Complementary Synthesis of Anti- and Syn-Hydroxymethyl 1,3-Diols via Regioselective Ring Opening of TIPS-Protected 2,3-Epoxy Alcohols: Toward Polypropionate Fragments
Abstract
1. Introduction
2. Materials and Methods
2.1. General Considerations
2.2. General Synthetic Procedures
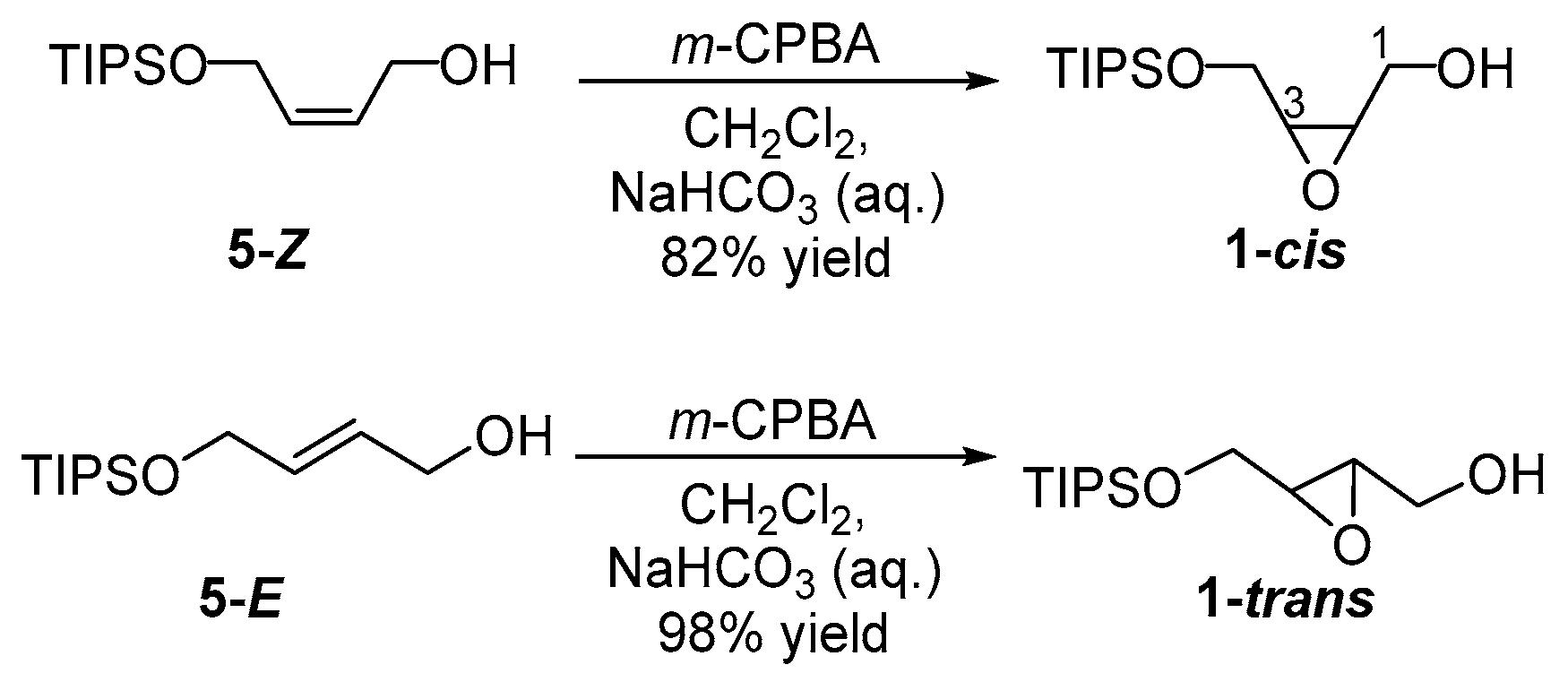
2.2.1. General Procedure A for the Synthesis of Optically Active Epoxy Alcohols via Sharpless Asymmetric Epoxidation
2.2.2. General Procedure B for the Epoxidation of Alkenes with Meta-Chloroperoxybenzoic Acid (m-CPBA) for the Synthesis of Racemic Epoxides
2.2.3. General Procedure C for the Microwave-Assisted VO(acac)2-Catalyzed Epoxidation of Alkenols with TBHP
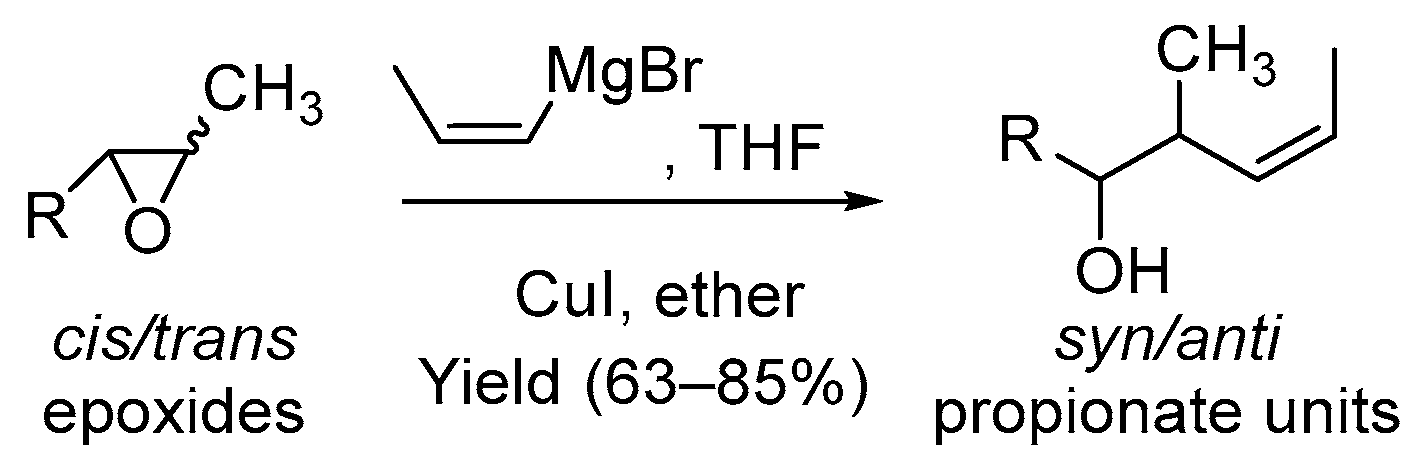
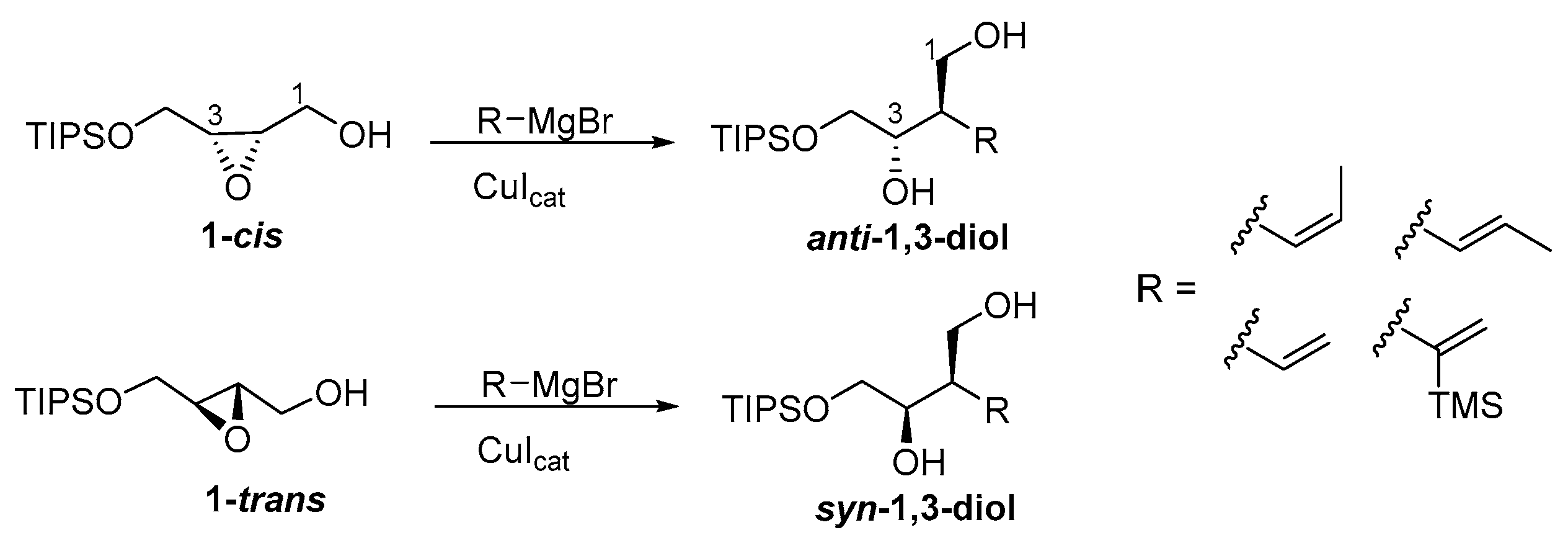
2.2.4. General Procedure D for the Copper-Catalyzed Cleavage of Epoxides Using Alkenylmagnesium Bromides
2.2.5. General Procedure E for the Alkynyl Substitution Reaction of Epoxy Alcohols (Alane Ate Procedure)
2.2.6. General Procedure F for the trans Reduction of Alkynes with Na°/NH3
2.2.7. General Procedure G for the Formation of Acetonides
2.2.8. General Procedure H for the Protection of Alcohols with TES-OTf
2.2.9. General Pro2cedure I for the Selective Deprotection of the 1° TES Silyl Ether in the Presence of a 2° TBS Ether
2.3. Experimental and Characterization Data
2.3.1. Preparation of (-)-(2S,3R)-4-(triisopropylsilyloxy)-2-3-epoxy-1-butanol ((-)-1-cis
2.3.2. Preparation of (+)-(2R,3S)-4-(triisopropylsilyloxy)-2-3-epoxy-1-butanol (+)-1-cis
2.3.3. Preparation of (-)-(2S,3S)-4-(triisopropylsilyloxy)-2-3-epoxy-1-butanol (-)-1-trans
2.3.4. Preparation of (+)-(2R,3R)-4-(triisopropylsilyloxy)-2-3-epoxy-1-butanol (+)-1-trans
2.3.5. Preparation of (±)-(2R,3S)-4-(triisopropylsilyloxy)-2,3-epoxy-1-butanol (1-cis)
2.3.6. Preparation of (±)-(2S,3S)-4-(triisopropylsilyloxy)-2,3-epoxy-1-butanol (1-trans)
2.3.7. Preparation of (±)-(2S,3R)-2-((Z)-prop-1-enyl)-4-(triisopropylsilyloxy)butane-1,3-diol (3a)
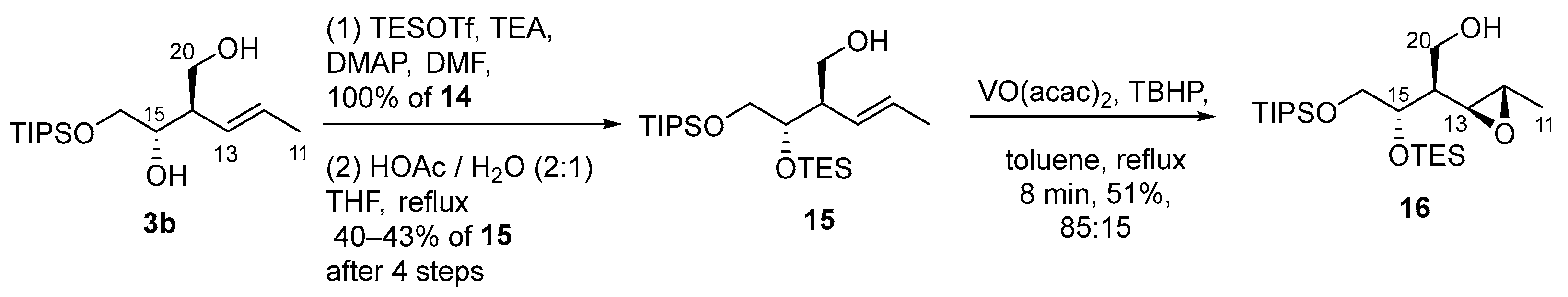
2.3.8. Preparation of (±)-(2R,3R)-2-[(Z)-1-propenyl]-4-[(triisopropylsilyl)oxy]-1,3-butanediol (3b)
2.3.9. Preparation of (±)-(2R,3S)-2-((E)-prop-1-enyl)-4-(triisopropylsilyloxy)butane-1,3-diol (3c)
2.3.10. Preparation of (±)-(2R,3R)-2-((E)-prop-1-enyl)-4-(triisopropylsilyloxy)butane-1,3-diol (3d)
2.3.11. Preparation of (±)-(2R,3S)-4-(triisopropylsilyloxy)-2-vinyl)butane-1,3-diol (3e)
