Efficient Degradation of Cis-Polyisoprene by GQDs/g-C3N4 Nanoparticles Under UV Light Irradiation
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Materials
2.2. Sample Preparation
2.2.1. Preparation of the GQDs/g-C3N4 Composite
2.2.2. Cis-Polyisoprene Photocatalytic Degradation
2.3. Characterization Method
3. Results and Discussion
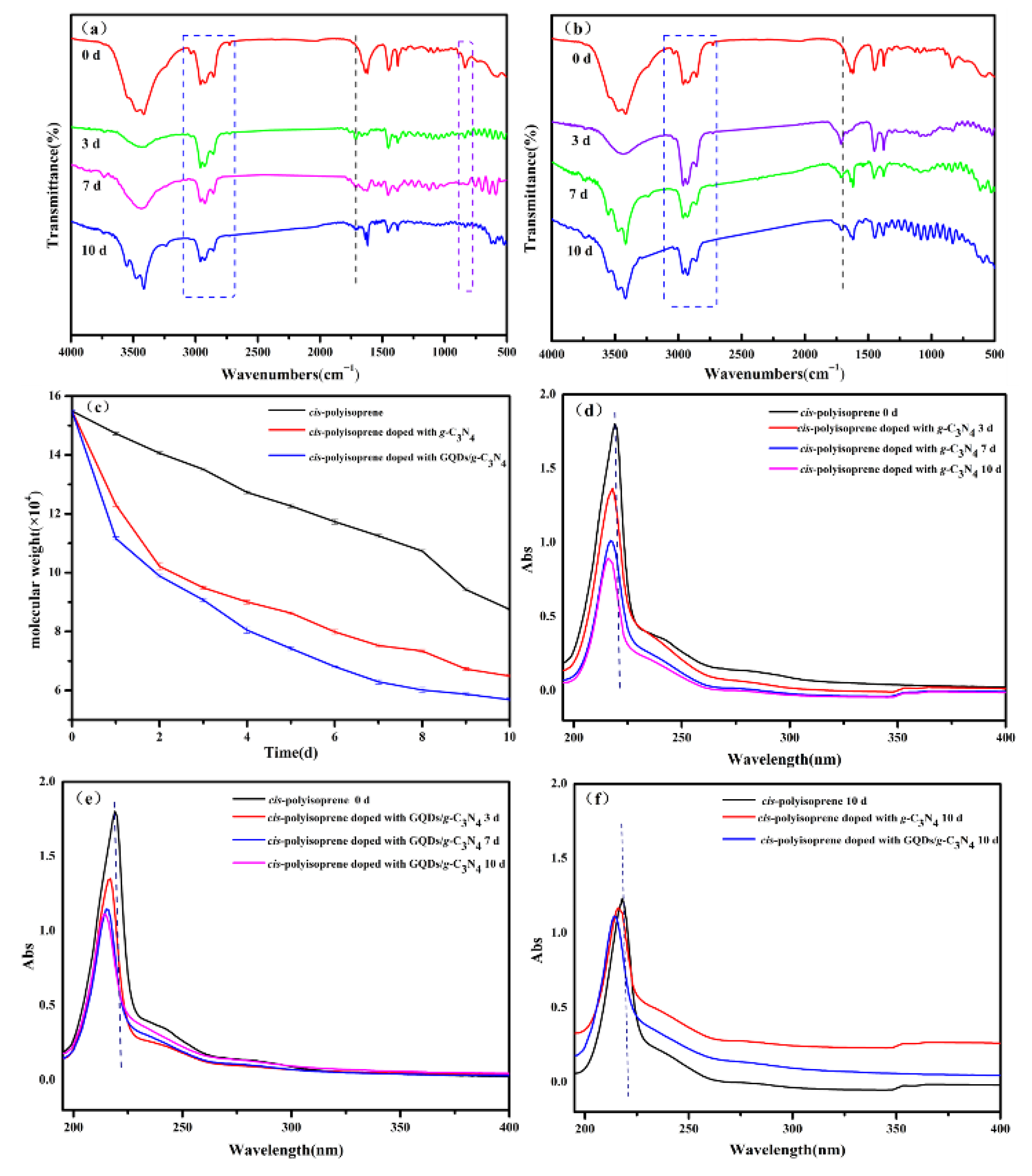
3.1. Aging of the Cis-Polyisoprene Under UV Light Irradiation
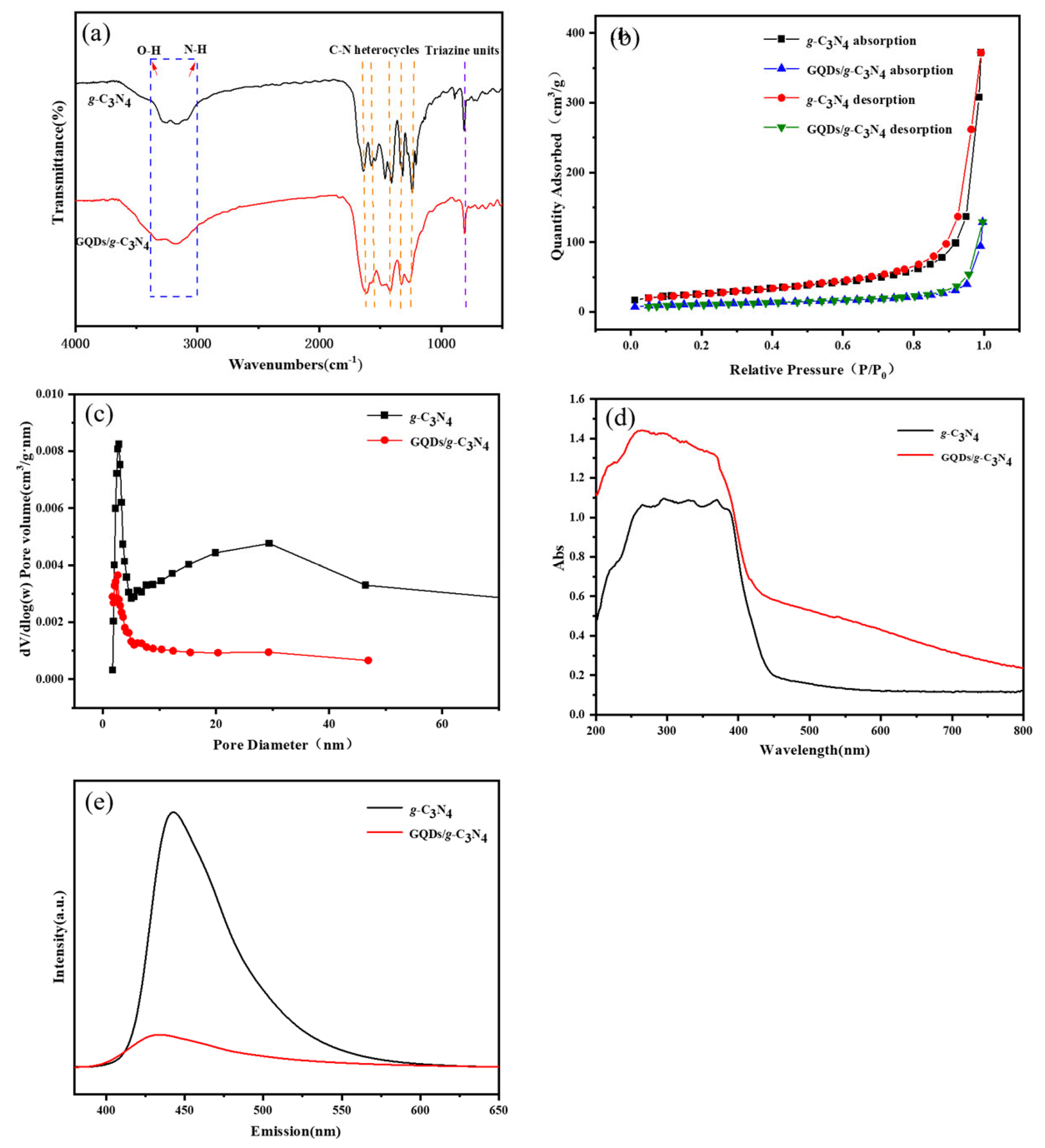
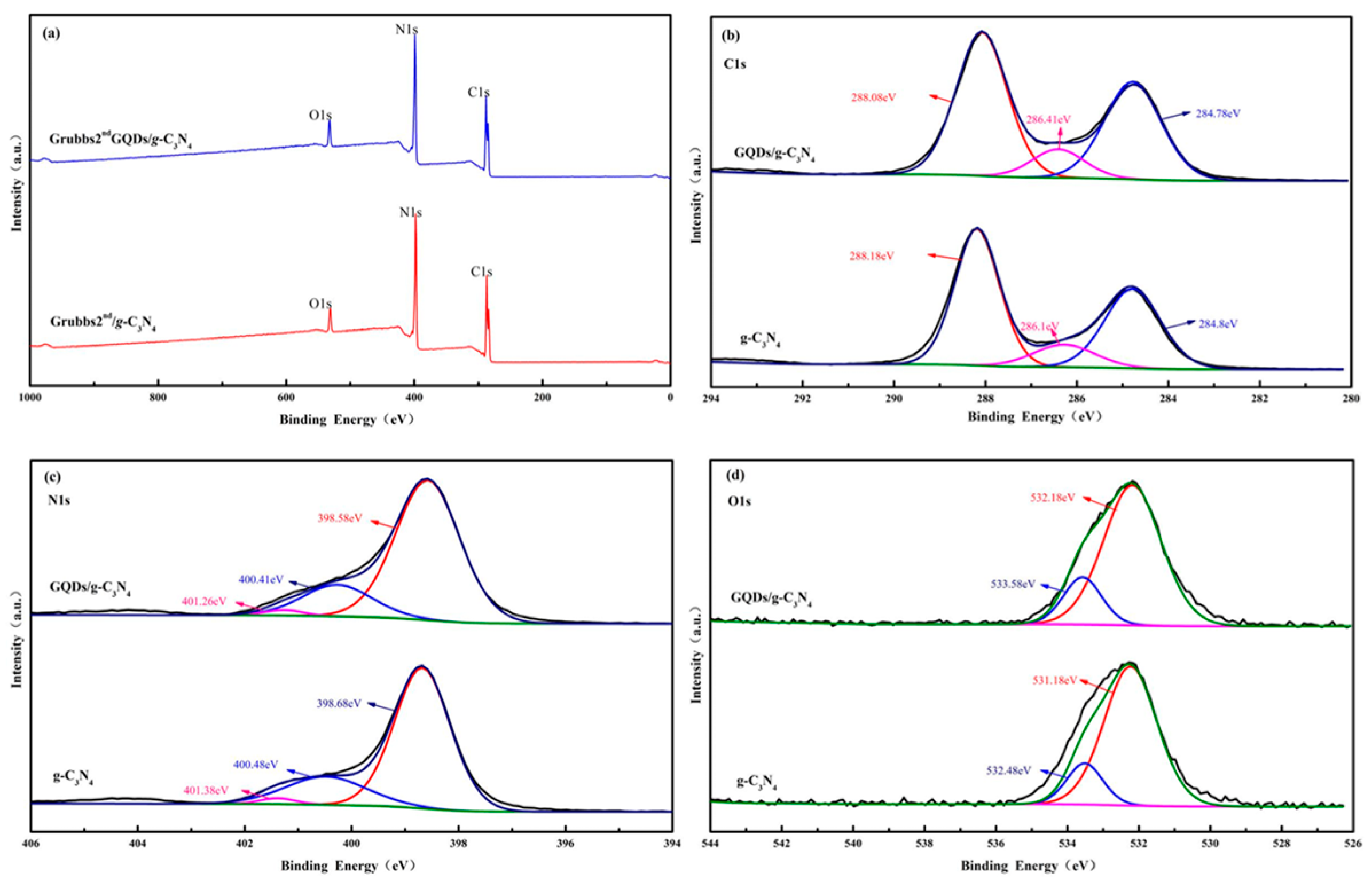
3.2. Preparation of the GQDs/g-C3N4 Composite
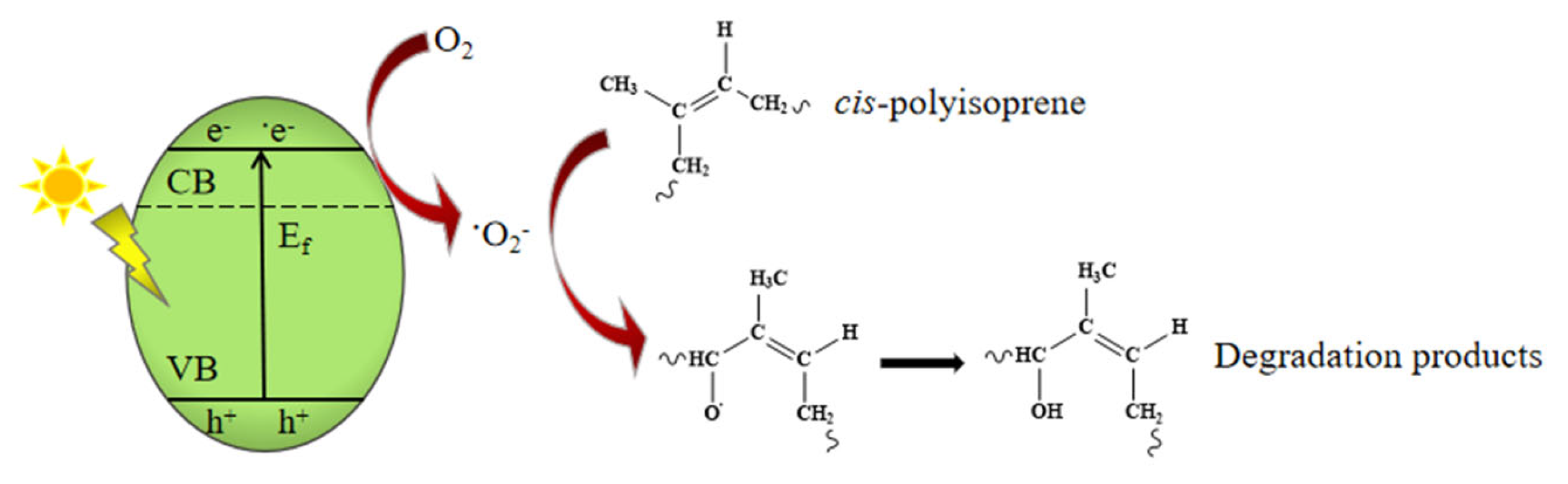
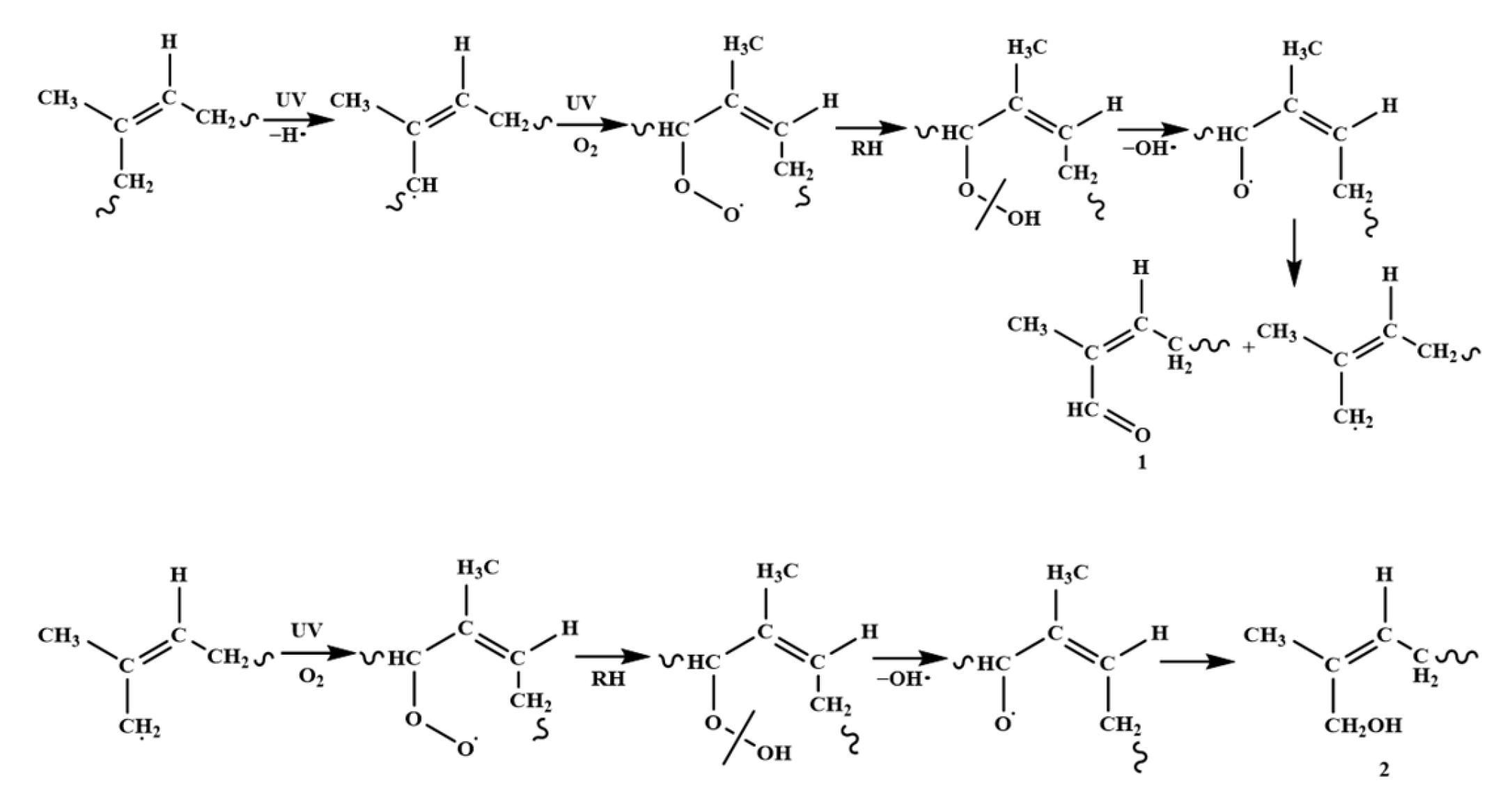
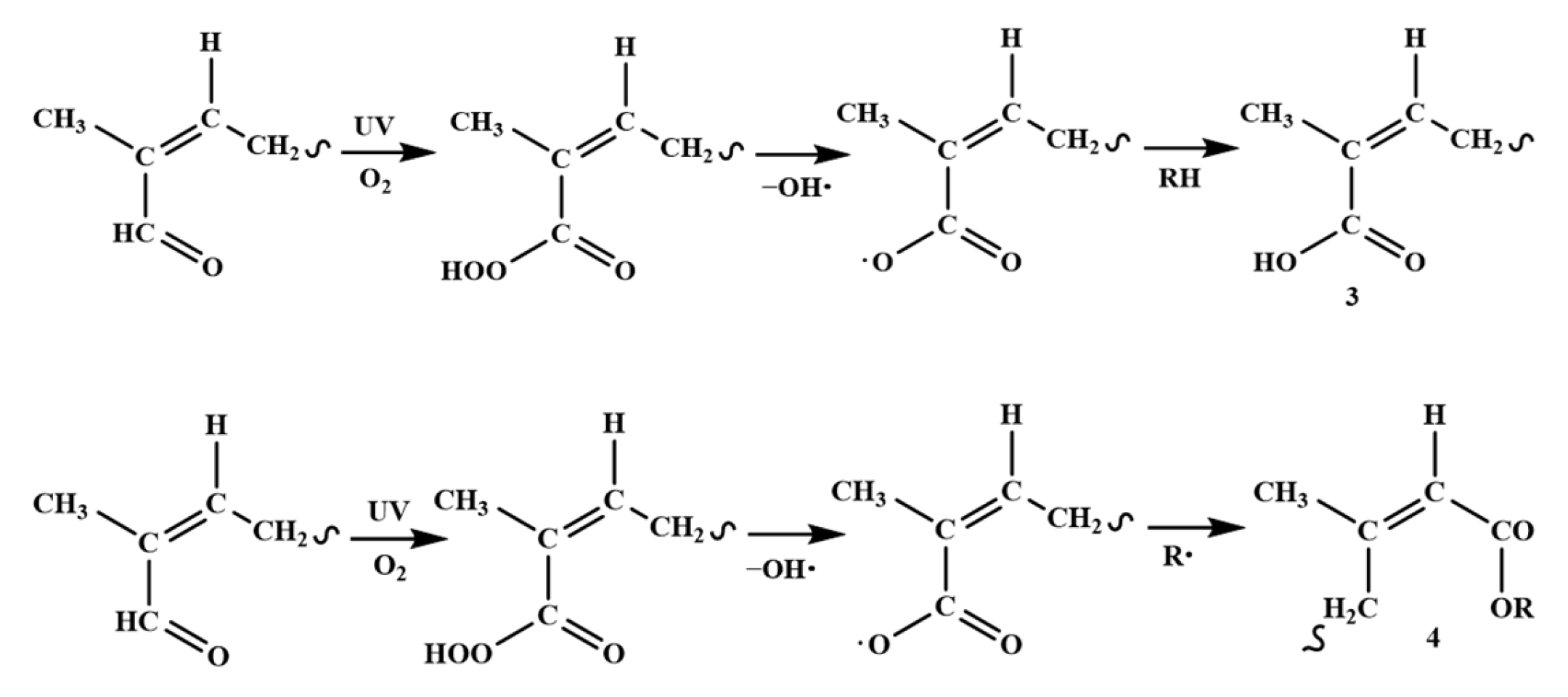
3.3. Degradation Mechanism of Cis-Polyisoprene by Ultraviolet Light
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, J.; Chen, C.; Wu, J.; He, R.; Liu, Y. Influence of ultraviolet aging on the structure, mechanical and gas permeability properties of hydrogenated nitrile butadiene rubber. J. Appl. Polym. Sci. 2021, 138, 50543. [Google Scholar] [CrossRef]
- Kolodziej, M.; Bokobza, L.; Bruneel, J.L. Investigations on natural rubber filled with multiwall carbon nanotubes. Compos. Interfaces 2007, 14, 215–228. [Google Scholar] [CrossRef]
- Vinod, V.S.; Varghese, S.; Kuriakose, B. Degradation behaviour of natural rubber–aluminium powder composites: Effect of heat, ozone and high energy radiation. Polym. Degrad. Stab. 2002, 75, 405–412. [Google Scholar] [CrossRef]
- Zheng, T.; Zheng, X.; Zhan, S.; Zhou, J.; Liao, S. Study on the ozone aging mechanism of Natural Rubber. Polym. Degrad. Stab. 2021, 186, 109514. [Google Scholar] [CrossRef]
