Grape Infusions: Between Nutraceutical and Green Chemistry
Abstract
:1. Introduction
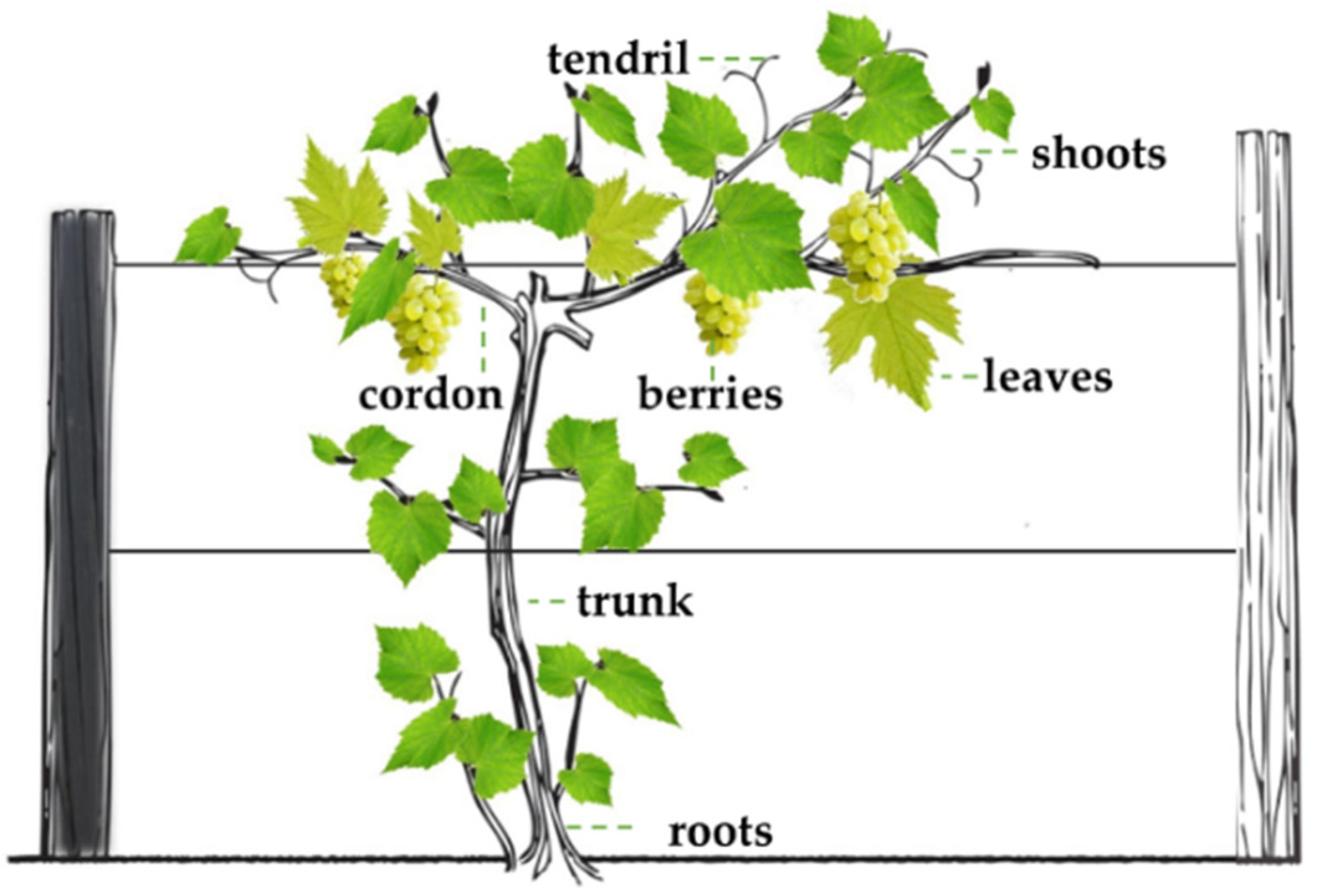
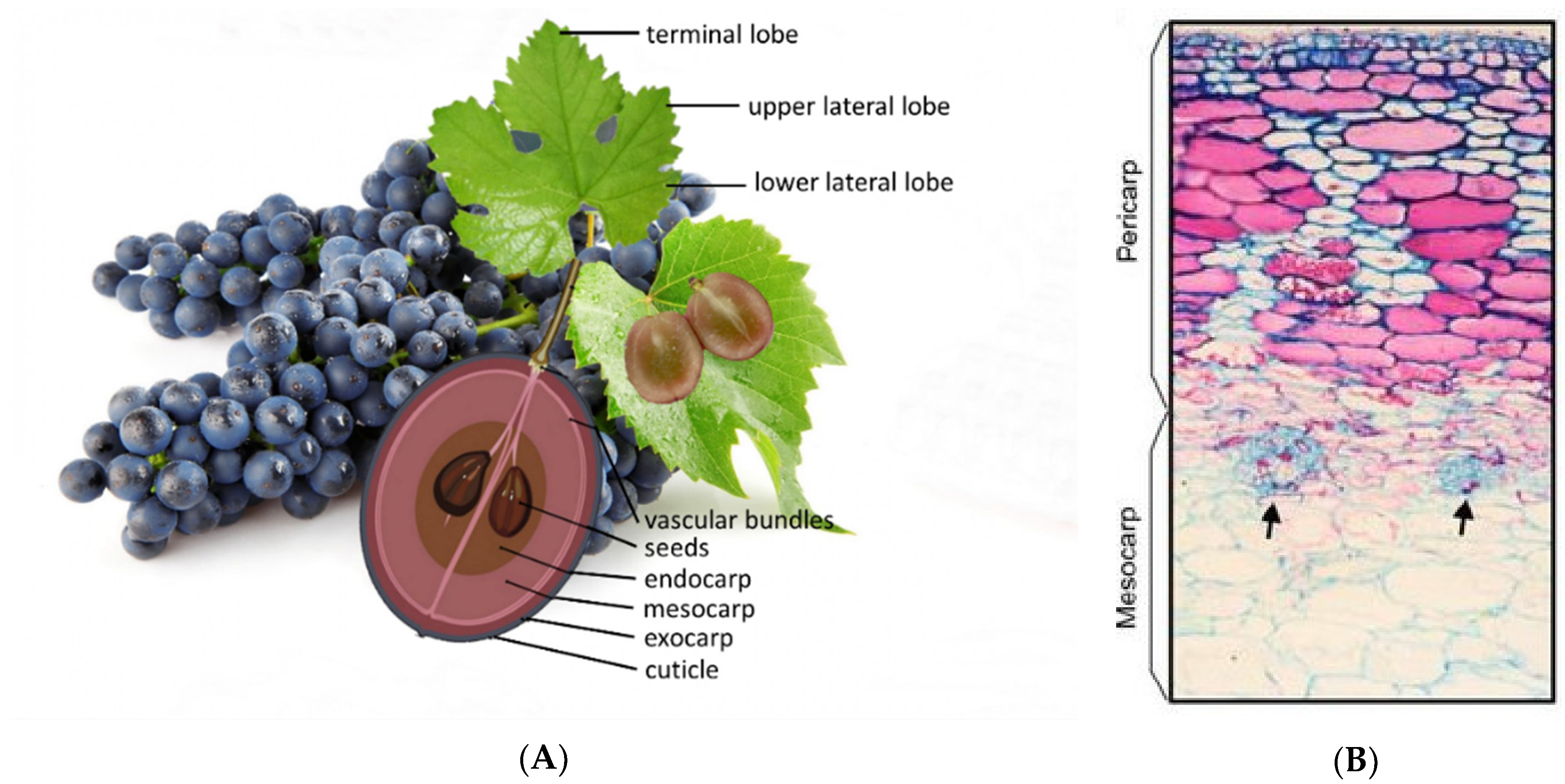
1.1. Grape Berry
- Exocarp—includes the cuticle (a waxy layer), epidermis, and hypodermis, consisting of 6 to 8 layers of cells smaller in size than the mesocarp cells [8]. However, between 5 to 18% of the fresh weight of the berry is attributed to the skin [14,15]. The epidermis is formed by tangentially elongated cells, one or two layers. The hypodermis can represent four to five layers of cells, in which the outermost layers of the cells have a rectangular shape, as opposed to the innermost layers in which the cells are polygonal [8,16].
- Mesocarp (Figure 2B)—rich in anthocyanins in red cultivars, occupies between 85–87% of the grape volume. Is made up of rounder and polygonal cells, with thin cell walls, very vacuolized [9,17]. It is exactly in these organelles, the vacuoles, where it is possible during the ripening of the grape to find sugars, organic acids, water, and ions [13].
