PANI-Based Thermoelectric Materials
Abstract
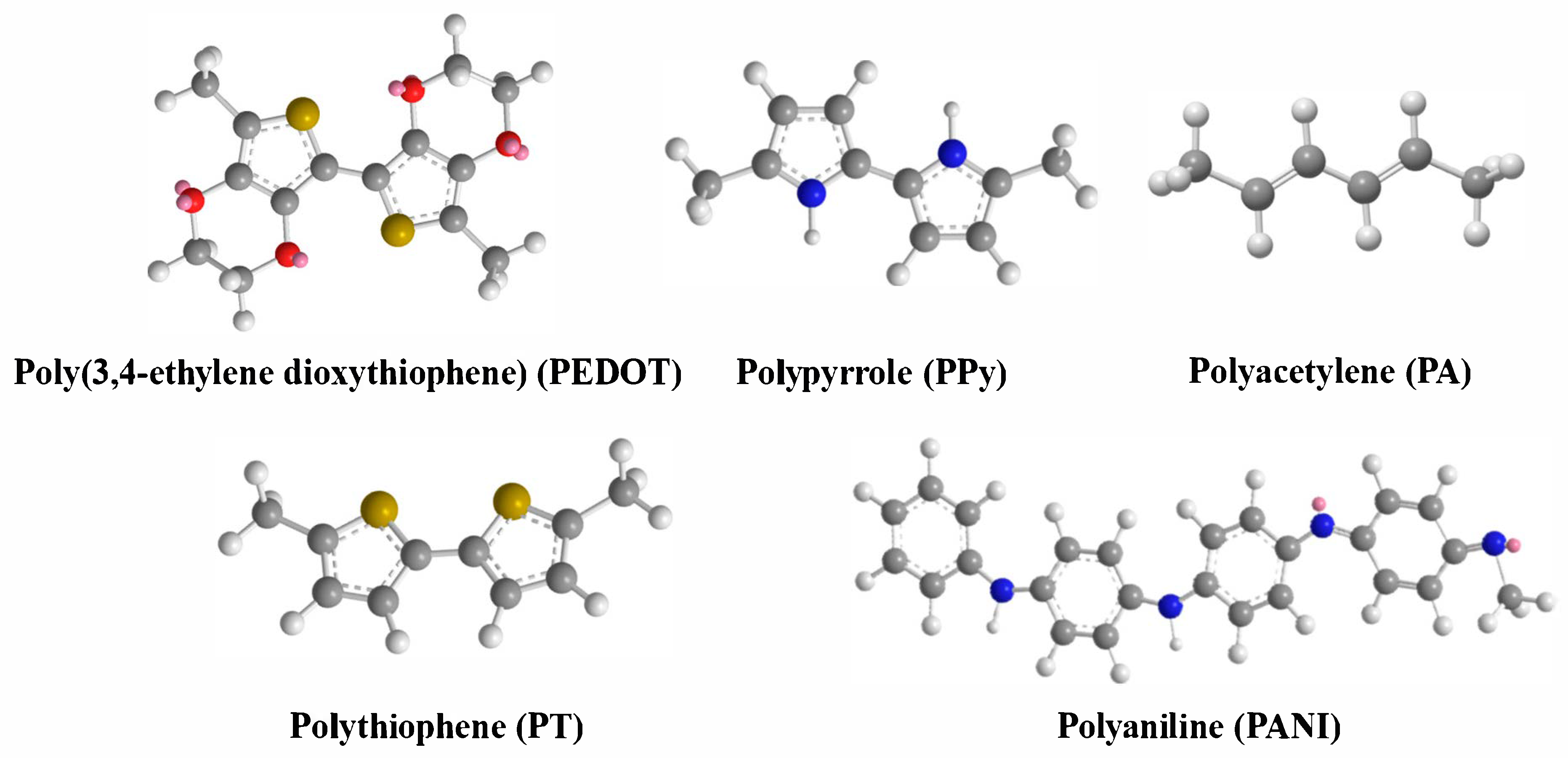
1. Introduction
2. Synthesis of PANI-Based Thermoelectric Materials
2.1. Mechanical Mixing
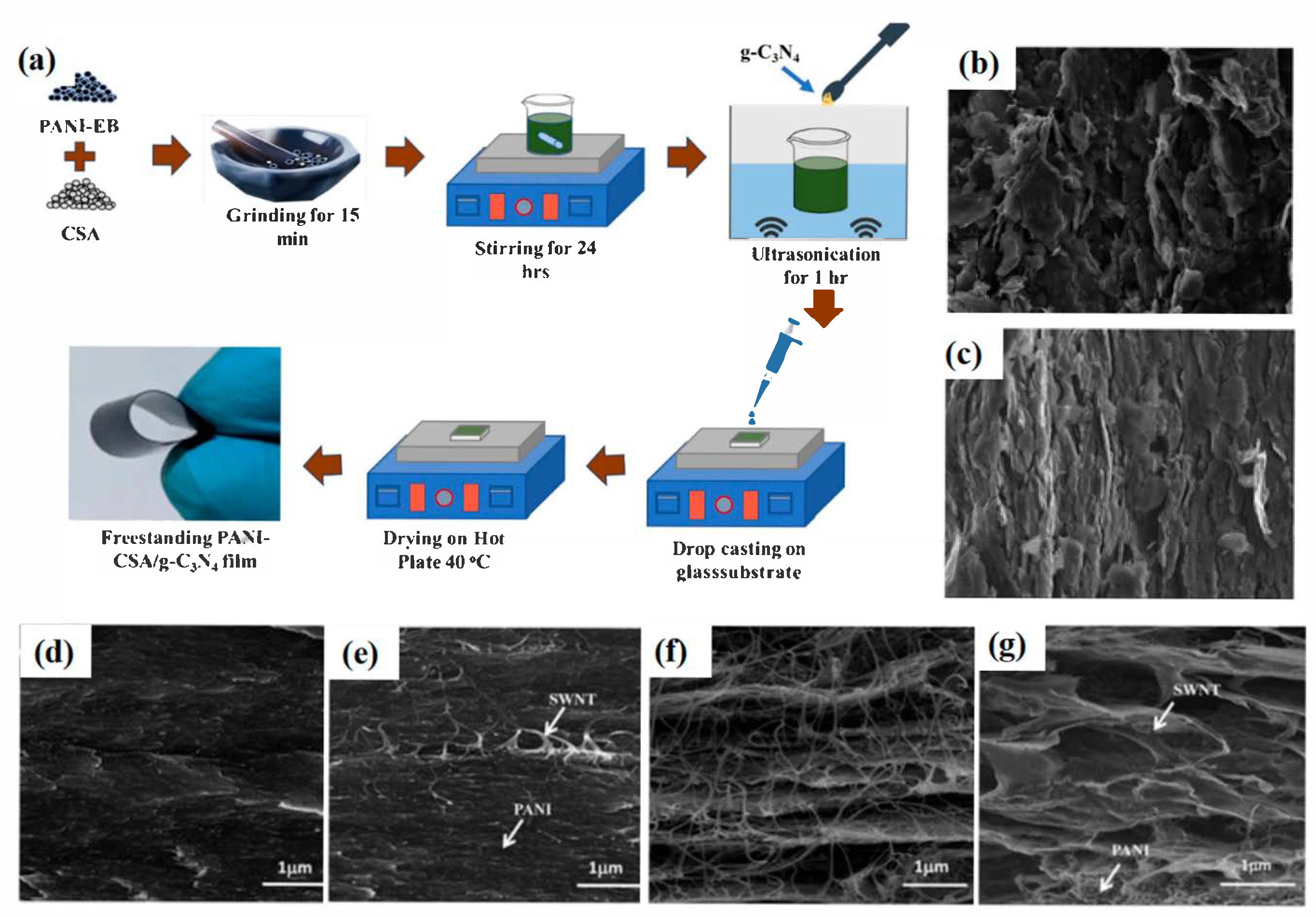
2.2. Solution-Mediated Mixing
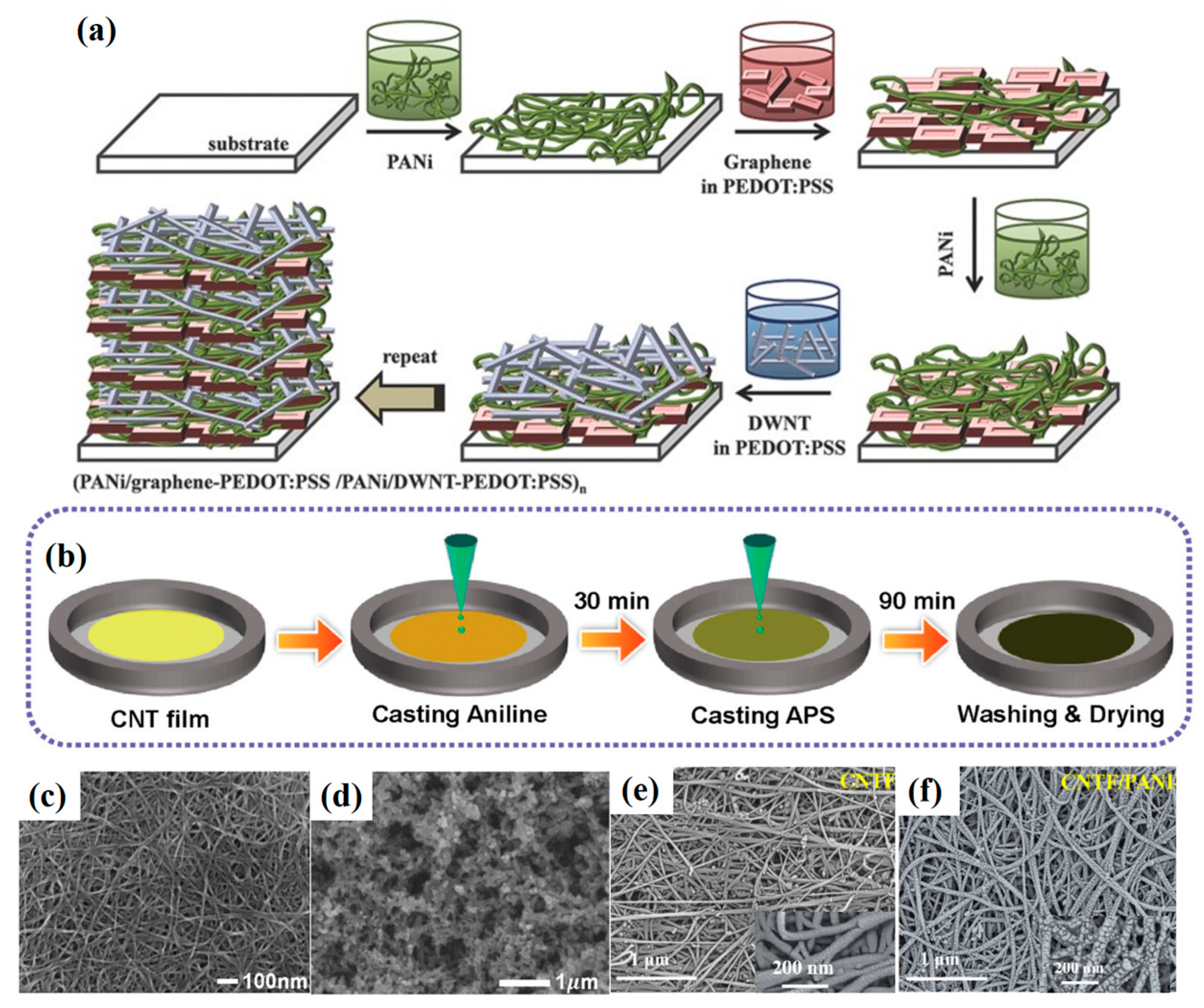
2.3. Layer-by-Layer Self-Assembly
2.4. In-Situ Polymerization
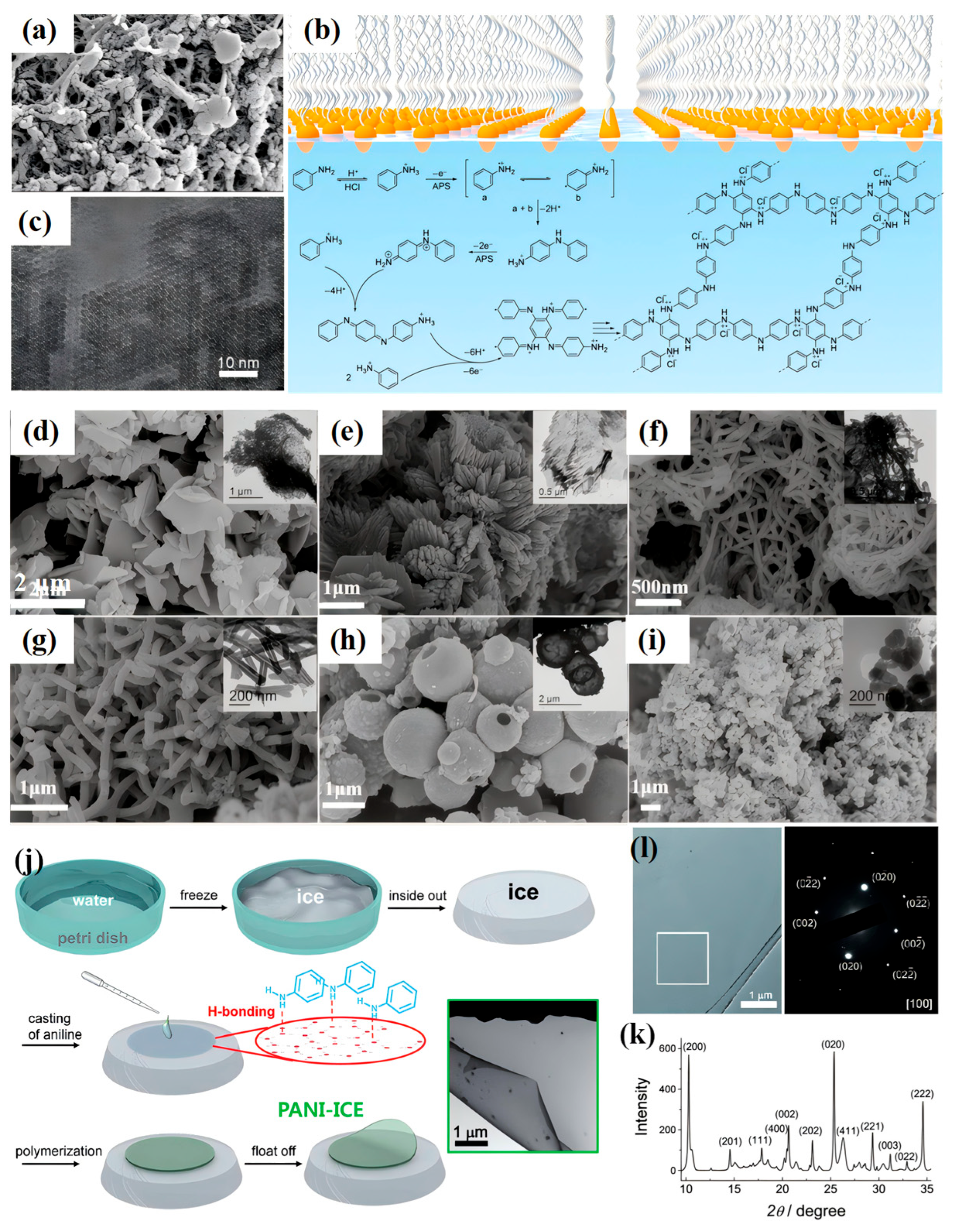
2.5. Interfacial Polymerization
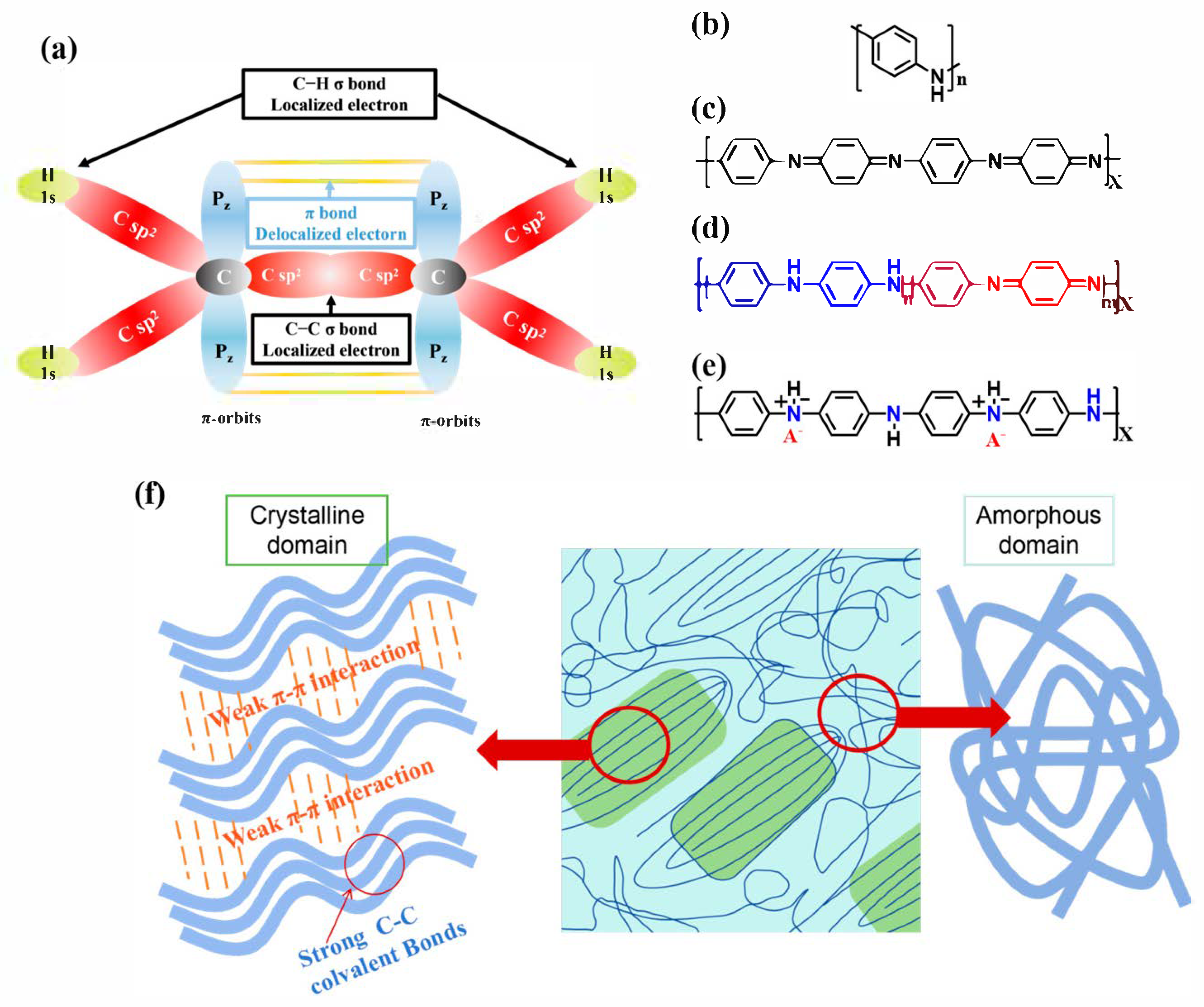
3. Mechanisms for Enhancing Thermoelectric Properties of PANI-Based Materials
3.1. Modulation of Doping Level
3.2. Ordering of Molecular Chains
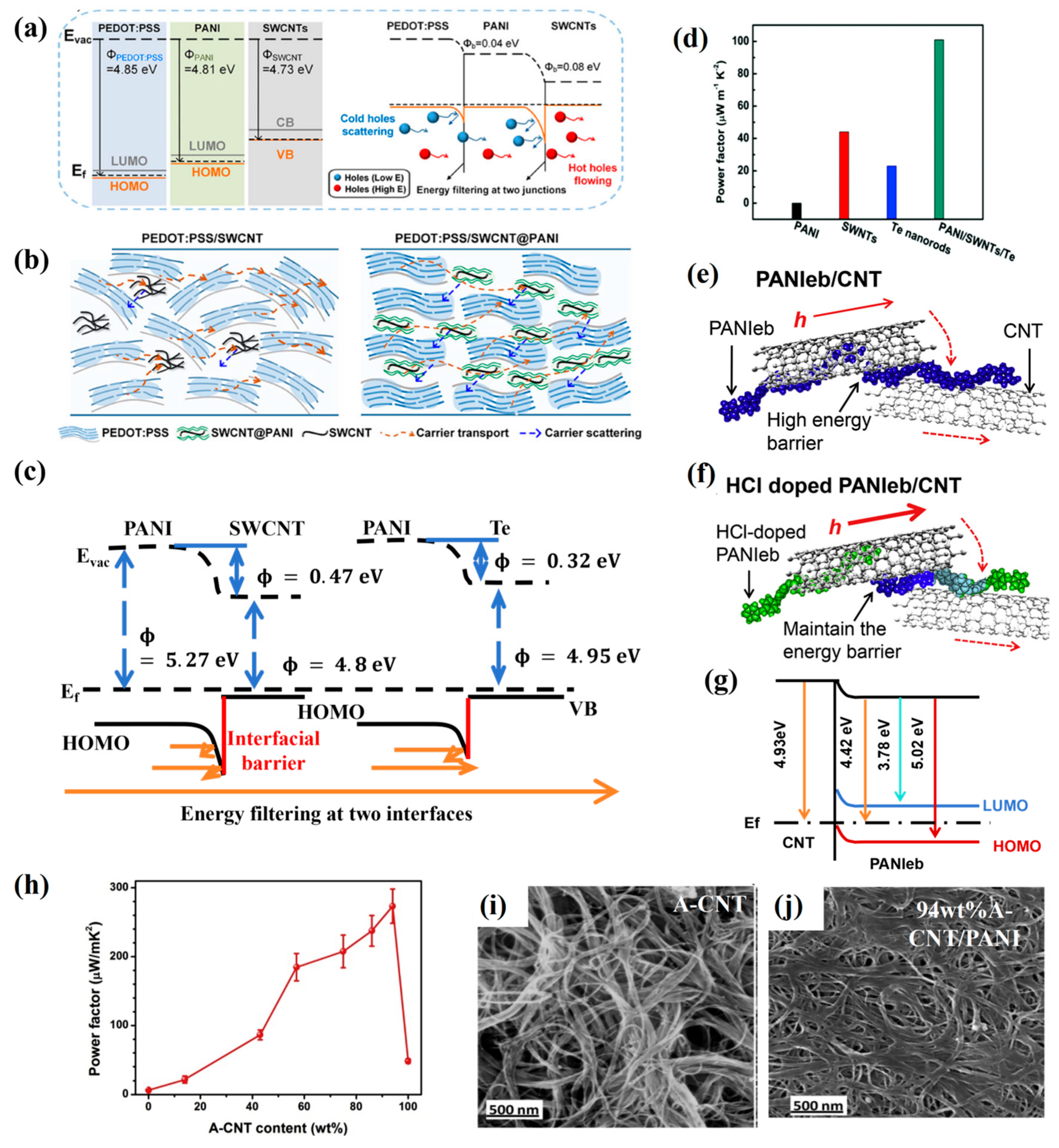
3.3. Organic–Inorganic Interface Effect
3.4. Bridging Effects
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zong, P.A.; Hanus, R.; Dylla, M.; Tang, Y.; Liao, J.; Zhang, Q.; Snyder, G.J.; Chen, L.D. Skutterudite with Graphene-modified Grain-boundary Complexion Enhances zT Enabling High-efficiency Thermoelectric Device. Energy Environ. Sci. 2017, 10, 183–191. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Ghosh, T.; Arora, R.; Samanta, M.; Xie, L.; Singh, N.K.; Soni, A.; He, J.Q.; Waghmare, U.V.; Biswas, K. Enhanced Atomic Ordering Leads To High Thermoelectric Performance in AgSbTe2. Science 2021, 371, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.B.; Yu, Y.; Cui, J.; Liu, X.X.; Xie, L.; Liao, J.C.; Zhang, Q.H.; Huang, Y.; Ning, S.C.; Jia, B.H.; et al. High-entropy-stabilized chalcogenides with high thermoelectric performance. Science 2021, 371, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, C.; Lilley, D.; Parez, P.S.; Prasher, R. Techno-economic analysis of waste-heat conversion. Joule 2021, 5, 3080–3096. [Google Scholar] [CrossRef]
- Anderson, K.; Brandon, N. Techno-economic analysis of thermoelectrics for waste heat recovery. Energy Sources Plan B 2019, 14, 147–157. [Google Scholar] [CrossRef]
- Shi, X.L.; Cao, T.Y.; Chen, W.Y.; Hu, B.X.; Sun, S.; Liu, W.D.; Li, M.; Lyu, W.Y.; Hong, M.; Chen, Z.G. Advances in flexible inorganic thermoelectrics. Eco Energy. 2023, 1, 296–343. [Google Scholar] [CrossRef]
- Xu, S.D.; Shi, X.L.; Dargusch, M.; Di, C.G.; Zou, J.; Chen, Z.G. Conducting polymer-based flexible thermoelectric materials and devices: From mechanisms to applications. Prog. Mater. Sci. 2021, 121, 100840. [Google Scholar] [CrossRef]
- LaLonde, D.A.; Pei, Y.; Wang, H.; Snyder, G.J. Lead telluride alloy thermoelectrics. Mat. Today 2011, 14, 526–532. [Google Scholar] [CrossRef]
- Cao, T.Y.; Shi, X.L.; Chen, Z.G. Advances in the design and assembly of flexible thermoelectric device. Prog. Mater. Sci. 2023, 131, 101003. [Google Scholar] [CrossRef]
