Redox Active Organic-Carbon Composites for Capacitive Electrodes: A Review
Abstract
1. Introduction
2. Redox Active Compounds and Carbon Substrates
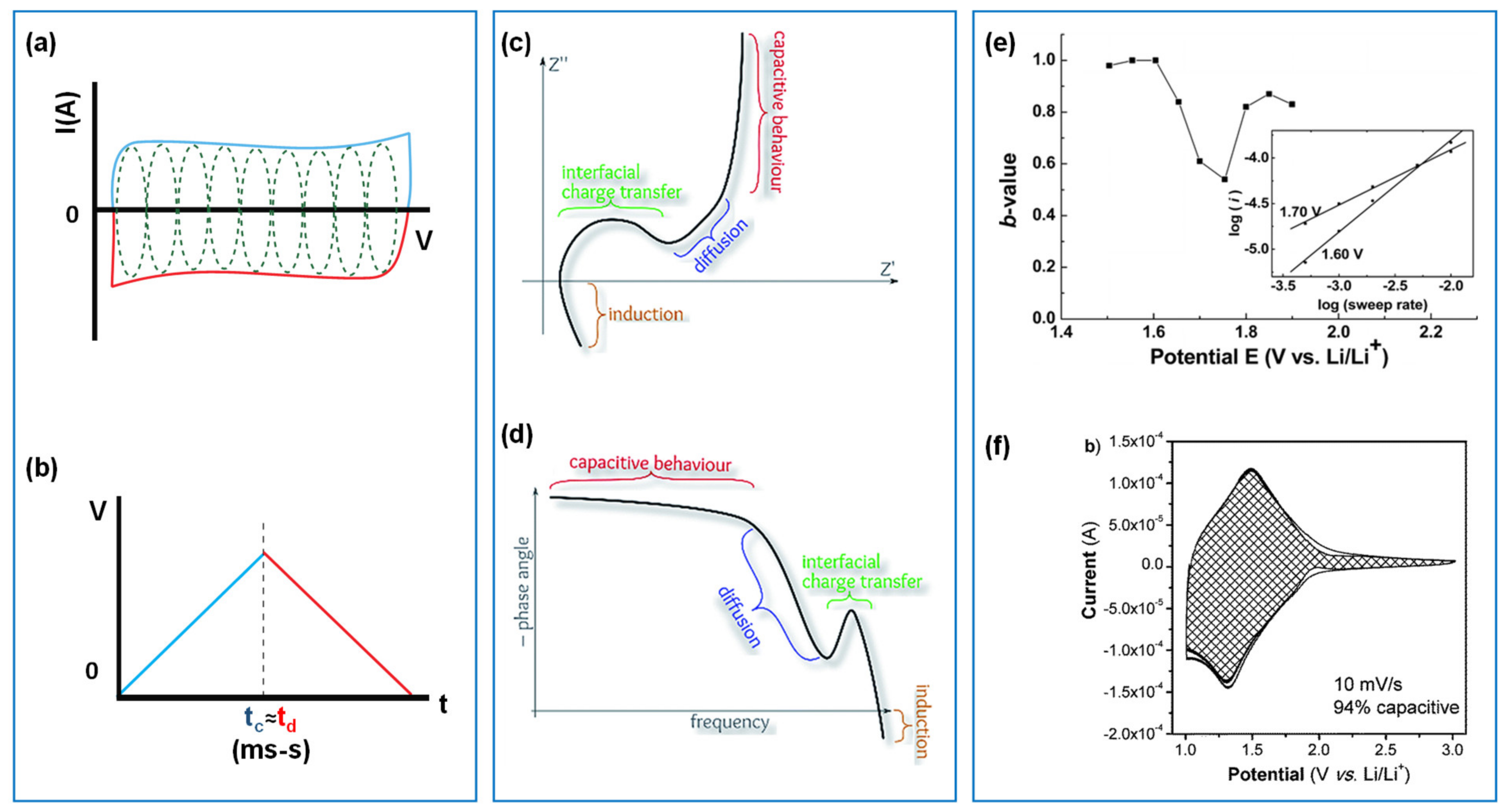
2.1. Characteristics of Capacitive Materials
2.2. Electrochemically Active Organic Materials
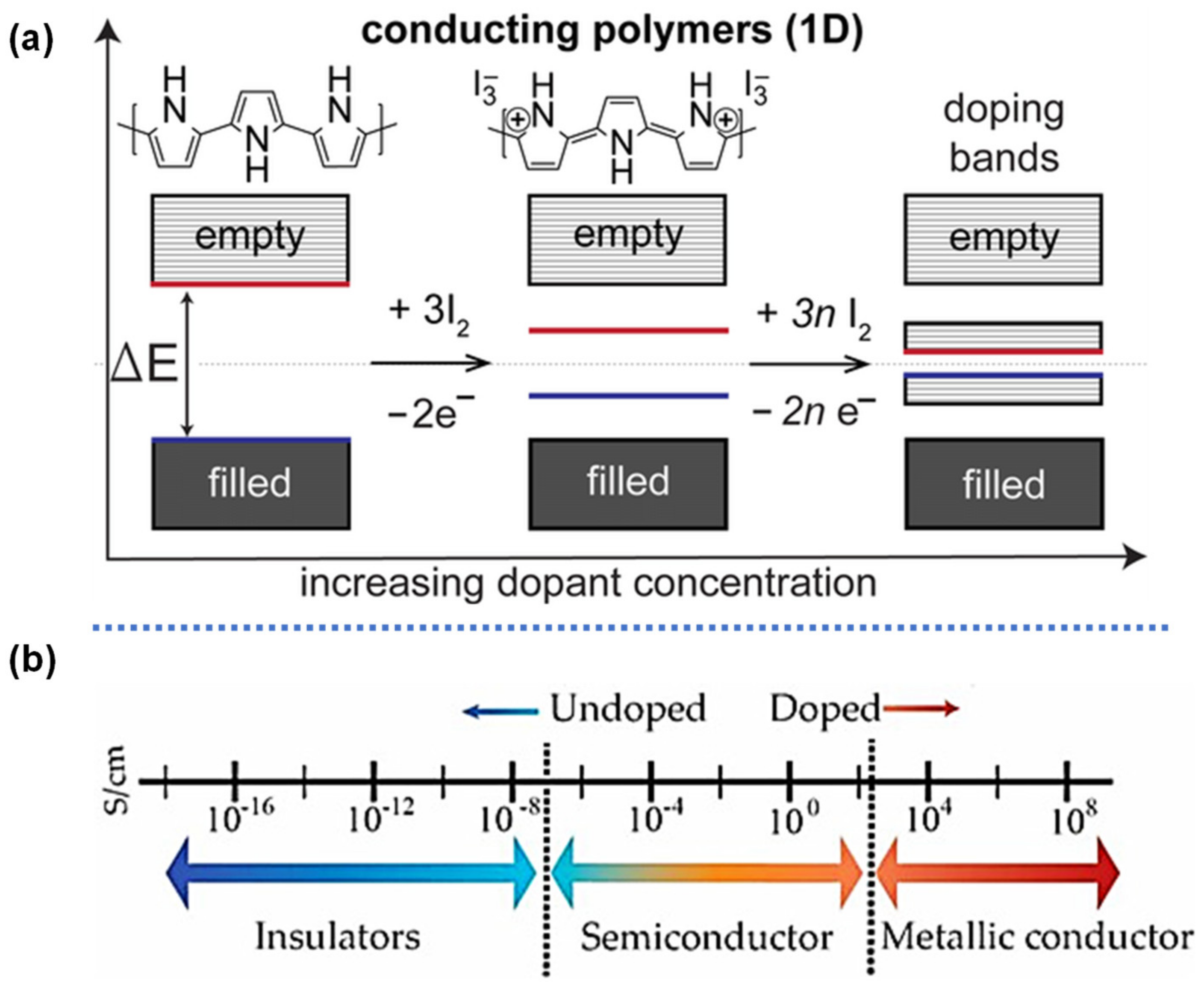
2.2.1. Redox Active Polymers
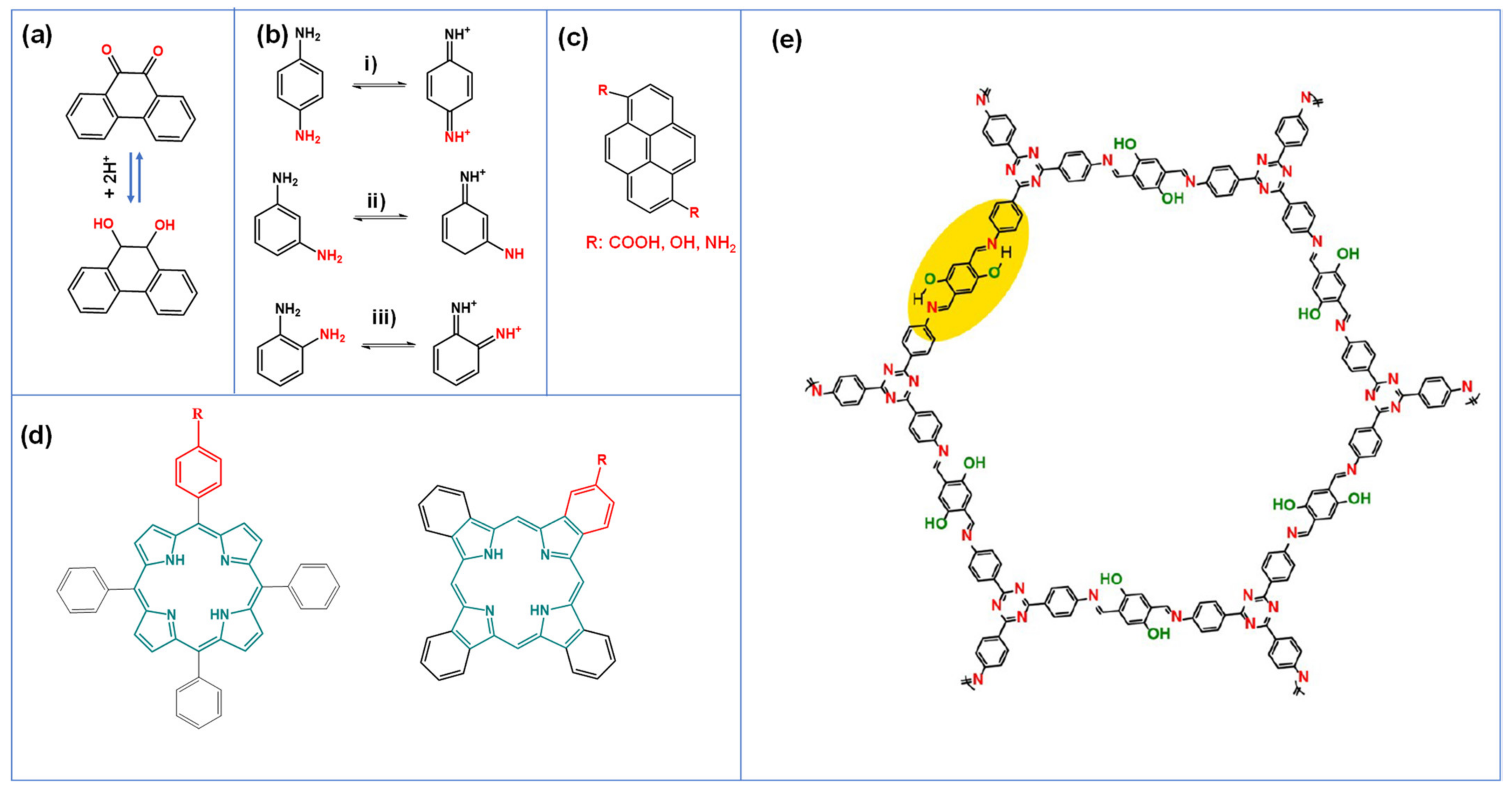
2.2.2. Redox Active Organic Compounds
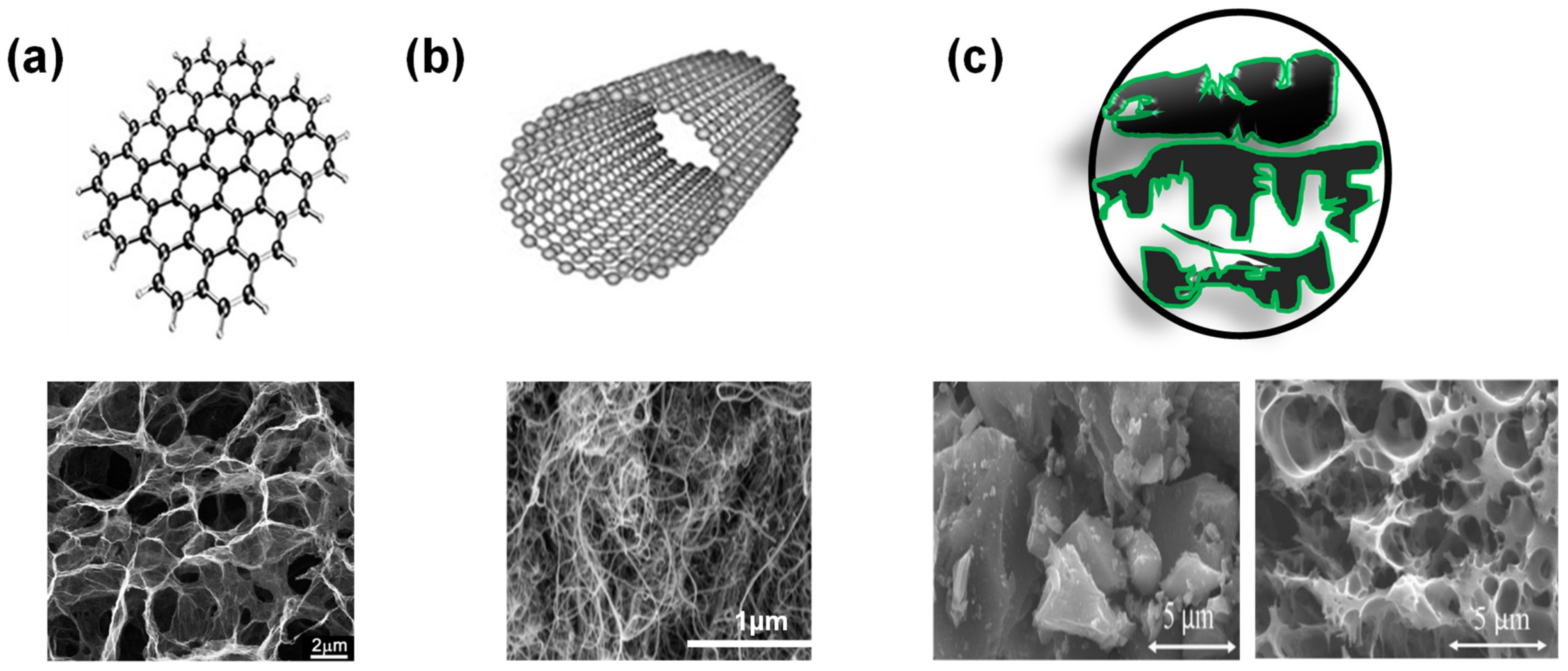
2.3. Carbon Substrates for Composite Electrodes
3. State-of-the-Art of Redox Active Organic-Carbon Composites
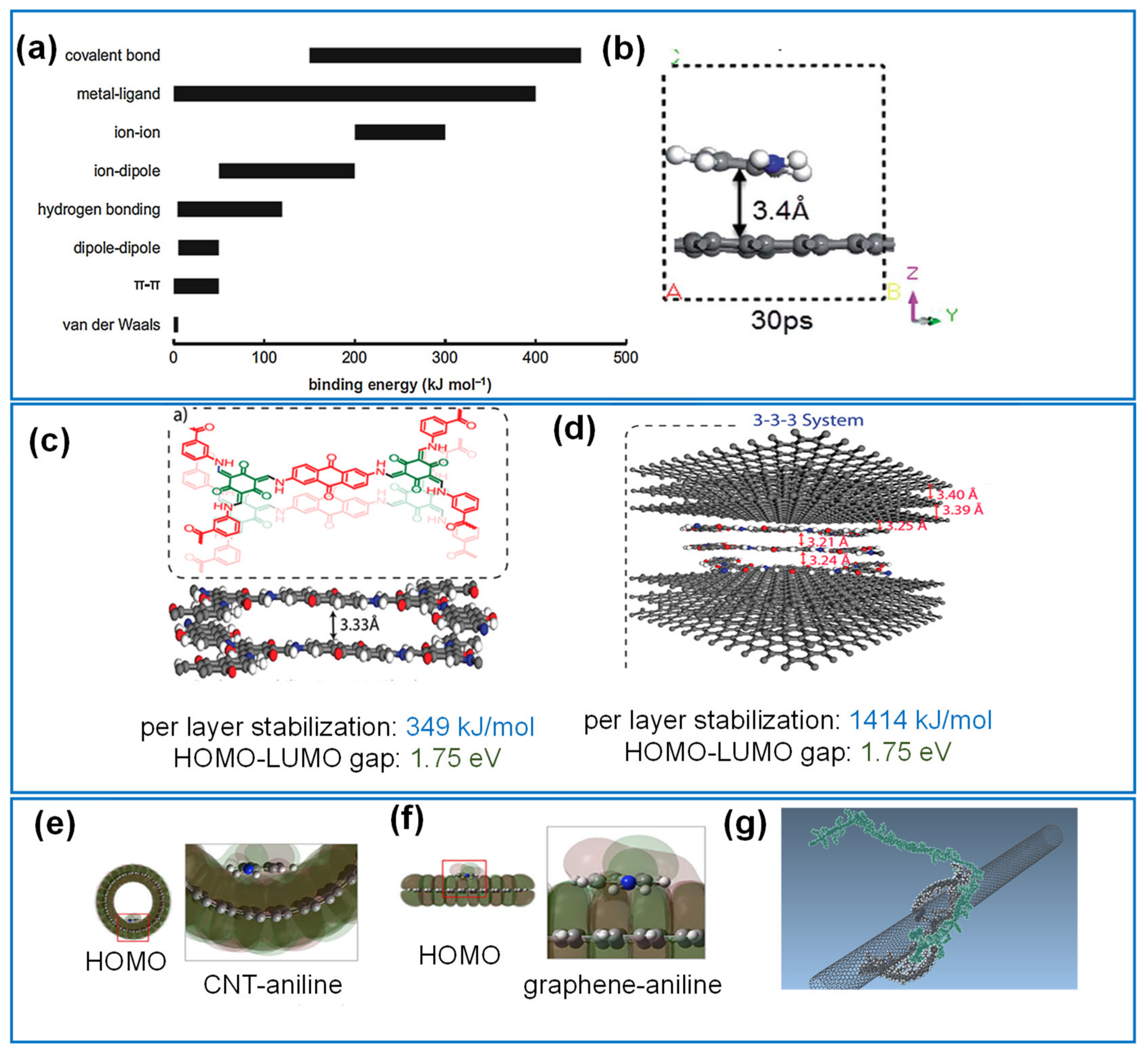
3.1. Computational Studies of the Interfacial Interactions between Components
3.1.1. Noncovalent Interactions
- Quantum chemical simulations
- Classical methods
3.1.2. Covalent Interactions
3.2. Fabrication Methods
3.2.1. Electropolymerization
3.2.2. In-Situ Chemical Polymerization
3.2.3. Hydro/Solvothermal Methods
3.2.4. Direct Deposition
4. Current Advances on Capacitive Organic-Carbon Composite Electrodes
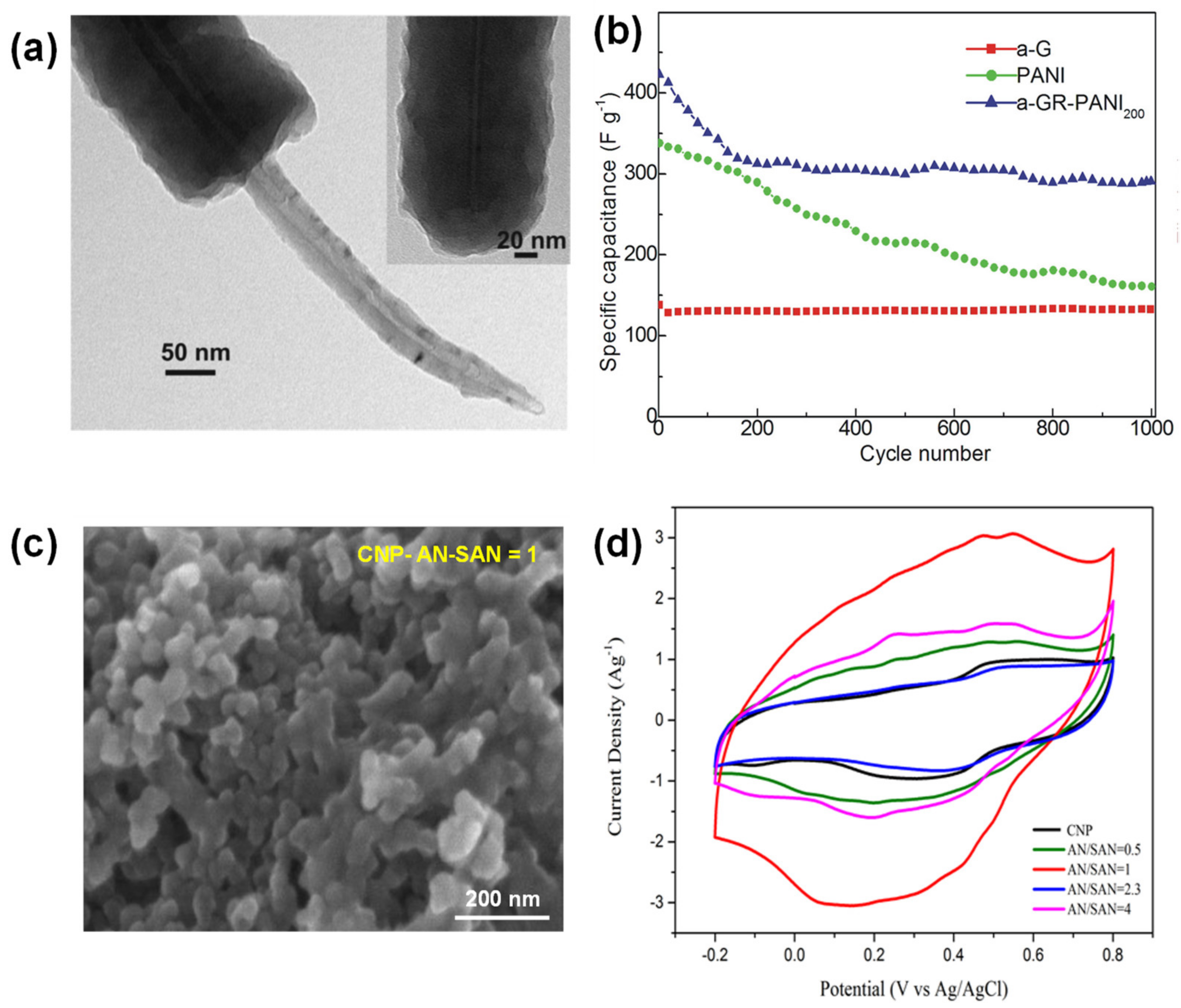
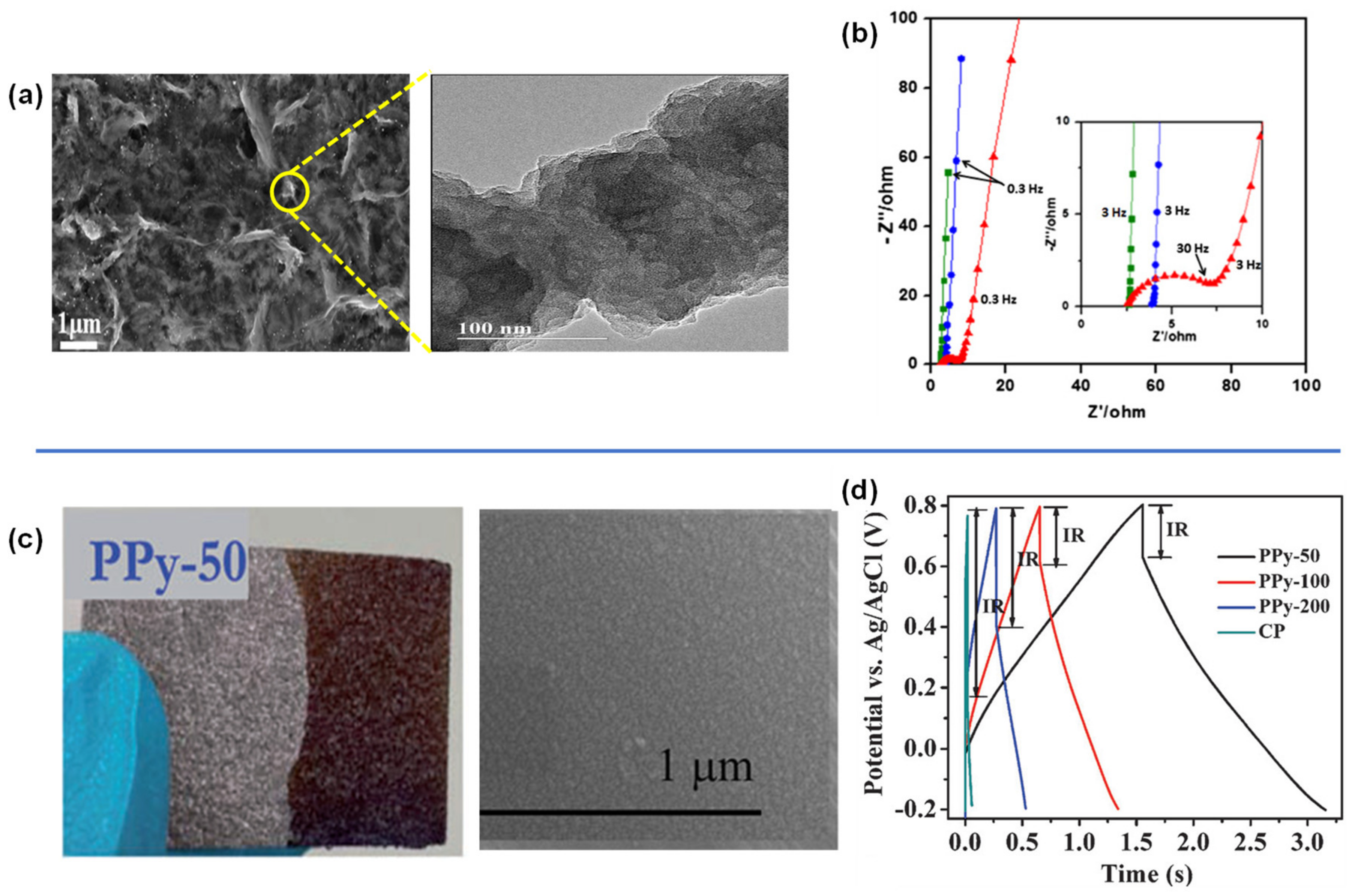
4.1. PANI-, PPy- and PEDOT-Carbon Composites
4.2. Redox Active Small Molecule-Carbon Composites
4.2.1. Phenylenediamine
4.2.2. Macrocycles (Porphyrin/Phthalocyanines)
4.3. Other Polymers
4.3.1. Pyrene Derivatives
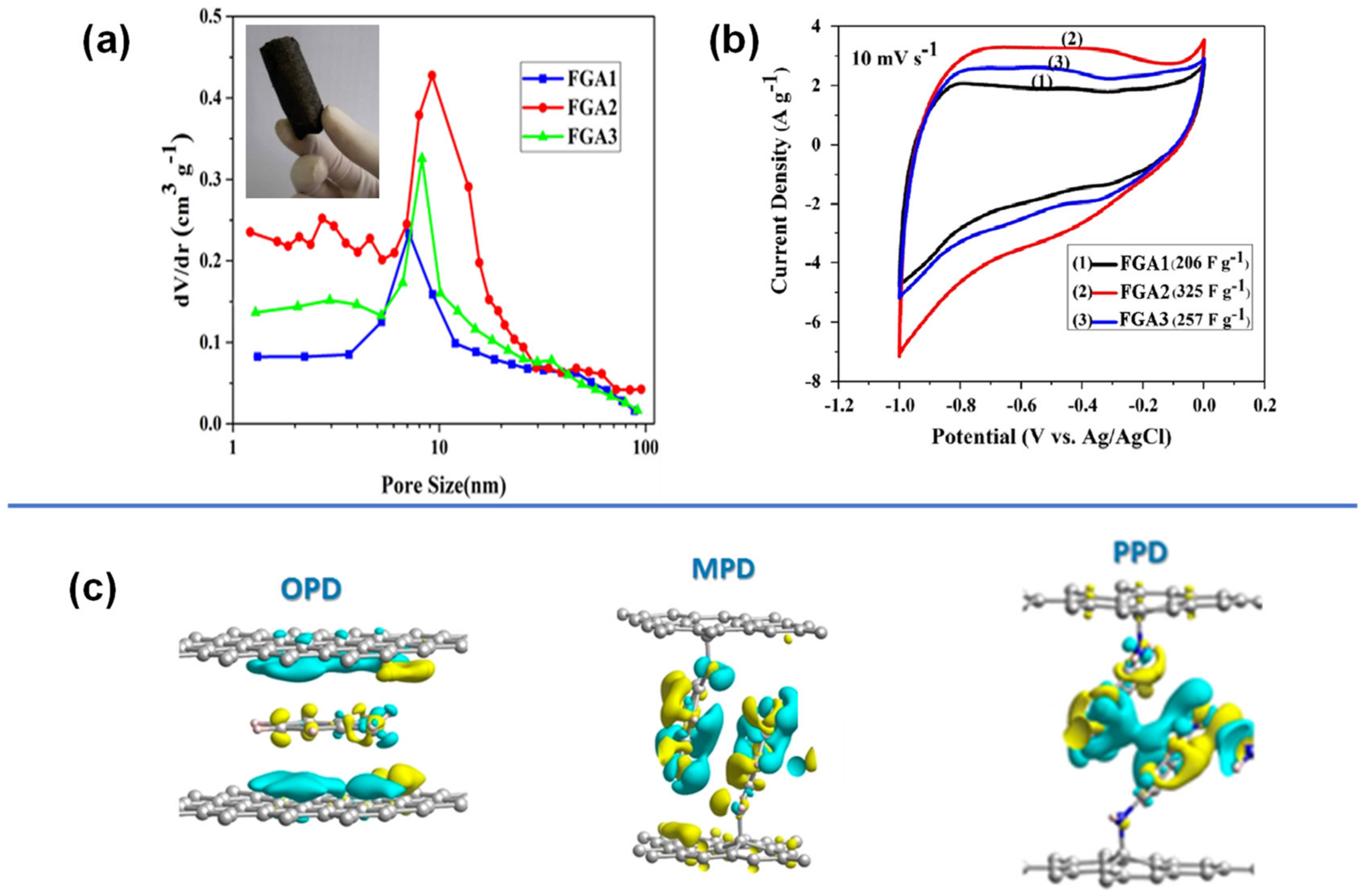
4.3.2. Poly-Phenylenediamine
4.3.3. Polyfuchsin and Polyluminol
4.4. Covalent Organic Frameworks (COF)
5. Perspectives and Conclusions
- Organic Materials
- Carbonaceous Substrates
- Computational Aspects
- Design and Fabrication of Composite Electrodes
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afif, A.; Rahman, S.M.H.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer: Boston, MA, USA, 1999; p. 685. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ge, Y.; Chao, Y.; Wang, C.; Wallace, G.G. Recent progress in 2D materials for flexible supercapacitors. J. Energy Chem. 2018, 27, 57–72. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “Supercapacitor” to “Battery” behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- Su, F.; Wu, Z.-S. A perspective on graphene for supercapacitors: Current status and future challenges. J. Energy Chem. 2021, 53, 354–357. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Li, Z.; Ma, Y.; Ma, L. Recent progress of biomass-derived carbon materials for supercapacitors. J. Power Sources 2020, 451, 227794. [Google Scholar] [CrossRef]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Kate, R.S.; Khalate, S.A.; Deokate, R.J. Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: A review. J. Alloys Compd. 2018, 734, 89–111. [Google Scholar] [CrossRef]
- Low, W.H.; Khiew, P.S.; Lim, S.S.; Siong, C.W.; Ezeigwe, E.R. Recent development of mixed transition metal oxide and graphene/mixed transition metal oxide based hybrid nanostructures for advanced supercapacitors. J. Alloys Compd. 2019, 775, 1324–1356. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincon, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical-Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Bakker, M.G.; Frazier, R.M.; Burkett, S.; Bara, J.E.; Chopra, N.; Spear, S.; Pan, S.; Xu, C. Perspectives on supercapacitors, pseudocapacitors and batteries. Nanomater. Energy 2012, 1, 136–158. [Google Scholar] [CrossRef]
- Niu, Z.; Liu, L.; Zhou, W.; Chen, X.; Xie, S. Carbon Nanotube-Based Thin Films for Flexible Supercapacitors. In Nanocarbons for Advanced Energy Storage; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 279–299. [Google Scholar] [CrossRef]
- Balli, B.; Şavk, A.; Şen, F. Graphene and polymer composites for supercapacitor applications. In Nanocarbon and Its Composites; Khan, A., Jawaid, M., Inamuddin Asiri, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 123–151. [Google Scholar] [CrossRef]
- Gao, Y. Graphene and Polymer Composites for Supercapacitor Applications: A Review. Nanoscale Res. Lett. 2017, 12, 387. [Google Scholar] [CrossRef]
- Magu, T.O.; Agobi, A.U.; Hitler, L.; Dass, P.M. A Review on Conducting Polymers-Based Composites for Energy Storage Application. J. Chem. Rev. 2019, 1, 19–34. [Google Scholar]
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano. 2010, 4, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards establishing standard performance metrics for batteries, supercapacitors and beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef]
- Taberna, P.L.; Simon, P.; Fauvarque, J.F. Electrochemical Characteristics and Impedance Spectroscopy Studies of Carbon-Carbon Supercapacitors. J. Electrochem. Soc. 2003, 150, A292. [Google Scholar] [CrossRef]
- Eftekhari, A. The mechanism of ultrafast supercapacitors. J. Mater. Chem. A 2018, 6, 2866–2876. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2(Anatase) Nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Cook, J.B.; Kim, H.-S.; Lin, T.C.; Lai, C.-H.; Dunn, B.; Tolbert, S.H. Pseudocapacitive Charge Storage in Thick Composite MoS2. Nanocrystal-Based Electrodes. Adv. Energy Mater. 2017, 7, 1601283. [Google Scholar] [CrossRef]
- Augustyn, V.; White, E.R.; Ko, J.; Grüner, G.; Regan, B.C.; Dunn, B. Lithium-ion storage properties of titanium oxide nanosheets. Mater. Horiz. 2014, 1, 219–223. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Kar, P. Classification of Dopants for the Conjugated Polymer. Doping Conjug. Polym. 2013, 19–46. [Google Scholar] [CrossRef]
- Kar, P. Role of Dopant on the Conduction of Conjugated Polymer. Doping Conjug. Polym. 2013, 63–79. [Google Scholar] [CrossRef]
