Engineering Nascent Disentangled Ultra-High-Molecular-Weight Polyethylene Based on Heterogeneous Catalytic Polymerization
Abstract
1. Introduction
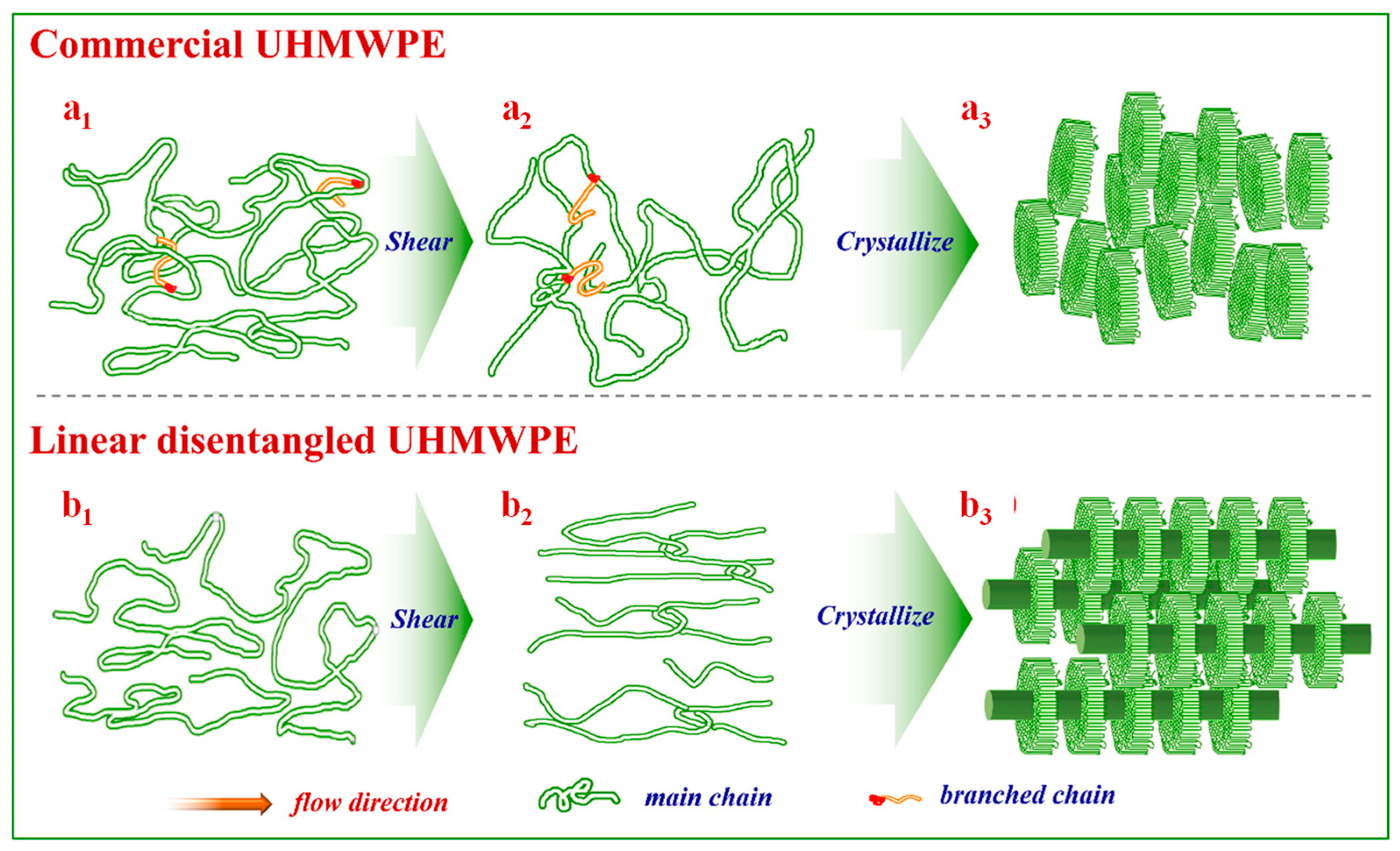
2. Entanglement Characterization of UHMWPE
2.1. Solid NMR Method
2.2. Melt Rheological Method
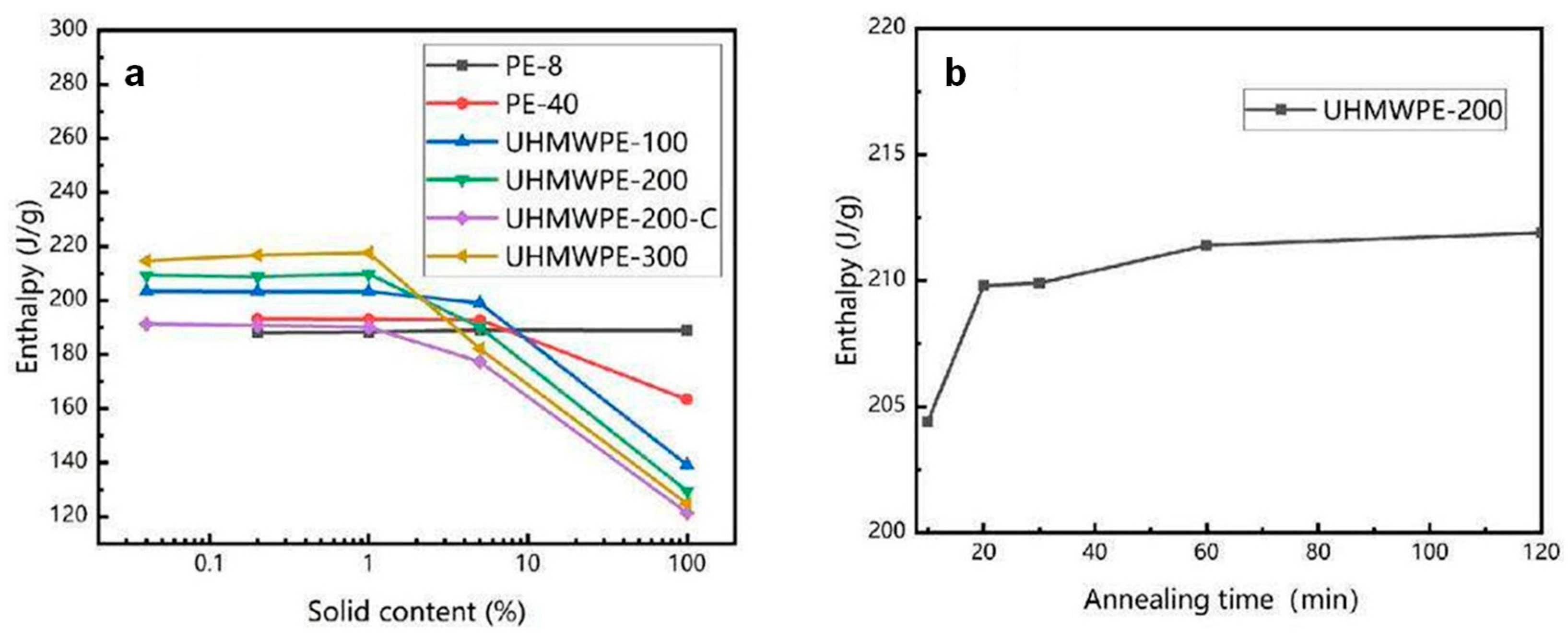
2.3. Thermodynamic Annealing Method
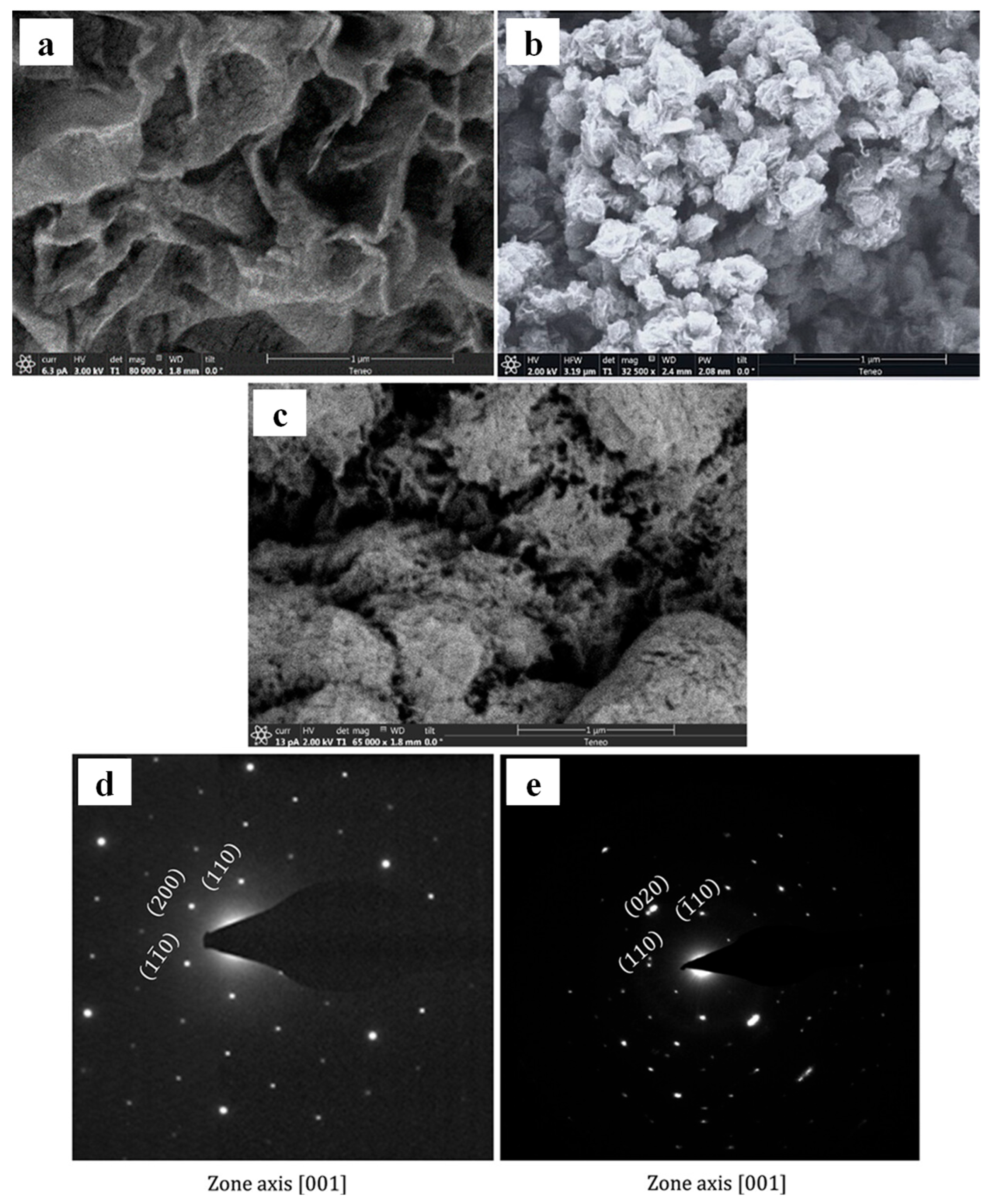
2.4. X-Ray Method
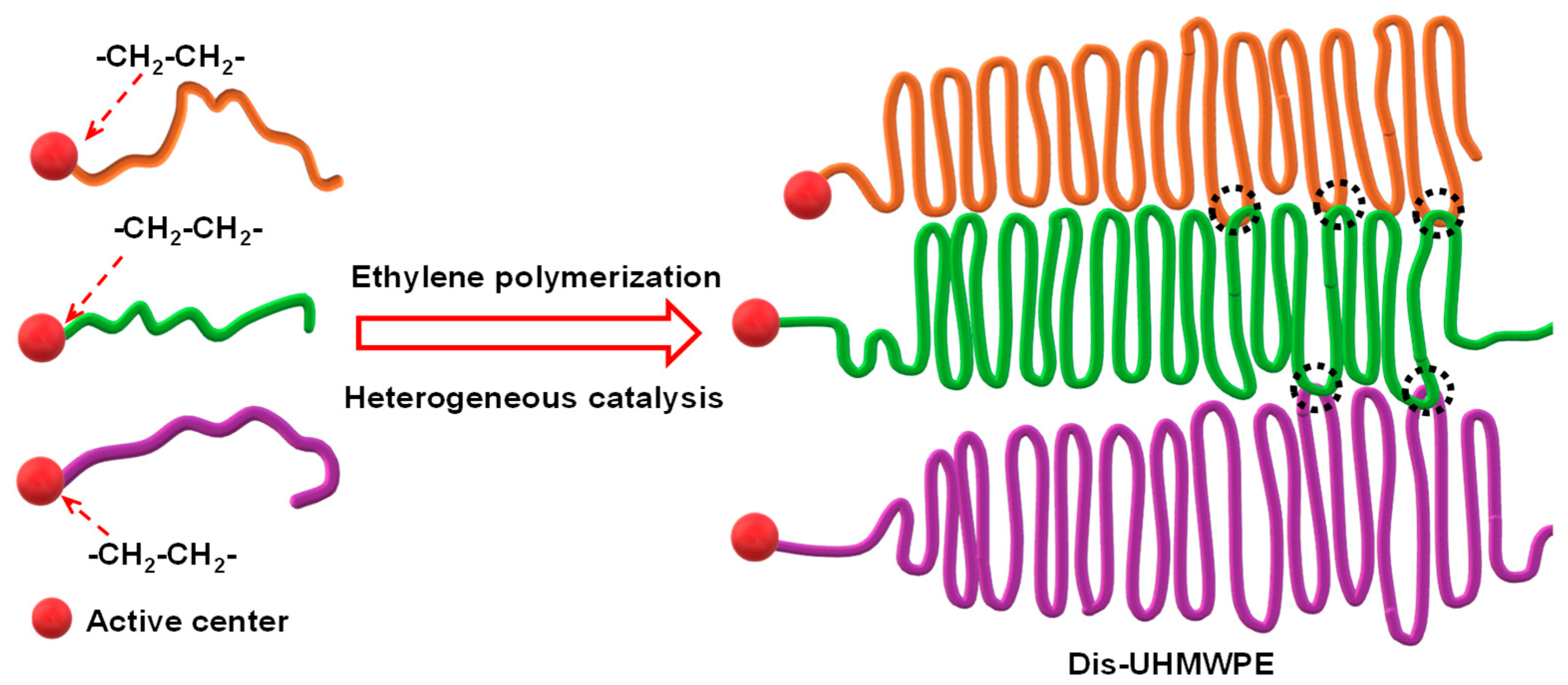
3. Synthesis of Dis-UHMWPE via Heterogeneous Catalytic Polymerization
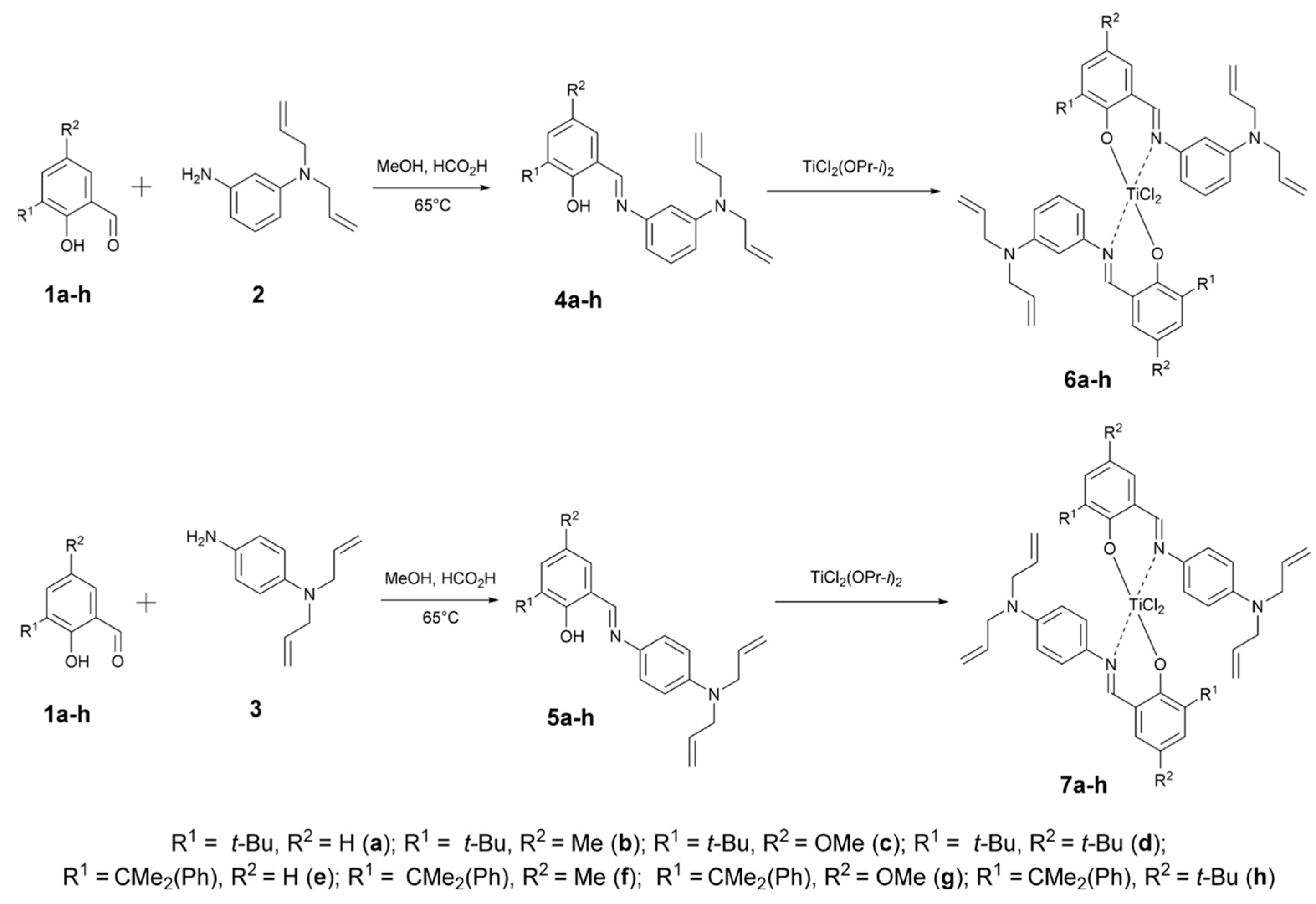
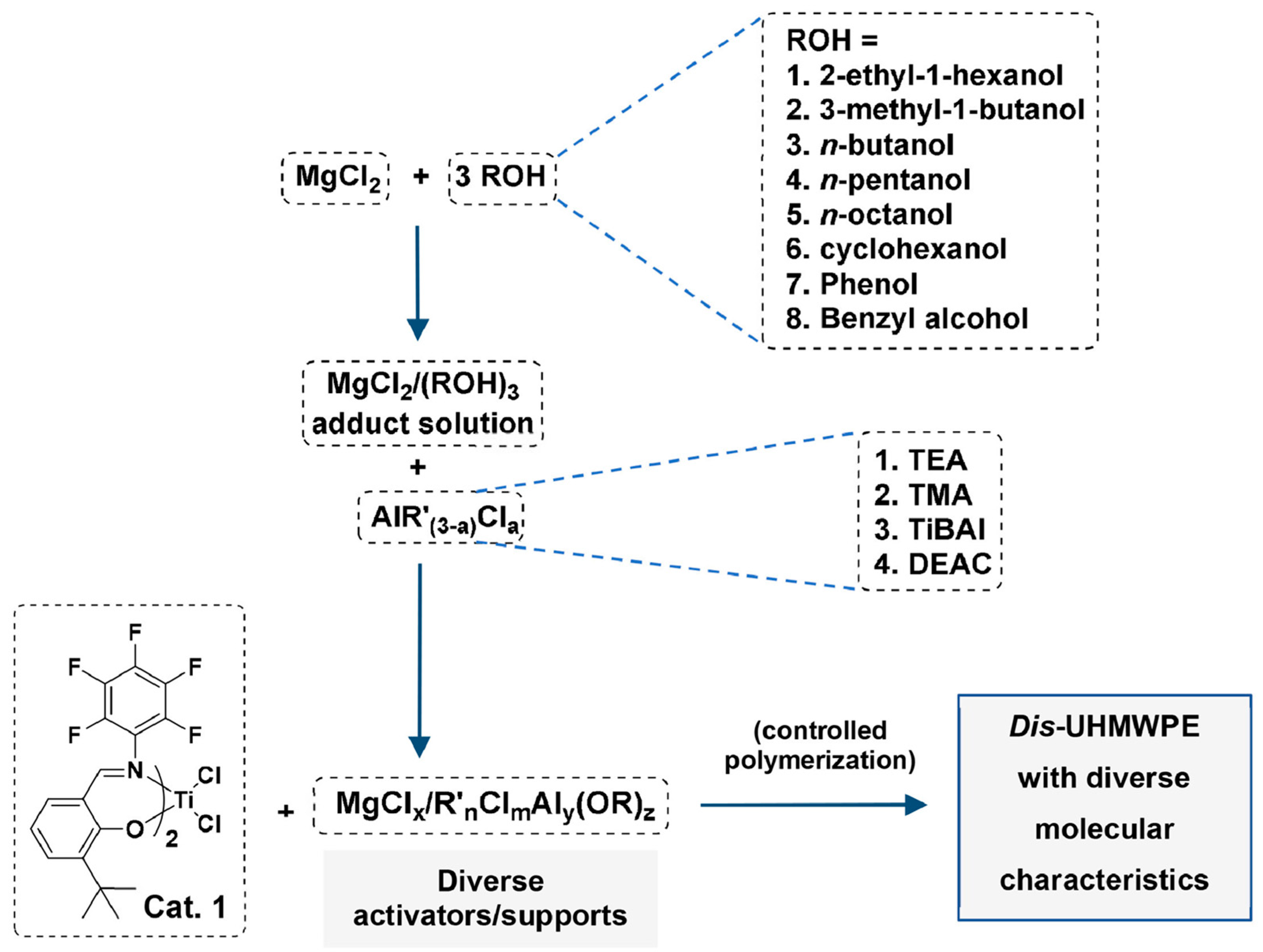
3.1. FI Catalyst-Based Heterogeneous Polymerization
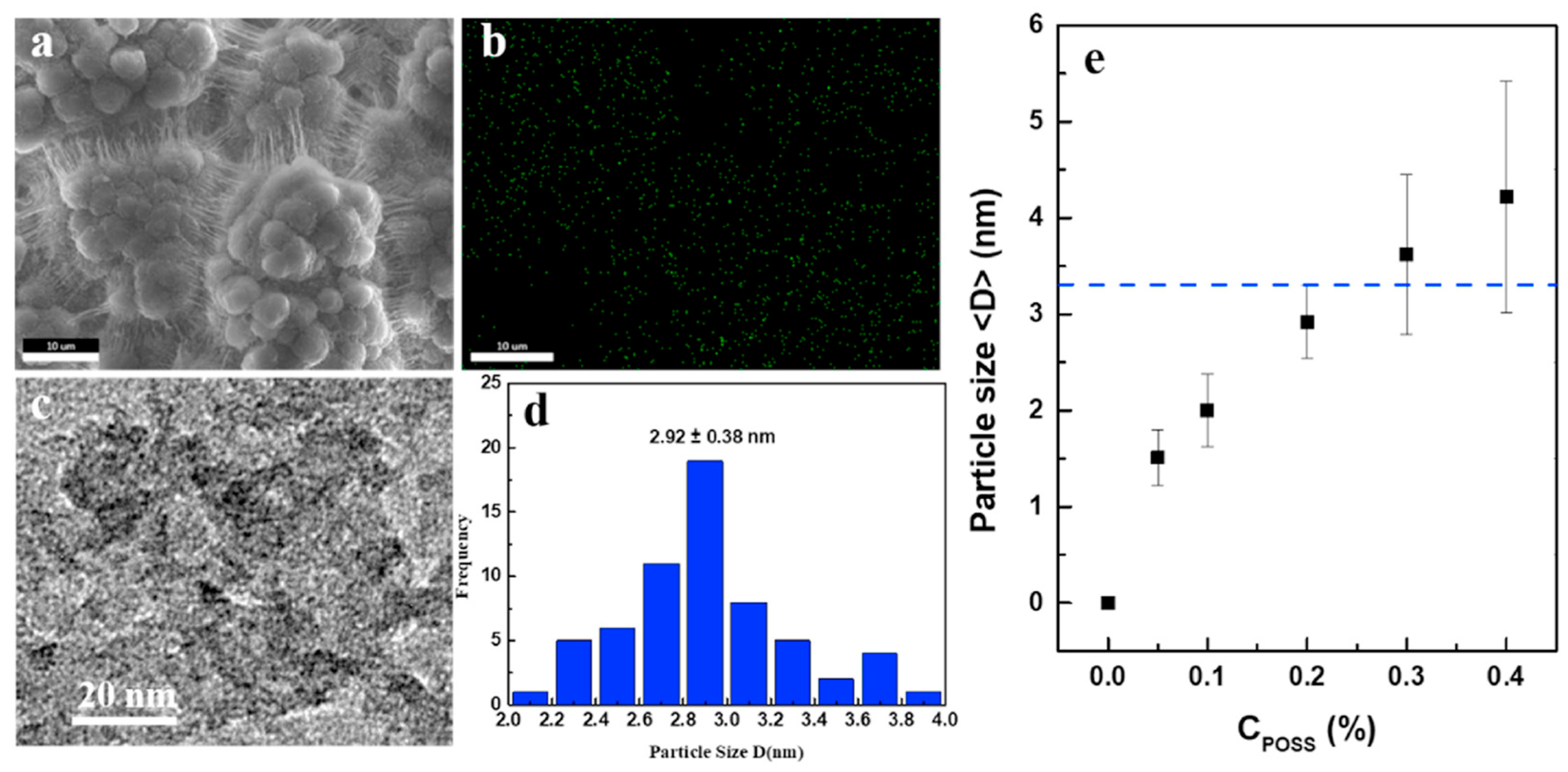
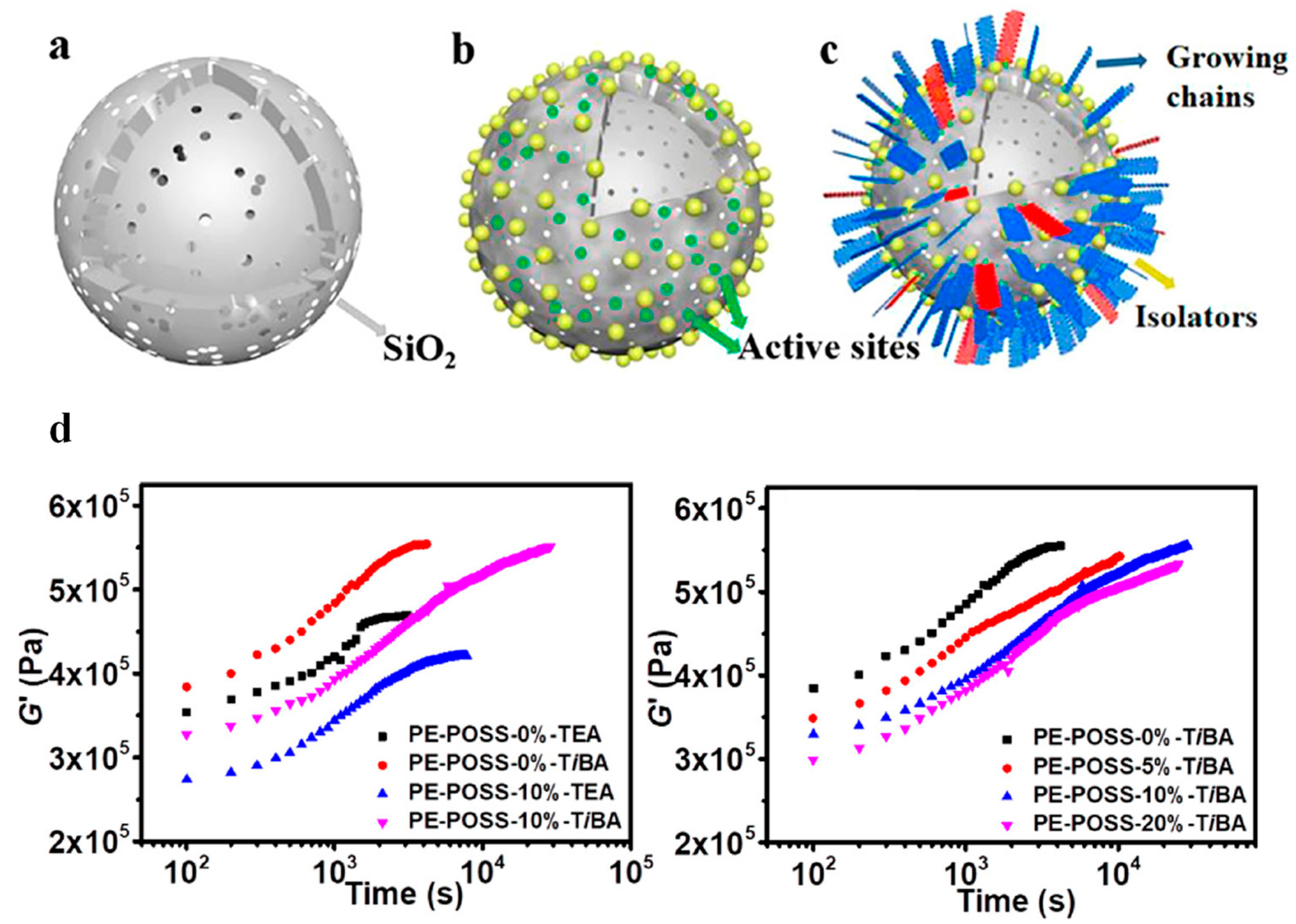
3.2. POSS-Modified Z-N Catalyst-Based Heterogeneous Polymerization
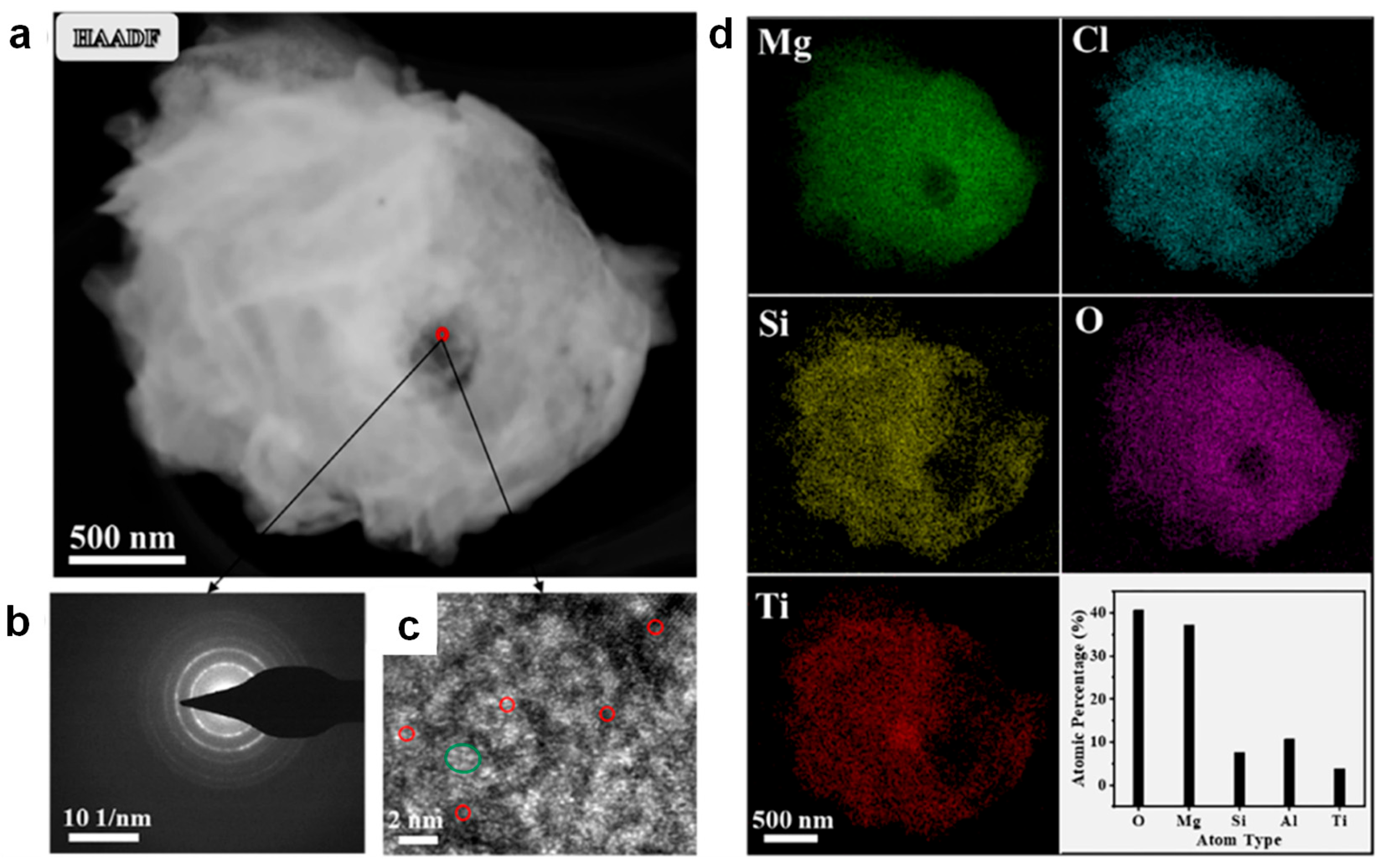
3.3. Other Novel Catalyst-Based Heterogeneous Polymerization
3.4. Optimization of Polymerization Conditions
4. Conclusions
Funding
Conflicts of Interest
References
- Drakopoulos, S.X.; Tarallo, O.; Guan, L.; Martin-Fabiani, I.; Ronca, S. Nanocomposites of Au/Disentangled UHMWPE: A Combined Optical and Structural Study. Molecules 2020, 25, 3225. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Drakopoulos, S.X.; Ronca, S.; Minnich, A.J. Origin of high thermal conductivity in disentangled ultra-high molecular weight polyethylene films: Ballistic phonons within enlarged crystals. Nat. Commun. 2022, 13, 2452. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, W.; Pang, W.; Liao, D.; Zou, C. Preparation of Toughened Bimodal Ultrahigh-Molecular-Weight Polyethylene by a Coanchoring Strategy. ACS Appl. Polym. Mater. 2024, 6, 13210–13216. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Xin, Z. The chain dis-entanglement effect of polyhedral oligomeric silsesquioxanes (POSS) on ultra-high molecular weight polyethylene (UHMWPE). Polymer 2020, 202, 122631. [Google Scholar] [CrossRef]
- Forster, A.L.; Forster, A.M.; Chin, J.W.; Peng, J.-S.; Lin, C.-C.; Petit, S.; Kang, K.-L.; Paulter, N.; Riley, M.A.; Rice, K.D.; et al. Long-term stability of UHMWPE fibers. Polym. Degrad. Stab. 2015, 114, 45–51. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Gu, T.; Zhang, F.; Niu, Y.; Liu, S. Effect of Hot-Pressing Process on Mechanical Properties of UHMWPE Fiber Non-Woven Fabrics. Materials 2024, 17, 2611. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-High-Molecular-Weight-Polyethylene (UHMWPE) as a Promising Polymer Material for Biomedical Applications: A Concise Review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Bashir, M.; Firdous, S.; Yasin, T.; Tariq, M.; Ikram, M.; Mehmood, M.S. Optical properties of ultra-high molecular weight polyethylene (UHMWPE): A material of choice for total joint applications. Radiat. Phys. Chem. 2016, 118, 102–106. [Google Scholar] [CrossRef]
- Tang, X.; Xing, J.; Yan, X.; Ye, C.; Zhang, L.; Zhang, Y.; Shu, B.; Mu, J.; Li, W.; Wang, J.; et al. Metallocene Polyolefins Reinforced by Low-Entanglement UHMWPE through Interfacial Entanglements. Adv. Polym. Technol. 2022, 2022, 9344096. [Google Scholar] [CrossRef]
- Tao, G.; Chen, Y.; Mu, J.; Zhang, L.; Ye, C.; Li, W. Exploring the entangled state and molecular weight of UHMWPE on the microstructure and mechanical properties of HDPE/UHMWPE blends. J. Appl. Polym. Sci. 2021, 138, 50741. [Google Scholar] [CrossRef]
- Li, L.; Kong, F.; Xiao, A.; Su, H.; Wu, X.; Zhang, Z.; Duan, Y. Current research status of high-performance UHMWPE fiber: A review. Mater. Technol. Rep. 2024, 2, 1518. [Google Scholar] [CrossRef]
- Mohammed, A.S.; Fareed, M.I. Improving the friction and wear of poly-ether-etherketone (PEEK) by using thin nano-composite coatings. Wear 2016, 364–365, 154–162. [Google Scholar] [CrossRef]
- Panin, S.V.; Kornienko, L.A.; Alexenko, V.O.; Buslovich, D.G.; Bochkareva, S.A.; Lyukshin, B.A. Increasing Wear Resistance of UHMWPE by Loading Enforcing Carbon Fibers: Effect of Irreversible and Elastic Deformation, Friction Heating, and Filler Size. Materials 2020, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Minn, M.; Sinha, S.K. DLC and UHMWPE as hard/soft composite film on Si for improved tribological performance. Surf. Coat. Technol. 2008, 202, 3698–3708. [Google Scholar] [CrossRef]
- Li, H.; Zong, S.; Xiong, X. The influence of penetration angle on anti-penetration performance and reverse penetration ricochet phenomenon of UHMWPE laminates. Int. J. Impact Eng. 2023, 182, 104780. [Google Scholar] [CrossRef]
