Synthesis of Biodiesel from Tall Oil Fatty Acids by Homogeneous and Heterogeneous Catalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Biodiesel via Homogeneous Catalysis
2.3. Synthesis of Biodiesel via Heterogeneous Catalysis
2.4. TOFA and Biodiesel Characterization
2.5. Design of Experiments
3. Results and Discussion
3.1. Elemental Analysis of TOFA
3.1.1. Physicochemical Properties of TOFA
3.1.2. FTIR Characterization of TOFA and Biodiesel
3.2. Homogeneous Catalysis
3.2.1. Biodiesel Yield
− 0.14 × Time + 0.03 × Ratio × Temperature + 0.81 × Ratio × Catalyst Conc. +
0.05 × Ratio × Time − Temperature × Time − 0.23 × Catalyst Conc. × Time.
3.2.2. Characterization of Homogenous Catalyzed TOFA Biodiesel Quality
3.3. Heterogeneous Catalysis
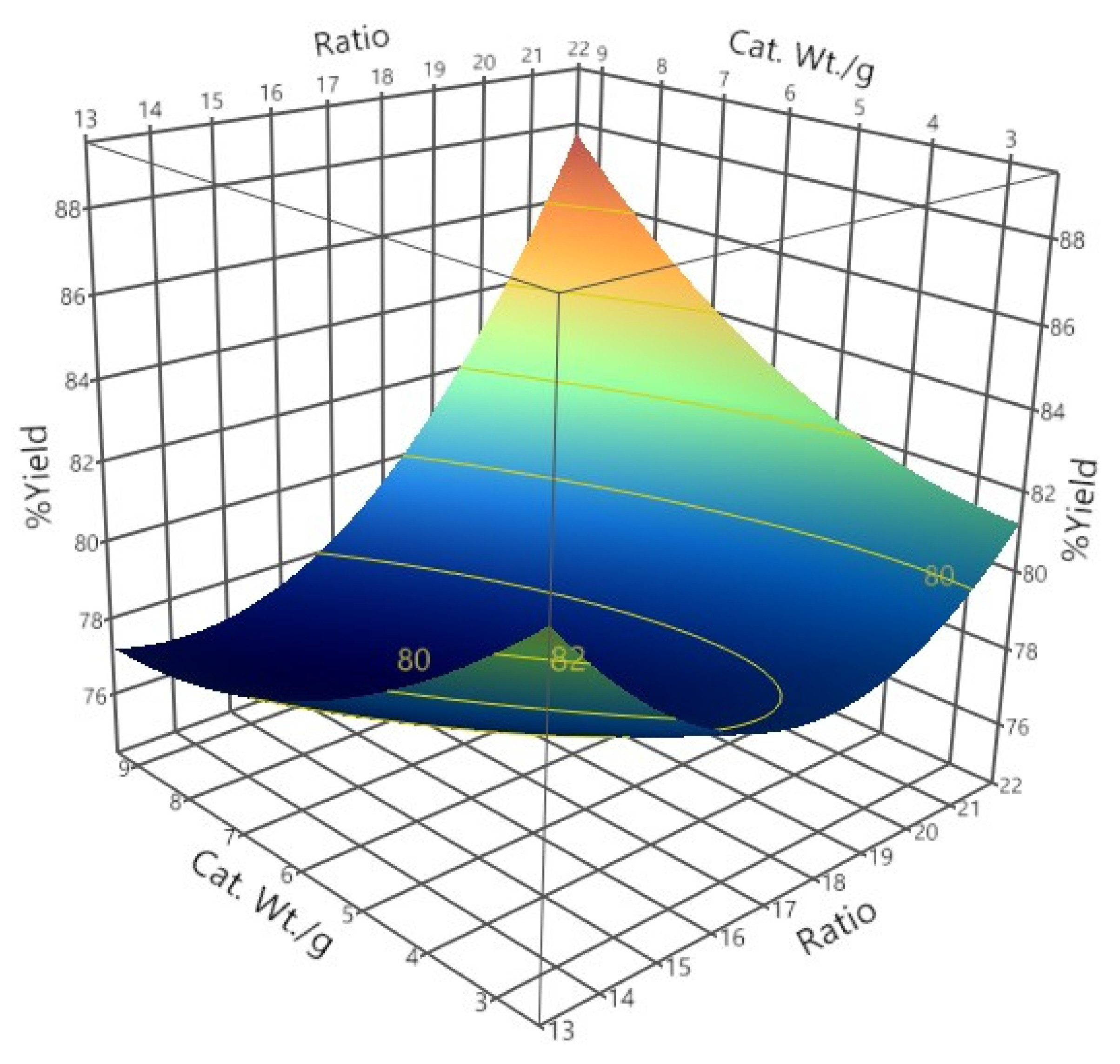