- Chen, S.; Zhitomirsky, I. Influence of dopants and carbon nanotubes on polypyrrole electropolymerization and capacitive behavior. Mater. Lett. 2013, 98, 67–70. [Google Scholar] [CrossRef]
- Kar, P. Doping Techniques for the Conjugated Polymer. Doping Conjug. Polym. 2013, 47–62. [Google Scholar] [CrossRef]
- Bryan, A.M.; Santino, L.M.; Lu, Y.; Acharya, S.; D’Arcy, J.M. Conducting Polymers for Pseudocapacitive Energy Storage. Chem. Mater. 2016, 28, 5989–5998. [Google Scholar] [CrossRef]
- Cheng, M.; Meng, Y.N.; Wei, Z.X. Conducting Polymer Nanostructures and their Derivatives for Flexible Supercapacitors. Isr. J. Chem. 2018, 58, 1299–1314. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-Active Polymers for Energy Storage Nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; You, J.; Park, M.-S.; Hossain, M.S.A.; Yamauchi, Y.; Kim, J.H. Conductive polymers for next-generation energy storage systems: Recent progress and new functions. Mater. Horiz. 2016, 3, 517–535. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Chu, Q.; Wang, Z.; Zhang, F.; Wang, S. Theoretical and experimental specific capacitance of polyaniline in sulfuric acid. J. Power Sources 2009, 190, 578–586. [Google Scholar] [CrossRef]
- Peng, C.; Hu, D.; Chen, G.Z. Theoretical specific capacitance based on charge storage mechanisms of conducting polymers: Comment on ‘Vertically oriented arrays of polyaniline nanorods and their super electrochemical properties’. Chem. Commun. 2011, 47, 4105–4107. [Google Scholar] [CrossRef]
- Lota, K.; Khomenko, V.; Frackowiak, E. Capacitance properties of poly(3,4-ethylenedioxythiophene)/carbon nanotubes composites. J. Phys. Chem. Solids 2004, 65, 295–301. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y. Addressing the Achilles’ heel of pseudocapacitive materials: Long-term stability. InfoMat 2020, 2, 807–842. [Google Scholar] [CrossRef]
- Li, J.; Jing, X.; Li, Q.; Li, S.; Gao, X.; Feng, X.; Wang, B. Bulk COFs and COF nanosheets for electrochemical energy storage and conversion. Chem. Soc. Rev. 2020, 49, 3565–3604. [Google Scholar] [CrossRef]
- Bachman, J.C.; Kavian, R.; Graham, D.J.; Kim, D.Y.; Noda, S.; Nocera, D.G.; Shao-Horn, Y.; Lee, S.W. Electrochemical polymerization of pyrene derivatives on functionalized carbon nanotubes for pseudocapacitive electrodes. Nat. Commun. 2015, 6, 7040. [Google Scholar] [CrossRef]
- Ghosh, S.; An, X.; Shah, R.; Rawat, D.; Dave, B.; Kar, S.; Talapatra, S. Effect of 1-Pyrene Carboxylic-Acid Functionalization of Graphene on Its Capacitive Energy Storage. J. Phys. Chem. C 2012, 116, 20688–20693. [Google Scholar] [CrossRef]
- Huangfu, C.; Liu, Z.; Zhou, Q.; Wang, L.; Wei, T.; Qiu, Z.; Zhang, S.; Fan, Z. Covalent grafting of p-phenylenediamine molecules onto a “bubble-like” carbon surface for high performance asymmetric supercapacitors. J. Mater. Chem. A 2020, 8, 1767–1778. [Google Scholar] [CrossRef]
- Song, B.; Choi, J.I.; Zhu, Y.; Geng, Z.; Zhang, L.; Lin, Z.; Tuan, C.-C.; Moon, K.-S.; Wong, C.-P. Molecular Level Study of Graphene Networks Functionalized with Phenylenediamine Monomers for Supercapacitor Electrodes. Chem. Mater. 2016, 28, 9110–9121. [Google Scholar] [CrossRef]
- Yadegari, H.; Heli, H.; Jabbari, A. Graphene/poly(ortho-phenylenediamine) nanocomposite material for electrochemical supercapacitor. J. Solid State Electrochem. 2013, 17, 2203–2212. [Google Scholar] [CrossRef]
- Jaffe, A.; Saldivar Valdes, A.; Karunadasa, H.I. Quinone-Functionalized Carbon Black Cathodes for Lithium Batteries with High Power Densities. Chem. Mater. 2015, 27, 3568–3571. [Google Scholar] [CrossRef]
- Suematsu, S.; Naoi, K. Quinone-introduced oligomeric supramolecule for supercapacitor. J. Power Sources 2001, 97–98, 816–818. [Google Scholar] [CrossRef]
- Tabor, D.P.; Gómez-Bombarelli, R.; Tong, L.; Gordon, R.G.; Aziz, M.J.; Aspuru-Guzik, A. Mapping the frontiers of quinone stability in aqueous media: Implications for organic aqueous redox flow batteries. J. Mater. Chem. A 2019, 7, 12833–12841. [Google Scholar] [CrossRef]
- Gao, P.; Chen, Z.; Zhao-Karger, Z.; Mueller, J.E.; Jung, C.; Klyatskaya, S.; Diemant, T.; Fuhr, O.; Jacob, T.; Behm, R.J.; et al. A Porphyrin Complex as a Self-Conditioned Electrode Material for High-Performance Energy Storage. Angew. Chem. Int. Ed. Engl. 2017, 56, 10341–10346. [Google Scholar] [CrossRef] [PubMed]
- Agboola, B.O.; Ozoemena, K.I. Synergistic enhancement of supercapacitance upon integration of nickel (II) octa [(3,5-biscarboxylate)-phenoxy] phthalocyanine with SWCNT-phenylamine. J. Power Sources 2010, 195, 3841–3848. [Google Scholar] [CrossRef]
- Chidembo, A.T.; Ozoemena, K.I. Electrochemical Capacitive Behaviour of Multiwalled Carbon Nanotubes Modified with Electropolymeric Films of Nickel Tetraaminophthalocyanine. Electroanalysis 2010, 22, 2529–2535. [Google Scholar] [CrossRef]
- Chidembo, A.T.; Ozoemena, K.I.; Agboola, B.O.; Gupta, V.; Wildgoose, G.G.; Compton, R.G. Nickel(ii) tetra-aminophthalocyanine modified MWCNTs as potential nanocomposite materials for the development of supercapacitors. Energy Environ. Sci. 2010, 3, 228–236. [Google Scholar] [CrossRef]
- Navarro-Suárez, A.M.; Carretero-González, J.; Rojo, T.; Armand, M. Poly(quinone-amine)/nanocarbon composite electrodes with enhanced proton storage capacity. J. Mater. Chem. A 2017, 5, 23292–23298. [Google Scholar] [CrossRef]
- Fan, L.-Z.; Maier, J. High-performance polypyrrole electrode materials for redox supercapacitors. Electrochem. Commun. 2006, 8, 937–940. [Google Scholar] [CrossRef]