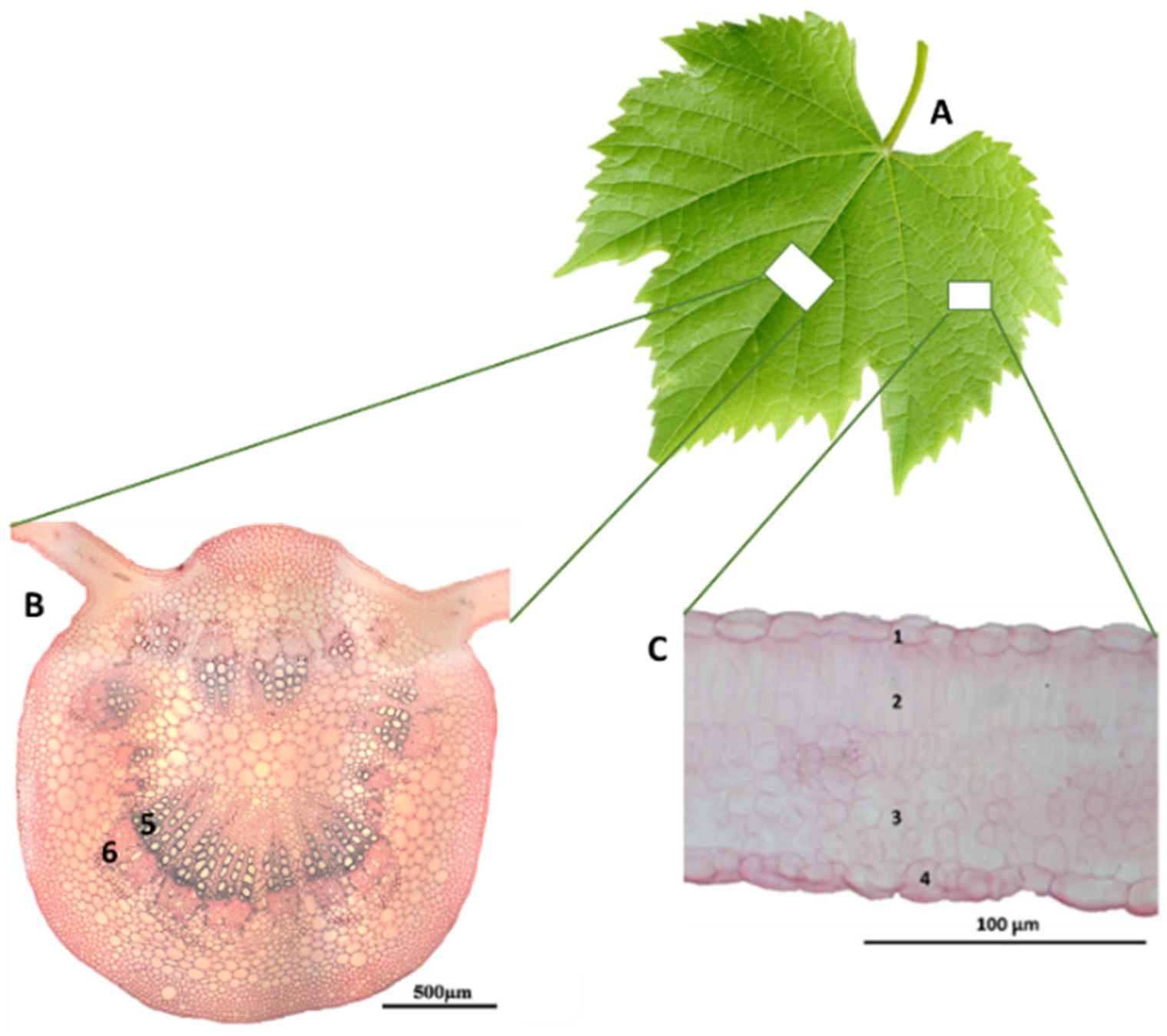
1.2. Grape Leaves
2. Nutraceutical Compounds of Grape Berries and Grape-Leaves
- Sugars such as glucose, fructose, and sucrose.
- Organic acids such as malic, tartaric, and citric acid.
- Aroma precursors, which could be volatile and non-volatile.
3. Ways of Extracting Nutraceutical Compounds from Grapes and Grapes by-Products
3.1. Ultrasound (UAE) and Microwave (MAE)—Assisted Extraction
3.2. Supercritical Fluid Extraction (SFE) and Pressurized Liquid Extraction (PLE)
3.3. Ionic Liquid (ILs) Solvents, Deep Eutectic Solvents (DESs), and Natural Eutectic Solvents (NADESs)
3.4. Enzyme-Assisted Extraction (EAE)
4. Grape Infusions as a Way of Extracting Nutraceutical and Antimicrobial Compounds
5. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grillo, G.; Boffa, L.; Talarico, S.; Solarino, R.; Binello, A.; Cavaglià, G.; Bensaid, S.; Telysheva, G.; Cravotto, G. Batch and Flow Ultrasound-Assisted Extraction of Grape Stalks: Process Intensification Design up to a Multi-Kilo Scale. Antioxidants 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Vivier, M.A.; Pretorius, I.S. Genetic Improvement of Grapevine: Tailoring Grape Varieties for the Third Millennium—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 5–26. [Google Scholar] [CrossRef] [Green Version]
- Dani, C.; Oliboni, L.S.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.A.; Salvador, M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol. In Vitro 2010, 24, 148–153. [Google Scholar] [CrossRef] [PubMed]
- OIV, International Organisation of Vine and Wine Intergovernmental Organisation. Statistical Report on World Vitiviniculture. 2019. Available online: https://www.oiv.int/en/oiv-life/oiv-2019-report-on-the-world-vitivinicultural-situation (accessed on 4 June 2021).
- Torregrosa, L.; Vialet, S.; Adivèze, A.; Iocco-Corena, P.; Thomas, M.R. Grapevine (Vitis vinifera L.). Methods Mol. Biol. 2015, 1224, 177–194. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science Principles and Applications, 4th ed.; Academic Press: New York, NY, USA, 2014; ISBN 9780123814692. [Google Scholar]
- Cosme, F.; Pinto, T.; Vilela, A. Oenology in the Kitchen: The Sensory Experience Offered by Culinary Dishes Cooked with Alcoholic Drinks, Grapes, and Grape Leaves. Beverages 2017, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages 2018, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, N. Viticulture Treaty: The Vine, the Vineyard and the Terroir; Chaves Ferreira Publications: Lisboa, Portugal, 2008; ISBN 9728987153. [Google Scholar]
- Keller, M. The Science of Grapevines: Anatomy and Physiology, 3rd ed.; Academic Press: London, UK, 2020; ISBN 978-0-12-816365-8. [Google Scholar]
- Zhang, X.Y.; Wang, X.L.; Wang, X.F.; Xia, G.H.; Pan, Q.H.; Fan, R.F.; Wu, F.Q.; Yu, X.C.; Zhang, P. A Shift of Phloem Unloading from Symplasmic to Apoplasmic Pathway Is Involved in Developmental Onset of Ripening in Grape Berry. Plant Physiol. 2006, 142, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Kuang, L.; Chen, S.; Guo, Y.; Ma, H. Quantitative Proteome Analysis Reveals Changes in the Protein Landscape During Grape Berry Development with a Focus on Vacuolar Transport Proteins. Front. Plant Sci. 2019, 10, 641. [Google Scholar] [CrossRef]
- Fontes, N.; Gerós, H.; Delrot, S. Grape Berry Vacuole: A Complex and Heterogeneous Membrane System Specialized in the Accumulation of Solutes. Am. J. Enol. Vitic. 2011, 62, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.; Strauss, C.R.; Williams, P.J. The distribution of free and glycosidically-bound monoterpenes among skin, juice, and pulp fractions of some white grape varieties. Am. J. Enol. Vitic. 1986, 37, 107–111. [Google Scholar]
- Schlosser, J.N.; Olsson, M.; Weis, K.; Reid, F.; Peng, S.; Lund, P.B. Expansão celular e expressão gênica na uva em desenvolvimento (Vitis vinifera L.). Protoplasma 2008, 232, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. The Microbiology of Wine and Vinifications. In Handbook of Enology, 1st ed.; Wiley: Chichester, UK, 2000; Volume 1. [Google Scholar]
- Hardie, W.J.; O’Brien, T.P.; Jaudzems, V.G. Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust. J. Grape Wine Res. 1996, 2, 97–142. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Güler, A.; Candemir, A. Total Phenolic and Flavonoid Contents, Phenolic Compositions, and Color Properties of Fresh Grape Leaves. Türk Tarım Doğa Bilim. Derg. 2014, 6, 778–782. [Google Scholar]
- Dogan, Y.; Nedelcheva, A.; Łuczaj, Ł.; Drăgulescu, C.; Stefkov, G.; Maglajlić, A.; Ferrier, J.; Papp, N.; Hajdari, A.; Mustafa, B.; et al. Of the importance of a leaf: The ethnobotany of sarma in Turkey and the Balkans. J. Ethnobiol. Ethnomed. 2015, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- El, S.N.; Kavas, A.; Karakaya, S. Nutrient Composition of Stuffed Vine Leaves: A Mediterranean Dietary. J. Food Qual. 1997, 20, 337–341. [Google Scholar] [CrossRef]
- Romero, M.J.; Madrid, J.; Hernández, F.; Cerón, J.J. Digestibility and voluntary intake of vine leaves (Vitis vinifera L.) by sheep. Small Rumin. Res. 2000, 38, 191–195. [Google Scholar] [CrossRef]
- Vilela, A.; Pinto, T. Grape Infusions: The Flavor of Grapes and Health-Promoting Compounds in Your Tea Cup. Beverages 2019, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Jaswal, B.S.; Sharma, J.; Rai, P.K. Analysis of stones formed in the human gall bladder and kidney using advanced spectroscopic techniques. Biophys. Rev. 2020, 12, 647–668. [Google Scholar] [CrossRef] [PubMed]
- Boso, S.; Gago, P.; Alonso-Villaverde, V.; Santiago, J.J.; Mendez, J.; Pazos, I.; Martínez, M.C. Variability at the electron microscopy level in leaves of members of the genus Vitis. Sci. Hortic. 2011, 128, 228–238. [Google Scholar] [CrossRef]
- Chitwood, D.H. The shapes of wine and table grape leaves: An ampelometric study inspired by the methods of Pierre Galet. Plants People Planet 2021, 3, 155–170. [Google Scholar] [CrossRef]
- Monteiro, A.; Teixeira, G.; Lopes, C.M. Comparative leaf micromorphoanatomy of Vitis vinifera ssp. Vinífera (Vitaceae) red cultivars. Ciênc. Téc. Vitivinic. 2013, 28, 19–28. [Google Scholar]
- Pinto, T.M.; Anjos, M.R.; Martins, N.M.; Gomes-Laranjo, J.; Ferreira-Cardoso, J.; Peixoto, F. Structural analysis of Castanea sativa Mill. leaves from diferent regions in the treetop. Braz. Arch. Biol. Technol. 2011, 54, 117–124. [Google Scholar] [CrossRef]
- Hogg, T.; Rebelo, J. Rumo Estratégico Para o Sector dos Vinhos do Porto e Douro, Síntese—Documento de Trabalho; IVDP, UTAD, Innovine and Wine: Vila Real, Portugal, 2017; ISBN 978-989-704-344-4. [Google Scholar]
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.; Kumar, N.V.A.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Peluso, I.; et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef] [Green Version]
