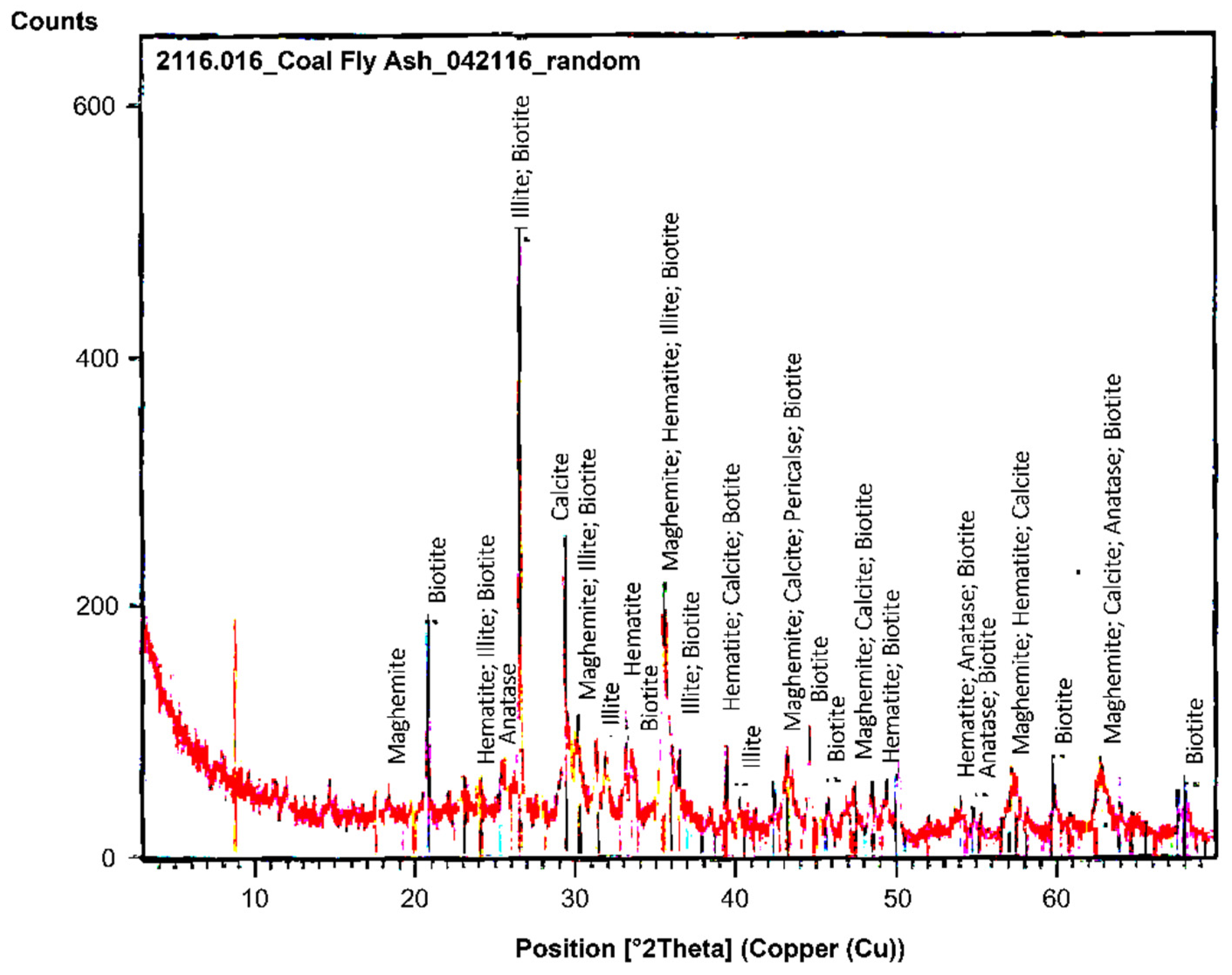
Figure 2 shows the XRD pattern of the CFA samples and it can be seen that the dominant minerals present are quartz (SiO
2), calcite (CaCO
3), iron oxide minerals such as magnetite (Fe
2O
4), hematite (Fe
2O
3) and maghemite (γ-Fe
2O
3), and aluminosilicates such as illite (
) and biotite (
).