2.3.12. Preparation of (±)-(2R,3R)-4-(triisopropylsilyloxy)-2-vinyl)butane-1,3-diol (3f)
2.3.13. Preparation of (±)-(2R,3S)-4-(triisopropylsilyloxy)-2-(1-(trimethylsilyl)vinyl)butane-1,3-diol (3g)
2.3.14. Preparation of (±)-(2R,3R)-4-(triisopropylsilyloxy)-2-(1-(trimethylsilyl)vinyl)butane-1,3-diol (3h)
2.3.15. Preparation of (±)-(2R,3S)-2-(prop-1-ynyl)-4-(triisopropylsilyloxy)butane-1,3-diol (2a) and the minor product (±)-(2R,3S)-3-((triisopropylsilyloxy)methyl)hex-4-yne-1,2-diol (6)
2.3.16. Preparation of (±)-(2R,3R)-2-(prop-1-ynyl)-4-(triisopropylsilyloxy)butane-1,3-diol (2b) and the minor product (±)-(2S,3S)-3-((triisopropylsilyloxy)methyl)hex-4-yne-1,2-diol
2.3.17. Preparation of (±)-(2R,3S)-2-((E)-prop-1-enyl)-4-(triisopropylsilyloxy)butane-1,3-diol (3c)
2.3.18. Preparation of (±)-(2R,3R)-2-((E)-prop-1-enyl)-4-(triisopropylsilyloxy)butane-1,3-diol (3d)
2.3.19. Preparation of (±)-(((4S,5S)-2,2-dimethyl-5-(prop-1-ynyl)-1,3-dioxan-4-yl)methoxy)triisopropylsilane (7)
2.3.20. Preparation of (±)-((R)-2-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)pent-3-ynyloxy)triisopropylsilane (8)
2.3.21. Preparation of (±)-(((4R,5R)-2,2-dimethyl-5-((Z)-prop-1-enyl)-1,3-dioxan-4-yl)methoxy)triisopropylsilane (9)
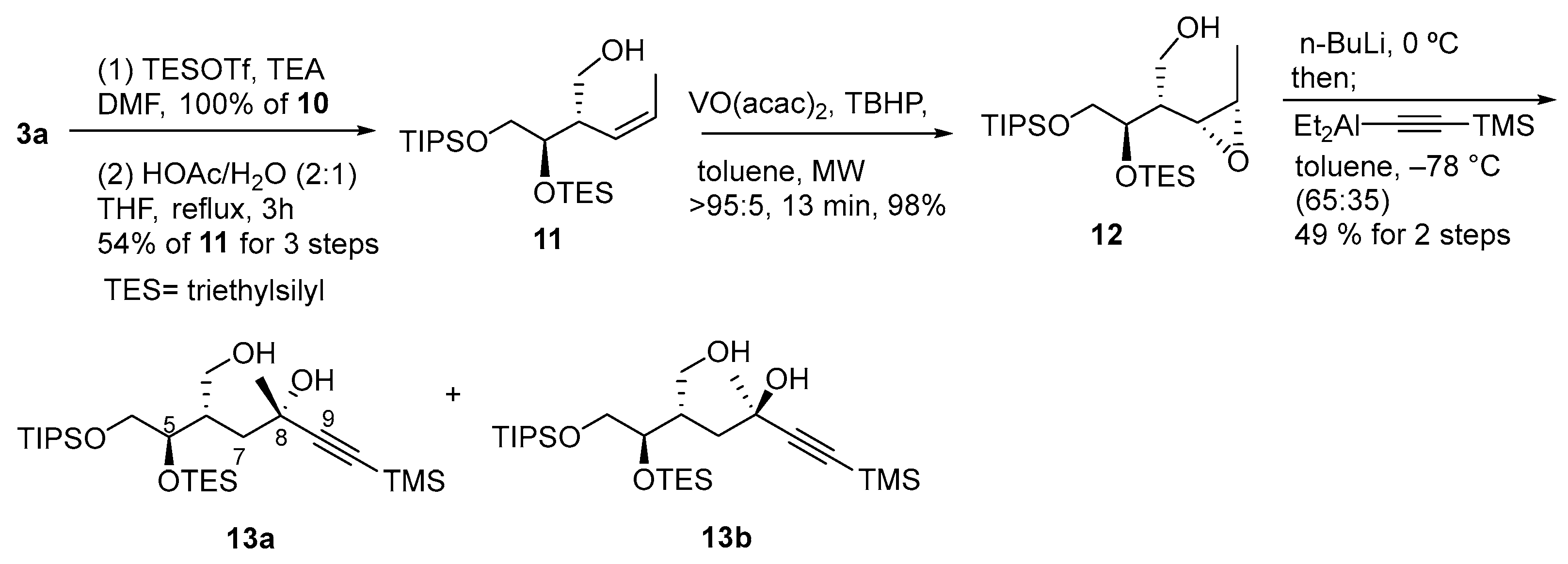
2.3.22. Preparation of (±)-(2Z,4S,5R)-4-(triethylsilyloxymethyl)-5-(triethylsilyloxy)-6-(triisopropylsilyloxy)-2-hexene (10)
2.3.23. Preparation of (±)-(2S,3R)-2-((Z)-prop-1-enyl)-3-(triethylsilyloxy)-4-(triisopropylsilyloxy)-1-butanol (11)
2.3.24. Preparation of (±)-(2R,3R)-2-((2S,3R)-3-methyloxiran-2-yl)-3-(triethylsilyloxy)-4-(triisopropylsilyloxy)butan-1-ol (12)
2.3.25. Preparation of (±)-(5S,6R)-5-(hydroxymethyl)-6-(triethylsilyloxy)-3-methyl-7-(triisopropylsilyloxy)-1-(trimethylsilyl)hept-1-yn-3-ol (13)
2.3.26. Preparation of (±)-(2E,4R,5S)-4-(triethylsilyloxymethyl)-5-(triethylsilyloxy)-6-(triisopropylsilyloxy)-2-hexene (14)
2.3.27. Preparation of (2S,3R)-2-((E)-prop-1-enyl)-3-(triethylsilyloxy)-4-(triisopropylsilyloxy)-1-butanol (15)
2.3.28. Preparation of (±)-(2S,3S)-2-((2S,3S)-3-methyloxiran-2-yl)-3-(triethylsilyloxy)-4-(triisopropylsilyloxy)butan-1-ol (16) and the Minor Isomer
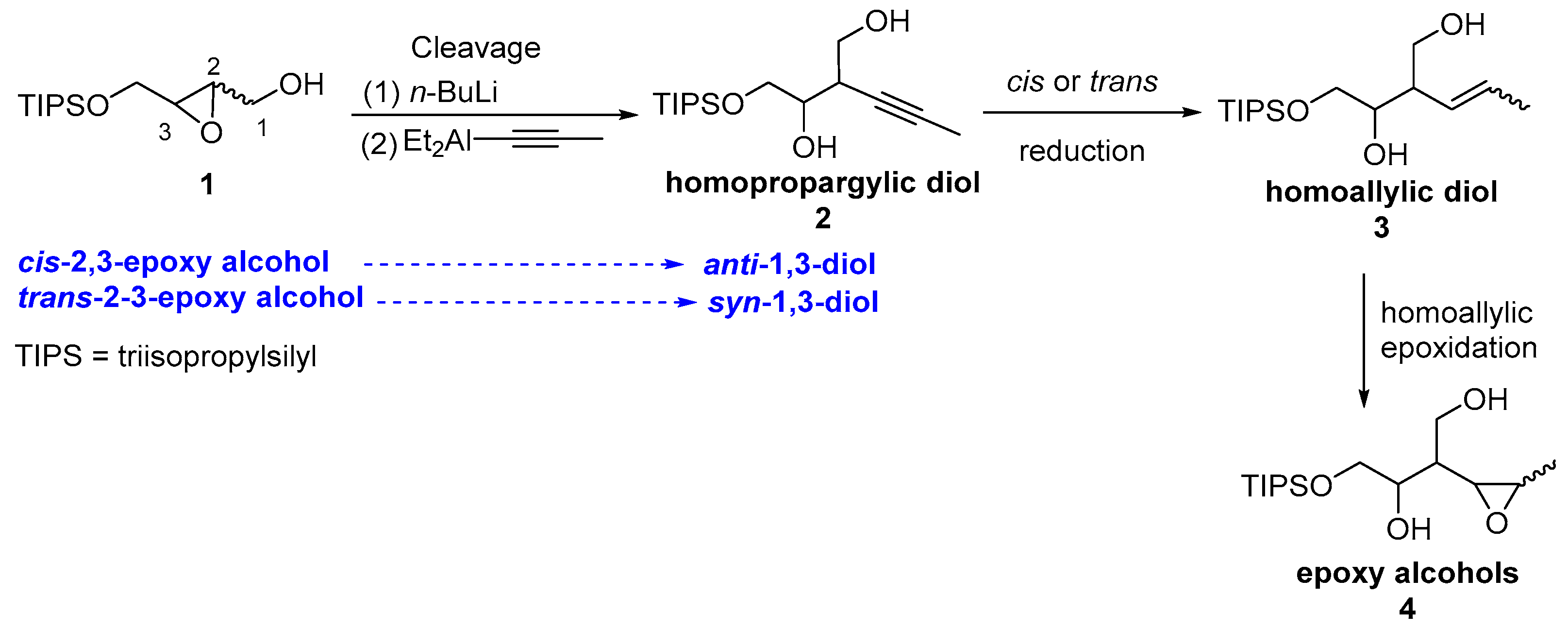
3. Results and Discussion
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Schönfeld, W.; Kirst, H.A. Macrolide Antibiotics; Springer Science & Business Media: Basel, Switzerland, 2002; ISBN 3-7643-6186-7. [Google Scholar]