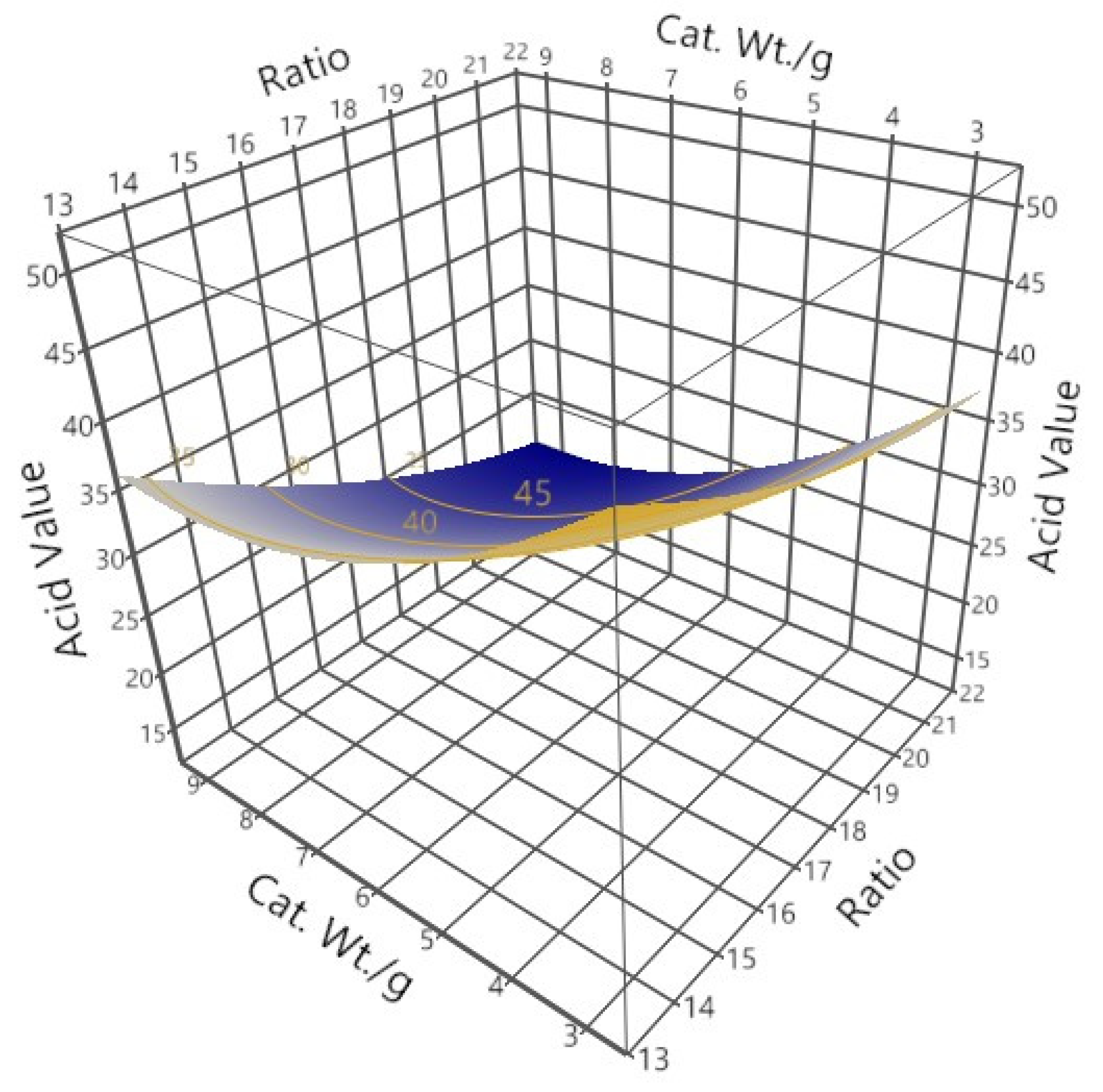
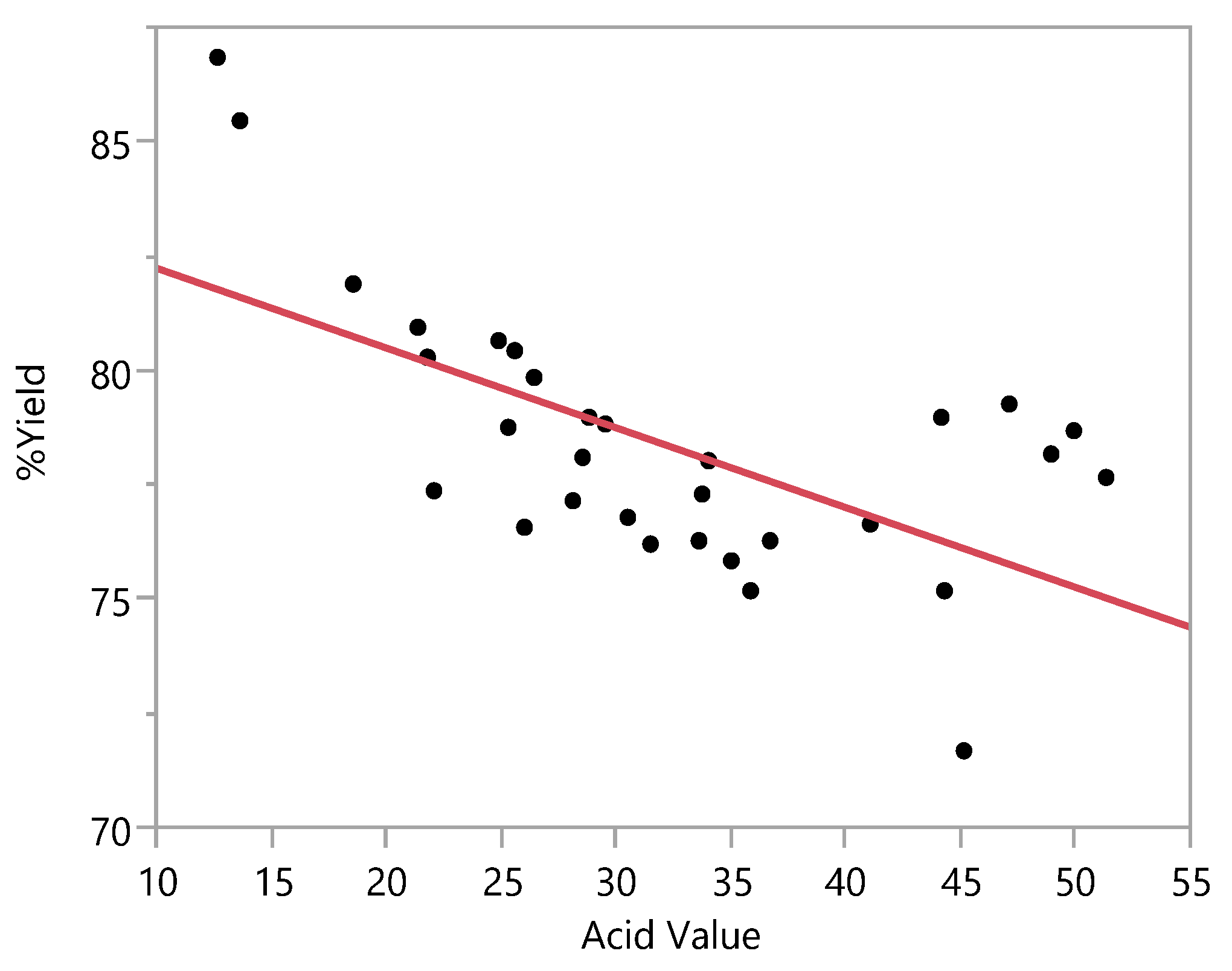
3.3.1. Biodiesel Yield
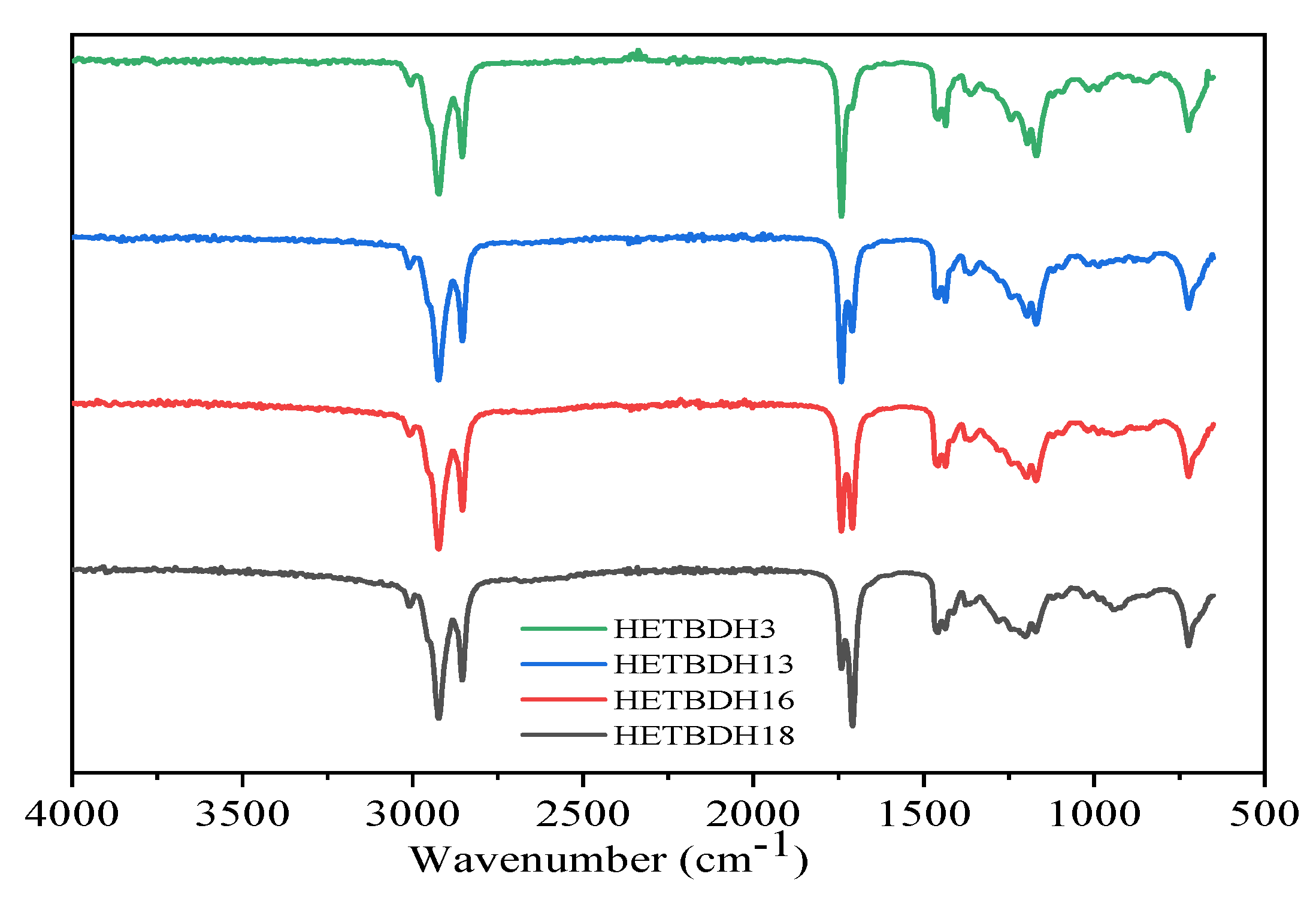
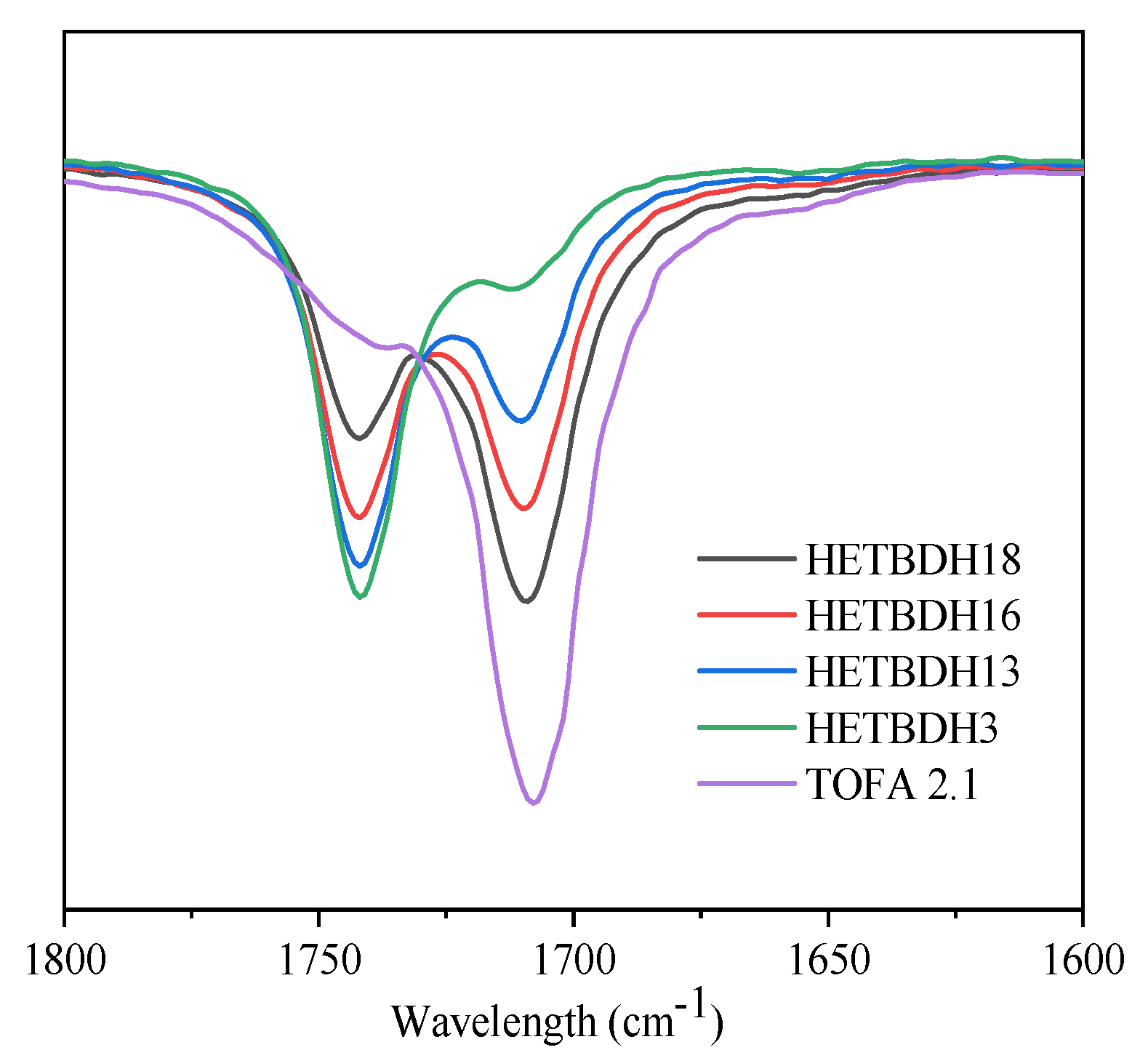
3.3.2. FTIR Characterization of Heterogeneous Catalysis of TOFA
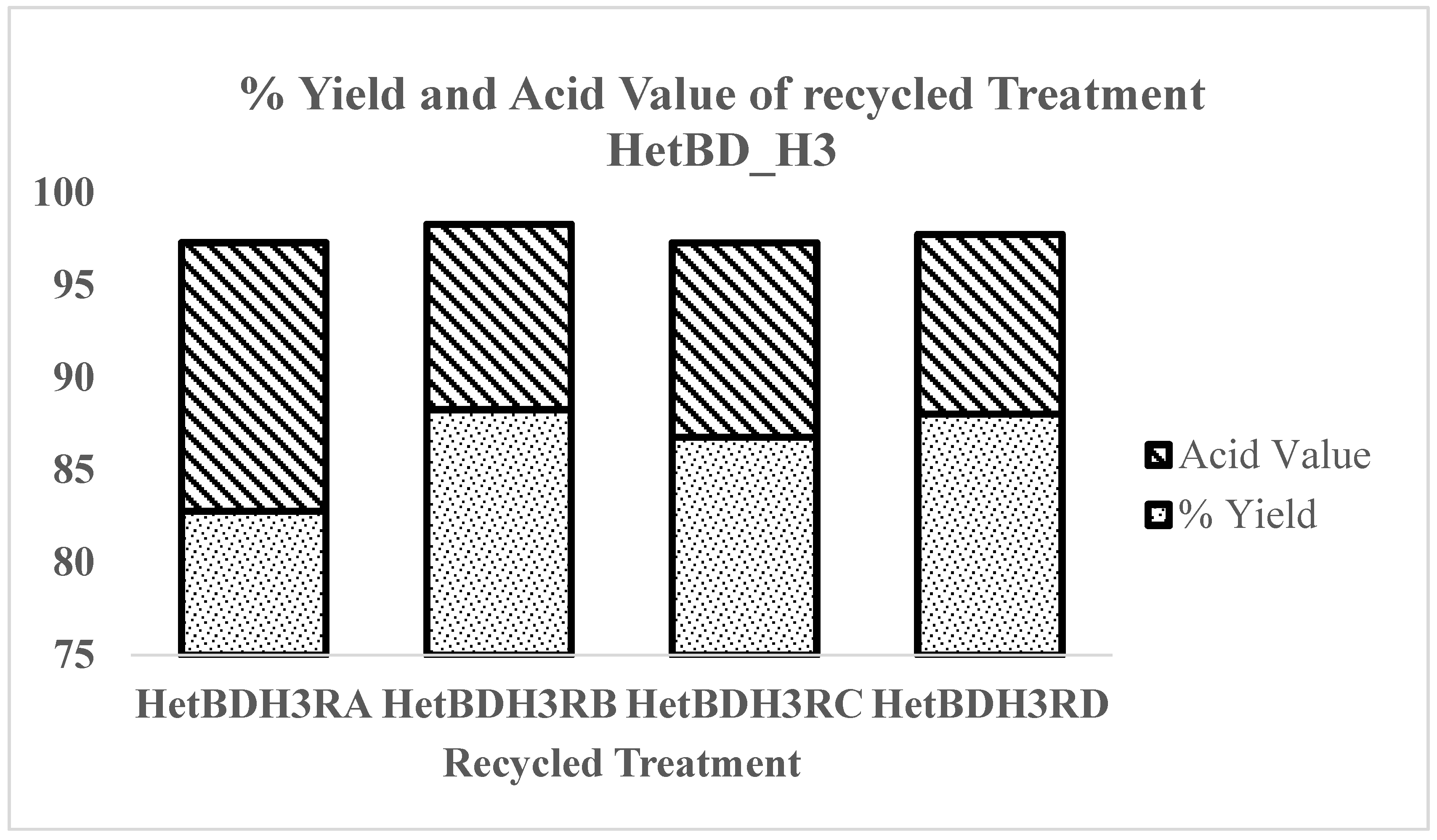
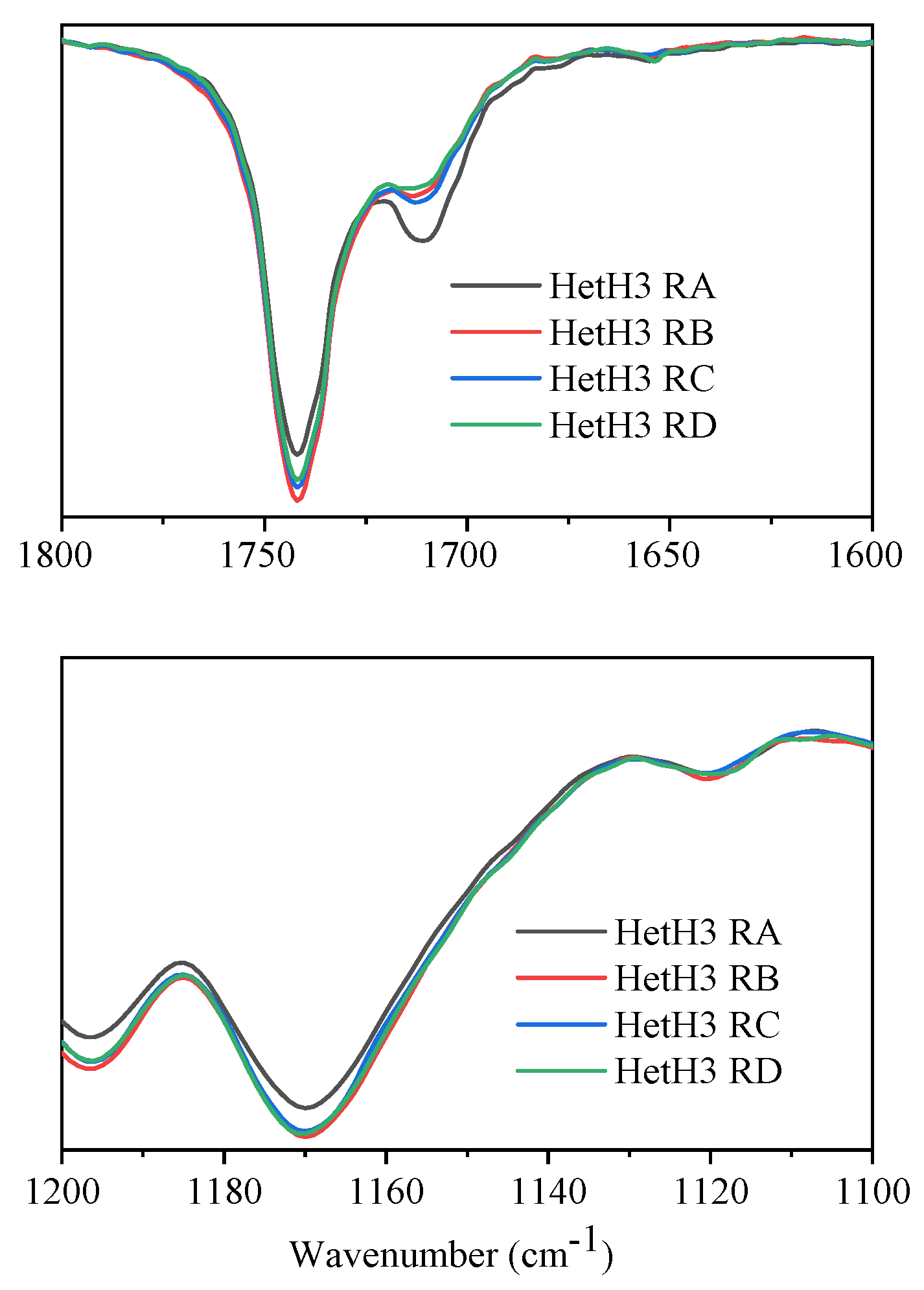
3.4. Recycling of Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kant Bhatia, S.; Kant Bhatia, R.; Jeon, J.-M.; Pugazhendhi, A.; Kumar Awasthi, M.; Kumar, D.; Kumar, G.; Yoon, J.-J.; Yang, Y.-H. An overview on advancements in biobased transesterification methods for biodiesel production: Oil resources, extraction, biocatalysts, and process intensification technologies. Fuel 2021, 285, 119117. [Google Scholar] [CrossRef]
- Aryan, V.; Kraft, A. The crude tall oil value chain: Global availability and the influence of regional energy policies. J. Clean. Prod. 2021, 280, 124616. [Google Scholar] [CrossRef]
- Pulidindi, K.; Prakash, A. Tall Oil Fatty Acid Market Size—Industry Trends Report 2024; Global Market Insights, Inc.: Delaware, DE, USA, 2017; Available online: https://www.gminsights.com/industry-analysis/tall-oil-fatty-acid-market (accessed on 16 June 2020).
