Synthesis of Fused Cyclic Aryl Amino Carbon Carbene Salt Precursors ([f-CArACH]+) Incorporating an Auxiliary Arene and Isolation of a Cu(I) Complex
Abstract
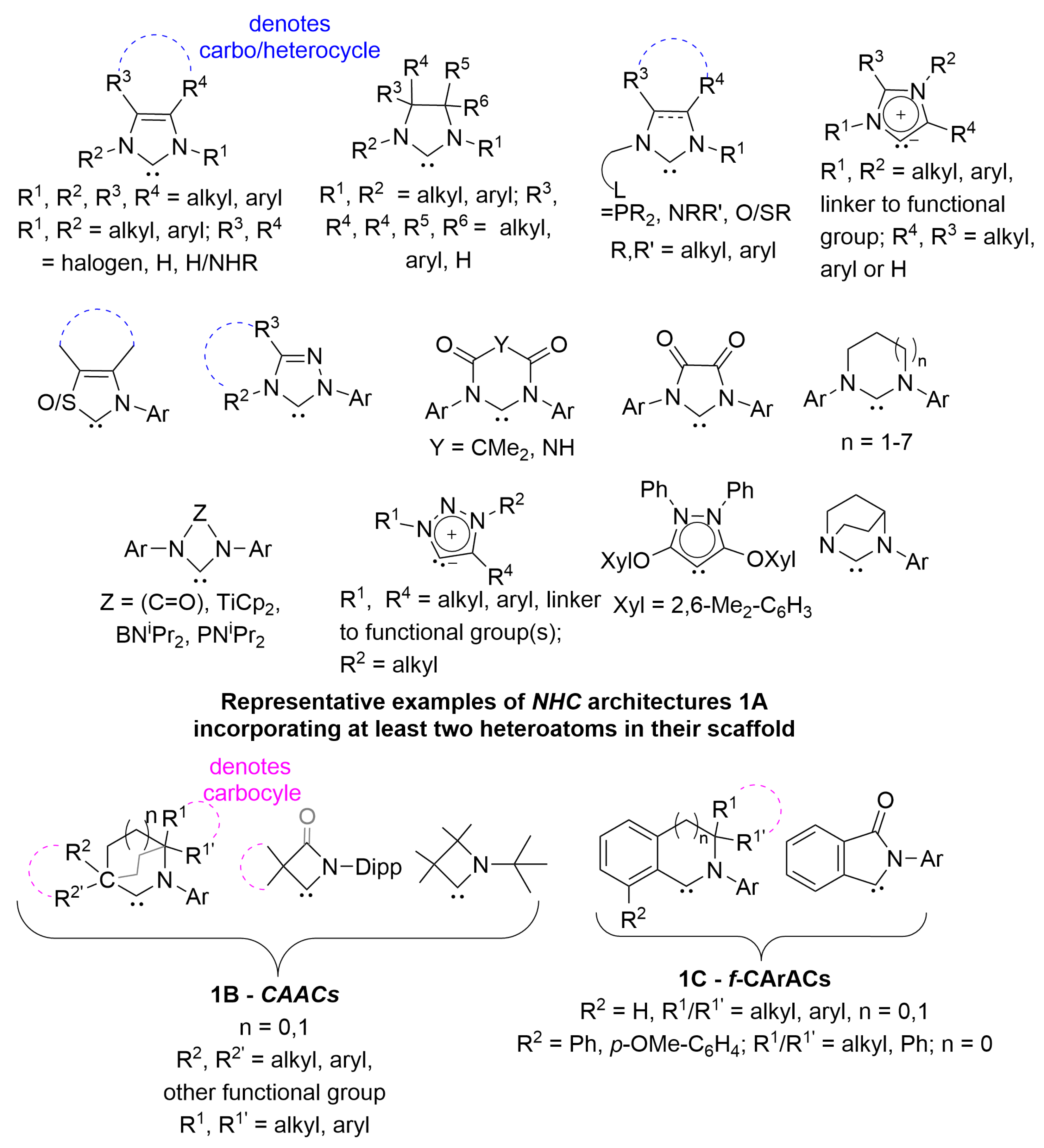
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Spectroscopic and Analytic Techniques
2.3. Single Crystal X-Ray Crystallography
3. Results and Discussion
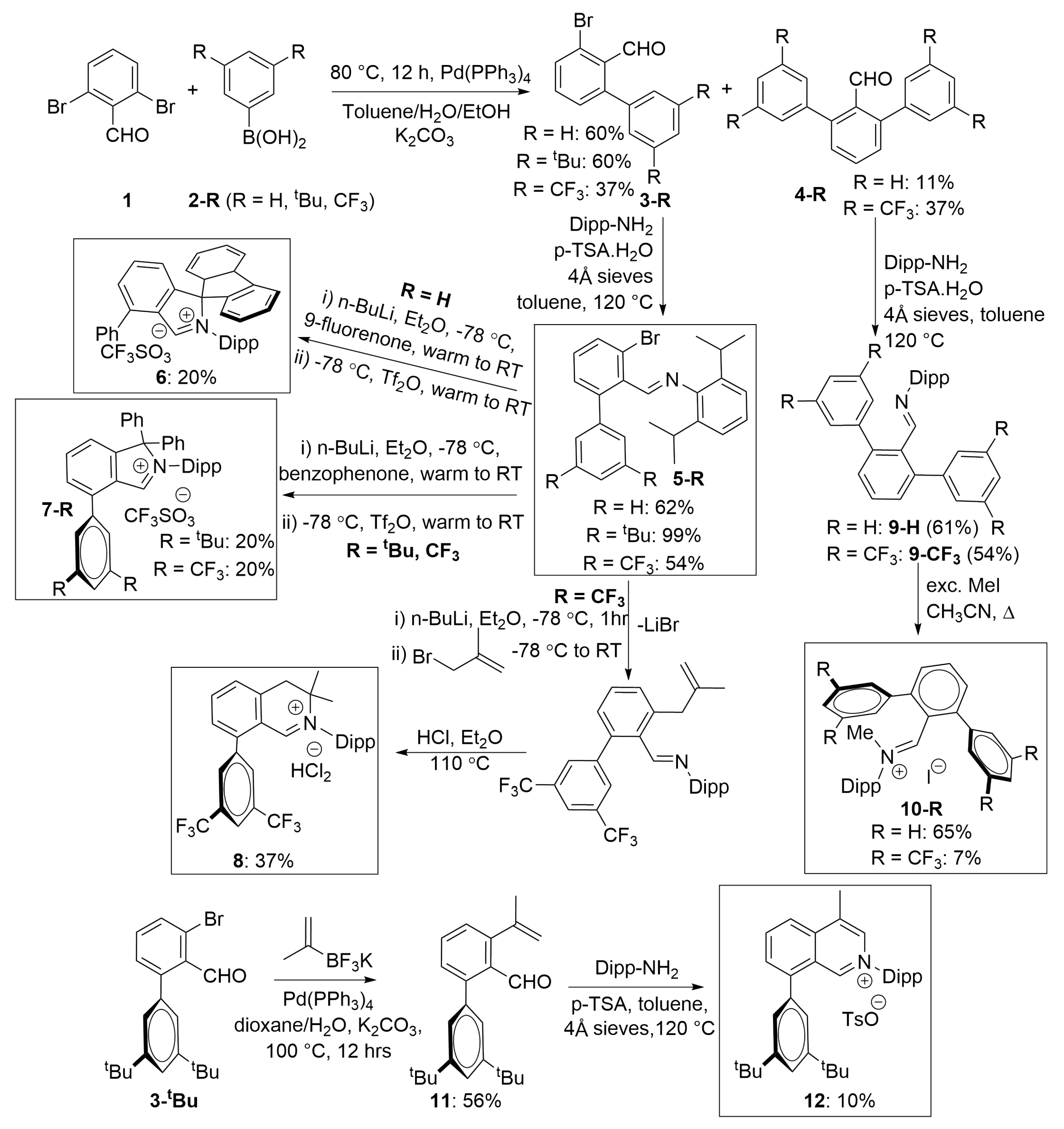
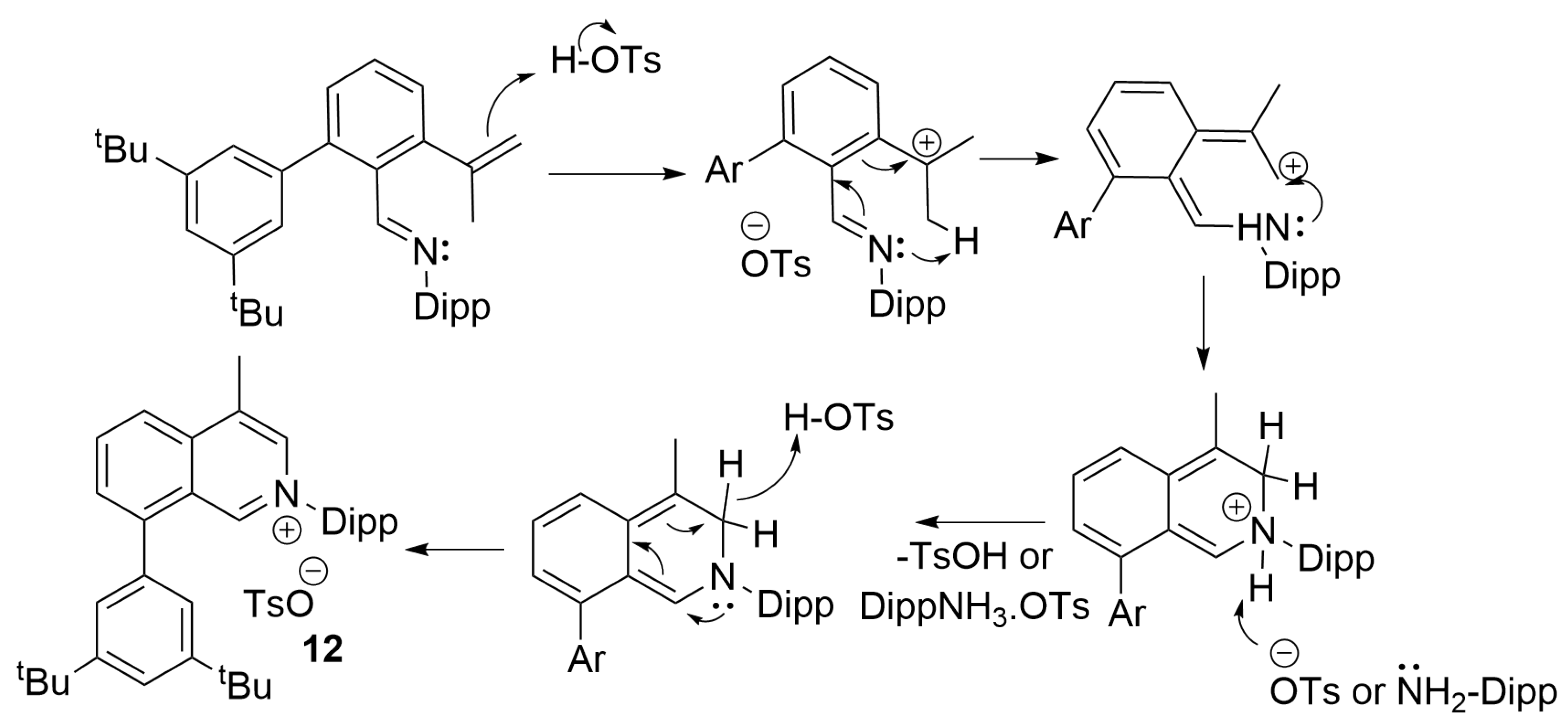
3.1. Synthesis of f-CArAC Salt Precursors
3.2. Characterisation of f-CArAC Salt Precursors
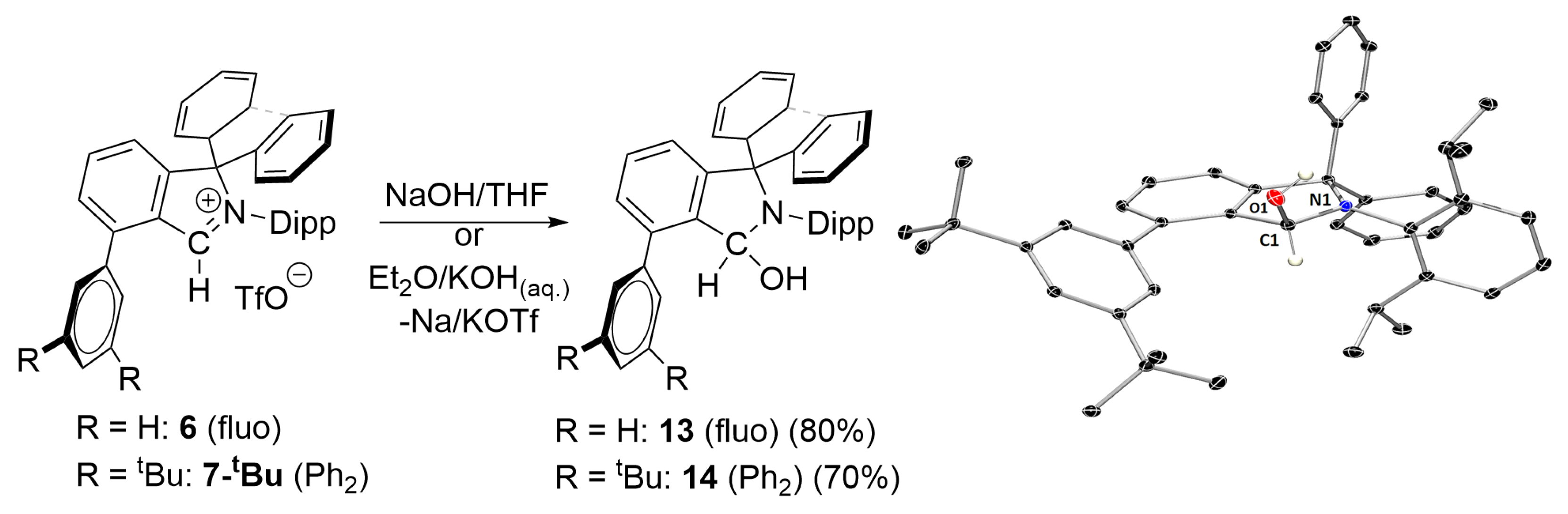
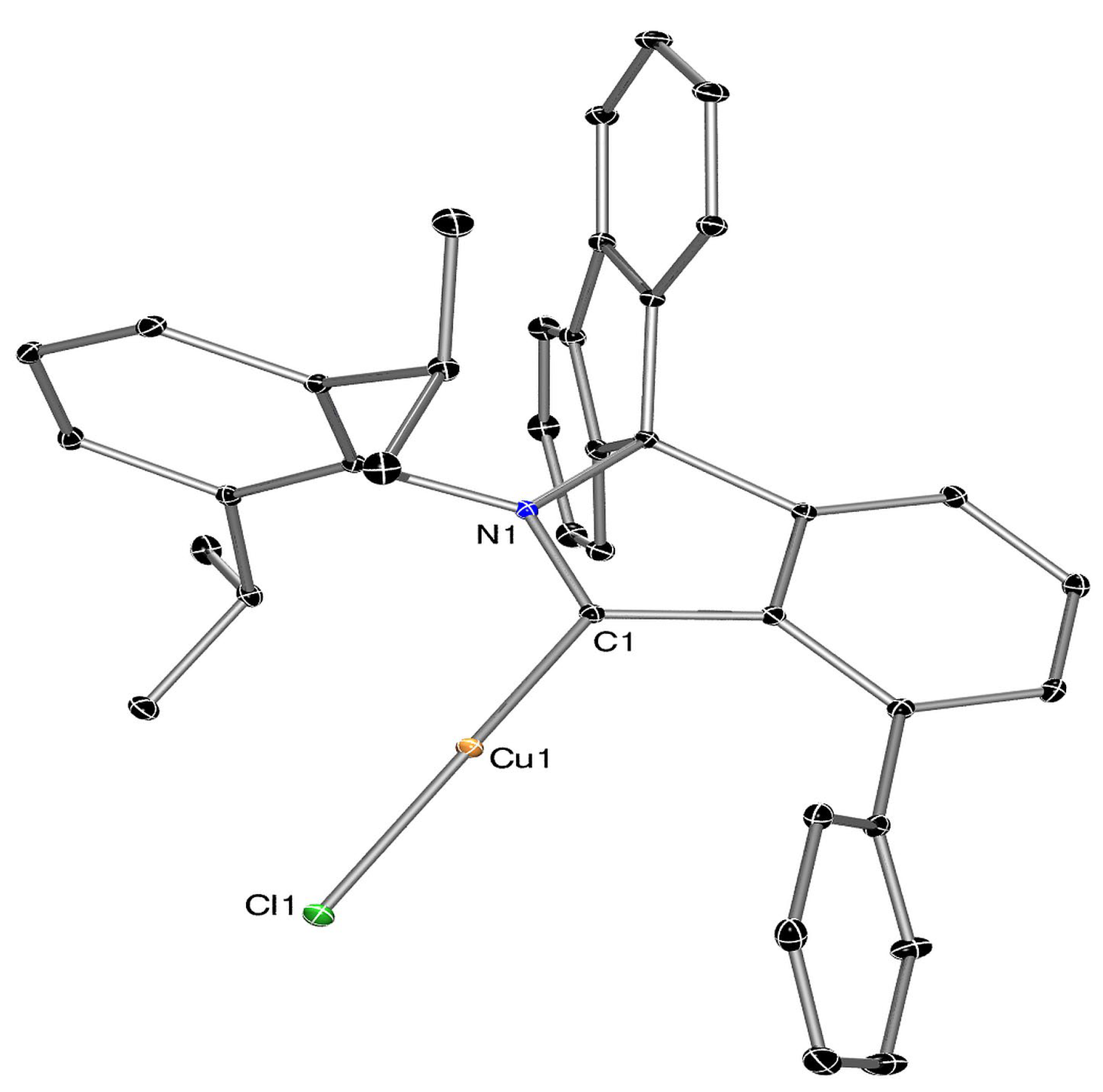
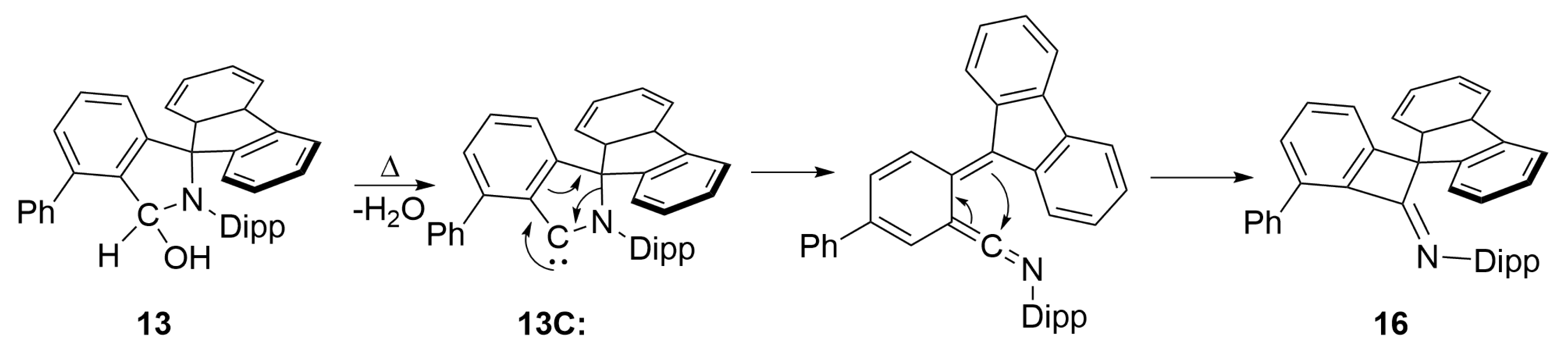
3.3. Attempts at Generating f-CArAC Free Carbenes and Synthesis of a Cu(I) Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, S.A.; Steinfort, R.; Hartmann, L. Progress, Challenges and Future Directions of Heterocycles as Building Blocks in Iterative Methodologies towards Sequence-Defined Oligomers and Polymers. Polym. Chem. 2021, 12, 4439–4450. [Google Scholar] [CrossRef]
- Murugan, R. Heterocycles in Polymers. Prog. Heterocycl. Chem. 2023, 35, 93–118. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909–1951. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Qadir, T.; Sharma, P.K.; Jeelani, I.; Abe, H. A Review on The Medicinal And Industrial Applications of N-Containing Heterocycles. Open Med. Chem. J. 2022, 16. [Google Scholar] [CrossRef]
- Cai, S.; Hu, X.; Han, J.; Zhang, Z.; Li, X.; Wang, C.; Su, J. Efficient Organic Dyes Containing Dibenzo Heterocycles as Conjugated Linker Part for Dye-Sensitized Solar Cells. Tetrahedron 2013, 69, 1970–1977. [Google Scholar] [CrossRef]
- Abbotto, A.; Beverina, L.; Bozio, R.; Facchetti, A.; Ferrante, C.; Pagani, G.A.; Pedron, D.; Signorini, R. Novel Heterocycle-Based Two-Photon Absorbing Dyes. Org. Lett. 2002, 4, 1495–1498. [Google Scholar] [CrossRef]
- MacMillan, D.W.C. The Advent and Development of Organocatalysis. Nature 2008, 455, 304–308. [Google Scholar] [CrossRef]
- Han, B.; He, X.H.; Liu, Y.Q.; He, G.; Peng, C.; Li, J.L. Asymmetric Organocatalysis: An Enabling Technology for Medicinal Chemistry. Chem. Soc. Rev. 2021, 50, 1522–1586. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Barik, S.; Biju, A.T. N-Heterocyclic Carbene (NHC) Organocatalysis: From Fundamentals to Frontiers. Chem. Soc. Rev. 2024, 54, 1102–1124. [Google Scholar] [CrossRef]
- Ha, H.-J. Recent Advances in Synthesizing and Utilizing Nitrogen-Containing Heterocycles. Front. Chem. 2023, 11, 1279418. [Google Scholar] [CrossRef]
- Galathri, E.M.; Kuczmera, T.J.; Nachtsheim, B.J.; Kokotos, C.G. Organocatalytic Friedel-Crafts Arylation of Aldehydes with Indoles Utilizing N-Heterocyclic Iod(Az)Olium Salts as Halogen-Bonding Catalysts. Green Chem. 2023, 26, 825–831. [Google Scholar] [CrossRef]
- Spyropoulou, C.K.; Skolia, E.; Flesariu, D.F.; Zissimou, G.A.; Gkizis, P.L.; Triandafillidi, I.; Athanasiou, M.; Itskos, G.; Koutentis, P.A.; Kokotos, C.G. 3H-Phenothiazin-3-One: A Photocatalyst for the Aerobic Photochemical Oxidation of Sulfides to Sulfoxides. Adv. Synth. Catal. 2023, 365, 2643–2650. [Google Scholar] [CrossRef]
- Zhilyaev, K.A.; Lipilin, D.L.; Kosobokov, M.D.; Samigullina, A.I.; Dilman, A.D. Preparation and Evaluation of Sterically Hindered Acridine Photocatalysts. Adv. Synth. Catal. 2022, 364, 3295–3301. [Google Scholar] [CrossRef]
- Rickertsen, D.R.L.; Crow, J.D.; Das, T.; Ghiviriga, I.; Hirschi, J.S.; Seidel, D. Acridine/Lewis Acid Complexes as Powerful Photocatalysts: A Combined Experimental and Mechanistic Study. ACS Catal. 2024, 14, 14574–14585. [Google Scholar] [CrossRef]
- Sideri, I.K.; Voutyritsa, E.; Kokotos, C.G. Photoorganocatalysis, Small Organic Molecules and Light in the Service of Organic Synthesis: The Awakening of a Sleeping Giant. Org. Biomol. Chem. 2018, 16, 4596–4614. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Harlow, R.L.; Kline, M. A Stable Crystalline Carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Bourissou, D.; Guerret, O.; Gabbaï, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–91. [Google Scholar] [CrossRef]
- Despagnet-Ayoub, E.; Grubbs, R.H. A Stable Four-Membered N-Heterocyclic Carbene. J. Am. Chem. Soc. 2004, 126, 10198–10199. [Google Scholar] [CrossRef]