- Kim, I.S.; Lee, B.W.; Sohn, K.S.; Yoon, J.; Lee, J.H. Characterization of the UV oxidation of raw natural rubber thin film Using Image and FT-IR Analysis. Elastomers Compos. 2016, 51, 1–9. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, H.; Wang, Y.; Deng, W.; Wang, P. Aging Characteristics of Constant Viscosity Natural Rubber. Polym. Mater. Sci. Eng. 2012, 28, 24–27. [Google Scholar]
- Dayang Habibah, A.I.H.; Devaraj, V.; Kamarularifin, H.; Suhawati, I. Cure Characteristics and Ageing Resistance of Recovered Waste Pre-Vulcanized Nitrile/Epoxidized Natural Rubber Latex Blends in Nitrile Butadiene Rubber Compounds. Adv. Mater. Res. 2015, 1119, 347–351. [Google Scholar] [CrossRef]
- Tuampoemsab, S. Influence of Amino Acids on Anti-Oxidative Properties of Green Natural Rubber and Natural Rubber Compound. Adv. Mater. Res. 2012, 747, 664–667. [Google Scholar]
- Pan, Q.; Wang, B.; Chen, Z. Application of antioxidant functionalized silicas in natural rubber. Acta Mater. Compos. Sin. 2013, 30, 1–8. [Google Scholar]
- Liu, S.M.; Yu, L.; Gao, J.X.; Zhang, X.D. Durability of rubber water stop in extreme environment: Effect and mechanisms of ultraviolet aging. Polym. Bull. 2021, 78, 4019–4032. [Google Scholar]
- Oda, H. The effect of hydroxyaryl benzotriazoles containing a singlet oxygen quenching moiety on the photofading of dyes on a polymer substrate. Dye. Pigment. 2001, 48, 233–238. [Google Scholar] [CrossRef]
- Layer, R.W. Reaction of ozone with p-phenylene-diamine and related compounds. Rubber Chem. Technol. 1966, 39, 1584–1592. [Google Scholar] [CrossRef]