- Avagyan, A.B.; Singh, B. Biodiesel from Plant Oil and Waste Cooking Oil. In Biodiesel: Feedstocks, Technologies, Economics and Barriers: Assessment of Environmental Impact in Producing and Using Chains; Avagyan, A.B., Singh, B., Eds.; Springer: Singapore, 2019; pp. 15–75. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Elsayed, M.; Esakkimuthu, S.; El-Sheekh, M.; Hanelt, D. Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci. 2020, 81, 100868. [Google Scholar] [CrossRef]
- Pinzi, S.; Garcia, I.L.; Lopez-Gimenez, F.J.; Luque de Castro, M.D.; Dorado, G.; Dorado, M.P. The Ideal Vegetable Oil-based Biodiesel Composition: A Review of Social, Economical and Technical Implications. Energy Fuels 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Sander, A.; Antonije Košćak, M.; Kosir, D.; Milosavljević, N.; Parlov Vuković, J.; Magić, L. The influence of animal fat type and purification conditions on biodiesel quality. Renew. Energy 2018, 118, 752–760. [Google Scholar] [CrossRef]
- Nasreen, S.; Nafees, M.; Qureshi, L.A.; Asad, M.S.; Sadiq, A.; Ali, S.D. Review of Catalytic Transesterification Methods for Biodiesel Production. Biofuels State Dev. 2018. [Google Scholar] [CrossRef]
- Bouaid, A.; Vázquez, R.; Martinez, M.; Aracil, J. Effect of free fatty acids contents on biodiesel quality. Pilot plant studies. Fuel 2016, 174, 54–62. [Google Scholar] [CrossRef]
- Brännström, H.; Kumar, H.; Alén, R. Current and Potential Biofuel Production from Plant Oils. Bioenergy Res. 2018, 11, 592–613. [Google Scholar] [CrossRef]
- Islam, M.S.; Christopher, L.P.; Alam, M.N. Separation and Purification of ω-6 Linoleic Acid from Crude Tall Oil. Separations 2020, 7, 9. [Google Scholar] [CrossRef]
- Govorin, A.S.; Gubanov, N.D.; Konovalov, N.P. Esterification of tall oil fatty acids using ion exchange resins in order to produce energy-efficient engine oil. IOP Conf. Ser. Earth Environ. Sci. 2020, 408, 012074. [Google Scholar] [CrossRef]
- Adewale, P.; Christopher, L.P. Thermal and Rheological Properties of Crude Tall Oil for Use in Biodiesel Production. Processes 2017, 5, 59. [Google Scholar] [CrossRef]
- Lawer-Yolar, G.S. Biodiesel from Tall Oil Fatty Acids and Physico Thermal Properties of Tropical Tree Fruit Oils as Thermal Energy Storage Systems. Ph.D. Thesis, West Virginia University, Morgantown, WV, USA, 2015. [Google Scholar] [CrossRef]
- White, K.; Lorenz, N.; Potts, T.; Penney, W.R.; Babcock, R.; Hardison, A.; Canuel, E.A.; Hestekin, J.A. Production of biodiesel fuel from tall oil fatty acids via high temperature methanol reaction. Fuel 2011, 90, 3193–3199. [Google Scholar] [CrossRef]
- Sakdasri, W.; Sawangkeaw, R.; Ngamprasertsith, S. Techno-economic analysis of biodiesel production from palm oil with supercritical methanol at a low molar ratio. Energy 2018, 152, 144–153. [Google Scholar] [CrossRef]
- Abstract: Biodiesel from Tall Oil Fatty Acids Using Homogeneous and Heterogeneous Catalysts (21st National Annual Meeting (7–12 June 2009)). Available online: https://nam.confex.com/nam/2009/webprogram/Paper2285.html (accessed on 28 May 2020).