- Wang, H.; Weerasinghe, D.; Hazell, P.J.; Mohotti, D.; Morozov, E.V.; Escobedo-Diaz, J.P. Ballistic impact response of flexible and rigid UHMWPE textile composites: Experiments and simulations. Def. Technol. 2023, 22, 37–53. [Google Scholar] [CrossRef]
- Zhang, R.; Song, X.-T.; Qiang, L.-S.; Xu, X.; Zheng, B.-Q.; Deng, J.; Zhou, Y.; Wang, X.; Ni, C.-Y. Ballistic performance of UHMWPE fiber laminates with pre-formed holes. Thin-Walled Struct. 2024, 201, 112011. [Google Scholar] [CrossRef]
- Gao, J.; Chen, L.; Zhong, Y.; Xing, C.; Li, Y.; Wang, Z. Structural evolution of low-entangled UHMWPE films with reserved shish crystals and different molecular weights during hot stretching. Polymer 2024, 312, 127657. [Google Scholar] [CrossRef]
- Jauffrès, D.; Lame, O.; Vigier, G.; Doré, F. Microstructural origin of physical and mechanical properties of ultra high molecular weight polyethylene processed by high velocity compaction. Polymer 2007, 48, 6374–6383. [Google Scholar] [CrossRef]
- Xing, C.; Chen, L.; Gao, J.; Zhong, Y.; Li, Y.; Wang, Z. Structural evolution of low-entangled UHMWPE gel films with reserved shish crystals and different entanglement degrees during stretching. Polymer 2024, 312, 127592. [Google Scholar] [CrossRef]
- Kuo, C.J.; Lan, W.L. 5—Gel spinning of synthetic polymer fibres. In Advances in Filament Yarn Spinning of Textiles and Polymers; Zhang, D., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 100–112. [Google Scholar]
- Wang, X.; Zheng, H.; Sun, Y. Study on structures and properties of ultra-hot drawing UHMWPE fibers fabricated via dry spinning method. J. Polym. Eng. 2018, 38, 863–870. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Christakopoulos, F.; Xie, K.; Zhu, C.; Xu, J.; Müller, A.J. Structure Formation and Unexpected Ultrafast Re-entanglement Dynamics of Disentangled Ultrahigh Molecular Weight Polyethylene. Macromolecules 2024, 57, 10240–10252. [Google Scholar] [CrossRef]
- Khalil, Y.; Hopkinson, N.; Kowalski, A.; Fairclough, J.P. Characterisation of UHMWPE Polymer Powder for Laser Sintering. Materials 2019, 12, 13496. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-L.; Qiao, J.; Guan, J.; Chen, H.-M.; Wang, T.; Wang, C.; Wang, Y. Nascent disentangled UHMWPE: Origin, synthesis, processing, performances and applications. Eur. Polym. J. 2023, 184, 111799. [Google Scholar] [CrossRef]
- Yilmaz, G.; Uslu, E. A new approach for high-quality production of UHMWPE by applying powder vibration densification before sintering. Powder Technol. 2023, 427, 118741. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, X.; Jiang, Y.; Cai, Z.; Li, S.; Cui, D. Access to Disentangled Ultrahigh Molecular Weight Polyethylene via a Binuclear Synergic Effect. Angew. Chem. Int. Ed. Engl. 2023, 62, e202215582. [Google Scholar] [CrossRef] [PubMed]
- Li, L. Catalytic strategies for synthesizing disentangled ultrahigh molecular weight polyethylene via homogeneous FI catalyst-based polymerization. Mater. Technol. Rep. 2025, 3, 3164. [Google Scholar] [CrossRef]
- Liang, P.; Chen, Y.; Ren, C.; Chen, M.; Jiang, B.; Wang, J.; Yang, Y.; Li, W. Efficient Synthesis of Low-Polydispersity UHMWPE by Elevating Active Sites on Anchored POSS Molecules. Ind. Eng. Chem. Res. 2020, 59, 19964–19971. [Google Scholar] [CrossRef]
- Yue, Z.; Wang, N.; Cao, Y.; Li, W.; Dong, C.-d. Reduced Entanglement Density of Ultrahigh-Molecular-Weight Polyethylene Favored by the Isolated Immobilization on the MgCl2 (110) Plane. Ind. Eng. Chem. Res. 2020, 59, 3351–3358. [Google Scholar] [CrossRef]
- Drakopoulos, S.X.; Psarras, G.C.; Forte, G.; Martin-Fabiani, I.; Ronca, S. Entanglement dynamics in ultra-high molecular weight polyethylene as revealed by dielectric spectroscopy. Polymer 2018, 150, 35–43. [Google Scholar] [CrossRef]
- Li, W.; Yue, Z.; Lozovoi, A.; Petrov, O.; Mattea, C.; Stapf, S. Heterogeneous distribution of chain mobility in nascent UHMWPE in the less entangled state. J. Polym. Res. 2018, 25, 239. [Google Scholar] [CrossRef]
- Vittoria, A.; Meppelder, A.; Friederichs, N.; Busico, V.; Cipullo, R. Demystifying Ziegler–Natta Catalysts: The Origin of Stereoselectivity. ACS Catal. 2017, 7, 4509–4518. [Google Scholar] [CrossRef]
- Huss-Hansen, M.K.; Hedlund, E.G.; Davydok, A.; Hansteen, M.; Overdijk, J.; de Cremer, G.; Roeffaers, M.; Knaapila, M.; Balzano, L. Local structure mapping of gel-spun ultrahigh-molecular-weight polyethylene fibers. Polymer 2022, 239, 124420. [Google Scholar] [CrossRef]
