Tranexamic Acid-Phenol Smart Scaffolds with Imine Linker: Unlocking Antimicrobial Potential Through In Vitro and In Silico Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedures and Materials
2.2. Characterization
General Procedure for Synthesis 3a–3k
2.3. Antimicrobial Activity
2.4. Molecular Docking Methodology
3. Results and Discussion
3.1. Synthesis and Characterization of Phenol-TXA Derivatives
3.2. Biological Assay
3.2.1. Antimicrobial Assay
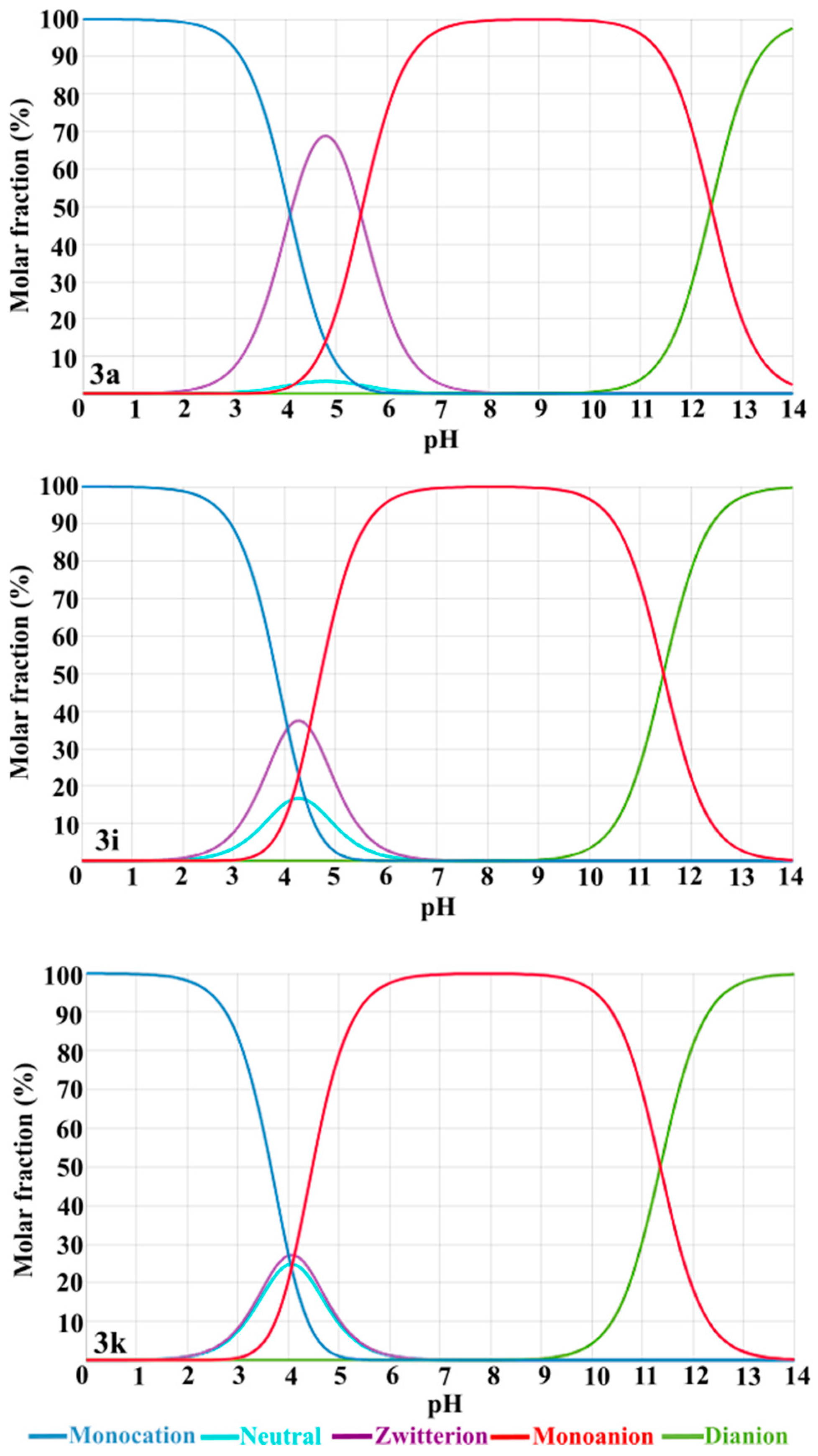
3.2.2. Stability Assay
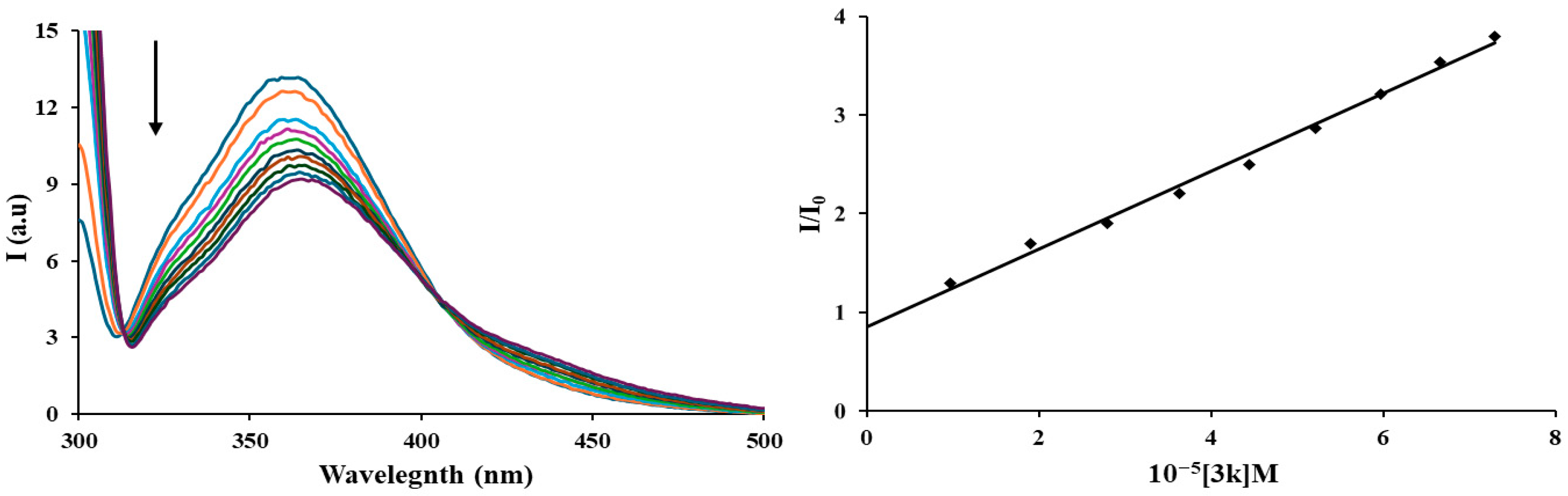
3.3. Protein Binding Studies—Fluorescence Spectroscopy of HSA
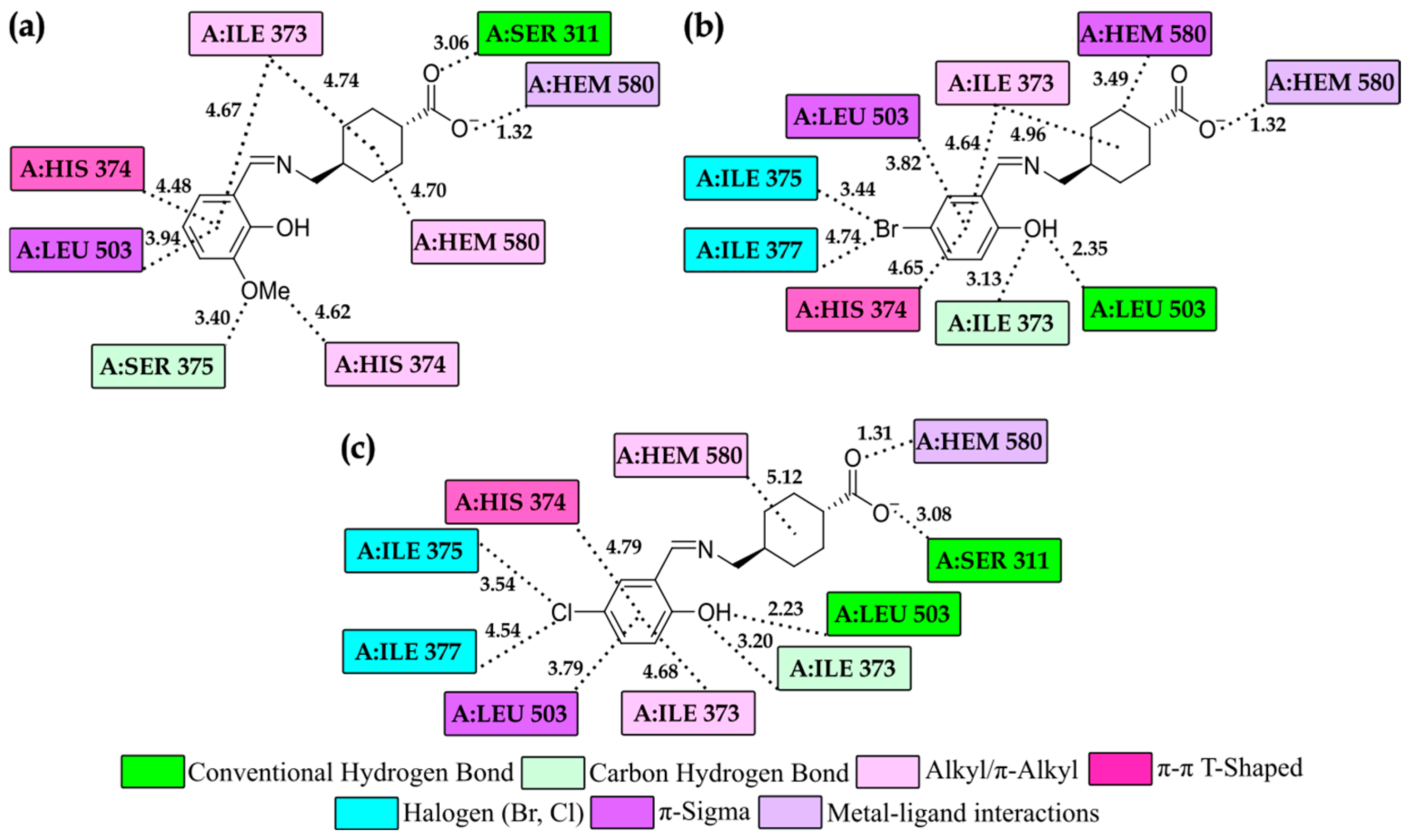
3.4. Molecular Docking Study
Molecular Docking Study of Complexes in the CYP51B
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franchini, M.; Mannucci, P.M. Adjunct agents for bleeding. Curr. Opin. Hematol. 2014, 21, 503–508. [Google Scholar] [CrossRef]
- Bouras, M.; Bourdiol, A.; Rooze, P.; Hourmant, Y.; Caillard, A.; Roquilly, A. Tranexamic acid: A narrative review of its current role in perioperative medicine and acute medical bleeding. Front. Med. 2024, 11, 1416998. [Google Scholar] [CrossRef]
- Cai, J.; Ribkoff, J.; Olson, S.; Raghunathan, V.; Al-Samkari, H.; DeLoughery, T.G.; Shatzel, J.J. The many roles of tranexamic acid: An overview of the clinical indications for TXA in medical and surgical patients. Eur. J. Haematol. 2020, 104, 79–87. [Google Scholar] [CrossRef]
- Hibbs, S.P.; Roberts, I.; Shakur-Still, H.; Hunt, B.J. Post-partum haemorrhage and tranexamic acid: A global issue. Br. J. Haematol. 2018, 180, 799–807. [Google Scholar] [CrossRef]
- Lier, H.; Maegele, M.; Shander, A. Tranexamic Acid for Acute Hemorrhage: A Narrative Review of Landmark Studies and a Critical Reappraisal of Its Use Over the Last Decade. Anesth. Analg. 2019, 129, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.J.; Spinella, P.C.; Bochicchio, G.V. Tranexamic Acid Update in Trauma. Crit. Care Clin. 2017, 33, 85–99. [Google Scholar] [CrossRef] [PubMed]
