Investigation on Porous Carbon-Loaded MnO for Removing Hexavalent Chromium from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
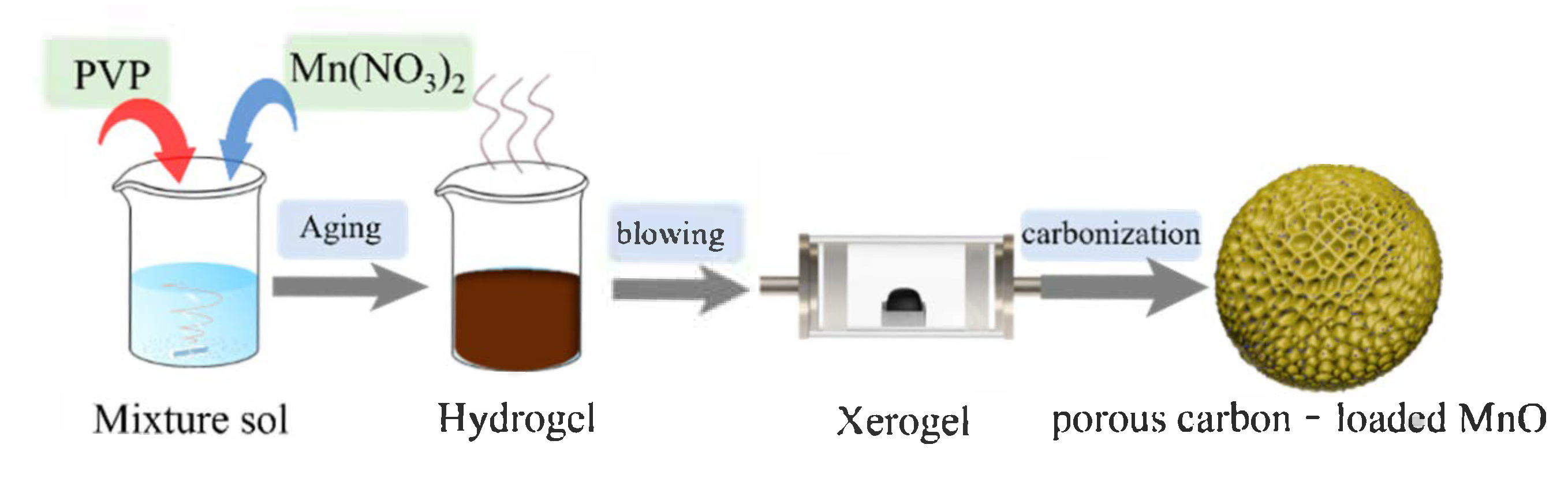
2.2. Preparation of Porous Carbon-Loaded MnO
2.3. Characterization of Porous Carbon-Loaded MnO
2.4. Adsorption Experiment
3. Results and Discussion
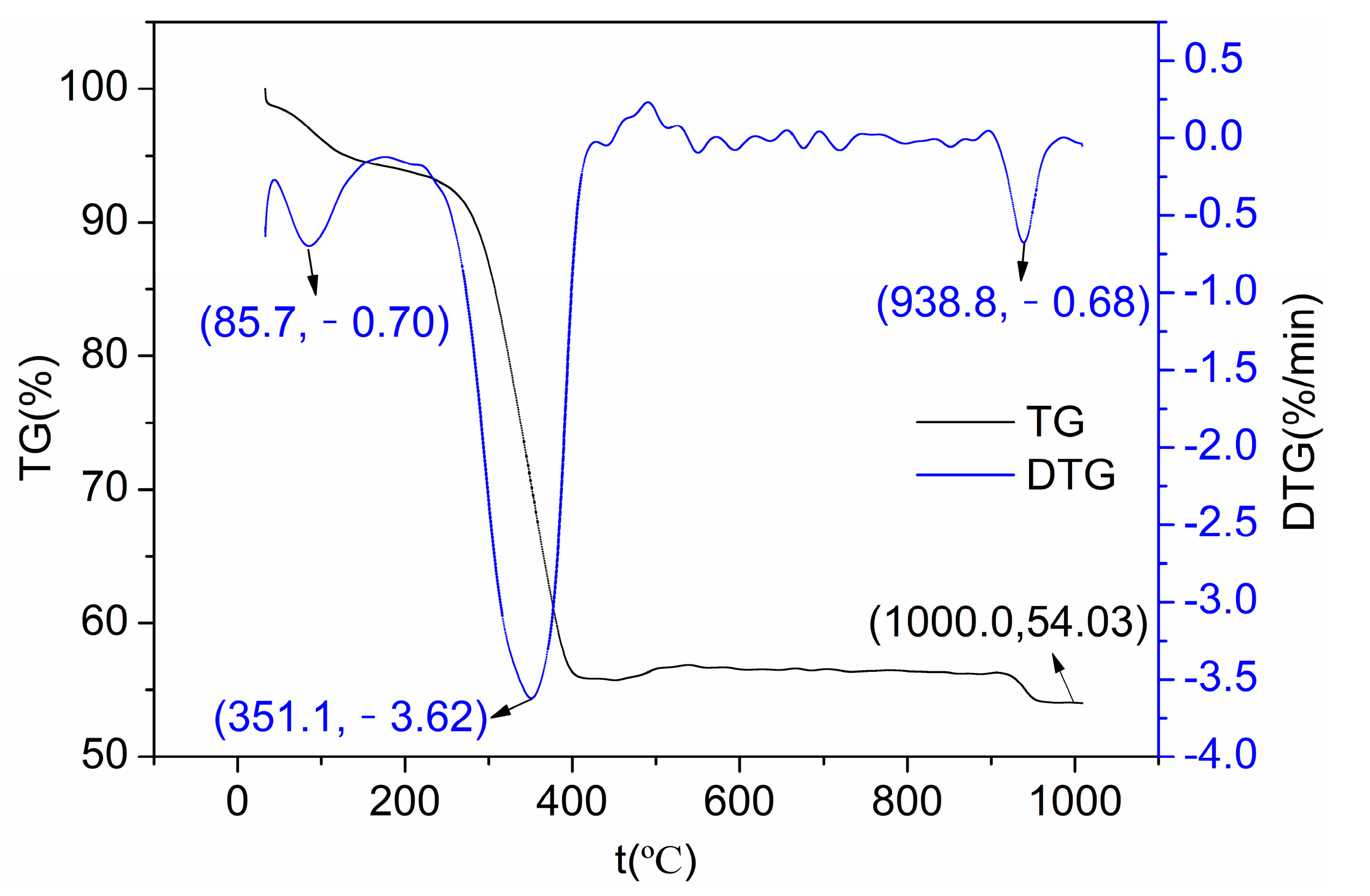
3.1. Structure Characterization
3.1.1. Phase Structure of the Material
3.1.2. Morphology and Elemental Distribution
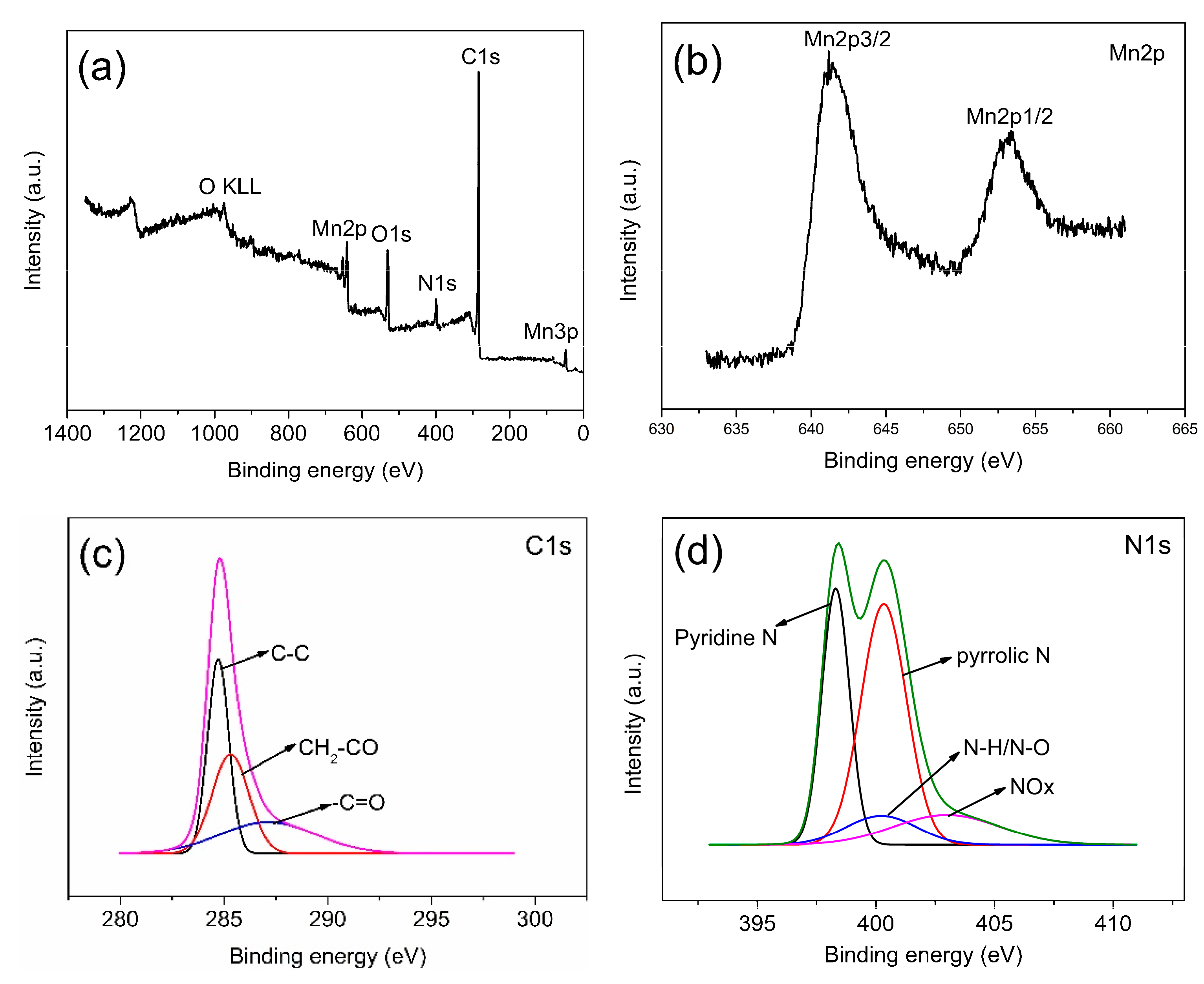
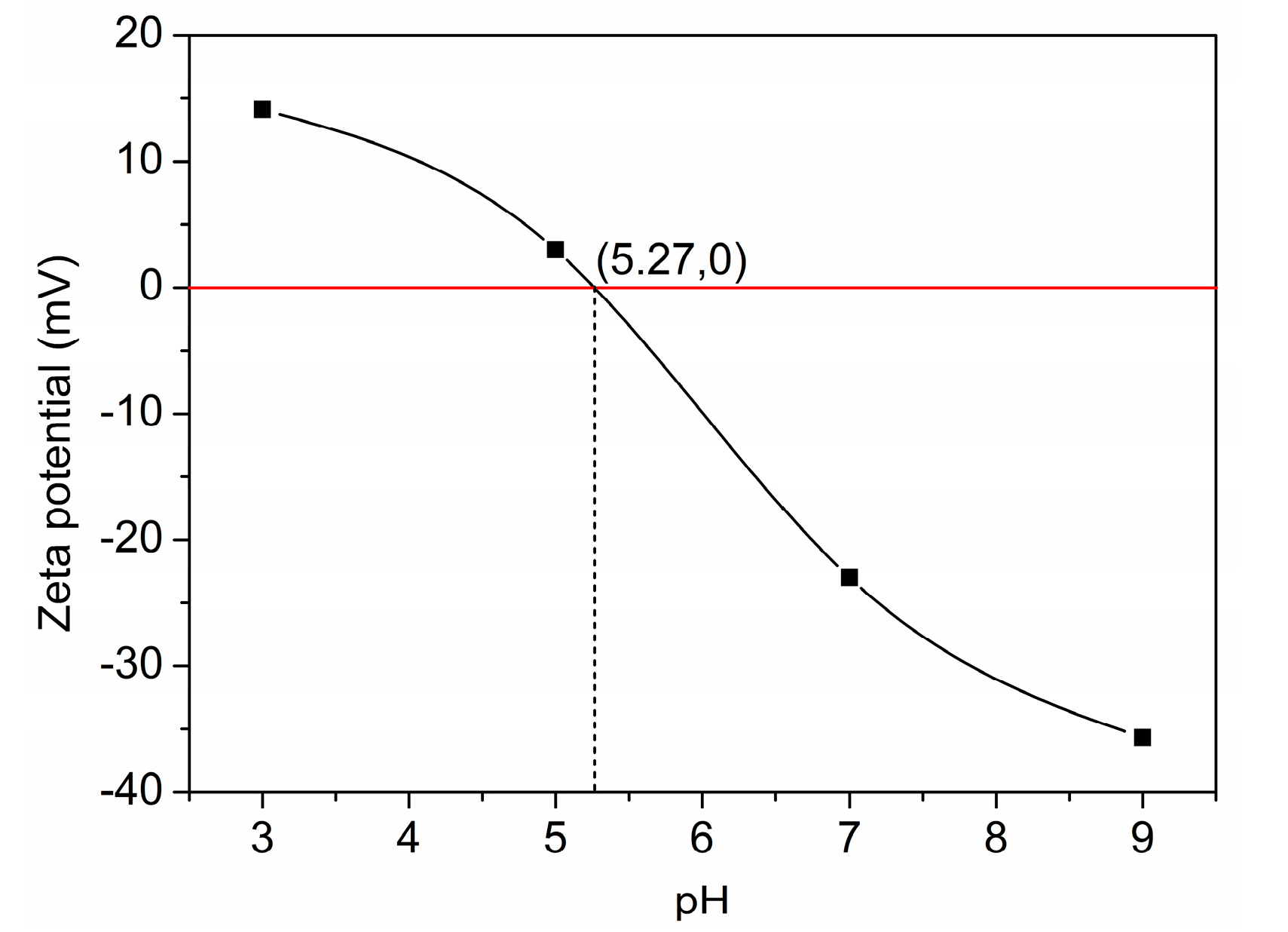
3.1.3. Surface Structure and Properties
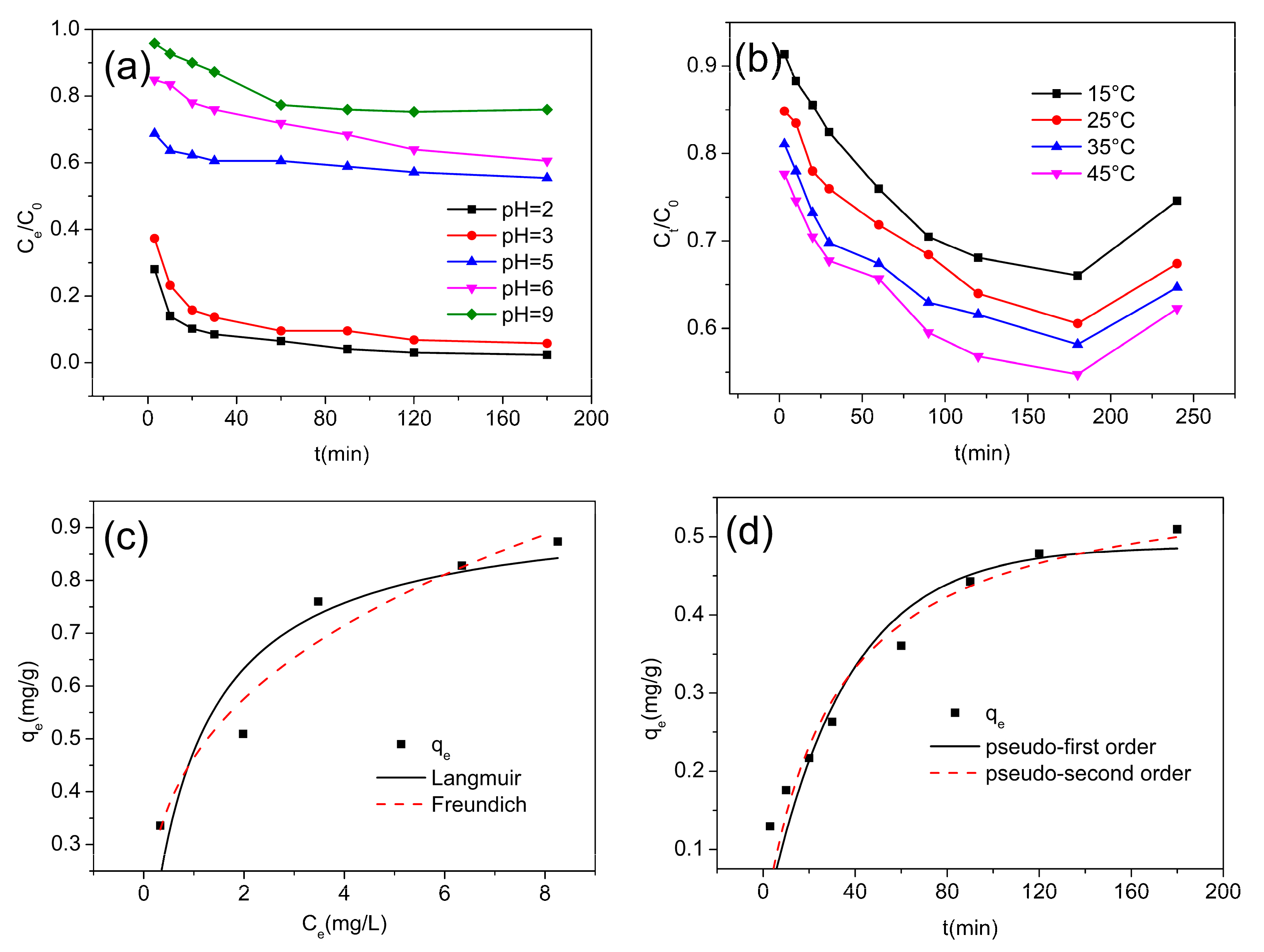
3.2. Adsorption Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; Wang, C.; Kwon, J.; Choi, Y.; Lee, J. Synthesis of 2D and 3D hierarchical β-FeOOH nanoparticles consisted of ultrathin nanowires for efficient hexavalent chromium removal. Appl. Surf. Sci. 2021, 543, 148823. [Google Scholar] [CrossRef]
- Hojabri, S.; Rajic, L.; Zhao, Y.; Alshawabkeh, A.N. Simulation of hexavalent chromium removal by electrocoagulation using iron anode in flow-through reactor. J. Hazard. Mater. 2024, 476, 135195. [Google Scholar] [CrossRef]
- Hou, B.; Pan, J.; Shi, T.; Dang, Z.; Yang, S.; Wang, L.; Gao, B. Efficient removal of hexavalent chromium by nano-cerium-based adsorbent: The critical role of valence state and oxygen vacancy. J. Hazard. Mater. 2024, 464, 133020. [Google Scholar] [CrossRef]