- Ji, Y.R.; Zhang, X.F.; Ai, W.; He, Z.X.; Lou, S.Z.; Tang, Z.; Hang, F.L.; Liu, Z.G.; Ou, Y.X.; Hu, X.H.; et al. Intercalation-deintercalation engineering of van der Waals stacked MXene films for wearable thermoelectrics and sensing. Chem. Eng. J. 2025, 512, 162603. [Google Scholar] [CrossRef]
- Chen, M.R.; Mao, Z.D.; Ji, Y.R.; Zong, P.A.; Zhang, Q.H. Bi2Te3-based flexible thermoelectrics. Mater. Today Energy 2024, 44, 101643. [Google Scholar] [CrossRef]
- Fan, Z.; Liang, J.S.; Chen, J.L.; Peng, Y.; Lai, H.J.; Nong, J.; Liu, C.Y.; Ding, W.Y.; Miao, L. Realizing high thermoelectric performance for p-type SiGe in medium temperature region via TaC compositing. J. Mater. 2023, 9, 984–991. [Google Scholar] [CrossRef]
- Shi, X.L.; Zou, J.; Chen, Z.G. Advanced thermoelectric design: From materials and structures to devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.X.; Shi, X.L.; Cao, T.Y.; Liu, S.Q.; Zhang, M.; Lyu, W.Y.; Yin, L.C.; Tesfamichael, T.Q.B.; Liu, Q.F.; Chen, Z.G. High-performing flexible Mg3Bi2 thin film thermoelectrics. Adv. Sci. 2024, 11, 2409788. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, X.L.; Chen, Z.G. Trends in GeTe Thermoelectrics: From fundamentals to Applications. Adv. Funct. Mater. 2024, 34, 2403498. [Google Scholar] [CrossRef]
- Shi, X.L.; Wang, L.J.; Lyu, W.Y.; Cao, T.Y.; Chen, W.Y.; Hu, B.X.; Chen, Z.G. Advancing flexible thermoelectrics for integrated electronics. Chem. Soc. Rev. 2024, 53, 9254–9305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Shi, X.L.; Shi, X.; Chen, L.D.; Dargusch, M.S.; Zou, J.; Chen, Z.G. Flexible thermoelectric materials and generators: Challenges and innovations. Adv. Mater. 2019, 31, 1807916. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.; Pathak, A.; Tripathi, A.; Qiao, T.; Lee, Y.; Lee, H.; Woo, H.Y. Recent progress in organic thermoelectric materials and devices. Macromol. Res. 2020, 28, 531–552. [Google Scholar] [CrossRef]
- Liu, W.D.; Yu, Y.; Dargusch, M.; Liu, Q.F.; Chen, Z.G. Carbon allotrope hybrids advance thermoelectric development and applications. Renew. Sustain. Energy Rev. 2021, 141, 110800. [Google Scholar] [CrossRef]
- Liu, S.Q.; Li, H.; Li, P.C.; Liu, Y.L.; He, C.B. Recent advances in polyaniline based thermoelectric composites. CCS Chem. 2021, 3, 2547–2560. [Google Scholar] [CrossRef]
- Bharti, M.; Singh, A.; Samanta, S.; Aswal, D.K. Conductive polymers for thermoelectric power generation. Prog. Mater. Sci. 2018, 93, 270. [Google Scholar] [CrossRef]