The quantity of the abundant minerals present was determined by XRF analysis. The results presented in
Figure 3 show that SiO
2 is the most abundant mineral, followed by Al
2O
3, Fe
2O
3 and CaO with small amounts of MgO, S, K
2O and TiO
2. A high amount of silica may not be desirable for leaching due to the formation of a silica gel layer which prevents diffusion of ions. However, the presence of calcium ions can somewhat counteract the gelling effect of the silica through the reaction of calcium ions with silica forming calcium silicates, thereby destabilizing the gel layer and reducing the amount of silicate [
32]. The CaO present in the CFA has to be dissolved so that it can react with the silica, and based on thermodynamics, CaO is easily dissolved by HCl [
44]. This is therefore one of the bases for the choice of HCl as lixiviant in this study.
To better understand how the other relatively abundant minerals present in the CFA may possibly interfere with the extraction of the REEs of interest, it is important to know the association of the REEs with these dominant minerals.
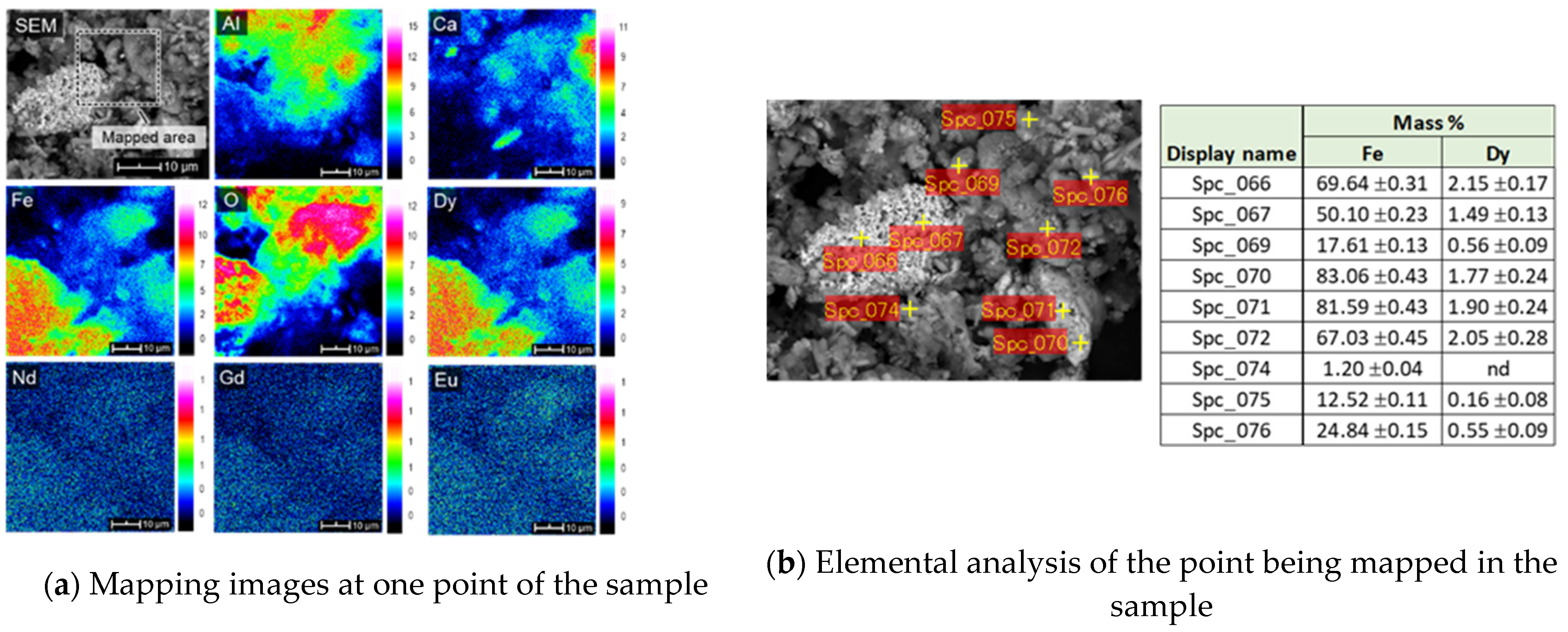
Figure 4 shows the SEM-EDS images with elemental map at different locations. It can be seen that the elemental map of Dy is almost identical with that of Fe, indicating a close association of Dy with Fe. Other REEs appear to be finely disseminated Throughout the matrix and show some associations with Al and Ca but they tend to appear more associated with Fe.