- Lin, X.; Xu, L.; Zhao, Z.; Liu, S.; Zhang, J.; Wu, D. Highly efficient removal of hexavalent chromium from electroplating wastewater by ferrous/ferric hydroxide complex: From lab-scale to pilot-scale study. Chem. Eng. J. 2023, 454, 140429. [Google Scholar] [CrossRef]
- Zha, S.; Yu, A.; Wang, Z.; Shi, Q.; Cheng, X.; Liu, C.; Deng, C.; Zeng, G.; Luo, S.; Zhao, Z.; et al. Microbial strategies for effective hexavalent chromium removal: A comprehensive review. Chem. Eng. J. 2024, 489, 151457. [Google Scholar] [CrossRef]
- Lai, Y.-R.; Hou, X.-X.; How, S.-C.; Lin, T.-H.; Wang, S.S.S. Development of two-dimensional amyloid fibril/carboxymethyl cellulose hybrid membranes for effective adsorption of hexavalent chromium. J. Environ. Chem. Eng. 2024, 12, 114134. [Google Scholar] [CrossRef]
- Das, S.; Behera, B.C.; Mohapatra, R.K.; Pradhan, B.; Sudarshan, M.; Chakraborty, A.; Thatoi, H. Reduction of hexavalent chromium by Exiguobacterium mexicanum isolated from chromite mines soil. Chemosphere 2021, 282, 131135. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Q.; Wang, Y.-X.; Li, Y.-M.; He, C.-S.; Lai, B.; Chen, F.; Wang, H.-J.; Zhou, X.-G.; Mu, Y. Metal organic framework decorated with molybdenum disulfide for visible-light-driven reduction of hexavalent chromium: Performance and mechanism. J. Clean. Prod. 2021, 318, 128513. [Google Scholar] [CrossRef]