- Pinto, T.; Vilela, A. Healthy Drinks with Lovely Colors: Phenolic Compounds as Constituents of Functional Beverages. Beverages 2021, 7, 12. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, drinks, and spice: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Avila, J.A.; Villa-Rodriguez, J.A.; Montiel-Herrera, M.; Pacheco-Ordaz, R.; Roopchand, D.E.; Venema, K.; González-Aguilar, G.A. Phenolic Compounds Promote Diversity of Gut Microbiota and Maintain Colonic Health. Dig. Dis. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Liu, H.; Hu, N.; Zhang, Y.; Wang, S. Grape seed extract ameliorates PhIP-induced colonic injury by modulating gut microbiota, lipid metabolism, and NF-κB signaling pathway in rats. J. Funct. Foods 2021, 78, 1–12. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Du, K.; McGill, M.R.; Xie, Y.; Bajt, M.L.; Jaeschke, H. Resveratrol Prevents Protein Nitration and Release of Endonucleases from Mitochondria During Acetaminophen Hepatotoxicity. Food Chem. Toxicol. 2015, 81, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Arts, I.C.; Hollman, P.C. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, U. Does Tea Affect Cardiovascular Disease? A Meta-Analysis. Am. J. Epidemiol. 2001, 154, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Lee, E.K.; Kim, D.H.; Yu, B.P.; Chung, H.Y. Kaempferol modulates pro-inflammatory NF-κB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. AGE 2010, 32, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.-P.; Nettleton, J.A.; Jacobs, D.R. Flavonoid Intake and Cardiovascular Disease Mortality: A Prospective Study in Postmenopausal Women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hessami, A.; Shamshirian, A.; Heydari, K.; Pourali, F.; Alizadeh-Navaei, R.; Moosazadeh, M.; Abrotan, S.; Shojaie, L.; Sedighi, S.; Shamshirian, D.; et al. Cardiovascular diseases burden in COVID-19: Systematic review and meta-analysis. Am. J. Emerg. Med. 2020, 46, 382–391. [Google Scholar] [CrossRef]
- Matsushita, K.; Ding, N.; Kou, M.H.; Hu, X.; Chen, M.K.; Gao, Y.M.; Honda, Y.; Zhao, D.; Dowdy, D.; Mok, Y.; et al. The Relationship of COVID-19 Severity with Cardiovascular Disease and Its Traditional Risk Factors: A Systematic Review and Meta-Analysis. Glob. Heart 2020, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, S.R.; Kim, M.N.; Shim, W.J.; Park, S.M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: A systematic review and meta-analysis. Heart 2021, 107, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Vakili, K.; Sayehmiri, F.; Mohamadkhani, A.; Hajiesmaeili, M.; Rezaei-Tavirani, M.; Eilami, O. The prognostic value of comorbidity for the severity of COVID-19: A systematic review and meta-analysis study. PLoS ONE 2021, 16, e0246190. [Google Scholar] [CrossRef]
- Naeini, M.B.; Sahebi, M.; Nikbakht, F.; Jamshidi, Z.; Ahmadimanesh, M.; Hashemi, M.; Ramezani, J.; Miri, H.H.; Yazdian-Robati, R. A meta-meta-analysis: Evaluation of meta-analyses published in the effectiveness of cardiovascular comorbidities on the severity of COVID-19. Obes. Med. 2021, 22, 100323. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef]
- Lin, S.C.; Ho, C.T.; Chuo, W.H.; Li, S.M.; Wang, T.T.; Lin, C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 144. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.H.; Wei, J.L.; Huang, T.; Lei, L.P.; Shen, C.G.; Lai, J.Z.; Yang, M.; Liu, L.; Yang, Y.; Liu, G.S.; et al. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phyther. Res. 2020, 35, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Wahedi, H.M.; Ahmad, S.; Abbasi, S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2020, 39, 3225–3234. [Google Scholar] [CrossRef]
- Giordo, R.; Zinellu, A.; Eid, A.H.; Pintus, G. Therapeutic Potential of Resveratrol in COVID-19-Associated Hemostatic Disorders. Molecules 2021, 26, 856. [Google Scholar] [CrossRef]
- Rafe, T.; Shawon, P.A.; Salem, L.; Chowdhury, N.I.; Kabir, F.; Bin Zahur, S.M.; Akhter, R.; Noor, H.B.; Mohib, M.M.; Sagor, M.A.T. Preventive Role of Resveratrol Against Inflammatory Cytokines and Related Diseases. Curr. Pharm. Des. 2019, 25, 1345–1371. [Google Scholar] [CrossRef] [PubMed]
- Giordo, R.; Nasrallah, G.K.; Al-Jamal, O.; Paliogiannis, P.; Pintus, G. Resveratrol Inhibits Oxidative Stress and Prevents Mitochon2drial Damage Induced by Zinc Oxide Nanoparticles in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2020, 21, 3838. [Google Scholar] [CrossRef]
- Mittra, I.; de Souza, R.; Bhadade, R.; Madke, T.; Shankpal, P.D.; Joshi, M.; Qayyumi, B.; Bhattacharjee, A.; Gota, V.; Gupta, S.; et al. Resveratrol and Copper for treatment of severe COVID-19: An observational study (RESCU 002). medRxiv 2020. [Google Scholar] [CrossRef]
- Emmanuel, R.D.; Lawrence, A.B.; Oluyomi, A.S. COVID 19: Resveratrol as a Potential Supplement to Mitigate the Cardiotoxicity Associated with Chloroquine and Hydroxychloroquine Treatment. Biointerface Res. Appl. Chem. 2021, 11, 11172–11186. [Google Scholar] [CrossRef]
- Kang, G.G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Santhakumar, A.B. Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review. Int. J. Mol Sci. 2020, 21, 140. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef] [Green Version]
- Montalbano, G.; Maugeri, A.; Guerrera, M.C.; Miceli, N.; Navarra, M.; Barreca, D.; Cirmi, S.; Germanà, A. A White Grape Juice Extract Reduces Fat Accumulation through the Modulation of Ghrelin and Leptin Expression in an In Vivo Model of Overfed Zebrafish. Molecules 2021, 26, 1119. [Google Scholar] [CrossRef]
- Visalli, G.; Ferlazzo, N.; Facciola, A.; Picerno, I.; Navarra, M.; Di Pietro, A. Ex vivo evaluation of the effects of a white grape juice extract on lymphocytic mitochondrial functions. Nat. Prod. Res. 2018, 34, 580–584. [Google Scholar] [CrossRef]
- Montalbano, G.; Mhalhel, K.; Briglia, M.; Levanti, M.; Abbate, F.; Guerrera, M.C.; D’Alessandro, E.; Laurà, R.; Germanà, A. Zebrafish and Flavonoids: Adjuvants against Obesity. Molecules 2021, 26, 3014. [Google Scholar] [CrossRef] [PubMed]
- Filocamo, A.; Bisignano, C.; Mandalari, G.; Navarra, M. In Vitro Antimicrobial Activity and Effect on Biofilm Production of a White Grape Juice (Vitis vinifera) Extract. Evid.-Based Complement. Altern. Med. 2015, 2015, 856243. [Google Scholar] [CrossRef] [Green Version]
- Giacoppo, S.; Galuppo, M.; Lombardo, G.E.; Ulaszewska, M.M.; Mattivi, F.; Bramanti, P.; Mazzon, E.; Navarra, M. Neuroprotective effects of a polyphenolic white grape juice ex-tract in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia 2015, 103, 171–186. [Google Scholar] [CrossRef]
- Monagas, M.; Hernández-Ledesma, B.; Gómez-Cordovés, C.; Bartolomé, B. Commercial dietary ingredients from Vitis vinifera L. leaves and grape skins: Antioxidant and chemical characterization. J. Agric. Food Chem. 2006, 54, 319–327. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Orhan, D.D.; Ergun, F.; Yeşilada, E. In-vivo assessment of antidiabetic and antioxidant activities of grapevine leaves (Vitis vinifera) in diabetic rats. J. Ethnopharmacol. 2006, 108, 280–286. [Google Scholar] [CrossRef]