- Kirst, H.A.; Wild, G.M.; Baltz, R.H.; Hamill, R.L.; Ott, J.L.; Counter, F.T.; Ose, E.E. Structure-Activity Studies among 16-Membered Macrolide Antibiotics Related to Tylosin. J. Antibiot. 1982, 35, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Takenaka, S.; Hayashi, M. Isolation of Proposed Intermediates in the Biosynthesis of Mycinamicins. J. Chem. Soc. Chem. Commun. 1988, 943–945. [Google Scholar] [CrossRef]
- Kinoshita, K.; Takenaka, S.; Suzuki, H.; Yamamoto, T.; Morohoshi, T.; Hayashi, M. Mycinamicin Biosynthesis: Isolation and Structural Elucidation of Novel Macrolactones and a Seco Acid Produced by a Mutant of Micromonospora Griseorubida. J. Chem. Soc. Chem. Commun. 1992, 957–959. [Google Scholar] [CrossRef]
- Kinoshita, K.; Takenaka, S.; Hayashi, M. Mycinamicins, New Macrolide Antibiotics. XII. Isolation and Structural Elucidation of Mycinamicins X and XI. J. Antibiot. 1991, 44, 1270–1273. [Google Scholar] [CrossRef][Green Version]
- Hayashi, M.; Ohno, M.; Katsumata, S.; Satoi, S. Mycinamicins, New Macrolide Antibiotics. IV. Structure of Mycinamicin III. J. Antibiot. 1981, 34, 276–281. [Google Scholar] [CrossRef]
- Späth, G.; Fürstner, A. Total Synthesis of Mycinamicin IV as Integral Part of a Collective Approach to Macrolide Antibiotics. Chem. A Eur. J. 2022, 28, e202104400. [Google Scholar] [CrossRef]
- Herlé, B.; Späth, G.; Schreyer, L.; Fürstner, A. Total Synthesis of Mycinolide IV and Path-Scouting for Aldgamycin N. Angew. Chem. Int. Ed. 2021, 60, 7893–7899. [Google Scholar] [CrossRef]
- Wang, C.-X.; Ding, R.; Jiang, S.-T.; Tang, J.-S.; Hu, D.; Chen, G.-D.; Lin, F.; Hong, K.; Yao, X.-S.; Gao, H. Aldgamycins J–O, 16-Membered Macrolides with a Branched Octose Unit from Streptomycetes Sp. and Their Antibacterial Activities. J. Nat. Prod. 2016, 79, 2446–2454. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tabudravu, J.; Jaspars, M.; Deng, H. Tianchimycins A–B, 16-Membered Macrolides from the Rare Actinomycete Saccharothrix Xinjiangensis. Tetrahedron 2013, 69, 6060–6064. [Google Scholar] [CrossRef]
- Franco, C.M.M.; Gandhi, J.N.; Chatterjee, S.; Ganguli, B.N. Swalpamycin, a New Macrolide Antibiotic. I. Taxonomy of the Producing Organism, Fermentation, Isolation and Biological Activity. J. Antibiot. 1987, 40, 1361–1367. [Google Scholar] [CrossRef]