- Siciliano, A.; Curcio, G.M.; Limonti, C. Hexavalent chromium reduction by zero-valent magnesium particles in column systems. J. Environ. Manag. 2021, 293, 112905. [Google Scholar] [CrossRef] [PubMed]
- Thiripelu, P.; Manjunathan, J.; Revathi, M.; Ramasamy, P. Removal of hexavalent chromium from electroplating wastewater by ion-exchange in presence of Ni(II) and Zn(II) ions. J. Water Process. Eng. 2024, 58, 104815. [Google Scholar] [CrossRef]
- Xu, X.; Xie, J.; Li, Y.; Wu, S.; Yang, X.; Eltzov, E.; Jia, K. Carboxylated polyarylene ether nitrile modified polysulfone membrane with enhanced anti-fouling for oily water separation and hexavalent chromium removal. Sep. Purif. Technol. 2025, 364, 132486. [Google Scholar] [CrossRef]
- Wei, X.-Z.; Gan, Z.-Q.; Shen, Y.-J.; Qiu, Z.-L.; Fang, L.-F.; Zhu, B.-K. Negatively-charged nanofiltration membrane and its hexavalent chromium removal performance. J. Colloid Interf. Sci. 2019, 553, 475–483. [Google Scholar] [CrossRef]
- Mondal, G.; Sahoo, P.; Banerjee, S.; Nandi, R.; Ghosh, C.; Mandal, J.; Bhattacharyya, P. Utilizing nano zero-valent iron impregnated biochar for removal of hexavalent chromium from water: An assessment through Box-Behnken optimization, kinetics, and isotherm studies. Groundw. Sust. Dev. 2025, 29, 101446. [Google Scholar] [CrossRef]
- Wu, B.; Hong, M.; Wu, Q.; Li, X.; Zhao, Y.; Wang, S.; Wang, Z. New Schiff base covalently bonded graphene oxide for removing chromium(VI) from surface runoff. Environ. Res. 2025, 264, 120360. [Google Scholar] [CrossRef]