- Pognon, G.; Cougnon, C.; Mayilukila, D.; Belanger, D. Catechol-modified activated carbon prepared by the diazonium chemistry for application as active electrode material in electrochemical capacitor. ACS Appl. Mater. Interfaces 2012, 4, 3788–3796. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Li, S.; Yang, W.; Xu, J.; Jiang, Y.; Wen, J. Electrochemical performance of conducting polymer and its nanocomposites prepared by chemical vapor phase polymerization method. J. Mater. Sci. Mater. Electron. 2013, 24, 2245–2253. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.S. Conducting Polymers Directly Coated on Reduced Graphene Oxide Sheets as High-Performance Supercapacitor Electrodes. J. Phys. Chem. C 2012, 116, 5420–5426. [Google Scholar] [CrossRef]
- Dai, G.; Liu, Y.; Niu, Z.; He, P.; Zhao, Y.; Zhang, X.; Zhou, H. The Design of Quaternary Nitrogen Redox Center for High-Performance Organic Battery Materials. Matter 2019, 1, 945–958. [Google Scholar] [CrossRef]
- Ji, L.; Krummenacher, I.; Friedrich, A.; Lorbach, A.; Haehnel, M.; Edkins, K.; Braunschweig, H.; Marder, T.B. Synthesis, Photophysical, and Electrochemical Properties of Pyrenes Substituted with Donors or Acceptors at the 4- or 4,9-Positions. J. Org. Chem. 2018, 83, 3599–3606. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Martínez, M.; Antonio Bautista-Martínez, J.; Rivera, E. Thermal, Optical, Electrochemical Properties and Conductivity of Pyrene Monomers. Des. Monomers Polym. 2008, 11, 173–186. [Google Scholar] [CrossRef]
- Dominska, M.; Jackowska, K.; Krysinski, P.; Blanchard, G.J. Probing interfacial organization in surface monolayers using tethered pyrene. 1. Structural mediation of electron and proton access to adsorbates. J. Phys. Chem. B 2005, 109, 15812–15821. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.C.; Posey, V.A.; Gray, J.; May, R.; Reed, D.A.; Zhang, H.; Marbella, L.E.; Steigerwald, M.L.; Yang, Y.; Roy, X.; et al. High-performance organic pseudocapacitors via molecular contortion. Nat. Mater. 2021, 20, 1136–1141. [Google Scholar] [CrossRef]
- Guo, C.X.; Lei, Y.; Li, C.M. Porphyrin Functionalized Graphene for Sensitive Electrochemical Detection of Ultratrace Explosives. Electroanalysis 2011, 23, 885–893. [Google Scholar] [CrossRef]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Lu, H.P.; Lan, C.M.; Huang, Y.L.; Liang, Y.R.; Yen, W.N.; Liu, Y.C.; Lin, Y.S.; Diau, E.W.; Yeh, C.Y. Novel zinc porphyrin sensitizers for dye-sensitized solar cells: Synthesis and spectral, electrochemical, and photovoltaic properties. Chemistry 2009, 15, 1403–1412. [Google Scholar] [CrossRef]
- Schlettwein, D.; Jaeger, N.I.; Oekermann, T. Photoelectrochemical Reactions at Phthalocyanine Electrodes. In The Porphyrin Handbook; Smith, K.M., Guilard, R., Eds.; Academic Press: Amsterdam, The Netherlands, 2003; pp. 247–283. [Google Scholar] [CrossRef]
- Tanaka, T.; Osuka, A. Conjugated porphyrin arrays: Synthesis, properties and applications for functional materials. Chem. Soc. Rev. 2015, 44, 943–969. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Lei, J.; Ju, H. Noncovalent nanoassembly of porphyrin on single-walled carbon nanotubes for electrocatalytic reduction of nitric oxide and oxygen. Electrochem. Commun. 2008, 10, 766–769. [Google Scholar] [CrossRef]
- Wang, C.-L.; Lan, C.-M.; Hong, S.-H.; Wang, Y.-F.; Pan, T.-Y.; Chang, C.-W.; Kuo, H.-H.; Kuo, M.-Y.; Diau, E.W.-G.; Lin, C.-Y. Enveloping porphyrins for efficient dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 6933–6940. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, C.M.; Li, J.; Cui, X.; Gervasio, D. Template-Synthesized Cobalt Porphyrin/Polypyrrole Nanocomposite and Its Electrocatalysis for Oxygen Reduction in Neutral Medium. J. Phys. Chem. C 2007, 111, 11216–11222. [Google Scholar] [CrossRef]
- Arıcı, M.; Arıcan, D.; Uğur, A.L.; Erdoğmuş, A.; Koca, A. Electrochemical and spectroelectrochemical characterization of newly synthesized manganese, cobalt, iron and copper phthalocyanines. Electrochim. Acta 2013, 87, 554–566. [Google Scholar] [CrossRef]
- Liao, M.-S.; Scheiner, S. Electronic structure and bonding in metal porphyrins, metal=Fe, Co, Ni, Cu, Zn. J. Chem. Phys. 2002, 117, 205–219. [Google Scholar] [CrossRef]
- Koca, A. Spectroelectrochemistry of Phthalocyanines. In Electrochemistry of N4 Macrocyclic Metal Complexes; Zagal, J.H., Bedioui, F., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 135–200. [Google Scholar] [CrossRef]
- Castro-Cruz, H.M.; Arias-Aranda, L.R.; Farfán, N.; Xochitiotzi-Flores, E.; Macías-Ruvalcaba, N.A. Elucidating the Electroreduction Mechanism of the Monoprotonated Octaethylporphyrin. A Comparative Study with the Diprotonated Octaethyl- and meso-Tetraphenyl-porphyrins. J. Electrochem. Soc. 2020, 167, 155507. [Google Scholar] [CrossRef]
- Su, B.; Li, F.; Partovi-Nia, R.; Gros, C.; Barbe, J.-M.; Samec, Z.; Girault, H.H. Evidence of tetraphenylporphyrin monoacids by ion-transfer voltammetry at polarized liquid|liquid interfaces. Chem. Commun. 2008, 5037–5038. [Google Scholar] [CrossRef][Green Version]
- Langhus, D.L.; Wilson, G.S. Spectroelectrochemistry and cyclic voltammetry of the EE mechanism in a porphyrin diacid reduction. Anal. Chem. 1979, 51, 1139–1144. [Google Scholar] [CrossRef]
- Mohammed, A.K.; Vijayakumar, V.; Halder, A.; Ghosh, M.; Addicoat, M.; Bansode, U.; Kurungot, S.; Banerjee, R. Weak Intermolecular Interactions in Covalent Organic Framework-Carbon Nanofiber Based Crystalline yet Flexible Devices. ACS Appl. Mater. Interfaces 2019, 11, 30828–30837. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef] [PubMed]