- Beaumier, E.P.; Gordon, C.P.; Harkins, R.P.; Mcgreal, M.E.; Wen, X.; Copéret, C.; Goodpaster, J.D.; Tonks, I.A. Cp2Ti(Κ2-tbuncntbu): A Complex with an Unusual Κ2 Coordination Mode of a Heterocumulene Featuring a Free Carbene. J. Am. Chem. Soc. 2020, 142, 8006–8018. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Donnadieu, B.; Bertrand, G. S2307 Four-Electron, Four-Membered Heterocyclic Cations and Carbenes. Proc. Natl. Acad. Sci. USA 2006, 12, 13585–13588. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, A.A.; Monakhov, K.Y.; Braunstein, P. Anionic N-Heterocyclic Carbene Ligands from Mesoionic Imidazolium Precursors: Remote Backbone Arylimino Substitution Directs Carbene Coordination. Chem. Eur. J. 2013, 19, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Reiß, G.; Ganter, C. The First Structurally Characterized N-Heterocyclic Carbene Complex with a Ligand Derived from Pyrimidine. J. Organomet. Chem. 2010, 695, 474–477. [Google Scholar] [CrossRef]
- Makhloufi, A.; Frank, W.; Ganter, C. Diamino- and Mixed Amino-Amido-N-Heterocyclic Carbenes Based on Triazine Backbones. Organometallics 2012, 31, 2001–2008. [Google Scholar] [CrossRef]
- Karl, L.; Meisner, J.; Ganter, C. Investigations on Novel 1,3-Diazetidine Based Four-Membered N-Heterocyclic Carbenes. Eur. J. Inorg. Chem. 2023, 26, e202300022. [Google Scholar] [CrossRef]
- Makhloufi, A.; Wahl, M.; Frank, W.; Ganter, C. A New Mixed Amino-Amido N-Heterocyclic Carbene Based on Anthranilic Acid. Organometallics 2013, 32, 854–861. [Google Scholar] [CrossRef]
- Braun, M.; Frank, W.; Reiss, G.J.; Ganter, C. An N-Heterocyclic Carbene Ligand with an Oxalamide Backbone. Organometallics 2010, 29, 4418–4420. [Google Scholar] [CrossRef]
- Brüggemann, P.; Wahl, M.; Schwengers, S.; Buhl, H.; Ganter, C. Access to a Cationic, Electron-Poor N-Heterocyclic Carbene with a Quinazolinium Core by Postsynthetic Modification of Related Neutral Derivatives. Organometallics 2018, 37, 4276–4286. [Google Scholar] [CrossRef]
- Bazinet, P.; Ong, T.G.; O’Brien, J.S.; Lavoie, N.; Bell, E.; Yap, G.P.A.; Korobkov, I.; Richeson, D.S. Design of Sterically Demanding, Electron-Rich Carbene Ligands with the Perimidine Scaffold. Organometallics 2007, 26, 2885–2895. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Winston, S.; Gelbrich, T.; Hursthouse, M.B.; Tooze, R.P. Synthesis and Structural Characterisation of Stable Pyridine- and Phosphine-Functionalised N-Heterocyclic Carbenes. Chem. Commun. 2002, 5, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, A.A.D.; Danopoulos, A.A.; Kleinhenz, S.; Light, M.E.; Hursthouse, M.B.; Eastham, G. Structural Diversity of Pyridine-N-Functionalized Carbene Copper(I) Complexes. Organometallics 2001, 20, 2027–2031. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Goerlich, J.R.; Marshall, W.J. A Stable Thiazol-2-Ylidene and Its Dimer. Liebigs Ann. 1997, 1997, 365–374. [Google Scholar] [CrossRef]
- Mendoza-Espinosa, D.; Ung, G.; Donnadieu, B.; Bertrand, G. Mesoionic Thiazol-5-Ylidenes as Ligands for Transition Metal Complexes. Chem. Commun. 2011, 47, 10614–10616. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Li, X.; Lv, A.; Li, X.; Wang, Z.; Wang, R.; Ma, Y.; Fang, R.; Szostak, R.; et al. Thiazol-2-Ylidenes as N-Heterocyclic Carbene Ligands with Enhanced Electrophilicity for Transition Metal Catalysis. Commun. Chem. 2022, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic, N.; César, V.; Lugan, N.; Lavigne, G. An Ambidentate Janus-Type Ligand System Based on Fused Carbene and Imidato Functionalities. Chem.—A Eur. J. 2011, 17, 13151–13155. [Google Scholar] [CrossRef]
- Perry, M.C.; Cui, X.; Powell, M.T.; Hou, D.R.; Reibenspies, J.H.; Burgess, K. Optically Active Iridium Imidazol-2-Ylidene-Oxazoline Complexes: Preparation and Use in Asymmetric Hydrogenation of Arylalkenes. J. Am. Chem. Soc. 2003, 125, 113–123. [Google Scholar] [CrossRef]
- Teng, Q.; Wu, W.; Duong, H.A.; Huynh, H.V. Ring-Expanded N-Heterocyclic Carbenes as Ligands in Iron-Catalysed Cross-Coupling Reactions of Arylmagnesium Reagents and Aryl Chlorides. Chem. Commun. 2018, 54, 6044–6047. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.J.; Wang, W.; Li, S.; Shi, M. Chiral NHC-Metal-Based Asymmetric Catalysis. Coord. Chem. Rev. 2012, 256, 804–853. [Google Scholar] [CrossRef]
- Glorius, F.; Altenhoff, G.; Goddard, R.; Lehmann, C. Oxazolines as Chiral Building Blocks for Imidazolium Salts and N-Heterocyclic Carbene Ligands. Chem. Commun. 2002, 2, 2704–2705. [Google Scholar] [CrossRef] [PubMed]
- Ros, A.; Monge, D.; Álcarazo, M.; Álvarez, E.; Lassaletta, J.M.; Fernández, R. Synthesis, Structure, and Applications of N-Dialkylamino-N′-Alkylimidazol-2-Ylidenes as a New Type of NHC Ligands. Organometallics 2006, 25, 6039–6046. [Google Scholar] [CrossRef]
- Massey, R.S.; Collett, C.J.; Lindsay, A.G.; Smith, A.D.; O’Donoghue, A.C. Proton Transfer Reactions of Triazol-3-Ylidenes: Kinetic Acidities and Carbon Acid p K a Values for Twenty Triazolium Salts in Aqueous Solution. J. Am. Chem. Soc. 2012, 134, 20421–20432. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Pearson, S. Abnormal N-Heterocyclic Carbenes. Coord. Chem. Rev. 2007, 251, 596–609. [Google Scholar] [CrossRef]