- Bubnova, O.; Crispin, X. Towards polymer-based organic thermoelectric generators. Energy Environ. Sci. 2012, 5, 9345–9362. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Heum Park, S.; Heeger, A.J.; Lee, C.W.; Lee, S.H. Metallic transport in polyaniline. Nature 2006, 441, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Stafström, S.; Brédas, J.L.; Epstein, A.J.; Woo, H.S.; Tanner, D.B.; Huang, W.S.; MacDiarmid, A.G. Polaron lattice in highly conducting polyaniline: Theoretical and optical studies. Phys. Rev. Lett. 1987, 59, 1464. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Treacy, G.M.; Smith, P.; Heeger, A.J. Optical-quality transparent conductive polyaniline films. Synth. Metal. 1993, 57, 3526–3531. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. “Synthetic Metals”: A Novel Role for Organic Polymers (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Park, Y.W.; Lee, Y.S.; Park, C.; Shacklette, L.W.; Baughman, R.H. Thermopower and conductivity of metallic polyaniline. Solid. State Commun. 1987, 63, 1063. [Google Scholar] [CrossRef]
- Nath, C.; Kumar, A.; Kuo, Y.K.; Okram, G.S. High thermoelectric figure of merit in nanocrystalline polyaniline at low temperatures. Appl. Phys. Lett. 2014, 105, 133108. [Google Scholar] [CrossRef]
- Wu, J.S.; Sun, Y.M.; Xu, W.; Zhang, Q.C. Investigating thermoelectric properties of doped polyaniline nanowires. Synth. Met. 2014, 189, 177–182. [Google Scholar] [CrossRef]
- Li, M.H.; Lin, X. Adapted Su-Schrieffer-Heeger Hamiltonian for polypyrrole. Phys. Rev. B. 2010, 82, 155141. [Google Scholar] [CrossRef]
- Yavas, Z.E.; Cevher, D.; Silis, H.T.; Cirpan, A.; Gulseren, O.; Franchini, C. Experimental and Ab-Initio Investigation of the Electrical Conductivity of Emeraldine Salt. J. Phys. Chem. C 2023, 127, 6813–6824. [Google Scholar] [CrossRef]
- Casanovas, J.; Canales, M.; Ferreira, C.A.; Alemán, C.A. First Principle Analysis of the Structure of Oligoanilines Doped with Alkylsulfonic Acids. J. Phys. Chem. A. 2009, 113, 8795–8800. [Google Scholar] [CrossRef] [PubMed]
- Scotland, K.M.; Strong, O.K.L.; Parnis, J.M.; Vreugdenhil, A.J. DFT modeling of polyaniline: A computational investigation into the structure and band gap of polyaniline. Can. J. Chem. 2021, 100, 162–167. [Google Scholar] [CrossRef]
- Reis, A.S.; Sanches, E.A.; Frota, H.O. Energy band structure and electronic transport properties of chlorine-doped polyaniline from ab initio calculations. Synth. Met. 2017, 231, 89–94. [Google Scholar] [CrossRef]
- Varela-Alvarez, A.; Sordo, J.A.; Scuseria, G.E. Doping of polyaniline by acid-base chemistry: Density functional calculations with periodic boundary conditions. J. Am. Chem. Soc. 2005, 127, 11318–11327. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.C.; MacDiarmid, A.G. “Polyaniline”: Protonic acid doping of the emeraldine form to the metallic regime. Synth. Met. 1986, 13, 193–205. [Google Scholar] [CrossRef]