- Chatterjee, S.; Reddy, G.C.S.; Franco, C.M.M.; Rupp, R.H.; Ganguli, B.N.; Fehlhaber, H.-W.; Kogler, H. Swalpamycin, a New Macrolide Antibiotic. II. Structure Elucidation. J. Antibiot. 1987, 40, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Asolkar, R.N.; Maskey, R.P.; Helmke, E.; Laatsch, H. Chalcomycin B, a New Macrolide Antibiotic from the Marine Isolate Streptomyces Sp. B7064. J. Antibiot. 2002, 55, 893–898. [Google Scholar] [CrossRef]
- Woo, P.W.K.; Dion, H.W.; Bartz, Q.R. The Structure of Chalcomycin. J. Am. Chem. Soc. 1964, 86, 2726–2727. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Gunasekera, S.P.; Yalamanchili, G.; Hossain, M.B.; Van Der Helm, D. Tedanolide: A Potent Cytotoxic Macrolide from the Caribbean Sponge Tedania Ignis. J. Am. Chem. Soc. 1984, 106, 7251–7252. [Google Scholar] [CrossRef]
- Taylor, R.E.; Hearn, B.R.; Ciavarri, J.P. A Divergent Approach to the Myriaporones and Tedanolide: Completion of the Carbon Skeleton of Myriaporone 1. Org. Lett. 2002, 4, 2953–2955. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Moore, R.E.; Patterson, G.M.L.; Xu, C.; Clardy, J. Scytophycins, Cytotoxic and Antimycotic Agents from the Cyanophyte Scytonema Pseudohofmanni. J. Org. Chem. 1986, 51, 5300–5306. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Tachibana, K. The 1994 Japan-U.S. Seminar on Bioorganic Marine Chemistry, Meeting Report. J. Nat. Prod. 1995, 58, 344–358. [Google Scholar] [CrossRef]
- Knöll, W.M.; Rinehart Jr, K.L.; Willey, P.F.; LI, L.H. Streptovaricin U, an Acyclic Ansamycin. J. Antibiot. 1980, 33, 249–251. [Google Scholar] [CrossRef][Green Version]
- Milavetz, B.I.; Carter, W.A. Streptovaricins. Pharmacol. Ther. Part A Chemother. Toxicol. Metab. Inhib. 1977, 1, 289–305. [Google Scholar] [CrossRef]
- Wang, B.-L.; Gao, H.-T.; Li, W.-D.Z. Total Synthesis of (+)-Iresin. J. Org. Chem. 2015, 80, 5296–5301. [Google Scholar] [CrossRef]
- Trost, B.M.; Nishimura, Y.; Yamamoto, K. A Total Synthesis of Aphidicolin. J. Am. Chem. Soc. 1979, 101, 1328–1330. [Google Scholar] [CrossRef]
- Su, B.-N.; Takaishi, Y.; Kusumi, T. Morinols A-L, Twelve Novel Sesquineolignans and Neolignans with a New Carbon Skeleton from Morina Chinensis. Tetrahedron 1999, 55, 14571–14586. [Google Scholar] [CrossRef]
- Gordaliza, M.; García, P.A.; Miguel Del Corral, J.M.; Castro, M.A.; Gómez-Zurita, M.A. Podophyllotoxin: Distribution, Sources, Applications and New Cytotoxic Derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sunaga, R.; Furihata, K.; Morisaki, N.; Iwasaki, S. Isolation and Structures of an Antifungal Antibiotic, Fusarielin A, and Related Compounds Produced by a Flusarium Sp. J. Antibiot. 1995, 48, 42–52. [Google Scholar] [CrossRef]