- Regnier, V.; Planet, Y.; Moore, C.E.; Pecaut, J.; Philouze, C.; Martin, D. Stable Di- and Tri-coordinated Carbon(II) Supported by an Electron-Rich Β-Diketiminate Ligand. Angew. Chem. Int. Ed. Engl. 2017, 129, 1051–1055. [Google Scholar] [CrossRef]
- Krahulic, K.E.; Tuononen, H.M.; Parvez, M.; Roesler, R. Isolation of Free Phenylide-like Carbanions with N-Heterocyclic Carbene Frameworks. J. Am. Chem. Soc. 2009, 131, 5858–5865. [Google Scholar] [CrossRef]
- Vermersch, F.; Wang, V.T.; Abdellaoui, M.; Jazzar, R.; Bertrand, G. Ambiphilicity of Ring-Expanded N-Heterocyclic Carbenes. Chem. Sci. 2024, 15, 3707–3710. [Google Scholar] [CrossRef]
- Hobbs, M.G.; Knapp, C.J.; Welsh, P.T.; Borau-Garcia, J.; Ziegler, T.; Roesler, R. Anionic N-Heterocyclic Carbenes with N,N′-Bis(Fluoroaryl) and N,N′-Bis(Perfluoroaryl) Substituents. Chem.—A Eur. J. 2010, 16, 14520–14533. [Google Scholar] [CrossRef]
- Enders, D.; Breuer, K.; Raabe, G.; Runsink, J.; Teles, J.H.; Melder, J.-P.; Ebel, K.; Brode, S. Preparation, Structure, and Reactivity of 1,3,4-Triphenyl-4,5-dihydro-1H-1,2,4-triazol-5-ylidene, a New Stable Carbene. Angew. Chem. Int. Ed. Engl. 1995, 34, 1021–1023. [Google Scholar] [CrossRef]
- Schuster, O.; Yang, L.; Raubenheimer, H.G.; Albrecht, M. Beyond Conventional N-Heterocyclic Carbenes: Abnormal, Remote, and Other Classes of NHC Ligands with Reduced Heteroatom Stabilization. Chem. Rev. 2009, 109, 3445–3478. [Google Scholar] [CrossRef]
- Martin, D.; Lassauque, N.; Donnadieu, B.; Bertrand, G. A Cyclic Diaminocarbene with a Pyramidalized Nitrogen Atom: A Stable N-Heterocyclic Carbene with Enhanced Electrophilicity. Angew. Chem. Int. Ed. Engl. 2012, 51, 6172–6175. [Google Scholar] [CrossRef] [PubMed]
- Fürstner, A.; Alcarazo, M.; Krause, H.; Lehmann, C.W. Effective Modulation of the Donor Properties of N-Heterocyclic Carbene Ligands by “Through-Space” Communication within a Planar Chiral Scaffold. J. Am. Chem. Soc. 2007, 129, 12676–12677. [Google Scholar] [CrossRef]
- Alder, R.W.; Butts, C.P.; Orpen, A.G. Stable Aminooxy- and Aminothiocarbenes. J. Am. Chem. Soc. 1998, 120, 11526–11527. [Google Scholar] [CrossRef]
- Tej Raviprolu, V.; Gregory, A.; Banda, I.; McArthur, S.G.; McArthur, S.E.; Goddard III, W.A.; Musgrave III, C.B.; Lavallo, V. Confirmation of Breslow’s Hypothesis: A Carbene Stable in Liquid Water. Sci. Adv. 2025, 11, 9681. [Google Scholar] [CrossRef]
- Alcarazo, M.; Roseblade, S.J.; Cowley, A.R.; Fernández, R.; Brown, J.M.; Lassaletta, J.M. Imidazo [1,5-a]Pyridine: A Versatile Architecture for Stable N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2005, 127, 3290–3291. [Google Scholar] [CrossRef]
- Lee, S.; Gabidullin, B.; Richeson, D. Distinct Palladium(II) Carbene Complexes Supported by Six-Membered 1,3-Disubstituted Permidin-2-Ylidene, Six-Membered N-Heterocyclic Carbenes. ACS Omega 2018, 3, 6587–6594. [Google Scholar] [CrossRef]
- Lee, B.C.; Liu, C.F.; Lin, L.Q.H.; Yap, K.Z.; Song, N.X.; Ko, C.H.M.; Chan, P.H.; Koh, M.J. N-Heterocyclic Carbenes as Privileged Ligands for Nickel-Catalysed Alkene Functionalisation. Chem. Soc. Rev. 2023, 52, 2946–2991. [Google Scholar] [CrossRef]
- Beig, N.; Goyal, V.; Bansal, R.K. Application of N-Heterocyclic Carbene–Cu(I) Complexes as Catalysts in Organic Synthesis: A Review. Beilstein J. Org. Chem. 2023, 19, 1408–1442. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; He, Y.M.; Pan, Y.; Li, F.; Li, D.; Hao, W.; Fan, Q.H. Tunable Unsymmetrical Chiral N-Heterocyclic Carbene Ligands for Highly Diastereo- and Enantioselective Copper-Catalyzed Tandem Borylative Cyclization: Ligand Controlled Diastereoselectivity Reversal. CCS Chem. 2023, 5, 2088–2100. [Google Scholar] [CrossRef]
- Chung, C.K.; Grubbs, R.H. Olefin Metathesis Catalyst: Stabilization Effect of Backbone Substitutions of N-Heterocyclic Carbene. Org. Lett. 2008, 10, 2693–2696. [Google Scholar] [CrossRef]
- Lavallo, V.; Dyker, C.A.; Donnadieu, B.; Bertrand, G. Synthesis and Ligand Properties of Stable Five-Membered-Ring Allenes Containing Only Second-Row Elements. Angew. Chem. Inter. Ed. Engl. 2008, 47, 5411–5414. [Google Scholar] [CrossRef] [PubMed]
- Zinner, S.C.; Herrmann, W.A.; Kühn, F.E. Synthesis and Characterization of Asymmetric NHC Complexes. J. Organomet. Chem. 2008, 693, 1543–1546. [Google Scholar] [CrossRef]