- Adams, P.N.; Laughlin, P.J.; Monkman, A.P.; Kenwright, A.M. Low temperature synthesis of high molecular weight polyani line. Polymer 1996, 37, 3411–3417. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, Q.; Wang, L.; Wang, Y.; Sun, J.; Zeng, H.; Jin, Z.; Huang, X.; Chen, L. The synergic regulation of conductivity and seebeck coefficient in pure polyaniline by chemically changing the ordered degree of molecular chains. J. Mater. Chem. A 2014, 2, 2634–2640. [Google Scholar] [CrossRef]
- Wang, H.; Yi, S.I.; Pu, X.; Yu, C.H. Simultaneously improving electrical conductivity and thermopower of polyaniline composites by utilizing carbon nanotubes as high mobility conduits. ACS Appl. Mater. Interfaces 2015, 7, 9589–9597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, Y.M.; Xu, W.; Zhu, D.B. Organic thermoelectric materials: Emerging green energy materials converting heat to electricity directly and efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef] [PubMed]
- Ao, D.W.; Liu, W.D.; Chen, Y.X.; Wei, M.; Jabar, B.; Li, F.; Shi, X.L.; Zheng, Z.H.; Liang, G.X.; Zhang, X.H. Novel thermal diffusion temperature engineering leading to high thermoelectric performance in Bi2Te3-based flexible thin-films. Adv. Sci. 2022, 9, 2103547. [Google Scholar] [CrossRef] [PubMed]
- Zong, P.A.; Liang, J.; Zhang, P.; Wan, C.L.; Wang, Y.F.; Koumoto, K. Graphene based thermoelectrics. ACS. Appl. Energy Mater. 2020, 3, 2224–2239. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, Y.C.; Zhan, B.; Lin, Y.H.; Lan, J.; Yang, X.P. Enhanced thermoelectric properties of BiCuSeO/Polyaniline composites. J. Electron. Mater. 2014, 43, 3695–3700. [Google Scholar] [CrossRef]
- Prabu, A.V.; Vijayaraghavan, G.V.; Suriakarthick, R.; Priscilla, J.; Shyju, T.S.; Mani, J. Electrical, electrochemical and thermoelectric properties of PANI/AgBiSe2 multi-functional polymeric composite material for energy storage and conversion applications. J. Alloys Compd. 2025, 1021, 179534. [Google Scholar] [CrossRef]
- Ugraskan, V.; Karaman, F. Polyaniline/Graphitic Carbon nitride nnanocomposites with improved ihermoelectric properties. J. Electron. Mater. 2021, 50, 3455–3461. [Google Scholar] [CrossRef]
- Singh, M.; Gautam, A.K.; Faraz, M.; Khare, N. Thermoelectric performance of (±) Camphor-10-sulfonic acid doped polyaniline/graphitic carbon nitride composite films. Eur. Phys. J. Plus 2022, 137, 1251. [Google Scholar] [CrossRef]
- Wang, L.M.; Yao, Q.; Xiao, J.X.; Zeng, K.Y.; Shi, W.; Qu, S.Y.; Chen, L.D. Enhanced Thermoelectric properties of polyaniline panofilms induced by self assembled supramolecules. Chem-A Sian. J. 2016, 11, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wang, Q.; Wang, L.M.; Chen, L.D. Abnormally enhanced thermoelectric transport properties of SWNT/PANI hybrid films by the strengthened PANI molecular ordering. Energy Environ. Sci. 2014, 7, 3801–3807. [Google Scholar] [CrossRef]