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.M.A.; Ahmad, A. State of the Art of Catalysts for Biodiesel Production. Front. Energy Res. 2020, 8, 8. [Google Scholar] [CrossRef]
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Islam, A.; Taufiq-Yap, Y.H.; Chu, C.-M.; Chan, E.-S.; Ravindra, P. Studies on design of heterogeneous catalysts for biodiesel production. Process Saf. Environ. Prot. 2013, 91, 131–144. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Adeniyi, O.D.; Olutoye, M.A.; Akpan, U.G. A Review on Application of Heterogeneous Catalyst in the Production of Biodiesel from Vegetable Oils. J. Appl. Sci. Process Eng. 2017, 4, 142–157. [Google Scholar] [CrossRef]
- Boz, N.; Degirmenbasi, N.; Kalyon, D.M. Esterification and transesterification of waste cooking oil over Amberlyst 15 and modified Amberlyst 15 catalysts. Appl. Catal. B Environ. 2015, 165, 723–730. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Wu, J.C.; Lau, S.K.; Cui, L.C.; Shimin, G.; Lim, A. Comparison of Novozym 435 and Amberlyst 15 as Heterogeneous Catalyst for Production of Biodiesel from Palm Fatty Acid Distillate. Energy Fuels 2009, 23, 1–4. [Google Scholar] [CrossRef]
- Schultz, A.K.; Hanlon, R.T.; Banavali, R. Heterogeneous Catalyst and Process for Production of Biodiesel; Dow Chemical: Spring House, PA, USA, 2011. [Google Scholar]
- Park, J.-Y.; Kim, D.-K.; Lee, J.-S. Esterification of free fatty acids using water-tolerable Amberlyst as a heterogeneous catalyst. Bioresour. Technol. 2010, 101, S62–S65. [Google Scholar] [CrossRef]
- Biodiesel Magazine. The Latest News and Data about Biodiesel Production. Available online: http://www.biodieselmagazine.com/articles/3193/rohm-and-haas-releases-new-technology (accessed on 2 July 2020).
- Keskin, A.; Yaşar, A.; Gürü, M.; Altıparmak, D. Usage of methyl ester of tall oil fatty acids and resinic acids as alternative diesel fuel. Energy Convers. Manag. 2010, 51, 2863–2868. [Google Scholar] [CrossRef]
- Keskin, A.; Gürü, M.; Altıparmak, D. Influence of tall oil biodiesel with Mg and Mo based fuel additives on diesel engine performance and emission. Bioresour. Technol. 2008, 99, 6434–6438. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpourpia, R.; Adamopoulos, S.; Parsland, C. Utilization of different tall oils for improving the water resistance of cellulosic fibers. J. Appl. Polym. Sci. 2019, 136, 47303. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2014; Available online: https://www.wiley.com/en-us/Spectrometric+Identification+of+Organic+Compounds%2C+8th+Edition-p-9780470616376 (accessed on 6 January 2021).
- Yadav, L.D.S. Organic Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Y.; Gong, Y.; Liu, X. Analysis of the oxidative degradation of biodiesel blends using FTIR, UV–Vis, TGA and TD-DES methods. Fuel 2017, 202, 23–28. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Fourier Transform Infrared Spectroscopy (FTIR) Method to Monitor Soy Biodiesel and Soybean Oil in Transesterification Reactions, Petrodiesel−Biodiesel Blends, and Blend Adulteration with Soy Oil. Energy Fuels 2009, 23, 3773–3782. [Google Scholar] [CrossRef]
- Praptijanto, A.; Sebayang, D.; Agustian, E.; Untoro, P. Rapid monitoring of fatty acid methyl ester in sonochemistry transesterification process using attenuated total reflection. In Proceedings of the International Conference on Environment (ICENV2010), Penang, Malaysia, 13–15 December 2010. [Google Scholar]
- Dymińska, L.; Calik, M.; Albegar, A.M.M.; Zając, A.; Kostyń, K.; Lorenc, J.; Hanuza, J. Quantitative determination of the iodine values of unsaturated plant oils using infrared and Raman spectroscopy methods. Int. J. Food Prop. 2017, 20, 2003–2015. [Google Scholar] [CrossRef]
- López, D.E.; Goodwin, J.G.; Bruce, D.A.; Lotero, E. Transesterification of triacetin with methanol on solid acid and base catalysts. Appl. Catal. A Gen. 2005, 295, 97–105. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Aishah, N.; Saidina Amin, N.A.; Zarei, A.; Noshadi, I. Transesterification of waste cooking oil by heteropoly acid (HPA) catalyst: Optimization and kinetic model. Appl. Energy 2012. [Google Scholar] [CrossRef]