- Dani, C.; Oliboni, L.S.; Pasquali, M.A.B.; Oliveira, M.R.; Umezu, F.M.; Salvador, M.; Moreira, J.C.F.; Henriques, J.A.P. Intake of purple grape juice as a hepatoprotective agent in Wistar rats. J. Med. Food 2008, 11, 127–132. [Google Scholar] [CrossRef]
- Suwannaphet, W.; Meeprom, A.; Yibchok-Anun, S.; Adisakwattana, S. Preventive effect of grape seed extract against high-fructose diet-induced insulin resistance and oxidative stress in rats. Food Chem. Toxicol. 2010, 48, 1853–1857. [Google Scholar] [CrossRef]
- Deliorman-Orhan, D.; Orhan, N.; Özçelik, B.; Ergun, F. Biological activities of Vitis vinifera L. leaves. Turk. J. Biol. 2009, 33, 341–348. [Google Scholar] [CrossRef]
- Goodrich, K.M.; Fundaro, G.; Griffin, L.E.; Grant, A.Q.; Hulver, M.W.; Ponder, M.A.; Neilson, A.P. Chronic administration of dietary grape seed extract increases colonic expression of gut tight junction protein occludin and reduces fecal calprotectin: A secondary analysis of healthy wistar furth rats. Nutr. Res. 2012, 32, 787–794. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Y.-Y. Grape phytochemicals and associated health benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentao, P.; Andrade, P.; Bento, A.; Pereira, J.A. Vitis vinifera leaves towards bioactivity. Ind. Crops Prod. 2013, 43, 434–440. [Google Scholar] [CrossRef]
- Lima, A.F. Caracterização da Bioatividade de Folhas de Diferentes Castas de Videira Quando Sujeitas a Processamento Alimentar. Bachelor’s Thesis, Universidade Tecnológica Federal do Paraná, Paraná, Brazil, 2015. [Google Scholar]
- Anđelković, M.; Radovanović, B.; Anđelković, A.M.; Radovanović, V.; Zarubica, A.; Stojković, N.; Nikolić, V. The determination of bioactive ingredients of grape pomace (Vranac variety) for potential use in food and pharmaceutical industries. Adv. Technol. 2015, 4, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Gülcü, M.; Uslu, N.; Özcan, M.M.; Gökmen, F.; Özcan, M.M.; Banjanin, T.; Gezgin, S.; Dursun, N.; Geçgel, Ü.; Ceylan, D.A.; et al. The investigation of bioactive compounds of wine, grape juice, and boiled grape juice wastes. J. Food Process. Preserv. 2019, 43, e13850. [Google Scholar] [CrossRef] [Green Version]
- Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Tit, O.; Lengyel, E.; Stegărus, D.I.; Săvescu, P.; Ciubara, A.B.; Constantinescu, M.A.; Tit, M.A.; Rat, D.; Ciubara, A. Identification and Quantification of Valuable Compounds in Red Grape Seeds. Appl. Sci. 2021, 11, 5124. [Google Scholar] [CrossRef]
- Garrido, T.; Gizdavic-Nikolaidis, M.; Leceta, I.; Urdanpilleta, M.; Guerrero, P.; de la Caba, K.; Kilmartin, P.A. Optimizing the extraction process of natural antioxidants from chardonnay grape marc using microwave-assisted extraction. Waste Manag. 2019, 88, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bail, S.; Stuebiger, G.; Krist, S.; Unterweger, H.; Buchbauer, G. Characterization of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols, and antioxidant capacity. Food Chem. 2008, 108, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, A.; Todasca, M.C.; Chira, N.A.; Maganu, M.; Rosca, S. The compositional characterization of Romanian grape seed oils using spectroscopic methods. Food Chem. 2012, 134, 2453–2458. [Google Scholar] [CrossRef]
- Ratnasooriya, C.C.; Rupasinghe, H.P.V. Extraction of phenolic compounds from grapes and their pomace using β-cyclodextrin. Food Chem. 2012, 134, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Goñi, I.; Viveros, A.; Hervert-Hernández, D.; Brenes, A. Changes in polyphenolic content and antioxidant activity after thermal treatments of grape seed extract and grape pomace. Eur. Food Res. Technol. 2012, 234, 147–155. [Google Scholar] [CrossRef]
- Dwyer, K.; Hosseinian, F.; Rod, M. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- Carmona-Jiménez, Y.; García-Moreno, M.V.; García-Barroso, C. Effect of Drying on the Phenolic Content and Antioxidant Activity of Red Grape Pomace. Plant Foods Hum. Nutr. 2018, 73, 74–81. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.d.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Choi, Y.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef] [Green Version]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef] [Green Version]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phytother. Res. 2009, 23, 1197–1204. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [Green Version]
- Ananga, A.; Georgiev, V.; Tsolova, V. Manipulation and engineering of metabolic and biosynthetic pathway of plant polyphenols. Curr. Pharm. Des. 2013, 19, 6186–6206. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Muñoz, N.; Fernández-González, M.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Red-color related phenolic composition of Garnacha Tintorera (Vitis vinifera L.) grapes and red wines. J. Agric. Food Chem. 2009, 57, 7883–7891. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Lu, J. Phenolic Contents and Compositions in Skins of Red Wine Grape Cultivars among Various Genetic Backgrounds and Originations. Int. J. Mol. Sci. 2012, 13, 3492–3510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharadze, M.; Japaridze, I.; Kalandia, A.; Vanidze, M. Anthocyanins and antioxidant activity of red wines made from endemic grape varieties. Ann. Agrar. Sci. 2018, 16, 181–184. [Google Scholar] [CrossRef]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC–DAD–MS-MS. J. Agric. Food Chem. 2002, 50, 5691–5696. [Google Scholar] [CrossRef] [PubMed]
- Peynaud, E.; Ribéreau-Gayon, P. The grape. In The Biochemistry of Fruits and Their Products; Hulme, A.C., Ed.; Academic Press: London, UK, 1971; Volume 2. [Google Scholar]
- Sat, I.G.; Sengul, M.; Keles, F. Use of Grape Leaves in Canned Food. Pak. J. Nutr. 2002, 1, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Radovanović, B.; Andjelkoví, M.; Radovanović, V.; Milenković-Andjelković, A.; Djekić, S. Polyphenols and Antioxidant Activity of Dierent Vinegrape Leaves. Zb. Rad. 2015, 20, 347–352. [Google Scholar]
- Balìk, J.; Kyseláková, M.; Vrchotová, N.; Triska, J.; Kumsta, M.; Veverka, J.; HÍc, P.; Totusek, J.; Lefnerová, D. Relations between polyphenols content and antioxidant activity in vine grapes and leaves. Czech J. Food Sci. 2008, 26, S25–S32. [Google Scholar] [CrossRef] [Green Version]
- Lacerda, D.S.; Costa, P.C.; Funchal, C.; Dani, C.; Gomez, R. Benefits of Vine Leaf on Different Biological Systems. In Grape and Wine Biotechnology; IntechOpen: London, UK, 2016; pp. 125–143. [Google Scholar] [CrossRef] [Green Version]
- Katalinic, V.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Teskera, A.; Konta, I.; Boban, M. Insight in the phenolic composition and antioxidative properties of Vitis vinifera leaves extracts. Croat. J. Food Sci. Technol. 2009, 1, 7–15. [Google Scholar]