- Le Comte, A.; Brousse, T.; Bélanger, D. Simpler and greener grafting method for improving the stability of anthraquinone-modified carbon electrode in alkaline media. Electrochim. Acta 2014, 137, 447–453. [Google Scholar] [CrossRef]
- Sun, B.; Liu, J.; Cao, A.; Song, W.; Wang, D. Interfacial synthesis of ordered and stable covalent organic frameworks on amino-functionalized carbon nanotubes with enhanced electrochemical performance. Chem. Commun. 2017, 53, 6303–6306. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Gu, W.; Yushin, G. Review of nanostructured carbon materials for electrochemical capacitor applications: Advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 424–473. [Google Scholar] [CrossRef]
- Hao, L.; Li, X.; Zhi, L. Carbonaceous Electrode Materials for Supercapacitors. Adv. Mater. 2013, 25, 3899–3904. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Li, Z.; Deng, Y.; Yang, Q.-H.; Kang, F. Graphene-based materials for electrochemical energy storage devices: Opportunities and challenges. Energy Storage 2016, 2, 107–138. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Recent advances in carbon-based supercapacitors. Mater. Adv. 2020, 1, 945–966. [Google Scholar] [CrossRef]
- Wang, J.-G.; Liu, H.; Zhang, X.; Shao, M.; Wei, B. Elaborate construction of N/S-co-doped carbon nanobowls for ultrahigh-power supercapacitors. J. Mater. Chem. A 2018, 6, 17653–17661. [Google Scholar] [CrossRef]
- Yang, L.; Wu, D.; Wang, T.; Jia, D. B/N-Codoped Carbon Nanosheets Derived from the Self-Assembly of Chitosan–Amino Acid Gels for Greatly Improved Supercapacitor Performances. ACS Appl. Mater. Interfaces 2020, 12, 18692–18704. [Google Scholar] [CrossRef]
- Tanguy, N.R.; N’Diaye, J.; Arjmand, M.; Lian, K.; Yan, N. Facile one-pot synthesis of water-dispersible phosphate functionalized reduced graphene oxide toward high-performance energy storage devices. Chem. Commun. 2020, 56, 1373–1376. [Google Scholar] [CrossRef]
- Xu, C.; Xu, B.; Gu, Y.; Xiong, Z.; Sun, J.; Zhao, X.S. Graphene-based electrodes for electrochemical energy storage. Energy Environ. Sci. 2013, 6, 1388–1414. [Google Scholar] [CrossRef]
- Tan, Y.B.; Lee, J.-M. Graphene for supercapacitor applications. J. Mater. Chem. A 2013, 1, 14814–14843. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, G. Self-Assembly of Graphene for Electrochemical Capacitors. In Nanocarbons for Advanced Energy Storage; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, G.; Zhu, Y. Carbon-Based Supercapacitors Produced by the Activation of Graphene. In Nanocarbons for Advanced Energy Storage; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 211–225. [Google Scholar] [CrossRef]
- Brisebois, P.P.; Siaj, M. Harvesting graphene oxide—Years 1859 to 2019: A review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Genovese, M.; Lian, K. Polyoxometalate modified pine cone biochar carbon for supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 3939–3947. [Google Scholar] [CrossRef]
- Genovese, M.; Wu, H.; Virya, A.; Li, J.; Shen, P.; Lian, K. Ultrathin all-solid-state supercapacitor devices based on chitosan activated carbon electrodes and polymer electrolytes. Electrochim. Acta 2018, 273, 392–401. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Weinstein, L.; Dash, R. Supercapacitor carbons. Mater. Today 2013, 16, 356–357. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Kharissova, O.V. General Data on Carbon Allotropes. In Carbon Allotropes: Metal-Complex Chemistry, Properties and Applications; Kharisov, B.I., Kharissova, O.V., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–8. [Google Scholar] [CrossRef]
- N’Diaye, J.; Hyun Chang, J.; Lian, K. The Capacitive Behavior of Polyluminol on Carbon Nanotubes Electrodes. ChemElectroChem 2019, 6, 5454–5461. [Google Scholar] [CrossRef]
- Wu, H.; Genovese, M.; Ton, K.; Lian, K. A Comparative Study of Activated Carbons from Liquid to Solid Polymer Electrolytes for Electrochemical Capacitors. J. Electrochem. Soc. 2019, 166, A821–A828. [Google Scholar] [CrossRef]
- Zhan, C.; Lian, C.; Zhang, Y.; Thompson, M.W.; Xie, Y.; Wu, J.; Kent, P.R.C.; Cummings, P.T.; Jiang, D.E.; Wesolowski, D.J. Computational Insights into Materials and Interfaces for Capacitive Energy Storage. Adv. Sci. (Weinh.) 2017, 4, 1700059. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Raimondo, M.; Naddeo, C.; Vertuccio, L.; Bonnaud, L.; Dubois, P.; Binder, W.H.; Sorrentino, A.; Guadagno, L. Multifunctionality of structural nanohybrids: The crucial role of carbon nanotube covalent and non-covalent functionalization in enabling high thermal, mechanical and self-healing performance. Nanotechnology 2020, 31, 225708. [Google Scholar] [CrossRef]
- Cho, Y.; Cho, W.J.; Youn, I.S.; Lee, G.; Singh, N.J.; Kim, K.S. Density functional theory based study of molecular interactions, recognition, engineering, and quantum transport in π molecular systems. Acc. Chem. Res. 2014, 47, 3321–3330. [Google Scholar] [CrossRef]
- Jonathan, W. Steed, J.L.A. Supramolecular Chemistry, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Sholl, D.S.; Steckel, J.A. Density Functional Theory; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–238. [Google Scholar] [CrossRef]
- Lazar, P.; Karlický, F.; Jurecka, P.; Kocman, M.; Otyepková, E.; Šafářová, K.; Otyepka, M. Adsorption of small organic molecules on graphene. J. Am. Chem. Soc. 2013, 135, 6372–6377. [Google Scholar] [CrossRef]