- Dos Santos, K.A.M.; Suarez, P.A.Z.; Rubim, J.C. Photo-degradation of synthetic and natural polyisoprenes at specific UV radiations. Polym. Degrad. Stab. 2005, 90, 34–43. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.; Podzorova, M.; Moskovskiy, M. Impact of water and UV irradiation on nonwoven polylactide/natural rubber fiber. Polymers 2021, 13, 461. [Google Scholar] [CrossRef] [PubMed]
- Tomer, N.S.; Delor-Jestin, F.; Singh, R.P.; Lacoste, J. Cross-linking assessment after accelerated ageing of ethylene propylene diene monomer rubber. Polym. Degrad. Stab. 2007, 92, 457–463. [Google Scholar] [CrossRef]
- Reowdecha, M.; Sukthaworn, C.; Dittanet, P.; Moonprasith, N.; Lampang, T.N.; Loykulnant, S.; Prapainainar, P. Degradation of silica-reinforced natural rubber by UV radiation and humidity in soil. Key Engineering Materials. Trans Tech Publ. Ltd. 2017, 751, 314–319. [Google Scholar]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability. Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Zhen, W.; Yuan, X.; Ning, X.; Gong, X.; Xue, C. Building oxime-Ni2+ complex on polymeric carbon nitride: Molecular-level design of highly efficient hydrogen generation photocatalysts. ACS Appl. Mater. Interfaces 2019, 12, 868–876. [Google Scholar] [CrossRef]
- Wang, Y.; Vogel, A.; Sachs, M.; Sprick, R.S.; Wilbraham, L.; Moniz, S.J.A.; Godin, R.; Zwijnenburg, M.A.; Durrant, J.R.; Cooper, A.I.; et al. Current understanding and challenges of solar-driven hydrogen generation using polymeric photocatalysts. Nat. Energy 2019, 4, 746–760. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A hierarchical Z-Scheme α-Fe2O3/g-C3N4 hybrid for enhanced photocatalytic CO2 reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Sun, J.; Rao, J.; Han, Z.; Hu, Y.; Xing, W.; Zhang, C. Doping effect of phosphate in Bi2WO6 and universal improved photocatalytic activity for removing various pollutants in water. Appl. Catal. B Environ. 2016, 188, 39–47. [Google Scholar] [CrossRef]
- Lau, V.W.; Moudrakovski, I.; Botari, T.; Weinberger, S.; Mesch, M.B.; Duppel, V.; Senker, J.; Blum, V.; Lotsch, B.V. Rational design of carbon nitride photocatalysts by identification of cyanamide defects as catalytically relevant sites. Nat. Commun. 2016, 7, 12165. [Google Scholar] [CrossRef] [PubMed]
- Hansupalak, N.; Srisuk, S.; Wiroonpochit, P.; Chisti, Y. Sulfur-free prevulcanization of natural rubber latex by ultraviolet irradiation. Ind. Eng. Chem. Res. 2016, 55, 3974–3981. [Google Scholar] [CrossRef]
- Stenberg, B.; Peterson, L.O.; Flink, P.; BjörK, F. Effect of butyl rubber coating on accelerated aging of natural rubber. Rubber Chem. Technol. 1986, 59, 70–76. [Google Scholar] [CrossRef]
- SchlögL, S.; Temel, A.; Schaller, R.; Holzner, A.; Kern, W. Prevulcanization of natural rubber latex by UV techniques: A process towards reducing type IV chemical sensitivity of latex articles. Rubber Chem. Technol. 2010, 83, 133. [Google Scholar] [CrossRef]
- Milman, B.L. Identification of chemical compounds. TRAC Trends Anal. Chem. 2005, 24, 493–508. [Google Scholar]
- Seentrakoon, B.; Junhasavasdikul, B.; Chavasiri, W. Enhanced UV-protection and antibacterial properties of natural rubber/rutile-TiO2 nanocomposites. Polym. Degrad. Stab. 2013, 98, 566–578. [Google Scholar] [CrossRef]