- da Silva Souza, I.D.; Lampe, L.; Winn, D. New topical tranexamic acid derivative for the improvement of hyperpigmentation and inflammation in the sun-damaged skin. J. Cosmet. Dermatol. 2021, 20, 561–565. [Google Scholar] [CrossRef]
- Lee, D.H.; Oh, I.Y.; Koo, K.T.; Suk, J.M.; Jung, S.W.; Park, J.O.; Kim, B.J.; Choi, Y.M. Reduction in facial hyperpigmentation after treatment with a combination of topical niacinamide and tranexamic acid: A randomized, double-blind, vehicle-controlled trial. Ski. Res. Technol. 2014, 20, 208–212. [Google Scholar] [CrossRef]
- Cruz, A.C.L.; Cassiano, D.P.; Bleixuvehl de Brito, M. Oral ketotifen associated with oral tranexamic acid for the treatment of facial melasma in women: A randomized, double-blind, placebo-controlled trial. J. Eur. Acad. Dermatol. Venereol. 2025, 39, e39–e41. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, M.; Chen, Y.; Xie, H.; Ding, P.; Zhang, K. Enhancing skin delivery of tranexamic acid via esterification: Synthesis and evaluation of alkyl ester derivatives. RSC Adv. 2024, 14, 34996–35004. [Google Scholar] [CrossRef] [PubMed]
- Anwer, J.; Ali, S.; Shahzadi, S.; Shahid, M.; Sharma, S.K.; Qanungo, K. Synthesis, characterization, semi-empirical study and biological activities of homobimetallic complexes of tranexamic acid with organotin(IV). J. Coord. Chem. 2013, 66, 1142–1152. [Google Scholar] [CrossRef]
- Shahzadi, S.; Ali, S.; Parvez, M.; Badshah, A.; Ahmed, E.; Malik, A. Synthesis, spectroscopy and antimicrobial activity of vanadium(III) and vanadium(IV) complexes involving Schiff bases derived from Tranexamic acid and X-ray structure of zwitter ion of Tranexamic acid. Russ. J. Inorg. Chem. 2007, 52, 386–393. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Sadeek, S.A.; Rashid, N.G.; Elshafie, H.S.; Camele, I. Synthesis, Characterization and Evaluation of the Antimicrobial and Herbicidal Activities of Some Transition Metal Ions Complexes with the Tranexamic Acid. Chem. Biodivers. 2024, 21, e202301970. [Google Scholar] [CrossRef]