| Factors | Levels for Homogeneous Catalysis | Levels for Heterogeneous Catalysis | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 | Methanol: oil ratio | 6:1 | 15:1 | 13.5 | 15 | 17.5 | 20 | 21.7 |
| X2 | Temperature °C | 55 | 75 | 75–80 | 75–80 | 75–80 | 75–80 | 75–80 |
| X3 | Catalyst concentration (w/w%) | 0.5 | 2 | 2.64 | 4 | 6 | 8 | 9.36 |
| X4 | Time (Min) | 30 | 60 | 1.3 | 2 | 3 | 4 | 4.7 |
| Element | Amount in Sample/mg/L |
|---|---|
| Al | 2.29 ± 0.73 |
| Fe | 2.42 ± 3.20 |
| Mg | 0.45 ± 0.34 |
| Ca | 5.25 ± 0.68 |
| K | 0 |
| Na | 5.97 ± 4.54 |
| Cu | 0 |
| Zn | 0.63 ± 0.14 |
| Fuel Properties | TOFA | Biodiesel |
| Kinematic Viscosity/cSt. | 20.61 ± 0.06 | 5.22 ± 0.02 |
| Specific Gravity @25/25 °C | 0.904 ± 0.00 | 0.97 ± 0.00 |
| Specific Gravity @60/25 °C | 0.89 ± 0.00 | 0.9707 ± 0.00 |
| Water Content/v/v | - | 0.00 |
| Acid Value/mg/g of oleic acid 113.18 ± 0.01 | 4.24 ± 0.12 | |
| Fatty acid composition of TOFA% | ||
| C15:1(cis-10) | 1.41 | |
| C16:1(cis-9) | 0.87 | |
| C18:1(cis-9) | 57.72 | |
| C18:2(cis-9,12) | 35.41 | |
| C20:0 | 2.61 | |
| Other Minor Fatty Acids (<0.5%) | 1.98 | |
| Runs | Levels of Factors | Mean %Yield (Stdev.) | |||
|---|---|---|---|---|---|
| Methanol: Oil Ratio | Temperature (°C) | Catalyst Concentration (%w/w) | Time (Min) | ||
| 1 | 6:1 | 55 | 0.5 | 30 | 92.20 (1.86) |
| 2 | 6:1 | 55 | 2 | 60 | 76.19 (10.9) |
| 3 | 15:1 | 55 | 0.5 | 60 | 91.87 (3.12) |
| 4 | 15:1 | 55 | 2 | 30 | 85.94 (4.84) |
| 5 | 6:1 | 75 | 0.5 | 60 | 83.58 (3.98) |
| 6 | 6:1 | 75 | 2 | 30 | 85.73 (1.25) |
| 7 | 15:1 | 75 | 0.5 | 30 | 85.27 (0.11) |
| 8 | 15:1 | 75 | 2 | 60 | 84.39 (3.96) |
| 9 | 15:1 | 55 | 2 | 60 | 93.89 (0.14) |
| 10 | 6:1 | 75 | 0.5 | 30 | 89.66 (3.33) |
| 11 | 6:1 | 55 | 0.5 | 60 | 92.58 (1.27) |
| 12 | 15:1 | 55 | 0.5 | 30 | 91.39 (1.67) |
| 13 | 6:1 | 55 | 2 | 30 | 87.01 (1.13) |
| 14 | 15:1 | 75 | 0.5 | 60 | 94.72 (0.26) |
| 15 | 6:1 | 75 | 2 | 60 | 0 |
| 16 | 15:1 | 75 | 2 | 30 | 93.32 (91.30) |
| Runs | Levels of Factors | Mean %Yield (Stdev.) | Mean Acid Value (Stdev.) | ||
|---|---|---|---|---|---|
| Methanol: Oil Ratio | Catalyst Concentration (%w/w) | Time (h.) | |||
| 1 | 17.5 | 2.64 | 3 | 77.33 (1.53) | 35.26 (8.16) |
| 2 | 15 | 8 | 2 | 81.18 (2.63) | 23.94 (2.98) |
| 3 | 20 | 8 | 4 | 86.18 (0.98) | 13.12 (2.01) |
| 4 | 21.7 | 6 | 3 | 81.42 (0.65) | 19.95 (2.01) |
| 5 | 17.5 | 6 | 1.3 | 74.22 (3.58) | 37.88 (10.32) |
| 6 | 17.5 | 6 | 3 | 77.38 (1.30) | 28.32 (3.10) |
| 7 | 15 | 4 | 2 | 78.57 (1.80) | 30.09 (5.17) |
| 8 | 17.5 | 6 | 4.7 | 76.64 (2.06) | 32.21 (5.13) |
| 9 | 20 | 4 | 2 | 80.51 (0.16) | 25.24 (0.43) |
| 10 | 20 | 8 | 2 | 77.62 (1.91) | 32.80 (5.61) |
| 11 | 17.5 | 9.36 | 3 | 77.13 (1.21) | 33.85 (0.28) |
| 12 | 15 | 8 | 4 | 76.62 (1.10) | 28.51 (9.16) |
| 13 | 15 | 4 | 4 | 78.96 (0.45) | 48.55 (1.97) |
| 14 | 20 | 4 | 4 | 77.91 (0.38) | 50.20 (1.67) |
| 15 | 20 | 4 | 4 | 77.08 (2.67) | 44.27 (0.13) |
| Recycle | Biodiesel Wt./g | %Yield | Acid Value |
|---|---|---|---|
| HetBDH3RA | 33.11 | 82.77 | 14.53 |
| HetBDH3RB | 35.30 | 88.26 | 10.03 |
| HetBDH3RC | 34.71 | 86.77 | 10.52 |
| HetBDH3RD | 35.21 | 88.02 | 9.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawer-Yolar, G.; Dawson-Andoh, B.; Atta-Obeng, E. Synthesis of Biodiesel from Tall Oil Fatty Acids by Homogeneous and Heterogeneous Catalysis. Sustain. Chem. 2021, 2, 206-221. https://doi.org/10.3390/suschem2010012
Lawer-Yolar G, Dawson-Andoh B, Atta-Obeng E. Synthesis of Biodiesel from Tall Oil Fatty Acids by Homogeneous and Heterogeneous Catalysis. Sustainable Chemistry. 2021; 2(1):206-221. https://doi.org/10.3390/suschem2010012
Chicago/Turabian StyleLawer-Yolar, Gideon, Benjamin Dawson-Andoh, and Emmanuel Atta-Obeng. 2021. "Synthesis of Biodiesel from Tall Oil Fatty Acids by Homogeneous and Heterogeneous Catalysis" Sustainable Chemistry 2, no. 1: 206-221. https://doi.org/10.3390/suschem2010012
APA StyleLawer-Yolar, G., Dawson-Andoh, B., & Atta-Obeng, E. (2021). Synthesis of Biodiesel from Tall Oil Fatty Acids by Homogeneous and Heterogeneous Catalysis. Sustainable Chemistry, 2(1), 206-221. https://doi.org/10.3390/suschem2010012