- Breitwieser, K.; Munz, D. Cyclic (Alkyl)(Amino)Carbene (CAAC) Ligands: Electronic Structure and Application as Chemically- and Redox-Non-Innocent Ligands and Chromophores. In Advances in Organometallic Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2022; Volume 78, pp. 79–132. ISBN 9780323990905. [Google Scholar]
- Dorta, R.; Scott, N.M.; Costabile, C.; Cavallo, L.; Hoff, C.D.; Nolan, S.P. Steric and Electronic Properties of N-Heterocyclic Carbenes (NHC): A Detailed Study on Their Interaction with Ni(CO)4. J. Am. Chem. Soc. 2005, 127, 2485–2495. [Google Scholar] [CrossRef]
- Hillier, A.C.; Sommer, W.J.; Yong, B.S.; Petersen, J.L.; Cavallo, L.; Nolan, S.P. A Combined Experimental and Theoretical Study Examining the Binding of N-Heterocyclic Carbenes (NHC) to the Cp*RuCl (Cp* = H5-C5Me5) Moiety: Insight into Stereoelectronic Differences between Unsaturated and Saturated NHC Ligands. Organometallics 2003, 22, 4322–4326. [Google Scholar] [CrossRef]
- Nelson, D.J.; Nolan, S.P. Quantifying and Understanding the Electronic Properties of N-Heterocyclic Carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [Google Scholar] [CrossRef] [PubMed]
- Kolychev, E.L.; Kronig, S.; Brandhorst, K.; Freytag, M.; Jones, P.G.; Tamm, M. Iridium(I) Complexes with Anionic N-Heterocyclic Carbene Ligands as Catalysts for the Hydrogenation of Alkenes in Nonpolar Media. J. Am. Chem. Soc. 2013, 135, 12448–12459. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.P.; Nasr, A.; Jones, P.G.; Altun, A.; Neese, F.; Bistoni, G.; Tamm, M. London Dispersion Interactions in Pnictogen Cations [ECl2]+ and [E=E]2+ (E=P, As, Sb) Supported by Anionic N-Heterocyclic Carbenes. Chem. Eur. J. 2018, 24, 18922–18932. [Google Scholar] [CrossRef] [PubMed]
- Hadei, N.; Kantchev, E.A.B.; O’Brien, C.J.; Organ, M.G. Electronic Nature of N-Heterocyclic Carbene Ligands: Effect on the Suzuki Reaction. Org. Lett. 2005, 7, 1991–1994. [Google Scholar] [CrossRef]
- Hu, X.; Castro-Rodriguez, I.; Olsen, K.; Meyer, K. Group 11 Metal Complexes of N-Heterocyclic Carbene Ligands: Nature of the Metal-Carbene Bond. Organometallics 2004, 23, 755–764. [Google Scholar] [CrossRef]
- Moerdyk, J.P.; Schilter, D.; Bielawski, C.W. N,N’-Diamidocarbenes: Isolable Divalent Carbons with Bona Fide Carbene Reactivity. Acc. Chem. Res. 2016, 49, 1458–1468. [Google Scholar] [CrossRef]
- Hudnall, T.W.; Bielawski, C.W. An N,N′-Diamidocarbene: Studies in C-H Insertion, Reversible Carbonylation, and Transition-Metal Coordination Chemistry. J. Am. Chem. Soc. 2009, 131, 16039–16041. [Google Scholar] [CrossRef]
- Powell, M.T.; Hou, D.R.; Perry, M.C.; Cui, X.; Burgess, K. Chiral Imidazolylidine Ligands for Asymmetric Hydrogenation of Aryl Alkenes. J. Am. Chem. Soc. 2001, 123, 8878–8879. [Google Scholar] [CrossRef]
- Sanderson, M.D.; Kamplain, J.W.; Bielawski, C.W. Quinone-Annulated N-Heterocyclic Carbene-Transition-Metal Complexes: Observation of π-Backbonding Using FT-IR Spectroscopy and Cyclic Voltammetry. J. Am. Chem. Soc. 2006, 128, 16514–16515. [Google Scholar] [CrossRef]
- César, V.; Lugan, N.; Lavigne, G. Electronic Tuning of a Carbene Center via Remote Chemical Induction, and Relevant Effects in Catalysis. Chem. Eur. J. 2010, 16, 11432–11442. [Google Scholar] [CrossRef]
- Sau, S.C.; Hota, P.K.; Mandal, S.K.; Soleilhavoup, M.; Bertrand, G. Stable Abnormal N-Heterocyclic Carbenes and Their Applications. Chem. Soc. Rev. 2020, 49, 1233–1252. [Google Scholar] [CrossRef]
- Vermersch, F.; Yazdani, S.; Junor, G.P.; Grotjahn, D.B.; Jazzar, R.; Bertrand, G. Stable Singlet Carbenes as Organic Superbases. Angew. Chem. Inter. Ed. Engl. 2021, 60, 27253–27257. [Google Scholar] [CrossRef]
- Pranckevicius, C.; Stephan, D.W. Three-Coordinate, Cyclic Bent Allene Iron Complexes. Organometallics 2013, 32, 2693–2697. [Google Scholar] [CrossRef]
- Takasao, G.; Maity, B.; Dutta, S.; Kancherla, R.; Rueping, M.; Cavallo, L. NHC-Cracker: A Platform for the In Silico Engineering of N-Heterocyclic Carbenes for Diverse Chemical Applications. ACS Catal. 2025, 15, 5915–5927. [Google Scholar] [CrossRef]
- Clavier, H.; Nolan, S.P. Percent Buried Volume for Phosphine and N-Heterocyclic Carbene Ligands: Steric Properties in Organometallic Chemistry. Chem. Commun. 2010, 46, 841–861. [Google Scholar] [CrossRef] [PubMed]
- Falivene, L.; Credendino, R.; Poater, A.; Petta, A.; Serra, L.; Oliva, R.; Scarano, V.; Cavallo, L. SambVca 2. A Web Tool for Analyzing Catalytic Pockets with Topographic Steric Maps. Organometallics 2016, 35, 2286–2293. [Google Scholar] [CrossRef]
- Dröge, T.; Glorius, F. The Measure of All Rings—N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. Engl. 2010, 49, 6940–6952. [Google Scholar] [CrossRef]