- Andrade, G.S.; Collard, D.M.; Schiraldi, D.A.; Hu, Y.; Baer, E.; Hiltner, A. Oxygen barrier properties of PET copolymers containing bis (2-hydroxyethyl) hydroquinones. J. Appl. Polym. Sci. 2003, 89, 934–942. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, H.; Weng, G.; Zhang, Y.; Cao, X.; Gu, Z.; Liu, Y.; Liu, R.; Zhou, Z.; Nie, Y. Effect of interface on bulk polymer: Control of glass transition temperature of rubber. J. Polym. Res. 2018, 25, 173. [Google Scholar] [CrossRef]
- Le Gac, P.Y.; Le Saux, V.; Paris, M.; Marco, Y. Ageing mechanism and mechanical degradation behaviour of polychloroprene rubber in a marine environment: Comparison of accelerated ageing and long term exposure. Polym. Degrad. Stab. 2012, 97, 288–296. [Google Scholar] [CrossRef]
- Boubakri, A.; Guermazi, N.; Elleuch, K.; Ayedi, H.F. Study of UV-aging of thermoplastic polyurethane material. Mater. Sci. Eng. A 2010, 527, 1649–1654. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, X.; Gao, J. Aging behavior and mechanism of ethylene–propylene-diene monomer (EPDM) rubber in fluorescent UV/condensation weathering environment. Polym. Degrad. Stab. 2009, 94, 339–343. [Google Scholar] [CrossRef]
- Ahmed, K.; Nizami, S.S.; Raza, N.Z.; Mahmood, K. Mechanical, swelling, and thermal aging properties of marble sludge-natural rubber composites. Int. J. Ind. Chem. 2012, 3, 21. [Google Scholar] [CrossRef]
- Bhowmick, A.K.; White, J.R. Thermal, UV-and sunlight ageing of thermoplastic elastomeric natural rubber-polyethylene blends. J. Mater. Sci. 2002, 37, 5141–5151. [Google Scholar] [CrossRef]
- Sperling, L.H.; Fay, J.J. Factors which affect the glass transition and damping capability of polymers. Polym. Adv. Technol. 1991, 2, 49–56. [Google Scholar] [CrossRef]