- Cho, C.; Stevens, B.; Hsu, J.H.; Bureau, R.; Hagen, D.A.; Regev, O.; Yu, C.; Grunlan, J.C. Completely organic multilayer thin film with thermoelectric power factor rivaling inorganic Tellurides. Adv. Mater. 2015, 27, 2996–3001. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Wallace, K.L.; Tzeng, P.; Hsu, J.H.; Yu, C.; Grunlan, J.C. Outstanding Low Temperature Thermoelectric power factor from completely organic thin films enabled by multidimensional Conjugated Nanomaterials. Adv. Energy Mater. 2016, 6, 1502168. [Google Scholar] [CrossRef]
- Cho, C.; Qin, S.; Choi, K.; Grunlan, J.C. Improved thermoelectric power pactor in completely organic nanocomposite enabled by l-ascorbic acid. ACS Appl. Polym. Mater. 2019, 1, 1942–1947. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, D.H.; Lee, K.; Lee, C.W. High performance polyaniline prepared via polymerization in a self-Stabilized dispersion. Adv. Funct. Mater. 2005, 15, 1495–1500. [Google Scholar] [CrossRef]
- Ji, D.Z.; Li, B.S.; Raj, B.T.; Li, X.; Zhang, D.W.; Rezeq, M.; Cantwell, W.; Zheng, L.X. In situ surface polymerization of PANI/SWCNT bilayer film: Effective composite for improving seebeck coefficient and power factor. Adv. Mater. Interfaces 2024, 12, 2400566. [Google Scholar] [CrossRef]
- Huang, J.; Liu, X.H.; Du, Y. Highly efficient and wearable thermoelectric composites based on carbon nanotube film/polyaniline. J. Mater. 2024, 10, 173–178. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.N.; Xiong, Z.M.; Jiang, C.; Zhang, Y.F.; Fu, P.; Du, F.P. Electrochemical polymerization of polyaniline/single walled carbon nanotube bilayer films with enhanced thermoelectric properties. Prog. Org. Coat. 2024, 194, 108612. [Google Scholar] [CrossRef]
- Park, D.; Kim, M.; Kim, J. Solution-mixed PANI-coated Bi2Si2Te6 nanosheet-based composite film for flexible thermoelectric energy harvesting. Appl. Surface Sci. 2023, 649, 159138. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, S.; Petkov, P.S.; Zhang, P.; Qi, H.Y.; Nguyen, N.N.; Zhang, W.J.; Yoon, J.; Li, P.N.; Brumme, T.; et al. Two-dimensional polyaniline crystal with metallic out-of-plane conductivity. Nature 2025, 638, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Wu, S.Q.; Yin, Q.J.; Jiang, B.; Mo, S.T. Tuning thermoelectric performance of Poly(3,4-ethylenedioxythiophene): Poly (styrene sulfonate)/Polyaniline composite films by nanostructure evolution of polyaniline. Polym. Test. 2021, 94, 107017. [Google Scholar] [CrossRef]
- Choi, I.Y.; Lee, J.; Ahn, H.; Lee, J.; Choi, H.C.; Park, M.J. High conductivity two dimensional polyaniline nanosheets developed on ice surfaces. Angew. Chem. Int. Ed. 2015, 54, 10377–10680. [Google Scholar] [CrossRef]