- Munz, D. Pushing Electrons—Which Carbene Ligand for Which Application? Organometallics 2018, 37, 275–289. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Grubbs, R.H. Synthesis and Activity of Ruthenium Olefin Metathesis Catalysts Coordinated with Thiazol-2-Ylidene Ligands. J. Am. Chem. Soc. 2008, 130, 2234–2245. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, A.A.; Simler, T.; Braunstein, P. N-Heterocyclic Carbene Complexes of Copper, Nickel, and Cobalt. Chem. Rev. 2019, 119, 3730–3961. [Google Scholar] [CrossRef]
- Charra, V.; de Frémont, P.; Braunstein, P. Multidentate N-Heterocyclic Carbene Complexes of the 3d Metals: Synthesis, Structure, Reactivity and Catalysis. Coord. Chem. Rev. 2017, 341, 53–176. [Google Scholar] [CrossRef]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef]
- Wang, Z.; Tzouras, N.V.; Nolan, S.P.; Bi, X. Silver N-Heterocyclic Carbenes: Emerging Powerful Catalysts. Trends Chem. 2021, 3, 674–685. [Google Scholar] [CrossRef]
- Jalal, M.; Hammouti, B.; Touzani, R.; Aouniti, A.; Ozdemir, I. Metal-NHC Heterocycle Complexes in Catalysis and Biological Applications: Systematic Review. Mater. Today Proc. 2020, 31, S122–S129. [Google Scholar] [CrossRef]
- Lehtonen, A. Metal Complexes of Redox Non-Innocent Ligand N,N′-Bis(3,5-Di-Tertbutyl-2-Hydroxy-Phenyl)-1,2-Phenylenediamine. Molecules 2024, 29, 1088–1102. [Google Scholar] [CrossRef]
- Lavallo, V.; Canac, Y.; Präsang, C.; Donnadieu, B.; Bertrand, G. Stable Cyclic (Alkyl)(Amino)Carbenes as Rigid or Flexible, Bulky, Electron-Rich Ligands for Transition-Metal Catalysts: A Quaternary Carbon Atom Makes the Difference. Angew. Chem. Int. Ed. Engl. 2005, 44, 5705–5709. [Google Scholar] [CrossRef]
- Jazzar, R.; Dewhurst, R.D.; Bourg, J.B.; Donnadieu, B.; Canac, Y.; Bertrand, G. Intramolecular “Hydroiminiumation” of Alkenes: Application to the Synthesis of Conjugate Acids of Cyclic Alkyl Amino Carbenes (CAACs). Angew. Chem. Int. Ed. Engl. 2007, 46, 2899–2902. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, C.M.; Junor, G.P.; Tolentino, D.R.; Jazzar, R.; Melaimi, M.; Bertrand, G. Highly Ambiphilic Room Temperature Stable Six-Membered Cyclic (Alkyl)(Amino)Carbenes. J. Am. Chem. Soc. 2018, 140, 9255–9260. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Mendivil, E.; Hansmann, M.M.; Weinstein, C.M.; Jazzar, R.; Melaimi, M.; Bertrand, G. Bicyclic (Alkyl)(Amino)Carbenes (BICAACs): Stable Carbenes More Ambiphilic than CAACs. J. Am. Chem. Soc. 2017, 139, 7753–7756. [Google Scholar] [CrossRef] [PubMed]
- Pichon, D.; Soleilhavoup, M.; Morvan, J.; Junor, G.P.; Vives, T.; Crévisy, C.; Lavallo, V.; Campagne, J.M.; Mauduit, M.; Jazzar, R.; et al. The Debut of Chiral Cyclic (Alkyl)(Amino)Carbenes (CAACs) in Enantioselective Catalysis. Chem. Sci. 2019, 10, 7807–7811. [Google Scholar] [CrossRef] [PubMed]
- Kumar Kushvaha, S.; Mishra, A.; Roesky, H.W.; Chandra Mondal, K. Recent Advances in the Domain of Cyclic (Alkyl)(Amino) Carbenes. Chem. Asian. J. 2022, 17, e202101301. [Google Scholar] [CrossRef]
- Tanaka, M.; Kamiyama, S.; Ishii, A.; Nakata, N. 2-[2,6-Diisopropylphenyl]-4-Phenyl-5H-5,9b[1′,2′]-Benzonaphtho[1,2-b]Pyrrol-2-Ium Tetrafluoroborate. Molbank 2023, 2023, M1601. [Google Scholar] [CrossRef]
- Tanaka, M.; Kamiyama, S.; Ota, K.; Nakata, N. Flash Communication: Exocyclic Alkenyl Tuning of Cyclic (Amino)Carbene: Enhancing π-Acceptor Property without Ring Unsaturation. Organometallics 2025, 44, 1510–1514. [Google Scholar] [CrossRef]
- Siwatch, R.K.; Ng, Z.J.G.; Lim, J.J.S.; Zhang, Z.F.; Su, M.D.; So, C.W. Strained Cyclic Alkyl(Amino)Carbene-Stabilized Tetraatomic Silicon(0) Cluster. J. Am. Chem. Soc. 2025, 147, 20251–20256. [Google Scholar] [CrossRef]
- Karl, L.; Deißenbeck, D.; Meisner, J.; Ganter, C. β-Lactam Ylidenes: An Overlooked Class of N-Heterocyclic Carbenes. Chem. Eur. J. 2025, 31, e202501320. [Google Scholar] [CrossRef]
- Serrato, M.R.; Melaimi, M.; Bertrand, G. Cyclic (Amino)(Barrelene)Carbenes: An Original Family of CAACs through a Novel Synthetic Pathway. Chem. Commun. 2022, 58, 7519–7521. [Google Scholar] [CrossRef]
- Majumder, C.; Sharma, A.; Das, B.; Yadav, R.; Kundu, S. Cyclic (Alkenyl)(Amino)Carbene (SMeCAenAC): Introducing a Member to the Cyclic (Alkyl)(Amino)Carbenes Family Featuring a Narrow Energy Gap. J. Am. Chem. Soc. 2025, 147, 6905–6913. [Google Scholar] [CrossRef]
- Volk, J.; Heinz, M.; Guthardt, R.; Yadav, S.; Bruhn, C.; Holthausen, M.C.; Siemeling, U. A Strongly Ambiphilic Ferrocene-Based Cyclic (Alkyl)(Amino)Carbene—Specific Decomposition to an Enamine by a 1,2-Phenyl Shift. Chem. Eur. J. 2024, 30, e202403028. [Google Scholar] [CrossRef] [PubMed]