Figure 5 shows a SEM-EDS image with elemental map and point analysis. The elemental map confirms the close association of Dy with Fe qualitatively and the point analysis quantifies the association and shows a general trend that the amount of Dy is directly proportional to the amount of Fe except at some points.
3.2. Optimization of Leaching Parameters Using Central Compiste Design-Response Surface Methodolgy
The highest extractions of Dy, Nd, Er, Eu and Tb were obtained at 3 M HCl, 65 °C and a leaching time of 270 min (
Table 4) while the lowest extraction occurred at the lowest acid concentration 0.3M where concentrations of Dy, Er, and Eu were no longer detected by ICP-MS. This implies that the acid concentration of HCl was too weak to leach out the REE in the CFA. Also, at this acid concentration, only calcium (Ca), among the major elements that existed in the CFA, was leached out (
Table 5). This inability of HCl at 0.3 M concentration to extract the major elements except Ca can be futher elucidated through the thermodynamic principles using the formula
. At
, the
corresponding to the following reactions are shown [
44]:
Among the four reactions, only the leaching reaction of calcium oxide (Equation (3)) has a negative , which means that it is the only spontaneous reaction at 0.3 M HCl concentration. The failure of the 0.3 M HCl concentration to leach out the REEs can be explained through the application of material balance where 5g of coal fly ash was leached with 50 mL of xM HCl. At very low HCl concentration, almost all of the ions were consumed by CaO which leaves a very small amount for the reaction with REE. Consequently, since no data was obtained at 0.3 M HCl concentration, it was excluded from the experimental design as including it would negatively affect the RSM analysis (ANOVA, diagnostics, Model graph, and optimization) in the Design Expert version 11 software.
To ensure a good model fitting, it was necessary to test for the significance of the regression model and its individual model coefficients. The significance of the factors were determined through the ANOVA where a factor or interaction between factors was considered significant if the p-value was less than 0.05. Otherwise, it was insignificant and thus excluded from the empirical model.
The results of the ANOVA for the quadratic model (Equation (1)) are shown in
Table 6,