- Ivan’kova, E.; Egorov, V.; Marikhin, V.; Myasnikova, L.; Boiko, Y.; Radovanova, E. Fundamental Structural and Kinetic Principals of High Strength UHMWPE Fibers Production by Gel-Technology. Polymers 2022, 14, 4771. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Severn, J.; Bastiaansen, C.W.M. Drawing behavior and mechanical properties of ultra-high molecular weight polyethylene blends with a linear polyethylene wax. Polymer 2018, 153, 354–361. [Google Scholar] [CrossRef]
- Yu, L.; Bao, J.; Wang, G.; Lu, W.; Chen, W. Structure and properties of gel-spun ultra-high molecular weight polyethylene fibers obtained from industrial production line. J. Appl. Polym. Sci. 2021, 138, 51317. [Google Scholar] [CrossRef]
- Bodkhe, D.V.; Chikkali, S.H. Ti-iminocarboxylate catalyzed polymerization of ethylene to highly crystalline, disentangled, ultrahigh molecular weight polyethylene. Eur. Polym. J. 2023, 182, 111725. [Google Scholar] [CrossRef]
- Forte, G.; Ronca, S. Synthesis of Disentangled Ultra-High Molecular Weight Polyethylene: Influence of Reaction Medium on Material Properties. Int. J. Polym. Sci. 2017, 2017, 7431419. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Gagieva, S.C.; Kurmaev, D.A.; Vasil’ev, V.G.; Kolosov, N.A.; Zubkevich, S.V.; Mikhaylik, E.S.; Golubev, E.K.; Nikiforova, G.G.; Zhizhko, P.A.; et al. Binuclear and Hexanuclear Ti(IV) Complexes Supported by [OOOO]4–-type Ligand for Preparing Disentangled UHMWPE. Chin. J. Polym. Sci. 2019, 37, 471–477. [Google Scholar] [CrossRef]
- Bally, F.; Serra, C.A.; Hessel, V.; Hadziioannou, G. Homogeneous Polymerization: Benefits Brought by Microprocess Technologies to the Synthesis and Production of Polymers. Macromol. React. Eng. 2010, 4, 543–561. [Google Scholar] [CrossRef]
- Bahri-Laleh, N.; Ghavampoor, A.H.; Karkhaneh, F. Chapter 2—Ethylene polymerization with homogeneous catalysts. In Homogeneous Polymerization and Oligomerization Reactions; Rahimpour, M.R., Makarem, M.A., Roostaei, T., Meshksar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; Volume 6, pp. 49–72. [Google Scholar]
- Chen, J.; Qu, S.; Li, X.; Wei, Y.; Li, Q.; Wen, Z.; Guo, Z. Single-Site Catalyst for the Synthesis of Disentangled Ultra-High-Molecular-Weight Polyethylene. Polymers 2025, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Seyed Dorraji, M.S.; Tabatabaei Rezaei, S.J.; Ramazani, A. Chapter 4—Homogeneous polymerization with metallocene catalysts. In Homogeneous Polymerization and Oligomerization Reactions; Rahimpour, M.R., Makarem, M.A., Roostaei, T., Meshksar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; Volume 6, pp. 89–110. [Google Scholar]
- Johannsmann, D.; Petri, J.; Leppin, C.; Langhoff, A.; Ibrahim, H. Particle fouling at hot reactor walls monitored In situ with a QCM-D and modeled with the frequency-domain lattice Boltzmann method. Results Phys. 2023, 45, 106219. [Google Scholar] [CrossRef]
- Bajya, M.; Majumdar, A.; Butola, B.S.; Jasra, R.V. Exploration of disentangled UHMWPE tape as a soft body armour material. Mater. Chem. Phys. 2023, 295, 127162. [Google Scholar] [CrossRef]
- Wencke, Y.L.; Luinstra, G.A.; Duchateau, R.; Proes, F.; Imgrund, P.; Evenson, J.S.; Emmelmann, C. Disentangled UHMWPE@silica powders for potential use in power bed fusion based additive manufacturing. Eur. Polym. J. 2022, 163, 110936. [Google Scholar] [CrossRef]
- Antonov, A.A.; Bryliakov, K.P. Post-metallocene catalysts for the synthesis of ultrahigh molecular weight polyethylene: Recent advances. Eur. Polym. J. 2021, 142, 110162. [Google Scholar] [CrossRef]
- Birajdar, R.S.; Dnyaneshwar, B.; Poonam, G.; Shaikha, M.H.; Rohan, R.; Chikkali, S.H. Emerging trends in olefin polymerization: A perspective. J. Macromol. Sci. Part A 2023, 60, 731–750. [Google Scholar] [CrossRef]
- Patel, K.; Chikkali, S.H.; Sivaram, S. Ultrahigh molecular weight polyethylene: Catalysis, structure, properties, processing and applications. Prog. Polym. Sci. 2020, 109, 101290. [Google Scholar] [CrossRef]
- Li, K.-T.; Wu, L.-H. Constrained Geometry Organotitanium Catalysts Supported on Nanosized Silica for Ethylene (co)Polymerization. Molecules 2017, 22, 751. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.K.; Dey, A.; Singh, A.; Tripathi, S.N.; Bonda, S.; Saha, S.; Iyer, P.K.; Srivastava, V.K.; Jasra, R.V. Disentangled ultrahigh molecular weight polyethylene thin film as a transparent substrate for flexible flat panel display. J. Appl. Polym. Sci. 2022, 139, e52932. [Google Scholar] [CrossRef]
- Dordinejad, A.K.; Sharif, F.; Ebrahimi, M.; Rashedi, R. Time-sweep rheometry for evaluating polyethylene degradation behavior: Effect of formulation and process conditions. Polym. Test. 2018, 70, 39–46. [Google Scholar] [CrossRef]