- Nikovskii, I.A.; Spiridonov, K.A.; Pavlov, A.A.; Nelyubina, Y.V.; Karnaukh, K.M.; Polezhaev, A.V. Synthetic Approaches to New Redox-Active Carbene Ligands. Russ. J Coord. Chem. 2021, 47, 117–126. [Google Scholar] [CrossRef]
- Madron du Vigné, A.; Cramer, N. Systematic Synthesis of Chiral CAACs with Three Independent Stereogenic Centers for Enantioselective Copper-Catalyzed Conjugate Borylation of Michael Acceptors. ACS Catal. 2025, 15, 15102–15111. [Google Scholar] [CrossRef]
- Frey, G.D.; Lavallo, V.; Donnadieu, B.; Schoeller, W.W.; Bertrand, G. Facile Splitting of Hydrogen and Ammonia by Nucleophilic Activation at a Single Carbon Center. Science (1979) 2007, 316, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Mondal, K.C.; Roesky, H.W. Cyclic Alkyl(Amino) Carbene Stabilized Complexes with Low Coordinate Metals of Enduring Nature. Acc. Chem. Res. 2016, 49, 357–369. [Google Scholar] [CrossRef]
- Arnold, N.; Braunschweig, H.; Brenner, P.B.; Celik, M.A.; Dewhurst, R.D.; Haehnel, M.; Kramer, T.; Krummenacher, I.; Marder, T.B. Correlations and Contrasts in Homo- and Heteroleptic Cyclic (Alkyl)(Amino)Carbene-Containing Pt0 Complexes. Chem. Eur. J. 2015, 21, 12357–12362. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, A.; Goswami, B.; Breitwieser, K.; Morgenstern, B.; Gimferrer, M.; Heinemann, F.W.; Momper, D.M.; Kay, C.W.M.; Munz, D. Palladium Terminal Imido Complexes with Nitrene Character. J. Am. Chem. Soc. 2022, 144, 8897–8901. [Google Scholar] [CrossRef] [PubMed]
- Goodner, S.J.; Grünwald, A.; Heinemann, F.W.; Munz, D. Carbon Dioxide Activation by a Palladium Terminal Imido Complex. Aust. J. Chem. 2019, 72, 900–903. [Google Scholar] [CrossRef]
- Martinez-Vollbert, E.; Anjana, S.S.; Dankert, F.; Morgenstern, B.; Munz, D. Gold(I) Terminal Imides: CH Auration and Dehydrogenation. ChemRxiv 2025. [Google Scholar] [CrossRef]
- Munz, D. How to Tame a Palladium Terminal Oxo. Chem. Sci. 2018, 9, 1155–1167. [Google Scholar] [CrossRef]
- Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl)(Amino)Carbenes (CAACs): Stable Carbenes on the Rise. Acc. Chem. Res. 2015, 48, 256–266. [Google Scholar] [CrossRef]
- Jazzar, R.; Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl)- And (Aryl)-(Amino)Carbene Coinage Metal Complexes and Their Applications. Chem. Rev. 2020, 120, 4141–4168. [Google Scholar] [CrossRef]
- Chu, J.; Munz, D.; Jazzar, R.; Melaimi, M.; Bertrand, G. Synthesis of Hemilabile Cyclic (Alkyl)(Amino)Carbenes (CAACs) and Applications in Organometallic Chemistry. J. Am. Chem. Soc. 2016, 138, 7884–7887. [Google Scholar] [CrossRef]
- Rao, B.; Tang, H.; Zeng, X.; Liu, L.L.; Melaimi, M.; Bertrand, G. Cyclic (Amino)(Aryl)Carbenes (CAArCs) as Strong Σ-Donating and Π-Accepting Ligands for Transition Metals. Angew. Chem. Int. Ed. Engl. 2015, 127, 15128–15132. [Google Scholar] [CrossRef]
- Puerta Lombardi, B.M.; Faas, M.R.; West, D.; Suvinen, R.A.; Tuononen, H.M.; Roesler, R. An Isolable, Chelating Bis[Cyclic (Alkyl)(Amino)Carbene] Stabilizes a Strongly Bent, Dicoordinate Ni(0) Complex. Nat. Commun. 2024, 15, 3417–3424. [Google Scholar] [CrossRef]
- Braunschweig, H.; Krummenacher, I.; Legare, M.A.; Matler, A.; Radacki, K.; Ye, Q. Main-Group Metallomimetics: Transition Metal-like Photolytic CO Substitution at Boron. J. Am. Chem. Soc. 2017, 139, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Böhnke, J.; Braunschweig, H.; Ewing, W.C.; Hörl, C.; Kramer, T.; Krummenacher, I.; Mies, J.; Vargas, A. Diborabutatriene: An Electron-Deficient Cumulene. Angew. Chem. Int. Ed. Engl. 2014, 53, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Légaré, M.-A.; Bélanger-Chabot, G.; Dewhurst, R.D.; Welz, E.; Krummenacher, I.; Engels, B.; Braunschweig, H. Nitrogen Fixation and Reduction at Boron. Science 2018, 359, 896–900. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Braunschweig, H.; Celik, M.A.; Dellermann, T.; Dewhurst, R.D.; Ewing, W.C.; Hammond, K.; Kramer, T.; Krummenacher, I.; Mies, J.; et al. Neutral Zero-Valent s-Block Complexes with Strong Multiple Bonding. Nat. Chem. 2016, 8, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gilliard, R.J.; Cummins, C.C. Arene Extrusion as an Approach to Reductive Elimination at Boron: Implication of Carbene-Ligated Haloborylene as a Transient Reactive Intermediate. Chem. Sci. 2024, 15, 17873–17880. [Google Scholar] [CrossRef]
- Wang, G.; Freeman, L.A.; Dickie, D.A.; Mokrai, R.; Benkő, Z.; Gilliard, R.J. Isolation of Cyclic(Alkyl)(Amino) Carbene–Bismuthinidene Mediated by a Beryllium(0) Complex. Chem. Eur. J. 2019, 25, 4335–4339. [Google Scholar] [CrossRef]
- Wang, G.; Walley, J.E.; Dickie, D.A.; Pan, S.; Frenking, G.; Gilliard, R.J. A Stable, Crystalline Beryllium Radical Cation. J. Am. Chem. Soc. 2020, 142, 4560–4564. [Google Scholar] [CrossRef]