- Bandini, M.; Cozzi, P.G.; Licciulli, S.; Umani-Ronchi, A. A Cross Metathesis Based Protocol for the Effective Synthesis of Function-alised Allyl Bromides and Chlorides. Synthesis 2004, 3, 409–414. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yang, J.H.; Kim, S.H.; Krische, M.J. Anti-Diastereo- and Enantioselective Carbonyl (Hydroxymethyl)Allylation from the Alcohol or Aldehyde Oxidation Level: Allyl Carbonates as Allylmetal Surrogates. J. Am. Chem. Soc. 2010, 132, 4562–4563. [Google Scholar] [CrossRef]
- Sasaki, M.; Tanino, K.; Miyashita, M. Stereospecific Alkyl and Alkynyl Substitution Reactions of Epoxy Sulfides with Organoaluminums with Double Inversion of the Configuration. J. Org. Chem. 2001, 66, 5388–5394. [Google Scholar] [CrossRef] [PubMed]
- Roush, W.R.; Adam, M.A.; Peseckis, S.M. Regioselectivity of the Reactions of Trialkylaluminum Reagents with 2,3-Epoxyalcohols: Application to the Synthesis of α-Chiral Aldehydes. Tetrahedron Lett. 1983, 24, 1377–1380. [Google Scholar] [CrossRef]
- Ahn, K.H.; Kim, J.S.; Jin, C.S.; Kang, D.H.; Han, D.S.; Shin, Y.S.; Kim, D.H. Regioselective Oxirane Ring Opening of Trans-2,3-Epoxy Alcohols with Arylaluminum Complexes. Synlett 1992, 1992, 306–308. [Google Scholar] [CrossRef]
- Chong, J.M.; Cyr, D.R.; Mar, E.K. Regioselective Opening of 2,3-Epoxy Alcohols with Organocuprates. Enhanced C-2 Selectivity through Solvent Effects. Tetrahedron Lett. 1987, 28, 5009–5012. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hirao, I. A Novel Alkynylation Reaction of Epoxy Alcohols: Use in the Synthesis of Erythro-6-Acetoxyhexadecan-5-Olide. J. Chem. Soc. Chem. Commun. 1984, 3, 202. [Google Scholar] [CrossRef]
- Prieto, J.A.; Torres, J.R.; Rodríguez-Berrios, R. Regiocontrolled Ring Opening of Monoprotected 2,3-Epoxy-1,4-Diols by Using Alkynyl Aluminum Reagents: Synthesis of Differentially Monoprotected Alkynyl Triol Derivatives. Synlett 2014, 25, 433–437. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Berríos, R.R.; Torres, G.; Prieto, J.A. Stereoselective VO (Acac) 2 Catalyzed Epoxidation of Acyclic Homoallylic Diols. Complementary Preparation of C2-Syn-3, 4-Epoxy Alcohols. Tetrahedron 2011, 67, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Tius, M.A.; Fauq, A.H. Copper(I)-Catalyzed Reactions of .Beta.,.Gamma.-Epoxy Alcohols with Grignard Reagents. J. Org. Chem. 1983, 48, 4131–4132. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Kozlowski, J.; Wilhelm, R.S. Chemistry of Higher Order Mixed Organocuprates. 2. Reactions of Epoxides. J. Am. Chem. Soc. 1982, 104, 2305–2307. [Google Scholar] [CrossRef]