- Honorato, L.R.; Visconte, L.L.Y.; Nunes, R.C.R. Effect of different cure systems on natural rubber/nanocellulose nanocomposites in rheological, physical-mechanical, aging, and mechanical properties. J. Elastomers Plast. 2022, 54, 676–692. [Google Scholar] [CrossRef]
- Li, C.; Sun, T.; Zhang, D.; Zhang, X.; Zhang, Y.; Lin, X.; Liu, J.; Zhu, L.; Wang, X.; Shi, Z.; et al. Microwave assisted-polymerization synthesis of 0D/2D CNQDs/g-C3N4 with the excellent visible light photocatalytic performance. Mater. Lett. 2022, 308, 131232. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, H.; Zhao, L.; He, X.; Du, Y.; Fang, W.; Li, W.; Tian, P. Constructing g-C3N4 quantum dots modified g-C3N4/GO nanosheet aerogel for UV-Vis-NIR driven highly efficient photocatalytic H2 production. Int. J. Hydrog. Energy 2019, 44, 31041–31052. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, X.; Li, J.; Zhou, P.; Yu, S.; Song, N.; Liu, C.; Che, G.; Li, C. Construction of morphology-controlled nonmetal 2D/3D homojunction towards enhancing photocatalytic activity and mechanism insight. Appl. Catal. B Environ. 2020, 263, 118270. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.; Zhou, H.; Li, X.-C.; Song, Y.; Zhang, C.; Li, Q.; He, C.; Luo, G.-N. Defect production and deuterium retention in quasi-homogeneously damaged tungsten. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 351, 23–26. [Google Scholar] [CrossRef]