- Freeman, L.A.; Obi, A.D.; Machost, H.R.; Molino, A.; Nichols, A.W.; Dickie, D.A.; Wilson, D.J.D.; Machan, C.W.; Gilliard, R.J. Soluble, Crystalline, and Thermally Stable Alkali CO2−and Carbonite (CO22−) Clusters Supported by Cyclic(Alkyl)(Amino) Carbenes. Chem. Sci. 2021, 12, 3544–3550. [Google Scholar] [CrossRef]
- Hollister, K.K.; Yang, W.; Mondol, R.; Wentz, K.E.; Molino, A.; Kaur, A.; Dickie, D.A.; Frenking, G.; Pan, S.; Wilson, D.J.D.; et al. Isolation of Stable Borepin Radicals and Anions. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202516. [Google Scholar] [CrossRef]
- Kretschmer, R.; Ruiz, D.A.; Moore, C.E.; Rheingold, A.L.; Bertrand, G. One-, Two-, and Three-Electron Reduction of a Cyclic Alkyl(Amino)Carbene- SbCl3 Adduct. Angew. Chem. Int. Ed. Engl. 2014, 53, 8176–8179. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.K.; Melaimi, M.; Gembicky, M.; Munz, D.; Bertrand, G. A Crystalline Doubly Oxidized Carbene. Nature 2023, 623, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Haimerl, M.; Schwarzmaier, C.; Riesinger, C.; Timoshkin, A.Y.; Melaimi, M.; Bertrand, G.; Scheer, M. Reactivity of Yellow Arsenic towards Cyclic (Alkyl)(Amino) Carbenes (CAACs). Chem. Eur. J. 2023, 29, e202300280. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mondal, K.C.; Roesky, H.W.; Zhu, H.; Stollberg, P.; Herbst-Irmer, R.; Stalke, D.; Andrada, D.M. Acyclic Germylones: Congeners of Allenes with a Central Germanium Atom. J. Am. Chem. Soc. 2013, 135, 12422–12428. [Google Scholar] [CrossRef]
- Brenton Gildner, M.; Hudnall, T.W. Cyclic (Aryl)(Amido)Carbenes: Pushing the π-Acidity of Amidocarbenes through Benzannulation. Chem. Commun. 2019, 55, 12300–12303. [Google Scholar] [CrossRef]
- Maity, S.; Muthig, A.M.T.; Sen, I.; Mrózek, O.; Belyaev, A.; Hupp, B.; Steffen, A. A [2.2]Isoindolinophanyl-Based Carbene (IPC) Ligand: Synthesis, Electronic and Photophysical Properties, and Application in Photocatalysis. Angew. Chem. Int. Ed. Engl. 2024, 63, e202409115. [Google Scholar] [CrossRef]
- Jazzar, R.; Bourg, J.B.; Dewhurst, R.D.; Donnadieu, B.; Bertrand, G. Intramolecular “Hydroiminiumation and -Amidiniumation” of Alkenes: A Convenient, Flexible, and Scalable Route to Cyclic Iminium and Imidazolinium Salts. J. Org. Chem. 2007, 72, 3492–3499. [Google Scholar] [CrossRef]
- Magriz, A.; Gómez-Bujedo, S.; Álvarez, E.; Fernández, R.; Lassaletta, J.M. Phthalazin-2-Ylidenes as Cyclic Amino Aryl Carbene Ligands in Rhodium(I) and Iridium(I) Complexes. Organometallics 2010, 29, 5941–5945. [Google Scholar] [CrossRef]
- Jothibasu, R.; Huynh, H.V. Versatile Coordination Chemistry of Indazole-Derived Carbenes. Chem. Commun. 2010, 46, 2986–2988. [Google Scholar] [CrossRef]
- Deck, E.; Reiter, K.; Klopper, W.; Breher, F. A Dinuclear Gold(I) Bis(Carbene) Complex Based on a Ditopic Cyclic (Aryl)(Amino)Carbene Framework. Z. Anorg. Allg. Chem. 2016, 642, 1320–1328. [Google Scholar] [CrossRef]
- Luliński, S.; Serwatowski, J. Regiospecific Metalation of Oligobromobenzenes. J. Org. Chem. 2003, 68, 5384–5387. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta. Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. Computer Program Abstracts ORTEP-3 for Windows-a Version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565–566. [Google Scholar] [CrossRef]
- Boga, S.B.; Alhassan, A.B.; Hesk, D. Efficient Synthesis of 2H & 13C Labeled Benzaldehydes via Regio-Selective Formylation. Tetrahedron Lett. 2014, 55, 4442–4444. [Google Scholar] [CrossRef]
- Al-Zoubi, R.M.; Jaradat, K.T.; Al-Jammal, W.K.; McDonald, R. Mild, Efficient, and Highly Regioselective Synthesis of 2,6-Diiodobenzaldehyde Derivatives. Synlett 2020, 31, 953–958. [Google Scholar] [CrossRef]
- Magis, D.; Cabrera-Trujillo, J.J.; Vignolle, J.; Sotiropoulos, J.M.; Taton, D.; Miqueu, K.; Landais, Y. Expedient Synthesis of Thermally Stable Acyclic Amino(Haloaryl)Carbenes: Experimental and Theoretical Evidence of “Push-Pull” Stabilized Carbenes. J. Am. Chem. Soc. 2024, 146, 16802–16813. [Google Scholar] [CrossRef]
- Lavallo, V.; Mafhouz, J.; Canac, Y.; Donnadieu, B.; Schoeller, W.W.; Bertrand, G. Synthesis, Reactivity, and Ligand Properties of a Stable Alkyl Carbene. J. Am. Chem. Soc. 2004, 126, 8670–8671. [Google Scholar] [CrossRef]
- Sole, S.; Gornitzka, H.; Schoeller, W.W.; Bourissou, D.; Bertrand, G. (Amino)(Aryl)Carbenes: Stable Carbenes Featuring a Spectator Substituent. Science 2001, 292, 1901–1903. [Google Scholar] [CrossRef]
- Slaughter, L.M. Acyclic Aminocarbenes in Catalysis. ACS Catal. 2012, 2, 1802–1816. [Google Scholar] [CrossRef]