- Rodríguez, D.; Mulero, M.; Prieto, J.A. Highly Regioselective Copper-Catalyzed Cis-and Trans-1-Propenyl Grignard Cleavage of Hindered Epoxides. Application in Propionate Synthesis. J. Org. Chem. 2006, 71, 5826–5829. [Google Scholar] [CrossRef]
- Rodríguez-Berríos, R.R.; Isbel, S.R.; Bugarin, A. Epoxide-Based Synthetic Approaches toward Polypropionates and Related Bioactive Natural Products. Int. J. Mol. Sci. 2023, 24, 6195. [Google Scholar] [CrossRef]
- Tirado, R.; Torres, G.; Torres, W.; Prieto, J.A. Regioselective Cleavage of Cis- and Trans-2-Methyl-3,4-Epoxy Alcohols with Diethylpropynyl Aluminum. Tetrahedron Lett. 2005, 46, 797–801. [Google Scholar] [CrossRef]
- Torres, W.; Torres, G.; Prieto, J.A. Synthesis of Stereotetrads by Regioselective Cleavage of Diastereomeric MEM-Protected 2-Methyl-3, 4-Epoxy Alcohols with Diethylpropynyl Aluminum. Synlett 2012, 23, 2534–2538. [Google Scholar]
- Cruz-Montañez, A.; Morales-Rivera, K.F.; Torres, W.; Valentín, E.M.; Rentas-Torres, J.; Prieto, J.A. Reiterative Epoxide-Based Strategies for the Synthesis of Stereo-n-Ads and Application to Polypropionate Synthesis. A Personal Account. Inorg. Chim. Acta 2017, 468, 28–37. [Google Scholar] [CrossRef]
- Izzo, I.; Scioscia, M.; Del Gaudio, P.; De Riccardis, F. First Enantioselective Non-Biological Synthesis of Asymmetrised Tris(Hydroxymethyl)Methane (THYM*) and Bis(Hydroxymethyl)Acetaldehyde (BHYMA*). Tetrahedron Lett. 2001, 42, 5421–5424. [Google Scholar] [CrossRef]
- Alexakis, A.; Frutos, J.C.; Mutti, S.; Mangeney, P. Chiral Diamines for a New Protocol To Determine the Enantiomeric Composition of Alcohols, Thiols, and Amines by 31P, 1H, 13C, and 19F NMR. J. Org. Chem. 1994, 59, 3326–3334. [Google Scholar] [CrossRef]
- Rentas-Torres, J.; Prieto, J.A. Studies Toward the Preparation of the Polypropionate Segments of Scytophycin C and Mycalolide A: First and Second Generation Epoxide-Based Approaches. Ph.D. Thesis, University of Puerto Rico, Rio Piedras Campus, San Juan, Puerto Rico, 2013. [Google Scholar]