- Mollenhauer, D.; Brieger, C.; Voloshina, E.; Paulus, B. Performance of dispersion-corrected DFT for the weak interaction between aromatic molecules and extended carbon-based systems. J. Phys. Chem. C 2015, 119, 1898–1904. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, J.; Zhang, Y.; Wang, T. First-principles calculations of graphene-based polyaniline nano-hybrids for insight of electromagnetic properties and electronic structures. RSC Adv. 2016, 6, 73915–73923. [Google Scholar] [CrossRef]
- N’Diaye, J.; Elshazly, M.; Lian, K. Capacitive Charge Storage of Tetraphenylporphyrin Sulfonate- CNT Composite Electrodes. Electrochim. Acta 2021, 389, 138593. [Google Scholar] [CrossRef]
- Ahn, D.H.; Park, C.; Song, J.W. Predicting whether aromatic molecules would prefer to enter a carbon nanotube: A density functional theory study. J. Comput. Chem. 2020, 41, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Han, Y.-K. How can we describe the adsorption of quinones on activated carbon surfaces? Curr. Appl. Phys. 2016, 16, 1437–1441. [Google Scholar] [CrossRef]
- Mousavi-Khoshdel, M.; Targholi, E.; Momeni, M.J. First-Principles Calculation of Quantum Capacitance of Codoped Graphenes as Supercapacitor Electrodes. J. Phys. Chem. C 2015, 119, 26290–26295. [Google Scholar] [CrossRef]
- Özkaya, S.; Blaisten-Barojas, E. Polypyrrole on graphene: A density functional theory study. Surf. Sci. 2018, 674, 1–5. [Google Scholar] [CrossRef]
- Pykal, M.; Jurečka, P.; Karlický, F.; Otyepka, M. Modelling of graphene functionalization. Phys. Chem. Chem. Phys. 2016, 18, 6351–6372. [Google Scholar] [CrossRef]
- Ravikumar, B.; Mynam, M.; Rai, B. Molecular dynamics investigation of electric field altered behavior of lithium ion battery electrolytes. J. Mol. Liq. 2020, 300, 112252. [Google Scholar] [CrossRef]
- Rissanou, A.N.; Harmandaris, V. A molecular dynamics study of polymer/graphene nanocomposites. Macromol. Symp. 2013, 331–332, 43–49. [Google Scholar] [CrossRef]
- Yang, M.; Koutsos, V.; Zaiser, M. Interactions between polymers and carbon nanotubes: A molecular dynamics study. J. Phys. Chem. B 2005, 109, 10009–10014. [Google Scholar] [CrossRef] [PubMed]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF reactive force-field: Development, applications and future directions. NPJ Comput. Mater. 2016, 2, 15011. [Google Scholar] [CrossRef]
- Elbaz, Y.; Furman, D.; Caspary Toroker, M. Modeling Diffusion in Functional Materials: From Density Functional Theory to Artificial Intelligence. Adv. Funct. Mater. 2020, 30, 1–18. [Google Scholar] [CrossRef]
- Benda, R.; Zucchi, G.; Cancès, E.; Lebental, B. Insights into the π–π interaction driven non-covalent functionalization of carbon nanotubes of various diameters by conjugated fluorene and carbazole copolymers. J. Chem. Phys. 2020, 152, 064708. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Liu, W. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polym. Sci. 2017, 71, 1–25. [Google Scholar] [CrossRef]
- Boukhvalov, D.W. DFT modeling of the covalent functionalization of graphene: From ideal to realistic models. RSC Adv. 2013, 3, 7150–7159. [Google Scholar] [CrossRef]
- Berisha, A. Interactions between the aryldiazonium cations and graphene oxide: A DFT study. J. Chem. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Oliveira, O.N.; Aoki, P.H.B.; Pavinatto, F.J.; Constantino, C.J.L. Controlled Architectures in LbL Films for Sensing and Biosensing. In Multilayer Thin Films; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 951–983. [Google Scholar] [CrossRef]
- Mokhtari, A.; Harismah, K.; Mirzaei, M. Covalent addition of chitosan to graphene sheets: Density functional theory explorations of quadrupole coupling constants. Superlattices Microstruct. 2015, 88, 56–61. [Google Scholar] [CrossRef]
- Gabe, A.; Mostazo-López, M.J.; Salinas-Torres, D.; Morallón, E.; Cazorla-Amorós, D. Synthesis of conducting polymer/carbon material composites and their application in electrical energy storage. In Hybrid Polymer Composite Materials; Thakur, V.K., Thakur, M.K., Gupta, R.K., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 173–209. [Google Scholar] [CrossRef]
- Zhu, D.; Mo, D.; Ma, X.; Zhou, Q.; Liu, H.; Xu, J.; Zhou, W.; Zhao, F. Effect of polymerization solvent, potential, and temperature on morphology and capacitance properties of poly(thieno[3,2-b]thiophene) films. Synth. Met. 2016, 220, 155–161. [Google Scholar] [CrossRef]
- Nabilah Azman, N.H.; Lim, H.N.; Sulaiman, Y. Effect of electropolymerization potential on the preparation of PEDOT/graphene oxide hybrid material for supercapacitor application. Electrochim. Acta 2016, 188, 785–792. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Jiang, F.; Zhou, W.; Wang, R.; Xu, J.; Duan, X.; Wu, Y.; Ding, Y. Electrochemical assembly of homogenized poly(3,4-ethylenedioxythiophene methanol)/SWCNT nano-networks and their high performances for supercapacitor electrodes. Ionics 2020, 26, 3631–3642. [Google Scholar] [CrossRef]
- Branzoi, F.; Branzoi, V.; Musina, A. Coatings based on conducting polymers and functionalized carbon nanotubes obtained by electropolymerization. Prog. Org. Coat. 2013, 76, 632–638. [Google Scholar] [CrossRef]
- Si, K.; Guo, X.Q.; Qiu, K.Y. Initiation Mechanism of Radical Polymerization Using Ammonium Persulfate and Polymerizable Amine Redox Initiators. J. Macromol. Sci. Part A 1995, 32, 1149–1159. [Google Scholar] [CrossRef]