- Naglah, A.M.; Almehizia, A.A.; Al-Wasidi, A.S.; Alharbi, A.S.; Alqarni, M.H.; Hassan, A.S.; Aboulthana, W.M. Exploring the Potential Biological Activities of Pyrazole-Based Schiff Bases as Anti-Diabetic, Anti-Alzheimer’s, Anti-Inflammatory, and Cytotoxic Agents: In Vitro Studies with Computational Predictions. Pharmaceuticals 2024, 17, 655. [Google Scholar] [CrossRef]
- Gul, S.; Alam, A.; Zainab; Assad, M.; Elhenawy, A.A.; Islam, M.S.; Shah, S.A.A.; Parveen, Z.; Shah, T.A.; Ahmad, M. Exploring the synthesis, molecular structure and biological activities of novel Bis-Schiff base derivatives: A combined theoretical and experimental approach. J. Mol. Struct. 2024, 1306, 137828. [Google Scholar] [CrossRef]
- Wajid, M.; Uzair, M.; Muhammad, G.; Siddique, F.; Ashraf, A.; Ahmad, S.; Alasmari, A.F. Biological Activities, DFT and Molecular Docking Studies of Novel Schiff Bases Derived from Sulfamethoxypyridazine. ChemistrySelect 2024, 9, e202400675. [Google Scholar] [CrossRef]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases—Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef] [PubMed]
- Musikavanhu, B.; Liang, Y.; Xue, Z.; Feng, L.; Zhao, L. Strategies for Improving Selectivity and Sensitivity of Schiff Base Fluorescent Chemosensors for Toxic and Heavy Metals. Molecules 2023, 28, 6960. [Google Scholar] [CrossRef]
- GÜLÇİN, İ.; Daştan, A. Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J. Enzym. Inhib. Med. Chem. 2007, 22, 685–695. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium. Stud. Mycol. 2004, 49, 1–174. [Google Scholar]
- Armbruster, C.E.; Mobley, H.L.T.; Pearson, M.M. Pathogenesis of Proteus mirabilis Infection. EcoSal Plus 2018, 8, 10–1128. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Wawrzak, Z.; Lamb, D.C.; Guengerich, F.P.; Lepesheva, G.I. Structure-Functional Characterization of Cytochrome P450 Sterol 14α-Demethylase (CYP51B) from Aspergillus fumigatus and Molecular Basis for the Development of Antifungal Drugs. J. Biol. Chem. 2015, 290, 23916–23934. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Fuhrmann, J.; Rurainski, A.; Lenhof, H.; Neumann, D. A new Lamarckian genetic algorithm for flexible ligand-receptor docking. J. Comput. Chem. 2010, 31, 1911–1918. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA Version 26; Dassault Systèmes: Vélizy-Villacoublay, France, 2024.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallin, CT, USA, 2019. [Google Scholar]
- Shah, F.A.; Ali, S.; Shahzadi, S. Spectral and biological studies of newly synthesized organotin(IV) complexes of 4-({[(E)-(2-hydroxyphenyl)methylidene]amino}methyl)cyclohexane carboxylic acid Schiff base. J. Iran. Chem. Soc. 2010, 7, 59–68. [Google Scholar] [CrossRef]
- Danish, M.; Akbar, S.; Tahir, M.N.; Butt, R.A.; Ashfaq, M. Crystal structure of 4-{[(2,4-dihydroxybenzylidene)amino]methyl}cyclohexanecarboxylic acid. Acta Crystallogr. Sect. E Crystallogr. Commun. 2015, 71, o995–o996. [Google Scholar] [CrossRef] [PubMed]
- Karan, J.; Kundu, A.; Gogoi, R.; Manjaiah, K.M.; Singh, A.K.; Mondal, K.; Kumar, R.; Kaushik, P.; Saini, P.; Rana, V.S.; et al. Synthesis of antifungal imines as inhibitors of ergosterol biosynthesis. J. Taibah Univ. Sci. 2025, 19, 2448897. [Google Scholar] [CrossRef]
- Login, C.C.; Bâldea, I.; Tiperciuc, B.; Benedec, D.; Vodnar, D.C.; Decea, N.; Suciu, Ş. A Novel Thiazolyl Schiff Base: Antibacterial and Antifungal Effects and In Vitro Oxidative Stress Modulation on Human Endothelial Cells. Oxid. Med. Cell Longev. 2019, 2019, 1607903. [Google Scholar] [CrossRef] [PubMed]
- Perz, M.; Szymanowska, D.; Kostrzewa-Susłow, E. The Influence of Flavonoids with -Br, -Cl Atoms and -NO2, -CH3 Groups on the Growth Kinetics and the Number of Pathogenic and Probiotic Microorganisms. Int. J. Mol. Sci. 2024, 25, 9269. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Matczak, K.; Olchowik-Grabarek, E.; Sękowski, S.; Nowicka, P.; Krawczyk-Łebek, A.; Kostrzewa-Susłow, E. The influence of the chlorine atom on the biological activity of 2′-hydroxychalcone in relation to the lipid phase of biological membranes—Anticancer and antimicrobial activity. Chem. Biol. Interact. 2024, 398, 111082. [Google Scholar] [CrossRef]