- Gao, Y.; Klunder, J.M.; Hanson, R.M.; Masamune, H.; Ko, S.Y.; Sharpless, K.B. Catalytic Asymmetric Epoxidation and Kinetic Resolution: Modified Procedures Including in Situ Derivatization. J. Am. Chem. Soc. 1987, 109, 5765–5780. [Google Scholar] [CrossRef]
- Katsuki, T.; Sharpless, K.B. The First Practical Method for Asymmetric Epoxidation. J. Am. Chem. Soc. 1980, 102, 5974–5976. [Google Scholar] [CrossRef]
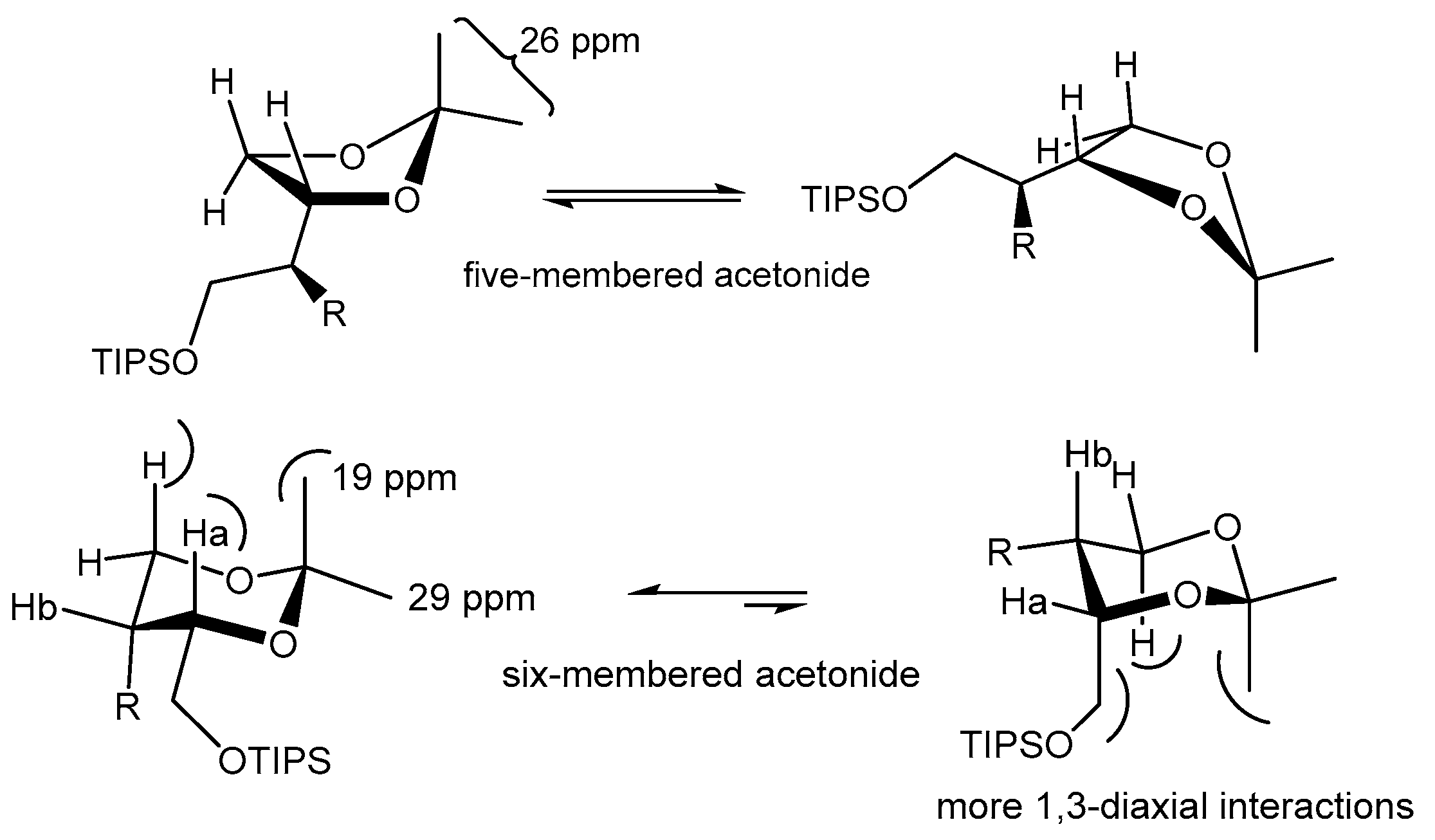
- Rychnovsky, S.D.; Skalitzky, D.J. Stereochemistry of Alternating Polyol Chains: 13C NMR Analysis of 1,3-Diol Acetonides. Tetrahedron Lett. 1990, 31, 945–948. [Google Scholar] [CrossRef]
- Evans, D.A.; Rieger, D.L.; Gage, J.R. 13C NMR Chemical Shift Correlations in 1,3-Diol Acetonides. Implications for the Stereochemical Assignment of Propionate-Derived Polyols. Tetrahedron Lett. 1990, 31, 7099–7100. [Google Scholar] [CrossRef]
- Morgans, D.J.; Sharpless, K.B.; Traynor, S.G. Epoxy Alcohol Rearrangements: Hydroxyl-Mediated Delivery of Lewis Acid Promoters. J. Am. Chem. Soc. 1981, 103, 462–464. [Google Scholar] [CrossRef]
- Maruoka, K.; Hasegawa, M.; Yamamoto, H.; Suzuki, K.; Shimazaki, M.; Tsuchihashi, G. Epoxy Silyl Ether Rearrangements: A New, Stereoselective Approach to the Synthesis of .Beta.-Hydroxy Carbonyl Compounds. J. Am. Chem. Soc. 1986, 108, 3827–3829. [Google Scholar] [CrossRef]
- Dávila, W.; Torres, W.; Prieto, J.A. Regioselective Cleavage of 3,4-Epoxy Alcohols with Substituted Alkynylaluminum Reagents: Application to the Stereoselective Synthesis of Polypropionates. Tetrahedron 2007, 63, 8218–8226. [Google Scholar] [CrossRef]
- Rodriguez-Berrios, R.; Prieto, A. Synthesis of Anti- and Syn-Hydroxymethyl 1,3-Diol Motifs Based on the Regioselective Cleavage of 2,3-Epoxy Diols Using Organomagnesium and Organoaluminum Reagents: Application to the Polypropionate Synthesis; ACS Spring: Philadelphia, PA, USA, 2020. [Google Scholar]


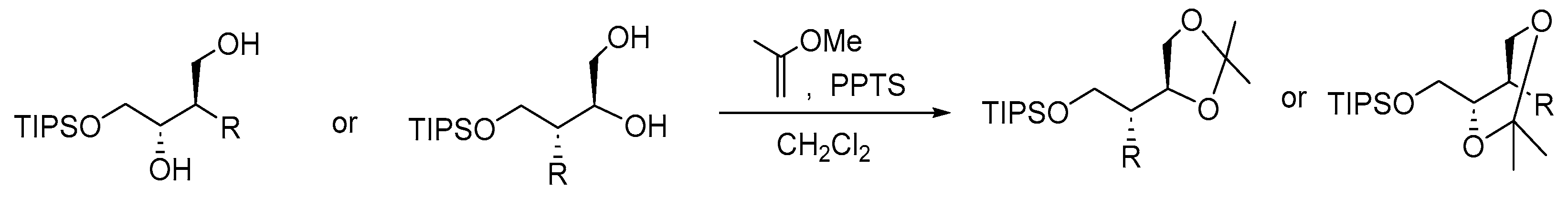
 | ||||||
| Entry | Allylic Alcohol | Tartrate | Product | ee% a | Yield% | |
|---|---|---|---|---|---|---|
| Entry 1 | 5-Z | L-DIPT |  (-)-1-cis | −9.9 | 80 | 83 |
| Entry 2 | D-DIPT | 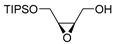 (+)-1-cis | +8.4 | 80 | 80 | |
| Entry 3 | 5-E | L-DIPT |  (-)-1-trans | −10.5 | 94 | 91 |
| Entry 4 | D-DIPT | 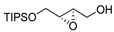 (+)-1-trans | +13.9 | 94 | 80 | |
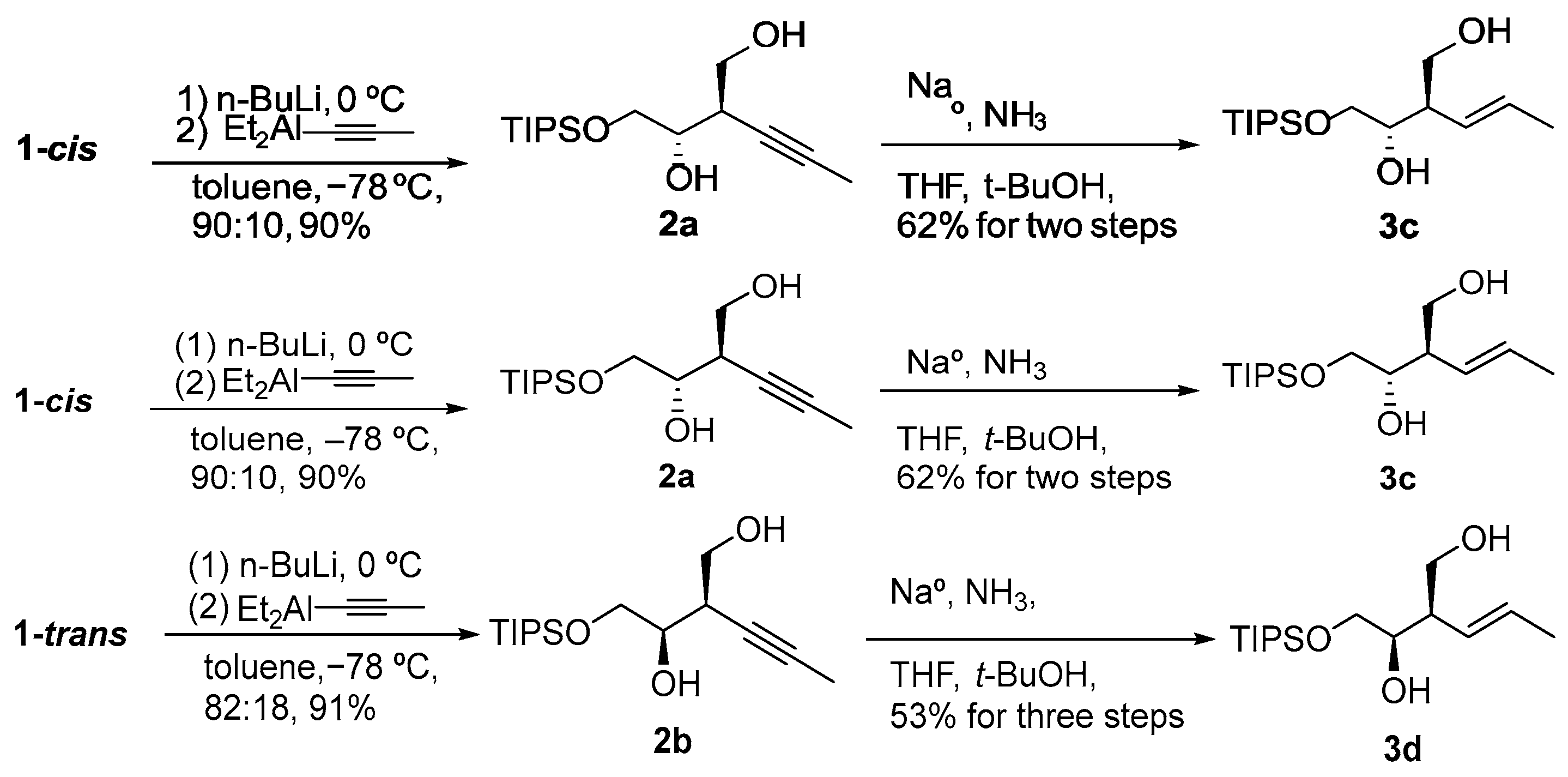