- Salehiyan, R.; Soleymani Eil Bakhtiari, S. A review on rheological approaches as a perfect tool to monitor thermal degradation of biodegradable polymers. Korea-Aust. Rheol. J. 2024, 36, 295–317. [Google Scholar] [CrossRef]
- Hawke, L.G.D.; Romano, D.; Rastogi, S. Nonequilibrium Melt State of Ultra-High-Molecular-Weight Polyethylene: A Theoretical Approach on the Equilibrium Process. Macromolecules 2019, 52, 8849–8866. [Google Scholar] [CrossRef]
- Gote, R.P.; Zhao, J.; Romano, D.; Rastogi, S. Solid-State Processing of In Situ Blended Prepolymer with Z–N Synthesized UHMWPE: Role of the Prepolymer. Macromolecules 2025, 58, 3604–3621. [Google Scholar] [CrossRef]
- Lai, S.-C.; Jin, J.; Luo, Z.-H. State-of-the-art heterogeneous polymerization kinetic modelling processes and their applications. React. Chem. Eng. 2025, 10, 942–952. [Google Scholar] [CrossRef]
- Liu, D.; He, J.; Zhang, L.; Tan, J. 100th Anniversary of Macromolecular Science Viewpoint: Heterogenous Reversible Deactivation Radical Polymerization at Room Temperature. Recent Advances and Future Opportunities. ACS Macro Lett. 2019, 8, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.C.; Martins, M.O.; Figueiras, A.O.; Martins, L.M.D.R.S. Introduction to Polymerization and Depolymerization. In Depolymerization: Concept, Progress, and Challenges Volume 1: Core Concepts and Fundamentals; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2025; Volume 1498, pp. 1–23. [Google Scholar]
- Shi, P.; Chen, G.; Chen, Q.; Wu, H.; Li, S.; Cao, X.; Yang, L.; Tian, Z. Heterogeneously catalyzed supramolecular polymerization: Essential roles of nucleation and fragmentation-induced autocatalysis in chiral transfer. Chem. Sci. 2025, 16, 5538–5546. [Google Scholar] [CrossRef] [PubMed]
- Romano, D.; Tops, N.; Andablo-Reyes, E.; Ronca, S.; Rastogi, S. Influence of Polymerization Conditions on Melting Kinetics of Low Entangled UHMWPE and Its Implications on Mechanical Properties. Macromolecules 2014, 47, 4750–4760. [Google Scholar] [CrossRef]
- Chen, T.; Yang, H.; Li, W. Phase structure and mechanical properties of disentangled ultra-high molecular weight polyethylene/polyhedral oligomeric silsesquioxane nanocomposites in a solid state. J. Polym. Res. 2015, 22, 223. [Google Scholar] [CrossRef]
- Li, W.; Chen, T.; Guan, C.; Gong, D.; Mu, J.; Chen, Z.-R.; Zhou, Q. Influence of Polyhedral Oligomeric Silsesquioxane Structure on the Disentangled State of Ultrahigh Molecular Weight Polyethylene Nanocomposites during Ethylene in Situ Polymerization. Ind. Eng. Chem. Res. 2015, 54, 1478–1486. [Google Scholar] [CrossRef]
- Romano, D.; Andablo-Reyes, E.; Ronca, S.; Rastogi, S. Aluminoxane co-catalysts for the activation of a bis phenoxyimine titanium (IV) catalyst in the synthesis of disentangled ultra-high molecular weight polyethylene. Polymer 2015, 74, 76–85. [Google Scholar] [CrossRef]
- Romano, D.; Ronca, S.; Rastogi, S. A Hemi-metallocene Chromium Catalyst with Trimethylaluminum-Free Methylaluminoxane for the Synthesis of Disentangled Ultra-High Molecular Weight Polyethylene. Macromol. Rapid Commun. 2015, 36, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Bravaya, N.M.; Faingol’d, E.E.; Mukhina, E.V.; Panin, A.N.; Perepelitsina, E.O.; Gagieva, S.C.; Tuskaev, V.A.; Bulychev, B.M. Effect of trimethylaluminum on the polymerization of ethylene with the catalytic system Bis[N-(3,5-di-tert-butylsalicylidene)-2,3,5,6-tetrafluoroanilinato]titanium(IV) dichloride-methylaluminoxane. Polym. Sci. Ser. B 2011, 52, 629–636. [Google Scholar] [CrossRef]
- Li, W.; Guan, C.; Xu, J.; Mu, J.; Gong, D.; Chen, Z.-r.; Zhou, Q. Disentangled UHMWPE/POSS nanocomposites prepared by ethylene in situ polymerization. Polymer 2014, 55, 1792–1798. [Google Scholar] [CrossRef]
- Makio, H.; Kashiwa, N.; Fujita, T. FI Catalysts: A New Family of High Performance Catalysts for Olefin Polymerization. Adv. Synth. Catal. 2002, 344, 477–493. [Google Scholar] [CrossRef]
- Matsui, S.; Fujita, T. FI Catalysts: Super active new ethylene polymerization catalysts. Catal. Today 2001, 66, 63–73. [Google Scholar] [CrossRef]
- Rastogi, S.; Yao, Y.; Ronca, S.; Bos, J.; van der Eem, J. Unprecedented High-Modulus High-Strength Tapes and Films of Ultrahigh Molecular Weight Polyethylene via Solvent-Free Route. Macromolecules 2011, 44, 5558–5568. [Google Scholar] [CrossRef]
- Ronca, S.; Romano, D.; Forte, G.; Andablo-Reyes, E.; Rastogi, S. Improving the performance of a catalytic system for the synthesis of ultra high molecular weight polyethylene with a reduced number of entanglements. Adv. Polym. Technol. 2012, 31, 193–204. [Google Scholar] [CrossRef]