- Shao, N.; Hou, Z.; Zhu, H.; Wang, J.; François-Xavier, C.P. Novel 3D core-shell structured CQDs/Ag3PO4@ Benzoxazine tetrapods for enhancement of visible-light photocatalytic activity and anti-photocorrosion. Appl. Catal. B Environ. 2018, 232, 574–586. [Google Scholar] [CrossRef]
- Ji, J.; Wen, J.; Shen, Y.; Lv, Y.; Chen, Y.; Liu, S.; Ma, H.; Zhang, Y. Simultaneous noncovalent modification and exfoliation of 2D carbon nitride for enhanced electrochemiluminescent biosensing. J. Am. Chem. Soc. 2017, 139, 11698–11701. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Feng, Y.; Zeng, Y.; Xie, Z.; Zhang, Q.; Su, Y.; Chen, P.; Liu, Y.; Yao, K.; et al. Novel ternary photocatalyst of single atom-dispersed silver and carbon quantum dots co-loaded with ultrathin g-C 3 N 4 for broad spectrum photocatalytic degradation of naproxen. Appl. Catal. B Environ. 2018, 221, 510–520. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Tang, L.; Zeng, G.; Wang, J.; Zhu, Y.; Feng, C.; Deng, Y.; He, W. Ultrathin Bi2WO6 nanosheets loaded g-C3N4 quantum dots: A direct Z-scheme photocatalyst with enhanced photocatalytic activity towards degradation of organic pollutants under wide spectrum light irradiation. J. Colloid Interface Sci. 2019, 539, 654–664. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Dong, H.; Liu, C.; Wu, H.; Che, H.; Chen, G. Z-scheme mesoporous photocatalyst constructed by modification of Sn3O4 nanoclusters on g-C3N4 nanosheets with improved photocatalytic performance and mechanism insight. Appl. Catal. B Environ. 2018, 238, 284–293. [Google Scholar] [CrossRef]
- Che, H.; Che, G.; Zhou, P.; Liu, C.; Dong, H.; Li, C.; Song, N.; Li, C. Nitrogen doped carbon ribbons modified g-C3N4 for markedly enhanced photocatalytic H2-production in visible to near-infrared region. Chem. Eng. J. 2020, 382, 122870. [Google Scholar] [CrossRef]