| Entry | Epoxide | Alkenyl Grignard a | Major Product | Regioselectivity b | Z/E Ratio c | % Yield d |
|---|---|---|---|---|---|---|
| entry 1 | 1-cis | 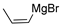 | 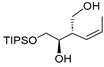 3a 3a | 95:5 | >95/5 | 66 |
| entry 2 | 1-trans | 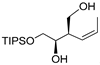 3b 3b | >95:5 | >95/5 | 88 | |
| entry 3 | 1-cis |  | 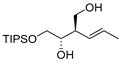 3c 3c | >95:5 | 34:66 | 42 |
| entry 4 | 1-trans | 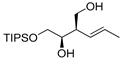 3d 3d | >95:5 | 37:63 | 63 | |
| entry 5 | 1-cis |  | 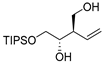 3e 3e | >95:5 | N/A | 81 |
| entry 6 | 1-trans | 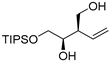 3f 3f | >95:5 | N/A | 51 | |
| entry 7 | 1-cis |  | 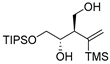 3g 3g | >95:5 | N/A | 54 |
| entry 8 | 1-trans | 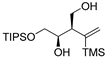 3h 3h | >95:5 | N/A | 46 |
| Entry | Diol | Product | Yield% | 13C (ppm) (CDCl3) | |
|---|---|---|---|---|---|
| Ketal carbon | Methyl carbons | ||||
| Entry 1 | 2a | 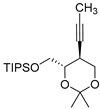 7 7 | 76 | 98.8 | 29.4, 19.0 |
| Entry 2 | 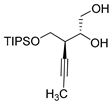 6 |  8 8 | 97 a | 108.8 | 26.5, 25.9 |
| Entry 3 | 3a | 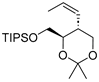 9 9 | 71 | 98.7 | 29.6, 19.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Berríos, R.R.; Prieto, J.A. Complementary Synthesis of Anti- and Syn-Hydroxymethyl 1,3-Diols via Regioselective Ring Opening of TIPS-Protected 2,3-Epoxy Alcohols: Toward Polypropionate Fragments. Organics 2025, 6, 29. https://doi.org/10.3390/org6030029
Rodríguez-Berríos RR, Prieto JA. Complementary Synthesis of Anti- and Syn-Hydroxymethyl 1,3-Diols via Regioselective Ring Opening of TIPS-Protected 2,3-Epoxy Alcohols: Toward Polypropionate Fragments. Organics. 2025; 6(3):29. https://doi.org/10.3390/org6030029
Chicago/Turabian StyleRodríguez-Berríos, Raúl R., and José A. Prieto. 2025. "Complementary Synthesis of Anti- and Syn-Hydroxymethyl 1,3-Diols via Regioselective Ring Opening of TIPS-Protected 2,3-Epoxy Alcohols: Toward Polypropionate Fragments" Organics 6, no. 3: 29. https://doi.org/10.3390/org6030029
APA StyleRodríguez-Berríos, R. R., & Prieto, J. A. (2025). Complementary Synthesis of Anti- and Syn-Hydroxymethyl 1,3-Diols via Regioselective Ring Opening of TIPS-Protected 2,3-Epoxy Alcohols: Toward Polypropionate Fragments. Organics, 6(3), 29. https://doi.org/10.3390/org6030029