- Sandaroos, R.; Zohuri, G.H.; Ahmadjo, S.; Damavandi, S. FI Catalyst for Polymerization of Olefin. In Polymerization; De Souza Gomes, A., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Talebi, S.; Duchateau, R.; Rastogi, S.; Kaschta, J.; Peters, G.W.M.; Lemstra, P.J. Molar Mass and Molecular Weight Distribution Determination Of UHMWPE Synthesized Using a Living Homogeneous Catalyst. Macromolecules 2010, 43, 2780–2788. [Google Scholar] [CrossRef]
- Chai, S.-C.; Xu, T.-Y.; Cao, X.; Wang, G.; Chen, Q.; Li, H.-L. Ultrasmall Nanoparticles Diluted Chain Entanglement in Polymer Nanocomposites. Chin. J. Polym. Sci. 2019, 37, 797–805. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Lichtenhan, J.; Reyes-Mayer, A.; Paredes-Pérez, M.; Yañez-Lino, M. Chain Disentanglements and Oxygen Transmission Reduction in LDPE/POSS Nanocomposites. Influence of POSS Size. Ind. Eng. Chem. Res. 2019, 58, 13145–13153. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Reyes-Mayer, A.; Paredes-Pérez, M.; Lichtenhan, J.; Yañez-Lino, M.; Sarmiento-Bustos, E. POSS driven chain disentanglements, decreased the melt viscosity and reduced O2 transmission in polyethylene. Polymer 2019, 165, 61–71. [Google Scholar] [CrossRef]
- Spronck, M.; Klein, A.; Blom, B.; Romano, D. Synthesis of Disentangled Ultra-High Molecular Weight Polyethylene using Vanadium(V)-Based Catalysts. Z. Für Anorg. Und Allg. Chem. 2018, 644, 993–998. [Google Scholar] [CrossRef]
- Kenyon, P.; Wörner, M.; Mecking, S. Controlled Polymerization in Polar Solvents to Ultrahigh Molecular Weight Polyethylene. J. Am. Chem. Soc. 2018, 140, 6685–6689. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shao, Y.; Liu, Z.; He, X.; Liu, B. Effect of short-chain branching on the tie chains and dynamics of bimodal polyethylene: Molecular dynamics simulation. Eur. Polym. J. 2018, 103, 312–321. [Google Scholar] [CrossRef]
- AlSalem, F.; Louhichi, A.; Rastogi, S. Melt blending of commercial linear polyethylene with low-entangled ultra-high molecular weight polyethylene: From dispersion compatibility to viscoelastic scaling laws. Polymer 2024, 311, 127563. [Google Scholar] [CrossRef]
- Orupattur, N.V.; Mushrif, S.H.; Prasad, V. Catalytic materials and chemistry development using a synergistic combination of machine learning and ab initio methods. Comput. Mater. Sci. 2020, 174, 109474. [Google Scholar] [CrossRef]
- Abedi, S.; Abdouss, M. A review of clay-supported Ziegler–Natta catalysts for production of polyolefin/clay nanocomposites through in situ polymerization. Appl. Catal. A Gen. 2014, 475, 386–409. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, H.; Li, B.-G. Functionalized Phenoxy-Imine Catalyst for Synthesizing Highly Crystalline Nascent UHMWPEs. 1. Molecular Weight Characteristics and Polymer Morphologies. Mater. Today Commun. 2020, 25, 101267. [Google Scholar] [CrossRef]
- Yao, Y.; Jiang, S.; Rastogi, S. 13C Solid State NMR Characterization of Structure and Orientation Development in the Narrow and Broad Molar Mass Disentangled UHMWPE. Macromolecules 2014, 47, 1371–1382. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Y.; Yao, Y.; Wang, H.; Lin, T.; Gao, Y.; Wang, X.; Xue, G. Chain Dynamics of Partially Disentangled UHMWPE around Melting Point Characterized by 1H Low-Field Solid-State NMR. Polymers 2023, 15, 1910. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; de Boer, E.L.; Yao, Y.; Romano, D.; Ronca, S.; Rastogi, S. Heterogeneous Distribution of Entanglements in a Nonequilibrium Polymer Melt of UHMWPE: Influence on Crystallization without and with Graphene Oxide. Macromolecules 2016, 49, 7497–7509. [Google Scholar] [CrossRef]
- Pandey, A.; Champouret, Y.; Rastogi, S. Heterogeneity in the Distribution of Entanglement Density during Polymerization in Disentangled Ultrahigh Molecular Weight Polyethylene. Macromolecules 2011, 44, 4952–4960. [Google Scholar] [CrossRef]
- Rastogi, S.; Lippits, D.R.; Höhne, G.W.H.; Mezari, B.; Magusin, P.C.M.M. The role of the amorphous phase in melting of linear UHMW-PE; implications for chain dynamics. J. Phys. Condens. Matter 2007, 19, 205122. [Google Scholar] [CrossRef]
- Ye, C.; Yang, T.; Li, Z.; Zhao, S.; Liu, Z.; Kang, D.; Zhou, J.; Li, J.; Xin, Z. Novel determining technique for the entanglement degree of ultra-high molecular weight polyethylene. Mater. Lett. 2023, 349, 134783. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, L.; Gao, J.; Guo, J.; Xing, C.; Li, Y.; Wang, Z. Structural Evolution of High-Entanglement Ultrahigh Molecular Weight Polyethylene Films with Reserved Shish Crystals during the Hot Stretching Process. Macromolecules 2024, 57, 2176–2190. [Google Scholar] [CrossRef]