- Horta-Romarís, L.; González-Rodríguez, M.V.; Lasagabáster, A.; Rivadulla, F.; Abad, M.J. Thermoelectric properties and intrinsic conduction processes in DBSA and NaSIPA doped polyanilines. Synth. Metal. 2018, 243, 44–50. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Liu, Y.; Liu, S.; Li, P.; He, C.B. Engineering doping level for enhanced thermoelectric performance of carbon nanotubes/polyaniline composites. Compos. Sci. Technol. 2021, 210, 108797. [Google Scholar] [CrossRef]
- Noby, H.; El-Shazly, A.H.; Elkady, M.F.; Ohshima, M. Strong acid doping for the preparation of conductive polyaniline nanoflowers, nanotubes, and nanofibers. Polymer 2019, 182, 121848. [Google Scholar] [CrossRef]
- Xiao, R.F.; Zhou, X.Y.; Zhang, C.; Liu, X.; Han, S.B.; Che, C.Y. Organic thermoelectric materials for wearable electronic devices. Sensors 2024, 24, 4600. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.Y.; Xu, Q.F.; Chu, C.W.; Yang, Y.; Li, G.; Shinar, J. On the mechanism of conductivity enhancement in poly (3,4-ethylenedioxythiophene): Poly(styrene sulfonate) film through solvent treatment. Polymer 2004, 45, 8443–8450. [Google Scholar] [CrossRef]
- Fan, Z.; Du, D.H.; Yu, Z.M.; Li, P.C.; Xia, Y.J.; Ouyang, J.Y. Significant enhancement in the thermoelectric properties of PEDOT:PSS films through a treatment with organic solutions of inorganic salts. ACS Appl. Mater. Interfaces 2016, 8, 23204–23211. [Google Scholar] [CrossRef] [PubMed]
- Ozlek, M.; Akbulut, M.S.; Burgaz, E. Exploring graphene’s impact on graphite/PANI matrix composites: High-pressure fabrication and enhanced thermal-electrical properties. Nanoscale 2025, 17, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.Y. Recent advances of intrinsically conductive polymers. Acta Phys.-Chim. Sin. 2018, 34, 1211–1220. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.L.; Li, P.C.; Liu, S.Q.; Du, F.P.; He, C.B. Enhanced thermoelectric performance of carbon nanotubes/polyaniline composites by multiple interface engineering. ACS Appl. Mater. Interfaces 2021, 13, 6650–6658. [Google Scholar] [CrossRef] [PubMed]
- Erden, F.; Li, H.; Wang, X.Z.; Wang, F.K.; He, C.B. High performance thermoelectric materials based on ternary TiO2/CNT/PANI composites. Phys. Chem. Chem. Phys. 2018, 20, 9411–9418. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Smith, P.; Heeger, A.J. Counter-ion induced processibility of conducting polyaniline and of conducting polyblends of polyaniline in bulk polymers. Synth. Met. 1992, 48, 91. [Google Scholar] [CrossRef]
- Kroon, R.; Mengistie, D.A.; Kiefer, D.; Hynynen, J.; Ryan, J.D.; Yu, L.Y.; Müller, C. Thermoelectric plastics: From design to synthesis, processing and structure property relationships. Chem. Soc. Rev. 2016, 45, 6147–6164. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.R.; Kim, B.J.; Lee, K. Highly conductive PEDOT:PSS nanofibrils induced by solution processed crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Garreau, S.; Louarn, G.; Buisson, J.P.; Froyer, G.; Lefrant, S. In situ spectroelectrochemical raman studies of poly(3,4-ethylenedioxythiophene) (PEDT). Macromolecules 1999, 32, 6807–6812. [Google Scholar] [CrossRef]