- Krawczyk-Łebek, A.; Żarowska, B.; Janeczko, T.; Kostrzewa-Susłow, E. Antimicrobial Activity of Chalcones with a Chlorine Atom and Their Glycosides. Int. J. Mol. Sci. 2024, 25, 9718. [Google Scholar] [CrossRef]
- Olanrewaju, R.O.; Lee, J.-H.; Kim, Y.-G.; Lee, J. Antimicrobial and antibiofilm activities of halogenated phenols against Staphylococcus aureus and other microbes. Chemosphere 2024, 367, 143646. [Google Scholar] [CrossRef] [PubMed]
- Vesović, M.; Jelić, R.; Nikolić, M.; Nedeljković, N.; Živanović, A.; Bukonjić, A.; Mrkalić, E.; Radić, G.; Ratković, Z.; Kljun, J.; et al. Investigation of the interaction between S-isoalkyl derivatives of the thiosalicylic acid and human serum albumin. J. Biomol. Struct. Dyn. 2025, 43, 4081–4094. [Google Scholar] [CrossRef]
- Dimiza, F.; Perdih, F.; Tangoulis, V.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Interaction of copper(II) with the non-steroidal anti-inflammatory drugs naproxen and diclofenac: Synthesis, structure, DNA- and albumin-binding. J. Inorg. Biochem. 2011, 105, 476–489. [Google Scholar] [CrossRef]
- Živković, N.; Mrkalić, E.; Jelić, R.; Tomović, J.; Odović, J.; Serafinović, M.Ć.; Sovrlić, M. The Molecular Recognition of Lurasidone by Human Serum Albumin: A Combined Experimental and Computational Approach. Molecules 2025, 30, 1420. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Gryczynski, I.; Gryczynski, Z.; Dattelbaum, J.D. Anisotropy-Based Sensing with Reference Fluorophores. Anal. Biochem. 1999, 267, 397–405. [Google Scholar] [CrossRef]
- Marjanović, J.S.; Matić, J.D.; Milanović, Ž.; Divac, V.M.; Kosanić, M.M.; Petković, M.R.; Kostić, M.D. Molecular modeling studies, in vitro antioxidant and antimicrobial assay and BSA affinity of novel benzyl-amine derived scaffolds as CYP51B inhibitors. Mol. Divers. 2024, 29, 5499–5521. [Google Scholar] [CrossRef]