- Mart, H. Oxidative polycondensation reaction. Des. Monomers Polym. 2012, 9, 551–588. [Google Scholar] [CrossRef]
- Lindfors, T.; Ivaska, A. pH sensitivity of polyaniline and its substituted derivatives. J. Electroanal. Chem. 2002, 531, 43–52. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Soni, R.; Bhange, S.N.; Kurungot, S. Novel scalable synthesis of highly conducting and robust PEDOT paper for a high performance flexible solid supercapacitor. Energy Environ. Sci. 2015, 8, 1339–1347. [Google Scholar] [CrossRef]
- Choi, H.; Ahn, K.-J.; Lee, Y.; Noh, S.; Yoon, H. Free-Standing, Multilayered Graphene/Polyaniline-Glue/Graphene Nanostructures for Flexible, Solid-State Electrochemical Capacitor Application. Adv. Mater. Interfaces 2015, 2, 1500117. [Google Scholar] [CrossRef]
- Assresahegn, B.D.; Brousse, T.; Bélanger, D. Advances on the use of diazonium chemistry for functionalization of materials used in energy storage systems. Carbon 2015, 92, 362–381. [Google Scholar] [CrossRef]
- Bensghaïer, A.; Mousli, F.; Lamouri, A.; Postnikov, P.S.; Chehimi, M.M. The Molecular and Macromolecular Level of Carbon Nanotube Modification Via Diazonium Chemistry: Emphasis on the 2010s Years. Chem. Afr. 2020, 3, 535–569. [Google Scholar] [CrossRef]
- Liu, X.; Shang, P.; Zhang, Y.; Wang, X.; Fan, Z.; Wang, B.; Zheng, Y. Three-dimensional and stable polyaniline-grafted graphene hybrid materials for supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 15273–15278. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; He, X.; Mao, X.; Zhou, Y.; Xu, J.; Yang, Y. Modifying Reduced Graphene Oxide by Conducting Polymer Through a Hydrothermal Polymerization Method and its Application as Energy Storage Electrodes. Nanoscale Res. Lett 2019, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Kuhn, P.; Weber, J.; Titirici, M.M.; Antonietti, M. Porous polymers: Enabling solutions for energy applications. Macromol. Rapid Commun. 2009, 30, 221–236. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, T.; Liu, Q.; Wang, Y.; Xiao, D. Preparation of quinone modified graphene-based fiber electrodes and its application in flexible asymmetrical supercapacitor. Electrochim. Acta 2020, 336, 135628. [Google Scholar] [CrossRef]
- Lu, X.; Li, L.; Song, B.; Moon, K.-S.; Hu, N.; Liao, G.; Shi, T.; Wong, C. Mechanistic investigation of the graphene functionalization using p-phenylenediamine and its application for supercapacitors. Nano Energy 2015, 17, 160–170. [Google Scholar] [CrossRef]
- Park, Y.T.; Grunlan, J.C. Carbon Nanotube-Based Multilayers. In Multilayer Thin Films; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 595–612. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Howden, R.M.; Borrelli, D.C.; Gleason, K.K. Vapor phase oxidative synthesis of conjugated polymers and applications. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1329–1351. [Google Scholar] [CrossRef]
- Asatekin, A.; Barr, M.C.; Baxamusa, S.H.; Lau, K.K.S.; Tenhaeff, W.; Xu, J.; Gleason, K.K. Designing polymer surfaces via vapor deposition. Mater. Today 2010, 13, 26–33. [Google Scholar] [CrossRef]
- Li, Q.; Horn, M.; Wang, Y.; MacLeod, J.; Motta, N.; Liu, J. A Review of Supercapacitors Based on Graphene and Redox-Active Organic Materials. Materials 2019, 12, 703. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True Performance Metrics in Electrochemical Energy Storage. Science 2011, 334, 917. [Google Scholar] [CrossRef]
- Ramana, G.V.; Srikanth, V.V.S.S.; Padya, B.; Jain, P.K. Carbon nanotube–polyaniline nanotube core–shell structures for electrochemical applications. Eur. Polym. J. 2014, 57, 137–142. [Google Scholar] [CrossRef]
- Wang, P.H.; Wang, T.L.; Lin, W.C.; Lin, H.Y.; Lee, M.H.; Yang, C.H. Enhanced Supercapacitor Performance Using Electropolymerization of Self-Doped Polyaniline on Carbon Film. Nanomaterials 2018, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, F.; Chang, J.; Wu, D.; Wang, X.; Wang, X.; Xu, F.; Gao, S.; Jiang, K. Chemically grafted graphene-polyaniline composite for application in supercapacitor. Electrochim. Acta 2014, 133, 325–334. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chang, C.-K.; Tsai, Y.-C.; Liao, C.-S. Electrochemically synthesized graphene/polypyrrole composites and their use in supercapacitor. Carbon 2012, 50, 2331–2336. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Y.; Guo, J.; Yan, X.; O’Connor, R.; Zhang, X.; Shen, N.Z.; Weeks, B.L.; Huang, X.; Wei, S.; et al. Electropolymerized Polypyrrole Nanocoatings on Carbon Paper for Electrochemical Energy Storage. ChemElectroChem 2015, 2, 119–126. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Wang, Y.; Pan, Q.; Gong, Q.; Xia, Z.; Li, Y. Remarkably enhanced performances of novel polythiophene-grafting-graphene oxide composite via long alkoxy linkage for supercapacitor application. Carbon 2019, 147, 519–531. [Google Scholar] [CrossRef]
- Teng, H.; Song, J.; Xu, G.; Gao, F.; Luo, X. Nitrogen-doped graphene and conducting polymer PEDOT hybrids for flexible supercapacitor and electrochemical sensor. Electrochim. Acta 2020, 355, 136772. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, H.; Huang, Z.; Wang, W.; Zeng, F.; Kuang, Y. Graphene covalently functionalized with poly(p-phenylenediamine) as high performance electrode material for supercapacitors. J. Mater. Chem. A 2013, 1, 3454–3462. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, H.; Huang, Z.; Li, H.; Fu, C.; Chen, L.; Li, M.; Liu, S.; Kuang, Y. In situ growth of single-stranded like poly (o-phenylenediamine) onto graphene for high performance supercapacitors. Electrochim. Acta 2017, 245, 41–50. [Google Scholar] [CrossRef]