- Chen, L.; Deng, B.; Li, X.; Wang, Z. Structural evolution of UHMWPE gel fibers as high degree plasticized system during stretching: An in-situ wide and small angle X-ray scattering study. Polymer 2022, 255, 125149. [Google Scholar] [CrossRef]
- Welzel, S.; Nieken, U. Fouling During Polymerization in Different Continuous Reactor Setups. Chem. Ing. Tech. 2024, 96, 1632–1641. [Google Scholar] [CrossRef]
- Mao, Z.; Liu, H.; Niu, B.; Bhagat, W.A.; Fan, W.; Liang, D.; Yang, L.; Zhao, Q.; Meng, S. Mitigation of fouling problem and optimization of treatment effect in the polyvinylidene fluoride (PVDF) based electrochemical membrane bioreactor (EMBR). Sep. Purif. Technol. 2024, 336, 126340. [Google Scholar] [CrossRef]
- Rohman, F.S.; Othman, M.R.; Muhammad, D.; Azmi, A.; Idris, I.; Ilyas, R.A.; Elkhatif, S.E.; Murat, M.N. Nonlinear Control of Fouling in Polyethylene Reactors. ACS Omega 2022, 7, 39648–39661. [Google Scholar] [CrossRef] [PubMed]
- Rust, S.; Osenberg, M.; Musch, T.; Pauer, W. Ultrasonic and conversion-based inline fouling measurements for continuous emulsion copolymerisation of vinyl acetate in a tubular reactor. Sci. Rep. 2024, 14, 4077. [Google Scholar] [CrossRef] [PubMed]
- Collins Rice, C.G.; Evans, A.; Turner, Z.R.; Wattoom, J.; O’Hare, D. Strategies for enhancing the processability of UHMWPE. Ind. Chem. Mater. 2025, 3, 178–190. [Google Scholar] [CrossRef]
- Fedorenko, E.; Luinstra, G.A. In Situ Polymerization and Synthesis of UHMWPE/Carbon Fiber Composites. Polymers 2025, 17, 90. [Google Scholar] [CrossRef] [PubMed]
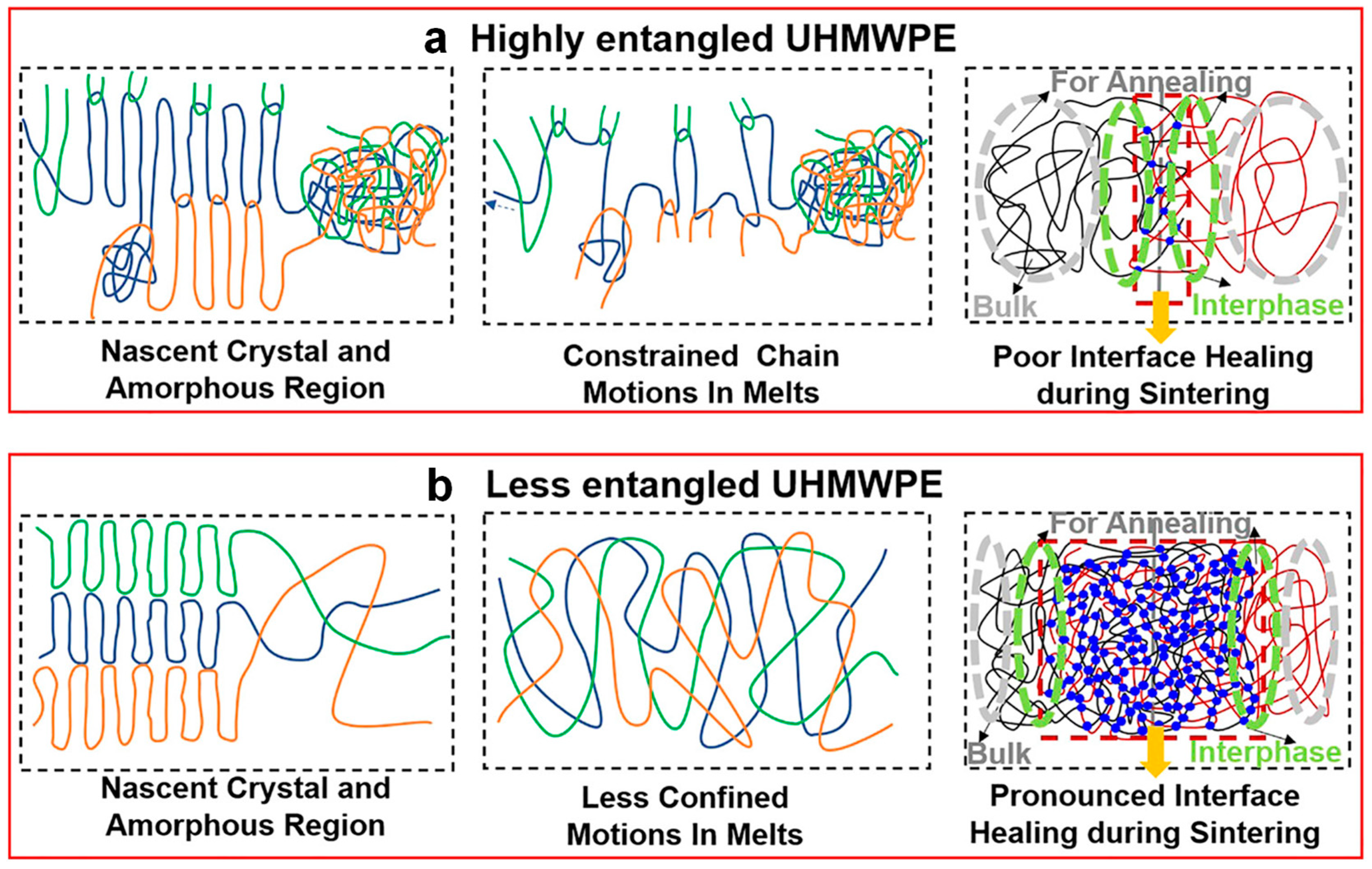
- Wang, W.; Wang, H.; Chen, J. Research on Chain Diffusion and Entanglement via Controlling the Sintering Process of Nascent UHMWPE. Macromolecules 2024, 57, 2205–2217. [Google Scholar] [CrossRef]
- Heidari, A.; Saeid, T.; Mostafa, R.; Hasan, K.-M.; Jafariyeh-Yazdi, E. In Situ Synthesis of Ultrahigh Molecular Weight Polyethylene/Graphene Oxide Nanocomposite Using the Immobilized Single-site Catalyst. Polym.-Plast. Technol. Eng. 2018, 57, 1313–1324. [Google Scholar] [CrossRef]
- Oleynik, I.V.; Shundrina, I.K.; Oleyinik, I.I. Highly active titanium(IV) dichloride FI catalysts bearing a diallylamino group for the synthesis of disentangled UHMWPE. Polym. Adv. Technol. 2020, 31, 1921–1934. [Google Scholar] [CrossRef]
- Hui, L.; Yue, Z.; Yang, H.; Chen, T.; Li, W. Influence of the Fragmentation of POSS-Modified Heterogeneous Catalyst on the Formation of Chain Entanglements. Ind. Eng. Chem. Res. 2018, 57, 9400–9406. [Google Scholar] [CrossRef]
- Yang, H.; Lolage, S.; van der Eem, J.; Rastogi, S.; Romano, D. Silica-supported catalyst for the synthesis of low entangled UHMWPE suitable for solid-state processing. Mol. Catal. 2024, 552, 113668. [Google Scholar] [CrossRef]
- Gote, R.P.; Romano, D.; van der Eem, J.; Zhao, J.; Zhou, F.; Rastogi, S. Unprecedented Mechanical Properties in Linear UHMWPE Using a Heterogeneous Catalytic System. Macromolecules 2023, 56, 361–378. [Google Scholar] [CrossRef]
- Gote, R.P.; Romano, D.; van der Eem, J.; Gaikwad, S.; Rastogi, S. Influence of diverse MgClx/R’nClmAly(OR)z activators/supports in tailoring of entangled state of UHMWPE. Mol. Catal. 2023, 551, 113580. [Google Scholar] [CrossRef]
- Li, W.; Yang, H.; Zhang, J.; Mu, J.; Gong, D.; Wang, X. Immobilization of isolated FI catalyst on polyhedral oligomeric silsesquioxane-functionalized silica for the synthesis of weakly entangled polyethylene. Chem. Commun. 2016, 52, 11092–11095. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, X.; Zhao, S.; Ye, C.; Zhang, Z.; Xin, Z. Chain disentanglement in POSS/UHMWPE composites prepared via in-situ polymerization. J. Polym. Res. 2022, 29, 97. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Zhao, S.; Ye, C.; Zhang, Z.; Kuo, S.-W.; Xin, Z. Study on the effects of soluble POSS on chain disentanglement in UHMWPE polymerization. Polymer 2022, 244, 124561. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Y.; Li, W.; Liang, P.; Ren, C.; Jiang, B.; Wang, J.; Yang, Y. Synthesis of Weakly Entangled Ultra-High-Molecular-Weight Polyethylene with a Fine Particle Size. Ind. Eng. Chem. Res. 2021, 60, 3354–3362. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, P.; Yue, Z.; Li, W.; Dong, C.; Jiang, B.; Wang, J.; Yang, Y. Entanglement Formation Mechanism in the POSS Modified Heterogeneous Ziegler–Natta Catalysts. Macromolecules 2019, 52, 7593–7602. [Google Scholar] [CrossRef]