Table 7,
Table 8,
Table 9 and
Table 10 for Dy, Er, Eu, Nd and Tb, respectively. As can be seen in the tables, the three main factors, namely, acid concentration, leaching temperature and leaching time, have significant effects on the extraction of Dy, Er, Eu, Nd and Tb. The interactions between acid concentration and leaching temperature (AB) for all the critical REEs investigated were significant while all interactions involving leaching time (AC and BC) were insignificant except for the case of Eu (
Table 8) where the interaction between acid concentration and leaching time (AC) was significant.
Table 11 shows the final empirical model which includes only the terms that were found to be significant. The negative values indicate antagonistic effects, whereas the positive values indicate synergistic effects [
39].
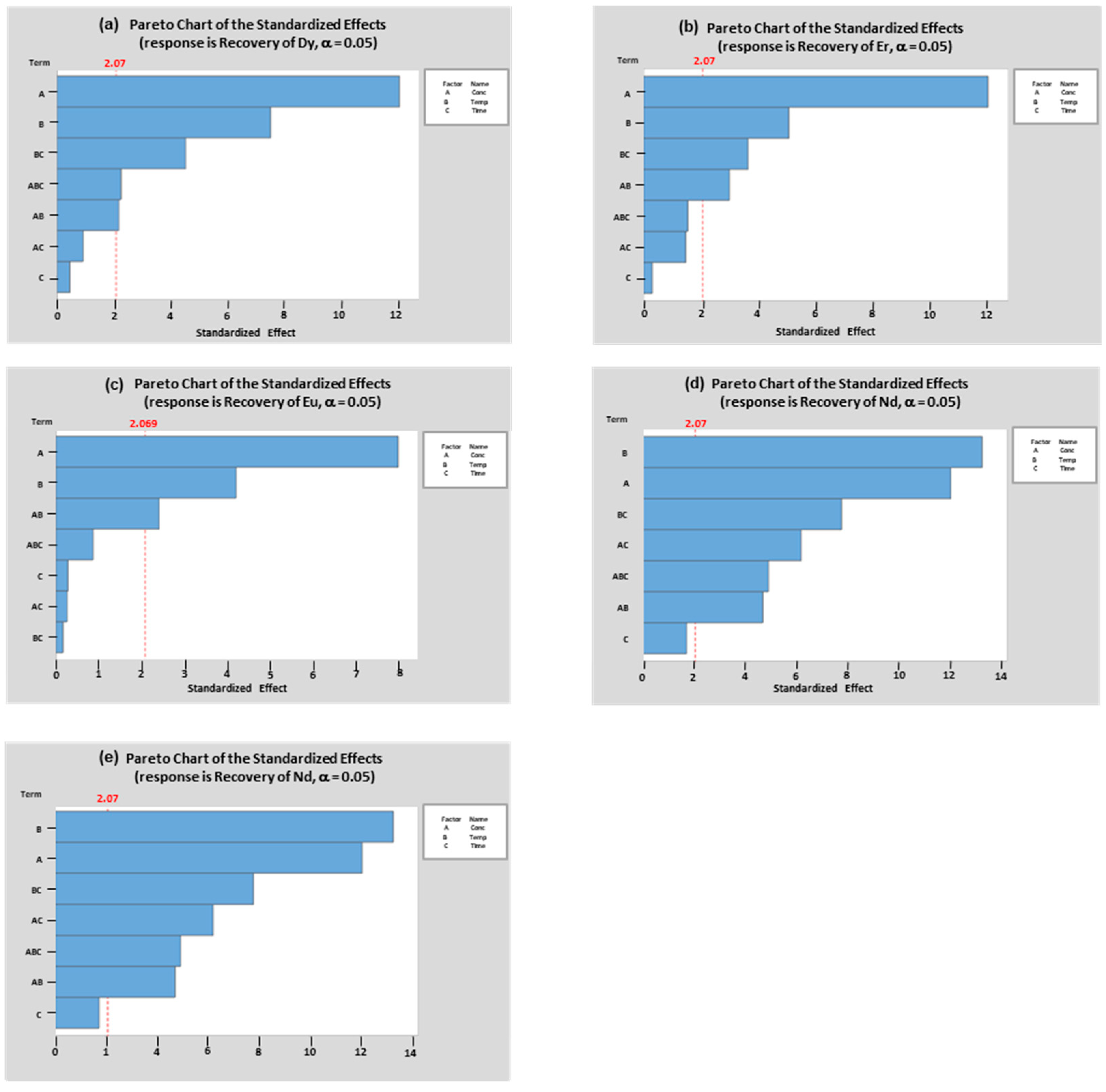
The magnitude of significance of the factors on the extraction of Dy, Er, Eu, Nd and Tb was measured through the Pareto chart of the standardized effects and is presented in
Figure 6. It can be seen that the greatest effect on the extraction of the REEs of interest was contributed by the acid concentration, except for Nd where the effect of leaching temperature was more significant than the acid concetration.
The adequacy of the developed model plays an important role in validating the analysis of the experimental data. Thus, fit statistics and diagnostics of the model were done and the results are summarized in
Table 12. The predicted
is a measure of how well the model predicts a response variable while the adjusted
is used to determine the reliability of the correlation and how much is determined by the addition of independent variables. A good model should have a difference between predicted
and adjusted
not exceeding 0.2. Based on the fit statistics in the table, all models for the extraction of the REEs of interest qualify for a good model.
Another value that should be considered is the adequate precision (Adeq Precision), which compares the range of the predicted values at the design points to the average prediction error. It is used to measure the signal to noise ratio and a ratio of greater than 4 is desirable. According to
Table 12, the Adeq Precision is greater than 17 for all extraction models indicating an adequate signal. Thus, the models can be used to navigate the design space.