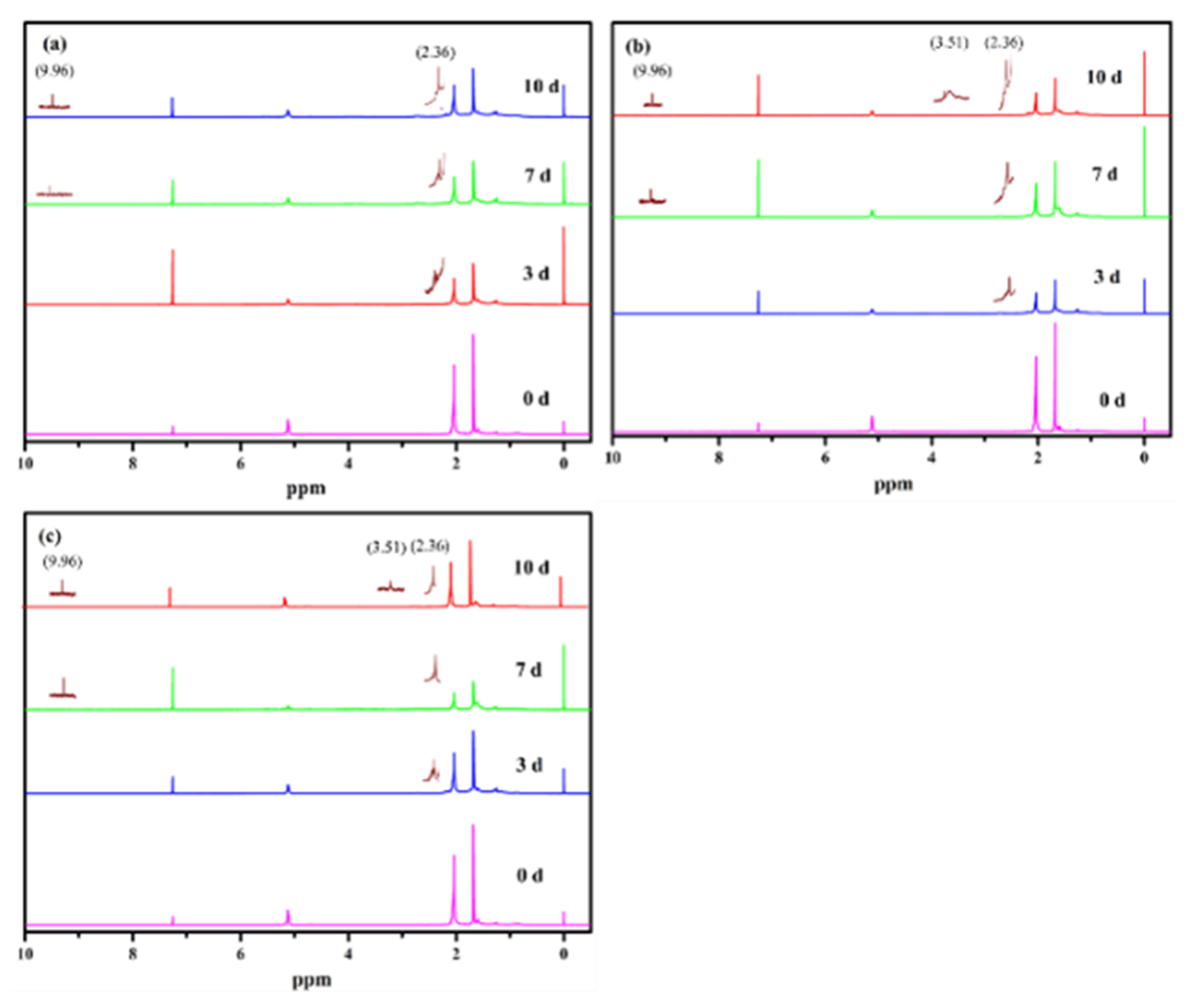
| Peak Evidence | Assignments | Origin of the Chemical Structures | Corresponding Legends |
|---|---|---|---|
| 1710–1730 cm−1 δ 9.96 | 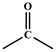 | aldehyde 1 | Figure 1a, Figure 6a,b and Figure 7a–c |
| δ 3.50–δ 3.54 | -OH | alcohol 2 | Figure 7b,c |
| 3351 cm−1–3428 cm−1 | 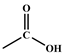 | carboxylic acid 3 | Figure 1a and Figure 6a,b |
| 1710–1730 cm−1 δ 4.10 | 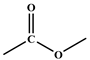 | ester 4 | Figure 1a, Figure 6a,b and Figure 7a–c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Liu, J.; Li, B.; Zhang, D.; Zhang, P.; Shi, J.; Shi, Z. Efficient Degradation of Cis-Polyisoprene by GQDs/g-C3N4 Nanoparticles Under UV Light Irradiation. Organics 2025, 6, 47. https://doi.org/10.3390/org6040047
Chen C, Liu J, Li B, Zhang D, Zhang P, Shi J, Shi Z. Efficient Degradation of Cis-Polyisoprene by GQDs/g-C3N4 Nanoparticles Under UV Light Irradiation. Organics. 2025; 6(4):47. https://doi.org/10.3390/org6040047
Chicago/Turabian StyleChen, Cilong, Jinrui Liu, Bangsen Li, Dashuai Zhang, Peisong Zhang, Jianjun Shi, and Zaifeng Shi. 2025. "Efficient Degradation of Cis-Polyisoprene by GQDs/g-C3N4 Nanoparticles Under UV Light Irradiation" Organics 6, no. 4: 47. https://doi.org/10.3390/org6040047
APA StyleChen, C., Liu, J., Li, B., Zhang, D., Zhang, P., Shi, J., & Shi, Z. (2025). Efficient Degradation of Cis-Polyisoprene by GQDs/g-C3N4 Nanoparticles Under UV Light Irradiation. Organics, 6(4), 47. https://doi.org/10.3390/org6040047