- Li, X.; Wu, Q.; Chen, D.; Tamjidur, R.S.; Fan, P.; Wang, G.; Zhang, W. Efficient chromium Cr (VI) removal from wastewater through modified cycad’s leaf biochar: Insights into adsorption-reduction mechanisms and kinetic analysis. Biomass Bioenergy 2025, 200, 107994. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, W.; Zhao, F.; Liu, F.; Zhao, T. Direct synthesis of N-doped porous carbon materials from EDTA dipotassium salt for the rapid adsorption of hexavalent chromium from aqueous solutions. Microporous Mesoporous Mater. 2025, 383, 113426. [Google Scholar] [CrossRef]
- Khdoor, Z.; Makharza, S.; Qurie, M.; Fohely, F.; Abu Taha, A.; Hampel, S. Removal of toxic hexavalent chromium via graphene oxide nanoparticles: Study of kinetics, isotherms, and thermodynamics. RSC Adv. 2024, 14, 24345–24351. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhou, L.; Wang, X.; Zhang, G.; Bao, X.; Yan, Z.; Ma, W. One-step synthesis of iron-rich biochar for efficient hexavalent chromium removal: Adsorption-reduction performance, mechanism and column experiments. J. Environ. Chem. Eng. 2025, 13, 115701. [Google Scholar] [CrossRef]
- Nguyen-Thi, N.Y.; Nguyen, C.Q.; Le Dang, Q.; De Tran, Q.; Do-Thi, T.N.; Vu Thanh, L.H. Extracting lignin from sugarcane bagasse for methylene blue and hexavalent chromium adsorption in textile wastewater: A facile, green, and sustainable approach††Electronic supplementary information (ESI) available. RSC Adv. 2024, 14, 4533–4542. [Google Scholar] [CrossRef]