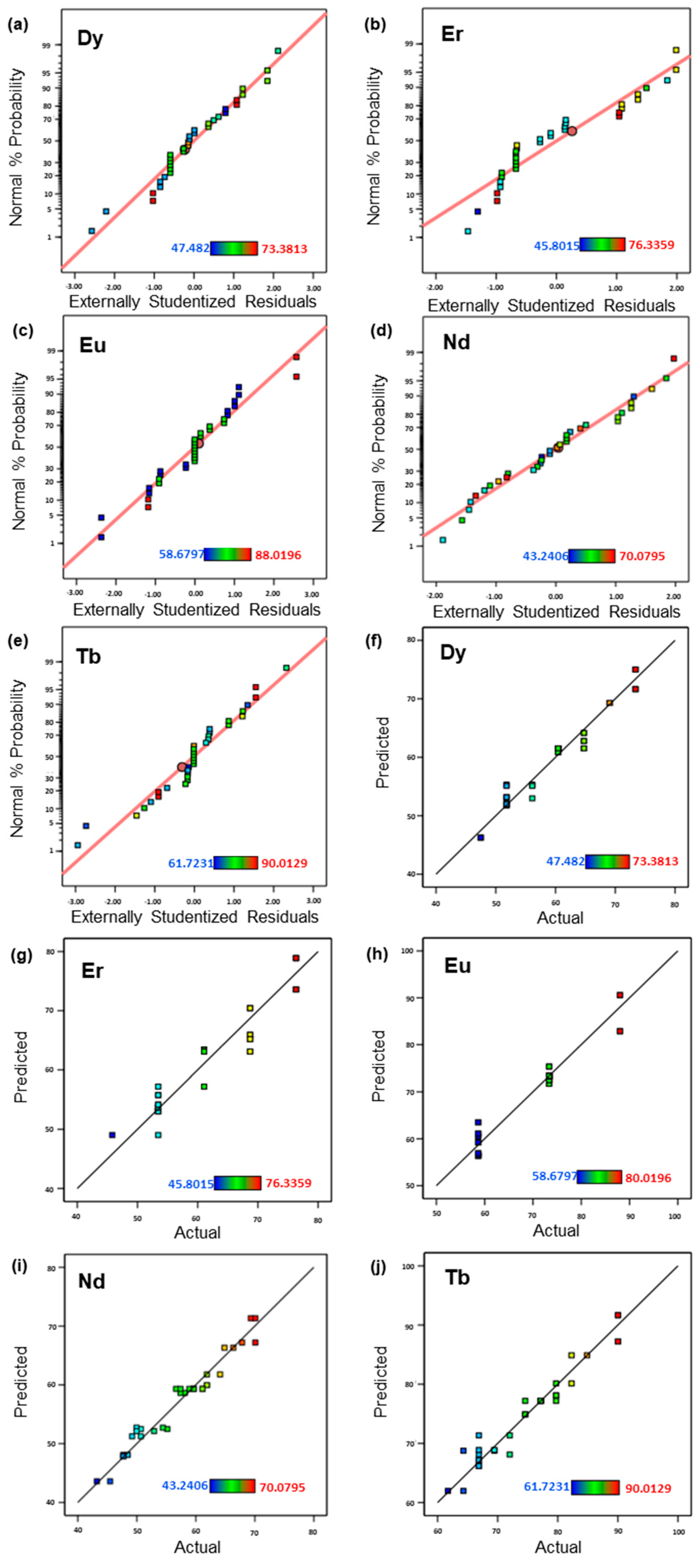
Diagnostic plots such as the normal probability plots and the plots for predicted vs actual are shown in
Figure 7. These plots are based on Externally Studentized Residuals method rather than on the Internally Studentized Residuals, as the former is more sensitive for finding the problems with the analysis compared to the latter. The Studentizing residuals map all the different normal distributions to a single standard normal distribution.
Figure 7a–e follow a straight line, which indicates that the data set follows a normal probability and thus there is no need for data transformations like logarithmic, square root or arc sine square root transformations. Moreover,
Figure 7f–j showed straight lines for the predicted vs actual plots which means that the data set can be easily predicted by the model.
3.3. Optimization of Factors by RSM
The most important part of this study is the determination of the optimum leaching process condition where maximum critical REE extraction can be obtained from CFA. The optimum leaching condition was generated by the Design Expert Software for the REEs of interest and was found to fall at 3 M HCl concentration, 65 °C and 270 min of leaching time, which are the highest levels for each factor. There was a consistent increase in the REE extractions as the levels of the factors were increased within the chosen range for each factor. While the optimum extraction is expected as the levels of the factors are increased, this may not be necessarily true if precipitation of the target REEs occur. In the case of this study, a significant decrease in the extraction was not observed as the levels of the factors were increased, indicating that leaching with HCl at the chosen range of concentration, temperature and time did not lead to any precipitation and the optimum condition was successfully achieved.
The accuracy of the empirical models was tested by comparing the experimental and the predicted values at the optimum conditions; the results are summarized in
Table 13. To be able to say that the model is accurate, the % error (i.e., the deviation of the predicted value from the experimental value) must be less than 5%. Based on the results in
Table 13, all models showed errors less than 5%, therefore it is safe to say that the models established predicted the extraction of the REEs accurately.