- N’Diaye, J.; Lian, K. Polymerized fuchsin and modified carbon nanotube electrodes for electrochemical capacitors. Nano-Struct. Nano-Objects 2018, 15, 173–179. [Google Scholar] [CrossRef]
- Gholipour-Ranjbar, H.; Ganjali, M.R.; Norouzi, P.; Naderi, H.R. Electrochemical investigation of functionalized graphene aerogel with different amount of p-phenylenediamine as an advanced electrode material for supercapacitors. Mater. Res. Express 2016, 3, 075501. [Google Scholar] [CrossRef]
- Bello, A.; Fabiane, M.; Momodu, D.Y.; Khamlich, S.; Dangbegnon, J.K.; Manyala, N. Functionalized graphene foam as electrode for improved electrochemical storage. J. Solid State Electrochem. 2014, 18, 2359–2365. [Google Scholar] [CrossRef]
- Manjunatha, N.; Imadadulla, M.; Lokesh, K.S.; Venugopala Reddy, K.R. Synthesis and electropolymerization of tetra-[β-(2-benzimidazole)] and tetra-[β-(2-(1-(4-aminophenyl))benzimidazole)] embedded cobalt phthalocyanine and their supercapacitance behaviour. Dyes Pigments 2018, 153, 213–224. [Google Scholar] [CrossRef]
- Sun, J.; Klechikov, A.; Moise, C.; Prodana, M.; Enachescu, M.; Talyzin, A.V. A Molecular Pillar Approach to Grow Vertical Covalent Organic Framework Nanosheets on Graphene: Hybrid Materials for Energy Storage. Angew. Chem. Int. Ed. 2018, 57, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Basnayaka, P.A.; Ram, M.K. A Review of Supercapacitor Energy Storage Using Nanohybrid Conducting Polymers and Carbon Electrode Materials. In Conducting Polymer Hybrids; Kumar, V., Kalia, S., Swart, H.C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 165–192. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Gul, H.; Shah, A.-u.-H.A.; Bilal, S. Fabrication of Eco-Friendly Solid-State Symmetric Ultracapacitor Device Based on Co-Doped PANI/GO Composite. Polymers 2019, 11, 1315. [Google Scholar] [CrossRef]
- Gul, H.; Shah, A.-u.-H.A.; Krewer, U.; Bilal, S. Study on Direct Synthesis of Energy Efficient Multifunctional Polyaniline–Graphene Oxide Nanocomposite and Its Application in Aqueous Symmetric Supercapacitor Devices. Nanomaterials 2020, 10, 118. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Wang, J.; Wang, J.; Bai, Y.; Du, X. Morphology controllable nano-sheet polypyrrole–graphene composites for high-rate supercapacitor. Phys. Chem. Chem. Phys. 2015, 17, 19885–19894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Li, H.; Huang, Y.; Zhi, C. Polymers for supercapacitors: Boosting the development of the flexible and wearable energy storage. Mater. Sci. Eng. R Rep. 2020, 139, 100520. [Google Scholar] [CrossRef]
- Rogers, R.E.; Bardsley, T.I.; Weinstein, S.J.; Landi, B.J. Solution-phase adsorption of 1-pyrenebutyric acid using single-wall carbon nanotubes. Chem. Eng. J. 2011, 173, 486–493. [Google Scholar] [CrossRef]
- Velasquez, J.D.; Tomczykowa, M.; Plonska-Brzezinska, M.E.; Chaur, M.N. Evaluation of the Covalent Functionalization of Carbon Nano-Onions with Pyrene Moieties for Supercapacitor Applications. Materials 2020, 13, 1141. [Google Scholar] [CrossRef]
- Putri, A.D.; Chotimah, N.; Ujjain, S.K.; Wang, S.; Futamura, R.; Vallejos-Burgos, F.; Khoerunnisa, F.; Morimoto, M.; Wang, Z.; Hattori, Y.; et al. Charge-transfer mediated nanopore-controlled pyrene derivatives/graphene colloids. Carbon 2018, 139, 512–521. [Google Scholar] [CrossRef]
- Wu, X.; Hong, J.J.; Shin, W.; Ma, L.; Liu, T.; Bi, X.; Yuan, Y.; Qi, Y.; Surta, T.W.; Huang, W.; et al. Diffusion-free Grotthuss topochemistry for high-rate and long-life proton batteries. Nat. Energy 2019, 4, 123–130. [Google Scholar] [CrossRef]
- Miyake, T.; Rolandi, M. Grotthuss mechanisms: From proton transport in proton wires to bioprotonic devices. J. Phys. Condens. Matter 2015, 28, 023001. [Google Scholar] [CrossRef]
- Mazari, S.A.; Ali, E.; Abro, R.; Khan, F.S.A.; Ahmed, I.; Ahmed, M.; Nizamuddin, S.; Siddiqui, T.H.; Hossain, N.; Mubarak, N.M.; et al. Nanomaterials: Applications, waste-handling, environmental toxicities, and future challenges—A review. J. Environ. Chem. Eng. 2021, 9, 105028. [Google Scholar] [CrossRef]
- Doan, H.A.; Agarwal, G.; Qian, H.; Counihan, M.J.; Rodríguez-López, J.; Moore, J.S.; Assary, R.S. Quantum Chemistry-Informed Active Learning to Accelerate the Design and Discovery of Sustainable Energy Storage Materials. Chem. Mater. 2020, 32, 6338–6346. [Google Scholar] [CrossRef]
- Schon, T.B.; McAllister, B.T.; Li, P.F.; Seferos, D.S. The rise of organic electrode materials for energy storage. Chem. Soc. Rev. 2016, 45, 6345–6404. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Li, C.; Yang, H.; Ostrikov, K.; Yan, J.; Cen, K. Design of Supercapacitor Electrodes Using Molecular Dynamics Simulations. Nano-Micro Lett. 2018, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, J.; Siddiqui, S.; Pak, K.L.; Lian, K. Layer-by-layer assembly of inorganic–organic molybdovanadogermanic (GeMoV)-polyluminol composite electrodes for capacitive charge storage. J. Mater. Chem. A 2020, 8, 23463–23472. [Google Scholar] [CrossRef]




| Structures | Redox Mechanism | Examples | References |
|---|---|---|---|
| Amine | 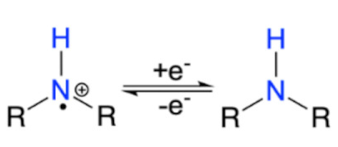 | PANI, PPy | [39,58] |
| Carbonyl | 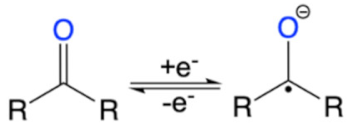 | Quinone | [59] |
| Thioether | 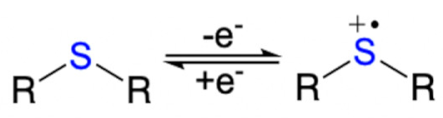 | PEDOT | [60,61] |
| Nitroxyl radical | 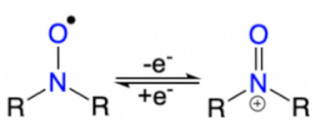 | [62] |