- Wei, S.S.; Liu, L.; Huang, X.; Zhang, Y.C.; Liu, F.S.; Deng, L.; Bilotti, E.; Chen, G.M. Flexible and foldable films of SWCNT thermoelectric composites and an S-shape thermoelectric generator with a vertical temperature gradient. ACS Appl. Mater. Interfaces 2022, 14, 5973–5982. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Gu, J.T.; Hao, Y.N.; Zheng, M.R.; Wang, L.M.; Yu, J.Y.; Qin, X.H. Continuous manufacture of stretchable and integratable thermoelectric nanofiber yarn for human body energy harvesting and self-powered motion detection. Chem. Eng. J. 2022, 450, 137937. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.L.; Liu, S.Q.; Li, P.C.; Zhang, C.; He, C.B. Wet spun flexible carbon nanotubes/polyaniline fibers for wearable thermoelectric energy harvesting. Compos. Part A 2023, 166, 107386. [Google Scholar] [CrossRef]
- Wang, H.; Yi, S.I.; Yu, C.H. Engineering electrical transport at the interface of conjugated carbon structures to improve thermoelectric properties of their composites. Polymer 2016, 97, 487–495. [Google Scholar] [CrossRef]
- Song, H.; Cai, K. Preparation and properties of PEDOT:PSS/Te nanorod composite films for flexible thermoelectric power generator. Energy 2017, 125, 519–525. [Google Scholar] [CrossRef]
- Wan, C.; Tian, R.; Azizi, A.B.; Huang, Y.; Wei, Q.; Sasai, R.; Wasusate, S.; Ishida, T.; Koumoto, K. Flexible thermoelectric foil for wearable energy harvesting. Nano Energy 2016, 30, 840–845. [Google Scholar] [CrossRef]
- Ko, D.K.; Kang, Y.; Murray, C.B. Enhanced thermopower via carrier energy filtering in ssolution processable Pt-Sb2Te3 nanocomposites. Nano. Lett. 2011, 11, 2841–2844. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.J.; Liu, J.; Qiu, X.K.; Chiechi, R.; Koster, L.J.A.; Portale, G. Engineering the thermoelectrical properties of PEDOT:PSS by alkali metal ion effect. ACS Appl. Mater. Interfaces 2021, 7, 647–654. [Google Scholar] [CrossRef]
- Ichikawa, S.; Toshima, N. Improvement of thermoelectric properties of composite films of PEDOT-PSS with xylitol by means of stretching and solvent treatment. Polym. J. 2015, 47, 522–526. [Google Scholar] [CrossRef]
- Wen, N.X.; Zuo, X.Q.; Zhou, J.J.; Sun, C.; Chen, C.; Jiang, D.Y.; Xu, H.; Wang, W.; Pan, L.J.; Fan, Z. Boosting thermoelectric performance of wet-spun PEDOT:PSS-based organic/inorganic composite fibers via a dual-Interfacial engineering approach. Small 2025, 21, 2500866. [Google Scholar] [CrossRef] [PubMed]