- Yi, Y.; Fu, Y.; Yang, W.; Chen, W.; Wang, Y.; Diao, Z.; Chen, Z.; Li, Z. Potassium chloride assisted pyrolysis to fabricate low-valent iron rich magnetic biochar for ultra-fast removal of hexavalent chromium: Performance and mechanism. J. Water Process Eng. 2025, 69, 106798. [Google Scholar] [CrossRef]
- Li, J.; Zhu, W.; Gao, L.; Liang, X.; Yang, Q. Removal of hexavalent chromium in water by chitosan-modified Enteromorpha prolifera biochar loaded with iron-manganese oxides: Application performances and reaction mechanisms. Mater. Chem. Phys. 2024, 317, 129189. [Google Scholar] [CrossRef]
- Liao, B.; Guo, M.; Zhao, S.; Lu, T. Efficient reduction of hexavalent chromium by Fe-Mn bimetallic nanoparticles: Performance and role of manganese. Surf. Interfaces 2024, 46, 103956. [Google Scholar] [CrossRef]
- Manzoor, Q.; Farrukh, M.A.; Qamar, M.T.; Sajid, A. Efficient adsorption and photocatalytic removal of hexavalent chromium using chitosan-functionalized graphene oxide-MnO2 nanocomposite. Int. J. Biol. Macromol. 2025, 311, 144009. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Slek, Y.; Amarray, A.; Salmi, M.; Rharib, M.E.; Zaroual, Z.; Ghachtouli, S.E. Amino-functionalized manganese oxide for effective hexavalent chromium adsorption. Environ. Sci. Pollut. Res. 2025, 32, 1480–1497. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.S.; Chen, D.-H. Fast removal of copper ions by gum arabic modified magnetic nano-adsorbent. J. Hazard. Mater. 2007, 147, 792–799. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Z.; Zhang, M.; Chai, B. Adsorption of methylene blue from aqueous solution on modified ACFs by chemical vapor deposition. Chem. Eng. J. 2012, 189–190, 168–174. [Google Scholar] [CrossRef]
- Tripathy, S.P.; Subudhi, S.; Das, S.; Ghosh, M.K.; Das, M.; Acharya, R.; Acharya, R.; Parida, K. Hydrolytically stable citrate capped Fe3O4@UiO-66-NH2 MOF: A hetero-structure composite with enhanced activity towards Cr (VI) adsorption and photocatalytic H2 evolution. J. Colloid Interf. Sci. 2022, 606, 353–366. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, F.; Li, L.; Ma, J. Adsorption-reduction synergistic effect for rapid removal of Cr (VI) ions on superelastic NH2-graphene sponge. Chem. Eng. J. 2021, 421, 129933. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Yue, C.; Song, L.; Lv, Y.; Liu, F.; Li, A. Insight into the efficient co-removal of Cr(VI) and Cr(III) by positively charged UiO-66-NH2 decorated ultrafiltration membrane. Chem. Eng. J. 2021, 404, 126546. [Google Scholar] [CrossRef]