| Materials | Fabrication Method | Type of Bonding | Thickness Coating (nm) | Electrolyte | Capacitance | Stability | Ref. |
|---|---|---|---|---|---|---|---|
| CNT-PANI on GCE | In-situ polymerization | Chemical interaction | 10–25 | 0.5 M H2SO4 vs. Ag/AgCl | CV: 354.3 F/g, GCD: 368.4 F/g | - | [157] |
| AN/SAN-CP (1:1) | Electro-polymerization | - | 30 | 1 M H2SO4 vs. Ag/AgCl | 273.3 F/g (0.5 A/g) | 96.1% (5000 cycles, 2 A/g) | [158] |
| PANI-Graphene | Chemical grafting | Covalent | - | 1 M H2SO4 vs. Ag/AgCl | 482.8 F/g (50 mV/s) | - | [159] |
| PPy-rGO | Electro-polymerization | - | 830 | 1 M H2SO4 vs. Ag/AgCl | 424 F/g (1 A/g) | - | [160] |
| PPy-CP-50 | Electro-polymerization | - | - | 1 M H2SO4 vs. Ag/AgCl | 13.42 mF/cm2 | 51.3% (3 mA/cm2, 1000 cycles) | [161] |
| PPy-CP-100 | 7.2 mF/cm2 | 59.3% (3 mA/cm2, 1000 cycles) | |||||
| PPy-CP-200 | 3.58 mF/cm2 | 63.5% (1000 cycles, 3 mA/cm2) | |||||
| PD4ET-g-GO | Chemical grafting | Covalent | - | 1 M KOH vs. Ag/AgCl | 561 F/g (5 mV/s) | 98% (10,000 cycles, 20 A/g) | [162] |
| NG/PEDOT | Solution Mixing | π-π stacking | - | 1. M H2SO4 vs. Ag/AgCl | 536 F/g (0.5 A/g) | 100% (10,000, 10 A/g) | [163] |
| poly-p-PD-rGO | Solvothermal | Covalent | - | 0.5 M H2SO4 vs. SCE | 342 F/g (100 mV/s), 347 F/g (0.5 A/g) | 90.1% (1000 cycles, 10 A/g,) | [164] |
| poly-o-PD-rGO | Hydrothermal | - | - | 2. M H2SO4 vs. Ag/AgCl | 515 mF/cm2 (1.6 mA/cm2), 308.3 F/g (1 A/g) | 99% (1500 cycles, 1 A/g) | [49] |
| poly-o-PD-rGO | In-situ polymerization | Covalent | 2–4 (dots) | 1. M H2SO4 vs. Ag/AgCl | 381 F/g (1 A/g) | 100% (5000 cycles, 250 mV/s) | [165] |
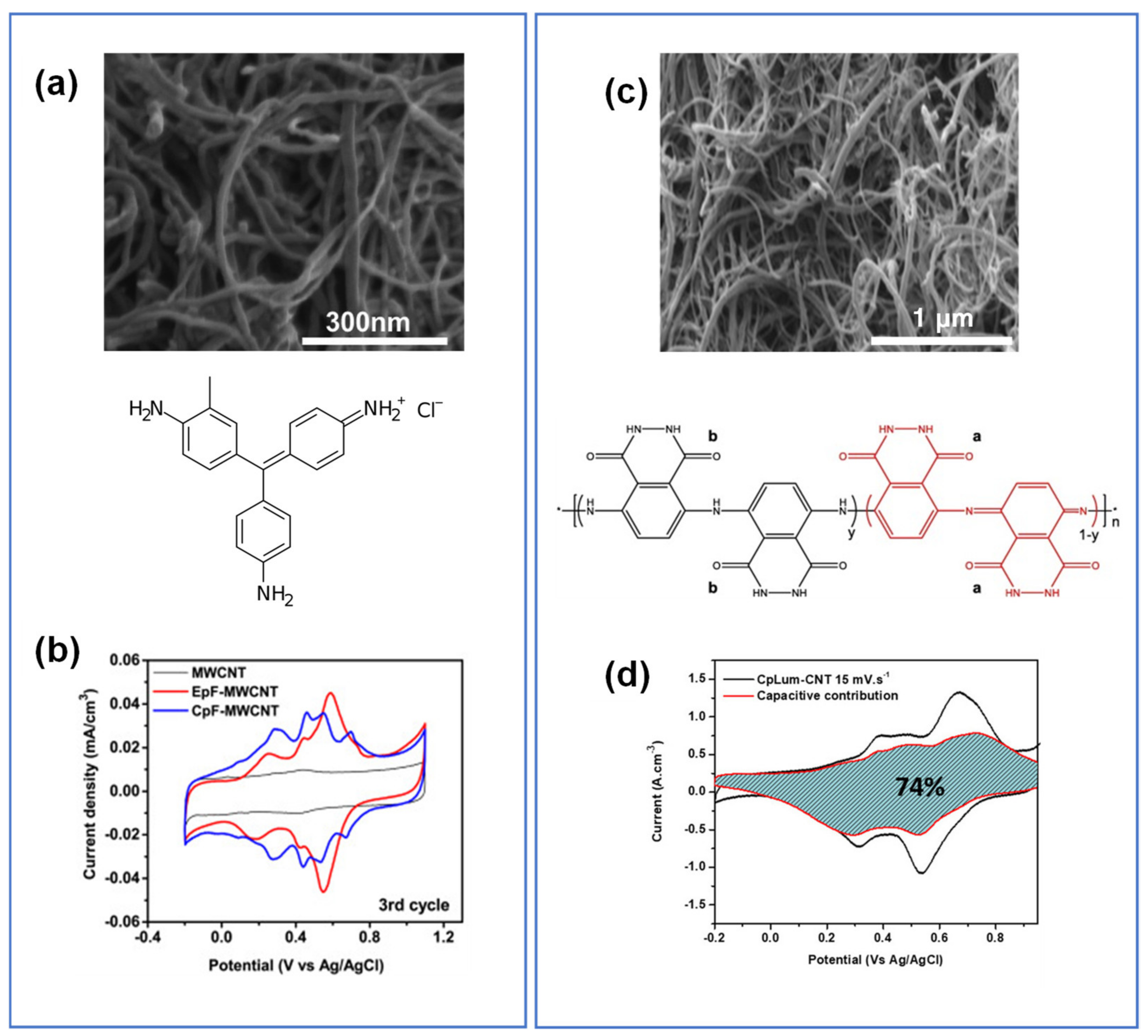
| CpF-CNT | Dip coating | π-π stacking | 1–2 | 0.5 M H2SO4 vs. Ag/AgCl | 30 F/cm3 (50 mV/s) | 95% (5000 cycles, 1 V/s) | [166] |
| EpF-CNT | Electro-polymerization | 1–2 | 32 F/cm3 (50 mV/s) | ||||
| CpLum-CNT | In-situ polymerization | π-π stacking | 4.5 | 1 M H2SO4 vs. Ag/AgCl | 48 F/cm3 (100 mV/s) | 95% (4000 cycles, 1 V/s) | [107] |
| GPDH-rGO | Hydrothermal | Covalent | - | 2 M H2SO4 | CV: 316 F/g at 10 mV/s, GCD: 303.88 F/g at 0.5 A/g | 93.66% (4000 cycles, 2 A/g) | [151] |
| GHPD-rGO | Hydrothermal | - | CV: 249 F/g at 10 mV/s, GCD: 231 F/g at 0.5 A/g | 87.14% (4000 cycles, 2 A/g) | |||
| o-PD-graphene | Hydrothermal | π-π stacking | - | 1 M H2SO4 vs. Ag/AgCl | 367 F/g at 50 mV/s | 83% (10,000 cycles, 2 A/g) | [48] |
| m-PD-graphene | Covalent | - | 372 F/g at 50 mV/s | 89% (10,000 cycles, 2 A/g) | |||
| p-PD-graphene | - | 473 F/g at 50 mV/s | |||||
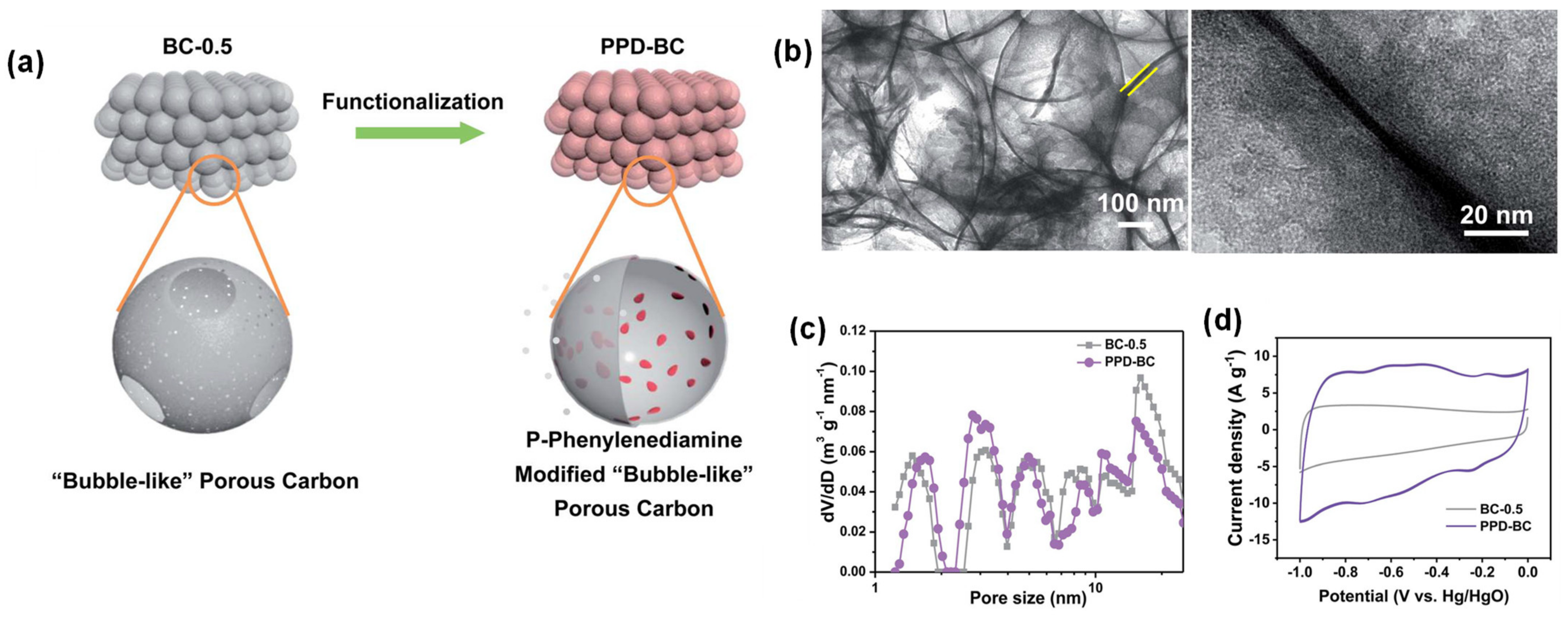
| p-PD-BC | Solvothermal | Covalent (CO–NH) | 4 | 2 M KOH vs. Hg/HgO | 451 F/g at 2 mV/s | 92% (5000 cycles, 1 00 mV/s) | [47] |
| 1 M H2SO4 vs. SCE | 442 F/g, 2 mV/s | - | |||||
| p-PD-FGA1 | Hydrothermal | Covalent bonding | - | 6 M KOH vs. Ag/AgCl | 206 F/g at 10 mV/s | 94% (5000 cycles, 250 mV s−1) | [167] |
| p-PD-FGA2 | 325 F/g at 10 mV/s | - | |||||
| p-PD-FGA3 | 257 F/g at 10 mV/s | 98% (5000 cycles, 250 mV s−1) | |||||
| 1-pyrenebutyric acid-MWCNT | Electrophoretic deposition | π-π stacking | 150 | 0.5 M Na2SO4 vs. Ag/AgCl | 125 F/g at 50 mV/s | - | [168] |
| pyrene -FWCNT | Electro-polymerization | π-π stacking | ˂ 5 | EC:DMC with 1 M LiPF6 vs. Li/Li+ | 60 F/g at 1 mV/s | - | [45] |
| COOH-pyrene-FWCNT | π-π stacking | 113 F/g at 1 mV/s | |||||
| NH2-pyrene-FWCNT | Electrostatic | 210 F/g at 1 mV/s | |||||
| Ni-Pc-CNT | - | Covalent (amide), π-π stacking | - | 1 M Na2SO4 vs. Ag/AgCl | 186 mF/cm2 at 138 µA/cm2 | 95% (1000 cycles) | [54] |
| Ni tetra NH-Pc-MWCNT | Direct mixing 1:1 ratio | π-π stacking | 50–100 Nano aggregates | 1 M H2SO4 SCE | 981 ± 57 F/g at 1 A/g | 83% (1500 cycles, 1 mA. cm−2) | [56] |
| Nickel Tetra-NH-Pc-CNT | Electro-polymreization | - | 100 | 1 M H2SO4 vs. Ag/AgCl | 673.2 F/g at 50 mV/s | negligeable (1500 cycles at 10 mA. cm−2) | [55] |
| Nickel Tetra-NH-Pc- OH-CNT | Electro-polymerization | 391.6 F/g at 50 mV/s | - | ||||
| Co-p-TBIm-Pc- rGO | Electro-polymerization | π-π stacking | - | 0.5 M H2SO4 vs. Ag/AgCl | 25.95 F/g at 5 mV/s | - | [169] |
| Co-p-TAPBIm-Pc-rGO | π-π stacking | - | 34.91 F/g at 5 mV/s | - | |||
| TPPS-CNT | Direct deposition | π-π stacking | 1.9 | 1 M H2SO4 vs. Ag/AgCl | 26.3 ± 1.3 F/cm3, 100 mV/s | 97% (10,000 cycles, 1000 mV/s) | [118] |
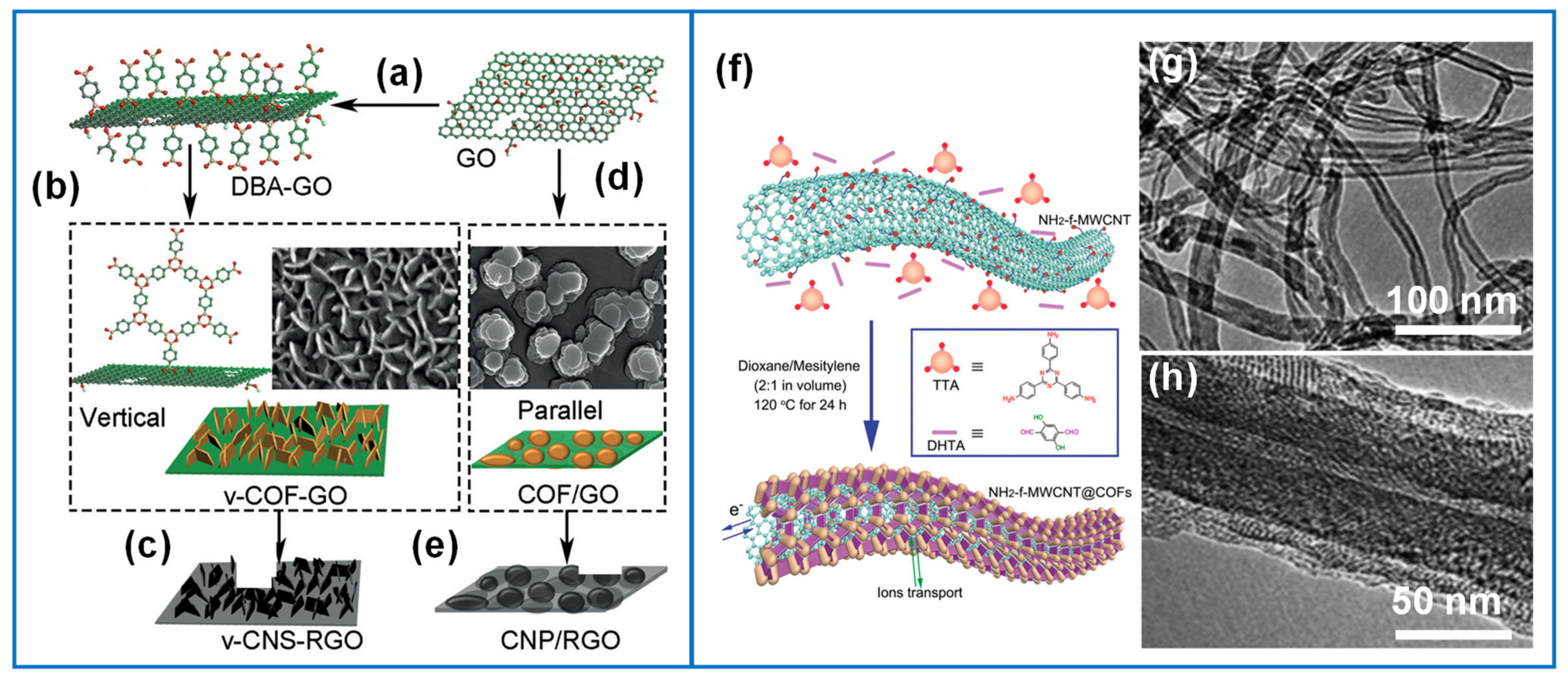
| COFTTA-DHTA-NH2-MWCNT | Solvothermal | Covalent (TTA-DHTA)π-π stacking | 3–15 | 1 M Na2SO4 vs. Ag/AgCl | - | 96% (1000 cycles) | [84] |
| V-CNS-GO | Solvothermal | Covalent | 13.6 ± 4.2 | 6 M KOH | 160 F/g at 1 A/g | 100% (3000 cycles, 10 A g−1) | [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
N’Diaye, J.; Bagchi, R.; Howe, J.Y.; Lian, K. Redox Active Organic-Carbon Composites for Capacitive Electrodes: A Review. Sustain. Chem. 2021, 2, 407-440. https://doi.org/10.3390/suschem2030024
N’Diaye J, Bagchi R, Howe JY, Lian K. Redox Active Organic-Carbon Composites for Capacitive Electrodes: A Review. Sustainable Chemistry. 2021; 2(3):407-440. https://doi.org/10.3390/suschem2030024
Chicago/Turabian StyleN’Diaye, Jeanne, Raunaq Bagchi, Jane Y. Howe, and Keryn Lian. 2021. "Redox Active Organic-Carbon Composites for Capacitive Electrodes: A Review" Sustainable Chemistry 2, no. 3: 407-440. https://doi.org/10.3390/suschem2030024
APA StyleN’Diaye, J., Bagchi, R., Howe, J. Y., & Lian, K. (2021). Redox Active Organic-Carbon Composites for Capacitive Electrodes: A Review. Sustainable Chemistry, 2(3), 407-440. https://doi.org/10.3390/suschem2030024