- Ferreira de Freitas, R. Schapira MAsystematic analysis of atomic protein–ligand interactions in the PDB. Medchemcomm 2017, 8, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Bissantz, C.; Kuhn, B.; Stahl, M. A Medicinal Chemist’s Guide to Molecular Interactions. J. Med. Chem. 2010, 53, 5061–5084. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Honda, K.; Uchimaru, T.; Mikami, M.; Tanabe, K. Origin of Attraction and Directionality of the π/π Interaction: Model Chemistry Calculations of Benzene Dimer Interaction. J. Am. Chem. Soc. 2002, 124, 104–112. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed]



| Tested Compounds | Bacillus subtilis | Staphylococcus aureus | Escherichia coli | Proteus mirabilis | Klebsiella pneumoniae |
|---|---|---|---|---|---|
| MIC (mg/mL) | |||||
| 3a | 0.234 | 0.937 | 0.468 | 0.234 | 0.234 |
| 3b | 0.937 | 1.875 | 1.875 | 0.468 | 0.468 |
| 3c | 0.937 | 1.875 | 0.937 | 0.468 | 0.468 |
| 3d | 0.117 | 1.875 | 0.234 | 0.117 | 0.234 |
| 3e | 0.468 | 1.875 | 0.937 | 0.468 | 0.937 |
| 3f | 1.875 | 3.75 | 1.875 | 0.937 | 0.937 |
| 3g | 7.5 | 7.5 | 7.5 | 3.75 | 7.5 |
| 3h | 1.875 | 3.75 | 3.75 | 0.468 | 3.75 |
| 3i | 0.234 | 0.234 | 0.468 | 0.058 | 0.234 |
| 3j | 0.234 | 0.234 | 0.937 | 0.234 | 3.75 |
| 3k | 0.117 | 0.234 | 0.468 | 0.058 | 0.234 |
| Streptomycine | 0.016 | 0.031 | 0.062 | 0.062 | 0.031 |
| Tested Compounds | Mucor mucedo | Cladosporium cladosporioides | Penicillium italicum | Aspergillus fumigatus | Rhizopus stolonifer |
|---|---|---|---|---|---|
| MIC (mg/mL) | |||||
| 3a | 0.234 | 0.117 | 0.058 | 0.058 | 0.234 |
| 3b | 0.234 | 0.234 | 0.117 | 0.117 | 0.468 |
| 3c | 0.234 | 0.234 | 0.117 | 0.234 | 0.468 |
| 3d | 0.468 | 0.234 | 0.117 | 0.234 | 0.468 |
| 3e | 0.234 | 0.117 | 0.058 | 0.117 | 0.468 |
| 3f | 0.234 | 0.234 | 0.058 | 0.234 | 0.468 |
| 3g | 0.468 | 0.468 | 0.234 | 0.117 | 0.234 |
| 3h | 0.937 | 0.234 | 0.058 | 0.117 | 0.937 |
| 3i | 0.234 | 0.058 | 0.029 | 0.029 | 0.117 |
| 3j | 0.468 | 0.029 | 0.014 | 0.029 | 0.234 |
| 3k | 0.058 | 0.029 | 0.014 | 0.058 | 0.117 |
| Ketokonazole | 0.156 | 0.039 | 0.156 | 0.156 | 0.156 |