- Li, W.; Hui, L.; Xue, B.; Dong, C.; Chen, Y.; Hou, L.; Jiang, B.; Wang, J.; Yang, Y. Facile high-temperature synthesis of weakly entangled polyethylene using a highly activated Ziegler-Natta catalyst. J. Catal. 2018, 360, 145–151. [Google Scholar] [CrossRef]
- Guo, T.; Zhou, Q.; Chen, Y.; Li, W.; Liang, P.; Wang, J.; Yang, Y. Preparation of Weakly Entangled and Fine-sized Ultra-High-Molecular Weight Polyethylene by a MgCl2-Based Ziegler–Natta Catalyst. Ind. Eng. Chem. Res. 2022, 61, 16711–16720. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Zhao, S.; Zhang, Z.; Ye, C.; Xin, Z. Influence of Modified Ziegler–Natta Catalyst on the Entanglement Behavior and Properties of Ultrahigh-Molecular-Weight Polyethylene (UHMWPE). Ind. Eng. Chem. Res. 2022, 61, 17512–17523. [Google Scholar] [CrossRef]
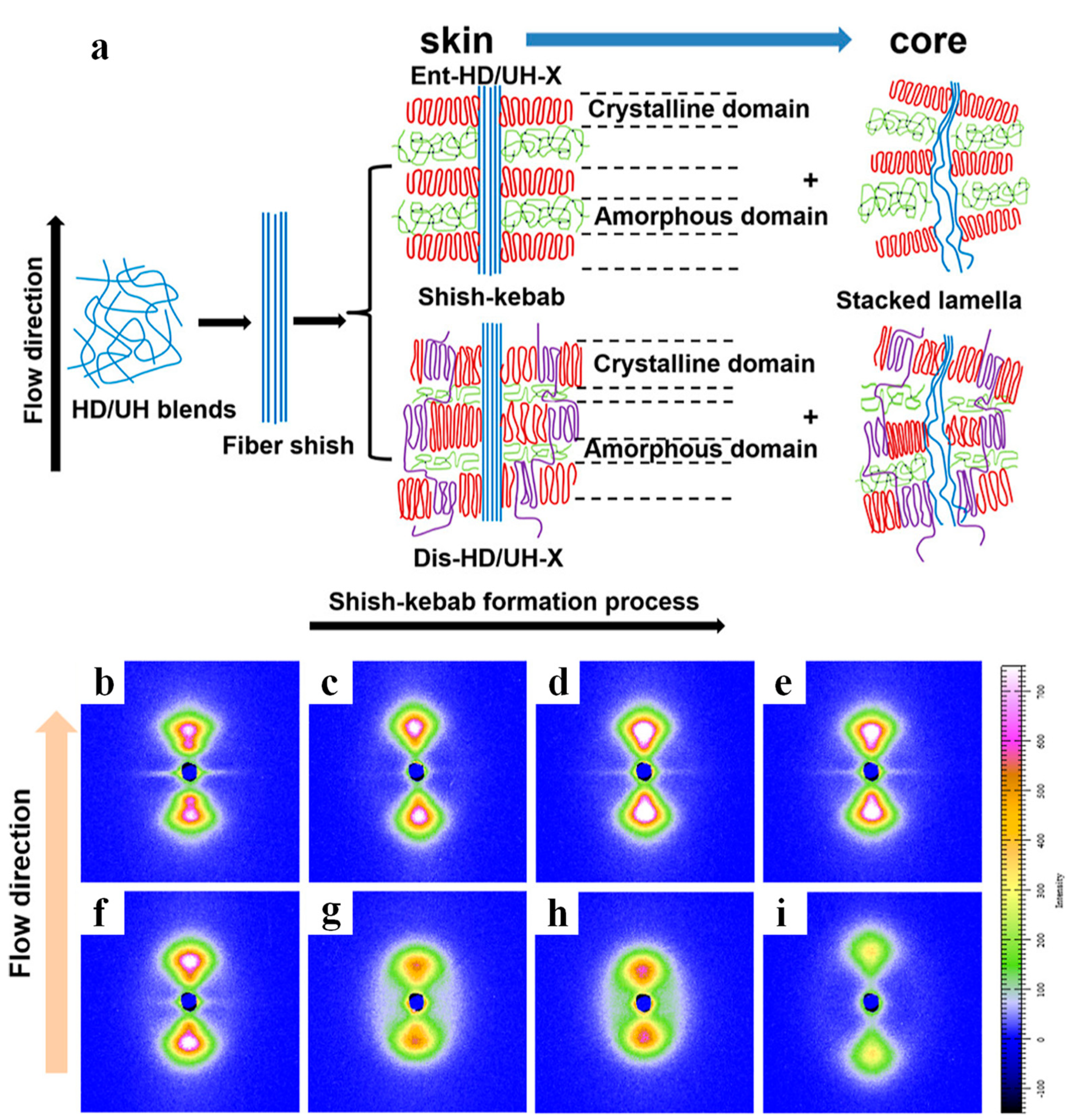
- Chen, Y.; Li, W.; Zhang, L.; Ye, C.; Tao, G.; Ren, C.; Jiang, B.; Wang, J.; Yang, Y. In Situ Synthesized Self-Reinforced HDPE/UHMWPE Composites with High Content of Less Entangled UHMWPE and High Gradient-Distributed Oriented Structures. ACS Appl. Polym. Mater. 2023, 5, 88–98. [Google Scholar] [CrossRef]
- Collins Rice, C.G.; Buffet, J.-C.; Turner, Z.R.; O’Hare, D. Supported permethylindenyl titanium catalysts for the synthesis of disentangled ultra-high molecular weight polyethylene (disUHMWPE). Chem. Commun. 2021, 57, 8600–8603. [Google Scholar] [CrossRef] [PubMed]
- Gote, R.P.; Mandal, D.; Patel, K.; Chaudhuri, K.; Vinod, C.P.; Lele, A.K.; Chikkali, S.H. Judicious Reduction of Supported Ti Catalyst Enables Access to Disentangled Ultrahigh Molecular Weight Polyethylene. Macromolecules 2018, 51, 4541–4552. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Y.; Tang, X.; Zhou, Q.; Stapf, S.; Mattea, C.; Li, W. Long-term efficiency for reducing entanglements of nascent polyethylene by a polystyrene-modified Ziegler-Natta catalyst. J. Appl. Polym. Sci. 2022, 139, 51790. [Google Scholar] [CrossRef]
- do Rosario, R.L.; Christakopoulos, F.; Tervoort, T.A.; Brunel, F.; McKenna, T.F.L. Gas-phase polymerization of ultra-high molecular weight polyethylene with decreased entanglement density. J. Polym. Sci. 2023, 61, 1183–1195. [Google Scholar] [CrossRef]
- Chammingkwan, P.; Bando, Y.; Mai, L.T.T.; Wada, T.; Thakur, A.; Terano, M.; Sinthusai, L.; Taniike, T. Less Entangled Ultrahigh-Molecular-Weight Polyethylene Produced by Nano-Dispersed Ziegler–Natta Catalyst. Ind. Eng. Chem. Res. 2021, 60, 2818–2827. [Google Scholar] [CrossRef]
- Wang, H.; Yan, X.; Tang, X.; Ma, Y.; Fan, X.; Li, W.; Yu, W.; Wang, J.; Yang, Y. Contribution of the Initially Entangled State and Particle Size to the Sintering Kinetics of UHMWPE. Macromolecules 2022, 55, 1310–1320. [Google Scholar] [CrossRef]
- Barrera, E.G.; dos Santos, J.H.Z. Designing polyethylene characteristics by modification of the support for FI catalyst. Mol. Catal. 2017, 434, 1–6. [Google Scholar] [CrossRef]
- Gagieva, S.C.; Magomedov, K.F.; Tuskaev, V.A.; Bogdanov, V.S.; Kurmaev, D.A.; Golubev, E.K.; Denisov, G.L.; Nikiforova, G.G.; Evseeva, M.D.; Saracheno, D.; et al. Effect of Activator and Outgoing Ligand Nature on the Catalytic Behavior of Bis(phenoxy-imine) Ti(IV) Complexes in the Polymerization of Ethylene and Its Copolymerization with Higher Olefins. Polymers 2022, 14, 4397. [Google Scholar] [CrossRef] [PubMed]
- Gagieva, S.C.; Tuskaev, V.A.; Magomedov, K.F.; Moskalenko, M.A.; Pavlov, A.A.; Meshchankina, M.Y.; Shcherbina, M.A.; Bulychev, B.M. Immobilized on MgCl2 bis(phenoxy-imine) complexes of Ti and Zr as catalysts for preparing UHMWPE and ethylene/higher α-olefin copolymers. Polym. Bull. 2022, 79, 8333–8351. [Google Scholar] [CrossRef]
- Li, T.; Kong, F.W.; Liu, R.; Li, Z.Y.; Zhu, F.M. Effect of cocatalysts on ethylene polymerization with fluorinated bisphenoxyimine titanium as a catalyst. J. Appl. Polym. Sci. 2011, 119, 572–576. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H. Ultra-high molecular weight polyethylene: Preparation and applications. J. Phys. Conf. Ser. 2022, 2229, 012006. [Google Scholar] [CrossRef]
- March, S.; Hand, M.; Morrissey, L.; Kelsey, D. Thermal buffering-controlled temperature variation between Mg–Al-rich rocks and migmatites. Sci. Rep. 2025, 15, 3038. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Zhou, L.; Ren, H.; Ran, L.; Xie, B. Study on thermal buffering effect of phase change material on press- pack IGBT. Int. J. Heat Mass Transf. 2020, 154, 119584. [Google Scholar] [CrossRef]