- Valcárcel-Muñoz, M.J.; Guerrero-Chanivet, M.; García-Moreno, M.V.; Rodríguez-Dodero, M.C.; Guillén-Sánchez, D.A. Comparative Evaluation of Brandy de Jerez Aged in American Oak Barrels with Different Times of Use. Foods 2021, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Lapuerta, M.; Rodríguez-Fernández, J.; Ramos, Á.; Donoso, D.; Canoira, L. WLTC and real-driving emissions for an autochthonous biofuel from wine-industry waste. Sci. Rep. 2021, 11, 7528. [Google Scholar] [CrossRef] [PubMed]
- Rasines-Perea, Z.; Teissedre, P.L. Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 2017, 22, 68–87. [Google Scholar] [CrossRef]
- Balli, D.; Cecchi, L.; Innocenti, M.; Bellumori, M.; Mulinacci, N. Food by-products valorisation: Grape pomace and olive pomace (pâté) as sources of phenolic compounds and fiber for enrichment of tagliatelle pasta. Food Chem. 2021, 355, 129642. [Google Scholar] [CrossRef]
- Sri Harsha, P.S.C.; Lavelli, V. Use of Grape Pomace Phenolics to Counteract Endogenous and Exogenous Formation of Advanced Glycation End-Products. Nutrients 2019, 11, 1917. [Google Scholar] [CrossRef] [Green Version]
- Morales-Prieto, N.; Huertas-Abril, P.V.; López de Lerma, N.; Pacheco, I.L.; Pérez, J.; Peinado, R.; Abril, N. Pedro Ximenez sun-dried grape must: A dietary supplement for a healthy longevity. Food Funct. 2020, 11, 4387–4402. [Google Scholar] [CrossRef]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef] [Green Version]
- Rahimmalek, M.; Goli, S.A.H. Evaluation of six drying treatments with respect to essential oil yield, composition, and color characteristics of Thymys daenensis subsp. daenensis. Celak leaves. Ind. Crops Prod. 2013, 42, 613–619. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Figueroa-Robles, A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Encapsulation of phenolic compounds with liposomal improvement in the cosmetic industry. Int. J. Pharm. 2021, 593, 120125. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Bi, J.; Ji, Q.; Zhao, X.; Zheng, Q.; Tan, S.; Gao, X. Effect of hot air drying on the polyphenol profile of Hongjv (Citrus reticulata Blanco, CV. Hongjv) peel: A multivariate analysis. J. Food Biochem. 2020, 44, e13174. [Google Scholar] [CrossRef]
- Weggler, B.A.; Gruber, B.; Teehan, P.; Jaramillo, R.; Dorman, F.L. Chapter 5—Inlets and sampling. In Separation Science and Technology; Nicholas, H.S., Ed.; Academic Press: London, UK, 2020; Volume 12, pp. 141–203. [Google Scholar] [CrossRef]
- Li, H.; Chen, B.; Nie, L.; Yao, S. Solvent effects on focused microwave-assisted extraction of polyphenolic acids from Eucommia ulmodies. Phytochem. Anal. 2004, 15, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Avello, D.; Mardones, C.; Saéz, V.; Riquelme, S.; von Baer, D.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Pilot-plant scale extraction of phenolic compounds from grape canes: Comprehensive characterization by LC-ESI-LTQ-Orbitrap-MS. Food Res. Int. 2021, 143, 110265. [Google Scholar] [CrossRef] [PubMed]
- Lončarić, A.; Lamas, J.P.; Guerra, E.; Kopjar, M.; Lores, M. Thermal stability of catechin and epicatechin upon disaccharides addition. Int. J. Food Sci. Technol. 2018, 53, 1195–1202. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Otero-Pareja, M.J.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C.; Martínez de la Ossa, E.J. Green extraction of antioxidants from different varieties of red grape pomace. Molecules 2015, 20, 9686–9702. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Montibeller, M.J.; Monteiro, P.L.; Stoll, L.; Tupuna-Yerovi, D.S.; Rodrigues, E.; Rodrigues, R.C.; Rios, A.O.; Manfroi, V. Improvement of enzymatic assisted extraction conditions on anthocyanin recovery from different varieties of V. vinifera and V. labrusca grape pomaces. Food Anal. Meth. 2019, 12, 2056–2068. [Google Scholar] [CrossRef]
- Maza, M.; Álvarez, I.; Raso, J. Thermal and Non-Thermal Physical Methods for Improving Polyphenol Extraction in Red Winemaking. Beverages 2019, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- De Sá, M.; Justino, V.; Spranger, M.I.; Zhao, Y.Q.; Han, L.; Sun, B.S. Extraction yields and antioxidant activity of proanthocyanidins from different parts of grape pomace: Effect of mechanical treatments. Phytochem. Anal. 2014, 25, 134–140. [Google Scholar] [CrossRef]
- Kang, W.; Bindon, K.A.; Wang, X.; Muhlack, R.A.; Smith, P.A.; Niimi, J.; Bastian, S.E.P. Chemical and Sensory Impacts of Accentuated Cut Edges (ACE) Grape Must Polyphenol Extraction Technique on Shiraz Wines. Foods 2020, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, A.M.; Holt, H.E.; Pearson, W.; Dambergs, R.G.; Close, D.C. Accentuated cut edges (ace): Effects of skin fragmentation on the composition and sensory attributes of Pinot Noir wines. Am. J. Enol. Vitic. 2016, 67, 169–178. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Labanca, F.; Faraone, I.; Nolè, M.R.; Hornedo-Ortega, R.; Russo, D.; García-Parrilla, M.C.; Chiummiento, L.; Bonomo, M.G.; Milella, L. New Insights into the Exploitation of Vitis vinifera L. cv. Aglianico Leaf Extracts for Nutraceutical Purposes. Antioxidants 2020, 9, 708. [Google Scholar] [CrossRef]
- Gerardi, C.; D’amico, L.; Migoni, D.; Santino, A.; Salomone, A.; Carluccio, M.A.; Giovinazzo, G. Strategies for Reuse of Skins Separated From Grape Pomace as Ingredient of Functional Beverages. Front. Bioeng. Biotechnol. 2020, 26, 645. [Google Scholar] [CrossRef]
- Vali Aftari, R.; Rezaei, K.; Mortazavi, A.; Bandani, A.R. Extraction Modeling to Optimize the Phycocyanin. J. Food Process. Preserv. 2015, 39, 3080–3091. [Google Scholar] [CrossRef]
- Álvarez, A.; Poejo, J.; Matias, A.A.; Duarte, C.M.M.; Cocero, M.J.; Mato, R.B. Microwave pretreatment to improve extraction efficiency and polyphenol extract richness from grape pomace. Effect on antioxidant bioactivity. Food Bioprod. Process. 2017, 106, 162–170. [Google Scholar] [CrossRef]
- Michailidis, D.; Angelis, A.; Nikolaou, P.E.; Mitakou, S.; Skaltsounis, A.L. Exploitation of Vitis vinifera, Foeniculum vulgare, Cannabis sativa and Punica granatum By-Product Seeds as Dermo-Cosmetic Agents. Molecules 2021, 26, 731. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Aguiar, A.C.; . Rostagno, M.A. Extraction of natural products using supercritical fluids and pressurized liquids assisted by ultrasound: Current status and trends. Ultrasonics Sonochemistry 2021, 74, 105584. [Google Scholar] [CrossRef]
- Tena, M.T.; Ríos, A.; Valcárcel, M. Supercritical fluid extraction of t-resveratrol and other phenolics from a spiked solid. Fresenius J. Anal. Chem. 1998, 361, 143–148. [Google Scholar] [CrossRef]