- Nakano, R.; Jazzar, R.; Bertrand, G. A Crystalline Monosubstituted Carbene. Nat. Chem. 2018, 10, 1196–1200. [Google Scholar] [CrossRef]
- Lorkowski, J.; Gojiashvili, L.; Yorkgitis, P.; Pichon, D.; Talcik, J.; Gembicky, M.; Roisnel, T.; Baslé, O.; Jazzar, R.; Mauduit, M.; et al. A Crystalline Annelated Pyridin-1-Ylidene and Its Isomerization into a Pyridin-3-Ylidene. J. Am. Chem. Soc. 2025, 147, 14972–14977. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bujedo, S.; Alcarazo, M.; Pichon, C.; Álvarez, E.; Fernández, R.; Lassaletta, J.M. Isoquinolin-1-Ylidenes as Electronically Tuneable Ligands. Chem. Commun. 2007, 11, 1180–1182. [Google Scholar] [CrossRef]
- Das, A.; Elvers, B.J.; Nayak, M.K.; Chrysochos, N.; Anga, S.; Kumar, A.; Rao, D.K.; Narayanan, T.N.; Schulzke, C.; Yildiz, C.B.; et al. Realizing 1,1-Dehydration of Secondary Alcohols to Carbenes: Pyrrolidin-2-Ols as a Source of Cyclic (Alkyl)(Amino)Carbenes. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202637. [Google Scholar] [CrossRef] [PubMed]
- Lorkowski, J.; Yorkgitis, P.; Serrato, M.R.; Gembicky, M.; Pietraszuk, C.; Bertrand, G.; Jazzar, R. Genuine Carbene versus Carbene-like Reactivity. Angew. Chem. Int. Ed. Engl. 2024, 63, e202401020. [Google Scholar] [CrossRef]
- Lorkowski, J.; Krahfuß, M.; Kubicki, M.; Radius, U.; Pietraszuk, C. Intramolecular Ring-Expansion Reaction (RER) and Intermolecular Coordination of In Situ Generated Cyclic (Amino)(Aryl)Carbenes (CAArCs). Chem. Eur. J. 2019, 25, 11365–11374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, S.; Zeng, X. Ring Contraction by Rearrangement of Sterically Congested Cyclic (Amino)(Aryl)Carbenes. J. Org. Chem. 2024, 89, 7795–7803. [Google Scholar] [CrossRef]
- Gernert, M.; Balles-Wolf, L.; Kerner, F.; Müller, U.; Schmiedel, A.; Holzapfel, M.; Marian, C.M.; Pflaum, J.; Lambert, C.; Steffen, A. Cyclic (Amino)(Aryl)Carbenes Enter the Field of Chromophore Ligands: Expanded π System Leads to Unusually Deep Red Emitting Cu(I) Compounds. J. Am. Chem. Soc. 2020, 142, 8897–8909. [Google Scholar] [CrossRef] [PubMed]
- O'Shea, D.F.; Sharp, T. Benzoxepine formation by the 1,7 electrocyclisation of diene-conjugated carbonyl ylides: Studies on relative rates of cyclisation via intramolecular competition reactions. J. Chem. Soc. Perkin Trans. 1997, 3025–3034. [Google Scholar] [CrossRef]
- Chao, B.; Bai, C.; Yan, H.; Zhao, R.; Liu, D.; Muschin, T.; Bao, A.; Eerdun, C.; Bao, Y.-S. Suzuki–Miyaura Type Regioselective C–H Arylation of Aromatic Aldehydes by a Transient Directing Strategy. Org. Lett. 2023, 25, 6823–6829. [Google Scholar] [CrossRef]
- Tredwell, M.J.; Gulias, M.; Bremeyer, N.G.; Johansson, C.C.C.; Collins, B.S.L.; Gaunt, M.J. Palladium(II)-Catalyzed C-H Bond Arylation of Electron-Deficient Arenes at Room Temperature. Angew. Chem. Int. Ed. 2011, 50, 1076–1079. [Google Scholar] [CrossRef]



| R | 3-R/4-R |
|---|---|
| H | 1/0.27 |
| tBu | 1/0.13 |
| CF3 | 1/0.71 |
| Compound |  | 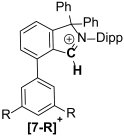 |  |
|---|---|---|---|
| 1H-NMR chemical shift (δ(CDCl3)) | 9.45 | 9.19 (R = tBu) 9.30 (R = CF3) | 8.11 |
| 13C{1H}-NMR chemical shift (δ(CDCl3)) | 175.01 | 173.77 (R = tBu) 175.08 (R = CF3) | 166.20 |
| Compound | 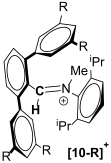 | 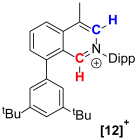 |
|---|---|---|
| 1H-NMR chemical shift (δ(CDCl3)) | 11.16 (R = H) 10.23 (R = CF3) | 8.67 8.58 |
| 13C{1H}-NMR chemical shift (δ(CDCl3)) | 185.44 (R = H) 184.26 (R = CF3) | 135.37 145.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannou, P.C.; Tsoureas, N.; Kotsaki, S.-P. Synthesis of Fused Cyclic Aryl Amino Carbon Carbene Salt Precursors ([f-CArACH]+) Incorporating an Auxiliary Arene and Isolation of a Cu(I) Complex. Organics 2025, 6, 51. https://doi.org/10.3390/org6040051
Ioannou PC, Tsoureas N, Kotsaki S-P. Synthesis of Fused Cyclic Aryl Amino Carbon Carbene Salt Precursors ([f-CArACH]+) Incorporating an Auxiliary Arene and Isolation of a Cu(I) Complex. Organics. 2025; 6(4):51. https://doi.org/10.3390/org6040051
Chicago/Turabian StyleIoannou, Polidoros Chrisovalantis., Nikolaos Tsoureas, and Sevasti-Panagiota Kotsaki. 2025. "Synthesis of Fused Cyclic Aryl Amino Carbon Carbene Salt Precursors ([f-CArACH]+) Incorporating an Auxiliary Arene and Isolation of a Cu(I) Complex" Organics 6, no. 4: 51. https://doi.org/10.3390/org6040051
APA StyleIoannou, P. C., Tsoureas, N., & Kotsaki, S.-P. (2025). Synthesis of Fused Cyclic Aryl Amino Carbon Carbene Salt Precursors ([f-CArACH]+) Incorporating an Auxiliary Arene and Isolation of a Cu(I) Complex. Organics, 6(4), 51. https://doi.org/10.3390/org6040051