| Compound | Ksv [M−1] | kq [M−1 s−1] |
|---|---|---|
| 3k | 3.9 × 104 | 3.9 × 1012 |
| Compounds | ΔGbind | Ki (µM) | ΔGinter | ΔGvdw+hbond+desolv | ΔGelec | ΔGtotal | ΔGtor | ΔGunb |
|---|---|---|---|---|---|---|---|---|
| CYP51B | ||||||||
| 3a− | −12.77 | 4.38 × 10−4 | −14.56 | −6.68 | −7.88 | −1.26 | 1.79 | −1.26 |
| 3i− | −13.76 | 8.23 × 10−5 | −15.25 | −7.49 | −7.76 | −0.46 | 1.49 | −0.46 |
| 3k− | −13.56 | 1.15 × 10−4 | −15.05 | −7.18 | −7.86 | −0.44 | 1.49 | −0.44 |
| * KET | −11.74 | 2.50 × 10−2 | −13.83 | −13.83 | −13.83 | −13.83 | −13.83 | −13.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragojević, J.S.; Milanović, Ž.; Milisavljević, K.; Petrović, N.; Petronijević, J.; Joksimović, N.; Divac, V.M.; Kosanić, M.; Kostić, M.D. Tranexamic Acid-Phenol Smart Scaffolds with Imine Linker: Unlocking Antimicrobial Potential Through In Vitro and In Silico Insights. Organics 2025, 6, 54. https://doi.org/10.3390/org6040054
Dragojević JS, Milanović Ž, Milisavljević K, Petrović N, Petronijević J, Joksimović N, Divac VM, Kosanić M, Kostić MD. Tranexamic Acid-Phenol Smart Scaffolds with Imine Linker: Unlocking Antimicrobial Potential Through In Vitro and In Silico Insights. Organics. 2025; 6(4):54. https://doi.org/10.3390/org6040054
Chicago/Turabian StyleDragojević, Jovana S., Žiko Milanović, Kristina Milisavljević, Nevena Petrović, Jelena Petronijević, Nenad Joksimović, Vera M. Divac, Marijana Kosanić, and Marina D. Kostić. 2025. "Tranexamic Acid-Phenol Smart Scaffolds with Imine Linker: Unlocking Antimicrobial Potential Through In Vitro and In Silico Insights" Organics 6, no. 4: 54. https://doi.org/10.3390/org6040054
APA StyleDragojević, J. S., Milanović, Ž., Milisavljević, K., Petrović, N., Petronijević, J., Joksimović, N., Divac, V. M., Kosanić, M., & Kostić, M. D. (2025). Tranexamic Acid-Phenol Smart Scaffolds with Imine Linker: Unlocking Antimicrobial Potential Through In Vitro and In Silico Insights. Organics, 6(4), 54. https://doi.org/10.3390/org6040054