- Jian, X.; Li, S.; Feng, Y.; Chen, X.; Kuang, R.; Li, B.; Sun, Y. Influence of Synthesis Methods on the High-Efficiency Removal of Cr(VI) from Aqueous Solution by Fe-Modified Magnetic Biochars. ACS Omega 2020, 5, 31234–31243. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]





| Element | Weight% | Atomic% |
|---|---|---|
| C K | 33.70 | 57.62 |
| N K | 2.29 | 3.36 |
| O K | 16.60 | 21.31 |
| Mn K | 47.40 | 17.71 |
| Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | KF | n | R2 |
| 0.9416 | 1.0277 | 0.8349 | 0.4643 | 3.214 | 0.9339 |
| Pseudo−First−Order Model | Pseudo−Second−Order Model | ||||
|---|---|---|---|---|---|
| qe (mg/g) | k1 | R2 | qe (mg/g) | k2 | R2 |
| 0.4878 | 0.0288 | 0.8933 | 0.5838 | 0.0565 | 0.9301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, M. Investigation on Porous Carbon-Loaded MnO for Removing Hexavalent Chromium from Aqueous Solution. Organics 2025, 6, 36. https://doi.org/10.3390/org6030036
Wang L, Zhang M. Investigation on Porous Carbon-Loaded MnO for Removing Hexavalent Chromium from Aqueous Solution. Organics. 2025; 6(3):36. https://doi.org/10.3390/org6030036
Chicago/Turabian StyleWang, Liping, and Mingyu Zhang. 2025. "Investigation on Porous Carbon-Loaded MnO for Removing Hexavalent Chromium from Aqueous Solution" Organics 6, no. 3: 36. https://doi.org/10.3390/org6030036
APA StyleWang, L., & Zhang, M. (2025). Investigation on Porous Carbon-Loaded MnO for Removing Hexavalent Chromium from Aqueous Solution. Organics, 6(3), 36. https://doi.org/10.3390/org6030036