- Manoj, S.; Amish, K.G.; Mohd, F.; Neeraj, K. Polyaniline/graphitic carbon nitride/reduced graphene oxide ternary nanocomposite film for flexible thermoelectric application. Nanotechnology 2024, 35, 495403. [Google Scholar] [CrossRef]
- Wang, L.M.; Yao, Q.; Shi, W.; Qu, S.Y.; Chen, L.D. Engineering carrier scattering at the interfaces in polyaniline based nanocomposites for high thermoelectric performances. Mater. Chem. Front. 2017, 1, 741–748. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.Q.; Li, P.C.; Yuan, D.; Zhou, X.; Sun, J.T.; Lu, X.H.; He, C.B. Interfacial control and carrier tuning of carbon nanotube/polyaniline composites for high thermoelectric performance. Carbon 2018, 136, 292–298. [Google Scholar] [CrossRef]

| Material | σ (S cm−1) | |S| μV K−1 | PF μW m−1 K−2 | Synthesis Methods | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| PANI | ~1000 | — | — | IP | OMC | [24] |
| PANI/BiCuSeO | 9.25 | 5~87 | 7 | MM | OIE | [43] |
| PANI/AgBiSe2 | — | 740 | — | SMM | OIE | [44] |
| PANI/g-C3N4 | ~5.8 | ~350 | 70.75 | SMM | OIE | [45] |
| PANI/g-C3N4 | ~12 | ~48 | ~2.8 | SMM | OIE | [46] |
| PANI/SAS | ~400 | ~28 | 31 | SMM | OMC | [47] |
| PANI/SWCNT | 769 | 65 | 176 | SMM | OIE | [48] |
| PANI/Graphene/DWNT | 1080 | 130 | 1825 | LbL | OIE | [49] |
| PANI/Graphene-PEDOT:PSS/PANI/DWNT-PEDOT:PSS | 1885 | 120 | 2710 | LbL | OIE | [50] |
| PANI/DWCNT/GO | 960 | 115 | 1260 | LbL | OIE | [51] |
| PANI (CSA doping) | 800 | — | — | IP | OMC | [52] |
| PANI/SWCNT | 1320 | ~26 | 90 | LbL | OIE | [53] |
| PANI/SWCNT | 2238 | 42.7 | 407 | ISP | MBL | [54] |
| PANI/SWCNT | 654.9 | 48.7 | 155.3 | ISP | OIE | [55] |
| PANI/TiO2/CNT | 2183 | 22.9 | 114.5 | ISP | OIE | [56] |
| 2DPANI | ~16 | — | — | IP | OMC | [57] |
| PANI/PEDOT:PSS | 2600 | 12.12 | 40.83 | ISP | MDL | [58] |
| PANI | 35 | — | — | IP | OMC | [59] |
| PANI/DBSA | ~27 | ~10.2 | 0.28 | ISP | MDL | [60] |
| PANI/SWCNT | 1856 | 30.8 | 176 | SMM | OMC | [61] |
| PANI/HCl | 3.7 | — | — | ISP | MDL | [62] |
| PANI/g-C3N4/rGO | 110.3 | 97.1 | 118.3 | SMM | OIE | [63] |
| EDOT:PSS/SWCNT@PANI | 2472 | 43.5 | 467.8 | ISP | OIE | [64] |
| PANI/CNT | ~2012 | ~37 | 273 | ISP | OIE | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Xie, D.; Zhou, H.; Zong, P. PANI-Based Thermoelectric Materials. Organics 2025, 6, 33. https://doi.org/10.3390/org6030033
Chen M, Xie D, Zhou H, Zong P. PANI-Based Thermoelectric Materials. Organics. 2025; 6(3):33. https://doi.org/10.3390/org6030033
Chicago/Turabian StyleChen, Mengran, Dongmei Xie, Hongqing Zhou, and Pengan Zong. 2025. "PANI-Based Thermoelectric Materials" Organics 6, no. 3: 33. https://doi.org/10.3390/org6030033
APA StyleChen, M., Xie, D., Zhou, H., & Zong, P. (2025). PANI-Based Thermoelectric Materials. Organics, 6(3), 33. https://doi.org/10.3390/org6030033