- Chafer, A.; Pascual-Martí, M.C.; Salvador, A.; Berna, A. Supercritical fluid extraction and HPLC determination of relevant polyphenolic compounds in grape skin. J. Sep. Sci. 2005, 28, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Zhang, K.; Wong, J.W. Solvent-Based Extraction Techniques for the Determination of Pesticides in Food, In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: London, UK, 2011; pp. 245–261. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, Z.; Palma, M.; Barroso, C.G. Determination of trans-resveratrol in grapes by pressurised liquid extraction and fast high-performance liquid chromatography. J. Chromatogr. A 2006, 1110, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Lantzouraki, D.Z.; Tsiaka, T.; Soteriou, N.; Asimomiti, G.; Spanidi, E.; Natskoulis, P.; Gardikis, K.; Sinanoglou, V.J.; Zoumpoulakis, P. Antioxidant Profiles of Vitis vinifera L. and Salvia triloba L. Leaves Using High-Energy Extraction Methodologies. J. AOAC Int. 2020, 103, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultur-al By-Products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which Is the Best Food Emerging Solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of Value-Added Components from Food Industry Based and Agro-Forest Biowastes by Deep Eutectic Solvents. J. Bio-Technol. 2018, 282, 46–66. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C. Enzyme-assisted extractions of polyphenols—a comprehensive review. Trends Food Sci Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Xavier-Machado, T.O.; Portugal, I.B.M.; Padilha, C.V.D.S.; Ferreira-Padilha, F.; Dos Santos Lima, M. New trends in the use of enzymes for the recovery of polyphenols in grape byproducts. J. Food Biochem. 2021, 45, e13712. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Wu, Z.; Liu, K.H.; Jang, C.H.; Kim, H.J.; Kim, J.S.; Kim, J.S. Improved extraction of resveratrol and antioxidants from grape peel using heat and enzymatic treatments. J. Sci. Food Agric. 2019, 99, 4043–4053. [Google Scholar] [CrossRef] [PubMed]
- Quesada, M.A.; Blanco-Portales, R.; Pose, S.; Garcia-Gago, J.A.; Jimenez-Bermudez, S.; Munoz-Serrano, A.; Caballero, J.L.; Pliego-Alfaro, F.; Mercado, J.A.; Blanco, J.M. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol. 2009, 150, 1022–1032. [Google Scholar] [CrossRef] [Green Version]
- Cutfield, S.M.; Davies, G.J.; Murshudov, G.; Anderson, B.F.; Moody, P.C.E.; Sullivan, P.A.; Cutfield, J.F. The structure of the exo-beta-(1,3)-glucanase from Candida albicans in native and bound forms: Relationship between a pocket and groove in family 5 glycosyl hydrolases. J. Mol. Biol. 1999, 294, 771–783. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Justo, M.L.; Claro, C.M.; Vila, E.; Parrado, J.; Herrera, M.D.; Alvarez de Sotomayor, M. Endothelium-dependent vasodilator and antioxidant properties of a novel enzymatic extract of grape pomace from wine industrial waste. Food Chem. 2012, 135, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Battestin, V.; Macedo, G.A.; de Freitas, V.A.P. Hydrolysis of epigallocatechin gallate using a tannase from Paecilomyces variotii. Food Chem. 2008, 108, 228–233. [Google Scholar] [CrossRef]
- Kabir, F.; Sultana, M.S.; Kurnianta, H. Polyphenolic Contents and Antioxidant Activities of Underutilized Grape (Vitis vinifera L.) Pomace Extracts. Prev. Nutr. Food Sci. 2015, 20, 210–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobar, P.; Moure, A.; Soto, C.; Chamy, R.; Zúñiga, M.E. Winery solid residue revalorization into oil and antioxidant with nutraceutical properties by an enzyme assisted process. Water Sci. Technol. 2005, 51, 47–52. [Google Scholar] [CrossRef]
- Štambuk, P.; Tomašković, D.; Tomaz, I.; Maslov, L.; Stupić, D.; Kontić, J.K. Application of pectinases for recovery of grape seeds phenolics. 3 Biotech 2016, 6, 224. [Google Scholar] [CrossRef] [Green Version]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017, 39, 51–58. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández-Sobrino, R.; Margalef, M.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Muguerza, B.; Bravo, F.I. Enzyme-Assisted Extraction to Obtain Phenolic-Enriched Wine Lees with Enhanced Bioactivity in Hypertensive Rats. Antioxidants 2021, 10, 517. [Google Scholar] [CrossRef]
- Zwingelstein, M.; Draye, M.; Besombes, J.-L.; Piot, C.; Chatel, G. Viticultural wood waste as a source of polyphenols of interest: Opportunities and perspectives through conventional and emerging extraction methods. Waste Manag. 2020, 102, 782–794. [Google Scholar] [CrossRef]
- Dimić, I.; Teslić, N.; Putnik, P.; Bursać Kovačević, D.; Zeković, Z.; Šojić, B.; Mrkonjić, Ž.; Čolović, D.; Montesano, D.; Pavlić, B. Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Thilakarathna, W.P.D.W.; Astatkie, T.; Rupasinghe, H.P.V. Optimization of Cate-chin and Proanthocyanidin Recovery from Grape Seeds Using Microwave-Assisted Ex-traction. Biomolecules 2020, 10, 243. [Google Scholar] [CrossRef] [Green Version]
- Floris, T.; Filippino, G.; Scrugli, S.; Pinna, M.B.; Argiolas, F.; Argiolas, A.; Murru, M.; Reverchon, E. Antioxidant compounds recovery from grape residues by a supercritical antisolvent assisted process. J. Supercrit. Fluids 2010, 54, 165–170. [Google Scholar] [CrossRef]
- Prado, J.M.; Dalmolin, I.; Carareto, N.D.D.; Basso, R.C.; Meirelles, A.J.A.; Oliveira, J.V.; Batista, E.A.C.; Meireles, M.A.A. Supercritical fluid extraction of grape seed: Process scale-up, extract chemical composition and economic evaluation. J. Food Eng. 2012, 109, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Ghafoor, K.; AL-Juhaimi, F.Y.; Choi, Y.H. Supercritical fluid extraction of phenolic compounds and antioxidants from grape (Vitis labrusca B.) seeds. Plant Foods Hum. Nutr. 2012, 67, 407–414. [Google Scholar] [CrossRef]
- Farías-Campomanes, A.M.; Rostagno, M.A.; Meireles, M.A.A. Production of polyphenol extracts from grape bagasse using supercritical fluids: Yield, extract composition and economic evaluation. J. Supercrit. Fluids 2013, 77, 70–78. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. The combined extraction of polyphenols from grape marc: Ultrasound assisted extraction followed by supercritical CO2 extraction of ultrasound-raffinate. LWT Food Sci. Technol. 2015, 61, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Aresta, A.; Cotugno, P.; De Vietro, N.; Massari, F.; Zambonin, C. Determination of polyphenols and vitamins in wine-making by-products by supercritical fluid extraction (SFE). Anal. Lett. 2020, 53, 2585–2595. [Google Scholar] [CrossRef]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical solvent extraction of procyanidins from dried red grape pomace. J. Agric. Food Chem. 2010, 58, 4014–4021. [Google Scholar] [CrossRef] [PubMed]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical solvent extraction of anthocyanins from dried red grape pomace. J. Agric. Food Chem. 2010, 58, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Pedras, B.; Salema-Oom, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Valorization of white wine grape pomace through application of subcritical water: Analysis of extraction, hydrolysis, and biological activity of the extracts obtained. J. Supercrit. Fluids 2017, 128, 138–144. [Google Scholar] [CrossRef]
- Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Huamán-Castilla, N.; Pedreschi, F.; Iglesias-Rebolledo, N.; Pérez-Correa, J.R. Impact of an integrated process of hot pressurised liquid extraction–macroporous resin purification over the polyphenols, hydroxymethylfurfural and reducing sugars content of Vitis vinifera ‘Carménère’pomace extracts. Int. J. Food Sci. Technol. 2018, 53, 1072–1078. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2019, 240, 105–113. [Google Scholar] [CrossRef]
- Panić, M.; Radić Stojković, M.; Kraljić, K.; Škevin, D.; Radojčić Redovniković, I.; Gaurina Srček, V.; Radošević, K. Ready-to-Use Green Polyphenolic Extracts from Food by-Products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Implementation of subcritical water extraction with natural deep eutectic solvents for sustainable extraction of phenolic compounds from winemaking by-products. Food Res. Int. 2020, 137, 109728. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Ra-dojčić Redovniković, I.; Jokić, S. Natural Deep Eutectic Solvents and Ultrasound-Assisted Extraction: Green Approaches for Extraction of Wine Lees Anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green Ex-traction of Polyphenols from Grapefruit Peels Using High Voltage Electrical Discharges, Deep Eutectic Solvents and Aqueous Glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; Yoo, D.E.; Lee, J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch. Pharm. Res. 2015, 38, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Claus, H.; Mojsov, K. Enzymes for Wine Fermentation: Current and Prospective Applications. Fermentation 2018, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound-assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Goulas, V.; Stavrou, K.; Michael, C.; Botsaris, G.; Barbouti, A. The Potential of Sun-Dried Grape Pomace as a Multi-Functional Ingredient for Herbal Infusion: Effects of Brewing Parameters on Composition and Bioactivity. Antioxidants 2021, 10, 586. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.; Cheng, V.J.; McConnell, M.; Zhao, J.H.; Sedcole, R.; Harrison, R. Antioxidant activities, sensory and anti-influenza activity of grape skin tea infusion. Food Chem. 2011, 129, 837–845. [Google Scholar] [CrossRef] [PubMed]
- HMPC (Herbal Medicinal Products Committee). Assessment report on Vitis vinifera L., folium. In European Medicines Agency; EMA/HMPC/464682/2016: London, UK, 2017; 44p. [Google Scholar]
- Rizzuti, A.; Caliandro, R.; Gallo, V.; Mastrorilli, P.; Chita, G.; Latronico, M. A combined approach for characterisation of fresh and brined vine leaves by X-ray powder diffraction, NMR spectroscopy and direct infusion high resolution mass spectrometry. Food Chem. 2013, 141, 1908–1915. [Google Scholar] [CrossRef]
- Koşar, M.; Kupeli, E.; Malyer, H.; Uylasüer, V.; Turkben, V.C.; Basüer, K.H.C. Effect of brining on biological activity of leaves of Vitis vinifera L. (Cv. Sultani Cekirdeksiz) from Turkey. J. Agric. Food Chem. 2007, 55, 4596–4603. [Google Scholar] [CrossRef]
- Ceyhan, N.; Keskin, D.; Zorlu, Z.; Ugur, A. In-vitro antimicrobial activities of different extracts of grapevine leaves (Vitis vinifera L.) from West Anatolia against some pathogenic microorganisms. J. Pure Appl. Microbiol. 2012, 6, 1303–1308. [Google Scholar]
- Fernandes, B.; Correia, A.C.; Cosme, F.; Nunes, F.M.; Jordão, A.M. Volatile components of vine leaves from two Portuguese grape varieties (Vitis vinifera L.), Touriga Nacional and Tinta Roriz, analysed by solid-phase microextraction. Nat. Prod. Res. 2015, 29, 37–45. [Google Scholar] [CrossRef]
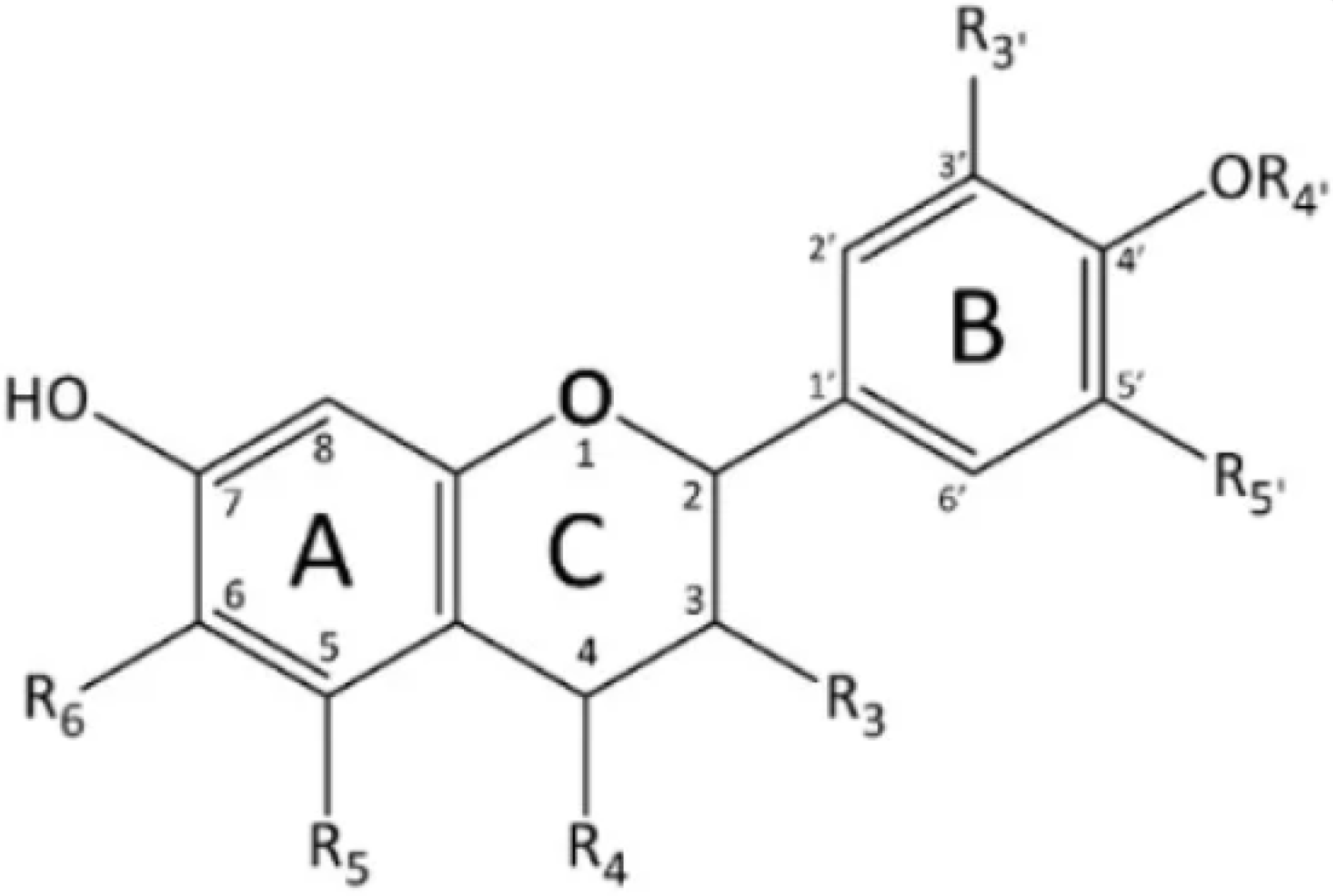
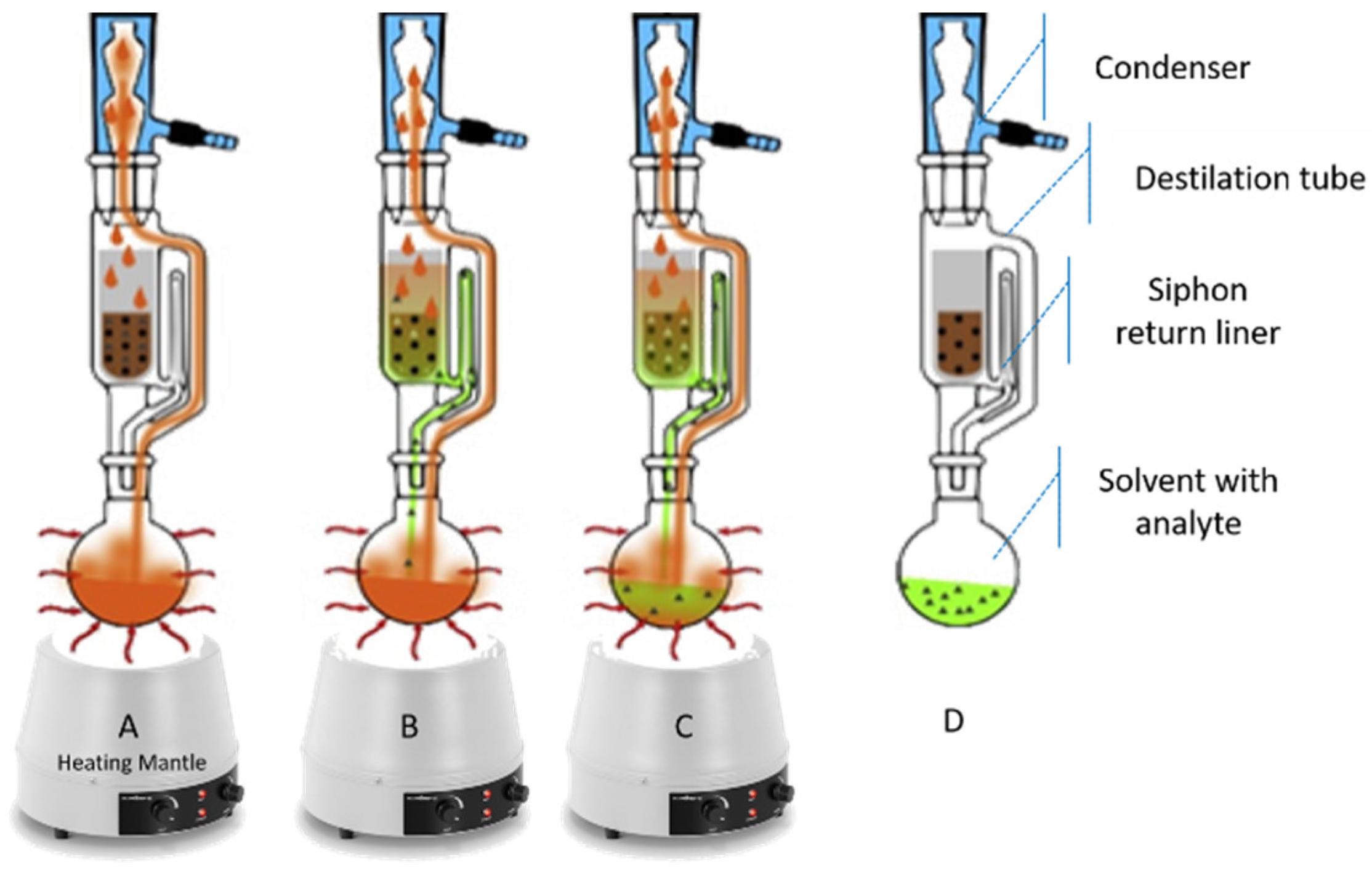
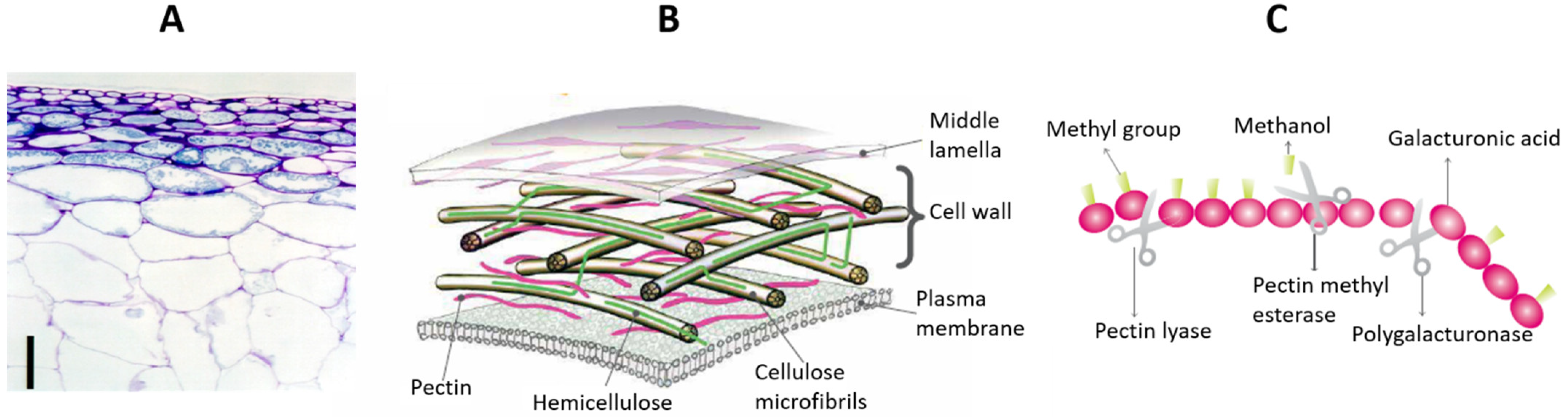

| Ext. Method | Product | Main Compounds and Analysis Methods | Extraction Conditions/Products Used and Quantity of Compounds Recovered | Ref. |
|---|---|---|---|---|
| MAE and/or UAE (Only at lab scale) | Grape canes | Trans-resveratrol and trans-ε-viniferin. Compounds were analyzed qualitatively by comparing their retention times and UV spectra with authentic standards. | Ultrasonic bath 20 kHz; Ethanol/water 80:20 (v/v) 10 mL·g−1, 5 min, 80 W. Microwave extractor 1500 W; Ethanol/water 60:40 (v/v) 200 mL·g−1, 20 min, 100 °C. | [159] |
| Grape seeds | Polyunsaturated fatty acids (linoleic acid); monounsaturated fatty acids oleic acid); Saturated fatty acids and tocopherols. The compound’s content was determined by high-pressure liquid chromatography (HPLC). | Ultrasonic bath 40 kHz. 30.0 g of grape seeds: 300 mL of n-hexane. T = 50 °C, t = 40 min, and sonication power at 60 W L−1. Tocopherol recovery of 7.92 (red grape seeds) and 2.18 (white grape-seeds) mg 100 g−1 Microwave extractor—10.0 g of seeds: 100 mL of n-hexane in a glass flask. Constant microwave irradiation power (600 W) for 15 min. Tocopherol recovery of 7.96 (red grape seeds) and 2.63 (white grape-seeds) mg 100 g−1 | [160] | |
| Vitis vinifera leaves | Polyphenolic compounds (caftaric acid, (+)-catechin, benzoic acid, rutin quercetin -3-O-glucuronide, quercetin-3-O-glycoside, and kaempferol-3-O-glucoside. Phytochemical profile was investigated by HPLC-DAD. | Ultrasonic bath 40 Hz, 10.27 g of dried leaves, and 170 mL of ultrapure water and ethanol (50:50, v/v) as solvent. T = 30 °C, 6 h. Extraction yield—13.81% of the total. | [130] | |
| Grape seed powder | Epicatechins, proanthocyanidins. Analysis by UPLC-ESI-MS. | Microwave-accelerated reaction system (800 W). Grape seed powder (0.5 g: 5 mL of ethanol (26–94%, v/v). T = 110–170 °C for 5–55 min. Total monomeric catechins and PAC were 8.15 ± 0.20 mg/g DW and 56.37 ± 8.37 mg CE/g DW, respectively. | [161] | |
| SFE | Grape residues | Polyphenols, anthocyanins. Analysis by HPLC. | Supercritical antisolvent extraction: methanol, Tc 40 °C, 11 MPa. The overall content of polyphenols and anthocyanins recovered from treated material was 521 mg/kg and 15,542 mg/kg, respectively. | [162] |
| Grape seeds | Phenolic Compounds and Antioxidants. Total phenolic analyzed using the Folin-Ciocalteu method. Total antioxidants evaluated by the phosphomolybdenum complex method. | 44 ~ 46 °C temperature and 153 ~ 161 bar CO2 pressure, along with ethanol (<7%) as a modifier. Extract yield—12.32% (2.45 mg GAE/mL total phenols and 7.08 mg AAE/mL antioxidants). | [163] | |
| Grape seeds | Linoleic, palmitic, stearic, and oleic acids. The fatty acid composition of the extracts was determined by GC. Before chromatographic analysis, the fatty samples were prepared in the form of fatty acid methyl esters (FAME). | The solvent used was carbon dioxide (99.9% purity), pressure 313 K/35 MPa. The total yield in 450 min of extraction was 13.42% (d.b.). | [164] | |