- Yang, H.; van Ingen, Y.; Blom, B.; Rastogi, S.; Romano, D. Structural modification of phenoxyimine titanium complexes and activation studies with alkylaluminum compounds. ChemCatChem 2020, 12, 5209–5220. [Google Scholar] [CrossRef]
- Sellinger, A.; Laine, R.M. Silsesquioxanes as Synthetic Platforms. Thermally Curable and Photocurable Inorganic/Organic Hybrids. Macromolecules 1996, 29, 2327–2330. [Google Scholar] [CrossRef]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar]
- Guo, M.; David, É.; Fréchette, M.; Demarquette, N.R. Polyethylene/polyhedral oligomeric silsesquioxanes composites: Dielectric, thermal and rheological properties. Polymer 2017, 115, 60–69. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Pielichowski, K. Segmental dynamics in hybrid polymer/POSS nanomaterials. Prog. Polym. Sci. 2016, 52, 136–187. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Xu, J.-Z.; Zhang, Z.-C.; Xu, L.; Li, L.-B.; Li, J.-F.; Li, Z.-M. Melt processing and structural manipulation of highly linear disentangled ultrahigh molecular weight polyethylene. Chem. Eng. J. 2017, 315, 132–141. [Google Scholar] [CrossRef]
- Heidari, A.; Zarghami, H.; Talebi, S.; Rezaei, M. A disentangled state using TiCl4/MgCl2 catalyst: A case study of polyethylene. Iran. Polym. J. 2018, 27, 701–708. [Google Scholar] [CrossRef]





| Catalyst | Support | Cocatalyst | Condition | Activity (106 g PE·mol−1[Ti]·h−1) | MW (106 g/mol) | Đ | Tm1 (°C) | Xc1 (%) | Tm2 (°C) | Xc2 (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluorinated FI | GO | MAO | 20 °C, n-hexane | 1.34 (Mv) | 143.1 | 63 | [99] | ||||
| Diallylamino modified FI | MAO | 40 °C, 4 bar, toluene | 3.44 | 4.1 (Mv) | 140.8 | 84.6 | 136.2 | 47.3 | [100] | ||
| FI | POSS/SiO2 | MAO | 30 °C, 10 bar, toluene | 7.6 | 2.0 (Mw) | 2.4 | 141.0 | 78.3 | 134.2 | 33.9 | [101] |
| FI | SiO2 | MAO | 10 °C, 2 bar, toluene | 1.24 | 11.8 (Mw) | 8.3 | 141.1 | 79 | [102] | ||
| FI | MgClx/EtnAly(2-ethyl-1-hexoxide)z | TEA | 40 °C, 4 bar, toluene | 2.01 | 4.6 (Mw) | 3.3 | 140.3 | 75 | [103] | ||
| FI | MgClx | R’nClmAly(OR)z | 40 °C, 1.2 bar, toluene | 2.41 | 4.4 (Mw) | 3.8 | 139.9 | 80 | [104] | ||
| FI | POSS/SiO2 | MAO | 30 °C, 10 bar, toluene | 7.8 | 2.0 (Mw) | 2.4 | [105] | ||||
| Z-N | TEA | 85 °C, 6 bar, n-hexane, 1.89% POSS | 14.2 | 2.8 (Mv) | 6.0 | 136.09 | 53.89 | [106] | |||
| Z-N | TEA | 85 °C, 6 bar, hexane, 0.2% POSS | 11.81 | 2.6 (Mv) | 5.9 | 140.2 | 66.89 | 135.8 | 49.64 | [107] | |
| Z-N | POSS/MgCl2 | TEA | 60 °C, 3 bar, n-heptane | 10.56 | 1.42 (Mw) | 6.17 | [108] | ||||
| Z-N | POSS/MgCl2 | TiBA | 85 °C, 10 bar, n-heptane | 2.72 | 2.25 (Mw) | 11.8 | 143.8 | 66.9 | 137 | 51 | [109] |
| Z-N | POSS/MgCl2 | TEA | 60 °C, 3 bar, toluene | 3.92 | 2.05 (Mw) | 9.5 | 144.2 | 65.5 | 137.2 | 53.3 | [110] |
| Z-N | POSS/MgCl2 | TEA | 60 °C, 7 bar, n-heptane | 6.052 | 2.46 (Mw) | 5.0 | 144.3 | 66.9 | 136.8 | 50.4 | [111] |
| Z-N | OH-POSS/MgCl2·(EtOH)1.28 | TEA | 85 °C, 6 bar, n-hexane | 18.83 | 1.53 (Mv) | 7.2 | 136.55 | 62.16 | 119.8 | [112] | |
| Z-N | POSS/MgCl2 | TEA | 60 °C/85 °C, 10 bar, n-heptane/n-hexane | 3.429 | 0.184 (Mw) | 5.7 | 136.3 | 60.5 | 135.3 | 62.5 | [113] |
| PHENI* complexes | sMAO | TIBA | 60 °C, 2 bar, n-hexane | 7.438 | 2.087 (Mw) | 5.2 | 133–135 | 68–83 | [114] | ||
| [Ti(OEt)4] | [Ti(OEt)4] | MMAO12 | 40 °C, 1 bar, toluene | 0.213 | 13.07 (Mw) | 2.17 | 144.1 | 69.7 | [115] | ||
| Z-N | SiO2/4%PS | TEA | 70 °C, 7 bar, n-heptane | 3.1 | 2.63 (Mv) | 144.2 | 66.5 | 138.8 | 47.2 | [116] | |
| Z-N | MgCl2 | TIBA | 50 °C, 4 bar, pentane, ICA | 5.45 (Mv) | 142.0 | 66.1 | [117] | ||||
| Z-N | MgO | TEA | 70 °C, 8 bar, n-heptane | 9.78 | 5.3 (Mv) | 142.9 | 75 | 135.4 | 58 | [118] | |
| Z-N | MgCl2 | 50 °C, 3 bar, | 2.4 | 5.693 (Mv) | 4.3 | 142.6 | 66.0 | 135.6 | 47.2 | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L. Engineering Nascent Disentangled Ultra-High-Molecular-Weight Polyethylene Based on Heterogeneous Catalytic Polymerization. Organics 2025, 6, 32. https://doi.org/10.3390/org6030032
Li L. Engineering Nascent Disentangled Ultra-High-Molecular-Weight Polyethylene Based on Heterogeneous Catalytic Polymerization. Organics. 2025; 6(3):32. https://doi.org/10.3390/org6030032
Chicago/Turabian StyleLi, Lei. 2025. "Engineering Nascent Disentangled Ultra-High-Molecular-Weight Polyethylene Based on Heterogeneous Catalytic Polymerization" Organics 6, no. 3: 32. https://doi.org/10.3390/org6030032
APA StyleLi, L. (2025). Engineering Nascent Disentangled Ultra-High-Molecular-Weight Polyethylene Based on Heterogeneous Catalytic Polymerization. Organics, 6(3), 32. https://doi.org/10.3390/org6030032