| Grape bagasse | Syringic, vanillic, gallic, p-hydroxybenzoic, protocatechuic and p-coumaric acids, and quercetin. Analyzed by thin-layer chromatography (TLC) and HPLC. | 20 g of bagasse as feed material, CO2+ 96% ethanol 10% (w/w) as a modifier, Tc 40 °C, 2 extraction cycles at Pc 20 and 35 MPa, S/F ratio 80 and 115, respectively. Extraction yields (5.5 ± 0.1%) achieved at 20 and 35 MPa. | [165] | |
| Grape marc | Polyphenols | Modifier ethanol (10%) with CO2, 40 °C, 8 MPa, the flow rate of CO2 at 6 Kg/h, ethanol 449.73 g/L. Extraction yield obtained by the combined process—3493 mg GAE/100 g D; Antioxidant activity—7503 mg α-tocopherol/100 g DM. | [166] | |
| Winemaking by-products | Polyphenols and Vitamins (trans-resveratrol, β-sitosterol, α-tocopherol, and ascorbic acid). The total polyphenols were determined using 4-benzoyl amino-2,5-dimethoxybenzenediazonium chloride salt, namely fast blue BB diazonium salt. The total antioxidant activity was evaluated using the 2,2-diphenyl-1-picrylhydrazyl test. | Modifier ethanol (20%) with CO2, 60 °C, 25 MPa, a flow rate of CO2 at 2 mL/min, a flow rate of ethanol at 0.4 mL/min. Extraction yield of 336 (seeds) to 603 (skins) μg/L GAE. | [167] | |
| PLE and ASE | Red grape pomace | Procyanidins Identified by HPLC-ESI-MS/MS. | Six solvents were tested 0, 10, 30, 50, 70, and 90% ethanol/water (v/v). Six temperatures (40, 60, 80, 100, 120, and 140 °C), pressure 6.8 MPa. The concentration and quality of procyanidins were dependent on solvent composition and temperature. | [168] |
| Anthocyanins Identified by HPLC-MS. | Four hydroethanolic solvents (10, 30, 50, and 70% ethanol in water, v/v) and six temperatures (40, 60, 80, 100, 120, and 140 °C), pressure 6.8 MPa. | [169] | ||
| White grape pomace | Phenolic compounds. Total phenolic content quantified by the Folin-Ciocalteu colorimetric method. | Temperature 170–210 °C, pressure 10 MPa, 30 min, 5–10 mL/min. Extraction yield of 1.67–2.62 g/100 g White grape pomace. | [170] | |
| Grape pomace | Proanthocyanidins Oligomeric distribution of proanthocyanidins was established by HILIC-FLD. | Extraction temperatures (60, 75 and 90 °C) and ethanol content (0%, 5%, 10% and 15%). Carménère pomace was also extracted by HPLE at 130, 150, and 200 °C without ethanol. Extraction yields were dependent on the conditions used. | [171] | |
| Grape marc | Anthocyanins Extracts analyzed by UHPLC-UV-Vis. | Ethanol and water mixtures (acidified or not) (50% w/w), pure ethanol, and acidified water at temperatures from 40 to 100 °C. The best extraction yield was 10.21 mg of malvidin-3-O-glucoside/g of dried grape marc (dr). | [172] | |
| NADES | Grape pomace | Phenolic acids, phenolic alcohols, vanillin (phenolic aldehyde), flavonoids, and pinoresinol. HPLC analysis of polyphenolic compounds. | Choline chloride:Ethyleneglycol (1:2; 20 mL; 20% water (v/v)). Other DESs were investigated: Choline chloride:xylitol (5:1), Choline chloride:glucose (1:1) and citric acid:glucose (1:1). Yielding between 2647.48–2892.07 mg total polyphenol kg−1 DW of grape-pomace. | [173]. |
| Tannins, hydroxycinnamic acids, and flavonols. HPLC-DAD-ESI-MS analysis. | Pressurized hot water extraction and eight combinations: Choline chloride: oxalic acid (1:1); Choline chloride: lactic acid (1:2) Choline chloride: fructose: water (2:1:1) Choline chloride: ethyleneglycol (1:2) Choline chloride:1,2-propanediol (1:2) Choline chloride: urea (1:2) Citric acid: maltose: water (4:1:5) Citric acid: fructose: water (1:1:2) The optimal conditions to maximize the extraction were ChClU at 30% and extraction temperature of 100 °C. | [174] | ||
| Wine lees | Anthocyanins Total anthocyanins extracted from the wine lees was determined by the bisulfite bleaching procedure and analyzed by HPLC. | Choline chloride:malic acid [1:1; -; 35.4% (w/w) water] The total anthocyanins in the extracts obtained varied from 2.89 mg g−1 DW to 6.42 mg g−1 DW. | [175] | |
| Grape skins | Polyphenols Total phenolic content quantified by the Folin-Ciocalteu colorimetric method. HPLC was used to identify the phenolic compounds. | Water, 50% (v/v) ethanol/water, 20% (w/v) aqueous glycerol, DES-6 (lactic acid: glucose), yielding 0.575, 2.092, 1.9695 and 3.42 g/100 g DM of naringin, respectively. | [176]. | |
| Phenolic compounds. Analyzed by HPLC. | Choline chloride:glycerol (ChGyl—1:2) Choline chloride:oxalic acid (ChOa—1:1) Choline chloride:malic acid (ChMa—1.5:1) Choline chloride:sorbose (ChSo—1:1) Choline chloride:proline:malic acid (ChMaPro—1:1:1) The best extraction efficiency was obtained with ChOa, followed by ChMa > ChMaPro > ChGyl > ChSor. | [177] | ||
| Anthocyanins Extracts qualitative analysis by UHPLC-Q-TOF–MS. Total anthocyanin contents (TACs) were measured using the pH differential spectrophotometric method. | Citric acid:D-(−)-fructose (1:1) Citric acid:maltose (2:1) Citric acid:maltitol (2:1) TACs of these solvents ranged from 9.3–23.5 mg g−1. | [178] | ||
| EAE | Wine lees | Phenolic compounds, anthocyanins, and flavanols Separation, identification, and quantification of anthocyanin and non-anthocyanin phenolic compounds were performed by UHPLC-ESI-Q-TOF-MS. | Hydrolysis of wine lees proteins with Flavourzyme® (endo- and exo-peptidases). The yield of flavanols (33.56%) and anthocyanin (33.52%). | [158] |
| White-grape pomace | Phenols, flavonoids, flavanols, and tannins. Extracts were characterized spectrophotometrically for phenolic, flavonoid, and flavanol contents and analyzed for phenolic compounds by HPLC-DAD. | Two-step enzymatic plus solvent-based process. Different concentrations (0.5, 1 or 2% enzyme volume/pomace DW) of Pectinex 3XL® (pectinase from Aspergillus niger), Pectinex Ultra SPL® (polygalacturonase); Termamyl® (endo-acting alpha amylase), Fungamyl® (α-Amylase from Aspergillus oryzae), Pentopan 500BG® (xylanase) or Celluclast® (cellulase). Solvents: water and ethanol. | [157] | |
| Grape seeds | Polyphenols, namely flavan-3-ols, catechin, and epicatechin. Extracts analyzed by HPLC. | Lallzyme HC® and Lallzyme EX-V® enzyme preparations isolated from Aspergillus niger. Enzyme’s preparation constitution-polygalacturonase, pectin lyase, pectin methylesterase, cellulase, and hemicellulase. | [156] | |
| Grapeseed oil with phenolic compounds. | Ultrazym®-Celluclast® (3:1) A pectic enzyme and a cellulase. | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, A.; Pinto, T. Grape Infusions: Between Nutraceutical and Green Chemistry. Sustain. Chem. 2021, 2, 441-466. https://doi.org/10.3390/suschem2030025
Vilela A, Pinto T. Grape Infusions: Between Nutraceutical and Green Chemistry. Sustainable Chemistry. 2021; 2(3):441-466. https://doi.org/10.3390/suschem2030025
Chicago/Turabian StyleVilela, Alice, and Teresa Pinto. 2021. "Grape Infusions: Between Nutraceutical and Green Chemistry" Sustainable Chemistry 2, no. 3: 441-466. https://doi.org/10.3390/suschem2030025
APA StyleVilela, A., & Pinto, T. (2021). Grape Infusions: Between Nutraceutical and Green Chemistry. Sustainable Chemistry, 2(3), 441-466. https://doi.org/10.3390/suschem2030025







